Abstract
BACKGROUND
Patients with prolonged neutropenia caused by chemotherapy or underlying marrow disorders are at risk of invasive bacterial and fungal infections. New treatment options alongside targeted antimicrobial therapy that might improve outcomes include granulocyte transfusions (GTX). To inform the research agenda, a prospective observational cohort study was performed in the Netherlands and United Kingdom. The aim was to describe the incidence, characteristics, and outcomes of patients developing invasive infections and assess patients fulfilling criteria for GTX.
STUDY DESIGN AND METHODS
All patients receiving myeloablative chemotherapy and anticipated to develop 7 or more days of neutropenia (<0.5 × 109/L) were eligible and followed for the development of invasive infections according to a defined algorithm and mortality up to 100 days. Secondary outcomes were types of infection and eligibility for GTX.
RESULTS
A total of 471 patients enrolled at six hematology‐oncology departments were followed for 569 neutropenic episodes. Overall, 32.5% of patients developed invasive infections during their first episode. Significant baseline risk factors for developing infections were high comorbidity scores (WHO performance status ≥ 2, hazard ratio [HR], 2.6 [1.7‐3.9]; and hematopoietic cell transplantation‐comorbidity index score ≥ 2 HR 1.3 [0.9‐1.8]). Infections were bacterial (59.4%) and fungal (22.3%). Despite 34 patients (6.3% of all episodes) appearing to meet criteria to receive GTX, only nine patients received granulocytes. The HR for death was 5.8 (2.5‐13.0) for patients with invasive infections.
CONCLUSION
This study documents that invasive infections are associated with significant mortality. There is a need for new strategies to prevent and treat infections, which may include better understanding of use GTX.
Short abstract
ABBREVIATIONS
- AML
acute myeloid leukemia
- GTX
granulocyte transfusions
- HCT‐CI
hematopoietic cell transplantation‐comorbidity index
- HR(s)
hazard ratio(s)
- MDS
myelodysplastic syndrome
- SCT(s)
stem cell transplantation(s).
Patients with prolonged neutropenia caused by chemotherapy or underlying marrow disorders are vulnerable to infections caused by bacteria and fungi. These infections can cause direct morbidity and mortality, but in addition, patients who survive may experience detrimental delays in receiving further curative chemotherapeutic treatment.1, 2 These problems might occur more frequently as increasing numbers of older patients with comorbidities are admitted for (multiple courses of) myeloablative treatment for hematologic malignancies.
Targeted use of antimicrobial drugs remains the cornerstone of management, but concerns are raised about the challenge of antimicrobial resistance to antibiotics and antifungal agents.3 Other treatment options might include granulocyte transfusions (GTX), although no significant differences in survival of patients receiving GTX could be established in reports of recent trials.4, 5, 6, 7
Recognizing there is little good prospective data on the outcomes of infections, this study was undertaken with the objectives to describe the incidence of invasive infections and outcomes of mortality in inpatients with a hematologic malignancy and to correlate these outcomes with baseline patient characteristics. A secondary aim was to focus on patients that based on current Dutch and British criteria could be considered eligible for GTX. The observed patterns and burden of severe infections in this large cohort of patients with hematologic malignancies would inform the research agenda.
MATERIALS AND METHODS
Study design overview and participating centers
We performed a multicenter prospective observational study termed Neptunis (NEutropenic PatienTs with UNresolved InfectionS). Patients were enrolled over an at least 6‐month period between December 2011 until June 2013 at three hematooncologic departments in the Netherlands (Leiden University Medical Centre adult and pediatric department, Leiden, and HAGA Hospital, The Hague, and three in the United Kingdom [University Hospitals Bristol NHS Foundation Trust, Bristol, Oxford; University Hospitals NHS Foundation Trust, Oxford; and Royal Free Hampstead, London). In five centers allogeneic stem cell transplantations (SCT) were performed; all centers supported induction and consolidation treatments for leukemia and autologous SCT.
Patient eligibility
All consecutive patients admitted to hematology wards that were at risk of developing neutropenia because of the underlying disease and/or the chemotherapy treatment were included. To capture the highest risk group of patients for infection, the inclusion criterion specified an anticipated period of neutropenia (granulocytes < 0.5 × 109/L) as 7 days or more. Patients who were sent home after myeloablative therapy and were readmitted during the neutropenic period were also eligible. Furthermore, patients could be reincluded with each additional neutropenic episode, e.g., because of new treatment courses. Patients with neutrophil functional disorders were not eligible.
Data collection
All recruited patients were followed up for the occurrence of invasive infections. A local experienced study nurse or study physician screened all new patients admitted to the ward. All data were collected as routinely available, and outcome data were retrieved from patient's files (electronic or paper) and via information from the treating physician.
At least weekly, data of inpatients were updated. Baseline data collection included diagnosis of the primary disease, treatment plans including chemotherapy or SCT conditioning regimen, current disease status, and performance status at start of therapy. For the performance status, the Eastern Cooperative Oncology Group8 score (called WHO performance score in this article) and the hematopoietic cell transplantation‐comorbidity index (HCT‐CI)–weighted score (derived from Sorror et al.9) were used. The person recording the data for the study was not involved in decision making with respect to the treatment of the patients. Data entry was undertaken via a secure, validated, and NEN7511‐certified Web‐based database.10
Ethical issues
All the patient data were stored anonymized. The medical ethical committee of the Leiden University Medical Centre gave permission for anonymous data collection. The protocol was reviewed and approved by the National Research Ethics Service in the United Kingdom and the Caldicott Guardians at the Trusts involved.
Outcomes
The primary outcomes were the proportion of patients who developed invasive infections during neutropenia and those who died up to 100 days. Secondary outcomes were the type of invasive infections and the response to treatment, the duration of hospital stay, the cause of death, and the number of patients who would fulfill current criteria for GTX.
An invasive infection was defined using an algorithm (Table 1A) and included patients with a microbiologically defined infection such as a bacteremia (excluding patients with positive blood cultures disappearing after removal of indwelling venous access catheters/devices); a microbiologically defined bacterial invasive site; or a possible, probable, or proven invasive tissue fungal disease (according to EORTC/MSG criteria 2008).11 The site of bacterial infection was defined when possible according to the involved tissue. Patients with longstanding fever and the suspicion of an invasive infection without microbiologic evidence were also included as having an invasive infection. The date of diagnosing the invasive infections was recorded as the date that there was a high clinical suspicion of the infection. Criteria for use of GTX were defined as having granulocytopenia (<0.5 × 109/L) for at least 72 hours and a life‐threatening infection not responding to systemic antimicrobial therapy given for at least 48 hours (Table 1B, Criteria 1‐3). Other used current criteria such as fever, reasonable life expectancy, and expectation of granulocyte recovery were not obligatory (Table 1B, Criteria 4‐6).
Table 1.
Invasive infection algorithm (A) and criteria for GTX (B)
| A. Algorithm for invasive infections | |
| 1 | Does the patient have a significant positive isolate from bacterial blood cultures with a disease‐causing pathogen (excluding positive blood cultures disappearing after removal of IV catheters/devices)? |
| 2 | Does the patient have clinical signs and symptoms compatible with an invasive tissue bacterial infection > 5 cm in diameter? |
| 3 | Does the patient have an (suspicion for) invasive fungal disease? |
| 4 | Does the patient have a strong clinical suspicion for invasive tissue infection despite not clearly fulfilling Criteria 1‐3 above? |
|
If 1 and/or 2 is yes: bacterial invasive infection record was scored. If 3 is yes: fungal invasive infection record was scored. If 4 is yes: both bacterial and fungal invasive infection records were scored. | |
| B. Criteria for GTX* | |
| 1. Granulocytes < 0.5 × 109/L ≥ 72 hr | |
| 2. Life‐threatening infection† | |
| 3. Infection is not responding to systemic antimicrobial therapy ≥48 hr | |
| 4. Fever (>38.0°C) | |
| 5. Life expectancy of more than three months (in absence of infection) | |
| 6. Expecting to recover from the granulocytopenia | |
When fulfilling all Criteria 1, 2, and 3, patients are regarded as eligible for GTX.
An infection was regarded as life‐threatening when there was a clinical deterioration of a patient requiring supportive measurements, like admittance to an intensive care unit, extensive oxygen support (>5 L O2/min), fluid resuscitation in case of severe sepsis (severe sepsis defined as sepsis plus sepsis‐induced organ dysfunction or tissue hypoperfusion), or severe organ dysfunction as a result of the infection.
Invasive infections
The response of the invasive infections was defined separately for bacterial and fungal infections. For patients with a microbiologically defined bacterial infection, success was defined as eradication of all signs, symptoms, and microbiologic evidence of infection.12 For fungal infections responses were defined as follows: complete response—resolution of all attributable clinical signs and symptoms, disappearance of all radiologic lesions, and no new clinical symptoms or signs or radiological abnormalities; partial response—major improvement of fever and attributable clinical signs and symptoms with no new clinical symptoms or signs or radiologic abnormalities and at least 50% decrease in the sum of the areas of the attributable measurable lesion; stable disease—the absence of complete or partial response or progression; and disease progression—worsening of baseline clinical signs and symptoms attributable to the invasive infection or appearance of new clinical signs or symptoms or new radiologic lesions and increase in the sum of areas of the attributable measurable clinical and radiologic lesions or death due to the infection or any cause at any time if response could not be assessed before death.13
GTX
All patients that developed an invasive infection were checked for eligibility to receive GTX according to the criteria in Table 1B. Information about actual use and indications for given GTX whether therapeutically or preemptive (as secondary prophylaxis) was recorded. In one center (HAGA) no GTX were available. Details of collection methods of granulocytes were recorded (apheresis from family donors after granulocyte–colony‐stimulating factor [G‐CSF] and/or dexamethasone or single or pooled buffy coats from whole blood donors).14 Reported adverse events from GTX were documented.
Statistical analysis
The main analysis was descriptive. Comparison between different subgroups was done with the chi‐square test for categorical infection variables. A p value of less than 0.05 was considered significant. For an unbiased estimate of the impact of baseline risk factors on the incidence of infections, and subsequently of those risk factors combined with infection occurrence on death, the time‐dependent nature of the data had to be taken into account. A Cox proportional hazards model for recurrent events was used to estimate the impact of disease, WHO performance status, and HCT‐CI score on the risk of infections for all patients. All episodes were included but only the first infection within an episode was counted as an event. A second Cox model was fitted to assess the impact of infections on death in the first 100 days after start of neutropenia, taking into account the same baseline risk factors and modeling infection as a time‐dependent covariate. For the acute myeloid leukemia (AML) patients, a separate model for infections was fitted, in which disease was replaced by treatment. All analyses were stratified by center to take into account the different level of infections between centers. The models were illustrated by means of predicted hazards and survival probabilities for reference patients. We calculated the proportions of invasive infections and patients fulfilling GTX criteria, using all patients with neutropenia as the denominator. All calculations were performed with computer software (IBM SPSS Statistics, Version 18, IBM Corp.; and R, Version 3.3.0, with package “survival”).
RESULTS
Recruitment
During the study period 513 patients were enrolled at the six hematooncologic departments for 633 expected neutropenic episodes. Patients with 47 neutropenic episodes failed to exceed 7 or more days of neutropenia. A total of 471 patients for 569 neutropenic episodes with complete data formed the final data set for the analysis (Fig. 1). The numbers of included neutropenic episodes per center varied from 70 to 148; in addition, 18 episodes from the pediatric department were added. The maximum number of neutropenic episodes in one patient was six; 85.6% of patients had only one neutropenic episode.
Figure 1.
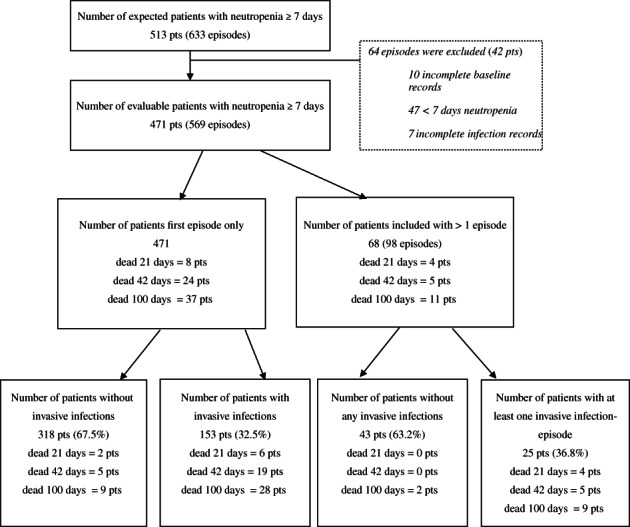
Patients with invasive infections in neutropenic episodes. Flow chart depicting all included patients with expected neutropenia of 7 days or longer and numbers of these patients developing invasive infections in the first neutropenic episode and in subsequent episodes. Numbers of patients who died are depicted.
Baseline characteristics of patients
Of the 471 patients, 297 were male (Table 2). Patients had a median age of 54 years; 18 children (age < 18) were included. AML was the most common underlying hematologic diagnosis (30.1%). A total of 305 patients (65% of all patients) were undergoing SCT at the time of the first neutropenic episode, of whom 141 patients received an autologous SCT and 166 patients received an allogeneic SCT (82 patients conditioned myeloablative and 84 nonmyeloablative or with reduced intensity; Table 2). The second most common underlying cause of neutropenia in this cohort was remission induction or consolidation chemotherapy for acute leukemia (124 patients). Most patients had a low comorbidity score at start of the treatment (88.7% WHO 0 or 1 score and 74.5% HCT‐CI scores of 0 or 1).
Table 2.
Baseline characteristics of patients in their first neutropenic episode*
| All patients | Patients without invasive infections | Patients with invasive infections | |
|---|---|---|---|
| Number | 471 | 318 (67.5) | 153 (32.5) |
| Sex (female/male %) | 36.9/63.1 | 36.2/63.8 | 39.0/61.0 |
| Age (years), median (range) | 54 (0‐91) | 54 (4‐91) | 52 (0‐76) |
| Diagnosis primary disease | |||
| AML | 142 (30.1) | 90 (28.3) | 52 (34.0) |
| ALL | 50 (10.6) | 32 (10.1) | 18 (11.8) |
| MDS | 39 (8.3) | 22 (6.9) | 17 (11.1) |
| NHL | 99 (21.0) | 69 (21.7) | 30 (19.6) |
| MM | 71 (15.1) | 58 ((18.2) | 13 (8.5) |
| other | 76 (16.1) | 50 (15.7) | 26 (17.0) |
| Disease status | |||
| Active/progressive | 162 (34.4) | 106 (33.3) | 56 (36.4) |
| Stable | 36 (7.6) | 24 (7.5) | 12 (7.8) |
| Partial response | 99 (21.0) | 75 (23.6) | 25 (16.2) |
| Complete remission | 163 (34.6) | 111 (34.9) | 52 (33.8) |
| NA | 11 (2.3) | 2 (0.6) | 9 (5.8) |
| Previous SCT | 76 (16.1) | 54 (17.0) | 22 (14.3) |
| Therapy this admission | |||
| Any stem cell transplant | 305 (65.0) | 216 (68.4) | 89 (58.2) |
| SCT‐MA | 82 (17.5) | 53 (16.8) | 29 (19.0) |
| SCT‐RIC | 84 (17.9) | 58 (18.4) | 26 (17.0) |
| SCT‐auto | 141 (30.1) | 106 (33.5) | 35 (22.9) |
| Induction/consolidation leukemia | 124 (26.4) | 75 (23.7) | 49 (32.0) |
| Intensive lymphoma treatment | 27 (5.8) | 18 (5.7) | 9 (5.9) |
| Other | 11 (2.3) | 6 (1.9) | 5 (3.3) |
| WHO performance score | |||
| 0 | 272 (58.5) | 198 (62.3) | 74 (50.3) |
| 1 | 146 (31.4) | 99 (31.1) | 47 (32.0) |
| 2 | 19 (4.1) | 12 (3.8) | 7 (4.8) |
| 3 | 22 (4.7) | 8 (2.5) | 14 (9.5) |
| 4 | 6 (1.3) | 1 (0.3) | 5 (3.4) |
| HCT‐CI comorbidity score | |||
| 0 | 283 (60.6) | 198 (62.9) | 85 (55.9) |
| 1 | 68 (14.6) | 48 (15.2) | 105 (13.2) |
| 2‐3 | 88 (18.8) | 55 (17.5) | 33 (21.7) |
| 4‐5 | 20 4.3) | 12 (3.8) | 8 (5.3) |
| ≥ 6 | 8 (1.7) | 2 (0.6) | 6 (3.9) |
Data are reported as number (%). Percentages in parentheses depict the distribution within diagnosis, disease status, etc.; these are corrected for missing data.
Incidence of invasive infections and risk factors
According to the algorithm for invasive infections, 153 patients (32.5%) evaluated at their first neutropenic episode developed an invasive infection (Fig. 1). This proportion remained at 32.0% when reported for all neutropenic episodes. Most patients experienced one infectious event per neutropenic episode (81.3% of 224 invasive infections); two patients suffered three infectious events during one episode of neutropenia (Table 3).
Table 3.
Characteristics and outcome of invasive infections*
| Invasive infections | Infections in patients NOT fulfilling GTX criteria | Infections in patients fulfilling GTX criteria | p value | |
|---|---|---|---|---|
| All infections | 224 (166 pts) | 173 (138 pts) | 51 (34 pts) | |
| Infectious events per neutropenic episode | 0.04 | |||
| 1 | 182 (81.3) | 146 (84.4) | 36 (70.6) | |
| 2 | 40 (17.8) | 25 (14.5) | 15 (29.4) | |
| 3 | 2 (0.9) | 2 (1.2) | 0 (0) | |
| Fever at diagnosis | 178 (80.9) | 131 (77.5) | 47 (92.2) | 0.02 |
| Bacterial infections | 133 (59.4) | 103 (59.5) | 28 (54.9) | |
| Bacteremia | 118 (89.4) | 95 (92.2) | 22 (78.6) | 0.08 |
| Invasive tissue infection | 21 (15.9) | 12 (11.7) | 9 (32.1) | 0.014 |
| Both | 7 (5.3) | 4 (3.9) | 3 (10.7) | |
| Fungal infections | 50 (22.3) | 33 (19.1) | 17 (33.3) | 0.99 |
| Possible | 27 (54.0) | 18 (54.5) | 9 (52.9) | |
| Probable | 17 (34.0) | 11 (33.3) | 6 (35.3) | |
| Proven | 6 (12.0) | 4 (12.1) | 2 (11.8) | |
| Suspicion of an invasive infection without proof | 41 (18.3) | 37 (21.4) | 6 (11.8) | |
| Outcome of infections after 21 days | ||||
| Blood cultures became negative | 103 (87.3) | 90 (94.7) | 13 (59.1) | 0.019 |
| Bacterial tissue infection | ||||
| CR | 10 (58.8) | 8 (72.2) | 2 (33.3) | |
| PR | 4 (23.5) | 2 (18.2) | 2 (33.3) | |
| SD | 0 | 0 | 0 | |
| PD | 1 (5.9) | 1 (9.1) | 0 | |
| Death | 2 (11.8) | 0 | 2 (33.3) | |
| Fungal tissue infection | ||||
| CR | 14 (31.8) | 13 (43.4) | 1 (7.1) | |
| PR | 19 (43.2) | 10 (33.3) | 7 (50.0) | |
| SD | 3 (6.8 | 3 (10.0) | 2 (14.3) | |
| PD | 4 (9.1) | 3 (10.0) | 1 (7.1) | |
| Death | 4 (9.1) | 1 (3.3) | 3 (21.4) | |
| Episodes with infections | 182 | 146 | 36 | |
| Time of infection after start neutropenia† | 6.0 | 6.0 | 4.5 | 0.118 |
| Considered life threatening | 47 (25.8) | 11 (7.5) | 36 (100) | <0.001 |
| Estimated life expectancy >3 months‡ | 163 (89.6) | 136 (93.2) | 28 (77.8) | <0.001 |
| Expectancy of bone marrow regeneration | 164 (90.1) | 137 (93.8) | 28 (77.8) | <0.001 |
| Infections unresponsive to antimicrobial therapy for | ||||
| 48 hr | 71 (39.0) | 35 (24.0) | 36 (100) | <0.001 |
| 96 hr | 53 (29.1) | 22 (15.1) | 31 (86.1) | <0.001 |
| G‐CSF use | 73 (40.1) | 53 (36.3) | 20 (55.6) | 0.059 |
| Treatment with GTX | 11 (6.0) | 2 (1.4) | 9 (25.0) | <0.001 |
| Length of hospital stay§ | 23.0 | 23.0 | 29 .0 | 0.224 |
Data are reported as number (%).
Median length in days after start neutropenia.
Without the presence of the infection.
Median length of hospital stay until discharge or death in days. Percentages are calculated taken into account missing items. p values are shown comparing infections fulfilling GTX criteria with infections not fulfilling GTX criteria.
CR = complete response; PR = partial response; PD = progressive disease; SD = stable disease.
Patients with myelodysplastic syndrome (MDS) or AML as underlying disease had a higher risk of invasive infections (hazard ratios [HRs], 1.6 and 1.4, respectively), although not significant (Table 4). Significantly fewer invasive infections occurred in patients with low comorbidity scores at baseline (Tables 2 and 4). The HR for developing invasive infections was 2.6 (1.7‐3.9) for patients with a WHO performance status of 2 or higher and 1.3 (0.9‐1.8) for patients with HCT‐CI scores of 2 and higher (Table 4). Taking time into account, the cumulative hazard of infections increased more steeply for patients with more baseline comorbidity (Fig. 2).
Table 4.
Cox proportional hazards models for the development of invasive infections and mortality
| Risk factor | Invasive infections | Mortality | ||
|---|---|---|---|---|
| HR* (95% CI) | p value | HR* (95% CI) | p value | |
| Disease | ||||
| MM | 1 | 1 | ||
| MDS | 1.6 (0.8‐3.3) | 0.2 | 1.1 (0.1‐8.7) | 1 |
| AML | 1.4 (0.8‐2.5) | 0.3 | 2.6 (0.6‐11.4) | 0.2 |
| Other | 1.4 (0.8‐2.5) | 0.3 | 2.6 (0.6‐11.7) | 0.2 |
| WHO | ||||
| 0 | 1 | 1 | ||
| 1 | 1.5 (1.0‐2.3) | 0.06 | 2.2 (1.0‐4.9) | 0.05 |
| ≥2 | 2.6 (1.7‐3.9) | <0.001 | 5.1 (2.1‐12.3) | <0.001 |
| HCT‐CI | ||||
| 0 | 1 | 1 | ||
| 1 | 1.0 (0.63‐1.5) | 0.9 | 1.7 (0.7‐4.1) | 0.3 |
| ≥2 | 1.3 (0.9‐1.8) | 0.16 | 2.4 (1.2‐5.2) | 0.02 |
| Infection† | ||||
| No | 1 | |||
| Yes | 5.8 (2.5‐13.0) | <0.001 | ||
HRs for the outcomes invasive infections and mortality were estimated in multivariable Cox models stratified for differences between centers.
Infection was included as a time‐dependent covariate into the Cox model for death. Its value switched from “no” to “yes” at the moment of onset of infection and remained “yes” afterward.
Figure 2.
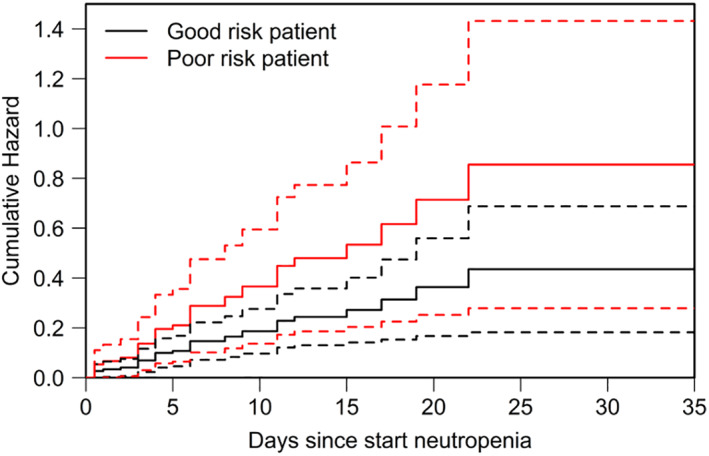
Cumulative hazard of infections, AML patient. In this model‐based figure the cumulative hazard of infections with 95% confidence intervals is depicted for reference patients with AML. The model is presented in Table 4. Good risk is defined as a patient with AML and WHO comorbidity score 0 and HCT‐CI score of 0. Poor risk, WHO score 1, HCT‐CI ≥ 2.
The diagnosis of invasive infections was established at a median of 6 days after start of neutropenia (Table 3). The median duration of neutropenia until granulocytes reached counts of 0.5 × 109/L or higher or death was 13 and 16 days in patients with episodes without and with invasive infections, respectively.
Type and outcome of infections
The infections reported were mainly bacterial infections (59.4%); 21 infections were tissue invasive infections (15.9%, Table 3). Fungal infections occurred in 22.3% of cases. From these, 54.0% were recorded as possible, 34.0% as probable, and 12.0% as proven invasive fungal infections. In 41 cases there was suspicion of an invasive infection without radiological and microbiologic proof. Infection outcome established at 21 days after diagnosing the infection revealed that most patients with bacteremia became blood culture negative upon treatment (87.3%). Tissue invasive infections and fungal disease often did not lead to complete disappearance of the (signs of) infection (Table 3).
Mortality and risk factors
The all‐cause mortality in patients with invasive infections was much higher in the infection group up to 100 days after neutropenia (Fig. 1, Table 4). In the patient group that developed invasive infections 28 patients died within 100 days compared to nine patients in the noninfection group. Using the Cox model for death, the HR for patients with invasive infections adjusted for baseline risk factors was 5.8 (2.5‐13.0).
High comorbidity scores at baseline predicted not only for infections but also for death (Table 4). The survival probability in neutropenic patients with high comorbidity scores at baseline declined further in those that developed invasive infections as illustrated by model‐based curves for reference patients with AML and with multiple myeloma (Fig. 3).
Figure 3.
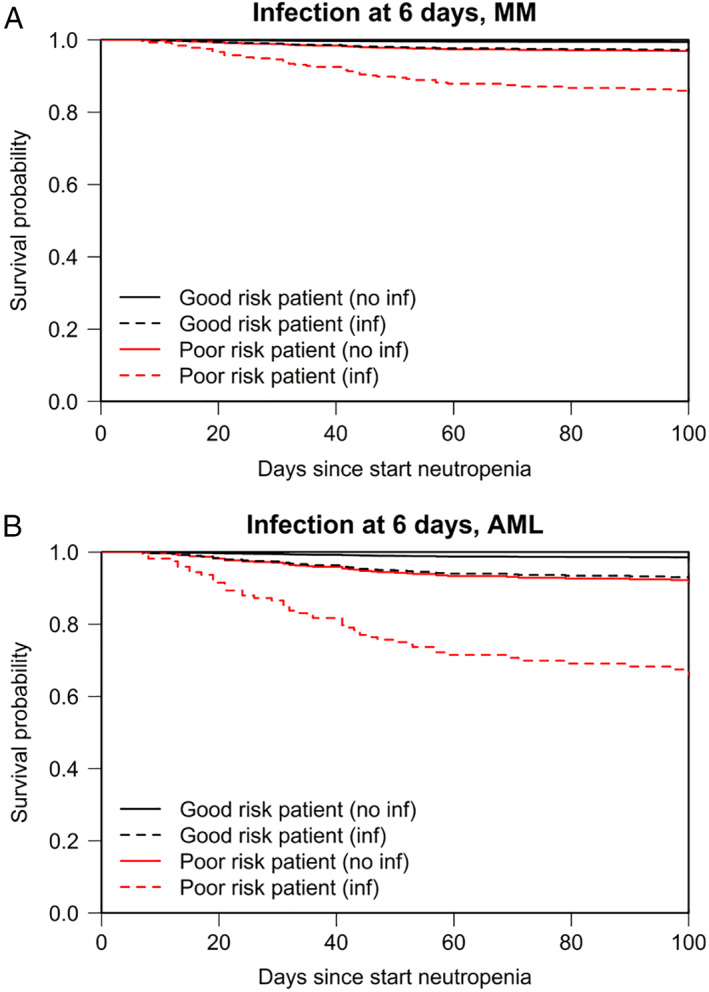
Predicted survival in reference patients. Model‐based predicted survival is shown for reference patients with multiple myeloma (A) and with AML (B). The model is presented in Table 4. The solid lines show the predicted survival for patients who do not develop an infection. The dashed lines show how the survival probabilities change if the patients develop an infection at 6 days after start of neutropenia. Six days was chosen as this is the median time to develop invasive infections after start of neutropenia. Good risk is defined as patients with WHO comorbidity score 0 and HCT‐CI score of 0. Poor risk, WHO score 1, HCT‐CI ≥ 2.
The reported causes of death up to 100 days after neutropenia were variable. Infections were the cause of death in 70% of all causes in the invasive infection group within the first 21 days after start granulocytopenia (total of 10 patients died) compared to 0% in the noninfection group (total of two patients died).
Granulocyte transfusion eligibility
Of the 182 neutropenic episodes with invasive infections, 36 (in 34 patients) fulfilled the criteria for therapeutic GTX (Table 5). In these patients, more invasive infections occurred per neutropenic episode (29.4% had more than one infection, compared to 15.7% in the infection group but not fulfilling the criteria for GTX), as shown in Table 3. Furthermore, invasive tissue bacterial infections per episode occurred more frequently (32.1% of all bacterial infections versus 11.7% in the infection group not fulfilling GTX criteria). In patients with positive blood cultures, significantly fewer patients attained culture negativity (59.1% vs. 94.7%). Patients with neutropenia episodes fulfilling criteria for GTX received G‐CSF in 55.6% of cases compared to 40.1% in the total infection group and had a significantly longer hospital stay: 29 days compared to 23 in the total invasive infection group (Table 3) and 17 days in the noninfections group (data not shown).
Table 5.
Treatments with GTX*
| GTX | All neutropenic episodes 569 episodes | Episodes with invasive infections 182 episodes | Episodes fulfilling GTX criteria 36 episodes |
|---|---|---|---|
| Number of patients | 15 | 11 | 9 |
| Indication | |||
| Therapeutic | 10 (1.8) | 9 (4.9) | 9 (25.0) |
| Preemptive/prophylactic | 5 (0.9) | 2 (1.1) | 0 |
| Source | |||
| Apheresis | 5 | 2 | 2 |
| Single/pooled buffy coats | 9 | 8 | 6 |
| Both | 1 | 1 | 1 |
| Adverse events reported† | 2 | 2 | 1 |
| Reason for stopping GTX | |||
| Granulocytes > 0.5 × 109/L and infection under control | 3 | 3 | 3 |
| Granulocytes > 0.5 × 109/L | 5 | 1 | 1 |
| No improvement/worsening patient | 3 | 3 | 3 |
| Adverse events | 0 | 0 | 0 |
| Death | 1 | 1 | 1 |
| Other‡ | 3 | 2 | 1 |
Data are reported as number (%).
Adverse events reported were febrile reactions (two), one in combination with an allergic reaction with moderately severe hypotension.
Other reasons to stop GTX support were switch to G‐CSF, new formation of HLA antibodies without increment, and one not specified.
For 15 neutropenic episodes GTX were administered, either therapeutically to treat the infection or prophylactically to prevent (flare of) invasive infections (Table 5). In five episodes granulocytes from apheresis donors were administered, in nine from single or pooled buffy coats and in one case from both donor sources. In two cases adverse events were reported (one febrile reaction, one case of moderately severe hypotension in combination with an allergic reaction). Four of nine patients treated therapeutically with GTX were alive after 100 days.
DISCUSSION
This large observational prospective cohort study describes the incidence, types and outcomes of invasive infection in a “real‐world setting” across six hematology wards in two countries. Approximately one‐third of neutropenic patients following intensive chemotherapy develop invasive infections. These infections were significantly associated with an increase in mortality up to 100 days after the start of neutropenia (HR, 5.8 for mortality related to invasive infections). The burden and outcomes of invasive infections is poor, and there is a pressing need to improve early and effective treatment of infections during neutropenia.
Patients with high comorbidity scores both according to the ZUBROD‐ECOG‐WHO Performance Status Scale and HCT‐CI–weighted score are known to have an increased risk for death.9 We show that these high‐risk patients are also more vulnerable to develop invasive infections compared to patients without comorbidity, which adds to their mortality risk. Further work may establish whether the worse comorbidity score is caused by previous or ongoing infection problems or whether invasive microorganisms have a higher likelihood to spread in patients with more comorbidity, such as well recognized for chronic obstructive pulmonary disease. Interestingly, the difference in survival in our cohort between patients that develop invasive infections and those who do not, keeps increasing up to 100 days after start of neutropenia when neutropenia and associated infections usually would have resolved. This could be due to other relevant differences between these groups at baseline such as underlying disease or comorbidity score. However, acquiring an invasive infection itself might induce new complications during or after the infectious episode like organ damage such as pulmonary dysfunction affecting the risk for morbidity and death in the future. In addition, a delay of a subsequent chemotherapy course could additionally increase the risk of death from relapsed disease.
Limitations of our study need to be recognized. The included population is heterogeneous and varies in hematologic diagnosis and reason for granulocytopenia. There may be center differences which we did not allow for, although we corrected for these differences by stratifying in the Cox models for center to get optimal estimates of the impact of the different risk factors. The attributing risk of high comorbidity scores on infection and death remained significant. A relatively high incidence of invasive infections was reported in the pediatric patient group (94%, not shown), although the numbers were small. Strengths of our study include a consecutive patient cohort reflecting the “real world population” best. Using the Cox models, we have tried to also prevent bias that could have occurred when time at risk for infection and death was not taken into account.
One treatment option for these patients might be GTX, although the supporting randomized trial evidence has been unclear.7 The recent randomized controlled trial by Price and colleagues6 included patients with fungemia, bacteremia, invasive tissue bacterial infections, and proven or probable invasive tissue fungal infection, and a high‐dose GTX was used. Although the desired accrual numbers were not met, no differences between the two arms in survival and outcome of infections could be detected. In a subgroup analyses, however, a GTX dose‐related beneficial effect was suggested. In our neutropenic patient cohort, in 6.3% of episodes, patients appeared to fulfill the criteria for GTX but only one‐fourth of them were actually treated with transfusions. This low proportion of patients actually receiving GTX may be due to the lack of availability of granulocyte products, an already poor prognosis or physicians doubting the effectiveness of GTX. Of note, patients fulfilling the criteria for GTX in our study had more invasive tissue infections compared to patients with less severe infections. Furthermore, the response to treatment of these infections was worse, reflecting a subpopulation of patients with severe infections that do not respond to given therapies.
In conclusion, we identified a high burden of invasive infections and poor outcomes. Strong risk factors for developing invasive infections were high comorbidity scores at baseline. The risk of death remained increased in the patients that developed invasive infections during neutropenia up to 100 days. The mortality risk is thus highest in patients with high comorbidity scores that develop invasive infections. Despite multiple advances in new antimicrobial therapies, death due to infections clearly remains a major problem. There is a need for alternative strategies in these patients, which may include a better understanding of the role of GTX.
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
ACKNOWLEDGMENTS
The design of the database build in ProMise was performed by Chantal te Marvelde.
This work was supported by a grant from Amgen Nederland (financial support of the database only).
REFERENCES
- 1. Lyman GH, Michels SL, Reynolds MW, et al. Risk of mortality in patients with cancer who experience febrile neutropenia. Cancer 2010;116:5555–63. [DOI] [PubMed] [Google Scholar]
- 2. Pfaller M, Neofytos D, Diekema D, et al. Epidemiology and outcomes of candidemia in 3648 patients: data from the prospective antifungal therapy (PATH Alliance(R)) registry, 2004‐2008. Diagn Microbiol Infect Dis 2012;74:323–31. [DOI] [PubMed] [Google Scholar]
- 3. Arendrup MC. Update on antifungal resistance in Aspergillus and Candida. Clin Microbiol Infect 2014;20(Suppl 6):42–8. [DOI] [PubMed] [Google Scholar]
- 4. Stanworth SJ, Massey E, Hyde C, et al. Granulocyte transfusions for treating infections in patients with neutropenia or neutrophil dysfunction. Cochrane Database Syst Rev 2005;3:Cd005339. [DOI] [PubMed] [Google Scholar]
- 5. Seidel MG, Peters C, Wacker A, et al. Randomized phase III study of granulocyte transfusions in neutropenic patients. Bone Marrow Transplant 2008;42:679–84. [DOI] [PubMed] [Google Scholar]
- 6. Price TH, Boeckh M, Harrison RW, et al. Efficacy of transfusion with granulocytes from G‐CSF/dexamethasone‐treated donors in neutropenic patients with infection. Blood 2015;126:2153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Estcourt LJ, Stanworth SJ, Hopewell S, et al. Granulocyte transfusions for treating infections in people with neutropenia or neutrophil dysfunction. Cochrane Database Syst Rev 2016;4:Cd005339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649–55. [PubMed] [Google Scholar]
- 9. Sorror ML, Giralt S, Sandmaier BM, et al. Hematopoietic cell transplantation specific comorbidity index as an outcome predictor for patients with acute myeloid leukemia in first remission: combined FHCRC and MDACC experiences. Blood 2007;110:4606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. ProMise database [monograph on the Internet] . 2017. Available from: http://www.msbi.nl/promise/
- 11. De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008;46:1813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. From the Immunocompromised Host Society . The design, analysis, and reporting of clinical trials on the empirical antibiotic management of the neutropenic patient. Report of a consensus panel. J Infect Dis 1990;161:397–401. [DOI] [PubMed] [Google Scholar]
- 13. Herbrecht R, Maertens J, Baila L, et al. Caspofungin first‐line therapy for invasive aspergillosis in allogeneic hematopoietic stem cell transplant patients: an European Organisation for Research and Treatment of Cancer study. Bone Marrow Transplant 2010;45:1227–33. [DOI] [PubMed] [Google Scholar]
- 14. Bashir S, Stanworth S, Massey E, et al. Neutrophil function is preserved in a pooled granulocyte component prepared from whole blood donations. Br J Haematol 2008;140:701–11. [DOI] [PubMed] [Google Scholar]


