Abstract
Background
Late stillbirth, which occurs ≥28 weeks’ gestation, affects 1.3‐8.8 per 1000 births in high‐income countries. Of concern, most occur in women without established risk factors. Identification of potentially modifiable risk factors that relate to maternal behaviors remains a priority in stillbirth prevention research. This study aimed to investigate, in an international cohort, whether maternal sleep practices are related to late stillbirth.
Methods
An Internet‐based case‐control study of women who had a stillbirth ≥28 weeks’ gestation within 30 days before completing the survey (n = 153) and women with an ongoing third‐trimester pregnancy or who had delivered a live born child within 30 days (n = 480). Bivariate and multivariate logistic regressions were used to determine unadjusted and adjusted odds ratios (OR and aOR, respectively) with 95% confidence intervals (95% CIs) for stillbirth.
Results
Sleeping >9 hours per night in the previous month was associated with stillbirth (aOR 1.75 [95% CI 1.10‐2.79]), as was waking on the right side (2.27 [1.31‐3.92]). Nonrestless sleep in the last month was also found to be associated with stillbirth (1.73 [1.03‐2.99]), with good sleep quality in the last month approaching significance (1.64 [0.98‐2.75]). On the last night of pregnancy, not waking more than one time was associated with stillbirth (2.03 [1.24‐3.34]). No relationship was found with going to sleep position during pregnancy, although very few women reported settling in the supine position (2.4%).
Conclusions
Long periods of undisturbed sleep are associated with late stillbirth. Physiological studies of how the neuroendocrine and autonomic system pathways are regulated during sleep in the context of late pregnancy are warranted.
Keywords: maternal sleep, sleep duration, stillbirth
1. INTRODUCTION
The mean annual rate of reduction of late stillbirth, at 28 weeks’ gestation or later, has been approximately 2.3% in high‐income countries and remains between 1.3 and 8.8 per 1000.1 If all high‐income countries were able to achieve stillbirth rates that were the same as the best six performing countries, almost 20 000 third‐trimester stillbirths could have been avoided in 2015 alone.1 Established risk factors for stillbirth include advanced maternal age, maternal obesity, smoking, and maternal medical and obstetric conditions such as diabetes and preeclampsia.2 However, given that most of these factors cannot be modified during pregnancy, there is a cogent need to identify modifiable risk factors, such as maternal behaviors and lifestyle.3
In 2011, the Auckland Stillbirth Study first reported that maternal sleep position was a significant risk factor for late stillbirth.4 This case‐control study demonstrated that women who reported settling to sleep in the supine position on the last night of pregnancy were significantly more likely to experience stillbirth, even after accounting for other factors (aOR 2.54 [95% CI 1.04‐6.18]). Indeed, compared with women who went to sleep on their left side, those who went to sleep in any other position had double the risk for stillbirth (2.03 [1.24‐3.29]). This finding has now been observed in three further studies using similar methodologies with similar effect sizes.5, 6, 7 These studies have also noted differences in maternal sleep duration and number of awakenings between stillbirths and live births.
Following the publication of the Auckland Stillbirth Study,4 an international group of researchers and clinicians—the Study of Trends and Associated Risks for Stillbirth Consortium—partnered with the Star Legacy Foundation and other stillbirth and parental support groups to conduct a web‐based survey of women who had experienced a stillbirth using a nested case‐control design within an uncontrolled cohort.8, 9 The overall goal was to investigate potentially modifiable risk factors, including maternal sleep practices, for late stillbirth in a large, international population. To understand maternal behaviors and symptoms during pregnancy, we sought to obtain information directly from women themselves including items that would not otherwise be documented in medical records. Our primary hypothesis for the case‐control arm of the study was that supine sleep and long sleep duration would increase the risk for late stillbirth.
2. METHODS
An anonymous online survey—Study of Trends and Risk Factors for Stillbirth—was developed during the first Stillbirth Summit in Minneapolis in 2011 by an international consortium of clinicians and academics, together with the Star Legacy Foundation and other stillbirth and parental support groups.8 The participant flow diagram for this case‐control study is presented in Figure 1. Briefly, the survey included questions related to established risk factors for stillbirth, including smoking, maternally perceived changes in fetal movements,9 and maternal health conditions, and novel questions relating to emerging risk factors, such as maternal sleep. Several questions about maternal sleep practices before pregnancy, in the last month of pregnancy, and on the last night were included in the survey. For cases, the last night referred to the last night before realization of the stillbirth. To allow similar gestational ages in cases and controls, control women included those who were currently in their third trimester and those who had recently delivered. For controls that were still pregnant, the last month and night referred to the previous month and night before completion of the survey, respectively. For controls that had recently delivered their baby, these questions referred to the last month and last night of their pregnancy.
Figure 1.
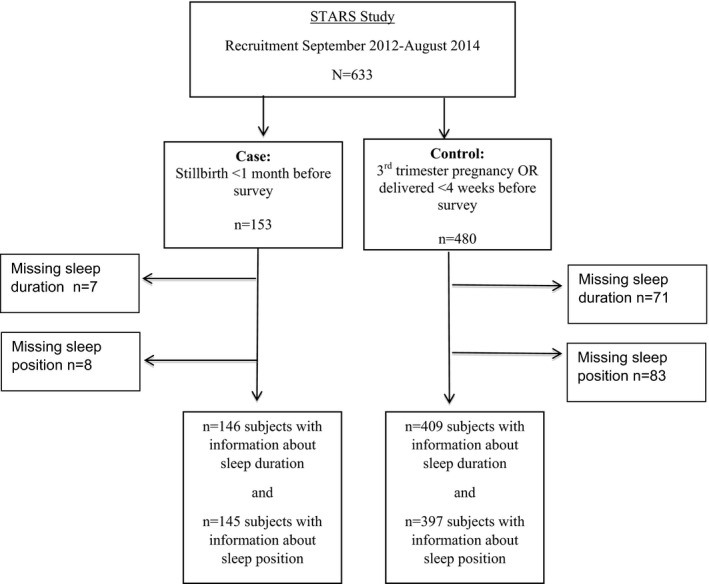
Participant flow diagram
In addition to sleep and wake position, women were asked about their typical nighttime sleep duration, daytime nap duration, number of awakenings, number of times out of bed to use the bathroom, restless sleep, sleep quality, and whether medications were used to aid sleep. Habitual snoring was defined as snoring at least three nights/week, and the Epworth Sleepiness Scale10 was used to determine excessive daytime sleepiness. The Brief Restless Legs Scale,11 a 4‐item questionnaire, was used to identify the presence of restless leg syndrome. Questions were included with open‐ended responses, categorical responses (ie, yes/no/don’t know), Likert scales, drop‐down menus with a single response, or checkboxes that allowed multiple responses. Free text boxes were also provided to allow women to provide any additional information.
The questionnaire is available as Appendices S1 and S2 to the manuscript. In reporting this study, guidelines from strengthening the Reporting of Observational Studies in Epidemiology12 group were followed.
2.1. Participants
Women were invited to participate between September 2012 and August 2014 by web‐based advertising and social media by means of the Star Legacy Foundation and word of mouth. Cases were women at least 18 years old, fluent in reading and writing English, who had delivered a singleton stillborn baby at least 28 weeks’ gestation within 1 month before completing the survey. Controls were women at least 18 years old who were either still pregnant (28 weeks or more) or had delivered a living baby within the month before survey completion. Women less than 18 years old, those with a multifetal gestation, a fetus with known congenital anomaly, and those who were not fluent in reading or writing English were excluded. This study was approved by the Institutional Review Board of the University of Michigan. Participants accessed the survey after reading the purpose of the study and clicking on the “I agree” button, thereby providing consent. They were also provided with contact information for a stillbirth support group (First Candle) if they considered completion of the survey to be upsetting.
2.2. Sample size
Sample size for maternal sleep practices was calculated based on the anticipated exposure of supine sleep. With an exposure frequency of 20%, a sample of 144 cases and controls would be required to detect a difference in odds ratio of at least 3.0 between women with late stillbirths compared with healthy controls, assuming a power of 80%, P = 0.05.
2.3. Data analysis
Data were cleaned by two authors (JW and LMO) before analysis. Women found not to fit the a priori inclusion criteria above were excluded. Categorical variables were reported as counts and proportions, whereas continuous variables were presented as mean (standard deviation) or median (interquartile range). All statistical analysis was performed with SPSS (version 24; IBM, Armonk, NY, USA) using cross‐tabulations, with chi‐squared tests and logistic regressions to find unadjusted and adjusted odds ratios (OR and aOR, respectively) with 95% confidence intervals (95% CIs). The level of statistical significance was set at P < 0.05. A multivariate logistic regression model was developed to include demographic variables reported to be associated with increased risk of stillbirth, based on prior knowledge and previous literature (maternal age, educational level, smoking, body mass index [BMI], parity). Country of respondent (United States vs non‐United States) and ethnicity of respondent (Caucasian vs non‐Caucasian) were also added to the model as they were significantly different between cases and controls. Customized birthweight centile was calculated using GROW software.13 The customized birthweight centile was not included in the final multivariate model as birthweight data were missing for half of controls. A sensitivity analysis was carried out to compare whether incorporating the customized birthweight centile altered the findings.
3. RESULTS
During the study period, 153 cases and 480 controls completed the survey. The median duration of time since stillbirth was 13 days (range 1‐29 days). Participant demographics are shown in Table 1. The median gestation at the time of the stillbirth was 37 weeks (range 28‐41 weeks), and 52% of stillborn babies were male.
Table 1.
Demographic information of an international group of women who had experienced a stillbirth of at least 28 weeks’ gestation compared with control women between 2012 and 2014
|
Stillbirth (n = 153) n (%), Mean ± SD or Median [IQR] |
Control (n = 480) n (%), Mean ± SD or Median [IQR] |
|
|---|---|---|
| Maternal age (years) | 31 ± 5.4 | 30 ± 4.8 |
| Maternal race | ||
| Caucasian | 122 (79.7)** | 430 (90.2) |
| Non‐Caucasian | 31 (20.3) | 47 (9.8) |
| Median maternal BMI (kg/m2) | 27 [23‐32] | 25 [23‐31] |
| Median gestational age (weeks) | 37 [34‐39] | 37 [32‐39] |
| Median number of prior pregnancies | 1 [0‐6]*** | 1 [0‐10] |
| Median number of prior live births | 0 [0‐4]** | 1 [0‐10] |
| Hypertension | 6 (3.9) | 35 (7.3) |
| Diabetes | 18 (11.8)* | 28 (5.8) |
| Smoking during pregnancy | 20 (13.1) | 41 (8.5) |
| Alcohol during pregnancy | 33 (21.6) | 134 (27.9) |
| Over‐the‐counter medication at least weekly | 22 (14.4)* | 111 (23.0) |
| Prescription medications at least weekly | 30 (19.6) | 127 (26.5) |
| Countryb | ||
| United States | 123 (80.4)** | 325 (67.7) |
| Other | 30 (19.6) | 148 (30.8) |
| Highest level of education | ||
| Graduate education | 35 (22.9) | 129 (26.9) |
| College‐level education | 81 (52.9) | 242 (50.4) |
| High school or lower | 37 (24.2) | 106 (22.1) |
| Activity level during pregnancy | ||
| Inactive | 17 (11.1) | 49 (10.3) |
| Lightly active | 96 (62.7) | 302 (62.9) |
| Moderately active | 28 (18.3) | 97 (20.2) |
| Active | 7 (4.6) | 18 (3.7) |
| Bedrest | 4 (2.6) | 10 (2.1) |
| Customized birthweight centilea | ||
| <10th centile | 36 (23.5)*** | 13 (2.7) |
| 10‐49.9th centile | 59 (38.5)*** | 72 (15.0) |
| 50‐89.9th centile | 35 (22.9) | 117 (24.4) |
| ≥90th centile | 20 (13.1) | 33 (6.9) |
| Missing | 3 (2.0) | 245 (51.0) |
IQR, interquartile range; SD, standard deviation.
Several variables have a small number of missing values not shown, except birth centile, which is shown in the table.
Customized birthweight centile is the birthweight‐for‐gestational‐age percentile that accounts for the influence of maternal characteristics on fetal growth.
Other countries included in the survey are as follows: the United Kingdom (n = 95), Canada (n = 44), Australia (n = 21), New Zealand (n = 2), Germany (n = 2), Greece (n = 2), India (n = 2), Philippines (n = 2), South Africa (n = 2), Finland (n = 1), Italy (n = 1), Sweden (n = 1), Switzerland (n = 1), Israel (n = 1), and Bahrain (n = 1).
*P < 0.05; **P < 0.01; ***P < 0.001.
Compared with controls, women in the stillbirth group were less likely to be Caucasian (90.2% vs 79.7%, P < 0.0002). Indeed, Caucasian race was protective for stillbirth (OR 0.41 [95% CI 0.25‐0.68]). However, nulliparity was associated with stillbirth (1.80 [1.22‐2.64]). In the control women for whom birthweight was available, compared with babies born between 50th and 89.9th customized centile, those with birthweight <10th customized centile, birthweight between 10th and 49.9th customized centile, and birthweight >90th customized centile were all associated with stillbirth (9.26 [4.43‐19.37], 2.74 [1.64‐4.57], and 2.03 [1.04‐3.97], respectively).
3.1. Sleep variables
Before pregnancy, no differences were found in self‐reported sleep practices between cases and controls (see Table S1). Nocturnal sleep duration was significantly longer in cases compared with controls in the last month of pregnancy, as was total 24‐hour sleep duration over the same period (Table 2). This was driven by the nocturnal sleep duration as nap durations were not different between groups. Women in the stillbirth group were at significantly higher odds (aOR 1.75 [95% CI 1.10‐2.79]) of having long sleep duration (≥9 hours) over the previous month (Table 3) after adjustment for other variables although no relationship was found with sleep duration on the last night and stillbirth (Table 4). Those who had a stillbirth were more likely to report that they did not wake up or woke up only once on the last night (aOR 2.03 [95% CI 1.24‐3.34]). Epworth Sleepiness Scale scores (Table 2) and clinical levels of daytime sleepiness increased from prepregnancy (see Table S1) to the last month of pregnancy (Table 3) although remained similar between groups.
Table 2.
Mean sleep variables before and during pregnancy in an international sample of women with and without a stillbirth, 2012‐2014
| Variable |
Stillbirth (n = 153) Mean ± SD |
Controls (n = 480) Mean ± SD |
|---|---|---|
| Sleep duration | ||
| Sleep duration before pregnancy (hours) | 7.7 ± 0.9 | 7.6 ± 1.0 |
| Sleep duration last month (hours) | 7.9 ± 1.3** | 7.5 ± 1.5 |
| Sleep duration last night (hours) | 6.4 ± 2.1 | 6.7 ± 2.1 |
| Nap duration | ||
| Nap duration before pregnancy (hours) | 0.9 ± 1.0 | 1.0 ± 0.9 |
| Nap duration last month (hours) | 1.6 ± 0.8 | 1.6 ± 0.9 |
| Total 24‐h sleep duration | ||
| Total sleep in 24 h before pregnancy (hours) | 8.7 ± 1.4 | 8.6 ± 1.4 |
| Total sleep in 24 h last month (hours) | 9.5 ± 1.5* | 9.1 ± 1.8 |
| Epworth Sleepiness Scale (ESS) | ||
| Total ESS before pregnancy | 3.9 ± 3.0 | 3.9 ± 3.1 |
| Total ESS last month | 7.5 ± 4.9 | 7.0 ± 4.5 |
P < 0.05;
P < 0.01.
Table 3.
Odds of stillbirth by sleep variable in the last month of pregnancy in an international sample of women with and without a stillbirth, 2012‐2014
| Variable |
Stillbirth (n = 153) n (%) |
Controls (n = 480) n (%) |
Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|---|---|
| Sleep duration last month | ||||
| ≤6 h | 15 (9.8) | 47 (9.8) | 1.05 (0.56‐1.97) | 1.11 (0.57‐2.16) |
| 6.5‐8.5 h | 86 (56.2) | 283 (59.0) | Reference | Reference |
| ≥9 h | 45 (29.4) | 79 (16.5) | 1.87 (1.21‐2.91) | 1.75 (1.10‐2.79) |
| Awakenings last month | ||||
| ≤1 awakening | 23 (15.0) | 61 (12.7) | 1.06 (0.63‐1.79) | 1.13 (0.65‐1.97) |
| ≥2 awakenings | 121 (79.1) | 341 (71.0) | Reference | Reference |
| Get up last month | ||||
| ≤1 time up | 47 (30.7) | 129 (26.9) | 1.00 (0.67‐1.51) | 1.16 (0.75‐1.79) |
| ≥2 time up | 98 (64.1) | 270 (56.3) | Reference | Reference |
| Restless last month | ||||
| None or little restless | 52 (34.0) | 97 (20.2) | 1.71 (1.02‐2.88) | 1.73 (1.03‐2.99) |
| Average restless | 32 (20.9) | 102 (21.3) | Reference | Reference |
| More than average or very restless | 62 (40.5) | 201 (41.9) | 0.98 (0.60‐1.60) | 0.91 (0.54‐1.53) |
| Fall asleep position last month | ||||
| Left | 81 (52.9) | 209 (43.5) | Reference | Reference |
| Supine | 1 (0.7) | 8 (1.7) | 0.32 (0.04‐2.62) | 0.37 (0.04‐3.12) |
| Right | 37 (24.2) | 95 (19.8) | 1.00 (0.64‐1.59) | 1.14 (0.70‐1.85) |
| Propped | 5 (3.2) | 14 (2.9) | 0.92 (0.32‐2.64) | 1.20 (0.39‐3.68) |
| Prone | 0 (0) | 5 (1.0) | NA | NA |
| Variable | 21 (13.7) | 66 (13.8) | 0.82 (0.47‐1.43) | 0.87 (0.48‐1.55) |
| Wake up position last month | ||||
| Left | 40 (26.1) | 128 (26.7) | Reference | Reference |
| Supine | 11 (7.2) | 42 (8.8) | 0.84 (0.40‐1.78) | 1.10 (0.50‐2.43) |
| Right | 47 (30.7) | 82 (17.1) | 1.83 (1.11‐3.04) | 2.27 (1.31‐3.92) |
| Propped | 2 (1.3) | 7 (1.5) | 0.91 (0.18‐4.58) | 0.98 (0.18‐5.30) |
| Prone | 1 (0.7) | 5 (1.0) | 0.64 (0.07‐5.64) | 0.51 (0.05‐4.87) |
| Variable | 36 (23.5) | 111 (23.1) | 1.04 (0.62‐1.74) | 1.16 (0.67‐2.00) |
| Naps last month | ||||
| Never/rare | 38 (24.8) | 125 (26.0) | Reference | Reference |
| Occasional | 41 (26.8) | 108 (22.5) | 1.25 (0.75‐2.08) | 1.32 (0.77‐2.77) |
| Often/almost always | 67 (43.8) | 168 (35.0) | 1.31 (0.83‐2.08) | 1.40 (0.86‐2.29) |
| Excessive daytime sleepiness last month | ||||
| No | 97 (63.4) | 281 (58.5) | Reference | Reference |
| Yes | 42 (27.5) | 107 (22.3) | 1.14 (0.74‐ 1.74) | 1.00 (0.64‐1.58) |
| Habitual snoring last month | ||||
| None/rare | 68 (44.4) | 196 (40.8) | Reference | Reference |
| Occasionally | 34 (22.2) | 95 (19.8) | 1.03 (0.64‐1.67) | 1.00 (0.59‐1.68) |
| Often/almost always | 31 (20.3) | 89 (18.5) | 1.00 (0.61‐1.64) | 0.95 (0.55‐1.66) |
| Restless leg syndrome last month | ||||
| No | 92 (60.1) | 255 (53.1) | Reference | Reference |
| Yes | 44 (28.8) | 128 (26.7) | 0.95 (0.63‐1.45) | 1.11 (0.71‐1.72) |
| Sleep quality last month | ||||
| Good/very good | 48 (31.4) | 89 (18.5) | 1.69 (1.04‐2.75) | 1.64 (0.98‐2.75) |
| Average | 50 (32.7) | 134 (27.9) | Reference | Reference |
| Poor/very poor | 47 (30.7) | 193 (40.2) | 0.65 (0.41‐1.03) | 0.65 (0.40‐1.06) |
| Medications to aid sleep last month | ||||
| No | 129 (84.3) | 345 (71.9) | Reference | Reference |
| Occasionally | 8 (5.2) | 25 (5.2) | 0.86 (0.38‐ 1.95) | 0.61 (0.25‐ 1.49) |
| Frequently | 5 (3.3) | 24 (5.0) | 0.56 (0.21‐ 1.49) | 0.64 (0.23‐ 1.45) |
All models were adjusted for maternal age, educational level, smoking, body mass index (BMI), parity, country of respondent (United States vs non‐United States), and ethnicity. Sleep duration was reported in half‐hour increments.
Table 4.
Odds of stillbirth by sleep variable in the last night of pregnancy in an international sample of women with and without a stillbirth, 2012‐2014
| Variable |
Stillbirth (n = 153) n (%) |
Controls (n = 480) n (%) |
Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|---|---|
| Sleep duration last night | ||||
| ≤6 h | 67 (43.8) | 153 (31.9) | 1.37 (0.91‐2.06) | 1.31 (0.84‐2.03) |
| 6.5‐8.5 h | 59 (38.6) | 184 (38.3) | Reference | Reference |
| ≥9 h | 16 (10.5) | 54 (11.3) | 0.92 (0.49‐1.74) | 0.75 (0.39‐1.46) |
| Awakenings last night | ||||
| ≤1 awakening | 41 (26.8) | 63 (13.1) | 2.16 (1.37‐3.41) | 2.03 (1.24‐3.34) |
| ≥2 awakenings | 94 (61.4) | 312 (65.0) | Reference | Reference |
| Get up last night | ||||
| ≤1 time up | 60 (39.2) | 132 (27.5) | 1.43 (0.96‐2.13) | 1.47 (0.96‐2.27) |
| ≥2 time up | 76 (49.7) | 239 (49.8) | Reference | Reference |
| Restless last night | ||||
| None or little restless | 47 (30.7) | 90 (18.8) | 1.24 (0.73‐2.10) | 1.16 (0.66‐2.04) |
| Average restless | 35 (22.9) | 83 (17.3) | Reference | Reference |
| More than average or very restless | 62 (40.5) | 214 (44.6) | 0.69 (0.42‐1.12) | 0.66 (0.40‐1.11) |
| Fall asleep position last night | ||||
| Left | 75 (49.0) | 193 (40.2) | Reference | Reference |
| Supine | 4 (2.6) | 11 (2.3) | 0.94 (0.29‐3.03) | 1.05 (0.32‐3.50) |
| Right | 45 (29.4) | 111 (23.1) | 1.04 (0.67‐1.62) | 1.11 (0.70‐1.77) |
| Propped up | 4 (2.6) | 15 (3.1) | 0.69 (0.22‐2.13) | 0.71 (0.22‐2.30) |
| Prone | 0 (0) | 4 (0.8) | NA | NA |
| Variable | 10 (6.5) | 39 (8.1) | 0.66 (0.31‐1.39) | 0.75 (0.34‐1.64) |
| Wake up position last night | ||||
| Left | 46 (30.1) | 147 (30.6) | Reference | Reference |
| Supine | 13 (8.5) | 39 (8.1) | 1.07 (0.52‐2.17) | 1.25 (0.58‐2.73) |
| Right | 44 (28.8) | 97 (20.2) | 1.45 (0.89‐2.36) | 1.54 (0.91‐2.61) |
| Propped up | 2 (1.3) | 11 (2.3) | 0.58 (0.12‐2.72) | 0.56 (0.12‐2.70) |
| Prone | 2 (1.3) | 3 (0.6) | 2.13 (0.35‐13.14) | 1.87 (0.27‐12.83) |
| Variable | 11 (7.2) | 46 (9.6) | 0.76 (0.37‐1.60) | 0.88 (0.41‐1.90) |
| Restless leg syndrome last night | ||||
| No | 100 (65.4) | 292 (60.8) | Reference | Reference |
| Yes | 20 (13.1) | 73 (15.2) | 0.80 (0.46‐1.38) | 0.92 (0.51‐1.65) |
| Sleep quality last night | ||||
| Good/very good | 37 (24.2) | 67 (14.0) | 1.53 (0.89‐2.60) | 1.40 (0.79‐2.47) |
| Average | 42 (27.5) | 116 (24.2) | Reference | Reference |
| Poor/very poor | 65 (40.5) | 202 (42.1) | 0.89 (0.57‐1.39) | 0.89 (0.55‐1.43) |
| Medications to aid sleep last night | ||||
| No | 138 (90.2) | 367 (76.5) | Reference | Reference |
| Yes | 6 (3.9) | 24 (5.0) | 0.67 (0.27‐1.66) | 0.65 (0.26‐1.67) |
All models were adjusted for maternal age, educational level, smoking, body mass index (BMI), parity, country of respondent (United States vs non‐United States), and ethnicity. Sleep duration was reported in half‐hour increments.
No relationship was found between reported position that women fell asleep in and stillbirth either in the last month or on the last night. However, the number of supine sleepers in this study was universally low (n = 1 for cases and n = 8 controls in the last month and n = 4 in the cases and n = 11 in the controls for the last night). Before pregnancy, the frequency of falling asleep supine was similar between groups (10.5% of cases and 12.7% of controls) but fell across both groups to 0.7% and 1.7% in the last month and 2.6% and 2.3% on the last night in cases and controls, respectively. This unexpected low frequency prevents full analysis. Wake up position was similar between groups before pregnancy, but cases were more likely to report waking up on their right side in the last month of pregnancy compared with controls (aOR 2.27 [95% CI 1.31‐3.92]) but not the last night (Tables 3 and 4). Position on going to sleep and position on waking up were highly correlated before pregnancy, in the last month, and on the last night (Pearson correlation coefficient r = 0.77, r = 0.81, and r = 0.83, respectively, P < 0.0001).
Both habitual snoring (snoring often or every night) and restless leg syndrome were similar in cases and controls at all time points and increased during pregnancy compared with before pregnancy (Table S1 and Tables 3 and 4). Compared with self‐report of an average amount of restless sleep, not having restless sleep was more likely in the stillbirth group in the last month, even after accounting for other risk factors (aOR 1.73 [95% CI 1.03‐2.99]).
Before pregnancy, sleep quality was similar between groups (Table S1), but poor sleep quality became more frequent during pregnancy (Tables 3 and 4). In the last month of pregnancy, the odds of good/very good sleep quality was higher in the stillbirth group (OR 1.69 [95% CI 1.04‐2.75]) although the difference was not significant after adjustment (Table 3). A minority of women reported use of medications during the last month and on the last night to help them sleep, but no relationship was found with stillbirth (Tables 3 and 4). A sensitivity analysis using customized birthweight centile as a covariate in the adjusted models described above did not appreciably change any of the findings.
To investigate whether there was a differential response to the survey from control women who had already delivered when they completed the survey compared with those who were still pregnant, a sensitivity analysis was conducted using only those women who were still pregnant compared with cases. This analysis did not alter the findings, and thus, all controls were retained. Moreover, a sensitivity analysis restricted to women in the United States only did not change any of the results.
No interactions were found between long sleep duration and fetal compromise, such as those born <10th centile or those exposed to maternal smoking. Furthermore, no interactions were found between long sleep duration and maternal perception of changes in fetal movement.
Women who had experienced a stillbirth were asked about their perception of the time that they believed their baby died. The time periods were “Morning, 6 am‐12 noon,” “Afternoon, 12 noon‐6 pm,” “Evening, 6 pm‐10 pm,” and “During the night, 10 pm‐6 am.” A total of n = 33 women (21.6%) were not sure and n = 8 women (5.2%) did not provide a response. However, out of the n = 112 women who provided a time period, n = 83 (74%) believed that their baby died during the night (Table 5).
Table 5.
Perceived time of death in an international sample of women with a stillbirth, 2012‐2014
| Time period |
N = 153 n (%) |
|---|---|
| Morning (6 am‐12 noon) | 10 (6.5) |
| Afternoon (12 noon‐6 pm) | 11 (7.2) |
| Evening (6 pm‐10 pm) | 8 (5.2) |
| During the night (10 pm‐6 am) | 83 (54.2) |
| Not sure | 33 (21.6) |
| Missing | 8 (5.2) |
4. DISCUSSION
This international study provides further evidence that maternal sleep practices are associated with a late stillbirth. In agreement with other studies, it suggests that long periods of undisturbed sleep, such as long sleep duration and not waking more than once, independent of other risk factors are associated with late fetal demise.
No evidence was found that maternal supine sleep position was a risk of stillbirth. Since the initial publication,4 one small cohort study from Ghana14 and several case‐control studies from Australia,5 New Zealand,6 and the United Kingdom7 have all demonstrated an association between supine sleep position and late stillbirth with odds ratios between 2.3 and 8.0. Although one of the goals of the current study was to investigate the role of supine sleep position, its reported frequency was much lower (2.3%) than the anticipated 20%. Thus, the study was underpowered to detect a difference at a low frequency of exposure.
As maternal sleep practices were of interest in the development of this case‐control study, a large number of questions about sleep were included. This allowed for exploration of changes in sleep across pregnancy since women were queried about sleep practices before pregnancy, in the last month, and on the last night. As expected, sleep disturbance increased during pregnancy. There is a large literature demonstrating that maternal sleep disturbance, such as sleep‐disordered breathing, short sleep duration, and poor sleep quality are common during pregnancy and have strong associations with poor outcomes such as gestational hypertension and preeclampsia,15, 16, 17, 18 gestational diabetes,16, 19, 20 fetal growth restriction,21, 22, 23 and preterm birth.17, 21, 24, 25 Importantly, the latter outcomes are known risk factors for stillbirth.26 Since nocturnal events may influence pregnancy outcomes, possibly by induction of inflammatory and oxidative stress responses, endothelial damage, and metabolic derangement,27, 28, 29 the link between maternal sleep and stillbirth warrants urgent investigation. Of note, consistent with other studies,5, 8 a large proportion of women (74%) perceived that their baby died during the night.
Our findings that long periods of undisturbed sleep were more frequent in the stillbirth group support other data. Indeed, the Auckland study4 reported higher odds of long sleep duration, one or fewer times getting up to use the bathroom, and regular daytime naps in the stillbirth group compared with controls, although short sleepers were also more common in the stillbirth group. This latter study was remarkably similar to the recent Midlands and North of England Stillbirth Study,7 which also reported frequent daytime napping, one or fewer awakenings to use the bathroom, and both long and short sleep as being more common in the stillbirth group. Short sleep—but not long sleep—was more common in the stillbirth group in the New Zealand multicenter stillbirth case‐control study,6 which also found a higher odds for not getting up to go to the toilet. Similar to the current study, McCowan et al6 did not find a relationship between daytime naps and stillbirth. Our novel findings of good sleep quality and lack of restless sleep being more likely in the stillbirth group compared with controls further add to this growing literature that suggests a role for undisturbed maternal sleep in stillbirth.
Prolonged sleep duration in older populations has been hypothesized to compensate for poor sleep quality30 although in the current study mothers who had a stillbirth were more likely to have good sleep quality. Long sleep durations have also been associated with a sedentary lifestyle, low levels of physical activity, socioeconomic status, and anxiety/depression.30, 31, 32 Nonetheless, in the context of stillbirth, the current study found no differences between cases and controls for reported levels of activity nor for educational attainment, but no measures of anxiety/depression were obtained.
Interestingly, otherwise healthy long sleepers have been reported to have elevated serum melatonin and cortisol, longer durations with low body temperature, and systematic differences in arousal.33 A strong relationship exists between arousal and an increase in sympathetic activity and thus blood pressure.34 Blood pressure decreases during sleep, with a gradual decrease during each stage of slow‐wave sleep irrespective of posture35 with the lowest pressure reached during deep sleep.36 During rapid eye movement sleep, blood pressure is transiently increased although not to awake levels. Indeed, the time spent in specific sleep states and the distribution of the sleep states across the night is known to affect blood pressure.37 Since arousals and awakenings from sleep cause surges in sympathetic activity with resultant increases in blood pressure, it is plausible that awakenings and periods of getting out of bed (such as to use the toilet) in pregnant women could serve to maintain blood pressure and prevent long periods of relative low pressures. This has particular relevance since maternal hypotension has been reported to be associated with fetal growth restriction, premature birth, and stillbirth.38, 39, 40 In a case‐control study designed to specifically investigate daytime maternal blood pressure in stillbirths compared with live births, Warland et al41 found that the stillbirth group were more likely to have borderline diastolic blood pressure (60‐70 mm Hg). Furthermore, the odds of stillbirth in women with at least three mean arterial pressure values ≤83 mm Hg was almost double that of controls (aOR 1.78 [95% CI 1.06‐2.99]). Moreover, long sleep duration may prolong inferior vena cava compression and lack of compression relief from not waking up could potentiate this effect.42
A strength of the current study is that it is the first to include an international group of women and the only one to include women from the United States; indeed, the majority of women received their care in the United States. The United States‐based Star Legacy Foundation hosted the online survey, and one possible explanation for the unexpectedly low prevalence of reported supine sleep potentially could have been because the results of the Auckland Stillbirth Study,4 the first to report an association between supine sleep and late stillbirth, were available on the website. Nonetheless, despite the international nature of this study we were unable to investigate the effect of race since the vast majority of women were of Caucasian background and insufficient women of other races were represented. Since non‐Hispanic black women are at 2‐3 times higher risk of stillbirth than are non‐Hispanic white women,43 there is an opportunity for future studies to explore sleep practices in the context of racial and ethnic background. Although part of the higher risk of fetal mortality for non‐Hispanic black women relates to their higher risk of preterm delivery, most of the disparity in fetal mortality remains unexplained.44
This study is not without limitation. Inherent in the design, it was only available to women who had Internet access. However, given that the Internet is widely available across the majority of household income brackets and that approximately one quarter of women had either a high school education or lower, the design is unlikely to limit generalizability. Although this was an international study, it is acknowledged that the majority of women were based in the United States. An additional potential limitation is recall bias, particularly with regard to sleep behaviors. However, care was taken to minimize recall bias by limiting the study to women who had delivered a stillborn baby within the previous month, when events can be recalled.45 Similarly, most controls were either still pregnant—to have a comparable range of gestational ages—or had delivered within the previous month. Although this approach cannot eliminate recall bias, use of similar time frames is unlikely to bias one group more than the other. Moreover, these data were based on subjective measures. Although objective measures of sleep such as polysomnography or actigraphy would allow a detailed understanding of nocturnal body position, it is extremely difficult to use such measures when stillbirth is the outcome of interest, since many thousands of women would be required for adequate power; this is cost‐prohibitive and logistically challenging. In addition, since the study included a large number of questions that were not related to sleep, it is unlikely that report of sleep practices would have been biased. Importantly, sleep practices before pregnancy were the same between groups.
In summary, long periods of undisturbed maternal sleep are associated with late stillbirth. Although no role for supine sleep was found—possibly because of very low numbers of women with reported supine sleep—findings of long sleep duration and few awakenings are consistent with other published data. Maternal sleep offers a modifiable risk factor for stillbirth. Physiological studies of how the neuroendocrine and autonomic system pathways are regulated during sleep in the context of late pregnancy are warranted.
Supporting information
ACKNOWLEDGMENTS
The authors acknowledge the invaluable assistance of the Star Legacy Foundation, especially Mrs Shauna Libsack and Mrs Lindsey Wimmer. We also thank the following organizations for assisting us in advertising and recruiting for this study: the Missing Grace Foundation, Group B Strep Foundation, First Candle. The authors also acknowledge all the participants who bravely shared their experiences to help us better understand stillbirth. STARS Consortium is listed in alphabetical order as follows: Collins JH, Heazell AEP, Huberty JL, Kliman HJ, McGregor JA, Mitchell EA, O’Brien LM, Parast M, Peesay M, Stacey T, Warland J, Wimmer LJ.
O’Brien LM, Warland J, Stacey T, Heazell AEP, Mitchell EA; on behalf of the STARS Consortium . Maternal sleep practices and stillbirth: Findings from an international case‐control study. Birth. 2019;46:344–354. 10.1111/birt.12416
Contributor Information
Louise M. O’Brien, Email: louiseo@med.umich.edu.
the STARS Consortium:
JH Collins, JL Huberty, HJ Kliman, JA McGregor, M Parast, M Peesay, and LJ Wimmer
REFERENCES
- 1. Flenady V, Middleton P, Smith GC, et al. Stillbirths: recall to action in high‐income countries. Lancet. 2016;387(10019):691‐702. [DOI] [PubMed] [Google Scholar]
- 2. Flenady V, Koopmans L, Middleton P, et al. Major risk factors for stillbirth in high‐income countries: a systematic review and meta‐analysis. Lancet. 2011;377(9774):1331‐1340. [DOI] [PubMed] [Google Scholar]
- 3. Heazell AE, Whitworth MK, Whitcombe J, et al. Research priorities for stillbirth: process overview and results from UK Stillbirth Priority Setting Partnership. Ultrasound Obstet Gynecol. 2015;46(6):641‐647. [DOI] [PubMed] [Google Scholar]
- 4. Stacey T, Thompson JM, Mitchell EA, et al. Association between maternal sleep practices and risk of late stillbirth: a case‐control study. BMJ. 2011;342:d3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gordon A, Raynes‐Greenow C, Bond D, Morris J, Rawlinson W, Jeffery H. Sleep position, fetal growth restriction, and late‐pregnancy stillbirth: the Sydney stillbirth study. Obstet Gynecol. 2015;125(2):347‐355. [DOI] [PubMed] [Google Scholar]
- 6. McCowan L, Thompson J, Cronin RS, et al. Going to sleep in the supine position is a modifiable risk factor for late pregnancy stillbirth; findings from the New Zealand multicentre stillbirth case‐control study. PLoS ONE. 2017;12(6):e0179396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heazell A, Li M, Budd J, et al. Association between maternal sleep practices and late stillbirth—findings from a stillbirth case‐control study. BJOG. 2018;125(2):254‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Warland J, O’Brien LM, Heazell AE, Mitchell EA; STARS Consortium . An international internet survey of the experiences of 1,714 mothers with a late stillbirth: the STARS cohort study. BMC Pregnancy Childbirth. 2015;15:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heazell A, Warland J, Stacey T, et al. Stillbirth is associated with perceived alterations in fetal activity—findings from an international case control study. BMC Pregnancy Childbirth. 2017;17(1):369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540‐545. [DOI] [PubMed] [Google Scholar]
- 11. Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health . Sleep Med. 2003;4(2):101‐119. [DOI] [PubMed] [Google Scholar]
- 12. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453‐1457. [DOI] [PubMed] [Google Scholar]
- 13. Gardosi J, Francis A. GROW Customised Weight Centile Calculator. Birmingham: Gestational Network; 2015. [Google Scholar]
- 14. Owusu JT, Anderson FJ, Coleman J, et al. Association of maternal sleep practices with pre‐eclampsia, low birth weight, and stillbirth among Ghanaian women. Int J Gynaecol Obstet. 2013;121(3):261‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O’Brien LM, Bullough AS, Owusu JT, et al. Pregnancy‐onset habitual snoring, gestational hypertension, and preeclampsia: prospective cohort study. Am J Obstet Gynecol. 2012;207(6):487 e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pamidi S, Pinto LM, Marc I, Benedetti A, Schwartzman K, Kimoff RJ. Maternal sleep‐disordered breathing and adverse pregnancy outcomes: a systematic review and metaanalysis. Am J Obstet Gynecol. 2014;210(1):52 e1‐52 e14. [DOI] [PubMed] [Google Scholar]
- 17. Bin YS, Cistulli PA, Ford JB. Population‐based study of sleep apnea in pregnancy and maternal and infant outcomes. J Clin Sleep Med. 2016;12(6):871‐877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Williams MA, Miller RS, Qiu C, Cripe SM, Gelaye B, Enquobahrie D. Associations of early pregnancy sleep duration with trimester‐specific blood pressures and hypertensive disorders in pregnancy. Sleep. 2010;33(10):1363‐1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Facco FL, Grobman WA, Reid KJ, et al. Objectively measured short sleep duration and later sleep midpoint in pregnancy are associated with a higher risk of gestational diabetes. Am J Obstet Gynecol. 2017;217(4):447 e1‐447 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reutrakul S, Anothaisintawee T, Herring SJ, Balserak BI, Marc I, Thakkinstian A. Short sleep duration and hyperglycemia in pregnancy: aggregate and individual patient data meta‐analysis. Sleep Med Rev. 2017;40:31‐42. [DOI] [PubMed] [Google Scholar]
- 21. Micheli K, Komninos I, Bagkeris E, et al. Sleep patterns in late pregnancy and risk of preterm birth and fetal growth restriction. Epidemiology. 2011;22(5):738‐744. [DOI] [PubMed] [Google Scholar]
- 22. Abeysena C, Jayawardana P, Seneviratne R. Maternal sleep deprivation is a risk factor for small for gestational age: a cohort study. Aust N Z J Obstet Gynaecol. 2009;49(4):382‐387. [DOI] [PubMed] [Google Scholar]
- 23. O’Brien LM, Bullough AS, Owusu JT, et al. Snoring during pregnancy and delivery outcomes: a cohort study. Sleep. 2013;36(11):1625‐1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kajeepeta S, Sanchez SE, Gelaye B, et al. Sleep duration, vital exhaustion, and odds of spontaneous preterm birth: a case‐control study. BMC Pregnancy Childbirth. 2014;14:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li R, Zhang J, Zhou R, et al. Sleep disturbances during pregnancy are associated with cesarean delivery and preterm birth. J Matern Fetal Neonatal Med. 2017;30(6):733‐738. [DOI] [PubMed] [Google Scholar]
- 26. Flenady V, Middleton P, Smith GC, et al. Stillbirths: the way forward in high‐income countries. Lancet. 2011;377(9778):1703‐1717. [DOI] [PubMed] [Google Scholar]
- 27. Okun ML, Hall M, Coussons‐Read ME. Sleep disturbances increase interleukin‐6 production during pregnancy: implications for pregnancy complications. Reprod Sci. 2007;14(6):560‐567. [DOI] [PubMed] [Google Scholar]
- 28. Okun ML, Roberts JM, Marsland AL, Hall M. How disturbed sleep may be a risk factor for adverse pregnancy outcomes. Obstet Gynecol Surv. 2009;64(4):273‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lavie L. Oxidative stress—a unifying paradigm in obstructive sleep apnea and comorbidities. Prog Cardiovasc Dis. 2009;51(4):303‐312. [DOI] [PubMed] [Google Scholar]
- 30. Suzuki E, Yorifuji T, Ueshima K, et al. Sleep duration, sleep quality and cardiovascular disease mortality among the elderly: a population‐based cohort study. Prev Med. 2009;49(2–3):135‐141. [DOI] [PubMed] [Google Scholar]
- 31. Patel SR, Malhotra A, Gottlieb DJ, White DP, Hu FB. Correlates of long sleep duration. Sleep. 2006;29(7):881‐889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Krueger PM, Friedman EM. Sleep duration in the United States: a cross‐sectional population‐based study. Am J Epidemiol. 2009;169(9):1052‐1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aeschbach D, Sher L, Postolache TT, Matthews JR, Jackson MA, Wehr TA. A longer biological night in long sleepers than in short sleepers. J Clin Endocrinol Metab. 2003;88(1):26‐30. [DOI] [PubMed] [Google Scholar]
- 34. Morgan BJ, Crabtree DC, Puleo DS, Badr MS, Toiber F, Skatrud JB. Neurocirculatory consequences of abrupt change in sleep state in humans. J Appl Physiol (1985). 1996;80(5):1627‐1636. [DOI] [PubMed] [Google Scholar]
- 35. Carrington MJ, Barbieri R, Colrain IM, Crowley KE, Kim Y, Trinder J. Changes in cardiovascular function during the sleep onset period in young adults. J Appl Physiol (1985). 2005;98(2):468‐476. [DOI] [PubMed] [Google Scholar]
- 36. Portaluppi F, Nabe B, Lies A, Kohl FV, Lohmann FW. Alterations of sleep and circadian blood pressure profile. Blood Press Monit. 1997;2(6):301‐313. [PubMed] [Google Scholar]
- 37. Javaheri S, Redline S. Sleep, slow‐wave sleep, and blood pressure. Curr Hypertens Rep. 2012;14(5):442‐448. [DOI] [PubMed] [Google Scholar]
- 38. Friedman EA, Neff RK. Hypertension‐hypotension in pregnancy. Correlation with fetal outcome. JAMA. 1978;239(21):2249‐2251. [PubMed] [Google Scholar]
- 39. Steer PJ, Little MP, Kold‐Jensen T, Chapple J, Elliott P. Maternal blood pressure in pregnancy, birth weight, and perinatal mortality in first births: prospective study. BMJ. 2004;329(7478):1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ng PH, Walters WA. The effects of chronic maternal hypotension during pregnancy. Aust N Z J Obstet Gynaecol. 1992;32(1):14‐16. [DOI] [PubMed] [Google Scholar]
- 41. Warland J, McCutcheon H, Baghurst P. Maternal blood pressure in pregnancy and stillbirth: a case‐control study of third‐trimester stillbirth. Am J Perinatol. 2008;25(5):311‐317. [DOI] [PubMed] [Google Scholar]
- 42. Humphries A, Ali Mirjalili S, Tarr GP, Thompson J, Stone P. The effect of supine positioning on maternal hemodynamics during late pregnancy. J Matern Fetal Neonatal Med. 2018. Jun 3:1-8. 10.1080/14767058.2018.1478958. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 43. MacDorman MF, Kirmeyer SE, Wilson EC. Fetal and perinatal mortality, United States, 2006. Natl Vital Stat Rep. 2012;60(8):344‐22. [PubMed] [Google Scholar]
- 44. Macdorman MF, Kirmeyer S. The challenge of fetal mortality. NCHS Data Brief. 2009;16:344‐8. [PubMed] [Google Scholar]
- 45. Gibbons LE, Ponsonby AL, Dwyer T. A comparison of prospective and retrospective responses on sudden infant death syndrome by case and control mothers. Am J Epidemiol. 1993;137(6):654‐659. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


