Summary
What is known
Currently, medication bottles with an electronic cap are frequently used to measure medication adherence. This system is termed medication event monitoring system (MEMS). To our knowledge, the optimal method to summarize data from MEMS has not yet been determined.
Objective
Look for best practices on how to quantify adherence data from MEMS.
Methods
Review of PubMed, Embase and Cochrane databases for the articles on medication adherence with MEMS.
Results
Of 1493 identified articles, 207 were included in this review. The MEMS cap was used for a median of 3 months (IQR: 4; range: 1 week to 24 months) in various health conditions. Many different outcome measures were used. Most studies computed an adherence score, expressed as the percentage of days on which the correct dose of medication was taken. The threshold to mark people as adherent was most frequently, arbitrarily, set at 80% (range: 67%‐95%). We found no data to support a specific threshold.
Discussion
Although the commonly used definition of adherence has face validity, we found no validation studies, and not all studies used the same cut‐off for adherence. Ideally, a cut‐off should be defined and validated in the context of the specific drug and its pharmacokinetic and dynamic characteristics, and perhaps other contextual factors, rather than generically. In addition, there was large heterogeneity in the definition of what “correct intake” of medication is.
What is new and conclusion
Outcome measures for MEMS data lacked standardization, and no demonstrable effort to validate any definition against a relevant clinical outcome is available. Consensus on the definition of adherence is urgently needed.
Keywords: adherence, literature review, outcome measures
Outcome measures for Medication Event Monitoring System (MEMS) data lacked standardization and no demonstrable effort to validate any definition against a relevant clinical outcome is available. A consensus effort on the definition of adherence is urgently needed.

1. WHAT IS KNOWN
Medication adherence can be measured noninvasively in different ways. These include questionnaires, pill counts and electronic monitoring1; medication bottles with an electronic cap are often seen as the preferred method to measure adherence.1 This method, termed medication event monitoring system (MEMS), comprises a cap that contains an electronic device which records the date and time of each opening and closing of the bottle.1 However, to our knowledge, there is no standard method to summarize the adherence data from MEMS.
2. OBJECTIVE
The rationale for this narrative literature review is to enable an informed choice on the preferred methods to summarize the adherence data from the currently running GLORIA trial.2 This trial, part of a project funded under the EU‐horizon 2020 programme, examines harm, benefit and costs of low‐dose glucocorticoids added to the standard treatment of rheumatoid arthritis patients of 65 years or older. Adherence is measured with MEMS throughout the trial.
In this literature review, the methods to summarize MEMS data will be described.
3. METHODS
3.1. Search strategy
A literature search was conducted in September and October 2016 in the databases, PubMed, Embase and Cochrane, and updated in July 2017. Search terms were related to the following main MESH search terms: medication (non)adherence/compliance, medication persistence, chronic disease/illness, chronically ill, medical electronics, treatment, (drug) therapy, data analysis and statistical study. For an additional search, the following terms were used: reminder system, smartphone and mobile/electronic app(lication). Synonyms of these search terms were also used. The main search terms and their synonyms were used in different combinations. Our search strategy is described in Appendix S1.
Study of title and abstract resulted in a first list of titles eligible for full‐text review. Articles not written in English or Dutch and those that did not describe electronic monitoring caps were excluded. All other articles were reviewed in full text. In this phase, articles lacking useful information for this review were excluded. A search of the reference lists of included articles did not provide extra articles.
An additional search was performed to find validation studies that compared the definitions that are used to summarize MEMS data.
3.2. Data extraction
One investigator (LH) extracted the following information: design of the study, sample size, mean age and health condition of participants, duration of monitoring and methods to calculate adherence (Appendix S2). Study results (eg, the effects of interventions on adherence) and the quality assessment of the study were not the object of study and thus not extracted.
4. RESULTS
4.1. Study selection
The search identified 1493 articles, of which 1127 off‐topic articles and 48 double entries were excluded after screening of title and abstract. Of the remainder, 71 articles were excluded because the subject was not about electronic monitoring (n = 34), the full text was not available (n = 29) or the article was not written in English or Dutch (n = 8). Of the 247 articles read in full, 40 contained no useful information for this review. In the end, 207 articles were included (see Figure 1 and Appendix S1). No validation studies were identified.
Figure 1.
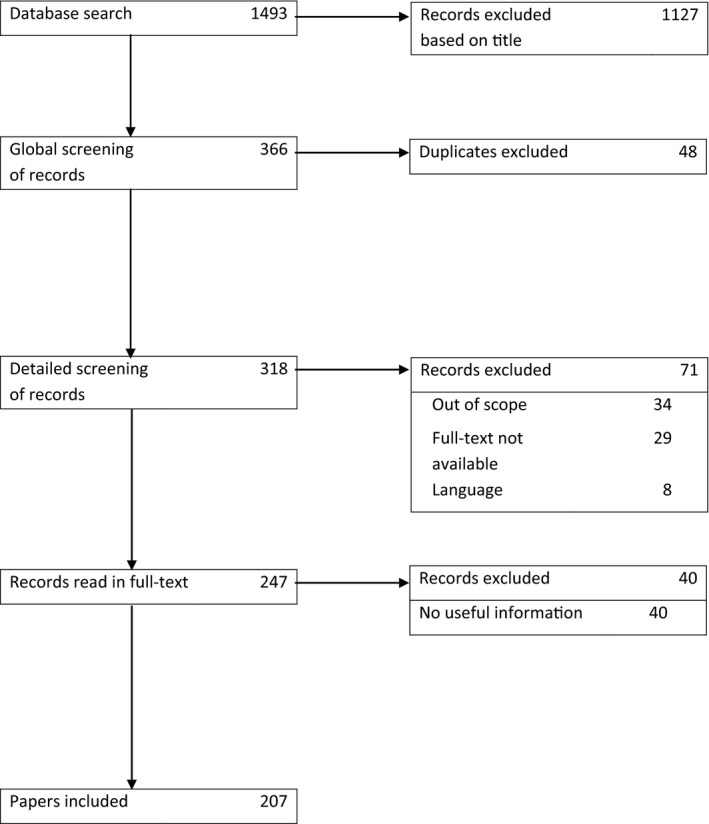
PRISMA flow diagram of article selection
4.2. Study characteristics
Most of the included studies had a prospective design; about one third was a randomized controlled trial. The sample size was a median of 83 patients (IQR: 106, range: 4‐3004). The mean age of the patients was 52 (SD 46) years, and 57% of them were male. A total of 62 different health conditions were studied. Most patients had HIV (29%) or heart failure (10%) (Table 1). The MEMS cap was used for a median of 3 months (IQR: 4; range: 1 week to 24 months).
Table 1.
Number of studies by health condition and adherence outcome measures
| Health condition | |
| HIV | 60 |
| Heart failure | 20 |
| Hypertension | 17 |
| Schizophrenia | 12 |
| Diabetes | 6 |
| Glaucoma | 6 |
| Depression | 5 |
| Type 2 diabetes and depression | 5 |
| Cancer | 4 |
| Kidney transplantation | 4 |
| Other conditions | |
| In 3 studies | 3 |
| In 2 studies | 10 |
| In 1 study | 39 |
| Adherence definitions | |
| Adherence score: percentage of days on which the correct dose was taken | 156 |
| In the week or month before the return date of the medication bottle | 3 |
| After intervention | 1 |
| Average change per month | 1 |
| Dose compliance: in drugs with multiple dosing on a day, the mean percentage of doses taken correctly per day | 48 |
| Timing compliance: percentage of doses taken at the appropriate time | 28 |
| Drug holiday: period of a certain number of days on which the medication bottle was not opened | 9 |
| Under (hypo‐)adherence: missing ≥10% of doses | 6 |
| Over (hyper‐)adherence: ≥10% more openings than expected | 5 |
| Dosing interval: the exact time between two openings (ie, doses) | 3 |
| Omissions: multiple missed doses | 2 |
| Noncompliance: percentage of skipped and extra doses | 1 |
| Patterns of missed doses: number of days without a dose, number of treatment interruptions lasting ≥48 hours, duration of the longest treatment interruption | 1 |
| Timing distribution index: indicates the regularity of the timing of drug intake | 1 |
4.3. Outcome measures
Medication event monitoring system systems can supply a wealth of information, including dates and times of openings, the intervals between two consecutive doses and a graph which presents the number of cap openings per day.3 The included studies reported several outcome measures (Table 1). Most studies computed an adherence score (n = 156), expressed as the percentage of days on which the correct dose of medication was taken.4 The choice of outcome measure was independent of the health condition (results not shown).
In 76 studies, a threshold was defined on the adherence score to mark people as adherent or nonadherent. The thresholds ranged from 67% to 95%, and in half of the studies, it was 80%; most frequently chosen alternatives included thresholds of 90% (n = 13), 88% (n = 8) and 95% (n = 8).
The dose compliance (ie, in drugs with multiple dosing on a day the mean percentage of doses taken correctly per day) and the timing compliance (ie, the percentage of doses taken at the appropriate time) were also calculated in several studies (in 14% and 23%, respectively). The time frame ranged from 2 to 4 hours in studies on glaucoma,5, 6, 7, 8 diabetes mellitus,9 HIV9, 10 and schizophrenia.11
A few studies (4%) calculated “drug holidays,” that is periods of a certain number of days on which the medication bottle was not opened, followed by a bottle opening.12, 13 In contrast, Israni et al excluded patients who had fewer than 14 days of usable adherence data.14 Olds et al considered MEMS data as missing if the bottle was not opened for a certain number of consecutive days.15
In some studies, multiple openings were counted as 1 opening if the bottle was opened several times within 1516 or 30 minutes17, 18 of the previous opening. Unexpected openings outside this time window were assumed to represent a taken dose.16
5. DISCUSSION
In this narrative literature review (the first to our knowledge), we looked for best practices on how to quantify adherence. We chose a broad scope, but were somewhat limited due to language restrictions and the unavailability of some studies.
The adherence score, that is the percentage of days correctly dosed, and its cut‐off of 80% were the most frequently used quantification and definition of sufficient adherence. Although this definition has face validity, we found no validation studies, and not all studies used this cut‐off. Ideally, a cut‐off should be defined and validated in the context of the specific drug and its pharmacokinetic and dynamic characteristics, and perhaps other contextual factors, rather than generically.
In addition, there was large heterogeneity in the definition of what “correct intake” is. This included definitions of the allowable time window between doses, overdosing and dealing with consecutive days with no bottle openings. Some studies mark these periods as drug holidays, whereas other studies consider these periods as missing data. Any definition should ideally be tested/validated against a clinically relevant outcome to be of use in the clinic. In addition, an array of definitions for adherence was used, indicating an urgent need for a consensus effort. Such efforts have been successful in rheumatology19 and are gaining traction in other fields.20
It is also remarkable that for so many health conditions, adherence studies with MEMS are rare or even nonexistent. Most studies were about HIV, heart failure, hypertension or schizophrenia. We did not find any relation between these health conditions and the methods that were used to summarize the MEMS data. MEMS is often seen as the reference standard to measure medication adherence, but it still assumes that one bottle opening equals the intake of one medication dose,3 a simplification that cannot be easily checked.16, 17, 18 For example, a patient could open the bottle and either not take any or more than the appropriate dose. Validation of MEMS data may become possible with compliance capsules with an ingestion sensor.21, 22 This is a new method, where the sensor signals when the drug is taken. Compliance capsules have the potential to become the new reference standard in the future.
6. WHAT IS NEW AND CONCLUSION
While adherence is clearly critical to treatment success, this review demonstrates a lack of consensus on a concrete working definition to be used in studies and no demonstrable effort to validate any one definition against a relevant clinical outcome. Progress in this field is unlikely unless these issues are addressed.
Supporting information
Hartman L, Lems WF, Boers M. Outcome measures for adherence data from a medication event monitoring system: A literature review. J Clin Pharm Ther. 2019;44:1–5. 10.1111/jcpt.12757
REFERENCES
- 1. El Alili M, Vrijens B, Demonceau J, Evers SM, Hiligsmann M. A scoping review of studies comparing the medication event monitoring system (MEMS) with alternative methods for measuring medication adherence. Br J Clin Pharmacol. 2016;82(1):268‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. ClinicalTrials.gov . The Glucocorticoid Low‐dose Outcome in RheumatoId Arthritis Study (Gloria) 2018. https://clinicaltrials.gov/ct2/show/NCT02585258. Accessed March 14, 2018.
- 3. Bachmann LH, Stephens J, Richey CM, Hook EW 3rd. Measured versus self‐reported compliance with doxycycline therapy for chlamydia‐associated syndromes: high therapeutic success rates despite poor compliance. Sex Transm Dis. 1999;26(5):272‐278. [DOI] [PubMed] [Google Scholar]
- 4. Eby J, Chapman J, Marukutira T, et al. The adherence‐outcome relationship is not altered by diary‐driven adjustments of microelectronic monitor data. Pharmacoepidemiol Drug Saf. 2015;24(12):1313‐1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barker GT, Cook PF, Schmiege SJ, Kahook MY, Kammer JA, Mansberger SL. Psychometric properties of the Glaucoma Treatment Compliance Assessment Tool in a multicenter trial. Am J Ophthalmol. 2015;159(6): 1092–1099 e2. [DOI] [PubMed] [Google Scholar]
- 6. Cook PF, Schmiege SJ, Mansberger SL, et al. Motivational interviewing or reminders for glaucoma medication adherence? Results of a multi‐site randomized controlled trial. Psychol Health. 2017;32(2):145–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cook PF, Schmiege SJ, Mansberger SL, Kammer J, Fitzgerald T, Kahook MY. Predictors of adherence to glaucoma treatment in a multisite study. Ann Behav Med. 2015;49(1):29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boland MV, Chang DS, Frazier T, Plyler R, Friedman DS. Electronic monitoring to assess adherence with once‐daily glaucoma medications and risk factors for nonadherence: the automated dosing reminder study. JAMA Ophthalmol. 2014;132(7):838–844. [DOI] [PubMed] [Google Scholar]
- 9. Rosen MI, Rigsby MO, Salahi JT, Ryan CE, Cramer JA. Electronic monitoring and counseling to improve medication adherence. Behav Res Ther. 2004;42(4):409–422. [DOI] [PubMed] [Google Scholar]
- 10. Magidson JF. Depressive symptoms, lifestyle structure, and art adherence among hiv‐infected individuals: a longitudinal mediation analysis. AIDS Behav. 2015;19(1):34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang J, Ko YH, Paik JW, et al. Symptom severity and attitudes toward medication: impacts on adherence in outpatients with schizophrenia. Schizophr Res. 2012;134(2–3):226–231. [DOI] [PubMed] [Google Scholar]
- 12. Santschi V, Wuerzner G, Schneider MP, Bugnon O, Burnier M. Clinical evaluation of IDAS II, a new electronic device enabling drug adherence monitoring. Eur J Clin Pharmacol. 2007;63(12):1179–1184. [DOI] [PubMed] [Google Scholar]
- 13. Deschamps AE, Graeve VD, van Wijngaerden E, et al. Prevalence and correlates of nonadherence to antiretroviral therapy in a population of HIV patients using Medication Event Monitoring System. AIDS Patient Care STDS. 2004;18(11):644–657. [DOI] [PubMed] [Google Scholar]
- 14. Israni AK, Weng FL, Cen YY, Joffe M, Kamoun M, Feldman HI. Electronically measured adherence to immunosuppressive medications and kidney function after deceased donor kidney transplantation. Clin Transplant. 2011;25(2):E124–E131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Olds PK, Kiwanuka JP, Nansera D, et al. Assessment of HIV antiretroviral therapy adherence by measuring drug concentrations in hair among children in rural Uganda. AIDS Care. 2015;27(3):327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Winkler A, Teuscher AU, Mueller B, Diem P. Monitoring adherence to prescribed medication in type 2 diabetic patients treated with sulfonylureas. Swiss Med Wkly. 2002;132(27–28):379–385. [DOI] [PubMed] [Google Scholar]
- 17. Lee JY, Kusek JW, Greene PG, et al. Assessing medication adherence by pill count and electronic monitoring in the African American Study of Kidney Disease and Hypertension (AASK) Pilot Study. Am J Hypertens. 1996;9(8):719–725. [DOI] [PubMed] [Google Scholar]
- 18. Alinia H, Moradi Tuchayi S, Smith JA, et al. Long‐term adherence to topical psoriasis treatment can be abysmal: a one‐year randomized intervention study using objective electronic adherence monitoring. Br J Dermatol. 2016;176(3):759–764. [DOI] [PubMed] [Google Scholar]
- 19. Tugwell P, Boers M, Brooks P, Simon L, Strand V, Idzerda L. OMERACT: an international initiative to improve outcome measurement in rheumatology. Trials. 2007;8:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gargon E, Williamson PR, Altman DG, Blazeby JM, Clarke M. The COMET Initiative database: progress and activities from 2011 to 2013. Trials. 2014;15:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chai PR, Carreiro S, Innes BJ, et al. Oxycodone ingestion patterns in acute fracture pain with digital pills. Anesth Analg. 2017;125(6):2105–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Browne SH, Peloquin C, Santillo F, et al. Digitizing medicines for remote capture of oral medication adherence using co‐encapsulation. Clin Pharmacol Ther. 2018;103(3):502–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


