Summary
Background
Temporomandibular joint (TMJ) arthralgia is a painful condition assumed to be associated with local inflammation.
Objective
The objective of the present study was to determine the efficacy for reducing pain of a single‐dose intra‐articular (IA) injection of methylprednisolone to the TMJ. The hypothesis was that methylprednisolone would effectively reduce TMJ pain.
Methods
This randomised, double‐blind, parallel‐group, multicentre, controlled study included visits for enrolment, treatment and 4‐week follow‐up. The study included patients 18 years and older who had been diagnosed with unilateral TMJ arthralgia. All participants were randomly assigned to receive 1 mL IA injections of methylprednisolone or saline. The primary outcome was change in recorded pain intensity on a visual analogue scale (VAS) at maximum jaw opening, analysed in the per protocol population.
Results
In total, 54 patients were randomly assigned to single‐dose IA injections with methylprednisolone (n = 27) or saline (n = 27). Between baseline and the 4‐week follow‐up, VAS‐rated pain intensity at maximum jaw opening decreased from a mean of 61.0 (95% confidence interval [CI]: 50.1; 70.7) to 33.9 (95% CI: 21.6; 46.2) in the methylprednisolone group and from 59.6 (95% CI: 50.7; 65.9) to 33.9 (95% CI: 23.8; 43.9) in the saline group. The between‐group difference was not significant (P = 0.812). Treatment‐related adverse events were doubled in the methylprednisolone group.
Conclusion
Methylprednisolone provided no additional benefit for reducing pain, but caused more harm compared with saline following a single‐dose IA injection in patients with TMJ arthralgia.
Keywords: arthralgia, corticosteroids, injection, intra‐articular, pain management, temporomandibular joint
1. INTRODUCTION
Temporomandibular joint (TMJ) arthralgia without associated general inflammatory disease is a painful condition assumed to be associated with local inflammation. Pain from the TMJ, jaws and face with aggravation at jaw opening and chewing is typical symptoms. Common active local therapeutic measures include occlusal splints, various methods with sensory stimulation, manipulation of the mandible and jaw exercise. Conventional analgesics and non‐steroidal anti‐inflammatory drugs (NSAIDs) are often prescribed. If treatment needs to be escalated because of persisting pain, intra‐articular (IA) injection of corticosteroids is another established treatment aimed at suppressing an assumed inflammation.1
Significant symptom and pain relief following IA injections of corticosteroids in the treatment of complaints from temporomandibular disorders (TMD) and osteoarthritis have been reported.2, 3 However, such data are difficult to interpret since placebo and comparison groups were not used in these studies. In the context of IA injections, it is worth mentioning that patients with TMJ arthralgia/osteoarthritis have been reported to achieve profound pain relief during the week following an IA injection of isotonic sodium chloride.4
In the national guidelines for adult dental care by the Swedish National Board of Health and Welfare,5 IA TMJ corticosteroids are considered to provide a moderate effect on pain and mouth opening in patients with arthralgia. However, the Swedish Agency for Health Technology Assessment and Assessment of Social Services (SBU) has concluded that more primary studies are needed and that systematic reviews are absent. The agency has judged that there is a knowledge gap regarding the efficacy of IA injections of corticosteroids in TMJ arthralgia.6
Thus, our hypothesis was that single‐dose IA methylprednisolone would effectively improve pain in TMJ arthralgia. The objective of the study was therefore to evaluate treatment efficacy during a 4‐week prospective follow‐up after a single‐dose IA injection of methylprednisolone vs saline among patients with unilateral TMJ arthralgia.
2. METHODS
2.1. Study design
This was a multicentre, randomised, controlled, double‐blind, parallel‐group study of patients who had a clinical diagnosis of arthralgia with complaints of unilateral TMJ area pain.
2.2. Patients
The study participants were patients from eight dental specialist clinics in Sweden. The inclusion criteria were unilateral TMJ arthralgia, at least 18 years of age, ability to understand and communicate in Swedish and providing informed consent. Exclusion criteria were TMJ sounds in the affected joint, connective tissue disease, bilateral arthralgia, fibromyalgia or other generalised pain, ongoing bacterial or viral infection, ongoing dental treatment, surgery on the affected joint, IA corticosteroid injection for the past 6 months, complex psychiatric profile judged by the investigator, employee at the clinic, allergy to local anaesthetics or methylprednisolone, haemophilia, methemoglobinemia, breastfeeding or taking any of the following drugs: cyclosporine, erythromycin, phenobarbital, itraconazole, ketoconazole, rifampicin, acetylsalicylic acid or oral anticoagulant. A protocol amendment was made allowing TMJ crepitation (but not clicking).
Diagnosis of arthralgia followed the diagnostic criteria/temporomandibular disorder (DC/TMD),1 that is a positive history of pain in the jaw, temple, in front of the ear or in the ear within the past 30 days, with examiner confirmation of pain in a masticatory structure and pain modified with jaw movement, function or parafunction. In addition, the examination of the TMJ had to elicit a report of familiar pain with at least one of the following provocation tests: palpation of the lateral pole or around the lateral pole and/or maximum unassisted or assisted opening, right or left lateral movements or protrusive movements.
2.3. Study protocol
The study included three clinic visits: (a) screening and diagnosis; (b) randomisation and treatment; and (c) evaluation. At the first visit and after establishing a diagnosis and administering informed consent, the patient received a diary to record their pain and use of analgesics/NSAIDs during the 3 days before treatment. At the second visit (1 week later), the diary was collected, and a baseline examination was collected immediately before treatment. An optional study route included merging the first and second visits into one visit, in which case the pretreatment diary was not obtained.
After the intervention, patients continued to make diary entries for another 5 days. Patients were called at 1 week post‐intervention to check for adverse events and to be reminded to return the diary to the clinic. The final visit was 4 weeks post‐intervention, when the questionnaire was repeated and a second clinician, blind to the therapy group, performed a clinical examination. Diary pain ratings were also recorded 3 days prior to the follow‐up visit.
Patients with remaining complaints at the end of the study were handled on an individual basis and outside the study protocol. The study protocol can be accessed at http://www.medfarm.uu.se/ckfvasteras/forskning/studieprotokoll.
2.4. Intervention
Participants were randomised to receive a single 1 mL IA injection of either methylprednisolone 40 mg/mL (Depo‐Medrol®, Pfizer, Sollentuna, Sweden) or saline (sodium chloride Braun for parental use, B. Braun, Melsungen, Germany). The intervention was initiated by blocking the auriculotemporal nerve with 1.8 mL of prilocaine‐felypressin 30 + 0.54 mg/mL (Citanest‐octapressin®, Dentsply, Weybridge, Great Britain). The deposit of the anaesthetic was made without interfering with the joint compartments. The skin surface over the test TMJ was then cleaned with a 70% alcohol solution and dried. The syringe and injection substance were prepared out of the patient's sight so that they remained unaware of which solution was injected. The lateral condylar pole of the TMJ was identified, and the patient was asked to open his/her jaw. The injection needle (gauge 0.7 mm) was moved towards the articular tubercle until contact was made with cartilage/bone, thereby identifying the upper compartment of the joint. The test solution was injected prior to an aspiration attempt and removal of any exudate.
Following the injection, rescue analgesics were allowed and, if used, both the dose and type of substance were registered in the patient diary. Those using an oral appliance were asked to continue doing so throughout the study period. No other treatment was allowed.
2.5. Assessments
The primary outcome measure was change in visual analogue scale (VAS)‐rated pain at maximum opening. This 100‐mm scale has end definitions of “no pain” and “worst pain imaginable.” Secondary measures were VAS‐rated pain at jaw rest. These pain measures were named VASpoint estimate and recorded directly before the intervention and at the follow‐up visit. In addition, a diary was used to rate pain on a VAS at maximal opening and at jaw rest three times daily during each of the 3 days before and 5 days after intervention, as well as the 3 days before the follow‐up visit (referred to as VASdiary). The amount and brand of analgesic used were also registered in the diary.
At baseline immediately before the intervention and at the follow‐up visit, a series of instruments was administered, and a clinical examination was completed. Jaw functional problems were evaluated using the 20‐item global Jaw Functional Limitation Scale (JFLS‐20), with possible mean scores ranging from 0 to 10,7 and the Patient Health Questionnaire‐9 (PHQ‐9), a multipurpose instrument used for screening, diagnosing and measuring depression severity, with possible scores ranging from 0 to 27.8
At the follow‐up visit, a second dentist working at the actual centre, blinded to the participants’ study group, examined the participants and administered the questionnaires. Participants rated the change in their overall status since beginning the study treatment using the Patient Global Impression of Change (PGIC), a 7‐point scaled instrument ranging from “very much improved” to “very much worse”.9
At the follow‐up examination, maximum mouth opening with/without pain, as well as operator‐assisted opening, was registered by measuring the distance between the lower and upper jaw incisal teeth edges.
Tenderness of the masseter and temporalis muscle, as well as the TMJs, was recorded according to the DC‐TMD manual.10 In addition, the presence of crepitation was recorded by palpation.
2.6. Safety
Spontaneously reported adverse experiences, as well as adverse events registered by the investigator, were recorded throughout the study. The investigator graded the event intensity (mild, moderate, severe) and relation to the study treatment (not related, probably related, definitely related). When the first 20 patients had completed the intervention, an independent safety committee with one anaesthesiologist and one statistician made an interim safety analysis and gave the green light to continue, a decision confirmed by the Medical Products Agency in Sweden.
2.7. Randomisation, blinding and monitoring
Randomisation was generated online11 by an assistant unaffiliated with the study at the Centre for Clinical Research (CCR), Västerås, with randomisation in blocks of four. Sealed envelopes with randomisation number and treatment choice were prepared by the same person, who also kept the randomisation list until “clean file” status was declared.
Each participant's randomisation envelope was opened immediately before the study intervention. After the intervention, the randomisation envelope was placed into another, larger envelope and sealed.
One study monitor checked all records and collected all case report forms before data management.
2.8. Statistics
The primary outcome measure, VASpoint estimate‐rated pain at maximum jaw opening, is a continuous response variable with an independent control. Sample size calculation was based on the assumption of 15‐mm VAS as a clinically meaningful difference.12 To find a mean difference in VASpoint estimate‐rated pain at maximum jaw opening of 15 (standard deviation 20) between the experimental and control groups with 5% alpha and 80% power, 32 patients were required in each group.
The treatment groups were compared using a non‐parametric Wilcoxon test and associated 95% confidence intervals (Cis) on the patients’ absolute change from baseline. The primary efficacy analysis was made on the per protocol population.
The median for diary assessments was calculated for each patient during each day. Fisher's exact probability test and the related‐sample McNemar test were used to analyse categorical variables. Student's t tests and paired groups t tests were used to analyse continuous variables, and the results were verified using a non‐parametric Wilcoxon Mann‐Whitney U test and the Wilcoxon signed‐rank test. SPSS (version 24, IBM Corp., Kista, Sweden) was used to conduct all statistical analyses, and a difference was considered statistically significant when P < 0.05 (two‐sided).
2.9. Ethical standards
The study was performed in accordance with the ethical standards of the Declaration of Helsinki. The Uppsala Regional Ethical Review Board (#2013/360) approved the study. Informed consent was obtained from each patient prior to participation.
3. RESULTS
3.1. Patients
Patients were enrolled to the study from December 2013 to February 2017. The study was prematurely stopped because of time constraints. At enrolment, informed consent was obtained from 56 patients, two of whom withdrew their consent before randomisation. The intention‐to‐treat (ITT) sample was n = 27 patients treated with methylprednisolone, and n = 27 was treated with saline. Figure 1 shows a summary profile of the trial including both the ITT and per protocol (PP) samples. Baseline demographics, jaw opening capacity, pain ratings and baseline instrument scores showed minor, non‐significant differences between the groups (Table 1).
Figure 1.
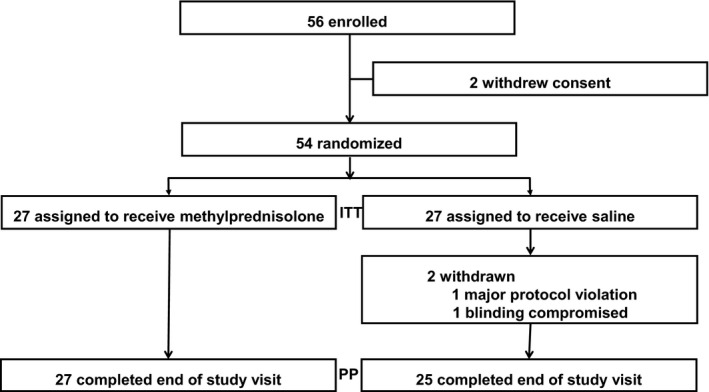
Profile of the randomised controlled trial. Intention‐to‐treat (ITT), per protocol (PP)
Table 1.
Patient baseline characteristics in the two groups, intention‐to‐treat population
| Methylprednisolone (n = 27) | Saline (n = 27) | |
|---|---|---|
| Female gender, No (%) | 23 (85%) | 21 (78%) |
| Age, y | 48 (18.6) | 56 (14.7) |
| BMI | 25 (4.4) | 27 (3.9) |
| VASpoint estimate at maximum openinga | 61 (25.3) | 60 (19.3) |
| VASpoint estimate when jaw at resta | 29 (22.7) | 27 (24.7) |
| Maximum jaw opening without pain, mm | 34 (10.4) | 35 (7.8) |
| Maximum jaw opening with pain, mm | 42 (9.6) | 42 (7.8) |
| Maximum jaw opening with assistance, mm | 45 (8.9) | 46 (8.1) |
| JFLS‐20 | 2.8 (1.6) | 2.7 (1.2) |
| PHQ‐9 | 5 (4.4) | 6 (5.3) |
Data are mean (standard deviation) unless specified otherwise.
BMI, body mass index; JFLS, Jaw Functional Limitation Scale (range, 0‐10); PHQ‐9, Patient Health Questionnaire‐9 (range, 0‐27); VAS, visual analogue scale.
Pain graded on a 100‐mm VAS with the end descriptions “No pain” and “Worst pain imaginable.”
3.2. Efficacy
From baseline to the 4‐week follow‐up, the primary efficacy variable VASpoint estimate pain score at maximum jaw opening decreased from a mean of 61.0 (95% CI: 50.1; 70.7) to 33.9 (95% CI: 21.6; 46.2) in the methylprednisolone group and from 59.6 (95% CI: 50.7; 65.9) to 33.9 (95% CI: 23.8; 43.9) in the saline group. There was no between‐group difference (P = 0.812) (Table 2).
Table 2.
Change from baseline to evaluation after 4 wk on the used instruments in the two groups, per protocol population
| Parameters | Methylprednisolone | Saline | Difference between groups (95% CI) | P | ||||
|---|---|---|---|---|---|---|---|---|
| nm | (95% CI) | P | ns | (95% CI) | P | |||
| Pain | ||||||||
| VASpoint estimate at maximum opening | 26 | −26.5 (−39.7; −13.2) | <0.001 | 25 | −24.4 (−35.7; −13.2) | <0.001 | −2.0 (−19.0; 14.9) | 0.812 |
| VASpoint estimate at jaw rest | 26 | −21.7 (−30.6; −12.8) | <0.001 | 25 | −13.8 (−23.3; −4.4) | 0.006 | −7.9 (−20.5; 4.8) | 0.218 |
| Jaw function | ||||||||
| JFLS‐20 | ||||||||
| Total score | 23 | −1.3 (−2.0; −0.5) | 0.002 | 24 | −1.0 (−1.6; −0.4) | 0.002 | −0.3 (−1.2; 0.7) | 0.588 |
| Limitations in mastication | 23 | −1.6 (−2.5; −0.6) | 0.003 | 24 | −1.4 (−2.2; −0.6) | 0.001 | −0.2 (−1.4; 1.0) | 0.780 |
| Limitation in mobility | 23 | −1.3 (−2.4; −0.2) | 0.018 | 24 | −1.5 (−2.4; −0.7) | 0.001 | 0.2 (−1.1; 1.5) | 0.742 |
| Limitation in verbal/emotional expression | 23 | −1.1 (−1.8; −0.4) | 0.003 | 24 | −0.6 (−1.1; −0.1) | 0.032 | −0.5 (−1.3; 0.3) | 0.246 |
| Patient health | ||||||||
| PHQ‐9 | 23 | −1.3 (−3.1; 0.6) | 0.176 | 24 | −1.7 (−3.8; 0.3) | 0.091 | 0.5 (−2.2; 3.2) | 0.718 |
mean graded pain difference on a 100‐mm VAS with 95% confidence intervals (CIs).
Within‐group differences tested by a paired t test. Differences between interventions tested by the Student's t test.
JFLS, Jaw Functional Limitation Scale; PHQ‐9, Patient Health Questionnaire‐9; VAS, 100‐mm visual analogue scale with the end definitions “No pain” and “Worst pain imaginable”.
At baseline, the VASpoint estimate pain score at jaw rest was considerably lower compared with that of maximum jaw opening. The score changed significantly from baseline to the 4‐week follow‐up within each group, but no difference was detected between the two interventions (P = 0.218) (Table 2).
The proportion of “responders,” defined as a ≥30% reduction in baseline VASpoint estimate pain score on maximum jaw opening, was 50% and 68% for the methylprednisolone and saline groups, respectively (P = 0.258). Using various definitions of responders, all tests were non‐significant between interventions (Table 3). The proportion of patients without the criteria of arthralgia at follow‐up was 22% and 32% in the methylprednisolone and saline groups, respectively. Defining responder as “much improved” or “very much improved” on the PGIC scale resulted in 41% and 28% in the methylprednisolone and saline groups, respectively.
Table 3.
Treatment outcome described as the proportion of responders with various definitions
| Methylprednisolone n/N (%) | Saline n/N (%) | P | |
|---|---|---|---|
| VASpoint estimate absolute value of <15 mm at maximum opening at evaluation | 10/26 (39) | 6/25 (23) | 0.376 |
| VASpoint estimate at jaw opening reduced by ≥30% of baseline | 13/26 (50) | 17/25 (68) | 0.258 |
| VASpoint estimate at jaw opening reduced by ≥50% of baseline | 11/26 (42) | 12/25 (48) | 0.781 |
| PGIC rating of much/very much improved | 11/27 (41) | 7/25 (28) | 0.392 |
| Proportion of patients without the TMJ arthralgia criteria at follow‐up | 6/27 (22) | 8/25 (32) | 0.536 |
Differences between methylprednisolone and saline tested by Fisher's exact test. Response alternatives were as follows: much worse, moderately worse, minimally worse, no change, minimally improved, moderately improved and much improved.
PGIC, Patient Global Impression of Change; TMJ, temporomandibular joint.
VASdiary pain scores were obtained from 37 patients (19 in the methylprednisolone group and 18 in the placebo group) during the 3 days before the intervention; 50 patients (26 in the methylprednisolone group and 24 in the placebo group) during the 5 days post‐intervention period; and 48 patients (27 in the methylprednisolone group and 21 in the placebo group) during 3 days prior to the 4‐week follow‐up. VASdiary pain scores at both maximum opening and at rest over these days are displayed in Figure 2. In the methylprednisolone group, there was a substantial increase in VASdiary pain scores during the days following the intervention compared with the saline intervention group.
Figure 2.
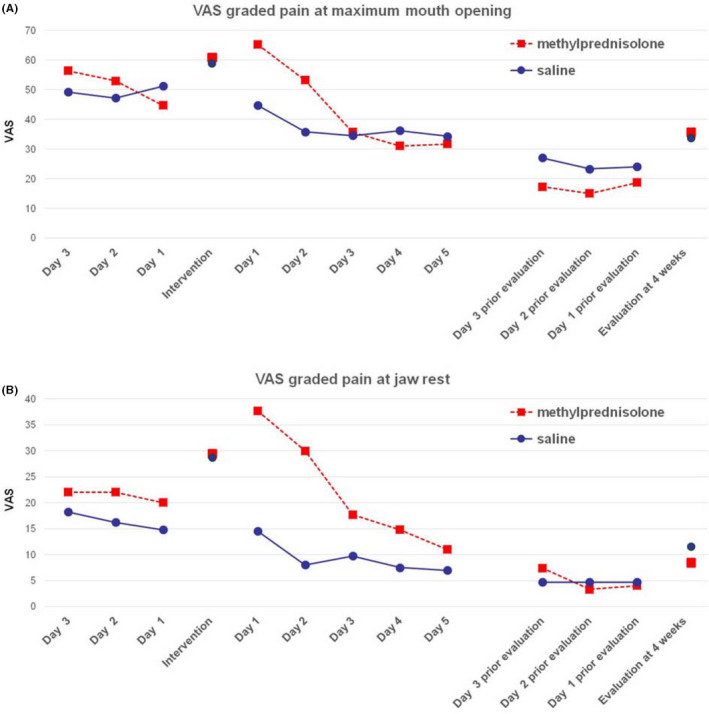
A, Visual analogue scale, VAS diary pain score at maximum jaw opening. B, VAS diary pain score at jaw rest. Daily median pain score 3 d before and 5 d after intervention, as well as 3 d before evaluation visit. The solitary marks are VAS point estimate pain directly before intervention and at evaluation [Colour figure can be viewed at wileyonlinelibrary.com]
Jaw function evaluated with the JFLS‐20 revealed a significant improvement in both total score and sub‐scores in both the methylprednisolone‐ and saline‐treated groups, but there were no significant between‐group differences (Table 2).
Baseline PHQ‐9 values were low and showed only small, non‐significant changes at follow‐up (Table 2).
At the follow‐up visit, only small and non‐significant changes were found for maximum jaw opening with/without pain or assisted opening, palpation tenderness of the TMJ and temporalis and masseter muscles (Tables S1 and S2).
The proportions of patients taking analgesics were 29% and 35% the day before the intervention in the methylprednisolone and saline groups, respectively. The day following the intervention, the number of patients taking analgesics was doubled in the methylprednisolone group, whereas the saline group remained at the same level. Data on analgesic consumption are presented in Tables S3 and S4.
3.3. Safety results
No withdrawals from the study were judged by the investigator to be related to the intervention. The overall incidence and treatment‐related adverse events were higher in the methylprednisolone group and were predominately from increased pain (Table 4).
Table 4.
Number and description of patients’ reported and observed adverse experiences during the period from treatment to evaluation. Per protocol population
| Methylprednisolone n = 27 n (%) | Saline n = 25 n (%) | |
|---|---|---|
| Any adverse eventa | 17 (63) | 7 (28) |
| Common cold/influenza | 2 (7) | 1 (4) |
| Treatment‐related adverse eventa | 16 (59) | 6 (24) |
| Increase in pain | 9 (33) | 4 (16) |
| Transient paraesthesia of the eyelid | 3 (11) | 0 (0) |
| Transient numbness | 2 (7) | 0 (0) |
| Rash/local allergic reaction | 2 (7) | 0 (0) |
| Difficulties in opening the jaw | 2 (7) | 0 (0) |
| TMJ sounds | 2 (7) | 0 (0) |
| Teeth do not fit together | 1 (4) | 1 (4) |
| Headache | 1 (4) | 1 (4) |
TMJ, temporomandibular joint.
Treatment‐emergent adverse events with onset on the day of commencing treatment until the evaluation visit.
Rated by the investigator as probably or definitely related to the intervention.
4. DISCUSSION
In the Guidelines for Assessment, Diagnosis and Management of Orofacial Pain,1 arthralgia is defined as inflammation of the synovial lining of the TMJ. In order to depress the inflammatory process and achieve relief from the joint pain, corticosteroid injections have been recommended5 on a limited basis, when other conservative treatments have been unsuccessful. The most prominent symptom in TMJ arthralgia is pain from jaw movement and pain at maximum mouth opening; on that basis, and for consistency with a previous study,4 we selected the primary outcome measure in our study. Baseline TMJ pain level slightly above 60 mm on a 100‐mm VAS scale was significantly reduced, by around 25 mm, at the 4‐week follow‐up, which is an absolute pain reduction of about 42% in both treatment groups. The clinical threshold for reducing chronic pain intensity following treatment has been defined as at least 13 mm on a 100‐mm VAS scale,12 which we reached in both groups. However, our study results show that methylprednisolone is not an improvement over saline for pain reduction over 4 weeks. On the contrary, IA methylprednisolone worsened pain on the days following intervention, and the incidence of adverse events was twice as high as the saline group.
The Initiative on Methods, Measurement, and Pain Assessment (IMMPACT) recommendations were set up for interpreting the clinical importance of treatment outcomes in pain trials.13 IMMPACT recommended four outcome domains: pain intensity, physical functioning, emotional functioning and participants’ ratings of overall improvement. In our study, all four domains were evaluated using the VAS, JFLS‐20, PHQ‐9 and PGIC, and all showed non‐significant differences between methylprednisolone and saline treatments.
The proportions of responders vary numerically depending on its definition but overall non‐significant. Using a definition of >30% pain reduction, 50% and 68% responders were found in the methylprednisolone and saline groups, respectively, whereas the reverse were found for the PGIC scale which showed 41% and 28%, respectively. The reason for the incongruence could be differences in the sensitivity to identify a true responder or that the numbers of patients are low and that the difference varies by chance. Also, the fact that our study did not have a lower limit of pain intensity as an inclusion criterion (easier to reach a 30% reduction of pain reduction in the lower bound) could be a source for the diverging outcomes.
Depression has been identified as a common disorder in the oro‐facial environment. It is reported that almost one out of three patients at an oro‐facial pain clinic has symptoms consistent with a diagnosis of depression.14 The PHQ‐9 baseline data in our study show scores in the lower range of mild depression which differs from a typical oro‐facial pain population. The reason could be that we included a “true” joint pain group or that our exclusion criteria of a “complex psychiatric profile” excluded many psychologically compromised patients. It is also reported that females from an exclusively myofascial pain group are significantly more depressed than those from a general population or from an exclusively joint pain group.15
Intra‐articular corticosteroid injections are commonly used to treat the signs and symptoms of rheumatic diseases, and methylprednisolone injections in the TMJ provide significant pain improvement in adult rheumatoid arthritis.16 In an open‐label study on TMJ arthritis of a specific nature (ie, rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and Sjögren syndrome), Alstergren et al17 found a significant treatment effect following a single IA dose of methylprednisolone for 2‐3 weeks post‐treatment. However, these investigators also noted that a group of patients with TMJ nonspecific inflammatory joint disease (ie, arthralgia) did not respond similarly to corticosteroid injections, consistent with our study, from which we excluded patients with general inflammatory diseases.
Corticosteroids are considered to induce synthesis of lipocortin, and in that way, block the inflammatory reaction18 and consequently reduce pain and improve jaw function. Our study showed no benefit of methylprednisolone over saline, though both substances led to significant within‐group effects. The question becomes whether TMJ arthralgia is caused by an inflammation triggered by IA agents, which is diluted at the IA intervention, or whether the nerve block anaesthetic stops a windup phenomenon. In addition, in arthrocentesis studies in which the intervention procedure was completed with or without deposition of corticosteroids for treatment of TMJ arthralgia, arthrosis and internal derangement have no significant effects on pain intensity.19, 20, 21, 22 Thus, it seems obvious that the combination of procedures, local anaesthetic and injected fluid reduce TMJ pain.
Summarised data suggest that local anaesthetics possess a wide range of anti‐inflammatory actions through their effects on the immune system.23 The anti‐inflammatory properties of local anaesthetics are in several aspects reported to be superior to traditional NSAIDs and steroid groups and proven successful in the treatment of arthritis. In the present study, local anaesthetics in terms of prilocaine were used to block the auriculotemporal nerve preceding the study drug intervention. Although injected extra‐articularly, the agent may have had an impact on the study results.
A shortcoming of our study was the inability to blind the opaque‐white methylprednisolone solution despite the consultation of pharmaceutical/galenic expertise. The use of an opaque syringe may disclose droplets of test substance at the tip of the needle. The routinely used aspiration procedure preceding the injection requires a transparent syringe that reveals the content. The blinding was thus obtained by the use of a study end examiner who was blind to the intervention group.
Temporomandibular joint arthralgia, ICD‐10 code M26.62, is a diagnosis based solely on pain symptoms in the affected joint; symptom aggravation with jaw movement and palpation tenderness confirms the diagnosis.1 In Sweden, TMJ arthralgia without associated symptoms and signs of a general inflammatory disease is commonly treated without examination of computerised tomography (CT) or magnetic resonance (MR) imaging. Osteoarthritis diagnosis is based on a combination of the criteria for arthralgia and osteoarthrosis (ie, deterioration of articular tissues detected with CT and the presence of joint crepitus). The lack of CT and/or MRI examination is a limitation of our study since we can assume that a certain proportion of the patients we included might have been classified as having osteoarthritis. In addition, disc displacement without reduction is a differential diagnosis to arthralgia, and MR examination is needed to verify this diagnosis.
5. CONCLUSION
In summary, our findings show that methylprednisolone was not superior to saline for reducing TMJ arthralgia pain. On the contrary, methylprednisolone caused greater pain compared with saline following the intervention. The combination of a nerve block with local anaesthetics and an IA deposition of saline provided substantial improvements in pain. The mechanism behind this phenomenon requires further evaluation.
CONFLICT OF INTEREST
All authors stated explicitly that there are no conflict of interests in connection with this article.
Supporting information
ACKNOWLEDGMENTS
The authors thank Drs. B. Adérn, M. Block, K. Bäck, B. Hedenberg‐Magnusson E. Lindfors, A. Minston, P. Miranda Burgos, S. Omrani, N. Piltan, E. Saghafi and D. Ström for contribution to the study.
Isacsson G, Schumann M, Nohlert E, Mejersjö C, Tegelberg Å. Pain relief following a single‐dose intra‐articular injection of methylprednisolone in the temporomandibular joint arthralgia—A multicentre randomised controlled trial. J Oral Rehabil. 2019;46:5–13. 10.1111/joor.12718
Funding information
This trial was funded by grants from the Uppsala‐Örebro Regional Research Council grant numbers RFR‐378481 and RFR‐477841 and Västmanland County Council grant numbers LTV‐472901, LTV‐376601, LTV‐370301 and LTV‐378361.
ClinicalTrials.gov identifier NCT01995019 and EudraCT 2013‐003365‐34.
REFERENCES
- 1. de Leeuw R, Klasser GD. Orofacial Pain: Guidelines for Assessment, Diagnosis, and Management, 5th edn Hanover Park, IL: Quintessence Publishing; 2013:155. [Google Scholar]
- 2. Wenneberg B, Kopp S. Short term effect of intra‐articular injections of a corticosteroid on temporomandibular joint pain and dysfunction. Swed Dent J. 1978;2(6):189‐196. [PubMed] [Google Scholar]
- 3. Bjornland T, Gjaerum AA, Moystad A. Osteoarthritis of the temporomandibular joint: an evaluation of the effects and complications of corticosteroid injection compared with injection with sodium hyaluronate. J Oral Rehabil. 2007;34(8):583‐589. [DOI] [PubMed] [Google Scholar]
- 4. List T, Tegelberg A, Haraldson T, Isacsson G. Intra‐articular morphine as analgesic in temporomandibular joint arthralgia/osteoarthritis. Pain. 2001;94(3):275‐282. [DOI] [PubMed] [Google Scholar]
- 5. Swedish National Board of Health and Welfare . National guidelines for adult dental care: national board of health and welfare: Vetenskapligt underlag; 2011. 458.
- 6. Swedish agency for health technology assessment and assessment of social services . Glukokortikoid intraartikulärt vid käkledssmärta [Intra‐articular glucocorticoid at TMJ artralgia]. 2012. http://www.sbu.se/sv/publikationer/kunskapsluckor/glukokortikoid-intraartikulart-vid-kakledssmarta-/Accessed February 20, 2018.
- 7. Ohrbach R, Larsson P, List T. The jaw functional limitation scale: development, reliability, and validity of 8‐item and 20‐item versions. J Orofac Pain. 2008;22(3):219‐230. [PubMed] [Google Scholar]
- 8. Kroenke K, Spitzer RL, Williams JB. The PHQ‐9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11‐point numerical pain rating scale. Pain. 2001;94(2):149‐158. [DOI] [PubMed] [Google Scholar]
- 10. International network for orofacial pain and related disorders methodology . Diagnostic Criteria for Temporomandibular Disorders. https://ubwp.buffalo.edu/rdc-tmdinternational/tmd-assessmentdiagnosis/dc-tmd/2014 Accessed February 20, 2018.
- 11. Randomization plan generators 2017. http://randomization.com/Accessed February 20, 2018.
- 12. Todd KH, Funk KG, Funk JP, Bonacci R. Clinical significance of reported changes in pain severity. Ann Emerg Med. 1996;27(4):485‐489. [DOI] [PubMed] [Google Scholar]
- 13. Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105‐121. [DOI] [PubMed] [Google Scholar]
- 14. Korszun A, Hinderstein B, Wong M. Comorbidity of depression with chronic facial pain and temporomandibular disorders. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82(5):496‐500. [DOI] [PubMed] [Google Scholar]
- 15. Giannakopoulos NN, Keller L, Rammelsberg P, Kronmuller KT, Schmitter M. Anxiety and depression in patients with chronic temporomandibular pain and in controls. J Dent. 2010;38(5):369‐376. [DOI] [PubMed] [Google Scholar]
- 16. Kopp S, Akerman S, Nilner M. Short‐term effects of intra‐articular sodium hyaluronate, glucocorticoid, and saline injections on rheumatoid arthritis of the temporomandibular joint. J Craniomandib Disord. 1991;5(4):231‐238. [PubMed] [Google Scholar]
- 17. Alstergren P, Appelgren A, Appelgren B, Kopp S, Lundeberg T, Theodorsson E. The effect on joint fluid concentration of neuropeptide Y by intra‐articular injection of glucocorticoid in temporomandibular joint arthritis. Acta Odontol Scand. 1996;54(1):1‐7. [DOI] [PubMed] [Google Scholar]
- 18. Lacronique J, Russo‐Marie F, Marsac J. Mechanism of action and effects of corticoids in asthma. Rev Mal Respir. 1989;6(1):15‐30. [PubMed] [Google Scholar]
- 19. Huddleston Slater JJ, Vos LM, Stroy LP, Stegenga B. Randomized trial on the effectiveness of dexamethasone in TMJ arthrocentesis. J Dent Res. 2012;91(2):173‐178. [DOI] [PubMed] [Google Scholar]
- 20. Comert Kilic S. Does injection of corticosteroid after arthrocentesis improve outcomes of temporomandibular joint osteoarthritis? a randomized clinical trial. J Oral Maxillofac Surg. 2016;74(11):2151‐2158. [DOI] [PubMed] [Google Scholar]
- 21. Bouloux GF, Chou J, Krishnan D, et al. Is hyaluronic acid or corticosteroid superior to lactated ringer solution in the short‐term reduction of temporomandibular joint pain after arthrocentesis? part 1. J Oral Maxillofac Surg. 2017;75(1):52‐62. [DOI] [PubMed] [Google Scholar]
- 22. Tabrizi R, Karagah T, Arabion H, Soleimanpour MR, Soleimanpour M. Outcomes of arthrocentesis for the treatment of internal derangement pain: with or without corticosteroids? J Craniofac Surg. 2014;25(6):571‐575. [DOI] [PubMed] [Google Scholar]
- 23. Cassuto J, Sinclair R, Bonderovic M. Anti‐inflammatory properties of local anesthetics and their present and potential clinical implications. Acta Anaesthesiol Scand. 2006;50(3):265‐282. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


