Abstract
Objectives
To obtain routine clinical practice data on cabazitaxel usage patterns for patients with metastatic castration‐resistant prostate cancer (mCRPC) and to describe physician‐assessed cabazitaxel effectiveness, health‐related quality of life (HRQoL) and safety.
Patients and Methods
CAPRISTANA was an international, observational cohort study examining cabazitaxel use for the treatment of patients with mCRPC. Effectiveness was assessed by overall survival (OS), progression‐free survival (PFS), time to treatment failure (TTF) and disease control rate. HRQoL was assessed using the Functional Assessment of Cancer Therapy‐Prostate questionnaire (FACT‐P) and the three‐level European Quality of Life questionnaire (EQ‐5D‐3L). Safety was assessed by adverse event (AE) reporting.
Results
A total of 189 patients were treated across 54 centres between April 2012 and June 2016. At baseline, 58.7% had ≥1 comorbidity, 93.7% had an Eastern Cooperative Oncology Group performance status ≤1, and 60.1% had a Gleason score at diagnosis of ≥8. Patients received a median of 6 cabazitaxel cycles; 84.7% received cabazitaxel as second‐line therapy. The median OS, PFS and TTF were 13.2, 5.6 and 4.4 months, respectively. Cabazitaxel led to disease control in 52.9% of patients. HRQoL was maintained (40.3%) or improved (32.2%) in 72.5% of patients based on total FACT‐P scores. Interestingly, 53.6% of patients reported pain improvement and a further 21.2% maintained pain control based on FACT‐P prostate cancer‐specific pain scores. The most common treatment‐related grade ≥3 AEs were neutropenia (7.9%) and anaemia (2.1%).
Conclusion
Patients in CAPRISTANA treated with cabazitaxel had similar disease outcomes and safety profiles compared with large phase III clinical trials. Most patients had maintained or improved HRQoL scores; >70% of patients had maintained or improved pain control.
Keywords: metastatic castration‐resistant prostate cancer, mCRPC, real‐world, cabazitaxel, health‐related quality of life, HRQoL, #PCSM, #ProstateCancer
Abbreviations
- mCRPC
metastatic castration‐resistant prostate cancer
- HRQoL
health‐related quality of life
- OS
overall survival
- PFS
progression‐free survival
- TTF
time to treatment failure
- FACT‐P
Functional Assessment of Cancer Therapy‐Prostate
- EQ‐5D‐3L
three‐level European Quality of Life questionnaire
- AE
adverse event
- ECOG‐PS
Eastern Cooperative Oncology Group performance status
- G‐CSF
granulocyte‐colony stimulating factor
- PCS
prostate cancer‐subscale of FACT‐P
- VAS
visual analogue scale
- TEAE
treatment‐emergent adverse event
Introduction
Prostate cancer is the second most frequently diagnosed cancer type in men 1. Although early diagnosis is associated with better prognosis, patients with metastatic castration‐resistant prostate cancer (mCRPC) typically have poorer outcomes 2. Several new treatments have been developed in the last 7 years including docetaxel, cabazitaxel, abiraterone acetate, enzalutamide, sipuleucel‐T and radium‐223 3, 4, 5, 6, 7, 8, 9. Cabazitaxel was approved in 2010 as a second‐line chemotherapy in combination with prednisone for treatment of patients with hormone‐refractory metastatic prostate cancer previously treated with a docetaxel‐containing treatment regimen based on the phase III TROPIC study 6, 9, 10. Cabazitaxel is a next‐generation taxane, selected for testing in clinical trials based on activity in docetaxel‐sensitive and ‐resistant cell lines 11. Cabazitaxel also exhibits stronger suppression of microtubule dynamics, faster cellular uptake and better intracellular retention than docetaxel in vitro 12.
Because of the exclusive design of clinical trials, patients with additional complicating factors, such as comorbidities and advanced age, are frequently not included. Real‐world studies provide an opportunity to validate the outcomes reported in large phase III trials using diverse patient populations and to identify unmet medical needs to better guide research and improve patient care. These studies help identify potential treatment risk factors, trends in healthcare service utilization, disparities in treatment access and health‐related quality of life (HRQoL), and costs associated with disease management 13.
The TROPIC and PROSELICA randomized clinical trials demonstrated the efficacy and safety of cabazitaxel; however, the effectiveness of cabazitaxel and its impact on HRQoL in routine clinical practice is still unknown.
CAPRISTANA, an international, multicentre, observational, prospective cohort study examined the use of cabazitaxel in conjunction with prednisone or prednisolone in routine clinical practice settings in patients with mCRPC. The primary objective was to observe the usage patterns of cabazitaxel; secondary objectives included further description of cabazitaxel usage patterns (subsequent therapies and use of granulocyte‐colony stimulating factor [G‐CSF]), clinical outcomes (overall survival [OS], progression‐free survival [PFS], time to treatment failure [TTF] and disease control rate), HRQoL and safety.
Patients and Methods
Study Design and Population
CAPRISTANA was a non‐interventional registry study (CABAZC 06092). Patients with mCRPC were enrolled across 54 centres in Lebanon, Czech Republic, Spain, Austria, Russia and Bulgaria. To be included in the study patients had to have a diagnosis of mCRPC, be aged ≥18 years, have received previous treatment with docetaxel and be scheduled to receive cabazitaxel as prescribed by their physician. Exclusion criteria included previous treatment with cabazitaxel or participation in another clinical study at the time of enrolment. All patients provided written consent prior to enrolment in the study, which was reviewed and approved by the appropriate Ethics Committee.
Data were recorded using an electronic case report form for ≤1.5 years after the first cabazitaxel dose or until death, whichever occurred first. The same electronic case report form was used across centres to gather information about patient and disease characteristics. Data were recorded at baseline, and every 3 months (±15 days) for endpoint analysis. Baseline data included: patient characteristics (age, body mass index, Charlson comorbidity index, Gleason score); disease characteristics (Eastern Cooperative Oncology Group performance status [ECOG‐PS], metastases, time since diagnosis); and treatment history (lines of prior therapy, response to docetaxel). Endpoint analyses included patient status, cabazitaxel usage, G‐CSF treatment, associated therapies, response to cabazitaxel and clinical symptoms.
Use of Cabazitaxel
Patients with mCRPC scheduled to receive cabazitaxel were prescribed the recommended dose at the time of 25 mg/m2, administered as a 1‐h intravenous infusion every 3 weeks in combination with oral prednisone or prednisolone 10 mg daily. Some patients received a starting dose of 20 mg/m2 as directed by their physician. Patients were allowed G‐CSF from cycle 1.
Efficacy Assessments
Overall survival was evaluated from date of first cabazitaxel administration to date of death from any cause. PFS was evaluated from the date of first cabazitaxel administration to the date of disease progression or death from any cause. Disease progression was defined as tumour or clinical progression or rising PSA level during or after cabazitaxel therapy. TTF was evaluated from the date of first administration of cabazitaxel to the date of cabazitaxel discontinuation, regardless of the cause. Criteria for response rate included PSA, clinical symptoms (including pain) and tumour imaging. Patients were assessed for efficacy at week 12, as recommended by Prostate Cancer Clinical Trials Working Group 2 guidelines.
Health‐related Quality of Life Assessments
Health‐related quality of life was assessed based on the results of the Functional Assessment of Cancer Therapy‐Prostate (FACT‐P) and the three‐level European Quality of Life (EQ‐5D‐3L) questionnaires provided at baseline and every two cycles until cabazitaxel discontinuation, then every 3 months until death or 1.5 years after treatment discontinuation.
The FACT‐P is a validated multidimensional prostate cancer‐specific patient‐reported outcome measure 14. The questionnaire consists of five subscales: physical, social, emotional and functional well‐being, and a prostate cancer‐subscale (PCS). The five subscales are summed to derive the FACT‐P total score, which ranges from a minimum of 0 to a maximum of 156, with higher values representing better HRQoL 15, 16. PCS‐pain is a pain‐related score ranging from 0 to 16 based on four questions from the FACT‐P questionnaire interrogating pain specifically (P1, P2, P3, GP4) 14. Differences in scores were clinically important if there was a change of ±10 points for the FACT‐P total score, ±3 points for the FACT‐P subscales (physical, social, emotional and functional well‐being, and PCS) and ±2 points for the FACT‐P PCS‐pain score 14.
The EQ‐5D‐3L is a widely used generic patient‐reported outcome instrument in clinical trials and observational studies 17, 18. The questionnaire assesses three levels of impairment in five dimensions of health: mobility; self‐care; usual activities; pain/discomfort; and anxiety/depression, and includes a visual analogue scale (VAS) 17, 18. EQ‐5D‐3L scores are converted into health state utility values using the UK 3L valuation set (with health state utility value scores ranging from −0.594 to 1.0). The EQ‐5D‐VAS ‘thermometer’ scores range from 0 to 100, with higher values representing a better health state 17, 18. Differences in score were clinically important if there was a change of ±7 points on the EQ‐5D VAS and ±0.074 points for the EQ‐5D health state utility value score 19, 20.
Safety Assessments
Adverse events (AEs) were collected from the time the patients signed the informed consent form to 30 days after the last administration of cabazitaxel. Treatment‐emergent adverse events (TEAEs) were defined as AEs that began, worsened or became serious between the first administration of cabazitaxel and 30 days after the last administration of cabazitaxel during the study. Serious AEs occurring >30 days after last treatment with cabazitaxel that were considered to be related to cabazitaxel treatment were also collected.
Statistical Considerations
The analyses of this study were descriptive, and the sample size was calculated based on 95% CIs associated with event rate estimations for individual countries to obtain sufficient data to fulfil post‐reimbursement requirements. All summaries and statistical analyses were generated using sas software version 9.2. Time‐to‐event outcomes were computed using the Kaplan–Meier estimates (95% CI), and the median survival times (with 95% CI) were provided.
Results
Patient Characteristics
A total of 191 patients were enrolled in the CAPRISTANA study, of whom 189 received cabazitaxel between April 2012 and June 2016. Patients had a median age of 69 years (Table 1). Most patients had an ECOG‐PS ≤1 (177 patients; 93.7%), a Gleason score at diagnosis of ≥8 (107 patients; 60.1%) and ≥1 comorbidity at baseline (111 patients; 58.7%). Most patients had bone metastases (165 patients; 87.3%). The median time from prostate cancer diagnosis to enrolment was 4.0 years, and 17.0 months from diagnosis of mCRPC to enrolment. All patients received at least one cycle of docetaxel before beginning treatment with cabazitaxel. Patients received a median of 6 docetaxel cycles prior to cabazitaxel, with 114 (60.3%) discontinuing docetaxel in the previous line because of disease progression. Patients also received non‐chemotherapy‐based treatments including 72 (38.1%) who received prostate cancer surgery, 100 (52.9%) who underwent radiotherapy and 178 (94.2%) who received some form of hormonal therapy.
Table 1.
Patient baseline characteristics
| N = 189 | |
|---|---|
| Median (range) age, years | 69 (47–87) |
| Age, n (%) | |
| <65 years | 55 (29.1) |
| 65–75 years | 89 (47.1) |
| ≥75 years | 45 (23.8) |
| Median (range) body mass index, kg/m2 | 27.65 (16.6–38.7) |
| ECOG PS, n (%) | |
| 0 | 73 (38.6) |
| 1 | 104 (55.0) |
| 2 | 11 (5.8) |
| 3 | 1 (0.5) |
| Median Charlson Comorbidity Index (range) | 4.0 (1–35) |
| At least one comorbidity, n (%) | 111 (58.7) |
| Gleason score at diagnosis, n (%) | |
| ≤6 | 25 (14.0) |
| 7 | 46 (25.8) |
| ≥8 | 107 (60.1) |
| Median time from prostate cancer diagnosis to inclusion, years (Q1–Q3) | 4.0 (2.1–6.0) |
| Median time from mCRPC diagnosis to inclusion, months (Q1–Q3) | 17.0 (10–29) |
| Median time since last progression, months (Q1–Q3) | 0.5 (0.2–1.0) |
| Metastatic sites, n (%) | |
| Bone | 165 (87.3) |
| Regional lymph node | 65 (34.4) |
| Visceral, other soft tissue | 42 (22.2) |
| Other | 12 (6.3) |
| Prior treatment, n (%) | 189 (100) |
| Received at least one prior surgery for prostate cancer, n (%) | 72 (38.1) |
| Prostatectomy | 46 (24.3) |
| Other | 20 (10.6) |
| Prostatectomy and other | 6 (3.2) |
| Received at least one prior radiotherapy, n (%) | 100 (52.9) |
| Received at least one prior hormonal therapy, n (%) | 178 (94.2) |
| Median number of prior hormonal therapies (range) | 2.0 (1.0–8.0) |
| Received at least one prior chemotherapy, n (%) | 189 (100) |
| Median number of docetaxel cycles (Q1–Q3) | 6 (5–10) |
| Response to last line of docetaxel, n (%) | |
| Complete response | 8 (4.2) |
| Partial response | 42 (22.2) |
| Stable disease | 23 (12.2) |
| Progressive disease | 114 (60.3) |
| Unknown or not evaluable | 2 (1.1) |
| N = 158 | |
|---|---|
| Baseline FACT‐P scores | |
| Physical well‐being, median (Q1–Q3)* | 19.9 (16.0–23.0) |
| Social well‐being, median (Q1–Q3)* , † | 21.0 (17.0–24.0) |
| Emotional well‐being, median (Q1–Q3)* | 15.0 (11.0–18.0) |
| Functional well‐being, median (Q1–Q3)* | 15.0 (12.0–19.0) |
| PCS, median (Q1–Q3)* , § | 27.0 (22.9–32.0) |
| PCS Pain, median (Q1–Q3)* | 8.0 (6.0–12.0) |
| FACT‐P Total, median (Q1–Q3)* , ‡ | 96.0 (83.0–114.5) |
ECOG PS, Eastern Cooperative Oncology Group performance status; FACT‐P, Functional Assessment of Cancer Therapy‐Prostate; HRQoL, health‐related quality of life; mCRPC, metastatic castration‐resistant prostate cancer; PCS, prostate cancer subscale; Q1–Q3, quartiles. *Higher values represent better HRQoL. The FACT‐P score scale ranged from 0 to 28 for physical, social and functional well‐being, 0–24 for social well‐being, 0–48 for prostate cancer subscale, 0–16 for prostate cancer subscale‐pain, and 0–156 for FACT‐P total score. † n = 157. ‡ n = 155. § n = 156.
Clinical Use of Cabazitaxel
For the 189 patients who were treated with cabazitaxel, 160 (84.7%) received cabazitaxel as second‐line chemotherapy (Table 2). Patients received a median (range) of 6 (1–24) cabazitaxel cycles. G‐CSF was received by 107 patients (56.6%) during cycle 1, with 100 patients (52.9%) receiving prophylactic G‐CSF and seven (3.7%) receiving therapeutic G‐CSF.
Table 2.
Description of cabazitaxel treatment and discontinuation
| Cabazitaxel use during study | N = 189 |
|---|---|
| Line of chemotherapy during the study, n (%) | |
| 2 | 160 (84.7) |
| 3 | 23 (12.2) |
| 4 | 4 (2.1) |
| 6 | 2 (1.1) |
| Median duration of exposure, weeks (Q1–Q3) | 18.6 (12.0–30.9) |
| Median number of cycles (Q1–Q3), [range] | 6 (4–9), [1–24] |
| Symptomatic overdose, n (%) | 0 (0) |
| G‐CSF use during cycle 1 of cabazitaxel therapy, n (%) | 107 (56.6) |
| Analgesic use associated with cabazitaxel therapy, n (%) | 88 (46.6) |
| At least one dose reduction, n (%) | 27 (14.3) |
| Haematological toxicity | 8 (4.2) |
| Non‐haematological toxicity | 10 (5.3) |
| Both haematological and non‐haematological toxicity | 3 (1.6) |
| Other reason | 7 (3.7) |
| At least one dose delay, n (%) | 96 (50.8) |
| Haematological toxicity | 14 (7.4) |
| Non‐haematological toxicity | 7 (3.7) |
| Both haematological and non‐haematological toxicity | 1 (0.5) |
| Other reason | 81 (42.9) |
| Reasons for cabazitaxel discontinuation, n (%) | |
| Progressive disease | 111 (58.7) |
| Patient's decision | 28 (14.8) |
| Other reason | 28 (14.8) |
| Adverse event | 22 (11.6) |
| Patients receiving treatment after cabazitaxel, n (%) | |
| Chemotherapy | 28 (14.8) |
| Hormonal therapy | 74 (39.2) |
G‐CSF, granulocyte‐colony stimulating factor; Q1–Q3, quartiles.
All patients discontinued cabazitaxel, with 111 (58.7%) discontinuing as a result of disease progression, 28 (14.8%) at patient request, and 28 (14.8%) for ‘other’ reasons, i.e. physician's direction, end of scheduled treatment or end of the 1.5‐year follow‐up period (Table 2). AEs were the least common reason for patients to discontinue cabazitaxel (22 patients; 11.6%). Dose modifications were required in 103 patients (54.5%), with dose delay being required in 96 of these patients. Non‐AE‐related events were the most frequent (42.9%) reasons for dose delay. Dose reduction was required in 27 patients (14.3%). After discontinuing cabazitaxel, 74 patients (39.2%) received hormonal therapy and 28 (14.8%) received chemotherapy.
Efficacy
The median OS was 13.2 months, median PFS was 5.6 months and median TTF was 4.4 months (Fig. 1). Cabazitaxel led to disease control in 52.9% of patients; of these, 1.1% achieved complete response, 22.2% achieved partial response and 29.6% achieved stable disease. Progressive disease was reported in 39.7% of patients. Efficacy was not evaluable in 14 patients (7.4%).
Figure 1.
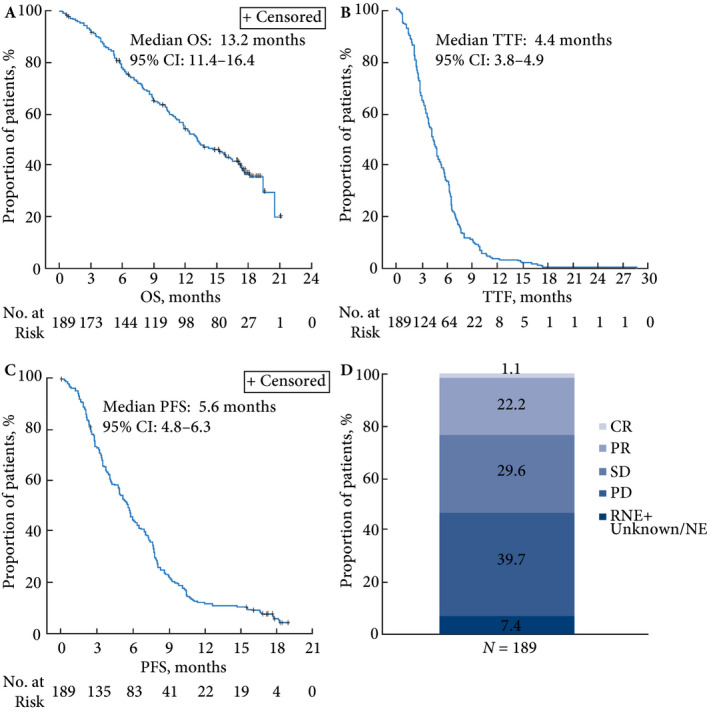
Cabazitaxel effect on patient survival and outcome. Response not evaluated in 13 patients and unknown/non‐evaluable in one patient. CR, complete response; NE, non‐evaluable; OS, overall survival; PD, progressive disease; PFS, progression‐free survival; PR, partial response; RNE, response not evaluated; SD, stable disease; TTF, time to treatment failure.
Health‐related Quality of Life
Of 189 patients, 158 (84%) completed HRQoL questionnaires. During the course of the study, HRQoL remained stable (40.3%) or improved (32.2%) in 72.5% of patients based on FACT‐P total score, and pain improved for over half of patients (53.6%) based on the PCS‐pain score (Table 3). There were no clinically relevant declines (≥10) in mean change in FACT‐P total and (≥3) subscale scores (Fig. 2); however, there was a clinically meaningful decline after cycle 10 for the <10 patients who remained on treatment (data not shown). There was a trend towards a clinically important improvement in FACT‐P PCS score (≥3) at cycle 3 and for FACT‐P PCS‐pain score (≥2) at cycle 8.
Table 3.
Health‐related quality of life responder analysis by FACT‐P questionnaire subscales and summary score
| Responders, n (%) | N = 151* | ||
|---|---|---|---|
| Improvement | Stable | Deterioration | |
| Physical well‐being | 45 (29.8) | 50 (33.1) | 56 (37.1) |
| Social well‐being† | 39 (26.0) | 69 (46.0) | 42 (28.0) |
| Emotional well‐being | 51 (33.8) | 52 (34.4) | 48 (31.8) |
| Functional well‐being | 45 (29.8) | 46 (30.5) | 60 (39.7) |
| PCS† | 75 (50.0) | 30 (20.0) | 45 (30.0) |
| PCS‐pain score | 81 (53.6) | 32 (21.2) | 38 (25.2) |
| FACT‐P total‡ | 48 (32.2) | 60 (40.3) | 41 (27.5) |
FACT‐P, Functional Assessment of Cancer Therapy‐Prostate; PCS, prostate cancer subscale; *Evaluable patients. † n = 150. ‡ n = 149.
Figure 2.
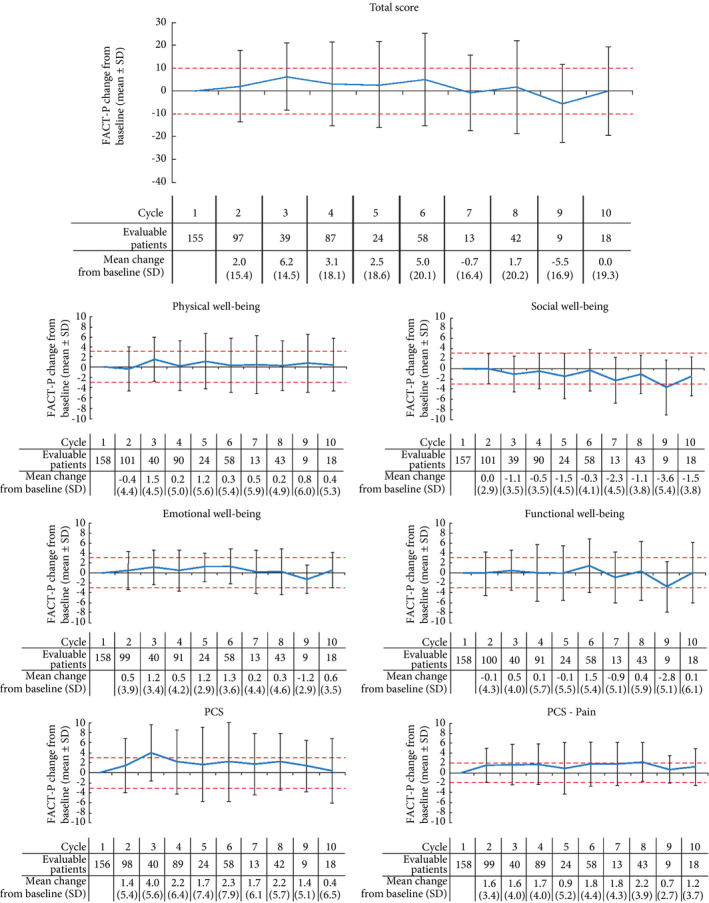
Change in mean Functional Assessment of Cancer Therapy‐Prostate questionnaire ( FACT‐P) total and subscale scores from baseline with cabazitaxel over time. Red lines at ±10 for total score, ±3 for physical, social, emotional and functional well‐being subscales and prostate‐cancer subscale (PCS) and ±2 for PCS‐pain subscale indicate limits of clinically important changes 14. Positive changes indicate improvement in health‐related quality of life, negative changes indicate deterioration.
Maintenance and improvement of HRQoL was also supported by the EQ‐5D‐VAS scores, which did not show clinically relevant changes (±7) from baseline during the course of treatment until cycles 6, 8 and 10, at which point clinically important improvements were seen (Fig. 3).
Figure 3.
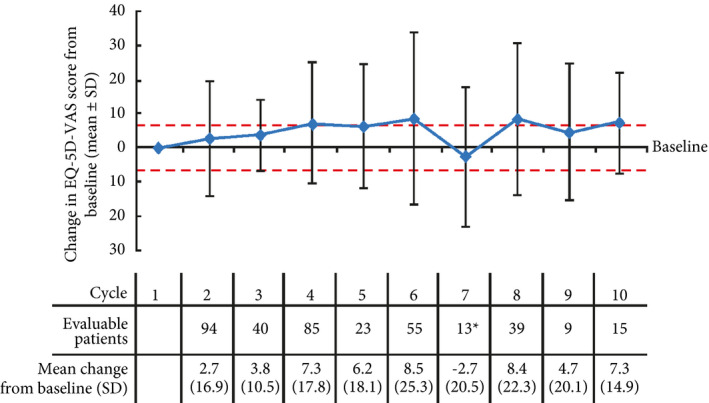
Change in EQ‐5D‐visual analogue scale (VAS) score from baseline with cabazitaxel over time. Red lines at ±7 indicate limits of clinically important changes; clinically important changes were reported at cycles 6, 8 and 10 (mean changes of 8.5, 8.4 and 7.3, respectively) 19, 20. Positive changes indicate improvement in health‐related quality of life, negative changes indicate deterioration. *Decline in EQ‐5D‐VAS score from baseline for cycle 7 may be a result of a low number of patients providing feedback.
Safety
Treatment‐emergent adverse events of any grade possibly related to cabazitaxel were reported in 37.6% of patients, and 13.8% of patients experienced TEAEs of grade ≥3 (Table 4). The most common clinical TEAEs of any grade possibly related to cabazitaxel were anaemia (10.6%), neutropenia (9.5%), diarrhoea (8.5%) and asthenia (7.9%). Clinical neutropenia and anaemia were the most common grade ≥3 TEAEs (7.9% and 2.1%, respectively). Serious TEAEs possibly related to cabazitaxel were reported in 12.2% of patients, the most common of which was neutropenia (5.8%).
Table 4.
Possibly related treatment‐emergent adverse events
| N = 189 | ||
|---|---|---|
| All grades | Grade ≥3 | |
| Possibly related TEAEs occurring in ≥2% of patients, n (%) | ||
| Any class | 71 (37.6) | 26 (13.8) |
| Anaemia | 20 (10.6) | 4 (2.1) |
| Neutropenia | 18 (9.5) | 15 (7.9) |
| Diarrhoea | 16 (8.5) | 2 (1.1) |
| Asthenia | 15 (7.9) | 1 (0.5) |
| Nausea | 10 (5.3) | 1 (0.5) |
| Fatigue | 10 (5.3) | 0 |
| Decreased appetite | 9 (4.8) | 0 |
| Vomiting | 7 (3.7) | 2 (1.1) |
| Constipation | 4 (2.1) | 0 |
| Stomatitis | 4 (2.1) | 0 |
| Peripheral neuropathy | 4 (2.1) | 0 |
| Possibly related serious TEAEs occurring in ≥2% of patients, n (%) | ||
| Any class | 23 (12.2) | 17 (9.0) |
| Neutropenia | 11 (5.8) | 9 (4.8) |
| Diarrhoea | 5 (2.6) | 2 (1.1) |
| Anaemia | 4 (2.1) | 3 (1.6) |
TEAE, treatment‐emergent adverse event.
Discussion
The CAPRISTANA study was a multinational, prospective, non‐interventional study designed to observe the use of cabazitaxel and evaluate real‐world effectiveness, HRQoL and safety. Patients enrolled in the CAPRISTANA study were similar to those enrolled in the pivotal phase III trials of cabazitaxel (TROPIC and PROSELICA). The proportion of patients with an ECOG‐PS ≤1 was similar across all three studies (CAPRISTANA: 93.7%, TROPIC: 91.9%, PROSELICA: 89.7%). The presence of bone metastases was also similar (CAPRISTANA: 87.3%, TROPIC: 83.6%, PROSELICA: 94.5%). Although patients’ median age in CAPRISTANA was similar to that in TROPIC, there were more patients aged ≥75 years in CAPRISTANA (23.8%) than TROPIC (18.4%) 9, 21, 22. The median number of treatment cycles administered, as well as discontinuation rates and rationales, TEAEs, rate and type of serious TEAEs (especially concerning grade ≥3 neutropenia and anaemia) were similar between CAPRISTANA and the previous clinical trials 9, 21.
Most patients in CAPRISTANA received cabazitaxel as a second‐line therapy as per the labelling instructions; however, ~15% of patients were prescribed cabazitaxel in third or later lines of therapy. Most patients discontinued cabazitaxel because of disease progression (58.7%); only 11.6% discontinued because of AEs. This contrasts with the TROPIC trial, where 47.6% discontinued treatment because of disease progression and 17.7% as a result of AEs 9. In CAPRISTANA, patients more often received hormonal therapies (39.2%) after cabazitaxel treatment than chemotherapy rechallenge (14.8%). Investigation into patient outcomes after these additional therapies is warranted because optimal treatment sequences are still unknown.
Patients enrolled in CAPRISTANA had similar clinical response rates to cabazitaxel as those included in TROPIC and PROSELICA 9, 19. The median OS was 13.2 months in CAPRISTANA, which was similar to that reported in TROPIC (15.1 months) and in PROSELICA (14.5 months) 9, 19. In CAPRISTANA, the best overall response was complete response in 1.1% and partial response in 22.2% of patients; in PROSELICA, 0.4% and 23.0% had complete response and partial response, respectively. Because of differences in how progression was measured, this result cannot be compared with TROPIC or PROSELICA.
The HRQoL improvements were maintained or improved in 72.5% of patients based on the FACT‐P total score and pain improved in over half of patients based on the FACT‐P PCS‐pain score, with most patients reporting improvements within the first 10 cycles of treatment. In TROPIC, 9.2% of patients had a pain response associated with cabazitaxel, compared with 7.7% of patients who received mitoxantrone, although this was not significantly different (P = 0.63), as assessed by the Present Pain Intensity score on the McGill‐Melzack scale 19, 23. The Present Pain Intensity score was also used in a Swiss registry study observing patients in the routine clinical practice setting 24. In CAPRISTANA, 53.6% reported pain improvement and a further 21.2% maintained pain control, as determined by FACT‐P PCS‐pain score. To our knowledge, this is the first report of maintained or improved pain control based on FACT‐P PCS‐pain score for cabazitaxel treatment in patients with mCRPC in routine clinical practice.
No new safety concerns were identified; patient toxicity profiles were similar to previous reports. Previously, the published incidence of grade ≥3 neutropenia in prospective phase III trials was based on laboratory assessments, and was 82% in TROPIC and 73.3% in PROSELICA 9, 19. Similarly, the rates of laboratory grade ≥3 anaemia were 11% and 14% in TROPIC and PROSELICA, respectively. In CAPRISTANA, neutropenia and anaemia rates were only recorded based on symptomatic, clinical AEs (as opposed to laboratory assessments), incidences of which were similar to those recorded in the clinical trials. The rates of possibly related grade ≥3 clinical neutropenia were 7.9% in CAPRISTANA, 21.3% in TROPIC and 9.6% in PROSELICA. Similarly, the rates of possibly related grade ≥3 clinical anaemia were 2.1% in CAPRISTANA, 2.7% in TROPIC and 2.4% in PROSELICA (Sanofi; data on file). G‐CSF use was permitted in PROSELICA, but avoided during the first cycle, and no restrictions were applied in CAPRISTANA. The question of whether early prophylactic use of G‐CSF may help reduce neutropenia rates associated with cabazitaxel administration merits further study.
Limitations of the present study include variability among the sites regarding use of electronic record forms, which could potentially lead to missing data and incomplete representation of cabazitaxel use in the clinical setting. There were also 14 patients who were not evaluable for efficacy. Additionally, only nine patients received >10 cycles of cabazitaxel; consequently, results after cycle 10 must be interpreted with caution.
In summary, cabazitaxel was predominately used as a second‐line chemotherapy, with patients receiving a median of 6 cycles. Patients enrolled in CAPRISTANA had similar disease outcomes compared with patients in TROPIC and PROSECLICA, which supports the effectiveness and safety of cabazitaxel for patients with mCRPC. Results of the study also show maintenance or improvement in HRQoL in the majority of patients and reduction of pain in over half of patients. These real‐world data help to better understand the effectiveness of cabazitaxel and its impact on mCRPC‐related HRQoL and pain in a routine clinical setting.
Conflicts of Interest
Ayse Özatilgan and Simon Hitier are employed by Sanofi. Denise Bury and Gisoo Barnes are contracted by Sanofi. Marwan Ghosn has provided a consulting/advisory role for Sanofi, Astellas and Janssen. Joan Carles has provided a consulting/advisory role for Johnson&Johnson, Astellas, Bayer, Sanofi, Pfizer and BMS, and has delivered lectures for Bayer and Johnson&Johnson. Irina Koroleva has received personal fees from AstraZeneca, Teva, MSD and Eisai, and grants from AstraZeneca and Teva. Angelika Pichler, Antoaneta Tomova, Fadi El Karak, Hana Korunkova, Jana Katolicka and Joseph Makdessi have no conflict of interests to disclose.
Acknowledgements
Research and analysis was supported by Sanofi. The authors were responsible for all content and editorial decisions, and received no honoraria for development of this manuscript. Editorial support was provided by Amber Wood and Anna Longjaloux of MediTech Media, funded by Sanofi. The authors would also like to thank Teri Michelini of Sanofi for support in preparing this manuscript.
References
- 1. Ferlay J, Soerjomataram I, Dikshit R et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–86 [DOI] [PubMed] [Google Scholar]
- 2. Kirby M, Hirst C, Crawford ED. Characterising the castration‐resistant prostate cancer population: a systematic review. Int J Clin Pract 2011; 65: 1180–92 [DOI] [PubMed] [Google Scholar]
- 3. National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Prostate Cancer (Version 2.2017), 2017.
- 4. de Bono JS, Logothetis CJ, Molina A et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011; 364: 1995–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scher HI, Fizazi K, Saad F et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012; 367: 1187–97 [DOI] [PubMed] [Google Scholar]
- 6. Tannock IF, de Wit R, Berry WR et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004; 351: 1502–12 [DOI] [PubMed] [Google Scholar]
- 7. Kantoff PW, Higano CS, Shore ND et al. Sipuleucel‐T immunotherapy for castration‐resistant prostate cancer. N Engl J Med 2010; 363: 411–22 [DOI] [PubMed] [Google Scholar]
- 8. Hoskin P, Sartor O, O'Sullivan JM et al. Efficacy and safety of radium‐223 dichloride in patients with castration‐resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: a prespecified subgroup analysis from the randomised, double‐blind, phase 3 ALSYMPCA trial. Lancet Oncol 2014; 15: 1397–406 [DOI] [PubMed] [Google Scholar]
- 9. de Bono JS, Oudard S, Ozguroglu M et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration‐resistant prostate cancer progressing after docetaxel treatment: a randomised open‐label trial. Lancet 2010; 376: 1147–54 [DOI] [PubMed] [Google Scholar]
- 10. Petrylak DP, Tangen CM, Hussain MH et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004; 351: 1513–20 [DOI] [PubMed] [Google Scholar]
- 11. Vrignaud P, Semiond D, Lejeune P et al. Preclinical antitumor activity of cabazitaxel, a semi‐synthetic taxane active in taxane‐resistant tumors. Clin Cancer Res 2013; 19: 2973–83 [DOI] [PubMed] [Google Scholar]
- 12. Azarenko O, Smiyun G, Mah J, Wilson L, Jordan MA. Antiproliferative mechanism of action of the novel taxane cabazitaxel as compared with the parent compound docetaxel in MCF7 breast cancer cells. Mol Cancer Ther 2014; 13: 2092–103 [DOI] [PubMed] [Google Scholar]
- 13. Gandaglia G, Bray F, Cooperberg MR et al. Prostate cancer registries: current status and future directions. Eur Urol 2016; 69: 998–1012 [DOI] [PubMed] [Google Scholar]
- 14. Cella D, Nichol MB, Eton D, Nelson JB, Mulani P. Estimating clinically meaningful changes for the Functional Assessment of Cancer Therapy–Prostate: results from a clinical trial of patients with metastatic hormone‐refractory prostate cancer. Value Health 2009; 12: 124–9 [DOI] [PubMed] [Google Scholar]
- 15. Cella DF, Tulsky DS, Gray G et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 1993; 11: 570–9 [DOI] [PubMed] [Google Scholar]
- 16. Esper P, Mo F, Chodak G, Sinner M, Cella D, Pienta KJ. Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy‐prostate instrument. Urology 1997; 50: 920–8 [DOI] [PubMed] [Google Scholar]
- 17. EuroQol Group . EuroQol – a new facility for the measurement of health‐related quality of life. Health Policy 1990; 16: 199–208 [DOI] [PubMed] [Google Scholar]
- 18. EuroQol Research Foundation . EQ‐5D‐3L User Guide: Basic information on how to use the EQ‐5D‐3L instrument, 2015.
- 19. Pickard AS, Wilke CT, Lin HW, Lloyd A. Health utilities using the EQ‐5D in studies of cancer. Pharmacoeconomics 2007; 25: 365–84 [DOI] [PubMed] [Google Scholar]
- 20. Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ‐5D and SF‐6D. Qual Life Res 2005; 14: 1523–32 [DOI] [PubMed] [Google Scholar]
- 21. Eisenberger M, Hardy‐Bessard AC, Kim CS et al. Phase III study comparing a reduced dose of cabazitaxel (20 mg/m2) and the currently approved dose (25 mg/m2) in postdocetaxel patients with metastatic castration‐resistant prostate cancer‐PROSELICA. J Clin Oncol 2017; 35: 3198–206 [DOI] [PubMed] [Google Scholar]
- 22. Sanofi PI. JEVTANA(R) (cabazitaxel) injection, Prescribing Information, FDA. Revised 2017
- 23. Bahl A, Oudard S, Tombal B et al. Impact of cabazitaxel on 2‐year survival and palliation of tumour‐related pain in men with metastatic castration‐resistant prostate cancer treated in the TROPIC trial. Ann Oncol 2013; 24: 2402–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stenner F, Rothschild SI, Betticher D et al. Quality of life in second‐line treatment of metastatic castration‐resistant prostate cancer using cabazitaxel or other therapies after previous docetaxel chemotherapy: Swiss observational treatment registry. Clin Genitourin Cancer 2017; 16: e151–9 [DOI] [PubMed] [Google Scholar]


