Abstract
Objective
To evaluate red blood cell (RBC) transfusion practices in preterm neonates before and after protocol change.
Methods
All preterm neonates (<32 weeks of gestation) admitted between 2008 and 2017 at our neonatal intensive care unit were included in this retrospective study. Since 2014, a more restrictive transfusion guideline was implemented in our unit. We compared transfusion practices before and after this guideline change. Primary outcome was the number of transfusions per neonate and the percentage of neonates receiving a blood transfusion. Secondary outcomes were neonatal morbidities and mortality during admission.
Results
The percentage of preterm neonates requiring a blood transfusion was 37·5% (405/1079) before and 32·7% (165/505) after the protocol change (P = 0·040). The mean number of transfusions given to each transfused neonate decreased from 2·93 (standard deviation (SD) ± 2·26) to 2·20 (SD ±1·29) (P = 0·007). We observed no association between changes in transfusion practices and neonatal outcome.
Conclusion
The use of a more restrictive transfusion guideline leads to a reduction in red blood cell transfusions in preterm neonates, without evidence of an increase in mortality or short‐term morbidity.
Keywords: erythrocyte transfusion, neonate, preterm
Introduction
Blood products, in particular red blood cell (RBC) transfusions, are frequently administered in preterm neonates during their stay in the neonatal intensive care unit (NICU). Up to 90% of extreme preterm neonates receive one or more transfusions in the first few weeks of life 1, 2. RBC transfusions in neonates are mostly administered prophylactically, when haemoglobin (Hb) levels drop below a certain level (transfusion trigger). However, the optimal transfusion trigger in neonates is not known and wide variance in Hb triggers and transfusion guidelines is used internationally 1. In the past decade, studies comparing liberal vs. restrictive transfusion guidelines in neonates showed conflicting results, in terms of short‐term outcome as well as long‐term neurodevelopmental outcome 3, 4, 5, 6. Various studies, both in adults and in children admitted to intensive care units, reported that restrictive RBC transfusion guidelines decrease transfusion requirements and the intrinsically associated patient burden, costs and labour. Importantly, the restrictive transfusion strategies were not associated with an increase in adverse outcomes 7. On the contrary, several studies suggest that less RBC transfusions should also reduce possible transfusion‐related immunomodulation 8, 9. Therefore, the lack of evidence for inferiority, together with the default advantages of restriction, led to ditto recommendations in the national transfusion guideline in 2014. We accordingly adapted our local transfusion protocol from a liberal to a more restrictive transfusion strategy.
The objective of this study was to evaluate the effect of the protocol change on the RBC transfusion practices and investigate the effect on neonatal morbidity and mortality.
Methods
Study design and population
This retrospective observational cohort study was conducted at the Leiden University Medical Centre (LUMC), a tertiary care centre in the Netherlands. We included all consecutive preterm neonates admitted to our NICU between 01‐01‐2008 and 31‐12‐2016, with gestational age at birth between 24 + 0 and 31 + 6 weeks.
The neonates were divided into two cohorts based on their year of birth, before and after the protocol change made in 2014. The study was approved by the Leiden Medical Ethics Committee (institutional review board) in July 2017 (G17·045).
Changes in protocol
Before 2014, the guidelines used in the LUMC for RBC transfusions in neonates were based on the Dutch national guidelines from 2004 10. Since 2014, our local guideline was changed, based on a Cochrane systematic review 11. The transfusion guidelines before 2014 were mainly based on specific Hb triggers (arterial or venous blood samples) and the need and type of respiratory support. The transfusion guidelines after 2014 were based on lower Hb triggers (also arterial or venous blood samples) in association with both the need of respiratory support and postnatal age in weeks (Table 1). The transfusion dosage, velocity of infusion and irradiation requirements remained the same during the two study periods. Routine transfusion dosage was 15 ml/kg administered in 3 h, using irradiated and leucocyte‐depleted product.
Table 1.
(a) Haemoglobin transfusion triggers before guideline change in 2014. (b) Haemoglobin transfusion triggers after guideline change in 2014
| Respiratory support | g/dl |
|---|---|
| (a) Transfusion guideline before 2014 | |
| Mechanical ventilation | <13 |
| CPAP and/or oxygen | <11·5 |
| No respiratory support | <10 |
| Postnatal age |
Respiratory support g/dl |
No respiratory support g/dl |
|---|---|---|
| (b) Transfusion guideline after 2014 | ||
| Day 0–6 (week 1) | <11·5 | <10 |
| Day 7–13 (week 2) | <10 | <8·5 |
| Day ≥ 14 (week ≥ 3) | <8·5 | <7·5 |
CPAP, continuous positive airway pressure.
After postnatal age ≥ 28 days, the recommended transfusion trigger was <7 g/dl (4·5 mmol/l).
Outcome measures
The primary outcome was the number of neonates requiring a RBC transfusion and the number of RBC transfusions per neonate. The secondary outcomes were changes in neonatal morbidities and mortality during admission. Data were furthermore analysed according to gestational age at birth and week of life. We recorded the following neonatal variables, including respiratory distress syndrome (RDS) defined as respiratory failure requiring ventilator support and surfactant treatment, severe intraventricular haemorrhage (IVH grade 3 or 4), cystic periventricular leukomalacia (PVL grade 2 or 3), symptomatic patent ductus arteriosus (PDA) requiring medical treatment (indomethacin or ibuprofen) or surgical closure, necrotizing enterocolitis (NEC) ≥ stage 2 12, proven neonatal sepsis defined as a clinically ill neonate with a positive bacterial blood culture and neonatal mortality and length of stay at our NICU. The recorded short‐term morbidities were not indications for transfusions, but used as secondary outcomes for this study.
Sample size
In order to detect 10% difference in transfusions before and after the protocol change (assuming 40% in the cohort before and 30% in the cohort after 2014), with a power of 80% and type one error of 5%, the sample size was estimated to be at least 354 neonates per cohort.
Statistical analysis
Results are expressed as percentages, mean (standard deviation (SD)) for normally distributed values (as calculated for with the Shapiro–Wilk test) or median (interquartile range (IQR)) for nonnormally distributed values. Results of categorical variables were compared using the chi‐squared test, whereas the independent Student's t‐test and Mann–Whitney U‐test were used for normally and nonnormally distributed continuous variables, as appropriate. A P‐value <0·05 was considered to indicate statistical significance. All statistical analyses were performed using spss version 23·0 (IBM, Chicago, IL, USA).
Results
A total of 1584 preterm neonates delivered before 32 weeks of gestation were admitted to our NICU between 1 January 2008 and 31 December 2016, of which 1079 in the first study period (01/01/2008‐31/12/2013) and 505 in the second study period (01/01/2014‐31/12/2016). The baseline characteristics of all included neonates from both cohorts are presented in Table 2. In the second period, significantly more children were born by caesarean section.
Table 2.
Baseline characteristics
| 2008‐2013 (n = 1079) | 2014‐2017 (n = 505) | P‐value | |
|---|---|---|---|
| Male gender | 581 (53·8%) | 271 (53·7%) | 0·957 |
| Gestational age (in weeks) | 29 (24‐31) | 29 (24‐31) | 0·669 |
| Inborna | 968 (89·7%) | 463 (91·7%) | 0·236 |
| Birthweight (in grams) | 1259 (375) | 1257 (377) | 0·921 |
| SGAb | 100 (9·3%) | 38 (7·5%) | 0·293 |
| Caesarean section | 510 (47·3%) | 267 (52·9%) | 0·052 |
| Multiple birth | 448 (41·5%) | 194 (38·4%) | 0·249 |
Values given as mean (SD), median (range) or n (%).
Inborn: born in the LUMC.
SGA: small for gestational age (birthweight below the 10th centile according to Dutch neonatal birthweight curves 28).
Primary outcome
The percentage of neonates receiving a RBC transfusion decreased significantly from 37·5% (405/1079) in the first period and 32·7% (165/505) in the second period (P = 0·040). The mean number of transfusions given to each transfused neonate decreased from 2·93 (± 2·26) transfusions per neonate in the first study period to 2·20 (± 1·29) in the second period (P = 0·007).
Secondary outcomes
In both cohorts, gestational age at birth was related to the need for RBC transfusions (Figs. 1 and 2). We found no difference in percentage of neonates requiring a transfusion nor in number of transfusions per neonate before and after protocol changes, when analysed by gestational age at birth.
Figure 1.
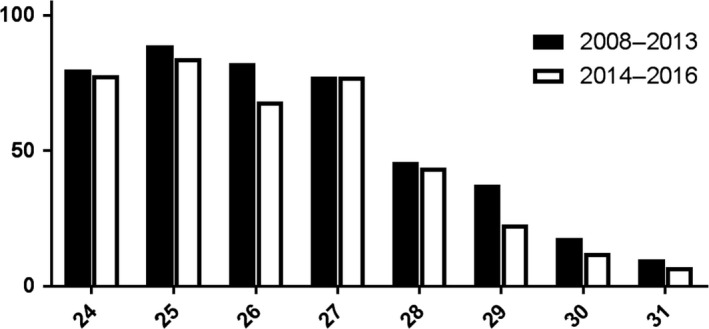
Percentage of neonates receiving RBC transfusion (Y‐axis) per gestational age at birth in weeks (X‐axis), before and after guideline change.
Figure 2.
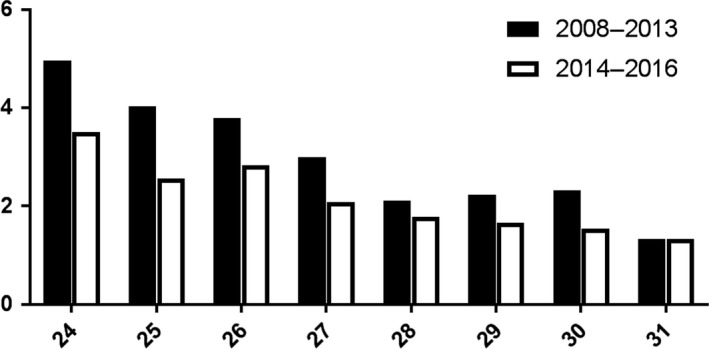
Mean number of transfusions per neonate (Y‐axis) per gestational age at birth in weeks (X‐axis), before and after guideline change.
RBC transfusions are mostly administered in the first week of life (Fig. 3). In the second study period, the need for RBC transfusion was significantly lower in the third week (P < 0·001) and fourth week of life (P = 0·036), but not in the first 2 weeks.
Figure 3.
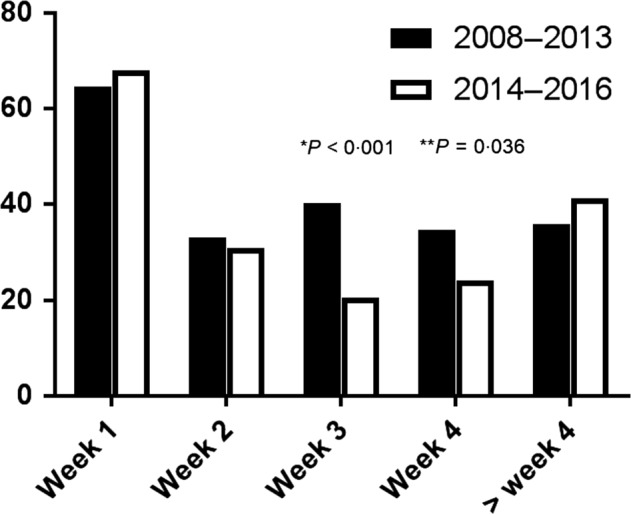
Percentage of neonates requiring a blood transfusion in the first 4 weeks of life and thereafter.
Table 3 shows the incidence of neonatal morbidity and mortality in both cohorts, both in the overall group of included neonates and in the subgroup receiving at least one RBC transfusion. When comparing the study periods, a significant decrease in incidence of severe IVH was found. In the second study period, the length of hospitalization was significantly higher compared to the first period.
Table 3.
Transfusion requirement and neonatal outcomes before and after transfusion guideline changes
| All neonates | Neonates receiving at least one RBC transfusion | |||||
|---|---|---|---|---|---|---|
| 2008‐2013 (n = 1079) | 2014‐2017 (n = 505) | P‐value | 2008‐2013 (n = 410) | 2014‐2017 (n = 165) | P‐value | |
| NEC | 3·7% | 5·1% | 0·181 | 5·9% | 8·5% | 0·254 |
| Proven sepsis | 24·2% | 26·3% | 0·357 | 43·7% | 44·2% | 0·917 |
| PDA | 13·2% | 12·9% | 0·874 | 27·6% | 32·7% | 0·224 |
| RDS | 37·5% | 36·4% | 0·699 | 64·1% | 62·4% | 0·754 |
| Severe IVH (grade 3/4) | 7·6% | 3·2% | 0·000 | 15·1% | 4·8% | 0·001 |
| Cystic PVL (≥ grade 2) | 1·0% | 1·6% | 0·337 | 2·0% | 3·0% | 0·431 |
| Mortality | 8·1% | 5·5% | 0·072 | 12·2% | 7·3% | 0·084 |
| Hospitalization, median number of days (IQR) | 12 (5–27) | 14 (7–32) | 0·001 | 29 (18–42) | 41 (21–56) | <0·001 |
| Receiving a RBC transfusion | 37·5% | 32·7% | 0·040 | 100% | 100% | ‐ |
| Number of transfusions per neonate | 1·11 (± 1·99) | 0·72 (± 1·27) | 0·009 | 2·93 (± 2·26) | 2·20 (± 1·29) | 0·007 |
| Number of transfusions per 100 days admission, mean | 5·3 (± 12·4) | 2·7 (± 7·4) | <0·001 | 13·9 (± 16·9) | 8·2 (± 11·0) | <0·001 |
Therefore, we calculated the number of transfusions per 100 admission days. The number of RBC transfusions per 100 days of hospitalization was 5·3 (SD ± 12·4) in the first period and 2·7 (SD ± 7·4) per 100 days in the second period (P < 0·001). For the neonates who received at least one RBC transfusion, the number of transfusions per 100 days of hospitalization was 13·9 (SD ±16·9) in the first period and 8·2 (SD ±11·0) in the second period (P < 0·001).
Discussion
In this large retrospective single centre study in more than 1500 very preterm neonates, we found a significant decrease in the rate of RBC transfusions. Since the introduction in 2014 of a more restrictive RBC transfusion guideline, the rate of neonates requiring a RBC transfusion dropped from 37·5 to 32·7% and the mean number of transfusions per transfused neonates was reduced from 2·93 to 2·20. The reduction in RBC transfusions using a more restrictive guideline did not result in an increase in mortality or morbidity. Recently, a possible association has been reported between red blood cell transfusions and NEC (transfusion‐associated NEC: TANEC 13, 14, 15). However, the causative relation is controversial and has not been proven yet. In this study, we found no significant changes in the incidence of NEC despite reduction in blood transfusions. Importantly, our study was not powered to detect changes in the incidence of NEC.
Various factors, besides the protocol changes, could have contributed to this decrease. Increased awareness of the possible adverse effects of RBC transfusions in the latter years may have increased the adherence to transfusion guidelines and reduced the rate of protocol violation (both effects were not measured in this study). Several studies have shown that strict adherence to transfusion protocols reduces the number of RBC transfusions in preterm neonates 16, 17.
Also, the reduced transfusion rate in the latter period could be related to an increased awareness on the deleterious impact of repeated blood testing on anaemia of prematurity 18, 19. Reducing the amount of blood drawn for laboratory testing in very preterm neonates reduces the risk of iatrogenic blood loss and the number of RBC transfusion 20, 21.
Another crucial factor on the Hb level at birth and the need for RBC transfusions is the timing of cord clamping at birth. Various studies have shown that delaying cord clamping will result in a higher Hb level at birth and reduce the initial need for RBC transfusions 22. Although our local guideline underscores the importance of delayed cord clamping, the timing of cord clamping is left to the discretion of the attending obstetrician, and the timing of cord clamping was unfortunately not routinely registered during the study period. As reported here above, it is conceivable that also other factors, besides the protocol change, may have contributed to the reduction in RBC transfusion. The baseline characteristics of the two cohorts were almost similar, except for a decrease in severe IVH and a longer period of hospitalization after the protocol change. The reduced incidence of severe IVH can be attributed to multiple factors. The longer stay in the more recent period may have led to an underestimation of the effects of restrictive transfusion guidelines since one could argue that the longer a neonate is admitted to a NICU, the sicker the neonate could be and the more at risk a neonate is for receiving a transfusion. We therefore calculated the rate of RBC transfusions per 100 hospitalization days and showed a stronger impact of restrictive transfusion guidelines on the reduction in transfusions.
This study also confirmed the positive correlation between extreme prematurity and need for RBC transfusions. The blood sparing effect of a more restrictive transfusion protocol was particularly pronounced in week 3 and 4 after birth, and not so much in the first 2 weeks of life. This difference in effect is probably due to the differences in triggers for blood transfusion between the two guidelines. The trigger for blood transfusion in week 3 and 4 was much lower compared to the initial guideline. We found no difference in the need of transfusions when analysed by gestational age at birth between the two periods. The lack of association between gestational age and transfusions could be due to a limited sample size since our study was not powered to detect differences related to gestational age at birth.
To date, international consensus on the optimal guideline for RBC transfusion in neonates is lacking and different protocol and transfusion triggers are being used around the world. The lack of consensus is partly due to contradicting results of various studies. Bell et al. 12 suggested that liberal RBC transfusions improved the short‐term neonatal outcome, but these findings were not confirmed in a larger study by Kirpalani et al. 3 (PINT study). Chen et al. 17 found an significant decrease in chronic lung disease due to restrictive transfusion threshold, without other significant changes in morbidity. Importantly, only one study assessed the effect of restrictive vs. liberal transfusions on the long‐term neurodevelopmental outcome. Although no major differences were found, a borderline statistical significant difference in cognitive delay favouring the liberal strategy was reported 4. In addition, Nopoulos et al. 23 showed that RBC transfusions affected the long‐term outcome of premature infants, inflicting reduced brain volumes especially in those who were transfused with a liberal strategy. In the Cochrane systematic review, Whyte and Kirpalani 11 concluded that the use of restrictive as compared to liberal transfusion guidelines resulted in modest reductions in exposure to transfusion, without a significant impact on death or major morbidities. In 2014, Ibrahim et al. 24 performed a meta‐analysis which reflects the results of the Cochrane review. These partly contradicting results highlight the need for more studies and clearer evidence to reach global consensus on transfusion thresholds and improve neonatal care 2, 25. Two large prospective studies (ETTNO study 26 and TOP study 27) comparing liberal vs. restrictive RBC transfusion strategies are currently being conducted. These studies also compare neurological outcomes up to 2 years of age. The results of these studies are eagerly awaited and will hopefully provide more information on the impact of different transfusion thresholds, contributing to an international consensus.
In conclusion, our study shows that the use of a more restrictive transfusion protocol has led to a reduction in RBC transfusions without an increase in morbidity or mortality. In addition to optimizing neonatal care, reducing the amount of transfusions also reduces the costs of neonatal medical care.
References
- 1. von Lindern JS, Lopriore E: Management and prevention of neonatal anemia: current evidence and guidelines. Expert Rev Hematol 2014; 7:195–202 [DOI] [PubMed] [Google Scholar]
- 2. Strauss RG: Anaemia of prematurity: pathophysiology and treatment. Blood Rev 2010; 24:221–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kirpalani H, Whyte RK, Andersen C, et al: The Premature Infants in Need of Transfusion (PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr 2006; 149:301–307 [DOI] [PubMed] [Google Scholar]
- 4. Whyte RK, Kirpalani H, Asztalos EV, et al: Neurodevelopmental outcome of extremely low birth weight infants randomly assigned to restrictive or liberal hemoglobin thresholds for blood transfusion. Pediatrics 2009; 123:207–213 [DOI] [PubMed] [Google Scholar]
- 5. Venkatesh V, Khan R, Curley A, et al: The safety and efficacy of red cell transfusions in neonates: a systematic review of randomized controlled trials. Br J Haematol 2012; 158:370–385 [DOI] [PubMed] [Google Scholar]
- 6. McCoy TE, Conrad AL, Richman LC, et al: Neurocognitive profiles of preterm infants randomly assigned to lower or higher hematocrit thresholds for transfusion. Child Neuropsychol 2011; 17:347–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lacroix J, Hébert PC, Hutchison JS, et al: Transfusion strategies for patients in pediatric intensive care units. N Engl J Med 2007; 356:1609–1619 [DOI] [PubMed] [Google Scholar]
- 8. Kleiber N, Lefebvre E, Gauvin F, et al: Respiratory dysfunction associated with RBC transfusion in critically ill children: a prospective cohort study. Pediatr Crit Care Med 2015; 16:325–334 [DOI] [PubMed] [Google Scholar]
- 9. Muszynski JA, Spinella PC, Cholette JM, et al: Transfusion‐related immunomodulation: review of the literature and implications for pediatric critical illness. Transfusion 2017; 57:195–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bloodtransfusion., D.i.f.h.c.i.C.G . (2004). Guideline Blood transfusion. Utrecht, The Netherlands: van Zuiden Communication BV: 133–139 [Google Scholar]
- 11. Whyte R, Kirpalani H: Low versus high haemoglobin concentration threshold for blood transfusion for preventing morbidity and mortality in very low birth weight infants. Cochrane Database Syst Rev. 2011;(11):CD000512. [DOI] [PubMed] [Google Scholar]
- 12. Bell EF, Strauss RG, Widness JA, et al: Randomized trial of liberal versus restrictive guidelines for red blood cell transfusion in preterm infants. Pediatrics 2005; 115:1685–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maheshwari A, Patel RM, Christensen RD: Anemia, red blood cell transfusions, and necrotizing enterocolitis. Semin Pediatr Surg 2018; 27:47–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mohamed A, Shah PS: Transfusion associated necrotizing enterocolitis: a meta‐analysis of observational data. Pediatrics 2012; 129:529–540 [DOI] [PubMed] [Google Scholar]
- 15. Paul DA, Mackley A, Novitsky A, et al: Increased odds of necrotizing enterocolitis after transfusion of red blood cells in premature infants. Pediatrics 2011; 127:635–641 [DOI] [PubMed] [Google Scholar]
- 16. Alagappan A, Shattuck KE, Malloy MH: Impact of transfusion guidelines on neonatal transfusions. J Perinatol 1998; 18:92–97 [PubMed] [Google Scholar]
- 17. Chen HL, Tseng HI, Lu CC, et al: Effect of blood transfusions on the outcome of very low body weight preterm infants under two different transfusion criteria. Pediatr Neonatol 2009; 50:110–116 [DOI] [PubMed] [Google Scholar]
- 18. Lin JC, Strauss RG, Kulhavy JC, et al: Phlebotomy overdraw in the neonatal intensive care nursery. Pediatrics 2000; 106:E19 [DOI] [PubMed] [Google Scholar]
- 19. Christensen RD, Carroll PD, Josephson CD: Evidence‐based advances in transfusion practice in neonatal intensive care units. Neonatology 2014; 106:245–253 [DOI] [PubMed] [Google Scholar]
- 20. Luban NL: Management of anemia in the newborn. Early Hum Dev 2008; 84:493–498 [DOI] [PubMed] [Google Scholar]
- 21. Bateman ST, Lacroix J, Boven K, et al: Anemia, blood loss, and blood transfusions in North American children in the intensive care unit. Am J Respir Crit Care Med 2008; 178:26–33 [DOI] [PubMed] [Google Scholar]
- 22. Rabe H, Reynolds G, Diaz‐Rossello J: A systematic review and meta‐analysis of a brief delay in clamping the umbilical cord of preterm infants. Neonatology 2008; 93:138–144 [DOI] [PubMed] [Google Scholar]
- 23. Nopoulos PC, Conrad AL, Bell EF, et al: Long‐term outcome of brain structure in premature infants: effects of liberal vs restricted red blood cell transfusions. Arch Pediatr Adolesc Med 2011; 165:443–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ibrahim M, Ho SK, Yeo CL: Restrictive versus liberal red blood cell transfusion thresholds in very low birth weight infants: a systematic review and meta‐analysis. J Paediatr Child Health 2014; 50:122–130 [DOI] [PubMed] [Google Scholar]
- 25. New HV, Berryman J, Bolton‐Maggs PH, et al: Guidelines on transfusion for fetuses, neonates and older children. Br J Haematol 2016; 175:784–828 [DOI] [PubMed] [Google Scholar]
- 26. Franz A: Effects of Transfusion Thresholds on Neurocognitive Outcome of Extremely Low Birth Weight Infants (ETTNO). 2011. 12‐05‐2016 [cited 2017 07‐07‐2017]; Available from: https://clinicaltrials.gov/ct2/show/NCT01393496 [Last accessed 07 July 2017]
- 27. Network NR : Transfusion of Prematures Trial (TOP). 2012. 26‐01‐2018; Available from: https://clinicaltrials.gov/ct2/show/NCT01702805. [Last accessed 07 July 2017]
- 28. Kloosterman GJ: Intrauterine growth and intrauterine growth curves. Maandschr Kindergeneeskd 1969; 37:209–225 [PubMed] [Google Scholar]


