Abstract
Objectives
To evaluate the long‐term safety (primary objective) and efficacy (secondary objective) of antimuscarinic add‐on therapy in patients receiving mirabegron.
Methods
During a 2‐week screening period, patients (aged ≥20 years, mirabegron treatment for ≥6 weeks, residual overactive bladder symptoms) received mirabegron 50 mg once daily. These patients were subsequently randomized to 52 weeks’ treatment with mirabegron 50 mg/day plus an antimuscarinic (solifenacin 5 mg, propiverine 20 mg, imidafenacin 0.2 mg, or tolterodine 4 mg) with the potential to double the antimuscarinic dose (except for tolterodine) at week 8. Safety assessments included treatment‐emergent adverse events, vital signs, 12‐lead electrocardiograms, post‐void residual volume, and laboratory evaluations. Efficacy was assessed using changes from baseline in overactive bladder symptom score total score; overactive bladder questionnaire short form score; micturitions, urgency episodes, urinary incontinence episodes, and urgency urinary incontinence episodes/24 h; mean volume voided per micturition; and number of night‐time micturitions.
Results
Overall, 80.2% of patients (88.1% women, mean age 65 years) experienced at least one treatment‐emergent adverse event, with similar rates for all treatments. The adverse events most commonly reported were dry mouth, nasopharyngitis, and constipation. No marked change was observed in systolic or diastolic blood pressure for any treatment, although pulse rate increased slightly in the mirabegron and propiverine, and mirabegron and tolterodine groups. For all treatments, significant improvements were observed in all efficacy parameters, including overactive bladder symptom score total and questionnaire short form scores.
Conclusions
Antimuscarinic add‐on therapy is well tolerated and effective after initial treatment with mirabegron in patients with overactive bladder symptoms.
Keywords: β3‐adrenoreceptor agonist, antimuscarinic therapy, combination therapy, mirabegron, overactive bladder
Abbreviations & Acronyms
- DBP
diastolic blood pressure
- ECG
electrocardiogram
- EoT
end of treatment
- FAS
full analysis set
- HRQoL
health‐related quality of life
- IMI
imidafenacin
- MIRA
mirabegron
- MVV
mean volume voided
- OAB
overactive bladder
- OAB‐q SF
overactive bladder questionnaire short form
- OABSS
overactive bladder symptom score
- PRO
propiverine
- PVR
post‐void residual
- QTcF
QT interval corrected for heart rate by Fridericia's formula
- SAF
safety analysis set
- SBP
systolic blood pressure
- SD
standard deviation
- SOLI
solifenacin
- TEAE
treatment‐emergent adverse event
- TOL
tolterodine
Introduction
OAB is characterized by urinary urgency, with or without urgency urinary incontinence, usually with increased daytime frequency and nocturia.1 The syndrome is known to have a substantial impact on HRQoL and rates of depression.2
Although antimuscarinics are the cornerstone of pharmacotherapy for OAB symptoms, they are associated with specific anticholinergic side‐effects, including dry mouth and constipation.3 Therefore, therapeutics that do not show these drawbacks could improve patient well being.
The β3‐adrenoreceptor agonist, MIRA, has a distinct mechanism of action from antimuscarinics4, 5 and is therefore a potential alternative treatment for OAB symptoms. The efficacy and safety of MIRA has been proved in several phase III clinical trials.6, 7 Additional studies have shown that MIRA appears to be as effective as antimuscarinics and shows a lower incidence of drug‐related TEAEs.8, 9 One‐year persistence rates of up to 66.0% have been reported, with improved persistence and adherence versus antimuscarinic therapy.10, 11
International urological associations recommend MIRA and antimuscarinics for treating patients with OAB symptoms.12, 13 However, even when favorable results are achieved in clinical studies, poor responses might be noted in the real‐world setting.14 Poor responders to initial treatment might achieve an improved outcome if they subsequently receive MIRA and an antimuscarinic in combination.
The favorable efficacy and safety profile of MIRA add‐on therapy was shown in patients with OAB symptoms who did not respond to initial SOLI treatment in the MILAI and BESIDE studies.15, 16 Alternatively, if MIRA is used as first‐line treatment, antimuscarinic add‐on therapy could be considered in patients experiencing a suboptimal response to MIRA.
Previous combination studies either involved add‐on treatment with MIRA15, 16 or concurrent use of both therapeutics17, 18 over a 12–16 week treatment period.15, 16, 17, 18 However, MIRA and antimuscarinics in combination might be used for long periods in clinical practice. Prolonged combination therapy might be associated with specific cumulative or delayed events and might have an additive effect on certain TEAEs. Therefore, the objectives of this MILAI II study were to evaluate the long‐term safety (primary objective) and efficacy (secondary objective) of antimuscarinic add‐on therapy to MIRA over 52 weeks in patients with OAB symptoms in Japan. The antimuscarinics investigated were the four main therapeutics used in Japan when the study was planned (SOLI, PRO, IMI, and TOL).
Methods
Study design
This was a multicenter, randomized, open‐label, phase IV study in patients with OAB symptoms treated with MIRA (ClinicalTrials.gov: NCT02294396) that was carried out from October 2014 to September 2016 at 60 sites in Japan (Fig. 1).
Figure 1.
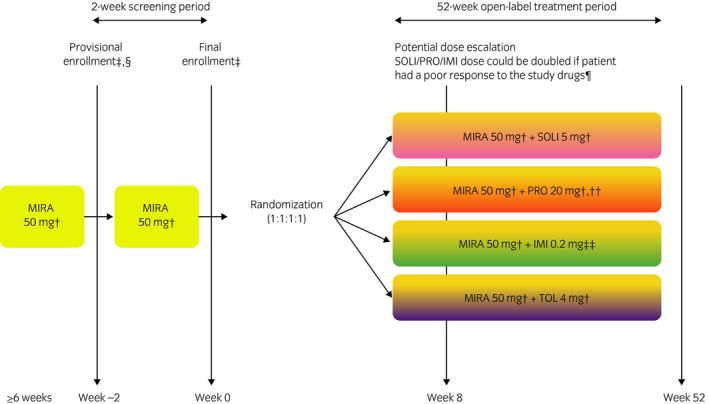
Study design. †Once daily. †Eligibility criteria were verified. §Informed consent was obtained. ¶Furthermore, the patient was considered by the investigator to have no safety concerns and agreed to the increased dose (in the event of a TEAE, the dose could be reduced to the initial dosage). ††If the PRO dose was doubled, patients received a 20‐mg dose twice daily. ††Twice daily (total daily dose shown).
The study was carried out in accordance with the Declaration of Helsinki and International Council for Harmonisation guidelines. The protocol was approved by the institutional review board for each site and all patients provided written informed consent.
Study duration was 54 weeks; a 2‐week screening period and a 52‐week treatment period. Eligible patients were aged ≥20 years, had received previous treatment with MIRA 50 mg for ≥6 weeks, and had residual OAB symptoms (OABSS total score ≥3 points, OABSS question 3 score ≥2 points). Full inclusion and exclusion criteria are in Table S1.
During screening, eligible patients received oral MIRA 50 mg once daily after breakfast. Using MIRA as a first choice therapeutic was based on daily clinical practice in Japan. Patients meeting the final eligibility criteria were subsequently randomized to receive a combination of MIRA 50 mg/day with SOLI 5 mg/day, PRO 20 mg/day, IMI 0.2 mg/day, or extended‐release TOL 4 mg/day for 52 weeks (1:1:1:1 ratio). All treatments were taken orally once daily after breakfast, except for IMI which was also taken after dinner. At week 8, the dose of SOLI, PRO, or IMI could be doubled (if a patient had a poor response to the study drugs, was considered by the investigator to have no safety concerns, and agreed to the dose increase [TOL dose could not be increased because of prescribing restrictions]). If a TEAE developed after the dose increase, the dose could be reduced to the original level at the investigator's discretion. Subsequent dose re‐escalations were not permitted.
Study assessments
Safety (primary objective) was assessed throughout the study using TEAEs; vital signs, measured by patients on awakening and 6 h post‐dose; 12‐lead ECGs, including QTcF measurements; PVR volume; and laboratory evaluations.
Efficacy assessments (secondary objective) included change from baseline in OABSS total score; OAB‐q SF score; micturitions, urgency episodes, urinary incontinence episodes, and urgency urinary incontinence episodes/24 h; MVV per micturition; and number of night‐time micturitions. Patients completed a paper micturition diary for the 3 days before each site visit. The diary included data on the number of micturitions, urgency episodes and urgency urinary incontinence episodes, and volume voided per micturition. Efficacy assessments were carried out at baseline; weeks 4, 8, 12, 16, 28, 40, and 52; and at EoT, except for the OABSS (no week 40) and the OAB‐q SF (only baseline; weeks 12, 28, and 52; and EoT).
Statistical analysis
The target number of patients was determined to be 150 in each group (600 patients altogether). This took into account the estimated number of patients discontinuing from the study during treatment.19 Randomization was carried out by the registration center (Bell Medical Solutions, Inc., Tokyo, Japan). Before treatment initiation, the site staff contacted the registration center to determine the treatment allocation.
Safety and demographic data were evaluated using the SAF (patients who received at least one dose of study drug). Efficacy data were evaluated using the FAS (patients who received at least one dose of study drug and provided data for at least one variable before and after treatment initiation).
Categorical data were summarized by the number and percentage of patients; descriptive statistics were used to analyze continuous variables. For efficacy variables, changes from baseline were evaluated using a one‐sample t‐test. Owing to the different nature of the antimuscarinics, it was judged that direct statistical comparison of the effectiveness of the combination regimens was not appropriate.
Results
Study population
Overall, 730 patients entered screening, of whom 649 were randomized and 647 were included in both the SAF and the FAS (Fig. 2). Most patients were women (570 [88.1%] patients), with a mean age of 65 years, and a mean OAB duration of 77.2 months (Table 1). All treatment groups were generally similar regarding patient demographics and baseline characteristics.
Figure 2.
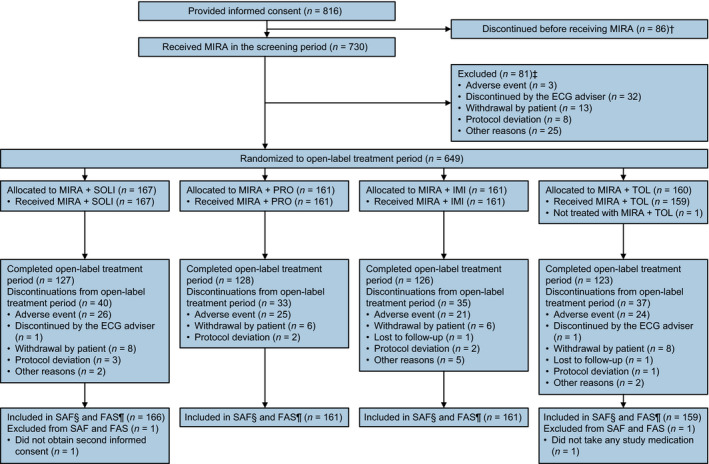
Patient disposition. †Patients who signed informed consent but discontinued before study treatment were defined as screen failures. †Patients who completed screening but discontinued before randomization were defined as run‐in failures. §Patients who received at least one dose of study drug. ¶Patients who received at least one dose of study drug and provided data for at least one variable before and after treatment initiation.
Table 1.
Patient demographics and baseline characteristics
| Variable | MIRA + SOLI (n = 166) | MIRA + PRO (n = 161) | MIRA + IMI (n = 161) | MIRA + TOL (n = 159) | Total (n = 647) |
|---|---|---|---|---|---|
| Sex, n (%) | |||||
| Male | 20 (12.0) | 17 (10.6) | 15 (9.3) | 25 (15.7) | 77 (11.9) |
| Female | 146 (88.0) | 144 (89.4) | 146 (90.7) | 134 (84.3) | 570 (88.1) |
| Age in years | |||||
| Mean (SD, range) | 64.6 (9.4, 45–89) | 64.0 (9.3, 42–82) | 65.7 (8.7, 47–85) | 65.7 (10.0, 40–85) | 65.0 (9.4, 40–89) |
| Age group, n (%) | |||||
| <65 years | 86 (51.8) | 82 (50.9) | 65 (40.4) | 65 (40.9) | 298 (46.1) |
| ≥65 years | 80 (48.2) | 79 (49.1) | 96 (59.6) | 94 (59.1) | 349 (53.9) |
| Duration of OAB in months | |||||
| Mean (SD) [n] | 69.3 (68.2) [162] | 78.8 (88.9) [158] | 83.3 (94.2) [156] | 77.9 (85.8) [155] | 77.2 (84.7) [631] |
| Median (range) | 49.0 (1–334) | 53.0 (1–602) | 59.0 (1–545) | 55.0 (1–565) | 55.0 (1–602) |
| Status of urinary incontinence, n (%) | |||||
| Absent | 35 (21.1) | 25 (15.5) | 16 (9.9) | 22 (13.8) | 98 (15.1) |
| Urgency urinary incontinence | 99 (59.6) | 100 (62.1) | 96 (59.6) | 91 (57.2) | 386 (59.7) |
| Mixed urinary incontinence | 32 (19.3) | 36 (22.4) | 49 (30.4) | 46 (28.9) | 163 (25.2) |
| OAB severity (mean no. micturitions at baseline), n (%) | |||||
| <10 | 85 (51.2) | 76 (47.2) | 85 (52.8) | 79 (49.7) | 325 (50.2) |
| ≥10 to <15 | 74 (44.6) | 74 (46.0) | 64 (39.8) | 69 (43.4) | 281 (43.4) |
| ≥15 | 7 (4.2) | 11 (6.8) | 12 (7.5) | 11 (6.9) | 41 (6.3) |
| Urinary incontinence episodes at baseline, n (%) | |||||
| No | 75 (45.2) | 66 (41.0) | 58 (36.0) | 63 (39.6) | 262 (40.5) |
| Yes | 91 (54.8) | 95 (59.0) | 103 (64.0) | 96 (60.4) | 385 (59.5) |
| OABSS total score, mean (SD) | 7.4 (2.6) | 7.7 (2.5) | 7.8 (2.3) | 7.7 (2.3) | 7.6 (2.4) |
| OAB‐q SF symptom severity score, mean (SD) | 32.81 (20.78) | 32.36 (21.23) | 32.92 (19.45) | 34.23 (22.60) | 33.08 (21.00) |
| OAB‐q SF total HRQoL score, mean (SD) | 75.16 (17.65) | 77.36 (16.11) | 74.85 (18.50) | 75.37 (19.33) | 75.68 (17.91) |
| Micturitions/24 h, mean (SD) | 10.06 (2.59) | 10.37 (2.65) | 10.13 (2.92) | 10.20 (2.62) | 10.19 (2.69) |
| Urgency episodes/24 h, mean (SD) [n] | 3.26 (2.46) [153] | 3.12 (2.67) [148] | 3.27 (2.20) [150] | 3.15 (2.54) [148] | 3.20 (2.47) [599] |
| Urinary incontinence episodes/24 h, mean (SD) [n] | 1.62 (1.62) [91] | 1.59 (1.83) [95] | 1.47 (1.35) [103] | 1.55 (1.76) [96] | 1.56 (1.64) [385] |
| Urgency urinary incontinence episodes/24 h, mean (SD) [n] | 1.55 (1.47) [80] | 1.39 (1.45) [82] | 1.30 (1.16) [86] | 1.31 (1.62) [85] | 1.38 (1.43) [333] |
| MVV in mL/micturition, mean (SD) | 166.600 (50.404) | 170.064 (63.781) | 169.309 (50.324) | 167.542 (54.320) | 168.368 (54.839) |
| Night‐time micturitions, mean (SD) [n] | 1.50 (0.96) [142] | 1.68 (1.08) [133] | 1.61 (1.29) [141] | 1.67 (1.04) [132] | 1.61 (1.10) [548] |
Data shown for the FAS (patients who received at least one dose of study drug and provided data for at least one variable before and after treatment initiation).
At week 8, most patients (595 [92.0%] patients) did not have their antimuscarinic dose increased. Dose increases were administered to 15 (9.0%) MIRA and SOLI patients, 15 (9.3%) MIRA and PRO patients, and 22 (13.7%) MIRA and IMI patients. Seven (4.2%), four (2.5%), and five (3.1%) patients from the SOLI, PRO, and IMI groups, respectively, had their dose decreased back to the initial dosage.
Safety
Overall, 519 (80.2%) patients experienced at least one TEAE (Table 2). Furthermore, 303 (46.8%) patients experienced at least one drug‐related TEAE with similar incidences for all groups. Drug‐related TEAEs leading to treatment withdrawal occurred in 47 (7.3%) patients; all occurrences were mild or moderate in severity.
Table 2.
TEAEs occurring during the course of the study
| TEAE, n (%) | Drug‐related TEAE, n (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIRA + SOLI (n = 166) | MIRA + PRO (n = 161) | MIRA + IMI (n = 161) | MIRA + TOL (n = 159) | Total (n = 647) | MIRA + SOLI (n = 166) | MIRA + PRO (n = 161) | MIRA + IMI (n = 161) | MIRA + TOL (n = 159) | Total (n = 647) | |
| TEAE | 131 (78.9) | 135 (83.9) | 133 (82.6) | 120 (75.5) | 519 (80.2) | 76 (45.8) | 81 (50.3) | 72 (44.7) | 74 (46.5) | 303 (46.8) |
| Serious TEAE | 10 (6.0) | 4 (2.5) | 5 (3.1) | 9 (5.7) | 28 (4.3) | 0 | 1 (0.6) | 0 | 1 (0.6) | 2 (0.3) |
| TEAE leading to withdrawal of treatment | 23 (13.9) | 19 (11.8) | 16 (9.9) | 18 (11.3) | 76 (11.7) | 12 (7.2) | 17 (10.6) | 10 (6.2) | 8 (5.0) | 47 (7.3) |
| TEAE leading to death | 0 | 0 | 0 | 1 (0.6) | 1 (0.2) | 0 | 0 | 0 | 0 | 0 |
| TEAEs (≥3.0% for any group) | ||||||||||
| Dry mouth | 32 (19.3) | 51 (31.7) | 40 (24.8) | 40 (25.2) | 163 (25.2) | 31 (18.7) | 51 (31.7) | 40 (24.8) | 40 (25.2) | 162 (25.0) |
| Nasopharyngitis | 35 (21.1) | 39 (24.2) | 34 (21.1) | 32 (20.1) | 140 (21.6) | 0 | 0 | 0 | 0 | 0 |
| Constipation | 37 (22.3) | 27 (16.8) | 24 (14.9) | 19 (11.9) | 107 (16.5) | 33 (19.9) | 26 (16.1) | 23 (14.3) | 18 (11.3) | 100 (15.5) |
| Cystitis | 18 (10.8) | 13 (8.1) | 25 (15.5) | 10 (6.3) | 66 (10.2) | 0 | 0 | 4 (2.5) | 0 | 4 (0.6) |
| Dysuria | 8 (4.8) | 4 (2.5) | 3 (1.9) | 7 (4.4) | 22 (3.4) | 8 (4.8) | 4 (2.5) | 3 (1.9) | 7 (4.4) | 22 (3.4) |
| Back pain | 3 (1.8) | 5 (3.1) | 6 (3.7) | 4 (2.5) | 18 (2.8) | 0 | 0 | 0 | 0 | 0 |
| Contusion | 4 (2.4) | 6 (3.7) | 3 (1.9) | 5 (3.1) | 18 (2.8) | 0 | 0 | 0 | 0 | 0 |
| Abdominal discomfort | 7 (4.2) | 4 (2.5) | 4 (2.5) | 2 (1.3) | 17 (2.6) | 5 (3.0) | 2 (1.2) | 3 (1.9) | 2 (1.3) | 12 (1.9) |
| Eczema | 5 (3.0) | 2 (1.2) | 6 (3.7) | 4 (2.5) | 17 (2.6) | 1 (0.6) | 0 | 1 (0.6) | 0 | 2 (0.3) |
| Gastroesophageal reflux disease | 2 (1.2) | 6 (3.7) | 4 (2.5) | 5 (3.1) | 17 (2.6) | 0 | 3 (1.9) | 2 (1.2) | 3 (1.9) | 8 (1.2) |
| Residual urine volume increased | 6 (3.6) | 8 (5.0) | 1 (0.6) | 2 (1.3) | 17 (2.6) | 6 (3.6) | 7 (4.3) | 1 (0.6) | 2 (1.3) | 16 (2.5) |
| Pharyngitis | 6 (3.6) | 4 (2.5) | 4 (2.5) | 2 (1.3) | 16 (2.5) | 0 | 0 | 0 | 0 | 0 |
| Dermatitis contact | 3 (1.8) | 7 (4.3) | 2 (1.2) | 2 (1.3) | 14 (2.2) | 0 | 0 | 0 | 0 | 0 |
| Gastroenteritis | 3 (1.8) | 2 (1.2) | 5 (3.1) | 2 (1.3) | 12 (1.9) | 0 | 0 | 0 | 0 | 0 |
| Osteoarthritis | 5 (3.0) | 2 (1.2) | 3 (1.9) | 2 (1.3) | 12 (1.9) | 0 | 0 | 0 | 0 | 0 |
| Gastritis | 2 (1.2) | 5 (3.1) | 4 (2.5) | 0 | 11 (1.7) | 0 | 1 (0.6) | 2 (1.2) | 0 | 3 (0.5) |
| Headache | 5 (3.0) | 4 (2.5) | 0 | 2 (1.3) | 11 (1.7) | 2 (1.2) | 2 (1.2) | 0 | 1 (0.6) | 5 (0.8) |
| Vomiting | 1 (0.6) | 2 (1.2) | 5 (3.1) | 3 (1.9) | 11 (1.7) | 0 | 0 | 1 (0.6) | 0 | 1 (0.2) |
| Dizziness | 2 (1.2) | 5 (3.1) | 0 | 3 (1.9) | 10 (1.5) | 1 (0.6) | 1 (0.6) | 0 | 1 (0.6) | 3 (0.5) |
| Dental caries | 0 | 6 (3.7) | 3 (1.9) | 0 | 9 (1.4) | 0 | 0 | 0 | 0 | 0 |
| ECG QT prolonged | 1 (0.6) | 2 (1.2) | 5 (3.1) | 1 (0.6) | 9 (1.4) | 1 (0.6) | 2 (1.2) | 4 (2.5) | 1 (0.6) | 8 (1.2) |
| Osteoporosis | 1 (0.6) | 5 (3.1) | 2 (1.2) | 1 (0.6) | 9 (1.4) | 0 | 0 | 0 | 0 | 0 |
| Cataract | 1 (0.6) | 1 (0.6) | 1 (0.6) | 5 (3.1) | 8 (1.2) | 0 | 0 | 0 | 0 | 0 |
| Dyspepsia | 0 | 5 (3.1) | 1 (0.6) | 1 (0.6) | 7 (1.1) | 0 | 2 (1.2) | 1 (0.6) | 1 (0.6) | 4 (0.6) |
Data shown for the SAF (patients who received at least one dose of study drug).
In total, 28 (4.3%) patients reported at least one serious TEAE. Two serious TEAEs were considered by the investigator to be possibly drug‐related. One patient treated with MIRA and PRO experienced a serious TEAE of atrial fibrillation, which resolved 10 days after treatment withdrawal. One patient treated with MIRA and TOL reported a serious TEAE of colitis ischemic, which resolved 23 days after treatment interruption and did not recur after restarting treatment. Another patient treated with MIRA and TOL died after a serious TEAE of acute respiratory distress syndrome. This event was considered to be unrelated to study treatment, as it occurred during drug withdrawal.
The most commonly reported TEAEs were dry mouth (163 [25.2%] patients), nasopharyngitis (140 [21.6%] patients), and constipation (107 [16.5%] patients). Compared with the other regimens, slightly higher incidences of dry mouth and constipation were observed in the MIRA and PRO and MIRA and SOLI groups, respectively. For drug‐related TEAEs, the most commonly reported events were dry mouth (162 [25.0%] patients) and constipation (100 [15.5%] patients). Time‐dependent changes were apparent in the prevalence of some TEAEs, with an overall higher prevalence in the earlier part of the study. In particular, dry mouth, constipation, dysuria, and residual urine volume increased were more commonly reported in the earlier stages of the study. There was no trend in the time onset of the other TEAEs (Table 3).
Table 3.
First onset of TEAEs (≥3.0% for any group) by time interval
| Time interval (days) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ≥1 to <7 (n = 647) | ≥7 to <14 (n = 645) | ≥14 to <28 (n = 642) | ≥28 to <56 (n = 628) | ≥56 to <84 (n = 598) | ≥84 to <112 (n = 579) | ≥112 to <196 (n = 561) | ≥196 to <280 (n = 538) | ≥280 to <365 (n = 517) | ≥365 (n = 129) | |
| Overall TEAEs, n (%) | 96 (14.8) | 38 (5.9) | 51 (7.9) | 107 (17.0) | 57 (9.5) | 33 (5.7) | 63 (11.2) | 46 (8.6) | 25 (4.8) | 3 (2.3) |
| Dry mouth | 70 (10.8) | 14 (2.2) | 9 (1.4) | 30 (4.8) | 17 (2.8) | 9 (1.6) | 7 (1.2) | 6 (1.1) | 1 (0.2) | 0 |
| Nasopharyngitis | 1 (0.2) | 4 (0.6) | 11 (1.7) | 17 (2.7) | 14 (2.3) | 6 (1.0) | 24 (4.3) | 37 (6.9) | 26 (5.0) | 0 |
| Constipation | 13 (2.0) | 13 (2.0) | 17 (2.6) | 20 (3.2) | 17 (2.8) | 8 (1.4) | 13 (2.3) | 4 (0.7) | 2 (0.4) | 0 |
| Cystitis | 1 (0.2) | 0 | 3 (0.5) | 4 (0.6) | 10 (1.7) | 5 (0.9) | 17 (3.0) | 17 (3.2) | 8 (1.5) | 1 (0.8) |
| Dysuria | 6 (0.9) | 2 (0.3) | 2 (0.3) | 4 (0.6) | 4 (0.7) | 1 (0.2) | 2 (0.4) | 1 (0.2) | 0 | 0 |
| Back pain | 0 | 0 | 0 | 1 (0.2) | 2 (0.3) | 1 (0.2) | 6 (1.1) | 5 (0.9) | 3 (0.6) | 0 |
| Contusion | 0 | 0 | 2 (0.3) | 3 (0.5) | 2 (0.3) | 0 | 3 (0.5) | 5 (0.9) | 3 (0.6) | 0 |
| Abdominal discomfort | 1 (0.2) | 3 (0.5) | 0 | 1 (0.2) | 3 (0.5) | 1 (0.2) | 4 (0.7) | 2 (0.4) | 2 (0.4) | 0 |
| Eczema | 2 (0.3) | 0 | 0 | 4 (0.6) | 1 (0.2) | 1 (0.2) | 4 (0.7) | 3 (0.6) | 2 (0.4) | 0 |
| Gastroesophageal reflux disease | 2 (0.3) | 1 (0.2) | 0 | 0 | 2 (0.3) | 1 (0.2) | 2 (0.4) | 7 (1.3) | 2 (0.4) | 0 |
| Residual urine volume increased | 1 (0.2) | 0 | 3 (0.5) | 10 (1.6) | 2 (0.3) | 0 | 1 (0.2) | 0 | 0 | 0 |
| Pharyngitis | 1 (0.2) | 0 | 2 (0.3) | 0 | 2 (0.3) | 1 (0.2) | 3 (0.5) | 4 (0.7) | 3 (0.6) | 0 |
| Dermatitis contact | 0 | 0 | 0 | 2 (0.3) | 1 (0.2) | 1 (0.2) | 5 (0.9) | 5 (0.9) | 0 | 0 |
| Gastroenteritis | 1 (0.2) | 1 (0.2) | 1 (0.2) | 0 | 0 | 0 | 4 (0.7) | 3 (0.6) | 2 (0.4) | 0 |
| Osteoarthritis | 0 | 0 | 1 (0.2) | 1 (0.2) | 0 | 0 | 5 (0.9) | 2 (0.4) | 3 (0.6) | 0 |
| Gastritis | 0 | 0 | 0 | 2 (0.3) | 0 | 1 (0.2) | 3 (0.5) | 3 (0.6) | 2 (0.4) | 0 |
| Headache | 3 (0.5) | 0 | 1 (0.2) | 1 (0.2) | 2 (0.3) | 2 (0.3) | 1 (0.2) | 0 | 1 (0.2) | 0 |
| Vomiting | 0 | 1 (0.2) | 1 (0.2) | 0 | 2 (0.3) | 4 (0.7) | 3 (0.5) | 0 | 0 | 0 |
| Dizziness | 1 (0.2) | 0 | 0 | 1 (0.2) | 1 (0.2) | 1 (0.2) | 3 (0.5) | 2 (0.4) | 1 (0.2) | 0 |
| Dental caries | 0 | 0 | 0 | 2 (0.3) | 2 (0.3) | 0 | 1 (0.2) | 2 (0.4) | 2 (0.4) | 0 |
| ECG QT prolonged | 0 | 0 | 0 | 4 (0.6) | 1 (0.2) | 1 (0.2) | 3 (0.5) | 0 | 0 | 0 |
| Osteoporosis | 0 | 0 | 1 (0.2) | 0 | 1 (0.2) | 1 (0.2) | 2 (0.4) | 2 (0.4) | 2 (0.4) | 0 |
| Cataract | 0 | 0 | 0 | 1 (0.2) | 0 | 0 | 5 (0.9) | 1 (0.2) | 1 (0.2) | 0 |
| Dyspepsia | 1 (0.2) | 2 (0.3) | 0 | 1 (0.2) | 0 | 0 | 1 (0.2) | 2 (0.4) | 0 | 0 |
Data shown for the SAF (patients who received at least one dose of study drug).
For pulse rate, no marked change from baseline to EoT was observed for any combination (Table 4). Over the treatment period, pulse rate remained constant in the MIRA and SOLI and MIRA and IMI groups and increased slightly in the MIRA and PRO and MIRA and TOL groups (Fig. S1). No marked change from baseline to EoT was observed in SBP or DBP for any group (Figs S2,S3).
Table 4.
Vital sign ECG, and PVR volume results
| Variable | MIRA + SOLI (n = 166) | MIRA + PRO (n = 161) | MIRA + IMI (n = 161) | MIRA + TOL (n = 159) |
|---|---|---|---|---|
| Vital signs | ||||
| Pulse rate in b.p.m. on awakening, mean (SD) [n] | ||||
| Baseline | 70.07 (8.15) [166] | 69.15 (8.19) [161] | 69.17 (7.25) [160] | 68.51 (8.15) [159] |
| EoT | 70.68 (7.55) [159] | 72.35 (9.68) [155] | 69.26 (6.76) [157] | 70.64 (8.39) [156] |
| Change from baseline to EoT | 0.68 (4.95) [159] | 3.19 (6.54) [155] | 0.09 (5.74) [156] | 2.11 (5.20) [156] |
| Pulse rate in b.p.m. 6 h post‐dose, mean (SD) [n] | ||||
| Baseline | 74.91 (8.80) [166] | 73.87 (9.16) [160] | 74.56 (8.08) [161] | 74.09 (8.68) [159] |
| EoT | 74.93 (7.99) [159] | 76.74 (10.09) [155] | 73.22 (7.94) [157] | 77.55 (9.25) [156] |
| Change from baseline to EoT | 0.17 (6.25) [159] | 2.86 (6.62) [154] | −1.27 (6.67) [157] | 3.40 (6.86) [156] |
| SBP in mmHg on awakening, mean (SD) [n] | ||||
| Baseline | 128.91 (16.03) [166] | 129.75 (17.22) [161] | 127.21 (16.34) [160] | 129.22 (16.41) [159] |
| EoT | 126.91 (15.02) [159] | 126.86 (15.87) [155] | 126.28 (15.24) [157] | 126.65 (14.62) [156] |
| Change from baseline to EoT | −1.60 (11.56) [159] | −2.88 (9.08) [155] | −0.86 (9.64) [156] | −2.31 (10.14) [156] |
| SBP in mmHg 6 h post‐dose, mean (SD) [n] | ||||
| Baseline | 125.45 (13.52) [166] | 125.44 (13.99) [160] | 125.20 (14.35) [161] | 127.15 (13.74) [159] |
| EoT | 125.06 (13.24) [159] | 123.78 (13.11) [155] | 123.45 (14.03) [157] | 124.66 (13.40) [156] |
| Change from baseline to EoT | −0.46 (10.53) [159] | −1.55 (9.27) [154] | −1.64 (9.23) [157] | −2.35 (9.86) [156] |
| DBP in mmHg on awakening, mean (SD) [n] | ||||
| Baseline | 80.65 (9.14) [166] | 80.45 (10.36) [161] | 79.08 (10.04) [160] | 79.40 (9.82) [159] |
| EoT | 79.40 (8.83) [159] | 79.46 (9.96) [155] | 78.52 (9.45) [157] | 78.72 (9.52) [156] |
| Change from baseline to EoT | −0.90 (6.48) [159] | −1.00 (6.07) [155] | −0.64 (6.31) [156] | −0.54 (6.81) [156] |
| DBP in mmHg 6 h post‐dose, mean (SD) [n] | ||||
| Baseline | 78.03 (8.38) [166] | 77.67 (9.23) [160] | 77.30 (9.32) [161] | 77.85 (8.76) [159] |
| EoT | 77.33 (8.40) [159] | 77.12 (9.24) [155] | 75.55 (9.13) [157] | 78.12 (8.89) [156] |
| Change from baseline to EoT | −0.54 (6.48) [159] | −0.56 (6.25) [154] | −1.79 (5.92) [157] | 0.33 (7.17) [156] |
| ECGs | ||||
| QTcF in ms, mean (SD) [n] | ||||
| Baseline | 418.5 (17.4) [164] | 419.2 (16.9) [161] | 416.4 (17.3) [160] | 415.4 (15.6) [158] |
| EoT | 420.5 (16.6) [164] | 418.0 (16.7) [160] | 416.0 (17.5) [160] | 418.5 (15.9) [159] |
| Change from baseline to EoT | 1.8 (11.6) [162] | −1.2 (10.8) [160] | −0.4 (12.8) [159] | 3.0 (10.6) [158] |
| Absolute QTcF at EoT, n (%) | ||||
| ≤450 ms | 158 (96.3) | 157 (98.1) | 158 (98.8) | 155 (97.5) |
| >450 to ≤480 ms | 6 (3.7) | 2 (1.3) | 2 (1.3) | 4 (2.5) |
| >480 to ≤500 ms | 0 | 1 (0.6) | 0 | 0 |
| Change in QTcF from baseline to EoT, n (%) | ||||
| <0 ms | 70 (43.2) | 86 (53.8) | 77 (48.4) | 54 (34.2) |
| >0 to ≤30 ms | 91 (56.2) | 73 (45.6) | 80 (50.3) | 104 (65.8) |
| >30 to ≤60 ms | 1 (0.6) | 1 (0.6) | 2 (1.3) | 0 |
| PVR volume | ||||
| PVR volume in mL, mean (SD) [n] | ||||
| Baseline | 11.02 (15.43) [166] | 10.43 (17.07) [161] | 9.74 (14.35) [161] | 9.10 (14.41) [159] |
| EoT | 19.31 (43.18) [164] | 17.27 (34.86) [160] | 14.26 (26.04) [161] | 15.04 (36.68) [159] |
| Change from baseline to EoT | 8.17 (39.42) [164] | 6.83 (32.20) [160] | 4.52 (23.51) [161] | 5.94 (35.83) [159] |
Data shown for the SAF (patients who received at least one dose of study drug).
The QTcF interval remained reasonably constant from baseline to EoT and the observed changes ranged from −1.2 to 3.0 ms. No patient had a change in QTcF interval >60 ms from baseline to EoT. One MIRA and PRO patient had a QTcF interval >480 ms at week 16 (489 ms) and was discontinued from the study.
No notable change from baseline was found for PVR volume in any group. Drug‐related residual urine volume increased was reported by 16 (2.5%) patients. No drug‐related urinary retention was noted during the study. No clinically significant changes from baseline were found for any laboratory parameter.
Efficacy
OABSS significantly improved by ≥3 points from baseline to EoT in all treatment groups (Table 5). From baseline to EoT, significant improvements of ≥10 points in both of the OAB‐q SF measures (symptom severity score and total HRQoL score) were observed in all treatment groups. Significant improvements in OABSS and OAB‐q SF were observed at the first time point evaluated (week 4 for OABSS, week 12 for OAB‐q SF) and were maintained throughout the entire 52‐week treatment period (Fig. 3).
Table 5.
Change from baseline to EoT in efficacy parameters
| Variable | MIRA + SOLI (n = 166) | MIRA + PRO (n = 161) | MIRA + IMI (n = 161) | MIRA + TOL (n = 159) |
|---|---|---|---|---|
| OABSS total score, mean (SD) [n] | −3.9 (2.7)* [164] | −4.1 (2.6)* [160] | −3.9 (2.6)* [161] | −4.2 (2.8)* [159] |
| OAB‐q SF symptom severity score, mean (SD) [n] | −18.92 (18.42)* [160] | −18.99 (19.14)* [159] | −18.89 (18.11)* [159] | −21.28 (20.99)* [154] |
| OAB‐q SF total HRQoL score, mean (SD) [n] | 14.38 (14.98)* [160] | 12.46 (13.89)* [159] | 13.99 (16.72)* [159] | 14.36 (17.51)* [154] |
| Micturitions/24 h, mean (SD) [n] | −2.18 (1.96)* [159] | −1.89 (2.08)* [155] | −1.75 (2.09)* [157] | −1.91 (2.22)* [156] |
| Urgency episodes/24 h, mean (SD) [n] | −2.03 (2.55)* [147] | −2.24 (2.41)* [143] | −2.04 (2.19)* [149] | −2.07 (2.23)* [146] |
| Urinary incontinence episodes/24 h, mean (SD) [n] | −1.25 (1.48)* [87] | −1.18 (1.59)* [92] | −1.03 (1.08)* [101] | −1.15 (1.52)* [93] |
| Urgency urinary incontinence episodes/24 h, mean (SD) [n] | −1.20 (1.32)* [76] | −1.12 (1.33)* [80] | −0.91 (0.93)* [85] | −1.05 (1.59)* [82] |
| MVV in mL/micturition, mean (SD) [n] | 40.004 (45.095)* [159] | 38.691 (46.429)* [155] | 32.854 (44.481)* [157] | 40.683 (46.566)* [156] |
| Night‐time micturitions, mean (SD) [n] | −0.47 (0.91)* [137] | −0.38 (0.88)* [131] | −0.48 (0.93)* [139] | −0.48 (0.88)* [130] |
*P < 0.001 vs baseline. Data shown for the FAS (patients who received at least one dose of study drug and provided data for at least one variable before and after treatment initiation).
Figure 3.
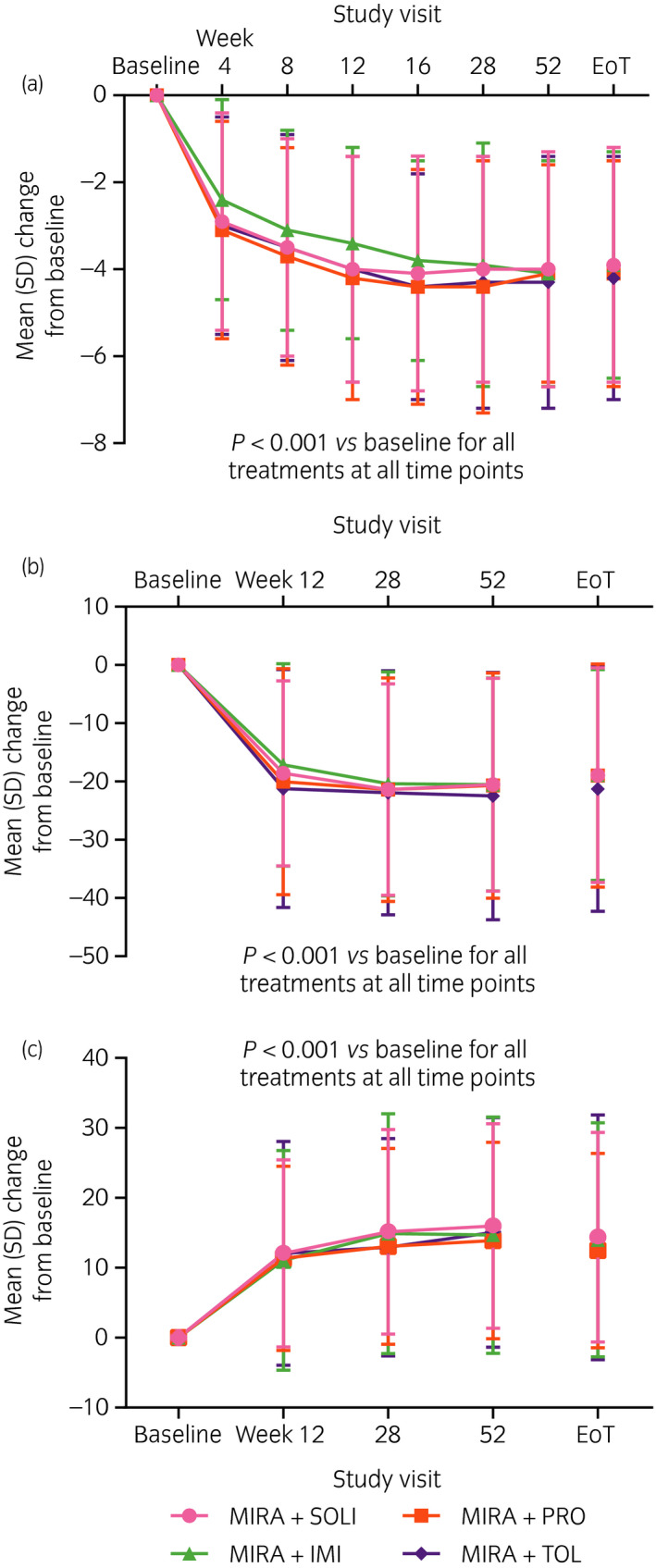
Mean change from baseline at each visit in (a) OABSS total score, (b) OAB‐q SF symptom severity score, and (c) OAB‐q SF total HRQoL score. Data shown for the FAS (patients who received at least one dose of study drug and provided data for at least one variable before and after treatment initiation).
For all combination treatments, significant improvements from baseline to EoT were observed in all parameters calculated from the micturition diary entries (micturitions, urgency episodes, urinary incontinence episodes, urgency urinary incontinence episodes, MVV, and night‐time micturitions).
Discussion
This is the first study to evaluate the long‐term safety and efficacy of add‐on therapy with four different antimuscarinics (SOLI, PRO, IMI, and TOL) in patients with OAB symptoms who were poor responders to initial MIRA treatment. Only one previous study has investigated MIRA in combination with a different antimuscarinic from SOLI; a small 8‐week study of 30 patients with OAB symptoms evaluated the use of PRO add‐on to MIRA therapy.20
In the present study, patients received add‐on treatment with antimuscarinics after ≥8 weeks’ treatment with MIRA (≥6 weeks before study start and 2 weeks during screening). The rationale for this time frame was based on findings from an efficacy analysis that found that clinical benefits can be achieved after just a few weeks of MIRA treatment.21
Add‐on therapy with antimuscarinics was well tolerated over 52 weeks in the present study. Similar incidences of TEAEs, drug‐related TEAEs, serious TEAEs, and the anticholinergic TEAEs of dry mouth and constipation were observed in all treatment groups. Although no monotherapy arms were included, the TEAE results from the present study are supported by findings from previous long‐term Japanese clinical studies investigating the use of antimuscarinics as single agents (except for PRO, where there are a lack of long‐term data).22, 23, 24, 25 For example, 45.8% of MIRA and SOLI patients reported a drug‐related TEAE in the present study and incidences of 58.8% and 64.6% were reported in a multicenter, open‐label study that involved the administration of SOLI monotherapy (5 or 10 mg) to 252 patients with OAB symptoms.22 In terms of anticholinergic TEAEs, the incidence of drug‐related constipation was 19.9% for the MIRA and SOLI group in the present study and incidences of 19.0% and 21.2% were reported in the Japanese SOLI monotherapy study.22 Furthermore, the incidence of dry mouth as a TEAE was 25.2% for the MIRA and TOL group in the present study and an incidence of 33.5% was reported in a 12‐month, open‐label TOL monotherapy study.23 Importantly, no new safety concerns were observed in the present study after the use of MIRA and antimuscarinics in combination versus previous long‐term studies involving MIRA or antimuscarinics as monotherapies.11, 22, 23, 24, 25 Additionally, no cumulative or delayed TEAEs were observed during the present study.
Compared with the present study, lower overall incidences of TEAEs have been reported in most previous SOLI and MIRA combination studies (78.9% vs 35.9–59.3%).15, 17, 18 Regarding drug‐related TEAEs, the overall incidence reported here (45.8%) was slightly higher than in earlier combination studies (17.7–44.4%).15, 16, 17, 18 In agreement with these findings, incidences of dry mouth (19.3%) and constipation (22.3%) after MIRA and SOLI in combination were slightly and noticeably higher, respectively, in the present study than in previous trials evaluating the safety of this combination regimen (dry mouth 5.9–17.3%, constipation 1.3–9.9%).15, 17, 18 The variations in safety findings between the present study and previous SOLI and MIRA combination studies are likely due to differences in study design. The present study involved a longer treatment period than previous trials, had a different order of administration (e.g. MIRA was used as add‐on therapy to SOLI in the BESIDE and MILAI studies15, 16), and involved potential increases in the antimuscarinic dose at week 8 (patients receiving increased doses experienced higher incidences of TEAEs than those receiving lower doses [data not shown]).
Previous investigations have indicated that MIRA might marginally increase heart rate,6, 7 although the clinical relevance of this is unknown. In the present study, no clinically significant differences in pulse rate from baseline to EoT were observed. For the MIRA and SOLI and MIRA and IMI groups, pulse rate remained constant over the treatment period. Increases in pulse rate of 2.86–3.19 b.p.m. and 2.11–3.40 b.p.m. were observed in the MIRA and PRO and MIRA and TOL groups, respectively. Previous clinical investigations have noted similar increases after the use of PRO (4.4 b.p.m.) or TOL (1.5–2.0 b.p.m.) as single agents.26, 27 Administration of the combination regimens did not have a notable effect on SBP or DBP. Supporting this finding, the results of the phase II Symphony study found that negligible changes in blood pressure were observed after the use of SOLI and MIRA in combination.17
Unanticipated cardiovascular events were not observed in the present study. This finding is supported by the results of a subanalysis from the BESIDE study, which found no synergistic effect on cardiovascular safety after SOLI and MIRA combination therapy.28 No clinically significant changes in QTcF intervals from baseline to EoT were observed, regardless of the combination treatment administered, and no patient experienced a QTcF interval >500 ms or a change in QTcF interval from baseline to EoT of >60 ms. Similar findings have been reported in both the BESIDE and MILAI studies.16, 28
Statistically significant improvements in efficacy were observed in the present study for all parameters investigated after the administration of all four combination regimens. In this study, clinically significant improvements in OABSS and OAB‐q SF were observed at EoT after the use of combination treatments (changes of ≥3 and ≥10 points denote clinically significant improvements in OABSS and OAB‐q parameters, respectively).29, 30 For both OABSS and OAB‐q SF, statistically significant differences from baseline were observed from the first time point evaluated and improvement was maintained throughout the entire treatment period. The significant improvements shown in OABSS and OAB‐q SF in the present study are supported by the SOLI and MIRA combination results from the short‐term MILAI study.16
Significant improvements from baseline were observed in all of the efficacy parameters that were assessed using data from the patient micturition diary. Similar findings have been observed in previous SOLI and MIRA combination studies.15, 16, 17, 18 Overall, similar levels of improvement were observed for all four combination therapies evaluated, regardless of the efficacy parameter investigated.
Although novel data were obtained, the present study does have some limitations. As 1‐year treatment with placebo is ethically problematic, a placebo arm was not included. Additionally, no monotherapy treatment arms were investigated. The trial was open label; a potential source of patient‐ and physician‐associated bias. In addition, 88.1% of the patients were women; a higher proportion than in previous SOLI and MIRA combination studies (66.4–83.9%)15, 16, 17, 18 and post‐marketing Japanese investigations (50.5–53.2%).11, 14 Furthermore, we believe that an antimuscarinic drug should be added to a patient's therapeutic regimen if they experience an insufficient response to treatment with MIRA. Alternatively, if a patient does not respond to MIRA, their treatment should be switched from MIRA to an antimuscarinic. However, patients who do not respond to MIRA treatment are infrequently encountered within the clinical setting. Therefore, in the present study, add‐on therapy with antimuscarinics was selected for use during the 52‐week treatment period. However, useful clinical data could be obtained if additional investigations are carried out to compare the efficacy and safety of switching to antimuscarinics with that of MIRA plus antimuscarinic add‐on therapy.
In conclusion, the present study is the first to show the safety and efficacy of long‐term antimuscarinic add‐on therapy in patients with OAB symptoms after initial MIRA treatment. The findings indicate that antimuscarinic add‐on treatment might become a potential clinical option for treating patients with OAB symptoms after the use of first‐line MIRA therapy. Results of additional studies examining the long‐term use of MIRA and antimuscarinics in combination are awaited with interest.
Conflict of interest
OYa, HK, YH, YI, MT, ON, MG, MY, and OYo received advisory board fees; OYa, HK, YH, YI, MT, ON, MG, MY, OYo, and NS received consultancy fees; OYa, HK, YH, YI, MT, ON, MG, MY, OYo, and NS received editorial assistance; HK and YI received grants; OYa, HK, YH, YI, MT, ON, MG, MY, OYo, and NS received lectureship fees; and MG received study funding from Astellas Pharma Inc. AO, TH, AK, and KK are employees/former employees of Astellas Pharma Inc. OYa received grants from Asahi Kasei Pharma Corporation. MT and MG received consultancy fees; HK, YI, and MG received grants; and YI, MT, MG, MY, and OYo received lectureship fees from Daiichi Sankyo Company, Limited. OYo received consultancy fees and lectureship fees from GlaxoSmithKline K.K. OYa received consultancy fees and grants; OYa and OYo received lectureship fees from Hisamitsu Pharmaceutical Co., Inc. MT and MY received advisory board fees; YI, MT, MG, MY, OYo, and NS received consultancy fees; HK and YI received grants; YI, MT, ON, MG, MY, OYo, and NS received lectureship fees; and HK received speaker fees from Kissei Pharmaceutical Co., Ltd. HK, MT, MG, MY, and OYo received advisory board fees; HK, MT, MG, MY, and OYo received consultancy fees; YI and MG received grants; and OYa, YI, MT, MG, MY, OYo, and NS received lectureship fees from KYORIN Pharmaceutical Co., Ltd. YI received consultancy fees; HK and YI received grants; YI, ON, and MY received lectureship fees; and HK received speaker fees from Nippon Shinyaku Co., Ltd. MY and OYo received advisory board fees; MT, MY, OYo, and NS received consultancy fees; YI and MG received grants; and YI, MT, MG, MY, OYo, and NS received lectureship fees from Ono Pharmaceutical Co., Ltd. MT received advisory board fees; YI, MT, and OYo received consultancy fees; YI and MG received grants; OYa, YI, MT, MG, OYo, and NS received lectureship fees; and HK received speaker fees from Pfizer Japan Inc. YI received grants from RaQualia. OYo received advisory board fees, consultancy fees, and lectureship fees from Sanwa Kagaku Kenkyusho Co., Ltd. MG, MY, and OYo received advisory board fees; OYa, MT, MG, MY, and OYo received consultancy fees; HK, YI, and MG received grants; YI, MT, MG, and OYo received lectureship fees; and HK received personal fees from Taiho Pharmaceutical Co., Ltd. HK received grants from Takeda Pharmaceutical Company Ltd.
Supporting information
Figure S1. Change from baseline in mean pulse rate (measured by patients) (a) on awakening and (b) 6 h post‐dose.
Figure S2. Change from baseline in mean SBP (measured by patients) (a) on awakening and (b) 6 h post‐dose.
Figure S3. Change from baseline in mean DBP (measured by patients) (a) on awakening and (b) 6 h post‐dose.
Table S1. Inclusion and exclusion criteria.
Appendix S1. MILAI II study group.
Acknowledgments
Study funding was provided by Astellas Pharma Inc. Medical writing support was provided by Michael Parsons of Envision Scientific Solutions and funded by Astellas Pharma Global Development. We thank Takao Katoh, M.D., Ph.D., Mita Hospital, Tokyo, Japan, for his advice/comments on the ECG, blood pressure, and pulse rate assessments. We also thank the MILAI II study group (see Appendix S1 for details).
References
- 1. Drake MJ. Do we need a new definition of the overactive bladder syndrome? ICI‐RS 2013. Neurourol. Urodyn. 2014; 33: 622–4. [DOI] [PubMed] [Google Scholar]
- 2. Coyne KS, Sexton CC, Irwin DE, Kopp ZS, Kelleher CJ, Milsom I. The impact of overactive bladder, incontinence and other lower urinary tract symptoms on quality of life, work productivity, sexuality and emotional well‐being in men and women: results from the EPIC study. BJU Int. 2008; 101: 1388–95. [DOI] [PubMed] [Google Scholar]
- 3. Chapple CR, Khullar V, Gabriel Z, Muston D, Bitoun CE, Weinstein D. The effects of antimuscarinic treatments in overactive bladder: an update of a systematic review and meta‐analysis. Eur. Urol. 2008; 54: 543–62. [DOI] [PubMed] [Google Scholar]
- 4. Yamaguchi O. Antimuscarinics and overactive bladder: other mechanism of action. Neurourol. Urodyn. 2010; 29: 112–5. [DOI] [PubMed] [Google Scholar]
- 5. Takasu T, Ukai M, Sato S et al Effect of (R)‐2‐(2‐aminothiazol‐4‐yl)‐4′‐{2‐[(2‐hydroxy‐2‐phenylethyl)amino]ethyl} acetanilide (YM178), a novel selective β 3‐adrenoceptor agonist, on bladder function. J. Pharmacol. Exp. Ther. 2007; 321: 642–7. [DOI] [PubMed] [Google Scholar]
- 6. Nitti VW, Auerbach S, Martin N, Calhoun A, Lee M, Herschorn S. Results of a randomized phase III trial of mirabegron in patients with overactive bladder. J. Urol. 2013; 189: 1388–95. [DOI] [PubMed] [Google Scholar]
- 7. Khullar V, Amarenco G, Angulo JC et al Efficacy and tolerability of mirabegron, a β3‐adrenoceptor agonist, in patients with overactive bladder: results from a randomised European‐Australian phase 3 trial. Eur. Urol. 2013; 63: 283–95. [DOI] [PubMed] [Google Scholar]
- 8. Torimoto K, Matsushita C, Yamada A et al Clinical efficacy and safety of mirabegron and imidafenacin in women with overactive bladder: a randomized crossover study (the MICRO study). Neurourol. Urodyn. 2017; 36: 1097–103. [DOI] [PubMed] [Google Scholar]
- 9. Otsuka A, Kageyama S, Suzuki T et al Comparison of mirabegron and imidafenacin for efficacy and safety in Japanese female patients with overactive bladder: A randomized controlled trial (COMFORT study). Int. J. Urol. 2016; 23: 1016–23. [DOI] [PubMed] [Google Scholar]
- 10. Chapple CR, Nazir J, Hakimi Z et al Persistence and adherence with mirabegron versus antimuscarinic agents in patients with overactive bladder: a retrospective observational study in UK clinical practice. Eur. Urol. 2017; 72: 389–99. [DOI] [PubMed] [Google Scholar]
- 11. Kato D, Tabuchi H, Uno S. Safety, efficacy, and persistence of long‐term mirabegron treatment for overactive bladder in the daily clinical setting: interim (1‐year) report from a Japanese post‐marketing surveillance study. Low. Urin. Tract Symptoms 2017; 10.1111/luts.12188. [DOI] [PubMed] [Google Scholar]
- 12. Takahashi S, Takei M, Nishizawa O et al Clinical guideline for female lower urinary tract symptoms. Low. Urin. Tract Symptoms 2016; 8: 5–29. [DOI] [PubMed] [Google Scholar]
- 13. Gormley EA, Lightner DJ, Faraday M, Vasavada SP. Diagnosis and treatment of overactive bladder (non‐neurogenic) in adults: AUA/SUFU guideline amendment. J. Urol. 2015; 193: 1572–80. [DOI] [PubMed] [Google Scholar]
- 14. Nozawa Y, Kato D, Tabuchi H, Kuroishi K. Safety and effectiveness of mirabegron in patients with overactive bladder in a real‐world clinical setting: a Japanese post‐marketing study. Low. Urin. Tract Symptoms 2018; 10: 122–30. [DOI] [PubMed] [Google Scholar]
- 15. Drake MJ, Chapple C, Esen AA et al Efficacy and safety of mirabegron add‐on therapy to solifenacin in incontinent overactive bladder patients with an inadequate response to initial 4‐week solifenacin monotherapy: a randomised double‐blind multicentre phase 3B study (BESIDE). Eur. Urol. 2016; 70: 136–45. [DOI] [PubMed] [Google Scholar]
- 16. Yamaguchi O, Kakizaki H, Homma Y et al Safety and efficacy of mirabegron as ‘add‐on’ therapy in patients with overactive bladder treated with solifenacin: a post‐marketing, open‐label study in Japan (MILAI study). BJU Int. 2015; 116: 612–22. [DOI] [PubMed] [Google Scholar]
- 17. Abrams P, Kelleher C, Staskin D et al Combination treatment with mirabegron and solifenacin in patients with overactive bladder: efficacy and safety results from a randomised, double‐blind, dose‐ranging, phase 2 study (Symphony). Eur. Urol. 2015; 67: 577–88. [DOI] [PubMed] [Google Scholar]
- 18. Herschorn S, Chapple CR, Abrams P et al Efficacy and safety of combinations of mirabegron and solifenacin compared with monotherapy and placebo in patients with overactive bladder (SYNERGY study). BJU Int. 2017; 120: 562–75. [DOI] [PubMed] [Google Scholar]
- 19. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline. The extent of population exposure to assess clinical safety for drugs intended for long‐term treatment of non‐life‐threatening conditions E1 (27 October 1994). [Cited 4 Sep 2018.] Available from URL: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E1/Step4/E1_Guideline.pdf
- 20. Shin JH, Kim A, Choo M‐S. Additional low‐dose antimuscarinics can improve overactive bladder symptoms in patients with suboptimal response to beta 3 agonist monotherapy. Investig. Clin. Urol. 2017; 58: 261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chapple CR, Nitti VW, Khullar V et al Onset of action of the β3‐adrenoceptor agonist, mirabegron, in Phase II and III clinical trials in patients with overactive bladder. World J. Urol. 2014; 32: 1565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yamaguchi O. Long‐term safety and efficacy of solifenacin succinate in patients with overactive bladder. Jpn. Pharmacol. Ther. 2006; 34(Suppl 1): S69–86. [Google Scholar]
- 23. Takei M, Homma Y. Long‐term safety, tolerability and efficacy of extended‐release tolterodine in the treatment of overactive bladder in Japanese patients. Int. J. Urol. 2005; 12: 456–64. [DOI] [PubMed] [Google Scholar]
- 24. Homma Y, Yamaguchi O. Long‐term safety, tolerability, and efficacy of the novel anti‐muscarinic agent imidafenacin in Japanese patients with overactive bladder. Int. J. Urol. 2008; 15: 986–91. [DOI] [PubMed] [Google Scholar]
- 25. Yamaguchi O, Homma Y. Long‐term efficacy and safety of dose increase study of imidafenacin in patients with overactive bladder. Jpn. Pharmacol. Ther. 2009; 37: 909–30. [Google Scholar]
- 26. Homma Y, Yamaguchi O. A randomized, double‐blind, placebo‐ and propiverine‐controlled trial of the novel antimuscarinic agent imidafenacin in Japanese patients with overactive bladder. Int. J. Urol. 2009; 16: 499–506. [DOI] [PubMed] [Google Scholar]
- 27. Chapple CR, Kaplan SA, Mitcheson D et al Mirabegron 50 mg once‐daily for the treatment of symptoms of overactive bladder: an overview of efficacy and tolerability over 12 weeks and 1 year. Int. J. Urol. 2014; 21: 960–7. [DOI] [PubMed] [Google Scholar]
- 28. Drake MJ, MacDiarmid S, Chapple CR et al Cardiovascular safety in refractory incontinent patients with overactive bladder receiving add‐on mirabegron therapy to solifenacin (BESIDE). Int. J. Clin. Pract. 2017; 71: e12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gotoh M, Homma Y, Yokoyama O, Nishizawa O. Responsiveness and minimal clinically important change in overactive bladder symptom score. Urology 2011; 78: 768–73. [DOI] [PubMed] [Google Scholar]
- 30. Coyne KS, Matza LS, Thompson CL, Kopp ZS, Khullar V. Determining the importance of change in the overactive bladder questionnaire. J. Urol. 2006; 176: 627–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Change from baseline in mean pulse rate (measured by patients) (a) on awakening and (b) 6 h post‐dose.
Figure S2. Change from baseline in mean SBP (measured by patients) (a) on awakening and (b) 6 h post‐dose.
Figure S3. Change from baseline in mean DBP (measured by patients) (a) on awakening and (b) 6 h post‐dose.
Table S1. Inclusion and exclusion criteria.
Appendix S1. MILAI II study group.


