Abstract
Background
Cardiovascular surgery patients with a prolonged intensive care unit (ICU) stay may benefit most from early nutrition support. Using established scoring systems for nutrition assessment and operative risk stratification, we aimed to develop a model to predict a prolonged ICU stay ≥5 days in order to identify patients who will benefit from early nutrition interventions.
Methods
This is a retrospective analysis of a prospective observational study of patients undergoing elective valvular, coronary artery bypass grafting, or combined cardiac surgery. The nutrition risk was assessed by well‐established screening tools. Patients’ preoperative EuroSCORE (European System for Cardiac Operative Risk Evaluation), primary disease, and intraoperative cardiopulmonary bypass (CPB) time were included as independent variables in a multivariate logistic regression analysis to predict a prolonged ICU stay (>4 days).
Results
The number of cardiac surgery patients included was 1193. Multivariate analysis revealed that for prediction of ICU stay >4 days, both Nutritional Risk Screening 2002 (area under the curve (AUC): 0.716, P = .020) and Mini Nutritional Assessment (MNA) score (AUC: 0.715, P = .037) were significant, whereas for prediction of ICU stay >5 days, only the MNA score showed significant results (AUC: 0.762, P = .011).
Conclusion
Present data provide first evidence about the combined use of EuroSCORE, primary disease, CPB time, and nutrition risk screening tools for prediction of prolonged ICU stay in cardiac surgery patients. If prospectively evaluated in adequately designed studies, this model may help to identify patients with prolonged ICU stay to initiate early postoperative nutrition therapy and thus, facilitate an enhanced recovery.
Keywords: cardiac disease, cardiac surgery, ICU stay, nutrition, nutrition assessment, nutrition screening tools, nutrition support practice, outcomes research, outcomes quality, parenteral nutrition, prediction model, research and diseases
Clinical Relevancy Statement
Emerging evidence indicates that adequate nutrition support is of special clinical significance for cardiac surgery patients with prolonged intensive care unit (ICU) stay. This small but resource‐intensive group are at increased risk to develop malnutrition, which is associated with increased morbidity and mortality. Our data demonstrate good ability of a model combining both preoperative risk stratification and nutrition risk screening tools to predict prolonged ICU length of stay (defined as ≥5 days) after cardiac surgery. These findings are clinically relevant for identifying patients with an estimated prolonged ICU stay, enabling clinicians to start early a postoperative nutrition support to prevent malnutrition and resulting delayed recovery.
Introduction
Adequate nutrition support is of special significance for critically ill patients with prolonged intensive care unit (ICU) stay as these patients are at increased risk to develop malnutrition and protein‐energy deficits, which in turn are associated with increased morbidity and mortality.1, 2, 3, 4, 5 Both protein and energy deficiency are commonly observed in ICUs, occurring in 43%–88% of critically ill patients.6, 7 Emerging evidence indicates that critically ill cardiac surgery patients are particularly prone to be at increased risk of malnutrition in the postoperative course.8, 9 In this setting, nutrition support was started later and had lowest total nutrition adequacy when compared with other surgical or medical ICU patients.8 Low nutrition adequacy during the postoperative course is associated with an increased rate of infection, poor wound healing, reduced respiratory muscle mass, delayed weaning from mechanical ventilation, increased length of ICU stay, increased readmission rates, and high healthcare costs and shorter survival time.6, 7, 10, 11, 12
While disease‐related malnutrition (DRM) represents a specific type of malnutrition caused by a concomitant disease,13 which cannot be easily treated by nutrition support only, the early identification of patients at nutrition risk may allow an early initiation of an adequate nutrition support during critical illness, to prevent a further aggravation of malnutrition. Although the cardiac surgery setting provides advantages due to its predictable inflammatory response and scheduled insult, valid assessment tools for the detection and evaluation of cardiac surgery patients at nutrition risk are still poorly investigated. While there are numerous risk scores and assessment tools that quantify the nutrition risk,14, 15, 16, 17, 18, 19 or which assist identifying critically ill patients most likely to benefit from nutrition therapy,20, 21, 22, 23 hitherto none have been specifically designed for cardiac surgery ICU patients. During the postoperative ICU stay, most would uniformly classify all critically ill patients to be at high nutrition risk.14, 21 As an alternative to the assessment of nutrition risk, the prediction of prolonged ICU stay may be a promising approach to identifying patients at risk for underfeeding. Faisy et al demonstrated that large, negative energy balance increases with duration of ICU stay, becoming most relevant during prolonged mechanical ventilation, in patients fed under standardized nutrition management protocol.24 In contrast to general ICU patients, elective cardiac surgery allows for pre‐insult nutrition risk assessment and preoperative risk stratification by well‐established scoring systems, such as EuroSCORE (European System for Cardiac Operative Risk Evaluation) and anticipated duration of cardiopulmonary bypass (CPB) time.25, 26, 27 The EuroSCORE is routinely used for preoperative risk stratification in cardiac surgery patients prior to surgery, which is based on patient factors, cardiac factors, and operation factors. In addition to these operative risk models, Lomivorotov et al provided first evidence about the predictive accuracy of nutrition screening and assessment tools for postoperative complications and ICU stay longer than 2 days.28 International nutrition guidelines recommend malnutrition risk screening and subsequent nutrition assessment in identified at‐risk patients in the ICU,29 as screening and subsequent assessment represent the basis for the diagnosis decisions, further therapeutic actions, and initiation of specific nutrition treatment. For cardiac surgery ICU patients, the effects of surgery obviously represent a further major determinant for postoperative outcomes; therefore, we developed a new conceptual model to consider these relevant factors for an early prediction of postoperative complications resulting in prolonged ICU stay in these patients (Figure 1).
Figure 1.
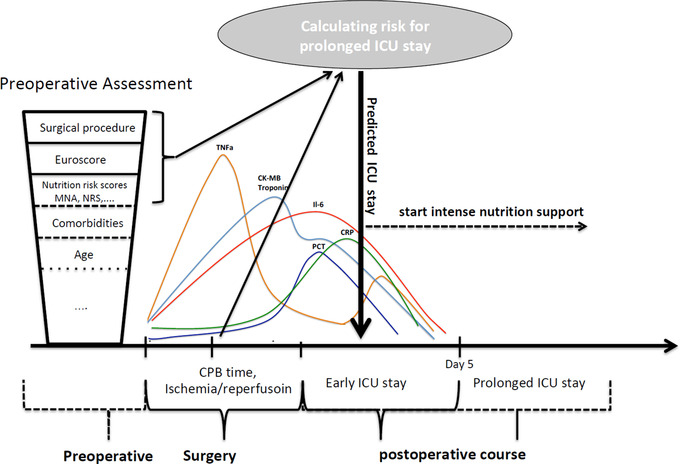
Conceptual model to identify patients with prolonged ICU stay and adapt ICU nutrition management accordingly. CK‐MB, creatine kinase MB; CPB, cardiopulmonary bypass; CRP, C‐reactive protein; EuroSCORE, European System for Cardiac Operative Risk Evaluation; ICU, intensive care unit; IL, interleukin; MNA, Mini Nutritional Assessment; NRS‐2002, Nutritional Risk Screening 2002; PCT, procalcitonin; TNF, tumor necrosis factor.
We followed the hypothesis that a simple prediction of prolonged ICU stay by combination of specific cardiac surgery risk scores (eg, EuroSCORE), key surgical information (eg, cardiopulmonary bypass (CPB) time), and nutrition risk measures could be an alternative approach to identify cardiac surgery patients with prolonged ICU stay to enable in future an early‐initiated postoperative intense nutrition therapy in these critically ill patients.
Methods
Study Design
The present study is a retrospective secondary analysis of a prospective observational study of nutrition practice in cardiac surgery patients. The original trial was focused on the short‐term prognostic value of different nutrition screening tools in patients undergoing CPB, with regard to the development of adverse clinical outcomes in the early postoperative course (48 hours).28 The study was approved by the local ethics committee and patients were consecutively enrolled after informed consent before surgery. The trial had been registered at clinicaltrials.gov as NCT01366807.
Patient Cohort
For this analysis, patients were included if they were >18 years and scheduled for cardiothoracic surgery with CPB, and underwent coronary artery bypass grafting (CABG), valvular, or combined cardiac surgery. The patients were assessed in the preoperative period within 48 hours of admission to the hospital. Both clinical management of patients and nutrition practice followed local clinical standards. Exclusion criteria were (i) emergency surgery, (ii) pulmonary thromboembolism in need of thrombectomy, (iii) aortic dissection (performed in deep hypothermic circulatory arrest), or (iv) off‐pump cardiac surgery. These exclusion criteria were established to provide time for the preoperative assessment.
General Data Collection
Data regarding baseline characteristics, including patient demographics, preoperative comorbidities, primary diagnosis, planned operative procedure, and baseline nutrition information were collected for each enrolled patient. Details on intraoperative events including CPB time and postoperative events including organ dysfunctions care processes such as length of stay and outcomes including in‐hospital mortality were also collected.
Definition of Prolonged ICU Stay
Prolonged postoperative ICU stay was defined as patients being treated at the ICU for 5 days or more. Patients requiring ICU treatment for >5 days most often suffer from severe postoperative complications along with prolonged weakness, mechanical ventilation and sedation time, and gastrointestinal dysfunction.30, 31 ICU residency of <4 days is often not associated with major morbidity, and these patients are more likely to quickly resume routine oral intake.
Nutrition Screening and Assessment
Nutrition screening was performed daily by Nutritional Risk Screening 2002 (NRS‐2002) and Malnutrition Universal Screening Tool (MUST). The nutrition assessment was performed in all subjects by Subjective Global Assessment (SGA), Mini Nutritional Assessment (MNA), and Short Nutritional Assessment Questionnaire (SNAQ) score. The preoperative assessment was performed by 2 trained specialists. The definitions for malnutrition or risk for malnutrition were classified as follows: SGA score of B or C, NRS‐2002 score ≥3, MUST score of 1 or 2, SNAQ score of 2 or 3, and MNA score of ≤7.
Statistical Methods
Data from a completed observational study were available for 1210 patients. This sample was selected pragmatical reasons and considered sufficient for the for planned exploratory analysis. Analysis was performed on available data by use of common statistical methods. Binary and categorical variables are presented as counts and percentages and compared between groups using Fisher's exact test in case of binary variables or χ2 test if >2 categories are compared. Continuous variables are presented as means with standard deviations and ranges and compared between groups using the Wilcoxon rank sum test. To assess the predictive value of different pre‐ and perioperative factors with regard to prolonged ICU stay, univariate analysis has been performed. Results of the univariate analyses are presented as odds ratios together with Wald asymptotic confidence limits and 2‐sided P‐values for the standard Wald test. Model selection was based on multivariate logistic regression analyses applying a backward selection strategy with a significance level of 0.1 required for a variable to stay in the model. Models were analyzed covering an ICU stay of >4 days with an additional sensitivity analysis of >5 days. The cutoff for EuroSCORE (9.4) and CPB time (>108 min) was calculated for prediction of ICU stay >5 days ICU by Youden Index. Given that the underlying disease significantly influences patients’ nutritional state, subsequent subgroup analysis we performed stratified analysis by primary disease (coronary artery disease (CAD) vs heart valvular disease (HVD)). As for cardiac surgery patients, the preoperatively assessed EuroSCORE as well as the CPB time are known relevant determinates for patients’ outcomes; therefore, we included these parameters in the multivariate analysis.
Model diagnostics include odds ratios together with 95% Wald confidence intervals and corresponding P‐values for factors in the model and the Hosmer‐Lemeshow goodness‐of‐fit test. Models were also compared by means of the area under the receiver operator characteristic curve. A 2‐sided P‐value < .05 was considered statistically significant.
Results
Patient Cohort and Baseline Characteristics
From 1210 patients initially screened and consented patients, 17 had to be excluded (13 patients underwent off‐pump surgery, 4 required deep hypothermic circulatory arrest), so that 1193 patients were included in the final analysis. Of these, 125 (10%) patients underwent combined CABG plus valvular surgery; 507 (43%) valvular surgery alone; and 561 (47%) CABG surgery alone (Table S1A). Demographics and patients’ baseline clinical characteristics are depicted in Tables S1A and S1B.
Univariate Analysis of the Prognostic Value of Nutrition Screening & Assessment Tools
Among the investigated nutrition screening and assessment tools, the NRS‐2002, MUST, and MNA were significant to predict an ICU stay >4 and >5 days (Table 1). Following these analyses, we performed a univariate analysis stratified by primary disease. The results showed that there were no significant predictive values for ICU stay >4 and 5 days when focusing on patients with CAD solely, whereas the predictive value remained significant when regarding patients with valvular disease alone (Tables S2–S7).
Table 1.
Univariate Analysis of the Prognostic Value of Nutrition Screening Tools
| ICU Stay >4 Days | ICU Stay >5 Days | |||||
|---|---|---|---|---|---|---|
| Nutrition Screening Tools | Odds Ratio (95% CI) | P | Odds Ratio (95% CI) | P | ||
| SNAQ (malnourished) | 1.273 | (0.811–1.998) | .294 | 1.217 | (0.721–2.054) | .463 |
| MUST (medium or high risk) | 1.853 | (1.231–2.789) | .003 | 1.831 | (1.148–2.922) | .011 |
| NRS‐2002 (malnourished) | 2.672 | (1.576–4.532) | .0003 | 2.264 | (1.227–4.180) | .009 |
| MNA (risk/malnourished) | 1.711 | (1.154–2.536) | .008 | 1.894 | (1.216–2.949) | .005 |
| SGA (Score B or C) | 1.896 | (0.982–3.661) | .057 | 1.662 | (0.767–3.601) | .198 |
ICU, intensive care unit; MNA, Mini Nutritional Assessment; MUST, Malnutrition Universal Screening Tool; NRS‐2002, Nutritional Risk Screening 2002; SGA, Subjective Global Assessment; SNAQ, Short Nutritional Assessment Questionnaire.
P, Wald χ2 test; significant values (P < .05) are depicted in bold.
Multivariate Analysis of the Prognostic Value of Nutrition Screening Tools
First, we entered model selection with different sets of baseline parameters and applied backward selection procedures. We found that all resulting models contained EuroSCORE and CPB time. Primary disease was also found as a relevant factor, and nutrition factors were selected as relevant in several models. EuroSCORE was found to be a good substitute for individual baseline parameters, and nutrition parameters were found to be interchangeable to some extent. CPB time was an independent factor in all models.
Next, we applied multivariate analyses to characterize the prognostic value of the well‐known risk variables EuroSCORE and CPB time, as well as primary disease and the nutrition screening tools. Both EuroSCORE and CPB time remained highly significant for prediction of ICU stay >4 and >5 days in this analysis. In addition, the NRS‐2002 (P = .020) and MNA score (P = .037) showed significant values to predict an ICU stay >4 days (Table 2). Regarding the prediction of ICU stay >5 days, the NRS‐2002 score showed a trend (P = .058) and MNA score was significant (P = .011) (Table 2).
Table 2.
Prognostic Value of Nutrition Screening Tools on Prolonged ICU Stay: A Multivariate Analysis
| ICU Stay >4 Days | ICU Stay >5 Days | |||||
|---|---|---|---|---|---|---|
| SNAQ Model | ||||||
| β | Odds Ratio (95% CI) | P | β | Odds Ratio (95% CI) | P | |
| Intercept | −3.759 | NA | < .0001 | −4.384 | NA | < .0001 |
| PrimPath CAD | 0.592 | 1.807 (1.120–2.915) | .015 | 0.771 | 2.160 (1.234–3.783) | .007 |
| EuroSCORE | 0.056 | 1.057 (1.027–1.089) | .0002 | 0.070 | 1.072 (1.039–1.107) | < .0001 |
| CPB time | 0.010 | 1.010 (1.006–1.013) | < .0001 | 0.010 | 1.010 (1.006–1.014) | < .0001 |
| SNAQ (malnourished) | 0.094 | 1.099 (0.628–1.922) | .742 | 0.083 | 1.086 (0.565–2.089) | .804 |
| HL = 0.142; AUC = 0.711 | HL = 0.552; AUC = 0.752 | |||||
| MUST Model | ||||||
|---|---|---|---|---|---|---|
| β | Odds Ratio (95% CI) | P | β | Odds Ratio (95% CI) | P | |
| Intercept | −3.855 | NA | < .0001 | −4.496 | NA | < .0001 |
| PrimPath CAD | 0.657 | 1.929 (1.194–3.116) | .007 | 0.846 | 2.329 (1.329–4.082) | .003 |
| EuroSCORE | 0.055 | 1.056 (1.025–1.088) | .0003 | 0.069 | 1.071 (1.038–1.106) | < .0001 |
| CPB time | 0.010 | 1.010 (1.006–1.013) | < .0001 | 0.010 | 1.010 (1.006–1.014) | < .0001 |
| MUST (medium or high risk) | 0.461 | 1.586 (0.951–2.644) | .077 | 0.483 | 1.620 (0.899–2.921) | .108 |
| HL = 0.146; AUC = 0.709 | HL = 0.601; AUC = 0.749 | |||||
| NRS‐2002 Model | ||||||
|---|---|---|---|---|---|---|
| β | Odds Ratio (95% CI) | P | β | Odds Ratio (95% CI) | P | |
| Intercept | −3.847 | NA | < .0001 | −4.480 | NA | |
| PrimPath CAD | 0.654 | 1.922 (1.193–3.098) | .007 | 0.837 | 2.309 (1.321–4.037) | .003 |
| EuroSCORE | 0.056 | 1.058 (1.027–1.090) | .0002 | 0.070 | 1.073 (1.039–1.108) | < .0001 |
| CPB time | 0.010 | 1.010 (1.006–1.013) | < .0001 | 0.010 | 1.010 (1.006–1.014) | < .0001 |
| NRS‐2002 (malnourished) | 0.812 | 2.252 (1.140–4.451) | .020 | 0.763 | 2.144 (0.975–4.718) | .058 |
| HL = 0.096; AUC = 0.716 | HL = 0.406; AUC = 0.755 | |||||
| MNA Model | ||||||
|---|---|---|---|---|---|---|
| β | Odds Ratio (95% CI) | P | β | Odds Ratio (95% CI) | P | |
| Intercept | −3.900 | NA | < .0001 | −4.618 | NA | < .0001 |
| PrimPath CAD | 0.668 | 1.949 (1.207–3.149) | .006 | 0.897 | 2.452 (1.395–4.310) | .002 |
| EuroSCORE | 0.053 | 1.055 (1.024–1.086) | .0004 | 0.067 | 1.069 (1.036–1.104) | < .0001 |
| CPB time | 0.010 | 1.010 (1.006–1.014) | < .0001 | 0.010 | 1.010 (1.006–1.014) | < .0001 |
| MNA (risk/malnourished) | 0.507 | 1.660 (1.030–2.676) | .037 | 0.702 | 2.018 (1.178–3.456) | .011 |
| HL = 0.446; AUC = 0.715 | HL = 0.428; AUC = 0.762 | |||||
| SGA Model | ||||||
|---|---|---|---|---|---|---|
| β | Odds Ratio (95% CI) | P | β | Odds Ratio (95% CI) | P | |
| Intercept | −3.796 | NA | < .0001 | −4.399 | NA | |
| PrimPath CAD | 0.632 | 1.881 (1.169–3.026) | .009 | 0.788 | 2.198 (1.264–3.822) | .005 |
| EuroSCORE | 0.056 | 1.058 (1.027–1.089) | .0002 | 0.070 | 1.073 (1.039–1.108) | < .0001 |
| CPB time | 0.010 | 1.009 (1.006–1.013) | < .0001 | 0.010 | 1.010 (1.006–1.014) | < .0001 |
| SGA (B or C) | 0.667 | 1.948 (0.894–4.246) | .094 | 0.403 | 1.496 (0.579–3.868) | .406 |
| HL = 0.688; AUC = 0.710 | HL = 0.243; AUC = 0.751 | |||||
AUC, area under the curve; CAD, coronary artery disease; CPB, cardiopulmonary bypass; EuroSCORE, European System for Cardiac Operative Risk Evaluation; HL, Hosmer and Lemeshow goodness‐of‐fit test; ICU, intensive care unit; MNA, Mini Nutritional Assessment; MUST, Malnutrition Universal Screening Tool; NA, not available; NRS‐2002, Nutritional Risk Screening 2002; PrimPath, primary pathology; SGA, Subjective Global Assessment; SNAQ, Short Nutritional Assessment Questionnaire.
β, parameter estimate; P, Wald χ2 test; significant values (P < .05) are depicted in bold.
Receiver Operating Characteristic (ROC) Curve for the Prediction of ICU Stay
Figure 2 illustrates the ROC curves for single and combined relevant factors to predict a prolonged ICU length of stay. In contrast to the well‐established risk factors EuroSCORE and CPB time, which demonstrated adequate predictive accuracy, the primary pathology alone and nutrition screening and assessment tools alone showed less predictive ability for prolonged ICU stays. The complete model using CPB time, EuroSCORE, underlying pathology, and nutrition risk scores showed improved predictive ability for prolonged ICU, when compared with CPB time or EuroSCORE alone. Results were comparable when using other scores than NRS‐2002 (data not shown). The calculation of ROC contrast estimation further confirmed a significantly different predictive value of the combined model compared with the single parameters.
Figure 2.
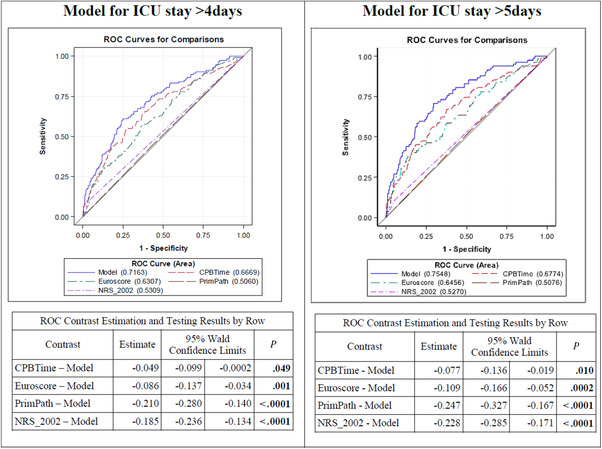
ROC curves for selected models including NRS‐2002 for prediction of ICU stay >4 and >5 days. CPB, cardiopulmonary bypass; EuroSCORE, European System for Cardiac Operative Risk Evaluation; ICU, intensive care unit; NRS‐2002, Nutritional Risk Screening 2002; PrimPath, primary pathology; ROC, receiver operating characteristic. P: Wald χ2 test; significant values (P < .05) are depicted in bold.
We also considered the area under the curve (AUC) separately for CABG and valvular patients. The EuroSCORE demonstrated more influence in the prediction model in CABG patients, whereas CPB time was more relevant for risk prediction in patients with valvular surgery. The influence of nutrition factors was seen predominantly in patients with HVD, with MNA showing the best results. In patients with HVD, the addition of MNA to EuroSCORE and CPB time further increased the AUC for prediction of ICU stay >5 days (AUC: 0.816; P = .004 vs .794; P = .354), whereas no increase of AUC could be detected when adding the MNA to CPB and EuroSCORE in patients with CAD (Figure 3, Table 3). The overall comparison is shown in Figure S1.
Figure 3.
ROC curves for predicting ICU stay >5 days by underlying pathology, including nutrition parameters, EuroSCORE, and CPB time. CAD, coronary artery disease; CPB, cardiopulmonary bypass; EuroSCORE, European System for Cardiac Operative Risk Evaluation; HVD, heart valvular disease; MNA, Mini Nutritional Assessment; NRS‐2002, Nutritional Risk Screening 2002; ROC, receiver operating characteristic. P: Wald χ2 test; significant values (P < .05) are depicted in bold.
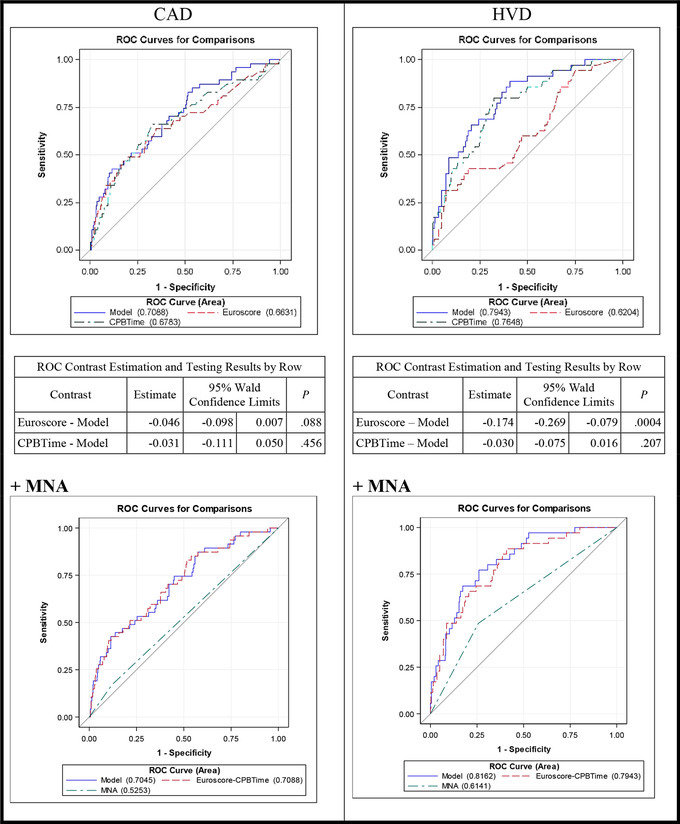
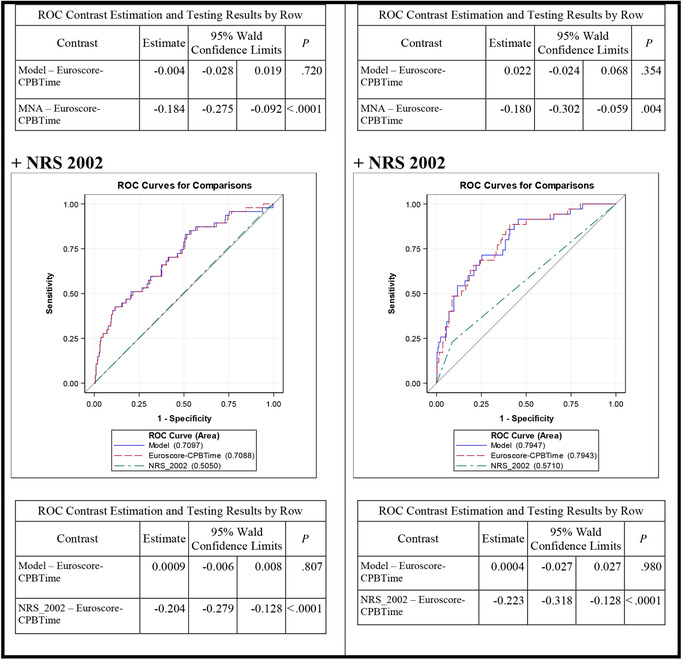
Table 3.
Predicting ICU Stay >5 Days, Separated by Underlying Pathology and Nutrition Screening Tools
| ICU Stay >5 Days | ||||||
|---|---|---|---|---|---|---|
| CAD | HVD | |||||
| Base Model | ||||||
| β | Odds Ratio (95% CI) | P | β | Odds Ratio (95% CI) | P | |
| Intercept | −3.321 | NA | < .0001 | −4.949 | NA | < .0001 |
| EuroSCORE | 0.090 | 1.094 (1.045–1.145) | .0001 | 0.057 | 1.058 (1.010–1.109) | .018 |
| CPB time | 0.005 | 1.005 (1.001–1.010) | .023 | 0.015 | 1.015 (1.009–1.020) | < .0001 |
| HL = 0.348; AUC = 0.709 | HL = 0.946; AUC = 0.794 | |||||
| MNA Model | ||||||
|---|---|---|---|---|---|---|
| β | Odds Ratio (95% CI) | P | β | Odds Ratio (95% CI) | P | |
| Intercept | −3.371 | NA | < .0001 | −5.316 | NA | < .0001 |
| EuroSCORE | 0.088 | 1.092 (1.043–1.143) | .0002 | 0.052 | 1.053 (1.004–1.104) | .033 |
| CPB time | 0.005 | 1.005 (1.001–1.010) | .021 | 0.015 | 1.015 (1.009–1.020) | < .0001 |
| MNA (risk/malnourished) | 0.340 | 1.405 (0.608–3.246) | .426 | 0.999 | 2.715 (1.268–5.809) | .010 |
| HL = 0.693; AUC = 0.705 | HL = 0.192; AUC = 0.816 | |||||
| NRS‐2002 Model | ||||||
|---|---|---|---|---|---|---|
| β | Odds Ratio (95% CI) | P | β | Odds Ratio (95% CI) | P | |
| Intercept | −3.314 | NA | < .0001 | −5.101 | NA | < .0001 |
| EuroSCORE | 0.089 | 1.093 (1.045–1.144) | .0001 | 0.056 | 1.058 (1.009–1.108) | .019 |
| CPB time | 0.005 | 1.005 (1.001–1.010) | .023 | 0.014 | 1.014 (1.009–1.020) | < .0001 |
| NRS‐2002 (malnourished) | −0.195 | 0.823 (0.106–6.401) | .852 | 1.072 | 2.920 (1.162–7.337) | .023 |
| HL = 0.308; AUC = 0.710 | HL = 0.183; AUC = 0.795 | |||||
AUC, area under the curve; CAD, coronary artery disease; CPB, cardiopulmonary bypass; EuroSCORE, European System for Cardiac Operative Risk Evaluation; HL, Hosmer and Lemeshow goodness‐of‐fit test; HVD, heart valvular disease; ICU, intensive care unit; MNA, Mini Nutritional Assessment; NA, not available; NRS‐2002, Nutritional Risk Screening 2002.
β, parameter estimate; P, Wald χ2 test; significant values (P < .05) are depicted in bold.
Lastly, we focused on a model without CPB time, as it is not always easy to predict preoperatively. Resulting data demonstrated that the preoperatively assessed EuroSCORE was only relevant in patients with CAD, whereas the MNA score was significant in the model for HVD patients. Overall, the AUC results were smaller than in the previous models, which included the CPB time (Figure S2).
Discussion
Numerous studies indicate that ICU patients with prolonged ICU stay experience greater protein and caloric deficits, which in turn is associated with deleterious effects. Thus, patients with prolonged ICU stays more likely may benefit from intense nutrition intake compared with patients with shorter stays.6, 7, 10, 11, 12, 32 DRM and its subtype cardiac cachexia represent a catabolic disorder, characterized by inflammation, malabsorption, metabolic dysfunction, and increased loss of nutrients, which is resistant to food intake alone and needs a multimodal therapeutic concept, including interventions such as physical exercise, improvement of bowel function, change of lifestyle, and adequate nutrition support.13 While adequate preoperative optimization strategies represent a clinical imperative requiring validation by adequately powered clinical studies,12 an early identification of patients at risk for a prolonged ICU stay may help to start an early initiation of adequate nutrition support postoperatively, to provide best‐possible treatment and prevent a further aggravation in these critically ill patients.
In comparison with the majority of critically ill non‐cardiac patients, elective cardiac surgery opens preoperatively a time window of 24 hours for an early risk assessment. This may enable an early postoperative initiation of nutrition support in such patients, who are supposed to benefit most from intense nutrition support. In this context, the present study provides first evidence about the clinical utility of combining the data of EuroSCORE, CPB duration, and nutrition assessment tools for the prediction of prolonged ICU stay in cardiac surgery patients.
In the majority of cardiac surgery patients undergoing elective single surgical procedures, oral feeding is resumed within 48–72 hours after surgery, and the patients are often discharged from ICU within 12–48 hours after termination of surgery. In contrast, 3%–35% of cardiac surgery patients show a more complicated and prolonged postoperative ICU stay with persistent organ dysfunction, resulting in an extended need of mechanical ventilation, ongoing vasopressor support, dialysis, or mechanical circulatory support.33 Especially in these high‐risk cardiac surgery patients with complex or combined surgical procedures and prolonged ICU stay, an early initiation of nutrition support may be helpful to prevent the frequently observed malnutrition with associated significant protein deficiency. One of the reasons for the late initiation is the commonly observed low cardiac output syndrome with resulting prolonged need of vasopressor support.34, 35 Despite lack of evidence, enteral nutrition (EN) is widely withheld during the early postoperative course of cardiac surgery patients and is often considered harmful, whereas current guidelines recommend only to withhold EN in hemodynamically unstable patients.29 Importantly, inadequate or delayed initiation of nutrition support, or both, may further aggravate pre‐existing malnutrition of cardiac surgery patients during the postoperative course. Therefore, an early identification of such patients at risk for prolonged ICU stay and early initiation of adequate postoperative nutrition support in such patients at risk may improve outcomes after cardiac surgery by maintaining gut integrity and improving wound healing.36
Until now, no validated methods have existed for the assessment of nutrition risk in cardiac surgery patients. As previous studies demonstrated that a prolonged ICU stay is associated with a significantly increased risk for underfeeding,24 we evaluated whether an early perioperative prediction of prolonged ICU stay may represent an alternative approach for an early identification of cardiac surgery patients at risk for underfeeding during the postoperative course. However, there are few empiric data to identify such patients with prolonged ICU stay, although previous studies demonstrated that such patients benefit most from postoperative nutrition support.32 Notably, there exist already well‐established scores for perioperative risk stratification (eg, EuroSCORE‐II score or CPB time), which may assist to identify patients at high risk for postoperative complications.25, 27 In addition, Lomivorotov et al provided first evidence about how nutritional status of patients was predictive for early postoperative complications. Therefore, we investigate the accuracy of these tools and their combination to predict a prolonged ICU stay. The NRS‐2002, MUST, and MNA score were significant for prediction of ICU length of stay longer than 4 and 5 days. As the underlying disease has previously been shown to significantly influence patients’ nutrition state,37 we performed a univariate analysis stratified by primary disease. Interestingly, we found that in patients with valvular disease and following surgery, the nutrition assessment tool MNA showed significant predictive accuracy. No adequate predictive accuracy was received for patients with CABG surgery alone, indicating the relevance of the pathophysiology from the underlying disease37 and the close link between malnutrition and heart valve diseases. In fact, previous studies already demonstrated that malnutrition occurred more often in patients with valvular disease,38 which may result from the significant alterations in hemodynamic state and inflammation observed in these patients.37
In extension, the following multivariate analysis confirmed the significant influence of EuroSCORE, CPB time, underlying physiopathology, and nutrition risk in models predicting a prolonged ICU stay. For the primary outcome, prediction of ICU stay >4 days, both NRS‐2002 and MNA score showed significant predictive accuracy. A following ROC analysis for prediction of such prolonged ICU stay demonstrated that the predictive value of the single relevant parameters, the EuroSCORE, CPB time, primary underlying pathology, and nutrition assessment tools, was moderate. The significantly higher AUC of the combined EuroSCORE and CPB time further increased after combination with the nutrition assessment. The duration of CPB showed the most important value in the model. As the underlying pathology was previously shown to be of high relevance for the nutrition risk, the following ROCs were categorized with respect to the underlying disease. While for the well‐known parameters—EuroSCORE and CPB time—a significant predictive accuracy was confirmed, in patients with valvular surgery the AUC for prediction of ICU stay >5 days further increased. It showed best predictive accuracy in a model after combining EuroSCORE, CPB time, and the nutrition assessment tool MNA; whereas, it remained unchanged in patients after CABG. When focusing on preoperative variables only and excluding variables such as CPB time, the predictive accuracy of the EuroSCORE significantly increased after combination with the MNA score, only in patients with valve surgery. Therefore, present findings highlight hitherto underestimated factors for prediction of ICU stay, which increased the predictive accuracy for prolonged ICU stay in cardiac surgery patients with valve surgery. To better visualize a potential approach regarding how to identify patients in which a nutrition support should be initiated, we developed the following cartoon (Figure 4). Yet, it must be recognized that the study is exploratory in nature and thus should be considered to be hypothesis generating, which is reflected in the statistical methodology. Therefore, an adequately designed prospective follow‐up study is needed to validate whether the presented model of CPB time, EuroSCORE, and MNA score may help to identify early the patients at risk for long ICU stay. If this concept is proven valid, it may assist decision making in clinical practice for dietitians, nurses, and physicians during the daily rounding to plan and initiate early an adequate nutrition support in cardiac surgery ICU patients.
Figure 4.
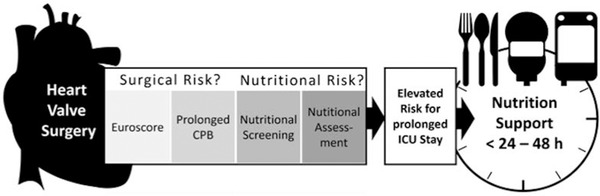
Schematic illustration of perioperative risk factors for a prolonged ICU stay of cardiac surgery patients. The figure depicts the perioperative variable, which should be considered to estimate an increased risk for a prolonged ICU stay (CPB time >108 min, EuroSCORE >9, nutrition risk should be assessed with MNA). CPB, cardiopulmonary bypass; EuroSCORE, European System for Cardiac Operative Risk Evaluation; ICU, intensive care unit; MNA, Mini Nutritional Assessment.
While the current ASPEN guidelines recommend withholding nutrition support in hemodynamically unstable patients and while no recommendations exist exclusively for cardiac surgery patients during the postoperative ICU stay, we hypothesize that a more precise discrimination between cardiac surgery patients with short vs prolonged ICU stay may assist to identify patients who may benefit from nutrition therapy and those who may not. Yet, we concede that in the present study we could not answer the question as to whether in patients identified with prolonged ICU stay, an early postoperative–initiated nutrition therapy would provide beneficial effects. Yet, based on present results, additional studies are encouraged to evaluate whether an early initiation of nutrition support in those patients with prolonged ICU stay may provide beneficial effects.
Conclusion
In conclusion, present data provided first evidence about the utility of a combined model of EuroSCORE, CPB, and the nutrition risk score MNA for the prediction of prolonged ICU stay in cardiac surgery patients. Present findings extended current knowledge about the predictive accuracy of CPB time and EuroSCORE, which further increased after combination with nutrition assessment tools in cardiac surgery patients with valvular surgery.
Supporting information
Supporting Information
Financial disclosures: None declared.
Conflicts of interest: None declared.
References
- 1. Wray CJ, Mammen JMV, Hasselgren P‐O. Catabolic response to stress and potential benefits of nutrition support. Nutrition. 2002;18(11‐12):971‐977. [DOI] [PubMed] [Google Scholar]
- 2. Plank LD, Hill GL. Energy balance in critical illness. Proc Nutr Soc. 2003;62(2):545‐52. [DOI] [PubMed] [Google Scholar]
- 3. Reid CL. Nutritional requirements of surgical and critically‐ill patients: do we really know what they need? Proc Nutr Soc. 2004;63(3):467‐472. [DOI] [PubMed] [Google Scholar]
- 4. Villet S, Chioléro RL, Bollmann MD, Revelly J‐P, Cayeux RN M‐C, Delarue J, et al. Negative impact of hypocaloric feeding and energy balance on clinical outcome in ICU patients. Clin Nutr. 2005;24(4):502‐509. [DOI] [PubMed] [Google Scholar]
- 5. Reid C. Frequency of under‐ and overfeeding in mechanically ventilated ICU patients: causes and possible consequences. J Hum Nutr Diet. 2006;19(1):13‐22. [DOI] [PubMed] [Google Scholar]
- 6. Rubinson L, Diette GB, Song X, Brower RG, Krishnan JA. Low caloric intake is associated with nosocomial bloodstream infections in patients in the medical intensive care unit. Crit Care Med. 2004;32(2):350‐357. [DOI] [PubMed] [Google Scholar]
- 7. Pingleton SK. Nutrition in chronic critical illness. Clin. Chest Med. 2001;22(1):149‐163. [DOI] [PubMed] [Google Scholar]
- 8. Drover JW, Cahill NE, Kutsogiannis J, et al. Nutrition therapy for the critically ill surgical patient: we need to do better! JPEN J Parenter Enteral Nutr. 2010;34(6):644‐652. [DOI] [PubMed] [Google Scholar]
- 9. Stoppe C, Goetzenich A, Whitman G, et al. Role of nutrition support in adult cardiac surgery: a consensus statement from an International Multidisciplinary Expert Group on Nutrition in Cardiac Surgery. Crit Care. 2017;21(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wei X, Day AG, Ouellette‐Kuntz H, Heyland DK. The association between nutritional adequacy and long‐term outcomes in critically ill patients requiring prolonged mechanical ventilation: a multicenter cohort study. Crit Care Med. 2015;43(8):1569‐1579. [DOI] [PubMed] [Google Scholar]
- 11. McClave SA, Lowen CC, Kleber MJ, et al. Are patients fed appropriately according to their caloric requirements? JPEN J Parenter Enteral Nutr. 1998;22(6):375‐381. [DOI] [PubMed] [Google Scholar]
- 12. Stoppe C, Goetzenich A, Whitman G, et al. Role of nutrition support in adult cardiac surgery: a consensus statement from an international multidisciplinary expert group on nutrition in cardiac surgery. Crit Care. 2017, 21(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jensen GL, Mirtallo J, Compher C, et al. Adult starvation and disease‐related malnutrition: a proposal for etiology‐based diagnosis in the clinical practice setting from the International Consensus Guideline Committee. Clin Nutr. 2010;29(2):151‐153. 10.1016/j.clnu.2009.11.010 [DOI] [PubMed] [Google Scholar]
- 14. Detsky AS, Baker JP, O'Rourke K, et al. Predicting nutrition‐associated complications for patients undergoing gastrointestinal surgery. JPEN J Parenter Enteral Nutr. 1987;11(5):440‐446. [DOI] [PubMed] [Google Scholar]
- 15. Malnutrition Advisory Group . A consistent and reliable tool for malnutrition screening. Nurs Times. 2003;99(46):26‐27. [PubMed] [Google Scholar]
- 16. Kruizenga HM, Seidell JC, de Vet HCW, Wierdsma NJ, van Bokhorst‐de van der Schueren MAE. Development and validation of a hospital screening tool for malnutrition: the short nutritional assessment questionnaire (SNAQ). Clin Nutr. 2005;24(1):75‐82. [DOI] [PubMed] [Google Scholar]
- 17. Ferguson M, Capra S, Bauer J, Banks M. Development of a valid and reliable malnutrition screening tool for adult acute hospital patients. Nutrition. 1999;15(6):458‐464. [DOI] [PubMed] [Google Scholar]
- 18. Lim S‐L, Tong C‐Y, Ang E, et al. Development and validation of 3‐Minute Nutrition Screening (3‐MinNS) tool for acute hospital patients in Singapore. Asia Pac J Clin Nutr. 2009;18(3):395‐403. [PubMed] [Google Scholar]
- 19. Anthony PS. Nutrition screening tools for hospitalized patients. Nutr Clin Pract. 2008;23(4):373‐382. [DOI] [PubMed] [Google Scholar]
- 20. Heyland DK, Dhaliwal R, Jiang X, Day AG. Identifying critically ill patients who benefit the most from nutrition therapy: the development and initial validation of a novel risk assessment tool. Crit Care. 2011;15(6):R268 10.1186/cc10546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kondrup J, Rasmussen HH, Hamberg O, Stanga Z, Ad Hoc ESPEN Working Group . Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22(3):321‐336. [DOI] [PubMed] [Google Scholar]
- 22. Jensen GL, Mirtallo J, Compher C, et al. Adult starvation and disease‐related malnutrition: a proposal for etiology‐based diagnosis in the clinical practice setting from the International Consensus Guideline Committee. JPEN J Parenter Enteral Nutr. 2010;34(2):156‐159. [DOI] [PubMed] [Google Scholar]
- 23. Rahman A, Hasan RM, Agarwala R, Martin C, Day AG, Heyland DK. Identifying critically‐ill patients who will benefit most from nutritional therapy: further validation of the “modified NUTRIC” nutritional risk assessment tool. Clin Nutr. 2016;35(1):158‐162. [DOI] [PubMed] [Google Scholar]
- 24. Faisy C, Lerolle N, Dachraoui F, et al. Impact of energy deficit calculated by a predictive method on outcome in medical patients requiring prolonged acute mechanical ventilation. Br J Nutr. 2009;101(7):1079‐1087. [DOI] [PubMed] [Google Scholar]
- 25. Nashef SAM, Sharples LD, Roques F, Lockowandt U. EuroSCORE II and the art and science of risk. Eur J Cardiothorac Surg. 2013;43(4):695–696. [DOI] [PubMed] [Google Scholar]
- 26. Gruenberg DA, Shelton W, Rose SL, Rutter AE, Socaris S, McGee G. Factors influencing length of stay in the intensive care unit. Am J Crit Care. 2006;15(5):502‐509. [PubMed] [Google Scholar]
- 27. Hein OV, Birnbaum J, Wernecke K, England M, Konertz W, Spies C. Prolonged intensive care unit stay in cardiac surgery: risk factors and long‐term‐survival. Ann Thorac Surg. 2006;81(3):880‐885. [DOI] [PubMed] [Google Scholar]
- 28. Lomivorotov VV, Efremov SM, Boboshko VA, et al. Prognostic value of nutritional screening tools for patients scheduled for cardiac surgery. Interact Cardiovasc Thorac Surg. 2013;16(6):612‐618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. 2016;40(2):159‐211. [DOI] [PubMed] [Google Scholar]
- 30. Atasever AG, Ozcan PE, Kasali K, Abdullah T, Orhun G, Senturk E. The frequency, risk factors, and complications of gastrointestinal dysfunction during enteral nutrition in critically ill patients. Ther Clin Risk Manag. 2018;14:385‐391. 10.2147/TCRM.S158492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Azarfarin R, Ashouri N, Totonchi Z, Bakhshandeh H, Yaghoubi A. Factors influencing prolonged ICU stay after open heart surgery. Res Cardiovasc Med. 2014;3(4):e20159 10.5812/cardiovascmed.20159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Compher C, Chittams J, Sammarco T, Nicolo M, Heyland DK. Greater protein and energy intake may be associated with improved mortality in higher risk critically ill patients: a multicenter, multinational observational study. Crit Care Med. 2017;45(2):156‐163. [DOI] [PubMed] [Google Scholar]
- 33. Stoppe C, McDonald B, Benstoem C, et al. Evaluation of persistent organ dysfunction plus death as a novel composite outcome in cardiac surgical patients. J Cardiothorac Vasc Anesth. 2016;30(1):30‐38. [DOI] [PubMed] [Google Scholar]
- 34. Lazar HL, Fitzgerald C, Gross S, Heeren T, Aldea GS, Shemin RJ. Determinants of length of stay after coronary artery bypass graft surgery. Circulation. 1995;92(9 suppl):II20‐24. [DOI] [PubMed] [Google Scholar]
- 35. Venkateswaran RV, Charman SC, Goddard M, Large SR. Lethal mesenteric ischaemia after cardiopulmonary bypass: a common complication? Eur J Cardiothorac Surg. 2002;22(4):534‐538. [DOI] [PubMed] [Google Scholar]
- 36. Tepaske R, Velthuis H, Oudemans‐van Straaten HM, et al. Effect of preoperative oral immune‐enhancing nutritional supplement on patients at high risk of infection after cardiac surgery: a randomised placebo‐controlled trial. Lancet. 2001;358(9283):696‐701. [DOI] [PubMed] [Google Scholar]
- 37. Gölbasi Z, Uçar O, Keles T, Sahin A, Cagli K, Camsari A, et al. Increased levels of high sensitive C‐reactive protein in patients with chronic rheumatic valve disease: evidence of ongoing inflammation. Eur J Heart Fail. 2002;4(5):593‐595. [DOI] [PubMed] [Google Scholar]
- 38. Lomivorotov VV, Efremov SM, Boboshko VA, et al. Evaluation of nutritional screening tools for patients scheduled for cardiac surgery. Nutrition. 2013;29(2):436‐442. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


