Abstract
Small heat shock proteins (sHSPs) are diverse and mainly function as molecular chaperones to protect organisms and cells from various stresses. In this study, hsp18.3, one Tribolium castaneum species‐specific shsp, has been identified. Quantitative real‐time polymerase chain reaction illustrated that Tchsp18.3 is expressed in all developmental stages, and is highly expressed at early pupal and late adult stages, while it is highly expressed in ovary and fat body at the adult period. Moreover, it was up‐regulated 4532 ± 396‐fold in response to enhanced heat stress but not to cold stress; meanwhile the lifespan of adults in ds‐Tchsp18.3 group reduced by 15.8% from control group under starvation. Laval RNA interference (RNAi) of Tchsp18.3 caused 86.1% ± 4.5% arrested pupal eclosion and revealed that Tchsp18.3 played an important role in insect development. In addition, parental RNAi of Tchsp18.3 reduced the oviposition amount by 94.7%. These results suggest that Tchsp18.3 is not only essential for the resistance to heat and starvation stress, but also is critical for normal development and reproduction in T. castaneum.
Keywords: development, reproduction, stress, Tchsp18.3, Tribolium castaneum
Introduction
Heat shock proteins (HSPs) were first discovered in 1962 by Ritossa and his co‐workers in overheated Drosophila melanogaster larvae (Ritossa, 1962), and subsequent studies found they are a set of evolutionarily conserved proteins present in virtually all living organisms, from bacteria to humans. It has been shown that most HSPs have strong cytoprotective effects and behave as molecular chaperones for other cellular proteins (Garrido et al., 2006). HSPs are commonly classified into five families based on their molecular weight and homologous relationship: HSP100, HSP90, HSP70, HSP60 and the small heat shock proteins (sHSPs) (Kim et al., 1998). Mainly because of the wide molecular mass range from 12 to 43 kDa and low homology, compared to other families of HSPs, sHSPs exhibit a greater variation in sequence, structure, size and function (Franck et al., 2004).
However, there are still some common characteristics in the structure and function of sHSPs, which have a relatively conserved α‐crystalling domain spanning about 80∼100 amino acid residues, located near the C‐terminal region (Caspers et al., 1995; Fu et al., 2006; Perez‐Morales & Espinoza, 2015). In the secondary structure, sHSP monomers are enriched in β‐strands organized in a β‐sheet sandwich responsible for dimer formation (Sun & MacRae, 2005). Most sHSPs display chaperone‐like activities, helping the unfolding proteins maintain their correct states, binding to denatured proteins and preventing irreversible protein aggregation during stresses, such as extreme temperatures, oxidation and heavy metals exposure (Haslbeck et al., 2005: Chen & Zhang, 2015). sHSPs have also been involved in a variety of cellular activities, including organization of the cytoskeletal integrity, maintaining signal transduction, modulating membrane lipid polymorphism (Arrigo, 2000; Tsvetkova et al., 2002; Bakthisaran et al., 2015; Haslbeck & Vierling, 2015). These proteins additionally participate in many other physiological processes, such as the regulation of cell cycle and differentiation, interfering with apoptosis and defending against diseases (Arrigo, 2000).
Recent studies showed that the sHSPs in insects also play various roles. For instance, in Apis cerana cerana, hsp22.6 was significantly up‐regulated by abiotic stresses, such as temperature (4°C, 16°C, 42°C), pesticides (cyhalothrin, pyridaben), oxidative stress (UV, H2O2) and heavy metals (CdCl2), as well as biotic stress, such as 20‐hydroxyecdysone (20E), Ascosphaera apis treatment (Zhang et al., 2014b). In D. melanogaster, over‐expressing the mitochondrial hsp22 increased the resistance to oxidative stress and extended lifespan by 32%. The longevity phenotype of a strain obtained by P‐element jump‐out was examined. It was found that the flies which did not express mitochondrial hsp22 led a 40% decrease in lifespan, and these flies died faster and displayed a decrease of 30% in locomotor activity compared with controls (Morrow et al., 2004). In addition, hsp21.3 which increased the expression from neo‐larval to pupal stage may play a critical role in the development of Grapholita molesta, and it was the most markedly up‐regulated one together with five other hsps during early diapause (Zhang et al., 2015).
In the red flour beetle, Tribolium castaneum (Coleoptera: Tenebrionidae), which has long been used as an important model organism for insect development, evolution, comparative genomics and pest science (Richards et al., 2008; Schroeder et al., 2008), ten shsps have been identified from the genome, and eight of them were T. castaneum species‐specific shsps (Li et al., 2009). Hsp18.3, one T. castaneum species‐specific shsp, was also mentioned as hsp27 (XM_969274) in Tribolium (Altincicek et al., 2008); here we called it Tchsp18.3 based on its predicted molecular weight (Li et al., 2009). Quantitative real‐time polymerase chain reaction (real‐time qPCR) analysis of transcriptional levels of this species‐specific shsp were up‐regulated when the beetles were in response to septic wounding, heat shock and UV‐A exposure, suggested that it was involved in immunity and stress responses (Altincicek et al., 2008; Sang et al., 2012). Additionally, when cell line BCIRL‐TcA‐CLG1 of T. castaneum was treated with heat shock, increased salinity, acidic pH and UV‐A light, hsp18.3 (hsp27) seems to be the most affected by 40°C and UV light other than hsp68a and hsp83 (Garcia‐Reina et al., 2017). Hsp18.3 seems to be an important one in terms of cell growth and viability of T. castaneum, but there are no studies carried out with its systematic functions in T. castaneum, and whether it has a functional differentiation is still unknown. Here, we further explore the diverse functions of hsp18.3 in T. castaneum, and provide experimental evidence and better understanding of the functions of insect shsps.
Materials and methods
Experimental insects
The Georgia‐1 (GA‐1) strain of T. castaneum was reared at 30 °C and 40% relative humidity in 5% yeasted flour under standard conditions (Haliscak & Beeman, 1983; Li et al., 2011).
Bioinformatic analysis of gene structure
The sHSPs sequences were obtained from Beetlebase (http://www.beetlebase.org/), FlyBase (http://flybase.org/), BeeBase (http://hymenopteragenome.org/beebase/), Silkworm Genome Database (http://silkworm.genomics.org.cn/), VectorBase (http://www.vectorbase.org/index.php). Sequences were aligned with ClustalW2 (http://www.ebi.ac.uk/Tools/msa/clustalw2/) and input to Boxshade program (http://www.ch.embnet.org/software/BOX_form.html). Genes structural prediction were analyzed online using PredictProtein (http://www.predictprotein.org/) and InterProScan (http://www.ebi.ac.uk/Tools/InterProScan/).
Quantitative real‐time PCR analysis
Total RNAs from pools of three individuals were extracted at each of the following developmental stages: early eggs (EE, 1 day old), late eggs (LE, 3 days old), early larvae (EL, 1 day old), late larvae (LL, last‐instar larvae), early pupae (EP, 1 day old), late pupae (LP, 5 days old), early adults (EA, 1 day old) and late adults (LA, 1 week old) with the RNAisoTMPlus reagent (TaKaRa, Kyoto, Japan). In addition, total RNAs from pools were extracted from various tissues of late adults: head, epidermis, gut, fat body, accessory gland, testis and ovary. Reverse transcription was performed using 1 μg total RNA. Real‐time qPCR was performed to check the temporal and spatial expression patterns with FastStart Universal SYBR Green Master (Roche, Indianapolis, IN, USA) following the manufacturer's instructions. The data were expressed here as the relative messenger RNA (mRNA) levels normalized to ribosomal protein S3 (rps3) in the same complementary DNA (cDNA) samples, using the 2−△△ method (Livak & Schmittgen, 2001). The primers are listed in Table 1.
Table 1.
Primers used in this study
| Primer name | Primer sequence (5′→3′) |
|---|---|
| Real time quantitative PCR | |
| Tchsp18.3 | CAGGACCATGGGTCTAGTGATA |
| TCCCATCGCCAACCTTCG | |
| Double‐stranded RNA | |
| Tchsp18.3 | TAATACGACTCACTATAGGGCGACCAGCTCATCGTTTCCT |
| TAATACGACTCACTATAGGGTTGTTGCACCGCTGGTGTA |
RNA interference (RNAi)
Double‐stranded RNA (dsRNA) primers containing gene‐specific sequences and the T7 polymerase promoter (TAATACGACTCACTATAGGG) at the 5′‐end of both the sense primer and anti‐sense primer were used (Table 1). The PCR products were used as templates for dsRNA synthesis with the TranscriptAidTM T7 High Yield Transcription Kit (Fermentas, Vilnius, Lithuania). In addition, dsRNA was treated with DNase I and purified using a chloroform extraction followed by ethanol precipitation and dissolved in nuclease‐free water to a concentration of 2 μg/μL. The quality of dsRNA was checked by running on an agarose gel electrophoresis and the concentration was measured using NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA, USA).
To explore the effects of Tchsp18.3 on the development of T. castaneum, about 200 ng of dsRNA in 200 nL solution was injected into each late larva (∼20 days old) (Tomoyasu & Denell, 2004). Negative controls consisted of non‐injection (wild type group, WT) or injection of an equal volume of buffer only (IB group). Since knocking down Tchsp18.3 in the late larval stage induced pupal mortality significantly, the parental RNAi experiment consisted of pupal injection, which did not cause any detectable defects in adult eclosion, was carried out to explore the effects of Tchsp18.3 on reproduction and starvation tolerance (Bucher et al., 2002; Sang et al., 2016). Mortality occurring less than 5 days after injection was attributed to injection injury rather than to target transcript knockdown (Begum et al., 2009). On the 6th day after dsRNA injection, insects were used to detect whether RNAi is effective. Every group had about 30 individuals. Three biological replications were carried out for each experiment.
Temperature and starvation stress treatment
To examine whether Tchsp18.3 is in response to thermal and cold stress, late larvae were reared at 45°C and 4°C, with an ambient temperature of 25°C serving as control, then the samples were collected after the insects were treated with 45°C, 25°C or 4°C for 1 h, 2 h, 4 h and 12 h, respectively. Thereafter, the expression level of Tchsp18.3 was detected.
For the starvation tolerance assay, parental RNAi was carried out. Then the adults (1 day old) in the WT group, IB group and ds‐Tchsp18.3 group were kept without food and the expression levels of Tchsp18.3 and the survival rates were investigated every day. Three biological replications were carried out for the experiments.
Behavior analysis
Larval injections were followed by the observation of the noticeable morphological defects and mortality. Parental RNAi experiments were followed by the assessment of oviposition rates. Individuals were utilized for single pair mattings (10 to 15 pairs, respectively). Three‐day ovipositions (13‐day‐old to 15‐day‐old females) were collected, counted and held for hatch‐rate measurement and their development observed. Numbers of offspring were counted 15 days after the eggs were collected.
Statistical analysis
The data analysis was done using SPSS version 13.0 by one‐way analysis of variance, followed by Tukey's Honestly Significant Difference test. All the data are presented as the mean ± SE. Error bars represent standard errors among three biological replications. P‐value <0.05 was regarded as statistically significant, while P‐value <0.001 was considered extreme significance.
Results
Identification of hsp18.3 from T. castaneum
Previous annotations for the gene encoding hsp18.3 in the T. castaneum genome (Li et al., 2009) was confirmed to be correct by full‐length cDNA cloning and sequencing in this study. Tchsp18.3 contains a 474 bp open reading frame (ORF) and locates on the LG8 chromosome. The predicted amino acid sequence of Tchsp18.3 exhibited 40.6%, 41.0%, 42.5%, 32.3% and 30.8% identity to hsp21.6 (XP_308606) from Anopheles gambiae, hsp22.5 (XP_001119884) from Apis mellifera, hsp20.4 (NP_001037038) from Bombyx mori, hsp23.0 (NP_523999) and hsp27.0 (NP_524000.1) from D. melanogaster, respectively. These shsps were the highest sequence identity screened out from other insects; all of them contained the strictly conserved α‐crystalline domain, which consists of approximately 100 amino acid residues and nine common β‐strands, with mild to low degrees of conservation in the remaining sequences. Identity of the α‐crystalline domain ranged from 45.4% to 61.5% between Tchsp18.3 and other shsps (Fig. 1).
Figure 1.
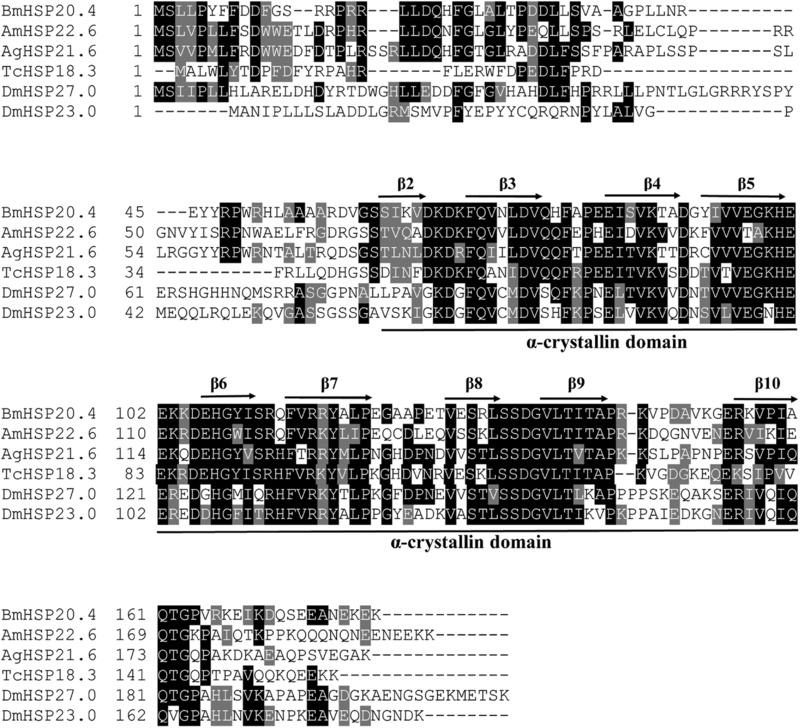
Amino acid sequence alignment of Tchsp18.3 and five selected shsps from Bombyx mori, Apis mellifera, Anopheles gambiae and Drosophila melanogaster. Identical residues in the sequences are in black and similar residues are in gray. Secondary structures, as indicated by arrows (β‐sheets) above and line (α‐crystallin domain) below the alignment, were predicted and numbered according to Aevermann and Waters (2008) and Li et al. (2009).
The temporal and spatial expression patterns of Tchsp18.3
Expression patterns of Tchsp18.3 were examined by real‐time qPCR. During all stages of development, Tchsp18.3 was expressed but displayed different expression patterns. It exhibited high expression levels at the early period of each developmental stage except the embryonic stage, while it was expressed the least at late larval stage and the most at the early pupal stage. Data showed that the expression of Tchsp18.3 at early pupal stage was 10.9 times more than that at the late larval stage (Fig. 2A), while the expression of Tchsp18.3 in the ovary showed the highest level, which was 49.7 ± 3.7‐fold higher than that in the accessory gland. Moreover, fat body also exhibited abundant expression of Tchsp18.3 (Fig. 2B).
Figure 2.

The expression patterns of Tchsp18.3 in different developmental stages and various late adult tissues. (A) Different developmental stages are EE: early eggs; LE: late eggs; EL: early larvae; LL: late larvae; EP: early pupae; LP: late pupae; EA: early adults; LA: late adults. (B) Various tissues from late adults are head, epidermis, gut, fat body, accessory gland, testis and ovary. Tribolium ribosomal protein S3 (rps3) transcript with the same complementary DNA (cDNA) template served as an internal control. Different lowercase letters indicate statistically significant differences (P < 0.05).
Responses to temperature stress
After heat stress, Tchsp18.3 expression level was significantly increased and it reached a peak after 4 h of treatment at 45°C, which was 4532 ± 396‐fold higher than that without the thermal treatment. Under 45°C for 12 h, the expression level was reduced but still much higher than that of control. Interestingly on the other hand, Tchsp18.3 did not show any response to cold stress (4 °C) (Fig. 3).
Figure 3.
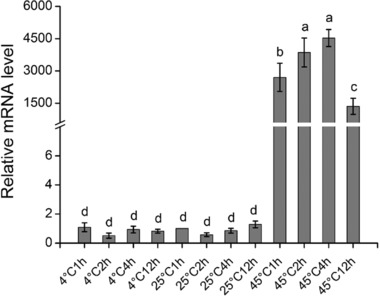
The expression patterns of Tchsp18.3 under cold (4°C) and heat (45°C) treatment, with an ambient temperature 25°C serving as control. Tribolium ribosomal protein S3 (rps3) transcript with the same complementary DNA (cDNA) template served as an internal control. Different lowercase letters indicate statistically significant differences (P<0.05).
Responses to starvation stress
After parental RNAi experiment, Tchsp18.3 gene has been strongly inhibited (Fig. S1A). Under starvation stress, Tchsp18.3 expression levels were significantly inhibited compared with the WT group until the beetles’ dying, and the relative expression levels were the lowest on the 4th day to the 8th day (Fig. 4A). From the 8th day, adults in the ds‐Tchsp18.3 group began to die, and the survival rate was significantly lower than the WT group and IB group from 12 days later (Fig. 4B). Finally, adults in the ds‐Tchsp18.3 group could only survive for 13.2 ± 0.2 days under starvation. It was significantly shorter than the WT group at 15.7 ± 0.2 days and IB group at 14.9 ± 0.2 days, which reduced by 15.8% compared to the WT group (P < 0.001) (Fig. 4C). These results showed that the beetles decreased the resistance to starvation when Tchsp18.3 was knocked down.
Figure 4.
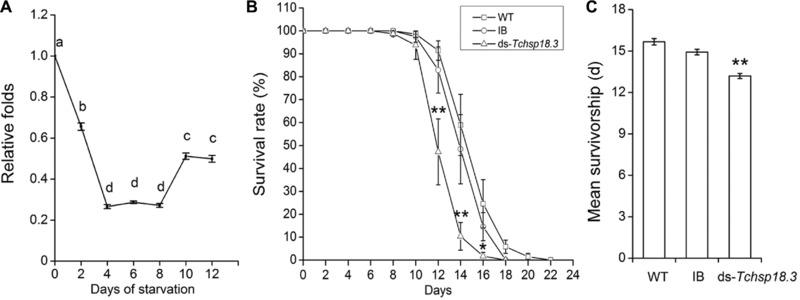
Effect of Tchsp18.3 on starvation treatment. (A) Time course analysis of Tchsp18.3 expression under starvation stress. Different lowercase letters indicate statistically significant differences (P < 0.05). (B) The differential survival rates of wild type (WT), IB and ds‐Tchsp18.3 beetles under starvation. (C) Mean survivals (days) of WT, IB and ds‐Tchsp18.3 beetles under starvation. WT, wild type, beetles received a non‐injection; IB, beetles injected with physiological buffer; ds‐Tchsp18.3, beetles injected with double‐stranded RNA in 2‐day‐old pupae. *P < 0.05; **P < 0.001.
RNAi phenotypes of Tchsp18.3
Injection of dsRNA of Tchsp18.3 during the late larval stage resulted in stagnation or defect in pupal‐adult metamorphosis (Fig. 5A) and suppression of transcript level for Tchsp18.3 (Fig. S1B; Fig. 5B). Interestingly, larval RNAi of Tchsp18.3 did not affect the insects’ pupation, but caused approximately 86.1% ± 4.5% of pupae to fail in eclosion (Fig. 5C). These insects with abnormal metamorphosis died at no more than 3 days.
Figure 5.
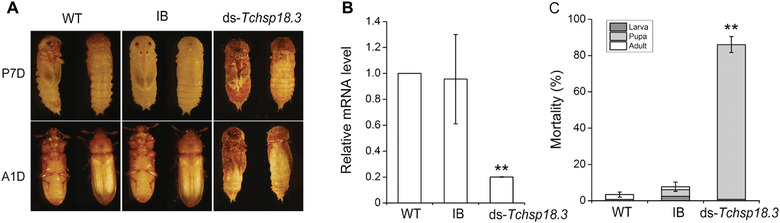
RNA interference (RNAi) phenotype of Tchsp18.3. (A) RNAi resulted in lethal phenotypes in pupal/adult stages. P7D, 7‐day‐old pupae; A1D, 1‐day‐old adult. (B) Expression levels of knocking down Tchsp18.3 using larval RNAi. (C) Mortality of wild type (WT), IB and ds‐Tchsp18.3 beetles. WT, wild type, beetles received a non‐injection; IB, beetles injected with physiological buffer; ds‐Tchsp18.3, beetles injected with double‐stranded RNA in late larval stage. **P < 0.001.
To investigate the effect of Tchsp18.3 on reproduction, parental RNAi was carried out. Five groups were set as follow: WT group, IB group, ds‐Tchsp18.3 group, ds‐Tchsp18.3♂ × WT♀ group and WT♂ × ds‐Tchsp18.3♀ group. One pair of adults of the WT group and IB group can lay an average of 6.8 and 6.6 eggs per day, respectively. When Tchsp18.3 was knocked down, one pair of the beetles can hardly spawn. The inhibited oviposition was also discovered when the female and male insects were injected separately, as one pair of beetles in the ds‐Tchsp18.3♂ × WT♀ group and WT♂ × ds‐Tchsp18.3♀ group can only lay about 3.4 eggs and 0.6 eggs per day, respectively (Fig. 6).
Figure 6.
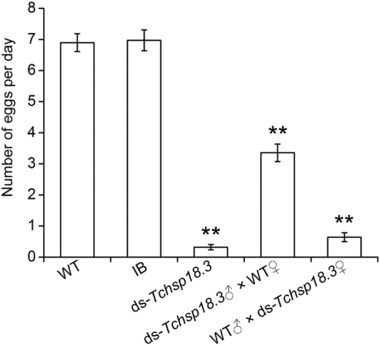
Effect of Tchsp18.3 on fertility. Wild type (WT), beetles received a non‐injection; IB, beetles injected with physiological buffer; ds‐Tchsp18.3, beetles injected with double‐stranded RNA in 2‐day‐old pupae. ds‐Tchsp18.3♂ × WT♀, ds‐Tchsp18.3 male crossed with WT female adult; WT♂ × ds‐Tchsp18.3♀, WT male crossed with ds‐Tchsp18.3 female adult. **P < 0.001.
Discussion
Although sHSPs are probably the most diverse in structure and function among the various superfamilies of stress proteins, they still have relatively conserved domains, the α‐crystallin domain. Usually, the amino‐terminal extension is variable in length and sequence, while in addition to the α‐crystallin domain, the region of the initial 29 amino acids in the amino‐terminal among these six aligned sHSPs showing 73.7% consensus is highly conserved than that of ten sHSPs in T. castanuem which only showed 40.7% consensus (Fig. 1). This domain is highly hydrophobic which can be involved in modulating oligomerization, subunit dynamics, and substrate binding (Sun & MacRae, 2005). The relatively higher homology of these sHSPs in the amino‐terminal suggests that Tchsp18.3 may also conserve in the structure as well as its function among differential organisms.
sHSPs have been shown to play important roles in insect development and always vary according to developmental stage. Here, Tchsp18.3 showed high expression levels at the early period of each developmental stage except the embryonic stage, and reaching a peak in the early pupal stage (Fig. 2A). The phenomenon of pupal high expression of shsps also can be found in other species, for instance, hsp19.5 in Plutella xylostella (Sonoda et al., 2006), hsp19.5, hsp20.8 and hsp21.7 in Liriomyza sativa (Huang et al., 2009). Hsp23, hsp26 and hsp27 mRNA of D. melanogaster are barely detectable in early third instar larvae but are major components of late third instar and early pupal stages (Mason et al., 1984). In the pupal stage, many tissues and organs are degraded and reconstructed during insect metamorphosis. It is likely that metamorphosis itself can serve as a factor to induce the expression of heat shock protein genes (Huang et al., 2009). This biological process was further regulated by steroid hormone 20E, which acts through a canonical nuclear receptor complex composed of the ecdysone receptor (EcR) and ultraspiracle (USP) heterodimer (Morrow & Tanguay, 2012). Cis‐acting elements involved in the ecdysterone regulation of several shsp genes have been mapped, and short sequence elements in the Dmhsp27 and Dmhsp23 promoters have been identified as binding sites for EcR (Amin et al., 1991). This suggested that expressions of Dmhsp27 and Dmhsp23 were regulated in part by the ecdysone hormone which regulated the binding of EcR to ecdysone response elements on the upstream of shsp genes. These results indicated that Tchsp18.3 may be like that of Dmhsp27 and Dmhsp23; its up‐regulated expression during early pupal stage may also involve the hormone regulated metamorphosis. Simultaneously, our RNAi experiment provided strong evidence that Tchsp18.3 is critical to pupal–adult metamorphosis (Fig. 5A). It showed about 86.1% ± 4.5% failure rate of adult eclosion in the ds‐Tchsp18.3 group, which indicated that Tchsp18.3 had a significant effect on metamorphosis and even the survival ability of the beetles.
Expression patterns of insect shsps usually show tissue specificity. In Oxya chinensis, hsp19.1, hsp20.4, hsp20.7 and hsp21.1 were more highly expressed in the ovary or testis than in the other tissues; meanwhile, the highest expression levels of hsp19.8 and hsp23.8 were found in the muscles (Kou et al., 2016). In B. mori, hsp19.1 and hsp22.6 showed relatively high expression levels in integument, head and midgut, while hsp20.1, hsp20.4 and hsp27.4 were highly expressed in ovary and testis (Li et al., 2009). Similar to hsp20.4 from B. mori, Tchsp18.3 showed the highest expression in ovaries than in other tissues. It was also highly expressed in fat body. These shsps expressed in special tissues without stress may play an important role in keeping the normal development of the cell and tissues. For example, Dmhsp27, which was highly expressed in ovaries in both unstressed and stressed conditions, played important roles in ovaries either related to the control of cell division/differentiation of germ cells during normal growth, or to the maintenance of ovarian integrity under environmental stresses (Marin and Tanguay, 1996). Hsp27 in the Mediterranean fruit fly, Ceratitis capitata, also was expressed in a stage and cell‐specific manner during oogenesis and spermatogenesis (Economou et al., 2017). Interestingly in our study, knockdown of Tchsp18.3 which had the highest expression in ovaries could cause a significantly declined reproductive capacity (Fig. 6), and since females in the ds‐Tchsp18.3 group appeared to have significant ovarian atrophy, Tchsp18.3 acted as a maternal effect gene which was similar to Tchsp90. RNAi of both of them led the female to have no offspring, and the male to have low reproduction rates (Zhang et al., 2014a). These phenomena implied Tchsp18.3 may also be involved in the oogenesis and early embryo development as it is likely involved in cell division/differentiation of germ cells during the reproductive process in T. castaneum. In addition, higher expression of Tchsp18.3 in fat body may also be another factor to affect reproduction. Fat body is not only a dynamic tissue involved in multiple metabolic functions and undergoes major functional changes during development and metamorphosis, but also an important site to synthesize and secrete insect yolk proteins, or vitellogenins (Hagedorn & Fallon, 1973). Further, fatty acids stored in the lipid droplets of fat body are mobilized for the provision of lipids to the ovaries, and then maintain the metabolic activity of the organ, because lipids comprise 30%–40% of the dry weight of insect oocytes and they are the main source of energy for the developing embryo (Arrese & Soulages, 2010). Thus, ds‐Tchsp18.3 affected the normal function of fat body and perhaps could impede the synthesis of vitellogenins or the accumulation of lipid in the ovaries, then declining the reproductive capacity as well.
Moreover, Tchsp18.3 was dramatically up‐regulated by heat stress. The relative expression of Tchsp18.3 in 45°C was approximately 4500‐fold higher than that of 25°C, which is much more significant than most shsps in other species. Meanwhile, this up‐regulation was more significant than Tchsp90 in response to heat stress (Zhang et al., 2014a). This is in agreement with recent findings. When the cells were is exposed to 40°C heat shock for 1 h, although Tchsp18.3, Tchsp68a and Tchsp83 were significantly up‐regulated as a similar expression pattern, Tchsp18.3 seems to be the most affected (Garcia‐Reina et al., 2017). This phenomenon could also be found when beetles were under mild heat shock; Tchsp18.3 was up‐regulated higher than Tchsp68 (Altincicek et al., 2008). However, Tchsp18.3 was insensitive to cold treatment (Fig. 3). It is worth mentioning that this is quite different from other structurally similar sHSPs. For example, both Dmhsp23 and Dmhsp27 were up‐regulated during recovery from cold stress (Colinet et al., 2010), and in Sarcophaga crassipalpis, hsp23 was developmentally up‐regulated during overwintering pupal diapause, the RNAi of which in older pupae even would cause a significant loss of cold tolerance (Rinehart et al., 2007). The different reactions to cold of shsps may be related to the specific living environments of the insects. Since T. castaneum is basically living in a warm cereal storage place and without diapause, then Tchsp18.3 gene only showed a highly sensitive response to high temperature stress. While Dipteran D. melanogaster exhibits diapause at low temperatures and short day lengths (Lumme et al., 1974; Saunders et al., 1989), it is necessary for D. melanogaster to contain certain shsps to resist cold stress. These results suggest that the insects as well as their chaperone molecules, shsp genes, could evolve according to their own survival environments and requirements.
In addition, when Tchsp18.3 was knocked down, the beetles decreased the ability to resist starvation, and all adults died within 18 days after starvation treatment. Their average lifespan was significantly shorter than the WT group (Fig. 4C). Meanwhile, Tchsp18.3 was significantly down‐regulated under starvation conditions, which is consistent with the early finding that a significant reduction in starvation resistance was associated with knockout hsp27 allele in D. melanogaster (Hao et al., 2007). In response to starvation, eukaryotic cells re‐absorb nutrition through autophagy, which occurs in the comprehensive reorganization of cellular activities aimed at surviving low nutrient levels (Scott et al., 2004). In mammals, phosphorylated dimers of HSP27 can block apoptosis induced by activation of the death receptor fas (Charette & Landry, 2000). It further intervened in modulating cell death pathways by interacting with various components of the cell death machinery upstream and downstream of the mitochondrial apoptotic events and can prevent apoptosis in lethal stress situations (Kanagasabai et al., 2010; Acunzo et al., 2012). Here, knockdown of Tchsp18.3 was associated with a significant decrease in response to starvation and insect survival rates, suggesting that it may also protect cells from the lethal condition perhaps mainly by its involvement in cell death pathways. Moreover, heat shock proteins could be immunoregulatory agents with potent and widely applicable therapeutic uses (Pockley, 2003). A sophisticated regulation of gene expression in response to different immune stimuli was determined in T. castaneum, and Tchsp18.3 was one of the immune‐induced genes (Altincicek et al., 2008). Thus under starvation, down‐regulated expression of Tchsp18.3 may cause in beetles a decrease in immunity, then dying faster than the control group.
Conclusion
In conclusion, the temporal and spatial expression patterns, and functions of Tchsp18.3 have been clarified in the present study. Tchsp18.3 showed high expression in early pupal stage and was the highest expressed in the ovary and followed the fat body. Knockdown of Tchsp18.3 in late larvae affected the pupal–adult metamorphosis, which led to 86.1% ± 4.5% of pupae failing in eclosion. Additionally, Tchsp18.3 silencing caused a significant reduction in reproduction in T. castaneum. These results indicated that Tchsp18.3 keeps certain similarities in expression patterns and several functions with other shsps, such as Dmhsp23 and Dmhsp27. However, Tchsp18.3 also differentiated from them. In addition to response to starvation, it was only strongly stimulated by high temperature stress but not in response to cold stress, which is likely to be an evolutional adaptation to survival environments and habits.
Disclosure
The authors declare that there is no conflict of interest.
Supporting information
Fig. S1 RNA interference efficiency after knockdown experiment in pupal stage (A) and larval stage (B).
Acknowledgments
The work was supported by grants from the National Natural Science Foundation of China (No. 31572326 & 31172146), the Natural Science Foundation of Jiangsu Province, China (No. BK2011785) and the PAPD of Jiangsu Higher Education Institutions.
The copyright line for this article was changed on 06 February 2019 after original online publication.
References
- Acunzo, J. , Katsogiannou, M. and Rocchi, P. (2012) Small heat shock proteins HSP27 (HspB1), alphaB‐crystallin (HspB5) and HSP22 (HspB8) as regulators of cell death. International Journal of Biochemistry and Cell Biology, 44, 1622–1631. [DOI] [PubMed] [Google Scholar]
- Aevermann, B.D. and Waters, E.R. (2008) A comparative genomic analysis of the small heat shock proteins in Caenorhabditis elegans and briggsae . Genetica, 133, 307–319. [DOI] [PubMed] [Google Scholar]
- Altincicek, B. , Knorr, E. and Vilcinskas, A. (2008) Beetle immunity: identification of immune‐inducible genes from the model insect Tribolium castaneum . Developmental and Comparative Immunology, 32, 585–595. [DOI] [PubMed] [Google Scholar]
- Amin, J. , Mestril, R. and Voellmy, R. (1991) Genes for Drosophila small heat shock proteins are regulated differently by ecdysterone. Molecular and Cellular Biology, 11, 5937–5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrese, E.L. and Soulages, J.L. (2010) Insect fat body: energy, metabolism, and regulation. Annual Review of Entomology, 55, 207–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo, A.P. (2000) sHsp as novel regulators of programmed cell death and tumorigenicity. Pathologie‐Biologie, 48, 280–288. [PubMed] [Google Scholar]
- Bakthisaran, R. , Tangirala, R. and Rao, C. (2015) Small heat shock proteins: role in cellular functions and pathology. Biochimica et Biophysica Acta, 1854, 291–319. [DOI] [PubMed] [Google Scholar]
- Begum, K. , Li, B. , Beeman, R.W. and Park, Y. (2009) Functions of ion transport peptide and ion transport peptide‐like in the red flour beetle Tribolium castaneum . Insect Biochemistry and Molecular Biology, 39, 717–725. [DOI] [PubMed] [Google Scholar]
- Bucher, G. , Scholten, J. and Klingler, M. (2002) Parental RNAi in Tribolium (Coleoptera). Current Biology, 12, R85–R86. [DOI] [PubMed] [Google Scholar]
- Caspers, G.J. , Leunissen, J.A. and de Jong, W.W. (1995) The expanding small heat‐shock protein family, and structure predictions of the conserved “alpha‐crystallin domain”. Journal of Molecular Evolution, 40, 238–248. [DOI] [PubMed] [Google Scholar]
- Charette, S.J. and Landry, J. (2000) The interaction of HSP27 with Daxx identifies a potential regulatory role of HSP27 in Fas‐induced apoptosis. Annals of the New York Academy of Sciences, 926, 126–131. [DOI] [PubMed] [Google Scholar]
- Chen, X.E. and Zhang, Y.L. (2015) Identification of multiple small heat‐shock protein genes in Plutella xylostella (L.) and their expression profiles in response to abiotic stresses. Cell Stress and Chaperones, 20, 23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colinet, H. , Lee, S.F. and Hoffmann, A. (2010) Temporal expression of heat shock genes during cold stress and recovery from chill coma in adult Drosophila melanogaster . The FEBS Journal, 277, 174–185. [DOI] [PubMed] [Google Scholar]
- Economou, K. , Kotsiliti, E. and Mintzas, A.C. (2017) Stage and cell‐specific expression and intracellular localization of the small heat shock protein Hsp27 during oogenesis and spermatogenesis in the Mediterranean fruit fly. Ceratitis capitata. Journal of Insect Physiology, 96, 64–72. [DOI] [PubMed] [Google Scholar]
- Franck, E. , Madsen, O. , van Rheede, T. , Ricard, G. , Huynen, M.A. and de Jong, W.W. (2004) Evolutionary diversity of vertebrate small heat shock proteins. Journal of Molecular Evolution, 59, 792–805. [DOI] [PubMed] [Google Scholar]
- Fu, X.M. , Jiao, W.W. and Chang, Z.Y. (2006) Phylogenetic and biochemical studies reveal a potential evolutionary origin of small heat shock proteins of animals from bacterial class A. Journal of Molecular Evolution, 62, 257–266. [DOI] [PubMed] [Google Scholar]
- Garcia‐Reina, A. , Rodriguez‐Garcia, M.J. , Ramis, G. and Galian, J. (2017) Real‐time cell analysis and heat shock protein gene expression in the TcA Tribolium castaneum cell line in response to environmental stress conditions. Insect Science, 24, 358–370. [DOI] [PubMed] [Google Scholar]
- Garrido, C. , Brunet, M. , Didelot, C. , Zermati, Y. , Schmitt, E. and Kroemer, G. (2006) Heat shock proteins 27 and 70: anti‐apoptotic proteins with tumorigenic properties. Cell Cycle, 5, 2592–2601. [DOI] [PubMed] [Google Scholar]
- Hagedorn, H.H. and Fallon, A.M. (1973) Ovarian control of vitellogenin synthesis by the fat body in Aedes aegypti . Nature, 244(5411), 103–105. [DOI] [PubMed] [Google Scholar]
- Haliscak, J.P. and Beeman, R.W. (1983) Status of malathion resistance in five genera of beetles infesting farm‐stored corn, wheat, and oats in the United States. Journal of Economic Entomology, 76, 717–722. [Google Scholar]
- Hao, X.M. , Zhang, S. , Timakov, B. and Zhang, P. (2007) The Hsp27 gene is not required for Drosophila development but its activity is associated with starvation resistance. Cell Stress and Chaperones, 12, 364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslbeck, M. , Franzmann, T. , Weinfurtner, D. and Buchner, J. (2005) Some like it hot: the structure and function of small heat‐shock proteins. Nature Structural and Molecular Biology, 12, 842–846. [DOI] [PubMed] [Google Scholar]
- Haslbeck, M. and Vierling, E. (2015) A first line of stress defense: small heat shock proteins and their function in protein homeostasis. Journal of Molecular Biology, 427, 1537–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, L.H. , Wang, C.Z. and Kang, L. (2009) Cloning and expression of five heat shock protein genes in relation to cold hardening and development in the leafminer. Liriomyza sativa. Journal of Insect Physiology, 55, 279–285. [DOI] [PubMed] [Google Scholar]
- Kanagasabai, R. , Karthikeyan, K. , Vedam, K. , Qien, W. , Zhu, Q. and Ilangovan, G. (2010) Hsp27 protects adenocarcinoma cells from UV‐induced apoptosis by Akt and p21‐dependent pathways of survival. Molecular Cancer Research, 8, 1399–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, K.K. , Kim, R. and Kim, S.H. (1998) Crystal structure of a small heat‐shock protein. Nature, 394(6693), 595–599. [DOI] [PubMed] [Google Scholar]
- Kou, L.H. , Wu, H.H. , Liu, Y.M. , Zhang, Y.P. , Zhang, J.Z. and Guo, Y.P. et al (2016) Molecular characterization of six small heat shock proteins and their responses under cadmium stress in Oxya chinensis (Orthoptera: Acridoidea). Environmental Entomology, 45, 258–267. [DOI] [PubMed] [Google Scholar]
- Li, B. , Beeman, R.W. and Park, Y. (2011) Functions of duplicated genes encoding CCAP receptors in the red flour beetle. Tribolium castaneum. Journal of Insect Physiology, 57, 1190–1197. [DOI] [PubMed] [Google Scholar]
- Li, Z.W. , Li, X. , Yu, Q.Y. , Xiang, Z.H. , Kishino, H. and Zhang, Z. (2009) The small heat shock protein (sHSP) genes in the silkworm, Bombyx mori, and comparative analysis with other insect sHSP genes. BMC Evolutionary Biology, 9, 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak, K.J. and Schmittgen, T.D. (2001) Analysis of relative gene expression data using real‐time quantitative PCR and the 2−△△ Method. Methods, 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Lumme, J. , Oikarinen, A. , Lakovaara, S. and Alatalo, R. (1974) The environmental regulation of adult diapause in Drosophila littoralis . Journal of Insect Physiology, 20, 2023–2033. [DOI] [PubMed] [Google Scholar]
- Marin, R. and Tanguay, R.M. (1996) Stage‐specific localization of the small heat shock protein Hsp27 during oogenesis in Drosophila melanogaster . Chromosoma, 105, 142–149. [DOI] [PubMed] [Google Scholar]
- Mason, P.J. , Hall, L.M.C. and Gausz, J. (1984). The expression of heat shock genes during normal development in Drosophila melanogaster (heat shock/abundant transcripts/developmental regulation). Molecular and General Genetics MGG, 194, 73–78. [Google Scholar]
- Morrow, G. , Battistini, S. , Zhang, P. and Tanguay, R.M. (2004) Decreased lifespan in the absence of expression of the mitochondrial small heat shock protein Hsp22 in Drosophila . Journal of Biological Chemistry, 279, 43382–43385. [DOI] [PubMed] [Google Scholar]
- Morrow, G. and Tanguay, R.M. (2012) Small heat shock protein expression and functions during development. International Journal of Biochemistry and Cell Biology, 44, 1613–1621. [DOI] [PubMed] [Google Scholar]
- Perez‐Morales, D. and Espinoza, B. (2015) The role of small heat shock proteins in parasites. Cell Stress and Chaperones, 20, 767–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockley, A.G. (2003) Heat shock proteins as regulators of the immune response. Lancet, 362(9382), 469–476. [DOI] [PubMed] [Google Scholar]
- Richards, S. , Gibbs, R.A. , Weinstock, G.M. , Brown, S.J. , Denell, R. , Beeman, R.W. et al (2008) The genome of the model beetle and pest Tribolium castaneum . Nature, 452(7190), 949–955. [DOI] [PubMed] [Google Scholar]
- Rinehart, J.P. , Li, A. , Yocum, G.D. , Robich, R.M. , Hayward, S.A.L. and Denlinger, D.L. (2007) Up‐regulation of heat shock proteins is essentail for cold survival during insect diapause. Proceedings of the National Academy of Sciences USA, 104, 11130–11137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritossa, F. (1962) A new puffing pattern induced by temperature shock and DNP in Drosophila . Experientia, 18(12), 571–573. [Google Scholar]
- Sang, M. , Li, C. , Wu, W. and Li, B. (2016) Identification and evolution of two insulin receptor genes involved in Tribolium castaneum development and reproduction. Gene, 585, 196–204. [DOI] [PubMed] [Google Scholar]
- Sang, W. , Ma, W.H. , Qiu, L. , Zhu, Z.H. and Lei, C.L. (2012) The involvement of heat shock protein and cytochrome P450 genes in response to UV‐A exposure in the beetle Tribolium castaneum . Journal of Insect Physiology, 58, 830–836. [DOI] [PubMed] [Google Scholar]
- Saunders, D.S. , Henrich, V.C. and Gilbert, L.I. (1989) Induction of diapause in Drosophila melanogaster: photoperiodic regulation and the impact of arrhythmic clock mutations on time measurement. Proceedings of the National Academy of Sciences USA, 86, 3748–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder, R. , Beermann, A. , Wittkopp, N. and Lutz, R. (2008) From development to biodiversity – Tribolium castaneum, an insect model organism for short germband development. Development Genes and Evolution, 218(3–4), 119–126. [DOI] [PubMed] [Google Scholar]
- Scott, R.C. , Schuldiner, O. and Neufeld, T.P. (2004) Role and regulation of starvation‐induced autophagy in the Drosophila fat body. Developmental Cell, 7, 167–178. [DOI] [PubMed] [Google Scholar]
- Sonoda, S. , Ashfaq, M. and Tsumuki, H. (2006) Cloning and nucleotide sequencing of three heat shock protein genes (hsp90, hsc70, and hsp19.5) from the diamondback moth, Plutella xylostella (L.) and their expression in relation to developmental stage and temperature. Archives of Insect Biochemistry and Physiology, 62(2), 80–90. [DOI] [PubMed] [Google Scholar]
- Sun, Y. and MacRae, T.H. (2005) Small heat shock proteins: molecular structure and chaperone function. Cellular and Molecular Life Sciences, 62(21), 2460–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoyasu, Y. and Denell, R.E. (2004) Larval RNAi in Tribolium (Coleoptera) for analyzing adult development. Development Genes and Evolution, 214, 575–578. [DOI] [PubMed] [Google Scholar]
- Tsvetkova, N.M. , Horvath, I. , Torok, Z. , Wolkers, W.F. , Balogi, Z. , Shigapova, N. et al (2002) Small heat‐shock proteins regulate membrane lipid polymorphism. Proceedings of the National Academy of Sciences USA, 99, 13504–13509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, B. , Zheng, J. , Peng, Y. , Liu, X. , Hoffmann, A.A. and Ma, C.S. (2015) Stress responses of small heat shock protein genes in Lepidoptera point to limited conservation of function across phylogeny. PLoS ONE, 10(7), e132700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Gu, S.S. , Li, C.J. , Sang, M. , Wu, W. , Yun, X.P. et al (2014a) Identification and characterization of novel ER‐based hsp90 gene in the red flour beetle. Tribolium castaneum. Cell Stress and Chaperones, 19, 623–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y.Y. , Liu, Y.L. , Guo, X.L. , Li, Y.L. , Gao, H.R. , Guo, X.Q. et al (2014b) sHsp22.6, an intronless small heat shock protein gene, is involved in stress defence and development in Apis cerana cerana . Insect Biochemistry and Molecular Biology, 53, 1–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 RNA interference efficiency after knockdown experiment in pupal stage (A) and larval stage (B).


