Abstract
In Mediterranean climate areas, the available scenarios for climate change suggest an increase in the frequency of heat waves and severe drought in summer. Grapevine (Vitis vinifera L.) is a traditional Mediterranean species and is the most valuable fruit crop in the world. Currently, viticulture must adjust to impending climate changes that are already pushing vine‐growers toward the use of irrigation, with the concomitant losses in wine quality, and researchers to study tolerance to stress in existing genotypes. The viticulture and winemaking worlds are in demand to understand the physiological potential of the available genotypes to respond to climate changes. In this review, we will focus on the cross‐talk between common abiotic stresses that currently affect grapevine productivity and that are prone to affect it deeper in the future. We will discuss results obtained under three experimental stress conditions and that call for specific responses: (1) acclimatization of in vitro plantlets, (2) stress combinations in controlled conditions for research purposes, (3) extreme events in the field that, driven by climate changes, are pushing Mediterranean species to the limit. The different levels of tolerance to stress put in evidence by the plasticity of phenotypic and genotypic response mechanisms, will be addressed. This information is relevant to understand varietal adaptation to impending climate changes and to assist vine growers in choosing genotypes and viticulture practices.
Abbreviations
- ABA
abscisic acid
- EL
electrolyte leakage
- HS
heat stress
- HSP
heat shock protein
- ROS
reactive oxygen species
- ZFP‐TF
zinc finger protein transcription factors
- sHSPs
small heat shock proteins
- ERF
ETHYLENE RESPONSE FACTOR
Introduction
The challenges of research on combined abiotic stresses
Abiotic stress is defined as an environmental factor potentially unfavorable to an organism, and ‘resistance’ is the capacity of an organism to overcome that environmental constraint (Levitt 1980). In the case of crop plants, the effect of the stress over the economically relevant part of the plant (the effect on the ‘harvest’) is also important to consider. The most common abiotic stresses that affect plants include drought, salinity, extreme temperatures, acid soils and chemical pollution (Tester and Bacic 2005, Vinocur and Altman 2005) and can impair plant growth and productivity worldwide by more than 50% (Bray et al. 2000). Drought, salinity and chemical pollution can lead to equivalent cellular responses, such as the production of stress proteins, upregulation of anti‐oxidants and accumulation of compatible solutes (reviewed in Wang et al. 2003). Temperatures above the optimum are sensed as heat stress (HS) by living organisms. HS disturbs cellular homeostasis and can lead to severe retardation in growth and development and even death (Kotak et al. 2007). All these primary stresses lead to secondary stresses, such as osmotic and oxidative stress. Oxidative stress, e.g. frequently accompanies high temperature and drought and may cause denaturation of functional and structural proteins (Mittler 2006).
In open field conditions, at any given moment, several abiotic stresses affect plants simultaneously (Mittler 2006). The combination of different stresses can induce synergistic or antagonistic responses. For example, in areas of the world with a Mediterranean climate, it is common that in the summer plants are exposed to high temperatures and/or drought and also to high levels of light causing photoinhibition. In the situation of simultaneous heat and drought, stomatal movements are opposite to those occurring upon heat alone (Rizhsky et al. 2002). Also, drought combined with high light can result in an enhanced production of reactive oxygen species (ROS) by the photosynthetic apparatus (Mullineaux et al. 2006). When photosynthesis is reduced, plants will likely experience the effects of the secondary type of stress termed oxidative stress. One example of the antagonistic effect of combined stresses is salinity and heat because the higher transpiration rates caused by heat could result in increases in the uptake of salt (Wang et al. 2003).
How will climate changes affect grapevine in the near future?
In the Mediterranean region where grapevine has been grown for a long‐time, the available scenarios for climate change over the pending decades suggest an increase in aridity and shifts in the amount, seasonality and distribution of precipitation (Pinto et al. 2011). The predicted higher frequency of summer heat waves together with a longer dry season will lead to extended and severe drought events. The ensuing overexploitation of water resources for agriculture purposes will limit plant growth and fruit development and therefore the fulfillment of their potential yields (Chaves 2002, Chaves et al. 2010). Drought and heat represent an excellent example of two different abiotic stresses occurring simultaneously (Knight and Knight 2001) in the Mediterranean surrounding areas, with severe consequences for viticulture and the range of wine characteristics that can be produced from the same variety, such as decrease in acidity (Escudier et al. 2017). Projection models indicate that, as early as 2040, viticulture will have moved its distribution northward and uphill, leading to changes in plant phenology, anticipating flowering and ripening (Ramos et al. 2015, Fraga et al. 2017), in order to avoid significant changes to the characteristics of the wines produced.
Experimental systems used to study the effects of climate changes
Taking the above scenario into account, it comes as no surprise that research on grapevine in the past years has focused on the effects of climate changes on plant productivity and grape quality, with work performed in controlled conditions and in the field.
In vitro artificial systems are helpful to elucidate specific questions at the metabolic level and also to extrapolate plant responses to specific stresses (e.g. light, salinity) using very simple layouts. However, to study individual and combined stresses and their effects in artificially controlled conditions, growth rooms/chambers that attempt to mimic ‘natural’ conditions must be used, or directly in field environments. Environmental control in greenhouses helps to focus on features of the plant's response and thus can elucidate important mechanisms of tolerance, but often results cannot be easily extrapolated to plants in field conditions when the whole environment is changing and affecting the plant. In the case of perennial species in which the fruit is the economically important product this is even more pressing, as in standard controlled conditions the reproductive cycles are hard to mimic. Grapevine is one of such species, mainly cultivated in areas of Mediterranean climate primarily for winemaking and table grapes.
Abiotic stress in the field: learning to adjust to climate changes
Genotype vs environment
Grapevine varieties are characterized by a distinct sensitivity to the environment. The metabolic characteristics of the berries show high phenotypic plasticity, summed up in the concept of terroir, which combines varietal attributes with the climate, soil and winemaking practices (Spielmann and Gélinas‐Chebat 2012). Furthermore, all the possible interactions among them offer advantages as evidenced by the adaptation of cultivated varieties to different growing regions (Keller 2010, Dai et al. 2011). Thus, there is a pressing need to understand the existing genetic diversity between and within varieties, and its influence on the physiological potential of the available genotypes to respond to climate changes and foster new terroirs. In light of this, an assembly of 65 differentially expressed genes (DEGs), known to respond to abiotic stresses in two well‐studied genotypes, ‘Touriga Nacional’ and ‘Trincadeira’ (Rocheta et al. 2016), was designed to scan gene expression in leaves of 10 traditional Portuguese varieties growing in two Portuguese regions with distinct environmental conditions: one with a typical Mediterranean climate and the other with a marked Atlantic influence (Carvalho et al. 2017). Due to the experimental set‐up behind the array design, the genotypes were characterized as ‘sensitive’ or ‘tolerant’ to abiotic stress and, furthermore, the DEGs were able to distinguish the main abiotic stress that each genotype/environment was subjected and responding to (drought, heat or excess light; Carvalho et al. 2017).
The relevance of the interactions between genotype and environment was also reported by Dal Santo et al. (2018) in berries of ‘Sangiovese’ and ‘Cabernet Sauvignon’ grown in Central Italy (in Tuscany and on the Adriatic coast). This study confirms that there is a huge berry transcriptomic plasticity, reflecting locations and vintages and that gene expression is differentially modulated in response to environmental cues (Dal Santo et al. 2013). In both studies, the location of the vineyard had a minor impact on the extent of transcriptome changes in grapes, while qualitative traits, such as the accumulation of secondary metabolites related to wine aroma and color, were significantly affected by the location (Dal Santo et al. 2013, 2018).
The regulation of the specialized metabolism of organic acids and sugars and of precursors of aromatic and phenolic compounds in berries of ‘Shiraz’ and ‘Cabernet Sauvignon’ also indicates a strong variety‐dependent response (Degu et al. 2014). Plants were grown in adverse environmental conditions and ‘Shiraz’ had greater upregulation of the entire polyphenol pathway and higher accumulation of piceid and coumaroyl anthocyanin forms than ‘Cabernet Sauvignon’ beginning at veraison, in concordance with transcript profiling of the genes encoding enzymes of key steps in the phenylpropanoid pathway. Overall, the enhanced response of stress‐related metabolites, such as trehalose, stilbene and abscisic acid (ABA) in ‘Shiraz’ berry‐skin are consistent with its relatively higher susceptibility to environmental cues than ‘Cabernet Sauvignon’ (Degu et al. 2014). The knowledge of these physiological modulations by the environment led to the recent research suggesting the use of cross‐breeding to exploit the diversity among varieties for berry size, sugar accumulation and malic to tartaric acid ratio and develop new varieties able to withstand adverse effects of climate warming on berry volume and quality (Bigard et al. 2018). In experiments performed in Montpellier, France, irrigated and nonirrigated plants of different varieties were analyzed for berry size and composition and a huge variability in the contents of malic and tartaric acids were found, together with some correlation with berry growth (Bigard et al. 2018).
The potential to adapt to climate changes
Summer drought together with heat waves is the most common abiotic stress combination in Mediterranean grapevine‐producing regions and the one that has given rise to a larger number of studies (Hannah et al. 2013). In addition to the increase in average temperature, the higher frequency of extreme events must be considered for a reliable climate change impact assessment on viticulture (Challinor et al. 2005). These conditions are reflected into two different trends, the first one is an increase of late spring frost at bud‐burst as the result of anticipated phenological states, reported for the first time in the region of Tuscany (Moriondo and Bindi 2007). On the other hand, phenological phases of fruit set and berry ripening will be more prone to heat waves in the summer (Moriondo and Bindi 2007). In a model developed for summer crops such as sunflower and wheat, the grain filling period was highly reduced (Moriondo et al. 2011). These data were used on a projection model for grapevine, supported by previous observations that higher temperatures shifted post‐bud break development to earlier, cooler conditions that countered the effects of increasing temperature (Moriondo and Bindi 2007). As a consequence, higher temperatures did not decrease the length of bud break to anthesis period whereas the length of anthesis to maturation time lapse was largely shortened (Moriondo and Bindi 2007). This model points to a major decrease in yield due to the combined effect of a shorter berry ripening period associated with summer drought (Moriondo et al. 2011). The phenological observations of Duchêne and Schneider (2005) over a long period in Alsace also fit well within the proposed model, the authors highlighted the pressure of high temperatures at ripening and the negative influences of anticipated flowering.
Heat waves occurring during key berry development stages can have devastating impacts on production even in case of overall favorable weather conditions for the rest of the season. It is well documented that heat stress (HS; Tmax >35°C) during berry ripening is associated with inhibition of photosynthesis and thus lower sugar accumulation and yield (Greer and Weston 2010, Greer et al. 2011). This is due to the decrease of berry size while the overall lower quality is a result of inhibition of color and flavor development (Greer and Weedon 2013). On the other hand, frost events at bud break are related to shoot death and lower yield (Friend et al. 2011). Precocity may be crucial for grapevine to escape intense drought and heat events during fruit ripening and the choice of the plant material is the first variable to consider for warmer climate adaptation. In both rootstocks and scions, genetic variability allows the choice of precocious genotypes tolerant to drought (Duchêne and Schneider 2005). However, for premium musts, adaptation may include the shift of varieties from their original areas of growth to new areas that match their traditional environmental requirements. Thus, an increase of temperature of 5°C (predicted for 2100; Fraga et al. 2017) would shift premium‐wine varieties toward altitudes above 400 m, whereas low elevation areas would likely be suitable for lower quality varieties, producing high alcoholic wines (Moriondo and Bindi 2007).
Climate changes are predicted to decrease the acidity of berries mostly through the decrease of malic acid, with significant costs on quality (Escudier et al. 2017). Grape malic and tartaric acid concentrations have poor stability and heritability and are dependent on genotype, environment and the respective interaction effects, that up to now have impaired the identification of markers (Houel et al. 2015). Therefore, the correlation found between berry size and malic/tartaric acids ratio (Bigard et al. 2018) is interesting to use as an indicator of berry quality. It was also found that phenological events throughout the growing season are responsible for the difference in size between table and wine grapes (Migicovsky et al. 2017; data collected for 17 years in 580 table and wine grape accessions).
The intensity and quality of light (e.g. the amount of UV‐B available) significantly affect grape quality as they modulate the production of phenolic compounds (mostly flavonoids) and cuticular waxes (Tilbrook et al. 2013). In a commercial vineyard of ‘Sauvignon Blanc’ in South Africa, plants were shaded with UV‐excluding acrylic panels applied to the bunch zone in order to monitor the differences on core metabolic processes in grapes due to the amount of UV‐B (Joubert et al. 2016). Metabolite profiles during berry developmental revealed specific adjustments to typical UV‐acclimation processes, such as the content of carotenoids and associated xanthophyll cycle metabolites in photosynthetically active green berries (Joubert et al. 2016). In ripe berries, on the other hand, UV‐B favored the production of volatile and polyphenolic compounds with direct antioxidant and/or ‘sunscreening’ abilities (Joubert et al. 2016), directly influencing grape composition. In ‘Malbec’ berries, UV‐B screening was shown to delay berry development and maturation, whereas UV‐B combined with ABA hastened berry sugar and phenol accumulation, anticipating ripening up to 20 days (Berli et al. 2011). Therefore, it is possible to notice that the increase of UV‐B radiation inherent to the forthcoming climate changes may in fact work in favor of viticulture by anticipating ripening and thus helping to decrease exposure to summer heat waves and also by countering the predicted decreases in grape quality.
Extreme events have become the norm all over winemaking regions. Despite the projections indicating that the Southern Hemisphere will suffer less than the northern with climate changes (Mozell and Thachn 2014), a survey of 92 vineyards from 10 winegrowing regions of Australia was undertaken after an intense heat wave (Webb et al. 2010). The authors report an absence of direct correlation between losses and the amount of heat above a certain threshold, whereas a strong correlation to viticultural practices was found, that led the authors to put forward several recommendations concerning water application, canopy cover, row orientation and an adequate choice of genotypes in order to successfully withstand severe heat events (Webb et al. 2010).
Controlled conditions: uncovering stress synergies and antagonisms
The trials under natural conditions, discussed above, are important to fully comprehend the whole picture of ‘stress tolerance’ mechanisms in the field. However, by addressing the same stresses in controlled environments, important mechanisms of resistance can be elucidated by allowing the separation of the effects of individual stresses. It is, however, important to bear in mind that when plants are studied under controlled conditions there are changes in phenotypic characteristics that are not derived from the stress applied but by growth under artificial conditions (Mishra et al. 2012).
Dissecting the Mediterranean summer
As the most common abiotic stresses affecting grapevine in the Mediterranean summer are drought, heat and excess light, the effects of those stresses individually and in all the combinations between them were analyzed in leaves of ‘Touriga Nacional’ and ‘Trincadeira’, two widely used Portuguese wine‐growing varieties, with distinct physiological behaviors in the field (Carvalho et al. 2016). The plants were evaluated for photosynthetic performance through several physiological parameters (Table 1). The main aim was to understand the response mechanisms and the antagonistic or synergistic effects that the combination of abiotic stresses can induce, and thus change the prior perceptions of ‘tolerance’ or ‘sensitivity’ of each variety (Carvalho et al. 2016). Common and distinct stress‐response features, namely regarding drought, were identified. Each genotype showed a specific response to water deficit, heat and light, pointing out that they can adapt to different environmental conditions. ‘Touriga Nacional’ showed higher capacity for heat dissipation via evaporative cooling than ‘Trincadeira’, thus being able to withstand HS as long as water was plentiful, indicating an adaptation to warmer climate conditions, as previously proposed by Costa et al. (2012). In ‘Touriga Nacional’, photosynthesis was unaffected under light stress, and this variety managed to keep the stomata open, a benefit for photosynthesis and for heat dissipation via evaporative cooling (Costa et al. 2012). ‘Trincadeira’, on the other hand, had its photosynthetic capacity hindered by individual stresses, water stress increasing respiration and HS reducing significantly the channeling of captured energy toward photosynthesis. This indicates that ‘Trincadeira’ is sensitive to abiotic stress in general, and that its use in warm regions should be carefully considered, while ‘Touriga Nacional’ will easily tolerate growth in such regions, as long as irrigation is provided.
Table 1.
Physiological and metabolic response to major abiotic stresses and their combinations in several grapevine varieties, applied in greenhouse conditions. LS, light stress; WS, drought, HS, heat stress, C/N ratio, carbon/nitrogen ratio.
| Stress | Variation of the parameters quantified in relation to the respective controls | Variety | Reference | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fv/Fm | Ψ | Pn | gs | ABA | EL | C/N ratio | Antioxidants | Primary metabolism | Secondary metabolism | |||
| WS | ↓↓ | ↓↓ | ↑ | ↓↓ | ↑ | ↓ | ↑↑ | Shiraz | Hochberg et al. (2013) | |||
| ↓ | ↓ | ↑ | ↓ | ↑↑ | ↓ | ↑ | Cabernet Sauvignon | |||||
| WS | ↓ | ↓ | ↓ | ↑↑ | ↑ | ↑ | Summer Black | Haider et al. (2017) | ||||
| HS | ↔ | ↑ | ↓↓ | ↑↑ | Shiraz | Hochberg et al. (2015) | ||||||
| ↓ | ↑ | ↓ | ↑ | Cabernet Sauvignon | ||||||||
| HS | ↔ | ↓↓ | Vitis vinifera | Zha et al. (2018) | ||||||||
| ↓ | V. vinifera × V. labrusca | |||||||||||
| HS + WS | ↓↓ | ↓↓ | ↓↓ | ↓ | ↑ | Pinot Noir | Griesser et al. (2015) | |||||
| WS + LS | ↓↓ | ↓ | ↓↓ | ↓↓ | ↔ | ↔ | Touriga Nacional | Carvalho et al. (2016) | ||||
| ↔ | ↓ | ↓ | ↓↓ | ↔ | ↔ | Trincadeira | ||||||
| HS + WS | ↓ | ↓ | ↓ | ↓↓ | ↓ | ↑ | Touriga Nacional | |||||
| ↓ | ↓ | ↓↓ | ↓↓ | ↔ | ↔ | Trincadeira | ||||||
| LS + HS | ↓ | ↔ | ↓ | ↓ | ↓ | ↑ | Touriga Nacional | |||||
| ↓ | ↔ | ↓ | ↓ | ↑↑ | ↔ | Trincadeira | ||||||
| WS + LS + HS | ↓↓ | ↓ | ↓↓ | ↓↓ | ↓ | ↑ | Touriga Nacional | |||||
| ↓ | ↓ | ↓↓ | ↓ | ↔ | ↑ | Trincadeira | ||||||
A comprehensive heat study, involving 68 varieties from several Vitis species was undertaken to assess the heat tolerance of those genotypes, that included rootstocks and hybrids (Zha et al. 2018). In this study, several heat‐related parameters were analyzed in leaves (Table 1) and genotypes were clustered into five tolerance categories. It was possible to assess that V. vinifera × V. labrusca hybrids were more heat tolerant than V. vinifera accessions (Fig. 1). Vitis vinifera accessions achieved tolerance by lowering electrolyte leakage (EL) values and increasing maximum photochemical efficiency of photosystem II (Fv/Fm) while V. vinifera × V. labrusca hybrids relayed mostly upon a tight control of EL and had lower Fv/Fm (Zha et al. 2018). In fact, quantum efficiency of photosynthesis drops upon HS (Wang et al. 2010) and decreases in Fv/Fm were also observed in leaves of ‘Touriga Nacional’ and ‘Trincadeira’ upon HS without previous acclimation (Carvalho et al. 2014). The capacity of some genotypes to keep high values of Fv/Fm is an indication of low energy dissipation in the photosynthetic electron transport chain (Demmig‐Adams et al. 2012), which allows photosynthesis to maintain its normal levels, and those genotypes can be considered more tolerant.
Figure 1.
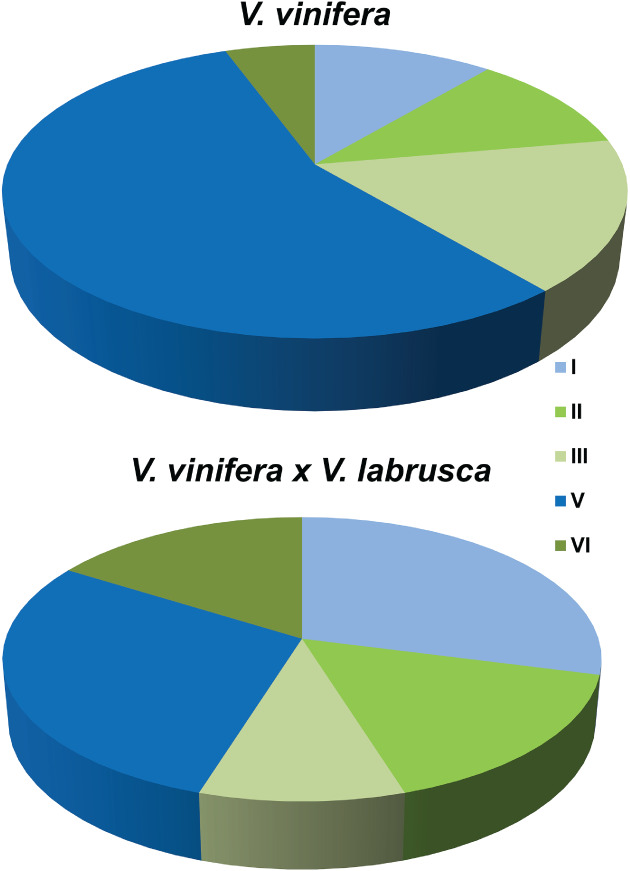
Distribution of 20 Vitis vinifera and 48 V. vinifera × V. labrusca accessions according to heat stress tolerance (Zha et al. 2018). Class I: high EL, low Fv/Fm; Class II: high EL, highest Fv/Fm; Class III: high EL, high Fv/Fm; Class V: low EL, high Fv/Fm; Class VI: highest EL, high Fv/Fm. Tolerant categories (I and V) are represented in blue tones and sensitive ones (II, III and VI) in green.
Another experiment focusing on leaf metabolite composition was assessed in water‐stressed potted plants of ‘Shiraz’ and ‘Cabernet Sauvignon’ (Hochberg et al. 2013). The authors found that lower leaf water potentials gave rise to a more intense response to stress in ‘Shiraz’ (Table 1), that showed a larger and more coordinated stress‐related response (Hochberg et al. 2013). However, the tighter regulation of stomata in ‘Cabernet Sauvignon’ upon stress induction was significant (Hochberg et al. 2013), and can be exploited in the same way as discussed for ‘Touriga Nacional’. The same varieties subjected to high temperatures but still within the optimal range (from 25 to 35°C) showed significant changes in leaf physiology, metabolite abundance and the metabolic network topology, revealing once more that ‘Shiraz’ is more sensitive to stress (Hochberg et al. 2015; Table 1). This study comes in line with several others, highlighting the importance of the genotype in establishing optimal growth conditions.
Climate change scenarios foresee increases of CO2 levels that will reach 550 μmol mol−1 in 2050 (Leakey et al. 2004). The combined effect of CO2 in the upregulation of antioxidant defense metabolism and in the decrease of photorespiration results in lower stress levels in Arabidopsis thaliana (Zinta et al. 2014). Photosynthesis rates, however, remained negatively affected in Pinus taeda and Quercus rubra (Ameye et al. 2012). In grapevine the negative impacts of drought and heat waves can be mitigated by high CO2 concentrations. Berry quality of red and white ‘Tempranillo’ (syn. ‘Aragonez’) subjected to 700 μmol mol−1 CO2 for three growing seasons in Spain was assessed (Kizildeniz et al. 2018). Elevated CO2 decreased pH due to high tartaric acid content in the red variety while lower contentrations of malic and tartaric acids reduced acidity in the white variety. As high temperature and drought decrease malic acid content and CO2 increases tartaric acid, must pH probably will not be significantly afected, as long as the increases in temperature are accompained by high CO2 in the atmosphere. Also, plant performance is impaired by drought and that effect can be mitigated, to some extent, by hight CO2 (Kizildeniz et al. 2015).
In more marginal grapevine growing regions such as central Europe, the main abiotic stress affecting plants in the summer is drought. In Austria, potted ‘Pinot Noir’ plants were subjected to moderate drought with mild temperatures and to severe drought with moderate heat (temperatures above 35°C; Griesser et al. 2015). The content of the majority of primary metabolites increased upon both treatments, whereas secondary metabolites were only induced upon prolonged drought with heat (Table 1).
Transcriptomic studies in stress response: the relevance of transcription factors and HSPs
Several transcriptomic experiments using cDNA microarrays and RNA‐Seq techniques to study abiotic stress effects on grapevine have been reported, and most revealed the involvement of heat shock proteins (HSPs) and transcription factors (TFs) in the stress response and also in the mitigation effects. (e.g. Tattersall et al. 2007, Xiao et al. 2008, Liu et al. 2012, Rocheta et al. 2016, Carvalho et al. 2017). The HSP20 family is known for a strong upregulation in response to abiotic stresses (Swindell et al. 2007), so the high expression levels of HSP20 found by Liu et al. (2012) in leaves of ‘Cabernet Sauvignon’ and of HSP17.9 found by Carvalho et al. (2014) in leaves of ‘Touriga Nacional’ upon HS were in line with findings from other species. Also, drought stress causes HSP upregulation, as found by Haider et al. (2017; Table 1) in leaves of ‘Summer Black’. The authors also reported that upregulated DEGs were about twofold as compared to those that were downregulated, modulating defense‐related pathways such as ROS defense system but also basic metabolism such as chlorophyll synthesis.
The responses to sudden short‐term and long‐term drought are different and correspond to pronounced and unexpected summer droughts and to average water scarcity, respectively. These differences were studied in shoots of greenhouse‐grown ‘Cabernet Sauvignon’ subjected to sudden and long‐term water and salinity stresses (Cramer et al. 2007, Tattersall et al. 2007). Although transcript changes after the sudden stresses were also found in the long‐term stresses, many changes in the latter were not reflected in the short‐term stress, as an indication that, when time is given to acclimate to stress, plants are able to withstand it better (Tattersall et al. 2007).
At least 5 of the 13 zinc finger protein transcription factors (ZFP‐TFs) present in grapevine are differentially regulated by water and salinity stresses and regulate the expression of other stress‐associated genes in several tissues and developmental stages (Wang et al. 2014a, Yu et al. 2016). It is therefore not surprising to find several ZFPs significantly upregulated by drought and salt in several varieties (Fig. 3), while none was significantly regulated upon UV‐B.
WRKY TFs are an important class of transcriptional regulators in higher plants (Rushton et al. 2010). Evidence has revealed the role of WRKYs in the regulation of signaling processes associated with abiotic stresses such as salinity, heat, osmotic stress, high CO2, ozone, cold and drought (Jiang and Deyholos 2009, Li et al. 2009, Wu et al. 2009). A comprehensive study to identify, classify and assess the response to abiotic stress of the members of this TF family in grapevine has associated members of subgroup II‐a as related with stress response (Wang et al. 2014b). In accordance, VvWRKY07, VvWRKY08 and VvWRKY28 were upregulated in response to drought and VvWRKY08 was also induced by cold, salinity, salicylic acid and ethylene treatments in several tissues of genotype PN40024 of ‘Pinot Noir’ (Wang et al. 2014b). Conversely, WRKY TF‐b, Myb‐related TF MybB1‐2, SPF1 protein (transcription activation) and NAC domain protein NAC1 were found to be downregulated by HS in leaves of ‘Cabernet Sauvignon’ (Liu et al. 2012; Fig. 2). Also Rocheta et al. (2016) reported downregulation of several WRKY TFs in leaves of ‘Trincadeira’ and ‘Touriga Nacional’ upon HS, while under UV‐B stress some WRKY TFs were upregulated in leaves of ‘Malbec’ (Pontin et al. 2010). In a greenhouse salt stress experiment involving the tolerant variety ‘Razegui’ and the susceptible variety ‘Syrah’, microarray analysis of leaves revealed several upregulated DEGs that included zinc finger TFs, ETHYLENE RESPONSE FACTOR (ERFs) and a NAM‐LIKE PROTEIN (NAC TF), with higher levels of upregulation in the tolerant ‘Razegui’ (Daldoul et al. 2010). Two ERFs found in this salt experiment (ERF3, ERF4) were also induced by salt and water stress in shoots of ‘Cabernet Sauvignon’ (Cramer et al. 2007; Fig. 2). ERF3 is known to modulate the abiotic stress response through the regulation of the antioxidative machinery (Daldoul et al. 2010).
Figure 2.
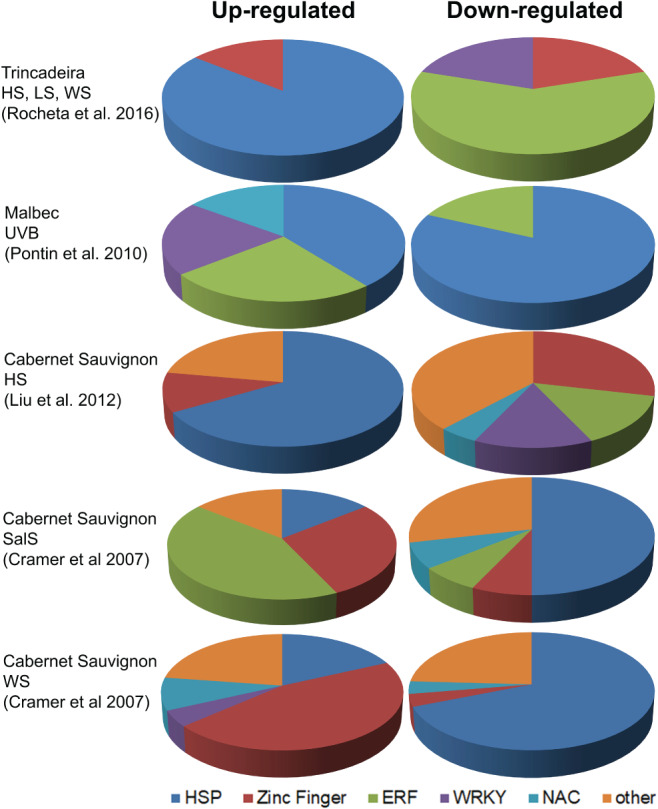
TFs and HSPs up‐ and downregulated in several abiotic stress conditions obtained through microarray analyses. The respective references are indicated in the figures. Stress conditions: WS, drought; LS, light stress; UVB, UV‐B light stress; SalS, salt stress.
Stress and recovery
Cycles of stress and recovery, such as rehydration or interchange between heat waves and mild temperatures, are the prevalent processes occurring under natural conditions during different seasons, and under agricultural practices such as irrigation. Thus, the degree of recovery from stress, as the basic mechanism enabling plant survival and grape production, is as relevant as the immediate response to stress. Liu et al. (2012) highlighted the relevance of transcriptional control in the response to HS in leaves of ‘Cabernet Sauvignon’ by showing that 8% of the Affymetrix GeneChip Array probe sets responded to HS and subsequent recovery, with specific gene expression changes, and that the number of HS‐regulated genes was almost twice that of recovery‐regulated genes. The genotype is also a key factor modulating HS response and recovery, as shown in ‘Touriga Nacional’ and ‘Trincadeira’ (Carvalho et al. 2014). Both varieties showed significant differences to stress applied in a stepwise increase of temperature, mimicking natural conditions as compared to sudden increase of temperature, once again drawing attention to the need to replicate natural field conditions when the objective is to dissect a natural response. Again, the response to stress was significantly different between varieties, ‘Touriga Nacional’ increased ascorbate and glutathione reduction levels, boosting the cell's redox buffering capacity while ‘Trincadeira’ needed to synthesize both metabolites, its response being insufficient to keep the redox state at working levels and affecting the plants for a longer period (Carvalho et al. 2014). Also, the upregulation pattern of antioxidative stress genes was more obvious in ‘Trincadeira’, while in ‘Touriga Nacional’, the canonical HS gene signature was not evident, nor the typical stress‐related shut‐down of the housekeeping metabolism (Carvalho et al. 2014). In ‘Cabernet Sauvignon’ the responsive genes also included those coding antioxidant enzymes and HSPs, especially small heat shock proteins (sHSPs), with HSF30 found to be activated upon HS, HSF7 and HSF1 triggered only after recovery (Liu et al. 2012).
Most abiotic stresses share common indicators
The global analysis of data obtained in several stress studies under controlled conditions reveals some similarities, such as low carbon assimilation rates (lower Pn), low levels of efficiency of use of available energy in severe stress combinations (lower Fv/Fm) but more significantly, and in all stresses by stomata closure (lower gs; Table 1), that negatively affect photosynthesis. The accumulation of antioxidative metabolites such as ascorbate and glutathione is common to drought and heat and their combinations, as well as the shift from primary to secondary metabolism, more forceful in sensitive genotypes (Table 1). Regulatory genes activated in various stresses belong to the families of ZFP‐TFs and HSPs while the activation or repression of other TF families is more dependent on the type and duration of stress (Fig. 2).
The use of in vitro systems to develop markers for abiotic stress
In addition to summer drought, grapevine routinely suffers from dry winters, especially in more marginal growing areas such as China or Germany (Su et al. 2015). In those regions of continental climate, winters are severe with low temperature and air humidity (Li et al. 2013) and very low water availability. Consequently, transpiration by woody tissues is high, even in a season of limited vegetative growth (Li et al. 2013). In the study by Su et al. (2015), the tolerance of tissue‐cultured plants of six grapevine varieties to drought and cold stresses was evaluated and validated. The evaluation was done by measuring EL and chlorophyll fluorescence parameters, particularly Fv/Fm, where a high correlation with EL was registered, making Fv/Fm measurement a good estimation of EL and thus of tolerance to drought and cold stress (Su et al. 2015). In ‘Chardonnay’, increases in EL were observed in frost‐treated organs and these measurements were put forward as indicators of plant sensitivity to cold (Ait‐Barka et al. 2006). However, in cold stress, as shown here for other abiotic stresses, there is also a marked varietal variability. It is well known that American (Vitis riparia) and eastern Asian (V. amurensis) species are more tolerant to cold than V. vinifera. However, some observations are contradictory within V. vinifera varieties, like the increase in EL observed in ‘Lagrein’ plants after low night temperature stress, while in ‘Müller‐Thurgau’ genotypes the permeability of the membrane did not changed (Bertamini et al. 2007). In leaf disks, EL and lipid peroxidation increased sharply when grapevine plants had been exposed to low night temperature with higher values in the varieties ‘Hatun Parmağı’ and ‘Ata Sarısı’ than in ‘Dimrit’ and ‘Razakı’ (Turfan et al. 2010).
Can acclimatization stress help understand acclimation to climate changes?
When the commercialization of in vitro plants, namely grafted scions, became widespread, stress studies of tissue‐cultured grapevine began focusing on the transition of the in vitro phase into ex vitro growth (i.e. acclimatization). Tissue‐cultured plantlets are developed in heterotrophic conditions subjected to low light intensities and low CO2 concentrations, that favor downregulation of photosynthesis (Carvalho and Amâncio 2002). During acclimatization, however, plantlets are transferred to autotrophic conditions and subjected to higher light intensities that induce photoinhibition and thus oxidative stress symptoms (Fig. 3). In fact, a transcriptomic study of in vitro plants in acclimatization (Carvalho et al. 2011) revealed striking similarities to those reported in excess‐light treatments in Arabidopsis (Mullineaux and Karpinski 2002, Ball et al. 2004) and to mechanisms controlling the expression of genes for antioxidative enzymes in grapevine (Carvalho et al. 2011). At the transfer to acclimatization, with its timing depending on the species (Carvalho et al. 2008), there is a moment of strong induction of the antioxidative defense, that occurs 48 h after transfer in grapevine (Carvalho et al. 2006), and that is parallel to the response of field plants to light in excess. Moreover, in vitro plants, due to their specific fragility, require smaller changes in light intensity and CO2 concentration to trigger specific response mechanisms. So, experimental approaches to find out the effects of high levels of atmospheric CO2 on plant growth and resilience can be made easier by manipulating acclimatization protocols (Carvalho et al. 2002). Therefore, such a simple, time and space saving experimental system can be a useful tool to understand the mechanisms of response to excess light that affect vineyards in the Mediterranean summer and also to anticipate the effects of the rising levels of atmospheric CO2.
Figure 3.
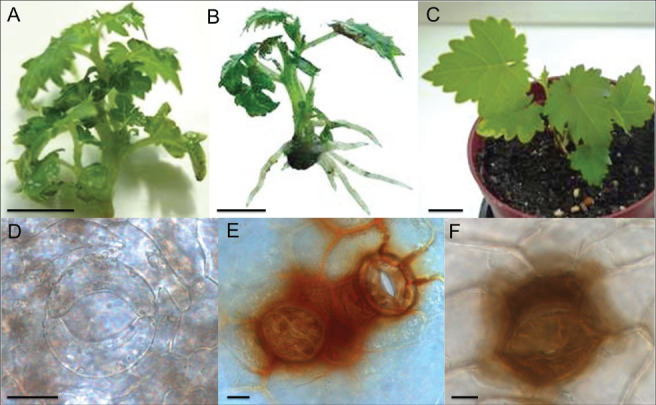
Plantlets of ‘Touriga Nacional’ at the moment of transfer to acclimatization (A), after 7 days, already showing an ex vitro expanded root system (B) and at 28 days, the end of acclimatization, with new fully expanded leaves (C). Progression of stomatal functioning during the first 7 days of ex vitro growth (magnification 630×), showing dysfunctional stomata with no H2O2 accumulation (as stained with DAB, 3,3' diaminobenzidine; Vilela et al. 2007) and no control over the opening/closure of stomata, at the moment of transfer to acclimatization (D), partially functional stomata with H2O2 accumulation on surrounding cells on day two of acclimatization (E) and functional stomata with H2O2 accumulation on guard cells after 7 days of acclimatization (F). Scale bars: A–C, bars = 1 cm; D–F, bars = 10 μm. (A–C, Carvalho and Amâncio, unpublished data; E, Vilela et al. 2007; D, F, Carvalho, Vilela and Amâncio, unpublished data).
Concluding remarks
Different forms of abiotic stresses are the foremost reason for losses in grapevine productivity and alterations in wine characteristics and quality (Hannah et al. 2013). In Mediterranean climate regions, climate changes are increasing the pressure on viticulture, through the higher frequency of heat waves and the aggravation of drought events (Mozell and Thachn 2014). Therefore, implementation of novel viticultural practices and identification of well‐adapted (tolerant) genotypes producing high quality wines are at the forefront of viticulture research. As a whole, a better adaptation can result from a genetic background giving rise to a more tolerant phenotype or can be achieved through a shift from the original areas of cultivation, thus escaping extreme events. Here, by assembling different recent results, it was possible to identify physiological, biochemical and molecular features that explain the capacity of adaptation of particular grapevine genotypes. Two widely studied genotypes that confirmed the mechanisms of tolerance to stress enabling to withstand heat waves are 'Touriga Nacional', ‘Cabernet Sauvignon’, and also, to a lower extent, ‘Summer Black’.
From the experiments reported here, it was possible to identify several similar responses to abiotic stress: (1) the negative effects on photosynthesis reflected by low carbon assimilation rates, due to low levels of energy use efficiency and to a significant impairment on stomata regulation, especially on the sensitive ‘Trincadeira’, ‘Shiraz’ and ‘Pinot Noir’; (2) the accumulation of antioxidant metabolites, mostly in the tolerant ‘Cabernet Sauvignon’; (3) the shift form primary to secondary metabolism, a feature common to all varieties; (4) a canonical gene expression stress signature including sHSPs and various families of transcription factors specific to the type of stress applied.
Climate changes, as a whole, do not just affect water availability and temperature, the more studied stress conditions, whether in the field or in controlled conditions (Cramer et al. 2011). Deriving from the fact that light intensity as well as quality and atmospheric CO2 concentrations will also change, there is the possibility that some of the negative impacts on quality might be countered by an increase in UV‐B, that can maintain the balance of phenolic compounds in grapes, and by the increase of CO2 that can overcome the negative impacts on vegetative growth. Also, economically relevant genotypes used in some of the works here presented, namely ‘Touriga Nacional’ and ‘Cabernet Sauvignon’, show capacity to withstand heat waves and average rises in temperature, as long as some viticulture practices are implemented, such as the use of tightly controlled deficit irrigation.
Acknowledgements
Fundação para a Ciência e Tecnologia (FCT), through LEAF Funding UID/AGR/04129/2013 and SFRH/BPD/109428/2015 to LC.
Contributor Information
Luísa C. Carvalho, Email: lcarvalho@isa.ulisboa.pt.
Sara Amâncio, Email: samport@isa.ulisboa.pt.
References
- Ait‐Barka E, Nowak J, Clément C (2006) Enhancement of chilling resistance of inoculated grapevine plantlets with a plant growth‐promoting rhizobacterium, Burkholderia phytofirmans strain PsJN. Appl Environ Microbiol 72: 7246–7252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameye M, Wertin TM, Bauweraerts I, Mcguire MA, Teskey RO, Steppe K (2012) The effect of induced heat waves on Pinus taeda and Quercus rubra seedlings in ambient and elevated CO2 atmospheres. New Phytol 196: 448–461 [DOI] [PubMed] [Google Scholar]
- Ball L, Accotto G‐P, Bechtold U, Creissen G, Funck D, Jimenez A, Kular B, Leyland N, Mejia‐Carranza J, Reynolds H, Karpinski S, Mullineaux PM (2004) Evidence for a direct link between glutathione biosynthesis and stress defense gene expression in Arabidopsis . Plant Cell 16: 2448–2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berli FJ, Fanzone M, Piccoli P, Bottini R (2011) Solar UV‐B and ABA are involved in phenol metabolism of Vitis vinifera L. increasing biosynthesis of berry skin polyphenols. J Agric Food Chem 59: 4874–4884 [DOI] [PubMed] [Google Scholar]
- Bertamini M, Zulini L, Muthuchelian K, Nedunchezhian N (2007) Low night temperature effects on photosynthetic performance on two grapevine genotypes. Biol Plant 51: 381–385 [Google Scholar]
- Bigard A, Berhe DT, Maoddi E, Sire Y, Boursiquot J‐M, Ojeda H, Péros J‐P, Doligez A, Romieu C, Torregrosa L (2018) Vitis vinifera L. fruit diversity to breed varieties anticipating climate changes. Front Plant Sci 9: 455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray EA, Bailey‐Serres J, Weretilnyk E (2000) Responses to abiotic stresses In: Gruissem W, Buchannan B, Jones R. (eds) Biochemistry and Molecular Biology of Plants. American Society of Plant Physiologists, Rockville, MD, pp 1158–1249 [Google Scholar]
- Carvalho LC, Amâncio S (2002) Antioxidant defence system in plantlets transferred from in vitro to ex vitro: effects of increasing light intensity and CO2 concentration. Plant Sci 162: 33–40 [Google Scholar]
- Carvalho LC, Santos P, Amancio S (2002) Effect of light intensity and CO2 concentration on growth and the acquisition of in vivo characteristics during acclimatization of grapevine regenerated in vitro . Vitis 41: 1–6 [Google Scholar]
- Carvalho LC, Vilela BJ, Vidigal P, Mullineaux PM, Amancio S (2006) Activation of the ascorbate‐glutathione cycle is an early response of micropropagated Vitis vinifera L. explants transferred to ex vitro . Int J Plant Sci 167: 759–770 [Google Scholar]
- Carvalho LC, Santos S, Vilela BJ, Amâncio S (2008) Solanum lycopersicon Mill. and Nicotiana benthamiana L. under high light show distinct responses to anti‐oxidative stress. J Plant Physiol 165: 1300–1312 [DOI] [PubMed] [Google Scholar]
- Carvalho LC, Vilela BJ, Mullineaux PM, Amâncio S (2011) Comparative transcriptomic profiling of Vitis vinifera under high light using a custom‐made array and the Affymetrix GeneChip. Mol Plant 4: 1038–1051 [DOI] [PubMed] [Google Scholar]
- Carvalho LC, Coito JL, Colaço S, Sangiogo M, Amâncio S (2014) Heat stress in grapevine: the pros and cons of acclimation. Plant Cell Environ 38: 777–789 [DOI] [PubMed] [Google Scholar]
- Carvalho LC, Coito JL, Gonçalves EF, Chaves MM, Amâncio S (2016) Differential physiological response of the grapevine varieties Touriga Nacional and Trincadeira to combined heat, drought and light stresses. Plant Biol 18: 101–111 [DOI] [PubMed] [Google Scholar]
- Carvalho LC, Silva M, Coito JL, Rocheta MP, Amancio S (2017) Design of a Custom RT‐qPCR Array for assignment of abiotic stress tolerance in traditional Portuguese grapevine varieties. Front Plant Sci 8: 1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challinor AJ, Wheeler TR, Craufurd PQ, Slingo JM (2005) Simulation of the impact of high temperature stress on annual crop yields. Agric For Meteorol 135: 180–189 [Google Scholar]
- Chaves MM (2002) How plants cope with water stress in the field? Photosynthesis and growth. Ann Bot 89: 907–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves MM, Zarrouk O, Francisco R, Costa JM, Santos T, Regalado AP, Rodrigues ML, Lopes CM (2010) Grapevine under deficit irrigation: hints from physiological and molecular data. Ann Bot 105: 661–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa JM, Ortuño MF, Lopes CM, Chaves MM (2012) Grapevine varieties exhibiting differences in stomatal response to water deficit. Funct Plant Biol 39: 179–189 [DOI] [PubMed] [Google Scholar]
- Cramer GR, Ergül A, Grimplet J, Tillett RL, Tattersall ER, Bohlman MC, Vincent D, Sonderegger J, Evans J, Osborne C, Quilici D, Schlauch K, Schooley D, Cushman JC (2007) Water and salinity stress in grapevines: early and late changes in transcript and metabolite profiles. Funct Integr Genomics 7: 111–134 [DOI] [PubMed] [Google Scholar]
- Cramer GR, Urano K, Delrot S, Pezzotti M, Shinozaki K (2011) Effects of abiotic stress on plants: a systems biology perspective. BMC Plant Biol 11: 163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai ZW, Ollat N, Gomès E, Decroocq S, Tandonnet J, Bordenave L, Pieri P, Hilbert G, Kappel C, Leeuwen CV, Vivin P, Delrot S (2011) Ecophysiological, genetic, and molecular causes of variation in grape berry weight and composition : a review. Am J Enol Vitic 62: 413–425 [Google Scholar]
- Dal Santo S, Tornielli GB, Zenoni S, Fasoli M, Farina L, Anesi A, Guzzo F, Delledonne M, Pezzotti M (2013) The plasticity of the grapevine berry transcriptome. Genome Biol 14: r54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Santo S, Zenoni S, Sandri M, Lorenzis GD, Magris G, Paoli ED, Di G, del FC, Morgante M, Brancadoro L, Grossi D, Fasoli M, Zuccolotto P, Tornielli GB, Pezzotti M (2018) Grapevine field experiments reveal the contribution of genotype, the influence of environment and the effect of their interaction (G X E) on the berry transcriptome. Plant J 93: 1143–1159 [DOI] [PubMed] [Google Scholar]
- Daldoul S, Guillaumie S, Reustle GM, Krczal G, Ghorbel A, Delrot S, Mliki A, Höfer MU (2010) Isolation and expression analysis of salt induced genes from contrasting grapevine (Vitis vinifera L.) cultivars. Plant Sci 179: 489–498 [DOI] [PubMed] [Google Scholar]
- Degu A, Hochberg U, Sikron N, Venturini L, Buson G, Ghan R, Plaschkes I, Batushansky A, Chalifa‐Caspi V, Mattivi F, Delledonne M, Pezzotti M, Rachmilevitch S, Cramer GR, Fait A (2014) Metabolite and transcript profiling of berry skin during fruit development elucidates differential regulation between Cabernet Sauvignon and Shiraz cultivars at branching points in the polyphenol pathway. BMC Plant Biol 14: 188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig‐Adams B, Cohu CM, Muller O, Adams WW (2012) Modulation of photosynthetic energy conversion efficiency in nature: from seconds to seasons. Photosynth Res 113: 75–88 [DOI] [PubMed] [Google Scholar]
- Duchêne E, Schneider C (2005) Grapevine and climatic changes: a glance at the situation in Alsace. Agron Sustain Dev 25: 93–99 [Google Scholar]
- Escudier H, Bigard A, Ojeda H, Samson A, Caillé S, Romieu C, Torregrosa L (2017) De la vigne au vin: des créations variétales adaptées au changement climatique et résistant aux maladies cryptogamiques 1/2: La résistance variétale. Rev Oenol 44: 16–18 [Google Scholar]
- Fraga H, García I, Atauri DC, Malheiro AC, Moutinho‐Pereira J, Santos JA (2017) Viticulture in Portugal: a review of recent trends and climate change projections. OENO One 51: 61–69 [Google Scholar]
- Friend AP, Trought MCT, Stushnoff C, Wells GH (2011) Effect of delaying budburst on shoot development and yield of Vitis vinifera L Chardonnay ‘Mendoza’ after a spring freeze event. Aust J Grape Wine Res 17: 378–382 [Google Scholar]
- Greer DH, Weedon MM (2013) The impact of high temperatures on Vitis vinifera cv Semillon grapevine performance and berry ripening. Front Plant Sci 4: 491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer DH, Weston C (2010) Heat stress affects flowering, berry growth, sugar accumulation and photosynthesis of Vitis vinifera cv Semillon grapevines grown in a controlled environment. Funct Plant Biol 37: 206–214 [Google Scholar]
- Greer DH, Weedon MM, Weston C (2011) Reductions in biomass accumulation, photosynthesis in situ and net carbon balance are the costs of protecting Vitis vinifera ‘Semillon’ grapevines from heat stress with shade covering. AoB Plants 2011: plr023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesser M, Weingart G, Schoedl‐Hummel K, Neumann N, Becker M, Varmuza K, Liebner F, Schuhmacher R, Forneck A (2015) Severe drought stress is affecting selected primary metabolites, polyphenols, and volatile metabolites in grapevine leaves (Vitis vinifera cv Pinot Noir). Plant Physiol Biochem 88: 17–26 [DOI] [PubMed] [Google Scholar]
- Haider MS, Zhang C, Pervaiz MMKT, Zheng T, Zhang C, Lide C, Shangguan L, Fang J (2017) Insights into grapevine defense response against drought as revealed by biochemical, physiological and RNA‐Seq analysis. Sci Rep 7: 13134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah L, Roehrdanz PR, Ikegami M, Shepard AV, Shaw MR, Tabor G, Zhi L, Marquet PA, Hijmans RJ (2013) Climate change, wine, and conservation. Proc Natl Acad Sci USA 110: 6907–6912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg U, Degu A, Toubiana D, Gendler T, Nikoloski Z, Rachmilevitch S, Fait A (2013) Metabolite profiling and network analysis reveal coordinated changes in grapevine water stress response. BMC Plant Biol 13: 184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg U, Batushansky A, Degu A, Rachmilevitch S, Fait A (2015) Metabolic and physiological responses of shiraz and cabernet sauvignon (Vitis vinifera L.) to near optimal temperatures of 25 and 35 °C. Int J Mol Sci 16: 24276–24294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houel C, Chatbanyong R, Doligez A, Rienth M, Foria S, Luchaire N, Roux C, Adivèze A, Lopez G, Farnos M, Pellegrino A, This P, Romieu C, Torregrosa L (2015) Identification of stable QTLs for vegetative and reproductive traits in the microvine (Vitis vinifera L.) using the 18 K Infinium chip. BMC Plant Biol 15: 205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Deyholos MK (2009) Functional characterization of Arabidopsis NaCl‐inducible WRKY25 and WRKY33 transcription factors in abiotic stresses. Plant Mol Biol 69: 91–105 [DOI] [PubMed] [Google Scholar]
- Joubert C, Young PR, Eyéghé‐Bickong HA, Castellarin SD (2016) Field‐grown grapevine berries use carotenoids and the associated xanthophyll cycles to acclimate to UV exposure differentially in high and low light (shade) conditions. Front Plant Sci 7: 786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M (2010) Managing grapevines to optimise fruit development in a challenging environment: a climate change primer for viticulturists. Aust J Grape Wine Res 16: 56–69 [Google Scholar]
- Kizildeniz T, Mekni I, Santesteban H, Pascual I, Morales F, Irigoyen JJ (2015) Effects of climate change including elevated CO2 concentration, temperature and water deficit on growth, water status, and yield quality of grapevine (Vitis vinifera L.) cultivars. Agric Water Manag 159: 155–164 [Google Scholar]
- Kizildeniz T, Pascual I, Irigoyen JJ, Morales F (2018) Using fruit‐bearing cuttings of grapevine and temperature gradient greenhouses to evaluate effects of climate change (elevated CO2 and temperature, and water deficit) on the cv. red and white Tempranillo. Yield and must quality in three consecutive growing seasons. Agric Water Manag 202: 299–310 [Google Scholar]
- Knight H, Knight MR (2001) Abiotic stress signalling pathways: specificity and cross‐talk. Trends Plant Sci 6: 262–267 [DOI] [PubMed] [Google Scholar]
- Kotak S, Larkindale J, Lee U, von Koskull‐Döring P, Vierling E, Scharf K‐D (2007) Complexity of the heat stress response in plants. Curr Opin Plant Biol 10: 310–316 [DOI] [PubMed] [Google Scholar]
- Leakey ADB, Bernacchi CJ, Dohleman FG, Ort DR, Long SP (2004) Will photosynthesis of maize (Zea mays) in the US Corn Belt increase in future [CO2] rich atmospheres? An analysis of diurnal courses of CO2 uptake under free‐air concentration enrichment (FACE). Glob Chang Biol 10: 951–962 [Google Scholar]
- Levitt J (1980) Responses of Plants to Environmental Stresses (2 Vol). Academic Press, New York: [Google Scholar]
- Li S, Fu Q, Huang W, Yu D (2009) Functional analysis of an Arabidopsis transcription factor WRKY25 in heat stress. Plant Cell Rep 28: 683–693 [DOI] [PubMed] [Google Scholar]
- Li J, Wang N, Xin H, Li S (2013) Overexpression of VaCBF4, a transcription factor from Vitis amurensis, improves cold tolerance accompanying increased resistance to drought and salinity in Arabidopsis. Plant Mol Biol Report 31: 1518–1528 [Google Scholar]
- Liu G‐T, Wang J‐F, Cramer G, Dai Z‐W, Duan W, Xu H‐G, Wu B‐H, Fan P‐G, Wang L‐J, Li S‐H (2012) Transcriptomic analysis of grape (Vitis vinifera L.) leaves during and after recovery from heat stress. BMC Plant Biol 12: 174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migicovsky Z, Sawler J, Gardner KM, Aradhya MK, Prins BH, Schwaninger HR, Bustamante CD, Buckler ES, Zhong G, Brown PJ, Myles S (2017) Patterns of genomic and phenomic diversity in wine and table grapes. Hortic Res 4: 17035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra Y, Jänkänpää HJ, Kiss AZ, Funk C, Schröder WP, Jansson S (2012) Arabidopsis plants grown in the field and climate chambers significantly differ in leaf morphology and photosystem components. BMC Plant Biol 12: 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R (2006) Abiotic stress, the field environment and stress combination. Trends Plant Sci 11: 15–19 [DOI] [PubMed] [Google Scholar]
- Moriondo M, Bindi M (2007) Impact of climate change on the phenology of typical mediterranean crops. Ital J Agrometeorol 2007: 5–12 [Google Scholar]
- Moriondo M, Giannakopoulos C, Bindi M (2011) Climate change impact assessment: the role of climate extremes in crop yield simulation. Clim Chang 104: 679–701 [Google Scholar]
- Mozell MR, Thachn L (2014) The impact of climate change on the global wine industry: challenges and solutions. Wine Econ Policy 3: 81–89 [Google Scholar]
- Mullineaux P, Karpinski S (2002) Signal transduction in response to excess light: getting out of the chloroplast. Curr Opin Plant Biol 5: 43–48 [DOI] [PubMed] [Google Scholar]
- Mullineaux PM, Karpinski S, Baker NR (2006) Spatial dependence for hydrogen peroxide‐directed signaling in light‐stressed plants. Plant Physiol 141: 346–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto C, Henriques MO, Figueiredo JP, David JS, Abreu FG, Pereira JS, Correia I, David TS (2011) Phenology and growth dynamics in Mediterranean evergreen oaks: effects of environmental conditions and water relations. For Ecol Manag 262: 500–508 [Google Scholar]
- Pontin M, Piccoli PN, Francisco R, Bottini R, Martinez‐Zapater JM, Lijavetzky D (2010) Transcriptome changes in grapevine (Vitis vinifera L.) cv Malbec leaves induced by ultraviolet‐B radiation. BMC Plant Biol 10: 224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos MC, Jones GV, Yuste J (2015) Spatial and temporal variability of cv Tempranillo phenology and grape quality within the Ribera del Duero DO (Spain) and relationships with climate. Int J Biometeorol 59: 1849–1860 [DOI] [PubMed] [Google Scholar]
- Rizhsky L, Liang H, Mittler R (2002) The combined effect of drought stress and heat shock on gene expression in tobacco. Plant Physiol 130: 1143–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocheta M, Coito JL, Ramos MJN, Carvalho LC, Becker JD, Carbonell‐Bejerano P, Amâncio S (2016) Transcriptomic comparison between two Vitis vinifera L. varieties (Trincadeira and Touriga Nacional) in abiotic stress conditions. BMC Plant Biol 16: 224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton PJ, Somssich IE, Ringler P, Shen QJ (2010) WRKY transcription factors. Trends Plant Sci 15: 247–258 [DOI] [PubMed] [Google Scholar]
- Spielmann N, Gélinas‐Chebat C (2012) Terroir? That's not how I would describe it. Int J Wine Bus Res 24: 254–270 [Google Scholar]
- Su L, Dai Z, Li S, Xin H (2015) A novel system for evaluating drought – cold tolerance of grapevines using chlorophyll fluorescence. BMC Plant Biol 15: 82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindell WR, Huebner M, Weber AP (2007) Transcriptional profiling of Arabidopsis heat shock proteins and transcription factors reveals extensive overlap between heat and non‐heat stress response pathways. BMC Genomics 8: 125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall EAR, Grimplet J, DeLuc L, Wheatley MD, Vincent D, Osborne C, Ergül A, Lomen E, Blank RR, Schlauch KA, Cushman JC, Cramer GR (2007) Transcript abundance profiles reveal larger and more complex responses of grapevine to chilling compared to osmotic and salinity stress. Funct Integr Genomics 7: 317–333 [DOI] [PubMed] [Google Scholar]
- Tester M, Bacic A (2005) Abiotic stress tolerance in grasses. From model plants to crop plants. Plant Physiol 137: 791–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilbrook K, Arongaus AB, Binkert M, Heijde M, Yin R, Ulm R (2013) The UVR8 UV‐B photoreceptor: perception, signaling and response. Arabidopsis Book 2013: e0164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turfan N, Aktaş LY, Güven A (2010) Low night temperature tolerance determining traits correlate with Paraquat tolerance in grapevine genotypes. YYU J Agric Sci 20: 194–200 [Google Scholar]
- Vilela BJ, Carvalho LC, Ferreira J, Amâncio S (2007) Gain of function of stomatal movements in rooting Vitis vinifera L. plants: regulation by H2O2 is independent of ABA before the protruding of roots. Plant Cell Rep 26: 2149–2157 [DOI] [PubMed] [Google Scholar]
- Vinocur B, Altman A (2005) Recent advances in engineering plant tolerance to abiotic stress: achievements and limitations. Curr Opin Biotechnol 16: 123–132 [DOI] [PubMed] [Google Scholar]
- Wang W, Vinocur B, Altman A (2003) Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218: 1–14 [DOI] [PubMed] [Google Scholar]
- Wang L‐J, Fan L, Loescher W, Duan W, Liu G‐J, Cheng J‐S, Luo H‐B, Li S‐H (2010) Salicylic acid alleviates decreases in photosynthesis under heat stress and accelerates recovery in grapevine leaves. BMC Plant Biol 10: 34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Yin X, Li X, Wang L, Zheng Y, Xu X, Zhang Y, Wang X (2014a) Genome‐wide identification, evolution and expression analysis of the grape (Vitis vinifera L.) zinc finger‐homeodomain gene family. Int J Mol Sci 15: 5730–5748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Vannozzi A, Wang G, Liang Y, Tornielli GB, Zenoni S, Cavallini E, Pezzotti M, Cheng ZM (2014b) Genome and transcriptome analysis of the grapevine (Vitis vinifera L.) WRKY gene family. Hortic Res 1: 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb L, Whiting J, Watt A, Hill T, Wigg F, Dunn G, Needs S (2010) Managing grapevines through severe heat : a survey of growers after the 2009 summer heatwave in South‐Eastern Australia. J Wine Res 21: 147–165 [Google Scholar]
- Wu X, Shiroto Y, Kishitani S, Ito Y, Toriyama K (2009) Enhanced heat and drought tolerance in transgenic rice seedlings overexpressing OsWRKY11 under the control of HSP101 promoter. Plant Cell Rep 28: 21–30 [DOI] [PubMed] [Google Scholar]
- Xiao H, Tattersall EAR, Siddiqua MK, Cramer GR, Nassuth A (2008) CBF4 is a unique member of the CBF transcription factor family of Vitis vinifera and Vitis riparia . Plant Cell Environ 31: 1–10 [DOI] [PubMed] [Google Scholar]
- Yu Y, Li X, Wu Z, Chen D, Li G, Li X, Zhang G (2016) VvZFP11, a Cys2His2‐type zinc finger transcription factor, is involved in defense responses in Vitis vinifera . Biol Plant 60: 292–298 [Google Scholar]
- Zha Q, Xi X, He Y, Jiang A (2018) Comprehensive evaluation of heat resistance in 68 Vitis germplasm resources. Vitis 57: 75–81 [Google Scholar]
- Zinta G, Abdelgawad H, Domagalska MA, Vergauwen L, Knapen D, Nijs I, Janssens I, Beemster G, Asard H (2014) Physiological, biochemical, and genome‐wide transcriptional analysis reveals that elevated CO2 mitigates the impact of combined heat wave and drought stress in Arabidopsis thaliana at multiple organizational levels. Glob Chang Biol 20: 3670–3685 [DOI] [PubMed] [Google Scholar]


