Abstract
We evaluated the efficacy and safety of a povidone‐iodine (PVP‐I) foam dressing (Betafoam) for donor site dressing versus a hydrocellular foam dressing (Allevyn) and petrolatum gauze. This prospective Phase 4 study was conducted between March 2016 and April 2017 at eight sites in Korea. A total of 106 consenting patients (aged ≥ 19 years, scheduled for split‐thickness skin graft) were randomised 1:1:1 to PVP‐I foam, hydrocellular, or petrolatum gauze dressings for up to 28 days after donor site collection. We assessed time to complete epithelialisation, proportion with complete epithelialisation at Day 14, and wound infection. Epithelialisation time was the shortest with PVP‐I foam dressing (12.74 ± 3.51 days) versus hydrocellular foam dressing (16.61 ± 4.45 days; P = 0.0003) and petrolatum gauze (15.06 ± 4.26 days, P = 0.0205). At Day 14, 83.87% of PVP‐I foam dressing donor sites had complete epithelialisation, versus 36.36% of hydrocellular foam dressing donor sites (P = 0.0001) and 55.88% of petrolatum gauze donor sites (P = 0.0146). There were no wound infections. Incidence rates of adverse events were comparable across groups (P = 0.1940). PVP‐I foam dressing required less time to complete epithelialisation and had a good safety profile.
Keywords: Betafoam, donor site dressing, povidone‐iodine, split‐thickness skin graft, wound healing
1. INTRODUCTION
Skin grafting is a common technique used to replace damaged or missing skin.1 A split‐thickness skin graft involves excision of the epidermis and part of the dermis, leaving a superficial to partial‐thickness donor site wound that generally heals by epithelialisation within 7 to 14 days.1 Proper donor site wound management after skin graft harvesting is important as donor site complications can lead to prolonged hospitalisation and excessive scarring.2 Selection of an appropriate wound dressing is thus key to donor site wound management after split‐thickness skin graft.
The ideal donor site dressing should accelerate the healing process and prevent infection while minimising discomfort and pain.3 Considering that the dressing requires frequent changing, it should also be cost‐effective and easy to apply and remove.3 Numerous types of wound dressings are commercially available, and many meet several of these criteria to varying degrees; however, no conclusive evidence on the superiority of a particular dressing type has been documented to date.3, 4 Traditionally, skin graft donor sites are covered with fine‐mesh gauze dressings and are allowed to dry. Newer wound dressings now provide a moist wound environment and prevent exudate desiccation by retaining moisture; this may help facilitate epithelialisation and cause less donor site discomfort and pain during dressing changes.5
Povidone‐iodine (PVP‐I) foam dressing (Betafoam)1 is a new polyurethane foam dressing impregnated with 3% PVP‐I.6 PVP‐I has been used in a wide range of wound‐healing applications for over 60 years. It has broad‐spectrum antiseptic activities (against bacteria, viruses, and fungi), anti‐inflammatory properties, and a well‐established safety profile.7, 8 In a recent study, PVP‐I foam dressing showed superior fluid handling and desirable antimicrobial activity with minimal cytotoxicity to host cells.6 These properties, coupled with its relatively low cost, suggest that PVP‐I foam dressing may be a good option for donor site dressing in split‐thickness skin grafts.
This prospective randomised study is the first study of PVP‐I foam dressing in donor site wounds. The purpose of the study was to evaluate the efficacy and safety of PVP‐I foam dressing in the management of split‐thickness skin graft donor sites compared with hydrocellular foam dressing (Allevyn) and conventional fine‐mesh petrolatum gauze.
2. MATERIALS AND METHODS
2.1. Study design and participants
This prospective, randomised, controlled, multicentre, open‐label, phase IV study was conducted between March 2016 and April 2017 at eight sites in the Republic of Korea. Patients who were scheduled to undergo skin grafting were assessed for study eligibility at the screening visit (Figure 1). Key inclusion criteria included: aged ≥ 19 years, planned to undergo split‐thickness skin graft harvest from the thighs or buttocks, and planned size of the donor site is between 25 and 150 cm2. Key exclusion criteria were: pregnancy, known hypersensitivity reactions to the investigational device and PVP‐I; hyperthyroidism, other thyroid dysfunctions, or treatment with radioiodine; comorbidities that may affect wound healing (eg, poorly controlled diabetes mellitus with HbA1c > 8%, acute/chronic renal failure, autoimmune disease, immunocompromised patient, etc.); planned treatment with anticoagulants, steroids, or immunosuppressants after enrolment; signs of systemic infections after enrolment; major skin injuries at or adjacent to the donor site; burn site >20% of body surface area; and previous skin graft from the same donor site. All patients provided written informed consent before study enrolment.
Figure 1.

Study design and visit schedule. Patients who were scheduled to undergo split‐thickness skin grafting were assessed for study eligibility at the screening visit. The randomised investigational device was applied to the donor site wound on Days 0, 1, 2, 4, and 7 after donor site collection. If complete epithelialisation was not achieved by Day 7, additional dressing changes were performed on Days 9, 11, 14, 17, 20, 23, 26, and 28 or until complete epithelialisation. Patients who agreed to undergo a follow‐up assessment were assessed at month 6. R, randomisation
The study protocol, case report forms, and documents used for informed consent were reviewed and approved by the site Institutional Review Board. All study procedures were conducted in accordance with the Korea Good Clinical Practice, the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use guideline, the Declaration of Helsinki, and applicable regulations in Korea. This study was registered at ClinicalTrials.gov (identifier NCT02543034).
2.2. Treatment allocation and schedule
Eligible patients were randomised to treatment with PVP‐I foam dressing (Betafoam, Genewel, Seongnam, Korea), hydrocellular foam dressing (Allevyn, Smith & Nephew, Hull, UK), or petrolatum gauze (SungKwang Pharm. Co., Ltd, Bucheon, Korea). Randomization was conducted in a 1:1:1 ratio by block randomisation, with allocation codes prepared in advance in the order of study participation. The pre‐generated randomisation list was sent to investigators prior to study initiation. Study treatment was not blinded.
The randomised investigational device was applied to the donor site wound on Days 0, 1, 2, 4, and 7 after donor site collection (Figure 1). If complete epithelialisation was not achieved by Day 7, additional dressing changes were performed on Days 9, 11, 14, 17, 20, 23, 26, and 28 or until complete epithelialisation. In the PVP‐I foam dressing and hydrocellular foam dressing groups, both the primary dressings (ie, PVP‐I foam or hydrocellular dressing) and secondary dressings (ie, regular gauze for fixing) were changed during dressing changes. In the petrolatum gauze group, only the secondary dressing (ie, regular gauze for fixing) was changed; the primary dressing (ie, petrolatum gauze) was changed only if it became detached during secondary dressing changes.
2.3. Study assessments and endpoints
Epithelialisation of the donor site, signs of inflammation and infection, pain before and after dressing changes, intensity of exudate from the bottom of the wound, and extent of acceptability were assessed during dressing changes.
The primary efficacy endpoint was the number of days to complete epithelialisation from the day of donor site collection. Secondary efficacy endpoints included the proportion of patients with complete epithelialisation within 14 days; rate of donor site infection; frequency of inflammatory signs visually assessed by the investigator; average number of dressing changes per day; intensity of exudate from the bottom of the donor site wound; intensity of pain before and after dressing changes; extent of acceptability during dressing changes (ease of application, ease of removal, adherence on removal, bleeding on removal, odour, maceration, leakage of exudate); and the rate of epithelialisation on Days 4, 7, and 14 (rate of epithelialisation = [area of epithelialisation] / [total area of donor site] × 100). Safety endpoints included the incidence of adverse events (AEs) and changes in clinical laboratory variables (haematology, blood chemistry, and urinalysis) and vital signs.
AE data were collected via spontaneous patient reporting, patient interview, and examination by the investigator. A three‐grade scale was used to assess the severity of AEs: mild—mild discomfort but does not interfere with daily activities; moderate—significant discomfort that interferes with daily activities; and severe—prevents normal daily activities. Serious AEs were any AEs that resulted in death, were life‐threatening, required inpatient hospitalisation or prolongation of existing hospitalisation, resulted in persistent or significant disability or incapacity, or other medically important events. Adverse device effects were any AEs that were deemed to be related to the application of the investigational device.
2.4. Statistical analyses
It was estimated that a sample size of 93 evaluable patients (31 patients per group) would provide approximately 90% power to detect a statistically significant difference between PVP‐I foam dressing and hydrocellular dressing and PVP‐I foam dressing and petrolatum gauze in time to complete epithelialisation. Assuming a 10% drop‐out rate, a total of 105 patients were planned to be enrolled for the study. Sample size assumptions were based on the results of earlier studies of foam dressing (Medifoam) and hydrocellular foam dressing9 and foam dressing and petrolatum gauze10; it was assumed that the effects of foam dressing would be similar to the effects of PVP‐I foam dressing.
The Safety Set consisted of all patients who applied the investigational device (ie, PVP‐I foam dressing, hydrocellular dressing, or petrolatum gauze) at least once; the Full Analysis Set (FAS) included patients in the Safety Set in whom efficacy endpoint data were obtained at least once after application of the investigational device; and the Per Protocol Set (PPS) included patients in the FAS who completed the study without any major protocol violations. Demographic baseline data, clinical medical history, and safety data were analysed in the Safety Set, and efficacy data were analysed in both the FAS and PPS.
Demographic variables, baseline donor site characteristics, efficacy, and safety outcomes were summarised by treatment group using descriptive statistics. Continuous variables were reported as means and standard deviations, while categorical data were expressed as n (%) in each treatment group. Differences between the treatment groups were analysed using t‐test for continuous data and χ 2 test or Fisher's exact test for categorical data. P‐values < 0.05 were considered statistically significant. All statistical analyses were performed using SAS statistical analysis software (SAS Institute, Cary, North Carolina, version 9.4).
3. RESULTS
3.1. Patient disposition and baseline characteristics
Patient flow through the study is shown in Figure 2. Of 121 patients who were screened, 106 eligible patients were randomised to PVP‐I foam dressing, hydrocellular dressing, or petrolatum gauze (Figure 2). Three patients discontinued the study before application of the investigational device. The remaining 103 patients applied the investigational device at least once and were included in the Safety Set (PVP‐I foam dressing: 34; hydrocellular dressing: 35; petrolatum gauze: 34). Among them, five patients withdrew prior to study completion; reasons for premature discontinuation are shown in Figure 2. The FAS consisted of 98 patients who had data for at least one efficacy endpoint (PVP‐I foam dressing: 31; hydrocellular dressing: 33; petrolatum gauze: 34). The PPS included 73 patients who completed the study without major protocol violations (PVP‐I foam dressing: 23; hydrocellular dressing: 25; petrolatum gauze: 25).
Figure 2.
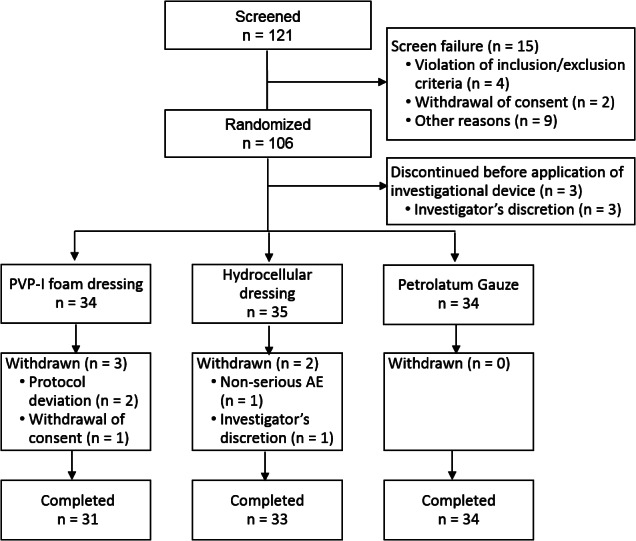
Flow of patients through the trial
Overall, the mean age of patients in the Safety Set was 53.82 years (range 17‐86 years), and the mean weight was 65.11 kg (SD 11.58 kg); 59.22% of the patients were male. The most common location of the donor site was the thigh (97.09%). Demographic variables and baseline donor site characteristics were comparable across treatment groups (Table 1).
Table 1.
Demographic variables and baseline characteristics (Safety Set)
| PVP‐I foam dressing (N = 34) | Hydrocellular dressing (N = 35) | Petrolatum gauze (N = 34) | |
|---|---|---|---|
| Age (y), median (range) | 55.5 (17.0–85.0) | 54.0 (19.0–86.0) | 59.0 (22.0–86.0) |
| Gender, n (%) | |||
| Male | 21 (61.76) | 20 (57.14) | 61 (59.22) |
| Female | 13 (38.24) | 15 (42.86) | 42 (40.78) |
| Weight (kg), mean (SD) | 67.09 (12.81) | 64.10 (12.52) | 64.18 (9.13) |
| Area of donor site (pixel), mean (SD) | 493 465.48 (447 945.60) | 406 194.40 (241 293.15) | 369 011.22 (189 994.64) |
| Depth of donor site (in.), mean (SD) | 0.011 (0.001) | 0.011 (0.001) | 0.011 (0.001) |
| Location of donor site, n (%) | |||
| Thigh | 34 (100.00) | 33 (94.29) | 33 (97.06) |
| Buttock | 0 (0.00) | 2 (5.71) | 1 (2.94) |
| Concurrent illnesses, n (%) | |||
| Yes | 25 (73.53) | 23 (65.71) | 28 (82.35) |
| No | 9 (26.47) | 12 (34.29) | 6 (17.65) |
3.2. Epithelialisation and infection
Time to complete epithelialisation was significantly shorter with PVP‐I foam dressing (12.74 ± 3.51 days) compared with hydrocellular foam dressing (16.61 ± 4.45 days; P = 0.0003) and petrolatum gauze (15.06 ± 4.26 days, P = 0.0205) (Figure 3A). At Day 14, 83.87% of PVP‐I foam dressing donor sites had complete epithelialisation vs 36.36% of hydrocellular foam dressing donor sites (P = 0.0001) and 55.88% of petrolatum gauze donor sites (P = 0.0146) (Figure 3B). The epithelialisation rate on Day 14 was significantly higher in the PVP‐I foam dressing group compared with the hydrocellular foam dressing group (97.37 ± 8.14% vs 80.95 ± 26.10%; P = 0.0014). There were no wound infections in any of the three groups. Inflammatory signs were observed in 41.94% of patients in the PVP‐I foam dressing group, 39.39% in the hydrocellular foam dressing group, and 41.18% in the petrolatum gauze group; in all three groups, the most frequent inflammatory sign was “Pain and Tenderness.” Frequencies of inflammatory signs were not significantly different between groups. Results for complete epithelialisation, infection, and inflammatory signs in the PPS were consistent with the FAS (data not shown).
Figure 3.
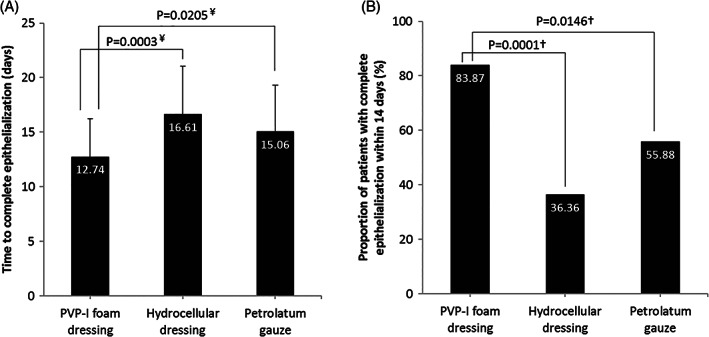
Epithelialisation after donor site collection in the PVP‐I foam dressing, hydrocellular dressing, and petrolatum gauze groups (Full Analysis Set). (A) Time to complete epithelialisation and (B) proportion of patients with complete epithelialisation within 14 days. ¥ t‐test, † χ 2 test
3.3. Ease and acceptability of dressing changes
The total number of dressing changes was significantly lower in PVP‐I foam dressing than with hydrocellular foam dressing (5.71 ± 1.57 vs 6.73 ± 1.88 times; P = 0.0224). No significant differences were found in the intensities of exudate from PVP‐I foam dressing and hydrocellular foam dressing donor sites; the majority of patients in both groups had only “scant” or “mild” exudate by Day 4. Changes in NRS pain scores before and after dressing change were also not significantly different between the PVP‐I foam dressing and hydrocellular foam dressing groups at all time points (Figure 4). Data obtained during dressing changes were not compared with the petrolatum gauze group because of differences in the dressing change protocol for petrolatum gauze.
Figure 4.
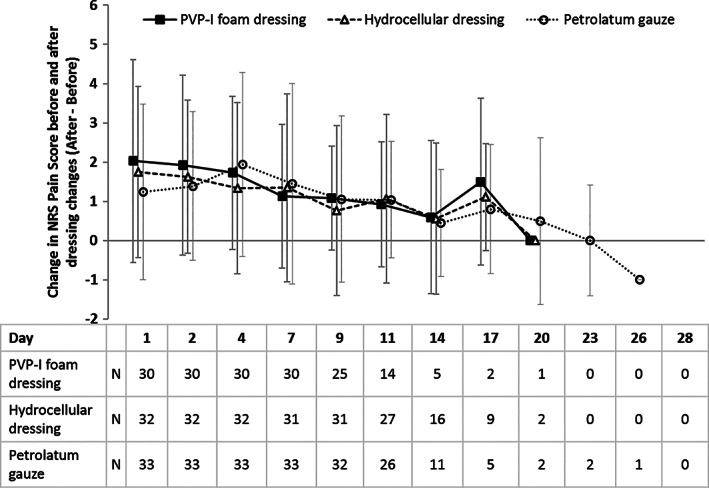
Change in NRS pain score before and after dressing change. Values are mean (standard deviation [SD]). NRS, numeric rating scale (0‐10 points, 0 = no pain and 10 = severe pain)
Some slight differences in the extent of acceptability during dressing changes were observed between treatment groups. There was a general tendency towards easier application and removal, lesser adherence and bleeding on removal, and lesser leakage of exudate with PVP‐I foam dressing compared with hydrocellular foam dressing and petrolatum gauze (data not shown).
3.4. Safety
The incidence rates of AEs were generally comparable between the PVP‐I foam dressing, hydrocellular dressing, and petrolatum gauze groups (17.65% vs 37.14% vs 29.41%; Table 2). All AEs reported were unexpected AEs. The most common AEs (incidence > 5%) were procedural pain (8.82%) in the PVP‐I foam dressing group; constipation (11.43%), cough (8.57%), and procedural pain (6.71%) in the hydrocellular foam dressing group; and cough (8.82%) and pyrexia (5.88%) in the petrolatum gauze group. Most of the AEs were mild in severity; one patient each from the PVP‐I foam dressing and hydrocellular foam dressing groups experienced AEs of moderate severity. There were no serious AEs, AEs resulting in withdrawal, adverse device effects, or skin‐related AEs in the PVP‐I foam dressing group. One case of application site haemorrhage each was observed in the hydrocellular foam dressing and petrolatum gauze groups—these were classified as adverse device effects as the causal relationship with the investigational device could not be ruled out. There were no significant differences in the clinical laboratory findings, vital signs, and physical examination results after the application of the investigational device between the PVP‐I foam dressing group, hydrocellular foam dressing group, and petrolatum gauze group (data not shown).
Table 2.
Adverse events (safety set)
| PVP‐I foam dressing (N = 34) | Hydrocellular dressing (N = 35) | Petrolatum gauze (N = 34) | ||||
|---|---|---|---|---|---|---|
| Incidence, n (%) | Events | Incidence, n (%) | Events | Incidence, n (%) | Events | |
| Adverse events | 6 (17.65) | 12 | 13 (37.14) | 21 | 10 (29.41) | 17 |
| Mild | 5 (14.71) | 8 | 12 (34.29) | 20 | 10 (29.41) | 17 |
| Moderate | 1 (2.94) | 4 | 1 (2.86) | 1 | 0 (0.00) | 0 |
| Severe | 0 (0.00) | 0 | 0 (0.00) | 0 | 0 (0.00) | 0 |
| Unexpected adverse events | 6 (17.65) | 12 | 13 (37.14) | 21 | 10 (29.41) | 17 |
| Serious adverse events | 0 (0.00) | 0 | 0 (0.00) | 0 | 0 (0.00) | 0 |
| Adverse events resulting in withdrawal | 0 (0.00) | 0 | 1 (2.86) | 1 | 0 (0.00) | 0 |
| Adverse device effects | 0 (0.00) | 0 | 1 (2.86) | 1 | 1 (2.94) | 1 |
| Skin‐related adverse event at the donor site | 0 (0.00) | 0 | 1 (2.86) | 1 | 1 (2.94) | 1 |
4. DISCUSSION
This is the first clinical study of the PVP‐I foam dressing for donor site wound management in split‐thickness skin grafts. The efficacy and safety of PVP‐I foam dressing was compared with two commonly used dressings, petrolatum gauze and hydrocellular dressing. As none of the donor site wounds became infected during the healing process, and the safety endpoints were generally comparable across groups, the main differentiating parameter used to rank the dressings was donor site epithelialisation.
Traditional wound dressings such as petrolatum gauze are dry and primarily function as a wound cover while absorbing exudates and fluid from the wound.11 In comparison, the three‐layer polyurethane foam in PVP‐I foam dressing is designed to absorb and retain wound exudate while maintaining a moist wound environment6; this keeps the wound from dehydration and is thought to promote healing. In the present study, PVP‐I foam dressing showed better wound‐healing efficacy compared with the conventional dry fine‐mesh petrolatum gauze dressing; time to complete epithelialisation was significantly shorter with PVP‐I foam dressing, and a higher proportion of patients achieved complete epithelialisation by Day 14. Although donor site complications such as pain, infection, and secretion were not significantly different between groups, there was a tendency towards better ease of use (application and removal), less bleeding and adherence on removal of dressing, and less leakage of exudate with PVP‐I foam dressing, thus indicating better exudate management. The superior fluid‐handling capacity of PVP‐I foam dressing has also been demonstrated in a previous study.6 Such properties can become particularly important when dressing wounds that are prone to pressure from the patient's own weight, such as donor sites located in the patient's back or buttocks. These findings are consistent with previous studies where moist dressings were found to have distinct clinical advantages over traditional non‐moist products in the management of split‐thickness skin graft donor sites.4, 12
Of the commercially available moist wound dressings, the hydrocellular foam dressing is a common choice for managing donor site wounds.13, 14, 15, 16, 17 Comparisons of the efficacy of PVP‐I foam dressing with hydrocellular foam dressing in the present study thus provide additional insight into the performance of PVP‐I foam dressing in donor site management. Fewer dressing changes were required overall with PVP‐I foam dressing, likely because of more rapid healing of the donor site wound. In Korea, PVP‐I foam dressings cost approximately three times less than hydrocellular foam dressings. Given that fewer dressing changes and faster healing were observed with PVP‐I foam dressings than with hydrocellular foam dressings, the total cost of dressings is likely to be significantly lower in patients managed with PVP‐I foam dressings.
Although the risk of donor site infection is low in split‐thickness skin grafts,3 an infected donor site can lead to prolonged hospitalisation and increase the cost of management.2 Several studies have explored the use of antimicrobial dressing products for donor site wounds with favourable outcomes.13, 14, 18, 19 PVP‐I foam dressing contains the antimicrobial agent PVP‐I. Compared with silver, a popular antimicrobial agent used in wound dressings, PVP‐I is effective against a broader range of wound pathogens, lacks bacterial resistance, and is less cytotoxic to host cells.20 PVP‐I foam may thus possess clinical advantages, particularly in the management of infected wounds. As none of the donor sites developed an infection in our study, the differences in the risk of infection between dressings could not be assessed. Nonetheless, it is noteworthy that the antimicrobial agent, 3% PVP‐I, in PVP‐I foam dressing does not appear to delay wound healing as has been seen with silver‐containing dressings.21 Future studies comparing the efficacy of PVP‐I foam dressing with those of other dressings in infected wounds may thus be important.
In conclusion, our comparison of PVP‐I foam dressing with hydrocellular foam dressing and petrolatum gauze suggests that PVP‐I foam dressing was more effective overall. PVP‐I foam dressing outperformed the two dressings in healing time and tended to be easier to use and less prone to leakage.
ACKNOWLEDGEMENTS
This study was funded by Mundipharma Korea Ltd. Medical writing and editorial support were funded by Mundipharma Korea Ltd and provided by Bao Hui Lee and Geraldine Toh of Tech Observer Asia‐Pacific Pte Ltd. Kyunghee Kwak is an employee of Mundipharma Korea Ltd. All other authors have no relevant relationships to disclose.
Pak CS, Park DH, Oh TS, et al. Comparison of the efficacy and safety of povidone‐iodine foam dressing (Betafoam), hydrocellular foam dressing (Allevyn), and petrolatum gauze for split‐thickness skin graft donor site dressing. Int Wound J. 2019;16:379–386. 10.1111/iwj.13043
Funding information Mundipharma Korea Ltd.
NOTE
Betafoam and Medifoam are registered trademark of Genewel, Seongnam, Korea. Allevyn is a registered trademark of Smith & Nephew, Hull, UK.
REFERENCES
- 1. Ratner D. Skin grafting. Semin Cutan Med Surg. 2003;22(4):295‐305. [DOI] [PubMed] [Google Scholar]
- 2. Otene CI, Olaitan PB, Ogbonnaya IS, Nnabuko RE. Donor site morbidity following harvest of split‐thickness skin grafts in south eastern Nigeria. J West Afr Coll Surg. 2011;1(2):86‐96. [PMC free article] [PubMed] [Google Scholar]
- 3. Voineskos SH, Ayeni OA, McKnight L, Thoma A. Systematic review of skin graft donor‐site dressings. Plast Reconstr Surg. 2009;124(1):298‐306. [DOI] [PubMed] [Google Scholar]
- 4. Wiechula R. The use of moist wound‐healing dressings in the management of split‐thickness skin graft donor sites: a systematic review. Int J Nurs Pract. 2003;9(2):S9‐S17. [DOI] [PubMed] [Google Scholar]
- 5. Broussard KC, Powers JG. Wound dressings: selecting the most appropriate type. Am J Clin Dermatol. 2013;14(6):449‐459. [DOI] [PubMed] [Google Scholar]
- 6. Jung JA, Han SK, Jeong SH, Dhong ES, Park KG, Kim WK. In vitro evaluation of Betafoam, a new polyurethane foam dressing. Adv Skin Wound Care. 2017;30(6):262‐271. [DOI] [PubMed] [Google Scholar]
- 7. Bigliardi PL, Alsagoff SAL, El‐Kafrawi HY, Pyon JK, Wa CTC, Villa MA. Povidone iodine in wound healing: a review of current concepts and practices. Int J Surg. 2017;44:260‐268. [DOI] [PubMed] [Google Scholar]
- 8. Cooper RA. Iodine revisited. Int Wound J. 2007;4(2):124‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lim SA, Lee DE. Comparison of usual applicating foam dressing materials in split thickness skin graft donor site. J Korean Burn Soc. 2003;6(1):45‐51. [Google Scholar]
- 10. Kim DH, Kim JH, Nam‐Koong Y, Kim DK, Park YK. Treatment of donor‐site wounds using foam dressing material. J Korean Soc Traumatol. 2002;15(1):1‐7. [Google Scholar]
- 11. Dhivya S, Padma VV, Santhini E. Wound dressings—a review. Biomedicine. 2015;5(4):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barnea Y, Amir A, Leshem D, et al. Clinical comparative study of aquacel and paraffin gauze dressing for split‐skin donor site treatment. Ann Plast Surg. 2004;53(2):132‐136. [DOI] [PubMed] [Google Scholar]
- 13. Argirova M, Hadjiski O, Victorova A. Acticoat versus Allevyn as a split‐thickness skin graft donor‐site dressing: a prospective comparative study. Ann Plast Surg. 2007;59(4):415‐422. [DOI] [PubMed] [Google Scholar]
- 14. Brenner M, Hilliard C, Peel G, Crispino G, Geraghty R, O'Callaghan G. Management of pediatric skin‐graft donor sites: a randomized controlled trial of three wound care products. J Burn Care Res. 2015;36(1):159‐166. [DOI] [PubMed] [Google Scholar]
- 15. Higgins L, Wasiak J, Spinks A, Cleland H. Split‐thickness skin graft donor site management: a randomized controlled trial comparing polyurethane with calcium alginate dressings. Int Wound J. 2012;9(2):126‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Karlsson M, Lindgren M, Jarnhed‐Andersson I, Tarpila E. Dressing the split‐thickness skin graft donor site: a randomized clinical trial. Adv Skin Wound Care. 2014;27(1):20‐25. [DOI] [PubMed] [Google Scholar]
- 17. Vaingankar NV, Sylaidis P, Eagling V, King C, Elender F. Comparison of hydrocellular foam and calcium alginate in the healing and comfort of split‐thickness skin‐graft donor sites. J Wound Care. 2001;10(7):289‐291. [DOI] [PubMed] [Google Scholar]
- 18. Bailey S, Carmean M, Cinat M, Burton K, Lane C, Malinoski D. A randomized comparison study of Aquacel ag and glucan II as donor site dressings with regard to healing time, cosmesis, infection rate, and patient's perceived pain: a pilot study. J Burn Care Res. 2011;32(6):627‐632. [DOI] [PubMed] [Google Scholar]
- 19. Demirtas Y, Yagmur C, Soylemez F, Ozturk N, Demir A. Management of split‐thickness skin graft donor site: a prospective clinical trial for comparison of five different dressing materials. Burns. 2010;36(7):999‐1005. [DOI] [PubMed] [Google Scholar]
- 20. EWMA . Position Document: Management of Wound Infection. Aylesford, UK: MEP Ltd; 2006.
- 21. Aziz Z, Abu SF, Chong NJ. A systematic review of silver‐containing dressings and topical silver agents (used with dressings) for burn wounds. Burns. 2012;38(3):307‐318. [DOI] [PubMed] [Google Scholar]


