ABSTRACT
Controversy persists about why so many large‐bodied mammal species went extinct around the end of the last ice age. Resolving this is important for understanding extinction processes in general, for assessing the ecological roles of humans, and for conserving remaining megafaunal species, many of which are endangered today. Here we explore an integrative hypothesis that asserts that an underlying cause of Late Quaternary megafaunal extinctions was a fundamental shift in the spatio‐temporal fabric of ecosystems worldwide. This shift was triggered by the loss of the millennial‐scale climate fluctuations that were characteristic of the ice age but ceased approximately 11700 years ago on most continents. Under ice‐age conditions, which prevailed for much of the preceding 2.6 Ma, these radical and rapid climate changes prevented many ecosystems from fully equilibrating with their contemporary climates. Instead of today's ‘striped’ world in which species' ranges have equilibrated with gradients of temperature, moisture, and seasonality, the ice‐age world was a disequilibrial ‘plaid’ in which species' ranges shifted rapidly and repeatedly over time and space, rarely catching up with contemporary climate. In the transient ecosystems that resulted, certain physiological, anatomical, and ecological attributes shared by megafaunal species pre‐adapted them for success. These traits included greater metabolic and locomotory efficiency, increased resistance to starvation, longer life spans, greater sensory ranges, and the ability to be nomadic or migratory. When the plaid world of the ice age ended, many of the advantages of being large were either lost or became disadvantages. For instance in a striped world, the low population densities and slow reproductive rates associated with large body size reduced the resiliency of megafaunal species to population bottlenecks. As the ice age ended, the downsides of being large in striped environments lowered the extinction thresholds of megafauna worldwide, which then increased the vulnerability of individual species to a variety of proximate threats they had previously tolerated, such as human predation, competition with other species, and habitat loss. For many megafaunal species, the plaid‐to‐stripes transition may have been near the base of a hierarchy of extinction causes whose relative importances varied geographically, temporally, and taxonomically.
Keywords: extinction, Late Quaternary, megafauna, climate change, terrestrial mammals, ecological disequilibrium, allometry
I. INTRODUCTION
A global wave of extinctions selectively removed many species of terrestrial megafauna (mammals weighing > 45 kg) around the end of the last ice age, and we are still not sure why. Although not as drastic as the End‐Cretaceous or End‐Permian extinctions, these Late Quaternary extinctions fundamentally changed the composition of mammalian faunas on continents other than Africa (Wallace, 1876; Romer, 1933; Barnosky et al., 2004b) and in some cases altered the functioning of entire ecosystems (Owen‐Smith, 1987; Gill et al., 2012; Johnson et al., 2016; Malhi et al., 2016; Galetti et al., 2017). What caused the Late Quaternary extinctions of megafauna, and what roles did humans play? Was there some shared factor that elevated the risk of extinction for megafauna everywhere? Answering these questions could help to manage the wave of human‐caused extinctions that is now underway (Barnosky & Lindsey, 2010; Barnosky et al., 2017).
Numerous explanations have been proposed for megafaunal extinctions during the Late Quaternary, roughly the last 100000 years of Earth's history (Stuart, 2015). These range from overkill by humans (Sandom et al., 2014; Miller et al., 2016; Surovell et al., 2016), to epidemic diseases (MacPhee & Marx, 1997), to cosmic collisions (Hagstrum et al., 2017), to some aspect of climate change (Cooper et al., 2015; Rabanus‐Wallace et al., 2017). Despite long‐running debate, numerous hypotheses, and a burgeoning literature, there is still no consensus within the scientific community about the cause of Late Quaternary megafaunal extinctions (Price et al., 2018).
Extinctions result from conspiracies of causes arranged in hierarchies according to their relative importances for the species concerned (Caughley, 1994; Barnosky et al., 2004a). Some of these synergistic causes are shared by multiple species (Caughley, 1994; Burney et al., 2004; Cardillo et al., 2005; Brook, Sodhi, & Bradshaw, 2008). The fact that so many megafaunal extinctions occurred during the Late Quaternary strongly suggests that one or more causes were widely shared.
Here we hypothesize that one of the most important of these underlying, shared causes of Late Quaternary extinctions was a fundamental shift in the spatio‐temporal ‘fabric’ of ecosystems that occurred during the ice age/Holocene transition. Rapidly changing climate during the ice age created widespread transient ecosystems in which the eco‐physiological traits associated with large body size endowed megafaunal species with special advantages. But these and other traits associated with being large became disadvantages within the more stable climate regime of the Holocene and thus lowered the extinction thresholds for many megafaunal species. Once these thresholds were lowered, individual species fell victim to a variety of proximate extinction causes, whether overkill by humans, disease, habitat loss, or disrupted trophic cascades.
II. BACKGROUND
(1). Temporal and geographic patterns of megafaunal extinctions
The present interglacial, the Holocene, which began 11700 calendar years before present (BP; hereafter 11.7 ka) is impoverished in megafauna compared to the preceding 50+ Ma of Earth's history (Alroy, 2000). This impoverishment is the result of some 64% of megafaunal genera worldwide going extinct sometime over the last roughly 100 ka (Koch & Barnosky, 2006). Although the fossil record is admittedly incomplete, so far there is no definitive evidence for comparable bouts of megafaunal extinction earlier in the Cenozoic (the last 66 Ma) (Alroy, 2000), nor is there evidence for comparable bouts of megafaunal extinctions during previous glacial–interglacial transitions (Stuart, 2015).
The magnitude of megafaunal extinction varied geographically. North America lost approximately 72% of its megafaunal genera (Barnosky et al., 2004b), while South America lost 83% (Barnosky & Lindsey, 2010). Northern Eurasia lost fewer genera, around 29% (Stuart, 2015), while Australia lost some 88% (Barnosky et al., 2004b), and the mega‐islands Madagascar and New Zealand both lost 100% (Burney et al., 2004; Perry et al., 2014). Africa alone has retained most of its megafauna; losses there ranged from 5 to 18% (Klein, 1984; Barnosky et al., 2004b). All four mega‐herbivore (>1000 kg) genera present in Africa during the Pleistocene survive today (Stuart, 1991).
Late Quaternary megafaunal extinctions occurred in a time‐transgressive manner across the planet and occupied varying time intervals. In Australia, they began approximately 400 ka and continued episodically until about 40 ka (Wroe et al., 2013). In Europe, most of the extinctions occurred between approximately 45 and 10 ka (Stuart, 2015), while in North America they seem to have been concentrated within several millennia each side of the Pleistocene–Holocene transition (Barnosky et al., 2004b; Grayson, 2016). South America's extinction window may have been wider, perhaps between about 15 and 8 ka (Turvey, 2009; Barnosky & Lindsey, 2010, pp. 24–26). Megafauna on islands worldwide experienced waves of extinction during Holocene times (Rick et al., 2013).
(2). Correlations with climate change
Correlations between the timing of megafaunal extinctions and climate changes are problematic. North America's extinctions and at least some of South America's and Europe's coincided with rapid climate changes during the Pleistocene–Holocene transition (Stuart, 1991; Barnosky et al., 2004b; Barnosky & Lindsey, 2010). By contrast, in Madagascar and New Zealand, peak extinction did not coincide with rapid climate changes (Burney et al., 2004; Duncan, Boyer, & Blackburn, 2013; Holdaway et al., 2014). In semi‐arid Australia, where bone preservation is often poor, palaeoenvironmental records fragmentary, and the extinctions occurred longer ago, heated debate continues over correlations between extinctions and climate change (Wroe et al., 2013; Cohen et al., 2015; Miller et al., 2016; Saltré et al., 2016; Price et al., 2018).
(3). Human roles
The evolution and global dispersal of hominins was relatively recent and rapid. Predatory, fire‐using hominins inhabited Africa and Eurasia roughly a million years before anatomically modern humans evolved and then dispersed out of Africa approximately 200 ka (Bae, Douka, & Petraglia, 2017; Hershkovitz et al., 2018). Homo sapiens reached Australia 50–45 ka (Wroe et al., 2013), colonized northern Eurasia after 45 ka (Bae et al., 2017), crossed from Siberia into North America 20–15 ka (Moreno‐Mayar et al., 2018), and spread across North and South America before 12 ka (Meltzer, 2015). The precise timing of human arrival on any continent will probably never be determined within 103–104 years because of the low probability of finding traces of the first colonists. The timing of human arrival on islands is more secure because the search area is smaller and in many cases the arrival was more recent. People reached Madagascar approximately 2.3 ka (Burney et al., 2004) and New Zealand about 0.7 ka (Wilmshurst et al., 2008). Small islands in the Caribbean and Oceania were settled at varying times in the middle and late Holocene (Turvey, 2009).
On many islands, the Holocene arrival of humans correlates closely with the timing of megafaunal extinctions. For instance, the Polynesian settlers in New Zealand exterminated nine species of ratite moa within several centuries of their arrival (Holdaway et al., 2014; Perry et al., 2014). In Madagascar, extinctions also commenced upon human arrival, but then took roughly 2 ka to conclude (Burney et al., 2004; Dewar et al., 2013).
On the continents, correlations between megafaunal extinctions and the timing of Homo sapiens arrival are less clear. In Africa, expansion of early hominins into scavenger/predator niches during the Pliocene may have triggered extinctions among competing carnivores (Werdelin & Lewis, 2013). On the other hand, the initial expansion of early hominins into Europe does not seem to have triggered widespread extinctions there. Instead, most megafaunal extinctions occurred some million years later and possibly accompanied the replacement of H. neanderthalensis by anatomically modern humans (Stuart, 1991). Depending on how Australia's records are interpreted, extinctions there were either distinctly out‐of‐phase with human arrival (Wroe et al., 2013), or lagged them by some 13 ka (Saltré et al., 2016). Human arrival coincided broadly with megafaunal extinctions in lower latitude North America (Stuart, 1991; Barnosky et al., 2004b); however, ‘broadly’ is the key word here because over half of the extinct fauna there are either not dated at all or have no dates younger than about 20 ka (Meltzer, 2015). In South America, it is possible some megafauna, including Smilodon, Doedicurus, Hippidion, several species of ground sloth, and possibly even gomphotheres, survived until 8 ka, which was at least 6–7 ka after human arrival (Hubbe, Hubbe, & Neves, 2007; Turvey, 2009; Barnosky & Lindsey, 2010, pp. 24–26). In summary, although human arrival is closely correlated with the timing of extinctions on islands during the Holocene, on the continents the correlation remains problematic because of compounding uncertainties about when people first arrived and when megafauna actually went extinct. Some of the causes for uncertainty about the timing of extinction are explored in Section II.4.
The roles played by humans in megafaunal extinctions varied geographically. Undoubtedly, people caused extinctions on many islands (Holdaway & Jacomb, 2000; Burney et al., 2004), but on continents their roles varied widely. Megafaunal extinctions occurred in continental ecosystems ranging from tropical rainforests to Arctic tundra and, in some cases, during periods when people were rare or absent. In Alaska, the extinctions of horse, bison, and mammoth probably occurred without human involvement (Guthrie, 2006; Mann et al., 2013). Similarly, Nikolskiy, Sulerzhitsky, & Pitulko (2011) argue that human hunting of mammoth was rare in Siberia, and at most was the coup de grâce for a species already in serious extinction debt (Kuussaari et al., 2009) caused by environmental changes. Even at lower latitudes in the western USA where people were more abundant than in the Arctic, archaeological evidence connecting megafaunal extinctions to hunting remains problematic (Grayson, 2001; Grayson & Meltzer, 2003; Meltzer, 2015). Archaeological evidence for overkill by humans is similarly ambiguous in Australia (Wroe et al., 2013; Jankowski et al., 2016). That said, it is unclear what convincing archaeological evidence for human overkill of megafauna would actually look like (Grayson, 2001), and whether archaeology is even capable of providing evidence for less direct, but nonetheless lethal impacts of humans (Burney et al., 2004): impacts like changes to fire regimes and disruption of food webs. Although extinction processes on islands and continents have been repeatedly conflated (Martin, 1984; Steadman & Martin, 2003; Johnson et al., 2016), the two settings are in fact biogeographically distinct (Grayson, 2001; Whittaker, Triantis, & Ladle, 2008; Wood et al., 2017), which means basic differences should be expected in the identities and scales of their extinction processes.
In historic times, humans have driven other species to extinction in a depressingly large number of ways (Wood et al., 2017), and only a handful of extinctions are known to have occurred without human involvement (Soulé, 1983; Caughley, 1994). In descending order of subtlety but not necessarily of effectiveness, humans are known to have caused extinctions by overkill (Perry et al., 2014), by habitat alteration (McWethy et al., 2014; Miller et al., 2016), and by trophic disruptions involving multiple processes (Diamond, 1984; Caughley, 1994; Atkinson, 1996; Burney et al., 2004; Brook et al., 2008; Ripple & Van Valkenburgh, 2010).
Humans excel at trophic disruption, in part because of our ability to tap into food chains at multiple levels. In ecosystems where herbivore populations are limited from the top‐down by predators rather than from the bottom‐up by food availability, human hunters can trigger sequential collapse of both predator and herbivore populations (Janzen, 1983; Ripple & Van Valkenburgh, 2010; Estes, 2016). Unlike most other species, humans can then sidestep the impacts of these extinctions by switching to other food sources (Grayson, 2001). Clearly, no discussion of megafaunal extinctions can ignore the possible roles of humans; however, these roles differed widely in space and time, were often indirect, and have rarely left clear traces in palaeoecological records.
(4). When did Late Quaternary megafaunal extinctions actually occur?
The Signor–Lipps effect, the decreasing probability of discovering the remains of individual organisms as the overall population of their taxon declines (Signor & Lipps, 1982), haunts all discussions of late‐Quaternary extinctions (Collen & Turvey, 2009). Because it is impossible to know when the last individual of a species died, estimates of extinction times are necessarily fuzzy. This temporal fuzziness is pertinent to the hypothesis we propose here because, while the ice age's distinctive climate regime did not end until the Holocene began, in some places at least, megafaunal extinctions appear to have occurred before the ice age ended.
How can a hypothesis that blames extinctions on climate changes that occurred during the last glacial–interglacial transition be valid if those extinctions actually occurred before the environmental plaidness of the ice age ended? The answer is, we think, that the extinction dates of many species are underestimated and that many Late Quaternary extinctions actually occurred in the early Holocene.
Numerous factors make it difficult to pinpoint when a prehistoric extinction actually occurred (Collen & Turvey, 2009). Fossil records fade rapidly into the past (Stuart, 1991; Alroy, 2000), and the accuracy and precision of dating are often poor, especially near the upper limit of the 14C technique (Zazula et al., 2014, 2017). Taphonomic processes can change over time, confounding assumptions about random sampling and the trajectories of population decline (Mann et al., 2013). As a result, the last‐appearance dates of most Late Quaternary megafaunal taxa tell us only when the animals were last abundant on the local landscape, not when they became globally extinct (Barnosky & Lindsey, 2010).
If you cannot track a species' descent into extinction, an alternative is to model the decline using probabilistic trends based on sighting records. This has become something of a cottage industry among mathematically minded palaeontologists (Solow, 1993, 2003; McInerny et al., 2006; Buck & Bard, 2007; Collen & Turvey, 2009; Rivadeneira, Hunt, & Roy, 2009; Bradshaw et al., 2012; Alroy, 2014; Saltré et al., 2016). Unfortunately, sighting records are inherently complex because extinction causes are diverse, collaborative, and can change through time. As the Signor–Lipps effect implies, sighting records become increasingly tenuous as populations retract into cryptic refugia (Bennett, Tzedakis, & Willis, 1991; Lister & Stuart, 2008; Kuzmin, 2010; Stewart et al., 2010), yet a population too small to be noticed by palaeontologists is still capable of giving rise to abundant descendants. Invasive species provide startling examples of the demographic resilience of small populations. For example, the 100–160 starlings (Sturnus vulgaris) Eugene Schieffelin released in New York City in 1890 and 1891 have increased to >200 million and populated much of North America.
Invasive species are not the only exemplars of demographic resilience. Lazarus taxa once thought to be extinct because of an absence of sightings include the coelocanth (Latimeria spp.), the dawn redwood (Metasequoia glyptostroboides), and the New Zealand Takahe (Porphyrio hochstetteri). Several ice‐age megafaunal species are now known to be Lazarus taxa. Irish elk (Megaloceros giganteus) were thought to have gone extinct in the terminal Pleistocene, but more recent discoveries reveal they persisted in the Ural Mountains until at least 7.6 ka (Stuart et al., 2004). Even more surprising is the late survival of woolly mammoth (Mammuthus primigenius). Prior to 1995, the last mammoth was thought to have died before 10 ka. Since then two, mid‐Holocene mammoth refugia have been discovered: one on Wrangel Island in northeastern Siberia (Vartanyan et al., 1995, 2008; Rogers & Slatkin, 2017), the other on Saint Paul Island in the Bering Sea (Guthrie, 2004; Graham et al., 2016). It turns out that mammoth extinction was a spatially complex phenomenon occupying some 10000 years spanning the Pleistocene–Holocene transition (Kuzmin, 2010), which is sobering given that proboscideans leave some of the most obvious fossil records of all megafauna.
Demographic resilience, undiscovered refugia, the Signor–Lipps effect, and the existence of Lazarus taxa imply that species' temporal persistence is routinely underestimated by their last dated fossils and therefore the extinctions of many megafaunal species occurred thousands of years after their youngest 14C‐dated remains. This means the global extinctions of many of these species actually occurred during the Holocene rather than the Pleistocene. This inference is guaranteed to raise the hackles of some palaeontologists and obviously warrants testing. Sadly, it also blunts the use of chronological correlation as a tool for testing hypotheses about extinction causes (Meltzer, 2015; Grayson, 2016).
III. THE PLAIDS AND STRIPES HYPOTHESIS
(1). Spatio‐temporal fabrics, the productivity paradox, and the mammoth steppe
The fabric analogy we employ here originates in Guthrie's (1984) attempt to dismiss the productivity paradox concerning the mammoth steppe, the now‐vanished biome that at various times during the ice age extended from Western Europe to the Yukon (Zimov et al., 2012). The productivity paradox arose from the apparent contradiction between palynological data that depicted full‐glacial, Arctic landscapes supporting only unproductive tundra vegetation, and the palaeontological data that depicted diverse communities of megafauna inhabiting these same landscapes. This mismatch triggered a long‐running controversy about the nature of the mammoth steppe (Ritchie & Cwynar, 1982; Guthrie, 2001; Zazula et al., 2006; Hofreiter & Stewart, 2009; Huntley et al., 2013; Mann et al., 2015).
Guthrie's (1984, 2001) solution to the paradox was twofold. First, he hypothesized that high‐latitude ecosystems in unglaciated regions possessed a different ‘ecological fabric’ during the ice age than in the Holocene as the result of ice‐age growing seasons being longer. Longer ice‐age summers accommodated a greater diversity of plant species with more complex phenologies, which then provided a greater variety of food resources for megafaunal herbivores. Guthrie then explained away the productivity paradox by asserting that the ice age's phenological ‘plaidness’ is largely invisible to the methods used to reconstruct ice‐age vegetation. It is invisible because the typical pollen record comes from a sediment core from a single lake (restricted spatial resolution), and this core is typically sampled for pollen at intervals corresponding to centuries to millennia (restricted temporal resolution). Compounding the dating‐resolution problem, many pre‐2010 studies of Arctic palynology relied on the 14C dating of bulk lake sediment, which often contains an admixture of ancient carbon derived from thawing permafrost in the surrounding watershed (Gaglioti et al., 2014). Further detracting from palynology's resolution is the fact that some of the most speciose plant taxa growing in the Arctic cannot be distinguished from one another based on their pollen (restricted taxonomic and ecological resolution). Guthrie's hypothesis has never been tested explicitly, but studies using precisely dated plant macrofossils rather than pollen to reconstruct vegetation are consistent with Guthrie's (1984) vision of diverse ice‐age plant communities (Goetcheus & Birks, 2001; Zazula et al., 2006, 2007).
Having disposed of the productivity paradox, Guthrie (1984, 1990) went on to blame late‐Quaternary extinctions on the mammoth steppe on a shift in growing‐season phenology. Megafaunal species became extinct because ‘…. the Pleistocene‐Holocene change was an unparalleled jolt across a threshold toward more constricted growth seasons which sharply reduced resources available for ungulate growth.’ (Guthrie, 1984, p. 262). This occurred, he speculated, because shorter, less‐variable growing seasons during the Holocene caused an increase in competition between plant species, which caused a decrease in species diversity. The remaining plant species tended to be K‐strategists with large investments in anatomy and chemistry that discouraged herbivory. These changes reduced herbivore diversity by restricting forage availability and reducing its protein content. Especially hard hit were caecalid–monogastric ungulates like mammoth, rhinoceros, and equids, which required diverse diets of high‐fibre/low‐protein plants relatively low in chemical defences. Guthrie (1984) suggested that by reducing the diversity of plant species, the Holocene's shorter growing seasons converted the mammoth steppe into the relatively unproductive and species‐poor ecosystems that occupy boreal regions today. Again, this hypothesis has never been directly tested, perhaps because of the difficulty of documenting prehistoric shifts in growing‐season length, plant phenology, and the physiological responses of ungulates.
In a brief contribution that caught the spirit if not the specifics of Guthrie's (1984) original idea, Lister & Sher (1995, p. 23) expanded the timescale of Guthrie's hypothesis by suggesting:
…. constant fluctuations of climate in the Late Pleistocene were partly responsible for maintaining a mosaic of plant communities, by a constant ‘stirring’, favoring a pioneering character of vegetation which supports grazing megafauna.”
Here we take Guthrie's ecological fabric analogy of seasonal plant phenology, expand it to the millennial scales that Lister & Sher (1995) had in mind, and then enlarge it even further to encompass not just vegetation but other aspects of environmental change (e.g. climate, geomorphology, soil development) that are relevant to the size‐dependent eco‐physiological attributes of ice‐age megafauna.
(2). The new Plaids and Stripes Hypothesis
We use the term ‘Plaids and Stripes hypothesis’ because it is shorter than ‘Temporal Simplification of Spatio‐temporal Dynamics Hypothesis’, but note that our version of Plaids and Stripes differs from Guthrie's (1984) by involving multiple time scales and more environmental variables than just summer climate and plant phenology. The expanded Plaids and Stripes Hypothesis states that during the last ice age (ca. 60–15 ka), short‐term climate fluctuations added a dimension of temporal complexity that is now missing from many modern ecosystems (Fig. 1). The ice age's spatio‐temporal fabric was fundamentally different from the Holocene's because it was created by large‐magnitude shifts in climate at decadal, centennial, and millennial time scales (Fig. 2). These climate changes were superimposed on the same geographic gradients arising from topography and latitude that are familiar to us in today's world.
Figure 1.
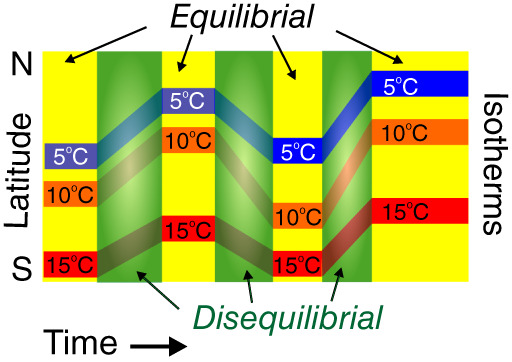
The expanded Plaids and Stripes Hypothesis. Imagine a landscape where species in three ecological communities (blue, orange, and red) live in equilibrium with a latitudinal gradient in temperature. This landscape is ‘striped’ in the sense that species' distributions parallel isotherms. Then a series of rapid (decades to millennia) climate changes occur. In response, species must disperse hundreds to thousands of kilometers to catch up with their new range limits, and ecological succession needs to occur before the new equilibria are reached. These processes involve time lags, which cause disequilibrial communities to occupy large areas over long periods. Together, these equilibrial and disequilibrial states create a spatio‐temporal fabric that is now ‘plaid’ rather than ‘striped’.
Figure 2.
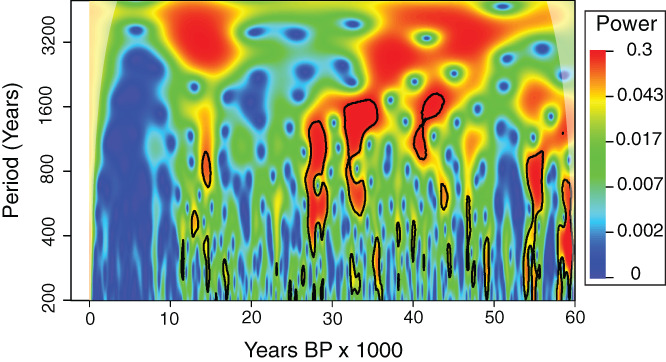
Holocene climate has been more complacent than that of the ice age. Morelet wavelet analysis (Torrence & Compo, 1998) of the NGRIP δ18O record interpolated at 50‐year intervals (Rasmussen et al., 2014). Blue indicates periods with little to no power in climate cycles of various wavelengths (y axis). Red indicates occurrence of pronounced climate cycles. Black lines outline zones falling outside (P < 0.05) the 95% range of 1000 Monte Carlo simulations of red‐noise time series using the same autoregressive model as for the NGRIP (AR1) data. White shading on the top corners is the ‘cone of influence’ where cycles become suspect due to edge effects.
The ice age's large and frequent climatic fluctuations (Fig. 2) kept ecosystems out of balance as plant and animal species struggled to keep up with repeated shifts in their environments. As a result of these lags, the ecological fabrics of ice‐age landscapes were more complex than most Holocene ones are. As detailed below, certain eco‐physiological attributes shared by megafaunal species including greater mobility, lower cost of locomotion, greater dietary breadth, and higher metabolic efficiency allowed them to flourish in these disequilibrial ‘plaid’ settings.
The plaid world of the ice age ended when millennial‐scale instabilities in climate largely ceased in most regions of the world approximately 11.7 ka as the Holocene began. Since then many regions of the planet have settled into new, ‘striped’ modes of ecology and biogeography, in which ecological fabrics are dominated by environmental gradients established by topography and latitude and that remain relatively stable over multiple millennia. Within this striped regime, the eco‐physiological traits of megafauna no longer give them as many advantages; in fact, certain attributes of being large pose distinct disadvantages. As detailed below, these disadvantages include megafaunal species' relatively low population densities (which can translate into small population sizes in striped landscapes), slow rates of reproduction, and the need for large daily food intakes. As a result, the shift from temporally complex, disequilibrial (plaid) ecologies to temporally simpler, equilibrial (striped) ones lowered the threshold for extinction for many terrestrial, megafaunal species (Fig. 3) and increased their vulnerabilities to a variety of species‐specific threats. On many landscapes worldwide, the small and the meek inherited the Holocene because climate stability favoured them.
Figure 3.
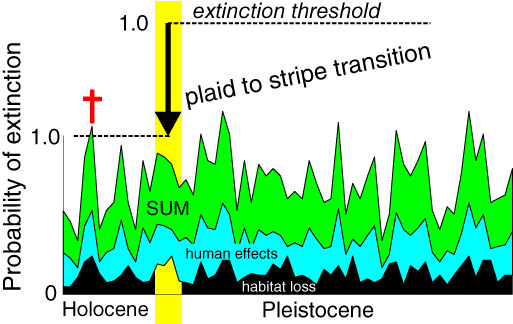
Extinction risks are hierarchical: they conspire to determine the risk of extinction for individual species. The transition from the mostly plaid ecosystems accompanying the tumultuous climatic regime of the ice age to the striped ecosystems of the climatically stable Holocene may have lowered the extinction thresholds for many megafaunal species because their life forms and life‐history strategies were no longer as adaptive as they had been during the ice age. Once these thresholds were lowered, individual species became more vulnerable to a variety of proximate threats, many of which had been only minor threats before.
(3). What do plaid and striped landscapes actually look like?
Striped landscapes occur today where plant communities are arranged along climatic gradients (Fig. 4). As long as climate is stable, the locations of these communities remain largely stationary because the ecophysiological requirements of their biota have equilibrated with average environmental conditions.
Figure 4.
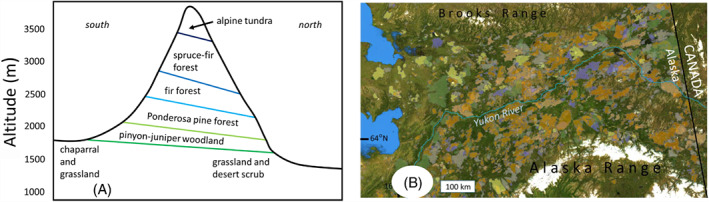
Modern examples of striped and plaid landscapes. (A) Striped: Altitudinal distribution of plant communities on San Francisco Peaks, Arizona (after Merriam, 1890). These communities have equilibrated with the Holocene's prolonged equable climate. (B) Plaid: Perimeters of wildland fires since AD 1940 in the boreal forest of Interior Alaska. Different colours depict fires occurring in different decades (https://afsmaps.blm.gov/imf/imf.jsp?site=firehistory). In this region, fire‐return intervals are 40–150 years (Gaglioti et al., 2016). Today, megafaunal herbivores (moose, Alces alces, and caribou, Rangifer tarandus) exploit vegetation growing during particular successional stages within these old burns (MacCracken & Viereck, 1990; Rupp et al., 2006).
By contrast, a plaid landscape is one where a large portion of the biota is out of equilibrium with contemporary climate. The novel communities resulting from transient combinations of species add an additional dimension to the ecological fabric – hence the image of plaids versus stripes. Reconstructions of shifting biomes during the Pleistocene–Holocene transition provide a glimpse of what ice‐age plaid landscapes looked like (Fig. 5). We suggest below that megafaunal herbivores are particularly well‐suited for exploiting transient ecosystems and thus were a favoured life form at times like the last ice age when plaid landscapes were widespread.
Figure 5.
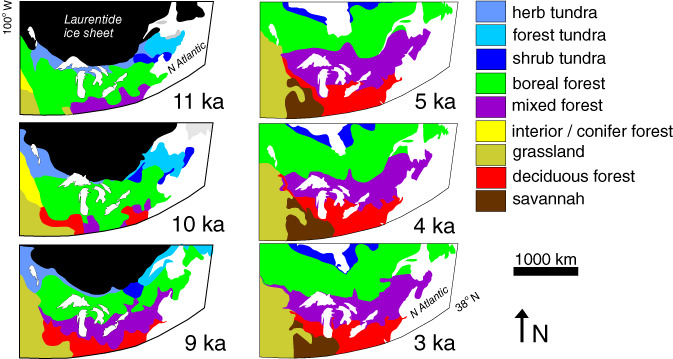
Ancient examples of plaid (left) and striped (right) landscapes based on the postglacial distribution of biomes in northeastern North America reconstructed from palaeobotanical records (redrawn from Dyke, 2005). Prior to 9 ka, rapid changes in the locations and extents of biomes occurred, at times with little regard for latitudinal climatic gradients. Palynological records suggest that between 11 and 5 ka the northern limits of a number of tree species in northeastern North America lagged behind their physiological range limits by as much as 1500 years because of dispersal lags (Prentice, Bartlein, & Webb, 1991). After approximately 5 ka, biome positions have remained relatively stable. Note that these biomes are defined by vegetation structure – not by the presence of certain indicator species.
(4). Requirements of a useful hypothesis
A useful hypothesis about a shared cause of Late Quaternary megafaunal extinctions has at least six requirements. First, it must be explicitly hierarchical and thus compatible both with extinction causes that act synergistically and with proximate causes of extinctions that vary among species. Second, it must invoke a special relationship between body size and some aspect of the ice‐age world that made being large more adaptive than it has been during the Holocene, at least on continents other than Africa. Requirements three, four, and five involve the hypothesis being consistent with the spatial and temporal patterns surrounding megafaunal extinctions. These patterns include lack of evidence for extinction bouts during previous glacial–interglacial transitions, early and staggered extinctions in Australia, and the African exception. The sixth requirement is that the hypothesis suggest new and interesting directions of research.
IV. TESTING THE HYPOTHESIS
(1). Large body size was more adaptive during the ice age
According to the Plaids and Stripes Hypothesis, large body size was more adaptive during the ice age because certain eco‐physiological traits associated with being large meshed favourably with the unique mode and tempo of the ice‐age climate. To develop this argument, we first describe the differences between the climates of the ice age and the Holocene, and then review the eco‐physiological implications of large body size in terrestrial mammals.
(a). A fundamentally different ice‐age climate
Ice‐age climate fluctuated dramatically in response to two different millennial‐scale drivers: Dansgaard–Oeschger (D‐O) and Heinrich events (Wolff et al., 2010) (Fig. 6). Between ∼ 60 and 11.7 ka, six Heinrich events occurred with a periodicity of about 7000 years (Hemming, 2004), and some 17 D‐O events occurred with periodicities of 1500–2000 years (Long & Stoy, 2013). Individual Heinrich events began over the space of several years to a decade and varied in duration from 200 to 2300 years (Hemming, 2004). In Greenland, D‐O events began with rapid warming lasting several decades (Capron et al., 2010; Rasmussen, Thomsen, & Moros, 2016). After several decades to several centuries of maximum warmth, gradual cooling then set in, concluding with a rapid return to full glacial conditions (Wolff et al., 2010; Rasmussen et al., 2014). Increasingly precise palaeo‐environmental chronologies have also identified sub‐millennial climate fluctuations occurring between and during D‐O events (Capron et al., 2010; Milner et al., 2013, 2016).
Figure 6.
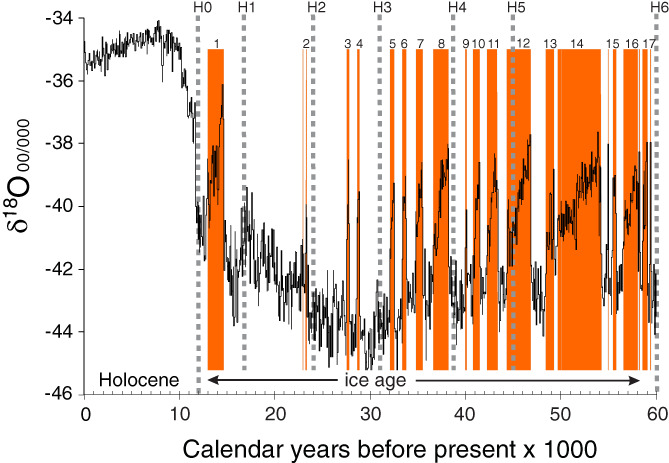
Changing δ18O concentrations in the NGRIP ice core are a proxy for air temperature over Greenland. Up is warmer in the graph, down is colder. The full amplitude of the change in mean annual air temperature between the ice age and the Holocene was about 20°C in Greenland. Since ice‐core records like this were first reconstructed (Dansgaard et al., 1993), it has been clear that the ice‐age climate had a fundamentally different mode and tempo than the Holocene. The x axis is in b2k‐calendar years (years before AD 2000). The δ18O data are from Rasmussen et al. (2014) and Seierstad et al. (2014). Timing of Dansgaard–Oeschger (D‐O) events (orange bars) is from Rasmussen et al. (2014); timing of Heinrich events (dashed lines) is from Hemming (2004).
Because so much palaeoclimatic research has been concentrated at middle and high latitudes, particularly in the North Atlantic region, the most detailed information we have about millennial climate changes comes from there. Nonetheless, the climatic impacts of D‐O and Heinrich events affected climate systems globally, in many cases with magnitudes comparable to those observed in the North Atlantic region (Wang et al., 2001; Grimm et al., 2006; Kanner et al., 2012; Denniston et al., 2013).
During Heinrich and D‐O events, mean annual temperatures in Greenland fluctuated by 5–17°C (Huber et al., 2006; Kindler et al., 2014), which was approximately half of the temperature difference between full glacial and full interglacial climate. Reconstructions of mean annual temperature based on the remains of beetles living in northwest Europe during D‐O events suggest similar temperature changes there (Atkinson, Briffa, & Coope, 1987; Walker, Coope, & Lowe, 1993; Ponel et al., 2005). Sea surface temperature (SST) in the North Atlantic varied by 3–8°C (Martrat et al., 2007; Maslin et al., 2014). In Norway and Switzerland, cooling in summer during the Younger Dryas, the last of the millennial‐scale cold events, was 3–4°C (Birks & Ammann, 2000). In southern France, Ampel et al. (2010) inferred only 0.5–2°C changes in July temperature between the warm and cold parts of D‐O events; however, in southern Italy D‐O events may have triggered changes of 20°C in winter (Allen et al., 1999). Though less well‐constrained than temperature, moisture availability also changed radically in many parts of the world during D‐O and Heinrich events (Wang et al., 2001; Benson et al., 2003; Shakun et al., 2007; Wang et al., 2007; Tierney et al., 2008; Ayliffe et al., 2013; Deplazes et al., 2013; Stockhecke et al., 2016). Compared to the late Pleistocene, Holocene climate changes across much of the planet have been markedly less frequent and less extreme, except in places like the drylands of Africa (Gasse, 2000).
(b). Ecological implications of ice‐age climate instability
The only constant feature of the ice‐age environment in many parts of the world, including low latitudes, was rapid and repeated change (Roy et al., 1996) (Fig. 6). Through their effects on precipitation, D‐O events altered wildland fire regimes in Iberia (Daniau et al., 2007) and North America (Fischer et al., 2015). In both southern Europe and Florida, D‐O and Heinrich events caused rapid (<240 year) alternation between forest and non‐forest vegetation (Allen et al., 1999; Grimm et al., 2006; Wohlfarth et al., 2008). In unglaciated regions of the Arctic, millennial‐scale climate changes were accompanied by population bottlenecks among megafaunal species (Mann et al., 2015). Records of vegetation history during post‐glacial times demonstrate that temperature changes with magnitudes similar to D‐O and Heinrich events caused biogeographical reorganization across much of North America (Williams et al., 2004; Fischer et al., 2015).
When climate changes rapidly, some species cannot keep up, resulting in ecological communities whose species compositions are not in equilibrium with climate (Davis, 1986; Huntley et al., 2013; Svenning & Sandel, 2013; Blonder et al., 2015; Svenning et al., 2015). These disequilibrial communities can result from slow rates of dispersal and/or delays in primary succession. Slow rates of soil development can delay primary succession by decades to centuries (Matthews, 1982). For instance, near the Klutlan Glacier in the Yukon, soil development was still proceeeding apace 250 years after deglaciation (Jacobson & Birks, 1980), and in Arctic Alaska, the development of a steady state, organic soil horizon requires over 500 years (Baughman et al., 2015). Even in temperate rainforests, soil development continues more than 5000 years after deglaciation (Klaar et al., 2015). Given these lags involving soil development, primary succession is unlikely to have reached its end point in many landscapes during the millennial‐scale climate fluctuations of the ice age (Svenning et al., 2015).
Lags in the dispersal of plant species add to delays in community assembly entailed by primary succession, and rapid climate changes during the ice age often exceeded the ability of tree species to keep up (Bennett et al., 1991). In post‐glacial times, the etablishment lags of different plants have varied from a few decades (Tinner & Kaltenrieder, 2005) to several millennia (Ammann et al., 2013). In post‐glacial times, the dispersal rates of most temperate‐zone tree species have been < 250 m year–1 (Feurdean et al., 2013) and in some cases <100 m year–1 (Svenning & Skov, 2007; Bradshaw, Kito, & Giesecke, 2010). As a result of these slow dispersal rates, even within the Holocene's 11700 years span of comparatively stable climate, numerous plant species have lagged several millennia or longer behind their climatic range limits in regions formerly buried by ice sheets (Johnstone & Chapin, 2003; Fang & Lechowicz, 2006; Tollefsrud et al., 2008; Gavin, 2009; Bradshaw et al., 2010; Elias, 2013; Svenning & Sandel, 2013). The key point here is that during the ice age few plant communities had sufficient time to assemble into equilibrial states before the climate changed again (Huntley et al., 2013).
Ice‐age animal communities also were frequently out of equilibrium (Graham & Lundelius, 1984). A reduced degree of latitudinal zonation during the ice age compared to the Holocene suggests that disequilibrial communities were widespread in Europe (Hofreiter et al., 2004). Similarly, boom and bust cycles of megafauna in Arctic Alaska imply disequilibria on the mammoth steppe (Mann et al., 2015), as do the frequent range shifts and population bottlenecks that ancient DNA (aDNA) documents for a number of mammal taxa (Shapiro et al., 2004; Enk et al., 2016; Chang et al., 2017; Froese et al., 2017).
Certain types of disequilibrial ecosystems may have favoured megafaunal herbivores precisely because they were transient. Early successional plant communities dominated by forbs and graminoids are capable of supporting dense populations of megafaunal herbivores (Owen‐Smith, 1988; Guthrie, 1990). One reason for this is that immature soils tend to be more fertile than older, more highly weathered ones (Huston, 2012). For example, soils in the drier, short‐grass region of the Serengeti have developed on recent volcanic deposits. They have higher fertility than the soils in surrounding wetter regions, whose soils are older and more weathered (Dobson, 2009). Today much of our grazing‐based agriculture takes advantage of the high productivity of recently disturbed plant communities and poorly developed soils.
Another reason why early successional communities dominated by forbs and graminoids can be highly productive for megafaunal grazers is because these communities are dominated by ruderal plant species. Weed species are often geographically widespread, complacent to climatic gradients, and masters of dispersal (Radosevich, Holt, & Ghersa, 1997). Many of them are minimally invested in anti‐herbivore defences, relying instead on fertile soils, transience, and wide geographic ranges to assure their persistence. These characteristics enabled them to thrive in the disequilibrial communities widespread during the ice age.
Frequent and large‐scale climate changes were not the only disturbances responsible for the plaid ecosystems of the ice age. Geomorphic disturbances associated with fluctuations of the cryosphere disrupted ice‐age soils and vegetation. At the peak of the last glacial maximum approximately 21–18 ka, glaciers covered roughly 1/4 of the northern hemisphere (Ehlers, Hughes, & Gibbard, 2016) and repeatedly fluctuated in extent (Dyke, 2005). Around ice‐sheet margins, broad regions were affected by shifting outwash streams, sand sheets, sand dunes, and rapidly accumulating loess. Low‐latitude regions of Africa, South America, southern Eurasia, and Australia also experienced repeated episodes of geomorphic disturbance caused by shifts in precipitation and temperature. Transfers of water between oceans and glaciers, coupled with isostatic adjustments of the crust beneath ice sheets, caused changes in relative sea level (RSL) (Grant et al., 2014), which alternately exposed and flooded millions of square kilometers of the continental shelves.
An idea of the magnitude of the impacts that ice age climate changes had on the global biota is gained by superimposing ice‐age temperature changes onto ordinations of modern biomes and climate (Fig. 7). We can also roughly estimate the distances organisms would have had to disperse in order to keep up with ice‐age climate. Today, mean annual temperature varies by approximately 1°C per degree latitude in the northern hemisphere. Since a degree of latitude corresponds roughly to 100 km, a 5–10°C warming over the first few decades of a D‐O cycle would cause isotherms to shift 500–1000 km poleward in a region of low topographic relief. Then the cold phase of the cycle would push the isotherms equatorward across a similar distance. Because of the slow rates of tree migration (Svenning et al., 2015), many of the plant species that determined vegetation structure, and hence the identity of biomes, may have had difficulty keeping up.
Figure 7.
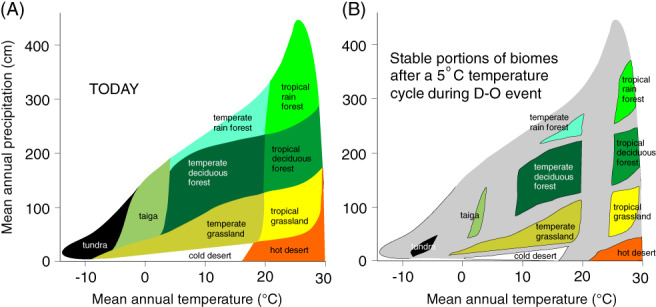
von Humboldt's nightmare: Only the core areas of biomes familiar to us during the Holocene (A) would have remained stable during the ice age (B) when millennial and sub‐millennial climate changes generated widespread ‘weed zones’ in which plant communities may never have had time to equilibrate with climate. We hypothesize that megafaunal species specialized in exploiting the transient, but often highly productive, weedy plant communities flourishing in the grey zones. D‐O is a Dansgaard–Oeschger event. Biome diagram redrawn from Whittaker (1970).
(c). The eco‐physiological correlates of body size: big is best in plaid
The ice ages' unique climate regimes created adaptive milieux that in many places were very different from those existing during the Holocene (Lister, 2004). In what ways might unstable climate and ecological disequilibrium favour megafaunal species? Numerous physiological, ecological, behavioural, and life‐history traits scale with body mass (Schmidt‐Nielsen, 1984) according to allometric relationships in the form Y = aM b, where Y is some biological variable such as metabolic rate or population density, M is body mass, a is an empirically derived constant, and b is the scaling exponent (Brown et al., 2004). Being large puts species at the extremes of several eco‐physiological niche axes (Fig. 8), and certain of these allometric extremes may be highly adaptive within plaid landscapes.
Figure 8.
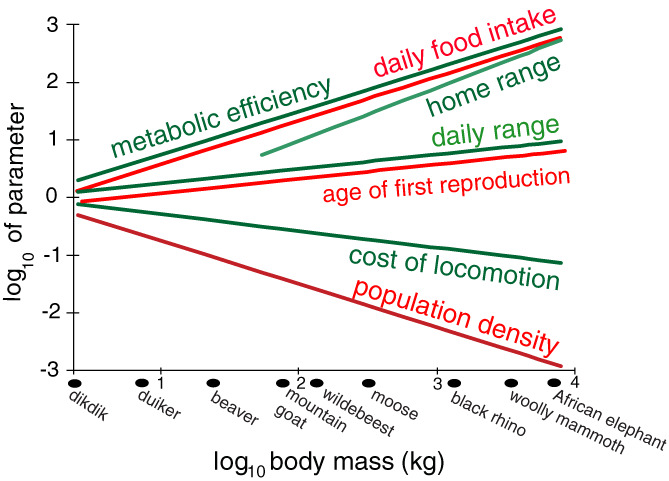
Allometric trends in ecophysiological traits associated with body mass among terrestrial mammals. Extreme differences exist in these traits between large and small species. For example, the African elephant and dikdik, a diminutive African antelope, differ by 1000‐fold in some traits. Because megafaunal species have greater locomotory efficiency, greater metabolic efficiency, increased resistance to starvation, longer life spans, and greater sensory ranges, they are able to exploit transient resource bonanzas widely scattered in space. These advantages come with trade‐offs including smaller population sizes, slower reproduction rates, and commitment to the life strategy of coping with local food shortages by dispersing to greener pastures.
(i). Greater metabolic efficiency
Larger animals are metabolically more efficient than smaller ones in terms of energy use per unit body mass (White & Seymour, 2005). Kleiber's Law states that basal metabolic rate (q 0) scales as q 0 ∼ M 0.75, although the exact value of the scaling exponent and its physical basis are still debated (West, Brown, & Enquist, 1999; Glazier, 2005). Food intake also scales as M 0.75 (Clauss et al., 2007). Hence instead of being 1000 times larger, the food requirements of a 5000 kg elephant (Loxodonta africana) are only 180 times larger than those of a 5 kg dikdik (Madoqua kirkii). Daily metabolic expenditure, which includes the cost of physical activity, also scales (although with a different normalization constant, a) as M 0.75 (Owen‐Smith, 1988), as do daily requirements for nitrogen and minerals (Müller et al., 2013). Thus, assuming no food limitations, the larger the mammalian herbivore, the more efficient it is at converting plants into animal tissue.
(ii). Broader diets, more‐diverse habitats, greater resiliency
Enhanced metabolic efficiency gives megafaunal herbivores several advantages over smaller species. Because their profit margin is higher, they can subsist on less nutritious forage (Müller et al., 2013). For instance, African elephants can subsist on a wider range of foods than the diminuitive dikdik, including fruits, dry leaves, grass stems, bark, and roots (Dobson, 2009). Their size‐derived metabolic advantage may be why, in the African savanna, the population metabolisms (mean population density × mean body mass × mass‐specific metabolic rate) of large herbivores like white rhinoceros (Ceratotherium simum) and elephant scale with body mass as M 0.45, but scale as M 0.65 among small herbivores like Thompson's gazelle (Eudorcas thomsonii) (du Toit & Owen‐Smith, 1989). As a result, effective food density can be higher for large herbivores than small ones living in the same landscape. This in turn implies that the habitats of megafaunal species may be more continuously distributed, with fewer unsuitable lacunae (Owen‐Smith, 1988). The tendency for larger species to exploit a wider range of resources holds for carnivores as well (Sinclair et al., 2007). Higher effective food density and broader habitats translate into greater resiliency of megafaunal populations to environmental challenges like droughts (Owen‐Smith, 1988). Overall, the greater metabolic efficiency of megafauna translates into a wider dietary breadth, a wider range of habitats, and possibly a heightened resiliency in the face of environmental change.
(iii). Large body size increases fasting endurance
The amount of stored fat increases with increasing body size in mammals as M 1.2, while survival time under starvation scales as M 0.44 (Lindstedt & Boyce, 1985). Overall, the maximum time between necessary refueling is about 200 times longer for an elephant than a mouse (Mus musculus), and this scaling of fasting endurance becomes even more pronounced at colder temperatures in accordance with Bergmann's Rule (Meiri & Dayan, 2003). Thus, larger mammals can persist better than smaller ones through extreme weather events that might limit feeding. They also can travel longer distances without food while seeking greener pastures.
(iv). Larger species have larger home ranges
Among mammals, home range size (km2) scales roughly with body mass (kg) as a 1:1 relationship (Jetz et al., 2004). Using only the body masses of mammals weighing > 0.1 kg, Burness, Diamond, & Flannery (2001) found that mass scales as 673 × home range 0.50. The body‐mass/home‐range relationship is complicated by the fact that ranges expand where/when primary productivity is low and contract where/when food is more plentiful (Owen‐Smith, 1988). The functional importance of the body‐size/home‐range relationship is poignantly illustrated by the decline of large mammal populations as African preserves are progressively isolated by human activities (Newmark, 2008).
(v). Bigger species are often most mobile
Given their large home ranges, it is no surprise that megafaunal species are the most mobile of non‐volant land animals (Sutherland et al., 2000). Species with larger body masses move about their home ranges more quickly than smaller species (Swihart, Slade, & Bergstrom, 1988). The day range, the distance an animal typically moves in the course of its daily activities, scales with body mass as M 0.25 (Carbone et al., 2004). Species with the largest home ranges also engage in the longest dispersals (Bowman, Jaeger, & Fahrig, 2002), and megafauna tend to walk faster than microfauna while engaged in day‐to‐day activities.
(vi). Bigger animals move more efficiently but at a higher net cost
The energetic cost of running in terrestrial animals scales as M –0.28 (Peters, 1986). Thus a 30 g rodent uses roughly 13 times the energy to move each gram of its weight as a 100 kg pony does over the same distance (Taylor & Heglund, 1982). Interestingly, once mammalian body mass exceeds ∼10 kg, the metabolic cost of running locomotion is about the same as the most efficient flight in birds. The energy savings achieved by larger mammals occur because the cost of transport scales as limb length–0.77 (Pontzer, 2007). On the other hand, the absolute cost of moving a larger body increases steadily as mass increases (Halsey, 2016), so the benefit of the enhanced efficiency of movement for megafauna is only accrued if food is not limiting.
(vii). Larger animals live longer, but reproduce later and more slowly
Both generation length and age of first reproduction scale as M 0.25 (Owen‐Smith, 1988; Brook & Bowman, 2005; Healy et al., 2014). While generation time and age of first reproduction increase with body mass, maximum rates of population increase decline according to M –0.25 (Fenchel, 1974; Fagan, Lynch, & Noon, 2010). These diverging trends have serious implications for the largest species in an ecological community that is suddenly exposed to a new source of mortality; large‐bodied species will probably have lower demographic resilience (Brook & Bowman, 2005), and in fact, low reproductive rates are correlated with increased probability of extinction during the Late Quaternary (Johnson, 2002).
(viii). Big animals are often rare with fewer subpopulations
Their larger home ranges imply that larger species living on continents have lower population densities. The general relationship between population density (D) and body mass (M) among large terrestrial mammals is D∝ M –0.75 (Brown et al., 2004). This amounts to a 5–6‐fold decrease in population density for every tenfold increase in body mass. While statistically significant, the relationship between population density and body mass contains considerable noise (Currie & Fritz, 1993). Where it has been studied among birds, the number of subpopulations scales to total population size (N) as N 0.75 (Keitt et al., 2002), suggesting that metapopulations of large‐bodied species contain fewer subpopulations.
(ix). Larger, rarer species are more prone to population fluctuations
As described by Taylor's Law, the rarer a species is, the more its population size varies through time (Keitt et al., 2002). This power law takes the form S 2 = aμ b where a is a sampling parameter, b is an index of the aggregation characteristics of the species, s 2 is the variance in population size, and μ is the mean. Empirical studies show the value of b varies between 1 and 2 (Taylor & Heglund, 1982).
(x). Adult megafauna often escape predation
The larger you are, the fewer predators you have. On the Serengeti, ungulate species weighing < 150 kg are vulnerable to more predator species than are larger ungulates (Sinclair et al., 2007). As a result, in many ecosystems, large‐bodied ungulates tend to be controlled from the bottom‐up by food supply rather than from the top‐down by predation (Mduma, Sinclair, & Hilborn, 1999; although see Ripple & Van Valkenburgh, 2010). In some situations, the vulnerability of young animals to large predators negates this trend (Grange et al., 2004).
(xi). Long‐distance movements are frequent among megafauna
In ecosystems where the quantity and quality of forage varies widely through time, some species move long distances tracking food supply (Fryxell, Greever, & Sinclair, 1988; Singh et al., 2010). These movements fall into two overlapping categories: nomadic behaviour and migration (Mueller et al., 2011). Migration is the seasonal, round‐trip movement of animals between discrete areas, while nomadism involves opportunistic and unpredictable wandering.
Most migratory and nomadic mammal species are large‐bodied ungulates. In today's world, 24 terrestrial mammal species weighing > 20 kg are migratory, all of whom are ungulates, of which 14 live in Africa (Harris et al., 2009). Globally, 17 of the 24 migratory species recognized by Harris et al. (2009) track seasonal shifts in the location of nutritious forage. In the Serengeti, millions of wildebeest (Connochaetes taurinus) and zebra (Equus burchellii) move into the drier, short‐grass area of the plains during the rainy season to exploit a flush of grass with high nitrogen content (Holdo, Holt, & Fryxell, 2009). As the dry season develops, they migrate to areas where green foliage persists year‐round, albeit at lower nutrient levels (McNaughton, 1990; Dobson, 2009). In Africa, family groups of elephants engage in episodic long‐distance movements in response to droughts and overgrazing (van Aarde & Jackson, 2007). Prior to their near‐extinction, many buffalo (Bison bison) led nomadic lives following greener pastures across the Great Plains of North America (Hart, 2001).
Today the movements of migratory and nomadic ungulates are increasingly constrained by people, but in recent times some of these movements were extensive. Saiga (Saiga tartarica) once migrated some 2000 km between the Volga River and Macedonia (Harris et al., 2009), and goitered gazelles (Gazella subgutturosa) once roamed 500–700 km year–1 across western Eurasia. Grevy's zebra (Equus grevyi) still move hundreds of km year–1 in response to droughts in northern Kenya (Harris et al., 2009). Some caribou (Rangifer tarandus) and Mongolian gazelle (Procapra gutturosa) routinely travel 1000 km year–1 during their migrations (Mueller et al., 2011).
(xii). Larger animals often have larger sensory ranges
To be successful at large spatial scales within a milieu of environmental change, successful herbivores need the high mobility afforded by large body size along with the ability to sense, learn, and remember complex landscapes (Owen‐Smith, Fryxell, & Merrill, 2010). In grazing ecosystems, the amount and nutritional quality of forage change continually through space and time. Because green flushes of young vegetation provide the highest nutrition, grazing species track these greener pastures, and they do so with an uncanny ability (McNaughton, 1985). For instance, wildebeest in the Serengeti seem to be able to compare grazing opportunities across distances of >80 km (Holdo et al., 2009).
(xiii). Being big could make you more responsive to climate change
Among mammals, body size is a strong predictor of responsiveness to climate change (McCain & King, 2014). There are multiple reasons for this, including the fact that climate grain size, mobility, and rarity all scale with body size. A positive correlation between extinction probability and body mass exists for late‐Quaternary extinctions (Lyons, Smith, & Brown, 2004; Lyons et al., 2016). Polishchuk (2010) found that the probability of extinction among terrestrial mammals scales with population density as D 0.75, which in turn scales with body mass as M 0.75.
In summary, megafaunal species tend to have higher metabolic and locomotory efficiencies, larger home ranges, greater resistance to starvation, longer lives, an ability to exploit a wider range of habitats and food types, and greater sensory ranges. Because of their mobility and various efficiencies, the vast majority of terrestrial animals engaging in long‐distance migration or nomadism in today's biologically impoverished world are megafaunal ungulates. Larger body volume, heightened metabolic efficiency, and tolerance for poorer forage give megafaunal species additional advantages in cold habitats where seasonal food shortages occur (Meiri & Dayan, 2003). These eco‐physiological traits associated with large bodies may have preadapted megafaunal species for efficiently tracking habitats that were continually shifting in response to the rapid environmental changes of the ice age.
In a striped environment, many of the advantages of being megafaunal disappear. Because ecotones are more stable, long‐distance tracking of shifting patches of suitable habitat is no longer needed. Where food resources are limited, the need for large amounts of food tends to negate metabolic efficiency. The ability to travel fast and efficiently become irrelevant when home ranges shrink, as does the ability to be nomadic or migratory. Being able to remember and learn is moot if environmental changes are few and the landscape remains in a stable, striped configuration. Simultaneously, the relatively small population sizes, low population densities, and slow reproductive rates associated with large body mass can reduce resilience in response to population bottlenecks. The probability of extinction varies inversely with population density, so the threat of extinction increases as ranges shrink when plaid landscapes shift into striped ones. By contrast, small‐bodied species are comparatively adept at coping with minor shifts in climate by tracking favoured microclimates over short distances while maintaining sizable populations within small areas (Ehrich & Stenseth, 2001). We suggest that the shift from widespread plaid landscapes to widespread striped ones at the end of the ice age lowered the extinction thresholds for many megafaunal species, making them more vulnerable to a myriad of proximate extinction causes (Fig. 3).
(2). Megafaunal extinctions during previous glacial–interglacial transitions
If the transition between two climate regimes possessing different modes and tempos of environmental change was a widely shared cause of Late Quaternary megafaunal extinctions, why didn't similar bouts of extinctions occur at the outsets of previous interglacials? After all, glacials and interglacials have alternated over the last 2.6 Ma, yet no clear evidence exists for bouts of megafaunal extinction during earlier interglacials in either Eurasia or North America (Stuart, 1991).
We suspect the apparent absence of megafaunal extinctions during earlier glacial‐interglacial transitions has three causes. The first relates to the Plaids and Stripes Hypothesis, namely that the climate of the early Holocene may have been more ‘stripe conducive’ than it was during the opening millennia of previous interglacials. The second concerns the fossil record, namely that limited temporal and taxonomic resolution make it difficult to quantify extinction rates during ancient interglacials. The third cause involves the presence/absence of humans, namely that people were absent from Australia, North America, and South America (the three continents where late Quaternary extinctions were most numerous) during all previous interglacials, but were present when the Holocene began. As detailed below, it is unlikely that any one of these explanations is sufficient on its own, but together they may explain the absence of evidence for megafaunal extinctions during earlier glacial–interglacial transitions.
Taken on its own, the Plaids and Stripes Hypothesis would suggest that more megafaunal extinctions occurred during the early Holocene than previous interglacials because the Holocene had an unusually stripe‐conducive climate. We currently lack the detailed chronological control and palaeoecological data from older glacial–interglacial transitions to definitively test this prediction. However, we do know that the climates of past interglacials varied widely (Tzedakis et al., 2009; Herold et al., 2012), mainly because their Milankovitch orbital drivers differed (Shakun et al., 2015; Yin & Berger, 2015), which caused markedly different trajectories of climate change (Fig. 9). The time intervals traditionally considered ‘interglacials’ often had complex temporal structures that in some cases included multiple ‘glacial’ interludes (Lang & Wolff, 2011). There have been numerous interglacials, but none possessed a climate regime exactly like the Holocene's.
Figure 9.
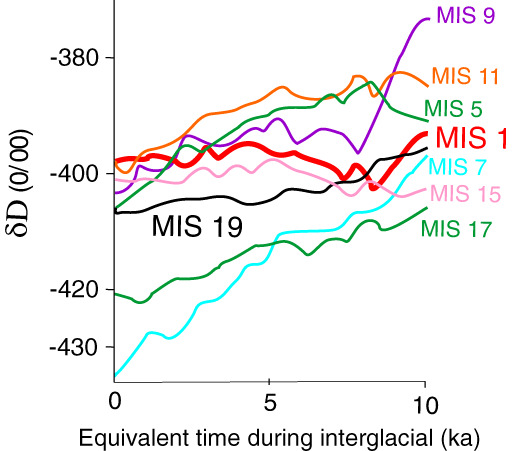
δD (deuterium/hydrogen) ratio records of air temperatures over Antarctica (Jouzel et al., 2003). δD levels have remained more constant during the Holocene (MIS 1) than during most other interglacial periods. Orbital forcing during MIS 19 (ca. 790–760 ka) was the most similar to the Holocene's, and MIS 15 (ca. 620–560 ka) has the most similar pattern of deuterium concentration, so it would be of interest to know what was happening to megafaunal species during these two interglacials (redrawn from Ruddiman et al., 2016).
An interesting illustration of the diversity of interglacial climates is provided by comparing the Holocene with the last interglacial, MIS 5e, the interglacial we know the most about because it is closest to us in time (Fig. 10). During MIS 5e, peak warmth occurred over a 5000‐year interval when global mean temperature was warmer than the Holocene's. Besides lasting a shorter time, MIS 5e differed from the Holocene in the mode and tempo of its climate changes. Atmospheric methane (CH4) concentrations declined after a peak lasting < 2000 years, and air temperatures cooled after <1000 years (Barker et al., 2011). By contrast, CH4 concentrations during the Holocene have followed a complicated trajectory involving multiple peaks that together have spanned > 10000 years. These comparisons illustrate the climatic diversity of interglacials by showing that in many regions of the world climate was more variable during MIS 5e than during the Holocene (Milner et al., 2013; Hansen et al., 2015).
Figure 10.
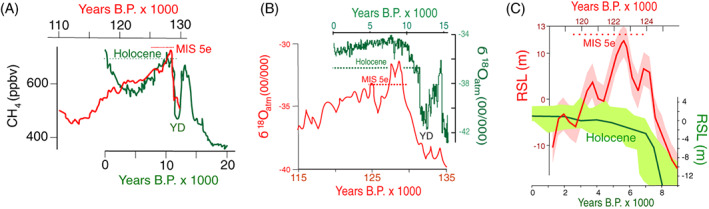
The mode and tempo of climate change during the last interglacial period, MIS 5e, (dashed horizontal red lines) was more like the ice age than the Holocene (dashed horizontal green lines). (A) Atmospheric methane concentration from the EDC ice core from Dome C in East Antarctica on the EDC3 timescale. YD is the Younger Dryas. Redrawn from Tzedakis (2010). (B) Holocene and last interglacial δ18O records from Greenland ice cores. The Holocene data are from the NGRIP core plotted on the GICC05 time scale (Rasmussen et al., 2014). The last interglacial time series is the Greenland synthetic record whose timescale is based on alignment with Chinese speleothem records (Barker et al., 2011). Redrawn from Tzedakis et al. (2012). (C) Relative sea level (RSL; red line) in the Red Sea during the warmest part of the last interglacial. The pink error envelope is ±6 m. The Holocene RSL curve (green line) is redrawn from a compilation by R.A. Rohde based on Fleming et al. (1998) and Milne, Long, & Bassett (2005). The error envelope joins the extremes of estimated errors.
Records of RSL tell a similar story of millennial‐scale instability of climate during MIS 5e (Rohling et al., 2008; Thompson et al., 2011). At sites distant from ice sheets, changes in RSL record fluctuations in the volumes of terrestrial ice sheets (Hearty et al., 2007), which in turn are controlled by climate. During the Holocene, rising RSL stabilized approximately 7 ka (Fig. 10C) and since then has risen slowly by 3–5 m in regions distant from former ice sheets (Peltier & Fairbanks, 2006). By contrast, RSL fluctuated by 6 m within one millennium or less during MIS 5e. Like the brief peaks of CH4 and δ18O during MIS 5e, RSL maintained its highest level for < 2000 years during MIS 5e (Rohling et al., 2008; NEEM, 2013; Hansen et al., 2015). Together, these data suggest the climate of the last interglacial was more plaid‐conducive than the Holocene's climate has been.
In addition to differences in Milankovitch forcing, the Holocene climate may be unusual because of anthropogenic effects that have imbued it with anomalous stability beginning in the early Holocene (Ruddiman, 2003, 2007, 2014). Human impacts on greenhouse processes may also have prolonged the present interglacial by several thousand years (Ruddiman et al., 2016), thus delaying the onset of ice‐sheet growth (Vavrus, Ruddiman, & Kutzbach, 2008; Vavrus et al., 2011) and the resumption of millennial‐scale climate fluctuations. It is possible that without the anthropogenic delaying of the next ice age, perhaps the woolly mammoths surviving on Wrangel Island until 4 ka would have had time to repopulate the boreal region (Fig. 11).
Figure 11.
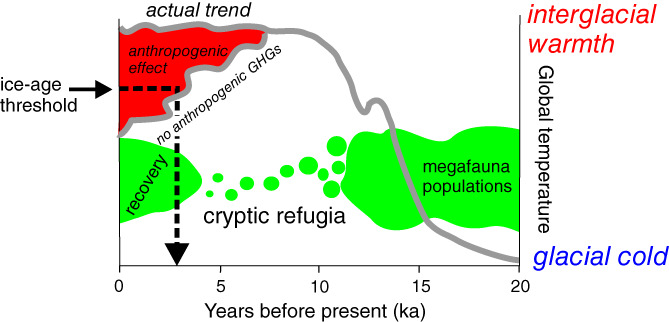
The trajectory of Holocene climate with and without human influences. Orbital changes triggered deglaciation starting approximately 20 ka and determined when the present interglacial began, but human agriculture and land clearance began to modify global climate in the early Holocene (Ruddiman, 2014). Without these anthropogenic greenhouse effects, the Holocene climate might already be cooling into the next ice age (Ruddiman et al., 2016). Shown in green are hypothetical responses of megafauna to a Holocene climate in the absence of anthropogenic greenhouse gases (GHGs). Like the woolly mammoth, many megafaunal species retracted into cryptic refugia as climate warmed, stabilized, and caused landscapes to shift from ice‐age plaids into striped configurations. If the Holocene had ended on schedule, some megafauna – possibly including woolly mammoth – might have re‐expanded from their refugia when the plaid world was reinstated by the return of ice‐age climate. Climate trends redrawn from Ruddiman et al. (2016).
A second reason for the lack of evidence for bouts of megafaunal extinction during previous glacial–interglacial transitions may be the limited temporal and geographical resolution of fossil records from older interglacials compared to the Holocene. For instance, there are <40 fossil vertebrate sites in North America pre‐dating the last ice age (>80 ka), compared to thousands from postglacial times (<15 ka) (Bell et al., 2004; Barnosky et al., 2004a). A similar disparity exists in Europe (Stuart, 1991; Schreve, 2001; Markova & Vislobokova, 2016), and it is even more pronounced in regions like Siberia and Alaska where Late Quaternary mammal fossil localities are legion, but only a handful of mid‐ and early Pleistocene ones are known.
An additional challenge when assessing extinction rates during previous glacial–interglacial transitions is the fact that chronological precision is often much less than ±10000 years in fossil deposits older than the upper limit of 14C dating (ca. 45 ka) (Bell et al., 2004). This makes it difficult to identify glacial–interglacial transitions precisely and to correlate megafaunal remains with the marine and ice‐core records that the glacial–interglacial chronology is based upon (Price et al., 2018). Fossil records from ancient interglacials like MIS 19 (790–760 ka), the interglacial whose orbital forcing and isotopic records most closely resemble the Holocene's (Yin & Berger, 2015), are too sparse and imprecisely dated to reject the possibility that population bottlenecks and heightened rates of megafaunal extinctions also occurred then.
Lack of taxonomic resolution in the fossil record adds to the difficulty of detecting bouts of megafaunal extinction in the deep past. Late Quaternary extinctions are usually quantified in terms of genera rather than species because of uncertainties in the species‐level taxonomy of many specimens (Stuart, 1991; Grayson, 2016). We suggest that the limited temporal, geographical, and taxonomic resolution in the fossil record make it impossible to say for certain that rates of megafaunal extinction did not increase during previous glacial–interglacial transitions compared to extinction rates during intervening glacial periods. That said, even if rates of megafaunal extinction did increase at the outsets of certain older interglacials, they certainly failed to reach the intensity of the Late Quaternary extinctions in the New World when entire higher taxa (e.g. proboscideans, equids, ground sloths) became extinct. Quantifying extinction rates during ancient glacial–interglacial transitions – perhaps by using DNA extracted from lake sediment – would be a major contribution to the megafaunal extinction debate.
A third contributing reason why more megafaunal extinctions occurred during the Pleistocene–Holocene transition than during earlier glacial–interglacial transitions may relate to the timing of human dispersal across the planet. On the three continents where late Quaternary megafaunal extinctions were most numerous (Australia, South America, and North America), people were absent during all previous interglacials except the last one. On these three continents, human impacts during a time of plaid to stripe transition may have been a key contributor to megafaunal extinctions.
In summary, we suspect three factors jointly explain the apparent lack of megafaunal extinctions during earlier interglacials. The first relates to the Plaids and Stripes Hypothesis in that the tempo and magnitude of climate change varied widely between different interglacials, and some interglacials were probably more conducive to plaid‐ness and hence to megafaunal survival than the Holocene has been. This possibility remains to be tested. The fading of the fossil record into the past may also have contributed, in particular the increasing scarcity of fossils and the declining precision of dating control beyond the limit of the 14C method. Another contributing factor may have been the absence of humans during earlier interglacials on the three continents where megafaunal extinctions were most frequent in Late Quaternary times.
(3). Australia's staggered extinctions
Unlike the Americas, where megafaunal extinctions were seemingly concentrated at the end of the last ice age, in Australia they began earlier in the Pleistocene and continued intermittently until approximately 40 ka (Wroe & Field, 2006; Wroe et al., 2013). This staggered schedule of extinctions is consistent with the Plaids & Stripes Hypothesis in that the adaptive advantages of megafauna within plaid environments are negated if aridification drives primary productivity too low. If there are no greener pastures anywhere on the continent, even the most wide‐ranging megafauna suffer.
Australia's physical geography has unique characteristics that make megafauna's ice‐age advantages susceptible to being trumped by aridity. Australia is the smallest, lowest, oldest, and tectonically most inactive continent, and as a result many of its soils are relatively infertile (Raupach et al., 2001; Owen‐Smith, 2013). Because of its low altitude, subdued topography, and location in the subtropical high‐pressure zone, Australia has a relatively simple climatic geography. Despite their similar surface areas, today Australia contains only 12 Köppen climate types, while the conterminous USA contains 21 (Peel, Finlayson, & McMahon, 2007).
A unique climate history has accentuated Australia's geographic features as related to megafaunal extinctions. Australia has experienced increasing aridification over the past 20 Ma as continental drift carried it northward into the subtropical high pressure zone (Wroe & Field, 2006; Byrne et al., 2008). This drying trend sped up after approximately 700 ka and has been particularly pronounced over the last 400 000 years (Nanson et al., 2008; Wroe et al., 2013; Miller et al., 2016). Today, 80% of Australia's land area has a mean annual precipitation (MAP) < 600 mm, and 50% has a MAP < 300 mm. During the ice age, the climate was even drier, resulting in deserts covering much of the continent's interior (Williams, 2001). Even while locked in ice‐age aridity, Australia experienced the millennial‐scale fluctuations that characterized ice‐age climates globally (Kershaw, Bretherton, & van der Kaars, 2007; Moss & Kershaw, 2007; Ayliffe et al., 2013; Denniston et al., 2013).
How did long‐term desertification affect Australia's megafauna? First, Australia's subdued topography and small size could have resulted in less ice‐age plaidness than in comparably sized areas of North America or Africa. Second, reduced primary productivity during times of ice‐age aridity may have negated the ecophysiological advantages of being large bodied. In dryland regions of Africa today, herbivore biomass correlates with above‐ground primary productivity (Fig. 12) (McNaughton, 1990; Hempson, Archibald, & Bond, 2015), which in turn is controlled by a combination of precipitation (Coe, Cumming, & Phillipson, 1976) and soil fertility (Fritz & Duncan, 1994). In Africa today, the number of ungulate species declines as mean annual precipitation decreases (Faith, 2013). Similarly in Australia today, a significant positive correlation exists between the number of mammal taxa living in an area and the mean annual precipitation there (Faith et al., 2017). Australia is exceptional because during the ice age it experienced aridity bottlenecks that reduced its overall carrying capacity for megafauna to low levels. During these bottlenecks, Australia's biogeographic simplicity ‐ accentuated by its generally infertile soils – may have failed to provide sufficient refugia for megafauna to survive.
Figure 12.
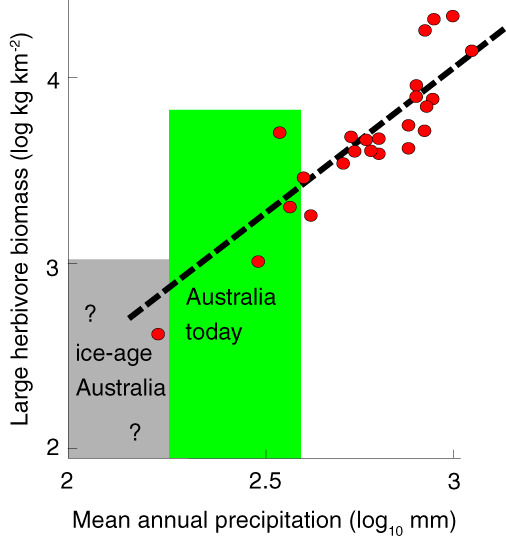
Relationship between rainfall and biomass of mammalian herbivores in wildlife reserves in eastern and southern Africa (redrawn from Coe et al., 1976). Megafaunal herbivores need large amounts of food, and primary productivity by plants depends strongly on precipitation. Soil fertility also plays a role, permitting higher carrying capacities in areas of higher fertility (Fritz & Duncan, 1994). Mean annual rainfall in interior regions of Australia today is shown in green. Much of the Australian continent became hyperarid during the coldest times of ice ages (grey).
(4). The African exception
Another test for the Plaids and Stripes Hypothesis is whether it can help explain why only Africa retains a terrestrial megafaunal assemblage comparable to its ice‐age one (Faurby & Svenning, 2015; Hempson et al., 2015). Undoubtedly there are multiple reasons. Perhaps same set of factors that made Africa a uniquely prolific evolutionary crucible over the last 66 Ma (Werdelin & Sanders, 2010) is somehow responsible for the persistence of megafauna there. A more specific possibility is that because African megafauna underwent lengthy coevolution with hominins, they have been better able to cope with human impacts than have species living on continents only recently colonized by people (Owen‐Smith, 1988). This implies that some megafaunal species in Africa were driven to extinction by early hominins, and those surviving have been evolutionarily and/or ecologically sorted for hominin resistance. Such sorting would have been a prolonged and subtle process, because early hominin population densities were low and their hunting technologies simple. Nonetheless, hominins' ability to exploit food chains at multiple levels was probably established early, and this possibly triggered a variety of trophic disruptions (Ripple & Van Valkenburgh, 2010; see also Estes, Burdin, & Doak, 2016). Unfortunately, evidence for such domino effects is subtle (Estes, 2016), and the fossil record of early hominin interactions with African megafauna is sparse. The study of Werdelin & Lewis (2013) comparing the timing of extinctions with the chronology of human evolution is a start at deciphering the long‐term sorting effects of hominins on the African fauna.
An additional explanation for the African exception is that Africa has preserved more of its ice‐age ‘plaid’ character than other continents. This is the case because Africa contains extensive low‐latitude, semi‐arid landscapes, and these dryland ecosystems are particularly susceptible to millennial‐scale boom‐and‐bust cycles in primary productivity (Marchant et al., 2009). These cycles result from a uniquely African hydrological regime that has persisted in being unstable at millennial and sub‐millennial time scales throughout the Holocene (Gasse, 2000).
Africa's persistent, ecological plaidness during the Holocene ultimately relates to its physical geography. It is the second largest continent and today has the largest area of arid (Köppen‐type B) climate of any continent crossed by the Equator (Peel et al., 2007). Unlike the extensive dryland regions of Australia, active tectonism and volcanism endow Africa with relatively fertile soils (Owen‐Smith, 2013). Dryland regions at low latitudes are particularly sensitive to changes in moisture availability because of the existence of particularly strong feedbacks between vegetation and climate (Brovkin, 2002; Hawkins et al., 2003; Good & Caylor, 2011). Large changes in primary productivity and hence in carrying capacity for megafauna are possible in the drylands of Africa following slight changes in effective precipitation (Huang et al., 2016; Wu et al., 2016). As a result, Africa's extensive savannas and deserts are prone to relatively large shifts in vegetation cover triggered by relatively minor climate changes. Droughts and pluvials have occurred on other continents during the Holocene, but seldom at the scale and frequency as in Africa's drylands (Gasse, 2000).
The climatic causes of Holocene plaidness in Africa's dryland regions relate to the dynamics of the Intertropical Convergence Zone (ITCZ), which controls African monsoon systems (Gasse et al., 2008; Maslin et al., 2014). The ITCZ is unstable at multiple time scales including millennial ones, and this was true both during the ice age and the Holocene (Tierney et al., 2008; Tierney, 2013; Willis et al., 2013). Throughout prehistory, the ITCZ has been strongly affected by the 23000‐year precession cycle. A peak in irradiance during the early and middle Holocene caused monsoonal rains to penetrate further poleward and intensify, greening the Sahara between 11 and 5 ka (Tierney, 2013). This was followed by a dry interval, and then another greening interval along the Sahara's southern margin between 4 and 3 ka (Willis et al., 2013). These greening episodes were accompanied by northward shifts in plant and animal ranges of >500 km (Watrin, Lézine, & Hély, 2009; Drake et al., 2011). Interestingly, some of the millennial‐scale shifts in African precipitation regimes may be caused by Milankovitch drivers unique to equatorial regions (Berger, Loutre, & Mélice, 2006).
If Africa retained its plaidness during the Holocene, why not South America? It too has sizable equatorial and dryland regions. The answer probably lies in differences in the physical geographies of the two continents. South America is significantly smaller, and a larger proportion of its surface area lies at higher latitudes (Fig. 13). The biggest difference is the greater extent of drylands in Africa: about four times the total present in South America today. Presumably, similar differences existed during the ice age as well. In contrast to the plaid‐prone drylands that predominate in Africa, tropical and temperate forests cover most of South America today. Testing this explanation for the differences in megafaunal extinctions between Africa and South America will require detailed, continent‐wide comparisons of hydrological changes and biome shifts between the two continents throughout the Holocene. A hint that megafauna in South America and Africa responded in similar ways to at least the beginning of the Holocene could be the survival of South American megafauna into the early Holocene (Hubbe et al., 2007; Turvey, 2009; Barnosky & Lindsey, 2010, p. 24–26). Perhaps South America's megafauna lingered because plaidness persisted in its low‐latitude savannahs.
Figure 13.
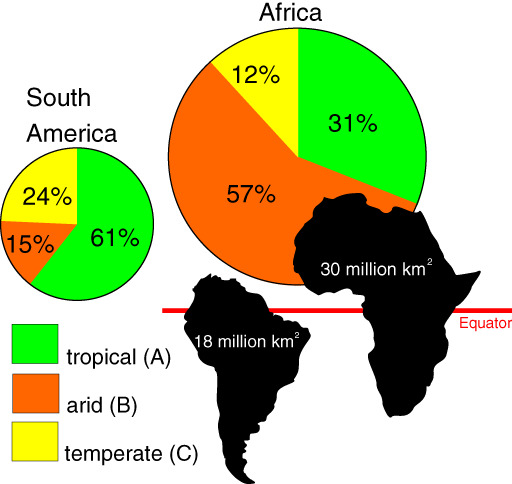
Africa and South America have very different physical geographies, which may have contributed to their different histories of megafaunal extinction. Africa is unique in having extensive dryland regions lying on both sides of the Equator, which are sensitive to major, episodic shifts in vegetation caused by repeated, millennial‐scale climate changes that have maintained extensive plaid landscapes during the Holocene. By contrast, South America is only 60% the size of Africa, lacks arid zones of comparable size, and has a large fraction of its total area lying at higher latitudes. Compilations of Köppen–Geiger climate zones by Peel et al. (2007).
V. PREDICTIONS OF THE PLAIDS AND STRIPES HYPOTHESIS
(1). Opposite bottlenecks
While megafauna coped with the ice age's tumultuous climate by tracking shifting habitats across continents, microfauna – for instance, rodents with limited dispersal abilities – would have been forced to either adapt to changing conditions in situ or be extirpated. It follows that megafauna and microfauna experienced population bottlenecks at different times during the Late Quaternary (Fig. 14). Megafauna are predicted to have flourished during times of rapid change and conditions of maximum plaidness. Their population sizes and degree of genetic panmixis would have peaked during these times. Population bottlenecks accompanied by lineage turnover would have occurred among megafauna during periods of stable climate when their size‐related advantage was less. Microfauna like small rodents should have experienced the opposite trends, with population bottlenecks and lineage turnovers occurring when the climate was changing rapidly. This prediction could be tested using aDNA analyses of precisely dated series of ancient bones belonging to megafauna and microfauna that lived in the same area during the same time interval.
Figure 14.
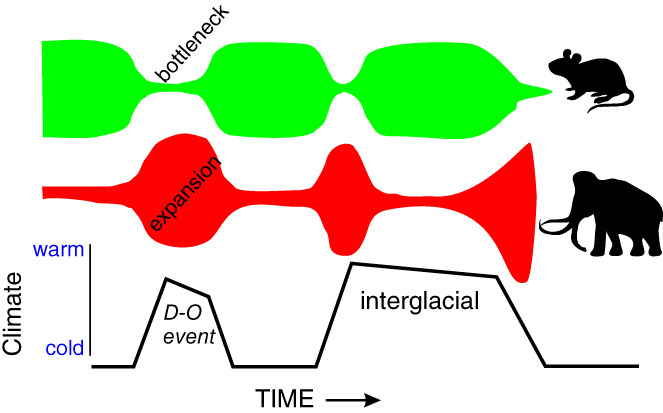
Opposite bottlenecks? The Plaids and Stripes Hypothesis predicts that megafauna flourished during times of rapid climate change, with coincident peaks in their population sizes and the degree of genetic panmixis (indicated by width of coloured lines). Microfauna that were unable to disperse fast enough to keep up with the changing climate would have experienced the opposite trends.
(2). Few megafaunal extinctions on islands without people
We have asserted that the adaptive value of being large changes according to how the eco‐physiological implications of body size mesh with temporal stability and spatial scale. Does this mean that megafauna living on islands always experienced striped landscapes – even during ice ages – and so evolved to conform with such settings? If so, this predicts that, in the absence of humans, megafaunal extinction rates did not increase on islands at the end of the ice age. Interestingly, no wave of late Quaternary megafaunal extinctions has been reported from either New Zealand (Wood et al., 2017) or Madagascar (Burney et al., 2004).
(3). Eternal plaidness for winged beetles?
A landscape that is alternately striped and plaid for a mammoth may always be plaid from the perspective of a dispersal‐prone beetle. In addition to possessing exoskeletons frequently preserved in the fossil record, many Coleoptera species have a high capacity for flight dispersal. Based on his interpretation of the fossil record of beetles, Coope (2004) remarked on the evolutionary stability of beetles compared to other organisms and suggested this resulted from their ability to track favoured habitats across great distances. By virtue of their out‐sized dispersal abilities, dispersal‐prone beetles should see the world as eternally plaid. If this is true, no late Quaternary wave of extinction should have occurred among beetles, a conclusion implicit in Coope's (2004) observations.
VI. SYNTHESIS
We are especially ill‐equipped to think hierarchically, and to juggle simultaneous influences from several nested levels upon the foci of our interests…I seriously wonder if some of the difficulties might not arise from limitations in the common mental machinery of Homo sapiens. Gould (2002, p. 598)
The Plaids and Stripes Hypothesis asserts that a temporal simplification of ecological dynamics accompanying the shift from unstable climates during the ice age to more equable ones during the Holocene contributed to the extinction of many megafaunal species. This happened because ice‐age ecosystems often existed in disequilibrial states as the result of plant and animal species being unable to reach their physiological range limits before the climate changed again. Because ecotones were continually shifting, the ice‐age world was azonal and ecologically plaid. By contrast, Holocene conditions in many regions have tended to be more zonal and ecologically striped. Size‐related ecological, physiological, and life‐history traits made large‐bodied herbivores particularly well suited for coping with the ice age's transient habitats.
The ice‐age advantages of megafauna included greater locomotory and metabolic efficiency, wider sensory ranges, the ability to migrate long distances, increased resistance to starvation, and longer life spans. Because of these traits, ice‐age megafauna may have been well‐suited to exploit transient resource bonanzas widely scattered in space. When the Holocene began, many of these advantages were lost. Where food resources are limited, the daily need for large amounts of food can negate metabolic efficiency. The ability to travel fast and efficiently becomes irrelevant when home ranges shrink, as does the ability to be nomadic or migratory. Other attributes of large body size may have posed disadvantages during the Holocene's striped conditions. As ranges shrank and settled into striped configurations, the low population densities and slow reproductive rates associated with large body size may have caused megafaunal populations to decline. Together these disadvantages would then have lowered the extinction thresholds of many megafaunal species in the striped world of the Holocene. This allowed a variety of proximate causes including overkill by humans, disease, competition with other species, and habitat loss to drive megafaunal species to extinction (Fig. 15).
Figure 15.
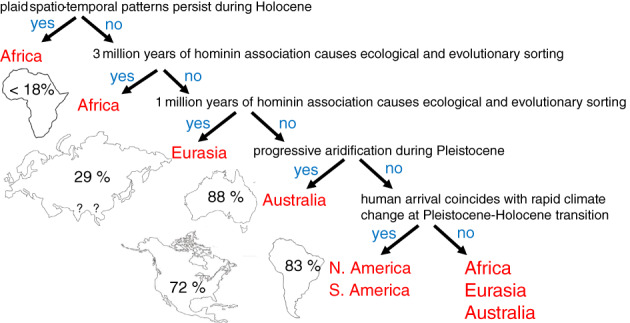
Late Quaternary extinctions on the continents resulted from conspiring processes whose roles varied according to geography and time. Percentages of megafaunal genera going extinct are shown within each continent's outline. On many continents, the transition from plaid to striped landscapes may have been basal within a hierarchy of extinction causes that included other kinds of climate‐change impacts and the impacts of human activities. The fewest extinctions occurred in the drylands of Africa because the spatio‐temporal fabric remained largely plaid throughout the Holocene, and possibly because its megafaunal ecosystems had lengthy prior exposure to hominins. Progressive aridification in Australia may have trumped the favourable effects of the ice‐age plaid for megafauna, causing extinctions there to be early and – after human arrival – to be severe. Multiple extinction drivers intersected in the Americas when initial human impacts coincided with the conversion from plaid to striped landscapes.
By being explicitly hierarchical, the Plaids and Stripes Hypothesis helps integrate divergent facets of the long‐running debate about the causes of Late Quaternary extinctions. Like many recent extinctions, megafaunal extinctions at the end of the last ice age resulted from conspiracies of causes whose relative importances varied across time, space, and taxa. Unfortunately, as the quote from S.J. Gould at the beginning of this section emphasizes, we have difficulty thinking hierarchically and find it easier to pursue singular explanations (e.g. human overkill, global drought, epidemic, or cosmic impact). The plaids to stripes transition may have been basal within the extinction–cause hierarchies of many megafaunal species during the last glacial–interglacial transition (Fig. 15). Depending on location and time, additional causes included a variety of other climate‐related phenomena and, in some cases, a variety of human impacts on ecosystem structure and function, the magnitude of which were probably modified by duration of prior exposure of the megafauna to hominins (Owen‐Smith, 1987).
The Plaids and Stripes Hypothesis may help explain two anomalies within the overall phenomenon of Late Quaternary extinctions: Australia's staggered extinctions and the persistence of Africa's megafauna to the present. Australia's staggered schedule of extinctions was probably related to its long‐term, progressive aridification. Lowered primary productivity during the driest intervals of the ice‐age plaid, combined with that continent's biogeographic simplicity (which made refugia hard to find) and infertile soils (which restricted primary productivity) negated the advantages the ice‐age plaid would have otherwise afforded megafauna.
One cause of the African exception may have been the retention of plaidness in its extensive drylands during Holocene times. Another may have been the large amount of ecological/ and evolutionary sorting that occurred over the long history of hominin evolution on that continent. Interestingly, Eurasia, which had the second lowest percentage of megafaunal extinctions during the Late Quaternary, also experienced the second‐longest history of hominin‐megafauna association (Fig. 15).
The Plaids and Stripes Hypothesis may be a part of the explanation for why bouts of megafaunal extinctions do not seem to have occurred during earlier glacial–interglacial transitions. Recent advances in reconstructing the palaeoclimates and palaeoenvironments of previous interglacials reveal they differed widely in their climate histories, and that some interglacials may have been more conducive to plaidness (and hence the retention of megafauna) than the Holocene has been. Another part of the explanation may be the increasing scarcity of fossils and the declining precision of dating control beyond the limit of the 14C method. A third cause for the apparent lack of megafaunal extinctions during earlier glacial–interglacial transitions may have been the absence of humans during earlier interglacials on the three continents (Australia, North America, South America) where megafaunal extinctions were most frequent in Late Quaternary times.
VII. CONCLUSIONS
We suggest that a widely shared cause for Late Quaternary megafaunal extinctions was a fundamental shift in the spatio‐temporal fabric of many ecosystems triggered by the loss of the millennial‐scale climate fluctuations that were characteristic of the ice age but that ceased approximately 11700 ka on most continents.
Under ice‐age conditions, radical and rapid climate changes prevented many ecosystems from equilibrating with their contemporary climates. Instead of today's ‘striped’ world where species' ranges have equilibrated with gradients of temperature, moisture, and seasonality, the ice‐age world was a disequilibrial ‘plaid’ where species' ranges shifted rapidly and repeatedly, rarely catching up with their contemporary climate.
Certain physiological, anatomical, and ecological attributes of megafauna pre‐adapted them for success in the ice age's transient ecosystems. These traits included greater metabolic and locomotory efficiency, increased resistance to starvation, longer life spans, greater sensory ranges, and the ability to be nomadic or migratory.
When the ice age's plaid world ended, many of the advantages of being large were either lost or became disadvantages. For instance, in the striped world, the low population densities and slow reproductive rates associated with large body size limited the resiliency of megafauna to population bottlenecks.
Disadvantages of being large in striped environments lowered the extinction thresholds for megafauna living on continents worldwide, which then increased the vulnerability of individual species to a wide range of proximate threats including human predation, competition with other species, and habitat loss.
For many megafaunal species living on the continents at the end of the last ice age, the plaid to stripes transition may have been positioned at the base of a hierarchy of extinction causes that included other climate‐change‐related effects as well as human impacts. The relative importance of these separate yet conspiring causes varied geographically, temporally, and taxonomically.
VIII. ACKNOWLEDGEMENTS
This work was supported by grants from the Bureau of Land Management and the National Science Foundation (PLR 1417611/1417036). We thank R. Reanier, D. Meltzer, G. Streveler, J. Olnes, R. Ruess, M. Kunz, K. Kielland, G. Zazula, and D. Froese for interesting discussions about the ideas presented here.
IX. REFERENCES
- Allen, J. R. , Brandt, U. , Brauer, A. , Hubberten, H.‐W. , Huntley, B. , Keller, J. , Kraml, M. , Mackensen, A. , Mingram, J. & Negendank, J. F. (1999). Rapid environmental changes in southern Europe during the last glacial period. Nature 400, 740–743. [Google Scholar]
- Alroy, J. (2000). New methods for quantifying macroevolutionary patterns and processes. Paleobiology 26, 707–733. [Google Scholar]
- Alroy, J. (2014). A simple Bayesian method of inferring extinction. Paleobiology 40, 584–607. [Google Scholar]
- Ammann, B. , van Leeuwen, J. F. , van der Knaap, W. O. , Lischke, H. , Heiri, O. & Tinner, W. (2013). Vegetation responses to rapid warming and to minor climatic fluctuations during the Late‐Glacial Interstadial (GI‐1) at Gerzensee (Switzerland). Palaeogeography, Palaeoclimatology, Palaeoecology 391, 40–59. [Google Scholar]
- Ampel, L. , Bigler, C. , Wohlfarth, B. , Risberg, J. , Lotter, A. F. & Veres, D. (2010). Modest summer temperature variability during DO cycles in western Europe. Quaternary Science Reviews 29, 1322–1327. [Google Scholar]
- Atkinson, I. A. (1996). Introductions of wildlife as a cause of species extinctions. Wildlife Biology 2, 135–141. [Google Scholar]
- Atkinson, T. C. , Briffa, K. R. & Coope, G. R. (1987). Seasonal temperatures in Britain during the past 22,000 years, reconstructed using beetle remains. Nature 325, 587–592. [Google Scholar]
- Ayliffe, L. K. , Gagan, M. K. , Zhao, J.‐X. , Drysdale, R. N. , Hellstrom, J. C. , Hantoro, W. S. , Griffiths, M. L. , Scott‐Gagan, H. , Pierre, E. S. , Cowley, J. A. & Suwargadi, B. W. (2013). Rapid interhemispheric climate links via the Australasian monsoon during the last deglaciation. Nature Communications 4, 2908. [DOI] [PubMed] [Google Scholar]
- Bae, C. J. , Douka, K. & Petraglia, M. D. (2017). Human colonization of Asia in the Late Pleistocene: an introduction to supplement 17. Current Anthropology 58, S373–S382. [Google Scholar]
- Barker, S. , Knorr, G. , Edwards, R. L. , Parrenin, F. , Putnam, A. E. , Skinner, L. C. , Wolff, E. & Ziegler, M. (2011). 800,000 years of abrupt climate variability. Science 334, 347–351. [DOI] [PubMed] [Google Scholar]
- Barnosky, A. D. , Bell, C. J. , Emslie, S. D. , Goodwin, H. T. , Mead, J. I. , Repenning, C. A. , Scott, E. & Shabel, A. B. (2004a). Exceptional record of mid‐Pleistocene vertebrates helps differentiate climatic from anthropogenic ecosystem perturbations. Proceedings of the National Academy of Sciences 101, 9297–9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnosky, A. D. , Hadly, E. A. , Gonzalez, P. , Head, J. , Polly, P. D. , Lawing, A. M. , Eronen, J. T. , Ackerly, D. D. , Alex, K. & Biber, E. (2017). Merging paleobiology with conservation biology to guide the future of terrestrial ecosystems. Science 355, eaah4787. [DOI] [PubMed] [Google Scholar]
- Barnosky, A. D. , Koch, P. L. , Feranec, R. S. , Wing, S. L. & Shabel, A. B. (2004b). Assessing the Causes of Late Pleistocene Extinctions on the Continents. Science 306, 70–75. [DOI] [PubMed] [Google Scholar]
- Barnosky, A. D. & Lindsey, E. L. (2010). Timing of Quaternary megafaunal extinction in South America in relation to human arrival and climate change. Quaternary International 217, 10–29. [Google Scholar]
- Baughman, C. A. , Mann, D. H. , Verbyla, D. L. & Kunz, M. L. (2015). Soil surface organic layers in Arctic Alaska: spatial distribution, rates of formation, and microclimatic effects. Journal of Geophysical Research: Biogeosciences 120, 1150–1164. [Google Scholar]
- Bell, C. J. , Lundelius, E. L. , Barnosky, A. D. , Graham, R. W. , Lindsay, E. H. , Ruez, D. R. , Semken, H. A. , Webb, S. D. & Zakrzewski, R. J. (2004). The Blancan, Irvingtonian, and Rancholabrean Land Mammal Ages In Late Cretaceous and Cenozoic Mammals of North America: Biostratigraphy and Geochronology (ed. Woodburne M. O.), pp. 232–314. Columbia University Press, New York. [Google Scholar]
- Bennett, K. , Tzedakis, P. & Willis, K. (1991). Quaternary refugia of north European trees. Journal of Biogeography 18, 103–115. [Google Scholar]
- Benson, L. , Lund, S. , Negrini, R. , Linsley, B. & Zic, M. (2003). Response of North American Great Basin Lakes to Dansgaard–Oeschger oscillations. Quaternary Science Reviews 22, 2239–2251. [Google Scholar]
- Berger, A. , Loutre, M.‐F. & Mélice, J. (2006). Equatorial insolation: from precession harmonics to eccentricity frequencies. Climate of the Past 2, 131–136. [Google Scholar]
- Birks, H. H. & Ammann, B. (2000). Two terrestrial records of rapid climatic change during the glacial–Holocene transition (14,000– 9,000 calendar years B.P.) from Europe. Proceedings of the National Academy of Sciences 97, 1390–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blonder, B. , Nogués‐Bravo, D. , Borregaard, M. K. , Donoghue, I. , John, C. , Jørgensen, P. M. , Kraft, N. J. , Lessard, J.‐P. , Morueta‐Holme, N. & Sandel, B. (2015). Linking environmental filtering and disequilibrium to biogeography with a community climate framework. Ecology 96, 972–985. [DOI] [PubMed] [Google Scholar]
- Bowman, J. , Jaeger, J. A. & Fahrig, L. (2002). Dispersal distance of mammals is proportional to home range size. Ecology 83, 2049–2055. [Google Scholar]
- Bradshaw, C. J. A. , Cooper, A. , Turney, C. S. M. & Brook, B. W. (2012). Robust estimates of extinction time in the geological record. Quaternary Science Reviews 33, 14–19. [Google Scholar]
- Bradshaw, R. H. W. , Kito, N. & Giesecke, T. (2010). Factors influencing the Holocene history of Fagus . Forest Ecology and Management 259, 2204–2212. [Google Scholar]
- Brook, B. W. & Bowman, D. M. (2005). One equation fits overkill: why allometry underpins both prehistoric and modern body size‐biased extinctions. Population Ecology 47, 137–141. [Google Scholar]
- Brook, B. W. , Sodhi, N. S. & Bradshaw, C. J. A. (2008). Synergies among extinction drivers under global change. Trends in Ecology and Evolution 23, 453–460. [DOI] [PubMed] [Google Scholar]
- Brovkin, V. (2002). Climate‐vegetation interaction. Journal de Physique IV (Proceedings) 12, 57–72. [Google Scholar]
- Brown, J. H. , Gillooly, J. F. , Allen, A. P. , Savage, V. M. & West, G. B. (2004). Toward a metabolic theory of ecology. Ecology 85, 1771–1789. [Google Scholar]
- Buck, C. E. & Bard, E. (2007). A calendar chronology for Pleistocene mammoth and horse extinction in North America based on Bayesian radiocarbon calibration. Quaternary Science Reviews 26, 2031–2035. [Google Scholar]
- Burness, G. P. , Diamond, J. & Flannery, T. (2001). Dinosaurs, dragons, and dwarfs: the evolution of maximal body size. Proceedings of the National Academy of Sciences 98, 14518–14523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burney, D. A. , Burney, L. P. , Godfrey, L. R. , Jungers, W. L. , Goodman, S. M. , Wright, H. T. & Jull, A. T. (2004). A chronology for late prehistoric Madagascar. Journal of Human Evolution 47, 25–63. [DOI] [PubMed] [Google Scholar]
- Byrne, M. , Yeates, D. , Joseph, L. , Kearney, M. , Bowler, J. , Williams, M. , Cooper, S. , Donnellan, S. , Keogh, J. S. & Leys, R. (2008). Birth of a biome: insights into the assembly and maintenance of the Australian arid zone biota. Molecular Ecology 17, 4398–4417. [DOI] [PubMed] [Google Scholar]
- Capron, E. , Landais, A. , Chappellaz, J. , Schilt, A. , Buiron, D. , Dahl‐Jensen, D. , Johnsen, S. J. , Jouzel, J. , Lemieux‐Dudon, B. & Loulergue, L. (2010). Millennial and sub‐millennial scale climatic variations recorded in polar ice cores over the last glacial period. Climate of the Past 6, 345–365. [Google Scholar]
- Carbone, C. , Cowlishaw, G. , Isaac, N. J. & Rowcliffe, J. M. (2004). How far do animals go? Determinants of day range in mammals. American Naturalist 165, 290–297. [DOI] [PubMed] [Google Scholar]
- Cardillo, M. , Mace, G. M. , Jones, K. E. , Bielby, J. , Bininda‐Emonds, O. R. , Sechrest, W. , Orme, C. D. L. & Purvis, A. (2005). Multiple causes of high extinction risk in large mammal species. Science 309, 1239–1241. [DOI] [PubMed] [Google Scholar]
- Caughley, G. (1994). Directions in conservation biology. Journal of Animal Ecology 63, 215–244. [Google Scholar]
- Chang, D. , Knapp, M. , Enk, J. , Lippold, S. , Kircher, M. , Lister, A. , MacPhee, R. D. , Widga, C. , Czechowski, P. & Sommer, R. (2017). The evolutionary and phylogeographic history of woolly mammoths: a comprehensive mitogenomic analysis. Scientific Reports 7, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss, M. , Schwarm, A. , Ortmann, S. , Streich, W. J. & Hummel, J. (2007). A case of non‐scaling in mammalian physiology? Body size, digestive capacity, food intake, and ingesta passage in mammalian herbivores. Comparative Biochemistry and Physiology, Part A: Molecular & Integrative Physiology 148, 249–265. [DOI] [PubMed] [Google Scholar]
- Coe, M. , Cumming, D. & Phillipson, J. (1976). Biomass and production of large African herbivores in relation to rainfall and primary production. Oecologia 22, 341–354. [DOI] [PubMed] [Google Scholar]
- Cohen, T. J. , Jansen, J. D. , Gliganic, L. A. , Larsen, J. R. , Nanson, G. C. , May, J.‐H. , Jones, B. G. & Price, D. M. (2015). Hydrological transformation coincided with megafaunal extinction in central Australia. Geology 43, 195–198. [Google Scholar]
- Collen, B. & Turvey, S. T. (2009). Probabilistic methods for determining extinction chronologies In Holocene Extinctions (ed. Turvey S. T.), pp. 181–192. Oxford Universisty Press, New York. [Google Scholar]
- Coope, G. R. (2004). Several million years of stability among insect species because of, or in spite of, Ice Age climatic instability? Philosophical Transactions of the Royal Society of London Series B: Biological Sciences 359, 209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper, A. , Turney, C. , Hughen, K. A. , Brook, B. W. , McDonald, H. G. & Bradshaw, C. J. A. (2015). Abrupt warming events drove Late Pleistocene Holarctic megafaunal turnover. Science 349, 602–606. [DOI] [PubMed] [Google Scholar]
- Currie, D. J. & Fritz, J. T. (1993). Global patterns of animal abundance and species energy use. Oikos 57, 56–68. [Google Scholar]
- Daniau, A. L. , Sánchez‐Goñi, M. F. , Beaufort, L. , Laggoun‐Défarge, F. , Loutre, M. F. & Duprat, J. (2007). Dansgaard–Oeschger climatic variability revealed by fire emissions in southwestern Iberia. Quaternary Science Reviews 26, 1369–1383. [Google Scholar]
- Dansgaard, W. , Johnsen, S. J. , Clausen, H. B. , Dahl‐Jensen, D. , Gundestrup, N. S. , Hammer, C. U. , Hvldberg, C. S. , Steffensen, J. P. , Sveinbjorndottir, A. E. , Jouzel, J. & Bond, G. (1993). Evidence for general instability of past climate from a 250‐kyr ice‐core record. Nature 364, 218–220. [Google Scholar]
- Davis, M. B. (1986). Climatic instability, time, lags, and community disequilibrium In Community Ecology (eds Diamond J. and Case T.), pp. 269–284. Harper & Row, New York. [Google Scholar]
- Denniston, R. F. , Wyrwoll, K.‐H. , Asmerom, Y. , Polyak, V. J. , Humphreys, W. F. , Cugley, J. , Woods, D. , LaPointe, Z. , Peota, J. & Greaves, E. (2013). North Atlantic forcing of millennial‐scale Indo‐Australian monsoon dynamics during the Last Glacial period. Quaternary Science Reviews 72, 159–168. [Google Scholar]
- Deplazes, G. , Luckge, A. , Peterson, L. C. , Timmermann, A. , Hamann, Y. , Hughen, K. A. , Rohl, U. , Laj, C. , Cane, M. A. , Sigman, D. M. & Haug, G. H. (2013). Links between tropical rainfall and North Atlantic climate during the last glacial period. Nature Geoscience 6, 213–217. [Google Scholar]
- Dewar, R. E. , Radimilahy, C. , Wright, H. T. , Jacobs, Z. , Kelly, G. O. & Berna, F. (2013). Stone tools and foraging in northern Madagascar challenge Holocene extinction models. Proceedings of the National Academy of Sciences 110, 12583–12588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond, J. (1984). "Normal" extinctions of isolated populations In Extinctions (ed. Nitecki M. H.), pp. 191–246. Chicago University Press, Chicago. [Google Scholar]
- Dobson, A. (2009). Food‐web structure and ecosystem services: insights from the Serengeti. Philosophical Transactions of the Royal Society B: Biological Sciences 364, 1665–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake, N. A. , Blench, R. M. , Armitage, S. J. , Bristow, C. S. & White, K. H. (2011). Ancient watercourses and biogeography of the Sahara explain the peopling of the desert. Proceedings of the National Academy of Sciences 108, 458–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Toit, J. T. & Owen‐Smith, N. (1989). Body size, population metabolism, and habitat specialization among large African herbivores. American Naturalist 133, 736–740. [Google Scholar]
- Duncan, R. P. , Boyer, A. G. & Blackburn, T. M. (2013). Magnitude and variation of prehistoric bird extinctions in the Pacific. Proceedings of the National Academy of Sciences 110, 6436–6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyke, A. (2005). Late Quaternary vegetation history of northern North America based on pollen, macrofossil, and faunal remains. Géographie physique et Quaternaire 59, 211–262. [Google Scholar]
- Ehlers, J. , Hughes, P. & Gibbard, P. (2016). The Ice Age. Wiley‐Blackwell, Oxford, UK. [Google Scholar]
- Ehrich, D. & Stenseth, N. C. (2001). Genetic structure of Siberian lemmings (Lemmus sibiricus) in a continuous habitat: large patches rather than isolation by distance. Heredity 86, 716–730. [DOI] [PubMed] [Google Scholar]
- Elias, S. A. (2013). The problem of conifer species migration lag in the Pacific Northwest region since the last glaciation. Quaternary Science Reviews 77, 55–69. [Google Scholar]
- Enk, J. , Devault, A. , Widga, C. , Saunders, J. , Szpak, P. , Southon, J. , Rouillard, J.‐M. , Shapiro, B. , Golding, G. B. & Zazula, G. (2016). Mammuthus Population Dynamics in Late Pleistocene North America: Divergence, Phylogeography, and Introgression. Frontiers in Ecology and Evolution 4, 1–13. [Google Scholar]
- Estes, J. A. (2016). Serendipity: An Ecologist's Quest to Understand Nature. University of California Press, Los Angeles. [Google Scholar]
- Estes, J. A. , Burdin, A. & Doak, D. F. (2016). Sea otters, kelp forests, and the extinction of Steller's sea cow. Proceedings of the National Academy of Sciences 113, 880–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan, W. F. , Lynch, H. J. & Noon, B. R. (2010). Pitfalls and challenges of estimating population growth rate from empirical data: consequences for allometric scaling relations. Oikos 119, 455–464. [Google Scholar]
- Faith, J. T. (2013). Ungulate diversity and precipitation history since the Last Glacial Maximum in the Western Cape, South Africa. Quaternary Science Reviews 68, 191–199. [Google Scholar]
- Faith, J. T. , Dortch, J. , Jones, C. , Schulmeister, J. & Travouillon, K. J. (2017). Large mammal species richness and late Quaternary precipitation change in south‐western Australia. Journal of Quaternary Science 32, 760–769. [Google Scholar]
- Fang, J. & Lechowicz, M. J. (2006). Climatic limits for the present distribution of beech (Fagus l.) species in the world. Journal of Biogeography 33, 1804–1819. [Google Scholar]
- Faurby, S. & Svenning, J. C. (2015). Historic and prehistoric human‐driven extinctions have reshaped global mammal diversity patterns. Diversity and Distributions 21, 1155–1166. [Google Scholar]
- Fenchel, T. (1974). Intrinsic rate of natural increase: the relationship with body size. Oecologia 14, 317–326. [DOI] [PubMed] [Google Scholar]
- Feurdean, A. , Bhagwat, S. A. , Willis, K. J. , Birks, H. J. B. , Lischke, H. & Hickler, T. (2013). Tree migration‐rates: narrowing the gap between inferred post‐glacial rates and projected rates. PLoS One 8, e71797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, H. , Schupbach, S. , Gfeller, G. , Bigler, M. , Rothlisberger, R. , Erhardt, T. , Stocker, T. F. , Mulvaney, R. & Wolff, E. W. (2015). Millennial changes in North American wildfire and soil activity over the last glacial cycle. Nature Geoscience 8, 723–727. [Google Scholar]
- Fleming, K. , Johnston, P. , Zwartz, D. , Yokoyama, Y. , Lambeck, K. & Chappell, J. (1998). Refining the eustatic sea‐level curve since the Last Glacial Maximum using far‐and intermediate‐field sites. Earth and Planetary Science Letters 163, 327–342. [Google Scholar]
- Fritz, H. & Duncan, P. (1994). On the carrying capacity for large ungulates of African savanna ecosystems. Proceedings of the Royal Society of London B: Biological Sciences 256, 77–82. [DOI] [PubMed] [Google Scholar]
- Froese, D. , Stiller, M. , Heintzman, P. D. , Reyes, A. V. , Zazula, G. D. , Soares, A. E. R. , Meyer, M. , Hall, E. , Jensen, B. J. L. , Arnold, L. J. , MacPhee, R. D. E. & Shapiro, B. (2017). Fossil and genomic evidence constrains the timing of bison arrival in North America. Proceedings of the National Academy of Sciences 114, 3457–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryxell, J. M. , Greever, J. & Sinclair, A. (1988). Why are migratory ungulates so abundant? American Naturalist 131, 781–798. [Google Scholar]
- Gaglioti, B. V. , Mann, D. H. , Jones, B. M. , Pohlman, J. W. , Kunz, M. L. & Wooller, M. J. (2014). Radiocarbon age‐offsets in an arctic lake reveal the long‐term response of permafrost carbon to climate change. Journal of Geophysical Research: Biogeosciences 119, 1630–1651. [Google Scholar]
- Gaglioti, B. V. , Mann, D. H. , Jones, B. M. , Wooller, M. J. & Finney, B. P. (2016). High‐resolution records detect human‐caused changes to the boreal forest wildfire regime in interior Alaska. The Holocene 26, 1064–1074. [Google Scholar]
- Galetti, M. , Brocardo, C. , Begotti, R. , Hortenci, L. , Rocha‐Mendes, F. , Bernardo, C. , Bueno, R. , Nobre, R. , Bovendorp, R. & Marques, R. (2017). Defaunation and biomass collapse of mammals in the largest Atlantic forest remnant. Animal Conservation 20, 270–281. [Google Scholar]
- Gasse, F. (2000). Hydrological changes in the African tropics since the Last Glacial Maximum. Quaternary Science Reviews 19, 189–211. [Google Scholar]
- Gasse, F. , Chalié, F. , Vincens, A. , Williams, M. A. & Williamson, D. (2008). Climatic patterns in equatorial and southern Africa from 30,000 to 10,000 years ago reconstructed from terrestrial and near‐shore proxy data. Quaternary Science Reviews 27, 2316–2340. [Google Scholar]
- Gavin, D. G. (2009). The coastal‐disjunct mesic flora in the inland Pacific Northwest of USA and Canada: refugia, dispersal and disequilibrium. Diversity and Distributions 15, 972–982. [Google Scholar]
- Gill, J. L. , Williams, J. W. , Jackson, S. T. , Donnelly, J. P. & Schellinger, G. C. (2012). Climatic and megaherbivory controls on late‐glacial vegetation dynamics: a new, high‐resolution, multi‐proxy record from Silver Lake, Ohio. Quaternary Science Reviews 34, 66–80. [Google Scholar]
- Glazier, D. S. (2005). Beyond the ‘3/4‐power law’: variation in the intra‐and interspecific scaling of metabolic rate in animals. Biological Reviews 80, 611–662. [DOI] [PubMed] [Google Scholar]
- Goetcheus, V. G. & Birks, H. H. (2001). Full‐glacial upland tundra vegetation preserved under tephra in the Beringia National Park, Seward Peninsula, Alaska. Quaternary Science Reviews 20, 135–147. [Google Scholar]
- Good, S. P. & Caylor, K. K. (2011). Climatological determinants of woody cover in Africa. Proceedings of the National Academy of Sciences 108, 4902–4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould, S. J. (2002). The Structure of Evolutionary Theory. Harvard University Press, Cambridge. [Google Scholar]
- Graham, R. W. , Belmecheri, S. , Choy, K. , Culleton, B. J. , Davies, L. J. , Froese, D. , Heintzman, P. D. , Hritz, C. , Kapp, J. D. & Newsom, L. A. (2016). Timing and causes of mid‐Holocene mammoth extinction on St. Paul Island, Alaska. Proceedings of the National Academy of Sciences 113, 9310–9314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham, R. W. & Lundelius, E. L. J. (1984). Coevolutionary disequilibrium and Pleistocene extinctions In Quaternary Extinctions: A Prehistoric Revolution (eds Martin P. S. and Klein R. G.), pp. 223–249. University of Arizona Press, Tuscon. [Google Scholar]
- Grange, S. , Duncan, P. , Gaillard, J.‐M. , Sinclair, A. R. , Gogan, P. J. , Packer, C. , Hofer, H. & East, M. (2004). What limits the Serengeti zebra population? Oecologia 140, 523–532. [DOI] [PubMed] [Google Scholar]
- Grant, K. M. , Rohling, E. J. , Ramsey, C. B. , Cheng, H. , Edwards, R. L. , Florindo, F. , Heslop, D. , Marra, F. , Roberts, A. P. , Tamisiea, M. E. & Williams, F. (2014). Sea‐level variability over five glacial cycles. Nature Communications 5, 5076. [DOI] [PubMed] [Google Scholar]
- Grayson, D. K. (2016). Giant Sloths and Sabertooth Cats: Archaeology of the Ice Age Great Basin. University of Utah Press, Salt Lake City, UT. [Google Scholar]
- Grayson, D. K. (2001). The archaeological record of human impacts on animal populations. Journal of World Prehistory 15, 1–68. [Google Scholar]
- Grayson, D. K. & Meltzer, D. J. (2003). A requiem for North American overkill. Journal of Archaeological Science 30, 585–593. [Google Scholar]
- Grimm, E. C. , Watts, W. A. , Jacobson, G. L. Jr. , Hansen, B. C. S. , Almquist, H. R. & Dieffenbacher‐Krall, A. C. (2006). Evidence for warm wet Heinrich events in Florida. Quaternary Science Reviews 25, 2197–2211. [Google Scholar]
- Guthrie, R. D. (1984). Mosaics, allelochemics and nutrients In Quaternary Extinctions, a Prehistoric Revolution (eds Martin P. S. and Klein R. G.), pp. 259–298. University of Arizona Press, Tucson. [Google Scholar]
- Guthrie, R. D. (1990). Frozen Fauna of the Mammoth Steppe; the Story of Blue Babe. University of Chicago Press, Chicago. [Google Scholar]
- Guthrie, R. D. (2001). Origin and causes of the mammoth steppe: a story of cloud cover, woolly mammal tooth pits, buckles, and inside‐out Beringia. Quaternary Science Reviews 20, 549–574. [Google Scholar]
- Guthrie, R. D. (2004). Radiocarbon evidence of mid‐Holocene mammoths stranded on an Alaskan Bering Sea island. Nature 429, 746–749. [DOI] [PubMed] [Google Scholar]
- Guthrie, R. D. (2006). New carbon dates link climatic change with human colonization and Pleistocene extinctions. Nature 441, 207–209. [DOI] [PubMed] [Google Scholar]
- Hagstrum, J. T. , Firestone, R. B. , West, A. , Weaver, J. C. & Bunch, T. E. (2017). Impact‐related microspherules in Late Pleistocene Alaskan and Yukon “muck” deposits signify recurrent episodes of catastrophic emplacement. Scientific Reports 7, 16620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halsey, L. G. (2016). Terrestrial movement energetics: current knowledge and its application to the optimising animal. Journal of Experimental Biology 219, 1424–1431. [DOI] [PubMed] [Google Scholar]
- Hansen, J. , Sato, M. , Hearty, P. , Ruedy, R. , Kelley, M. , Masson‐Delmotte, V. , Russell, G. , Tselioudis, G. , Cao, J. & Rignot, E. (2015). Ice melt, sea level rise and superstorms: evidence from paleoclimate data, climate modeling, and modern observations that 2 C global warming is highly dangerous. Atmospheric Chemistry and Physics Discussions 15, 20059–20179. [Google Scholar]
- Harris, G. , Thirgood, S. , Hopcraft, J. G. C. , Cromsigt, J. P. & Berger, J. (2009). Global decline in aggregated migrations of large terrestrial mammals. Endangered Species Research 7, 55–76. [Google Scholar]
- Hart, R. H. (2001). Where the buffalo roamed—or did they? Great Plains Research 11, 83–102. [Google Scholar]
- Hawkins, B. A. , Field, R. , Cornell, H. V. , Currie, D. J. , Guégan, J.‐F. , Kaufman, D. M. , Kerr, J. T. , Mittelbach, G. G. , Oberdorff, T. & O'Brien, E. M. (2003). Energy, water, and broad‐scale geographic patterns of species richness. Ecology 84, 3105–3117. [Google Scholar]
- Healy, K. , Guillerme, T. , Finlay, S. , Kane, A. , Kelly, S. B. , McClean, D. , Kelly, D. J. , Donohue, I. , Jackson, A. L. & Cooper, N. (2014). Ecology and mode‐of‐life explain lifespan variation in birds and mammals. Proceedings of the Royal Society B: Biological Sciences 281, 20140298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearty, P. J. , Hollin, J. T. , Neumann, A. C. , O'Leary, M. J. & McCulloch, M. (2007). Global sea‐level fluctuations during the Last Interglaciation (MIS 5e). Quaternary Science Reviews 26, 2090–2112. [Google Scholar]
- Hemming, S. R. (2004). Heinrich events: massive late Pleistocene detritus layers of the North Atlantic and their global climate imprint. Reviews of Geophysics 42, RG1005. [Google Scholar]
- Hempson, G. P. , Archibald, S. & Bond, W. J. (2015). A continent‐wide assessment of the form and intensity of large mammal herbivory in Africa. Science 350, 1056–1061. [DOI] [PubMed] [Google Scholar]
- Herold, N. , Yin, Q. , Karami, M. & Berger, A. (2012). Modelling the climatic diversity of the warm interglacials. Quaternary Science Reviews 56, 126–141. [Google Scholar]
- Hershkovitz, I. , Weber, G. W. , Quam, R. , Duval, M. , Grün, R. , Kinsley, L. , Ayalon, A. , Bar‐Matthews, M. , Valladas, H. , Mercier, N. , Arsuaga, J. L. , Martinón‐Torres, M. , Bermúdez de Castro, J. M. , Fornai, C. , Martín‐Francés, L. , Sarig, R. , et al. (2018). The earliest modern humans outside Africa. Science 359, 456–459. [DOI] [PubMed] [Google Scholar]
- Hofreiter, M. , Serre, D. , Rohland, N. , Rabeder, G. , Nagel, D. , Conard, N. , Münzel, S. & Pääbo, S. (2004). Lack of phylogeography in European mammals before the last glaciation. Proceedings of the National Academy of Sciences 101, 12963–12968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofreiter, M. & Stewart, J. (2009). Ecological change, range fluctuations and population dynamics during the Pleistocene. Current Biology 19, R584–R594. [DOI] [PubMed] [Google Scholar]
- Holdaway, R. N. , Allentoft, M. E. , Jacomb, C. , Oskam, C. L. , Beavan, N. R. & Bunce, M. (2014). An extremely low‐density human population exterminated New Zealand moa. Nature Communications 5, 5436. [DOI] [PubMed] [Google Scholar]
- Holdaway, R. N. & Jacomb, C. (2000). Rapid extinction of the moas (Aves: Dinornithiformes): model, test, and implications. Science 287, 2250–2254. [DOI] [PubMed] [Google Scholar]
- Holdo, R. M. , Holt, R. D. & Fryxell, J. M. (2009). Opposing rainfall and plant nutritional gradients best explain the wildebeest migration in the Serengeti. American Naturalist 173, 431–445. [DOI] [PubMed] [Google Scholar]
- Huang, J. , Ji, M. , Xie, Y. , Wang, S. , He, Y. & Ran, J. (2016). Global semi‐arid climate change over last 60 years. Climate Dynamics 46, 1131–1150. [Google Scholar]
- Hubbe, A. , Hubbe, M. & Neves, W. (2007). Early Holocene survival of megafauna in South America. Journal of Biogeography 34, 1642–1646. [Google Scholar]
- Huber, C. , Leuenberger, M. , Spahni, R. E. , Fluckiger, J. , Schwander, J. , Stocker, T. F. , Johnsen, S. , Landais, A. & Jouzel, J. (2006). Isotope calibrated Greenland temperature record over Marine Isotope Stage 3 and its relation to CH4 . Earth and Planetary Science Letters 243, 504–519. [Google Scholar]
- Huntley, B. , Allen, J. R. , Collingham, Y. C. , Hickler, T. , Lister, A. M. , Singarayer, J. , Stuart, A. J. , Sykes, M. T. & Valdes, P. J. (2013). Millennial climatic fluctuations are key to the structure of last glacial ecosystems. PLoS One 8, e61963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huston, M. A. (2012). Precipitation, soils, NPP, and biodiversity: resurrection of Albrecht's curve. Ecological Monographs 82, 277–296. [Google Scholar]
- Jacobson, G. & Birks, H. J. B. (1980). Soil development on recent end moraines of the Klutlan Glacier, Yukon Territory, Canada'. Quaternary Research 14, 87–100. [Google Scholar]
- Jankowski, N. R. , Gully, G. A. , Jacobs, Z. , Roberts, R. G. & Prideaux, G. J. (2016). A late Quaternary vertebrate deposit in Kudjal Yolgah Cave, south‐western Australia: refining regional late Pleistocene extinctions. Journal of Quaternary Science 31, 538–550. [Google Scholar]
- Janzen, D. H. (1983). The Pleistocene hunters had help. The American Naturalist 121, 598–599. [Google Scholar]
- Jetz, W. , Carbone, C. , Fulford, J. & Brown, J. H. (2004). The scaling of animal space use. Science 306, 266–268. [DOI] [PubMed] [Google Scholar]
- Johnson, C. N. (2002). Determinants of loss of mammal species during the Late Quaternary ‘megafauna’ extinctions: life history and ecology, but not body size. Proceedings of the Royal Society of London B: Biological Sciences 269, 2221–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, C. N. , Rule, S. , Haberle, S. G. , Kershaw, A. P. , McKenzie, G. M. & Brook, B. W. (2016). Geographic variation in the ecological effects of extinction of Australia's Pleistocene megafauna. Ecography 39, 109–116. [Google Scholar]
- Johnstone, J. F. & Chapin, F. S. (2003). Non‐equilibrium succession dynamics indicate continued northern migration of lodgepole pine. Global Change Biology 9, 1401–1409. [Google Scholar]
- Jouzel, J. , Vimeux, F. , Caillon, N. , Delaygue, G. , Hoffmann, G. , Masson‐Delmotte, V. & Parrenin, F. (2003). Magnitude of isotope/temperature scaling for interpretation of central Antarctic ice cores. Journal of Geophysical Research: Atmospheres 108, D12. [Google Scholar]
- Kanner, L. C. , Burns, S. J. , Cheng, H. & Edwards, R. L. (2012). High–latitude forcing of the South American summer monsoon during the last glacial. Science 335, 570–573. [DOI] [PubMed] [Google Scholar]
- Keitt, T. H. , Amaral, L. A. , Buldyrev, S. V. & Stanley, H. E. (2002). Scaling in the growth of geographically subdivided populations: invariant patterns from a continent‐wide biological survey. Philosophical Transactions of the Royal Society of London B: Biological Sciences 357, 627–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershaw, A. P. , Bretherton, S. C. & van der Kaars, S. (2007). A complete pollen record of the last 230 ka from Lynch's Crater, north‐eastern Australia. Palaeogeography, Palaeoclimatology, Palaeoecology 251, 23–45. [Google Scholar]
- Kindler, P. , Guillevic, M. , Baumgartner, M. , Schwander, J. , Landais, A. & Leuenberger, M. (2014). Temperature reconstruction from 10 to 120 kyr b2k from the NGRIP ice core. Climate of the Past 10, 887–902. [Google Scholar]
- Klaar, M. J. , Kidd, C. , Malone, E. , Bartlett, R. , Pinay, G. , Chapin, F. S. & Milner, A. (2015). Vegetation succession in deglaciated landscapes: implications for sediment and landscape stability. Earth Surface Processes and Landforms 40, 1088–1100. [Google Scholar]
- Klein, R. G. (1984). Mammalian extinctions and Stone Age people In Quaternary Extinctions (eds Martin P. S. and Klein R. G.), pp. 553–573. University of Arizona Press, Tuscon. [Google Scholar]
- Koch, P. L. & Barnosky, A. D. (2006). Late Quaternary extinctions: state of the debate. Annual Review of Ecology, Evolution, and Systematics 37, 215–250. [Google Scholar]
- Kuussaari, M. , Bommarco, R. , Heikkinen, R. K. , Helm, A. , Krauss, J. , Lindborg, R. , Öckinger, E. , Pärtel, M. , Pino, J. & Rodà, F. (2009). Extinction debt: a challenge for biodiversity conservation. Trends in Ecology & Evolution 24, 564–571. [DOI] [PubMed] [Google Scholar]
- Kuzmin, Y. V. (2010). Extinction of the woolly mammoth (Mammuthus primigenius) and woolly rhinoceros (Coelodonta antiquitatis) in Eurasia: review of chronological and environmental issues. Boreas 39, 247–261. [Google Scholar]
- Lang, N. & Wolff, E. W. (2011). Interglacial and glacial variability from the last 800 ka in marine, ice and terrestrial archives. Climate of the Past 7, 361. [Google Scholar]
- Lindstedt, S. L. & Boyce, M. S. (1985). Seasonality, fasting endurance, and body size in mammals. American Naturalist 125, 873–878. [Google Scholar]
- LISTER, A. M. (2004). The impact of Quaternary ice ages on mammalian evolution. Philosophical Transactions of the Royal Society of London B: Biological Sciences 359, 221–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister, A. M. & Sher, A. V. (1995). Ice cores and mammoth extinction. Nature 378, 23–24. [Google Scholar]
- Lister, A. M. & Stuart, A. J. (2008). The impact of climate change on large mammal distribution and extinction: evidence from the last glacial/interglacial transition. Comptes Rendus Geoscience 340, 615–620. [Google Scholar]
- Long, J. A. & Stoy, P. C. (2013). Quantifying the periodicity of Heinrich and Dansgaard–Oeschger events during Marine Oxygen Isotope Stage 3. Quaternary Research 79, 413–423. [Google Scholar]
- Lyons, S. K. , Miller, J. H. , Fraser, D. , Smith, F. A. , Boyer, A. , Lindsey, E. & Mychajliw, A. M. (2016). The changing role of mammal life histories in Late Quaternary extinction vulnerability on continents and islands. Biology Letters 12, 20160342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons, S. K. , Smith, F. A. & Brown, J. H. (2004). Of mice, mastodons and men: human‐mediated extinctions on four continents. Evolutionary Ecology Research 6, 339–358. [Google Scholar]
- MacCracken, J. G. & Viereck, L. A. (1990). Browse regrowth and use by moose after fire in interior Alaska. Northwest Science 64, 11–18. [Google Scholar]
- MacPhee, R. D. & Marx, P. A. (1997). The 40,000‐year plague. Humans, hyperdisease , and first‐contact extinctions In Natural Change and Human Impact in Madagascar (eds Goodman S. and Patterson B.), pp. 169–217. Smithsonian Institution Press, Washington. [Google Scholar]
- Malhi, Y. , Doughty, C. E. , Galetti, M. , Smith, F. A. , Svenning, J.‐C. & Terborgh, J. W. (2016). Megafauna and ecosystem function from the Pleistocene to the Anthropocene. Proceedings of the National Academy of Sciences 113, 838–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann, D. H. , Groves, P. , Kunz, M. L. , Reanier, R. E. & Gaglioti, B. V. (2013). Ice‐age megafauna in Arctic Alaska: extinction, invasion, survival. Quaternary Science Reviews 70, 91–108. [Google Scholar]
- Mann, D. H. , Groves, P. , Reanier, R. E. , Gaglioti, B. V. , Kunz, M. L. & Shapiro, B. (2015). Life and extinction of megafauna in the ice‐age Arctic. Proceedings of the National Academy of Sciences 112, 14301–14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant, R. , Brewer, S. , Webb, T. III & Turvey, S. T. (2009). Holocene deforestation: a history o f human–environmental interactions, climate change, and extinction In Holocene Extinctions (ed. Turvey S. T.), pp. 213–234. Oxford University Press, New York. [Google Scholar]
- Markova, A. K. & Vislobokova, I. A. (2016). Mammal faunas in Europe at the end of the Early–Beginning of the Middle Pleistocene. Quaternary International 420, 363–377. [Google Scholar]
- Martin, P. S. (1984). Prehistoric overkill: the global model In Quaternary Extinctions, A Prehistoric Revolution (eds Martin P. S. and Klein R. G.), pp. 354–403. University of Arizona Press, Tuscon. [Google Scholar]
- Martrat, B. , Grimalt, J. O. , Shackleton, N. J. , de Abreu, L. , Hutterli, M. A. & Stocker, T. F. (2007). Four climate cycles of recurring deep and surface water destabilizations on the Iberian Margin. Science 317, 502–507. [DOI] [PubMed] [Google Scholar]
- Maslin, M. A. , Brierley, C. M. , Milner, A. M. , Shultz, S. , Trauth, M. H. & Wilson, K. E. (2014). East African climate pulses and early human evolution. Quaternary Science Reviews 101, 1–17. [Google Scholar]
- Matthews, J. V. (1982). East Beringia during late Wisconsin time: a review of the biotic evidence In Paleoecology of Beringia (eds Hopkins D. M., Matthews J. V., Schweger C. E. and Young S. B.), pp. 127–150. Academic Press, New York. [Google Scholar]
- McCain, C. M. & King, S. R. (2014). Body size and activity times mediate mammalian responses to climate change. Global Change Biology 20, 1760–1769. [DOI] [PubMed] [Google Scholar]
- McInerny, G. , Roberts, D. L. , Davy, A. J. & Cribb, P. J. (2006). Significance of sighting rate in inferring extinction and threat. Conservation Biology 20, 562–567. [DOI] [PubMed] [Google Scholar]
- McNaughton, S. (1985). Ecology of a grazing ecosystem: the Serengeti. Ecological Monographs 55, 259–294. [Google Scholar]
- McNaughton, S. (1990). Mineral nutrition and seasonal movements of African migratory ungulates. Nature 345, 613–615. [Google Scholar]
- McWethy, D. B. , Wilmshurst, J. M. , Whitlock, C. , Wood, J. R. & McGlone, M. S. (2014). A high‐resolution chronology of rapid forest transitions following Polynesian arrival in New Zealand. PLoS One 9, e111328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mduma, S. A. , Sinclair, A. & Hilborn, R. (1999). Food regulates the Serengeti wildebeest: a 40‐year record. Journal of Animal Ecology 68, 1101–1122. [Google Scholar]
- Meiri, S. & Dayan, T. (2003). On the validity of Bergmann's rule. Journal of Biogeography 30, 331–351. [Google Scholar]
- Meltzer, D. J. (2015). Pleistocene overkill and North American mammalian extinctions. Annual Review of Anthropology 44, 33–53. [Google Scholar]
- Merriam, C. H. (1890). Results of a biological survey of the San Francisco Mountain region and desert of the Little Colorado in Arizona. North American Fauna 3, 1–4. [Google Scholar]
- Miller, G. H. , Fogel, M. L. , Magee, J. W. & Gagan, M. K. (2016). Disentangling the impacts of climate and human colonization on the flora and fauna of the Australian arid zone over the past 100 ka using stable isotopes in avian eggshell. Quaternary Science Reviews 151, 27–57. [Google Scholar]
- Milne, G. A. , Long, A. J. & Bassett, S. E. (2005). Modelling Holocene relative sea‐level observations from the Caribbean and South America. Quaternary Science Reviews 24, 1183–1202. [Google Scholar]
- Milner, A. M. , Müller, U. C. , Roucoux, K. H. , Collier, R. E. , Pross, J. , Kalaitzidis, S. , Christanis, K. & Tzedakis, P. C. (2013). Environmental variability during the Last Interglacial: a new high‐resolution pollen record from Tenaghi Philippon, Greece. Journal of Quaternary Science 28, 113–117. [Google Scholar]
- Milner, A. M. , Roucoux, K. H. , Collier, R. E. L. , Müller, U. C. , Pross, J. & Tzedakis, P. C. (2016). Vegetation responses to abrupt climatic changes during the Last Interglacial Complex (Marine Isotope Stage 5) at Tenaghi Philippon, NE Greece. Quaternary Science Reviews 154, 169–181. [Google Scholar]
- Moreno‐Mayar, J. V. , Potter, B. A. , Vinner, L. , Steinrücken, M. , Rasmussen, S. , Terhorst, J. , Kamm, J. A. , Albrechtsen, A. , Malaspinas, A.‐S. & Sikora, M. (2018). Terminal Pleistocene Alaskan genome reveals first founding population of Native Americans. Nature 553, 203–207. [DOI] [PubMed] [Google Scholar]
- Moss, P. T. & Kershaw, A. P. (2007). A late Quaternary marine palynological record (oxygen isotope stages 1 to 7) for the humid tropics of northeastern Australia based on ODP Site 820. Palaeogeography Palaeoclimatology Palaeoecology 251, 4–22. [Google Scholar]
- Mueller, T. , Olson, K. A. , Dressler, G. , Leimgruber, P. , Fuller, T. K. , Nicolson, C. , Novaro, A. J. , Bolgeri, M. J. , Wattles, D. & DeStefano, S. (2011). How landscape dynamics link individual‐to population‐level movement patterns: a multispecies comparison of ungulate relocation data. Global Ecology and Biogeography 20, 683–694. [Google Scholar]
- Müller, D. W. , Codron, D. , Meloro, C. , Munn, A. , Schwarm, A. , Hummel, J. & Clauss, M. (2013). Assessing the Jarman–Bell principle: scaling of intake, digestibility, retention time and gut fill with body mass in mammalian herbivores. Comparative Biochemistry and Physiology, Part A: Molecular & Integrative Physiology 164, 129–140. [DOI] [PubMed] [Google Scholar]
- Nanson, G. C. , Price, D. M. , Jones, B. G. , Maroulis, J. C. , Coleman, M. , Bowman, H. , Cohen, T. J. , Pietsch, T. J. & Larsen, J. R. (2008). Alluvial evidence for major climate and flow regime changes during the middle and late Quaternary in eastern central Australia. Geomorphology 101, 109–129. [Google Scholar]
- NEEM (2013). Eemian interglacial reconstructed from a Greenland folded ice core. Nature 493, 489–494. [DOI] [PubMed] [Google Scholar]
- Newmark, W. D. (2008). Isolation of African protected areas. Frontiers in Ecology and the Environment 6, 321–328. [Google Scholar]
- Nikolskiy, P. A. , Sulerzhitsky, L. D. & Pitulko, V. V. (2011). Last straw versus Blitzkrieg overkill: climate‐driven changes in the Arctic Siberian mammoth population and the Late Pleistocene extinction problem. Quaternary Science Reviews 30, 2309–2328. [Google Scholar]
- Owen‐Smith, R. N. (1987). Pleistocene extinctions: the pivotal role of megaherbivores. Paleobiology 13, 352–362. [Google Scholar]
- Owen‐Smith, R. N. (1988). Megaherbivores: The Influence of Very Large Body Size on Ecology. Cambridge University Press, Cambridge, UK. [Google Scholar]
- Owen‐Smith, N. (2013). Contrasts in the large herbivore faunas of the southern continents in the late Pleistocene and the ecological implications for human origins. Journal of Biogeography 40, 1215–1224. [Google Scholar]
- Owen‐Smith, N. , Fryxell, J. & Merrill, E. (2010). Foraging theory upscaled: the behavioural ecology of herbivore movement. Philosophical Transactions of the Royal Society of London B: Biological Sciences 365, 2267–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peel, M. C. , Finlayson, B. L. & McMahon, T. A. (2007). Updated world map of the Köppen–Geiger climate classification. Hydrology and Earth System Sciences Discussions 4, 439–473. [Google Scholar]
- Peltier, W. & Fairbanks, R. G. (2006). Global glacial ice volume and Last Glacial maximum duration from an extended Barbados sea level record. Quaternary Science Reviews 25, 3322–3337. [Google Scholar]
- Perry, G. L. W. , Wheeler, A. B. , Wood, J. R. & Wilmshurst, J. M. (2014). A high‐precision chronology for the rapid extinction of New Zealand moa (Aves, Dinornithiformes). Quaternary Science Reviews 105, 126–135. [Google Scholar]
- Peters, R. H. (1986). The Ecological Implications of Body Size. Cambridge University Press, New York. [Google Scholar]
- Polishchuk, L. V. (2010). The three‐quarter‐power scaling of extinction risk in Late Pleistocene mammals, and a new theory of the size selectivity of extinction. Evolutionary Ecology Research 12, 1–22. [Google Scholar]
- Ponel, P. , Coope, R. , Antoine, P. , Limondin‐Lozouet, N. , Leroyer, C. , Munaut, A.‐V. , Pastre, J.‐F. & Guiter, F. (2005). Lateglacial palaeoenvironments and palaeoclimates from Conty and Houdancourt, northern France, reconstructed from Beetle remains. Quaternary Science Reviews 24, 2449–2465. [Google Scholar]
- Pontzer, H. (2007). Effective limb length and the scaling of locomotor cost in terrestrial animals. Journal of Experimental Biology 210, 1752–1761. [DOI] [PubMed] [Google Scholar]
- Prentice, I. C. , Bartlein, P. J. & Webb, T. (1991). Vegetation and climate change in eastern North America since the last glacial maximum. Ecology 72, 2038–2056. [Google Scholar]
- Price, G. , Louys, J. , Faith, J. , Lorenzen, E. & Westaway, M. (2018). Big data little help in megafauna mysteries. Nature 558, 23. [DOI] [PubMed] [Google Scholar]
- Rabanus‐Wallace, M. T. , Wooller, M. J. , Zazula, G. D. , Shute, E. , Jahren, A. H. , Kosintsev, P. , Burns, J. A. , Breen, J. , Llamas, B. & Cooper, A. (2017). Megafaunal isotopes reveal role of increased moisture on rangeland during late Pleistocene extinctions. Nature Ecology & Evolution 1, 0125. [DOI] [PubMed] [Google Scholar]
- Radosevich, S. R. , Holt, J. S. & Ghersa, C. (1997). Weed Ecology: Implications for Management. John Wiley & Sons, New York. [Google Scholar]
- Rasmussen, S. O. , Bigler, M. , Blockley, S. P. , Blunier, T. , Buchardt, S. L. , Clausen, H. B. , Cvijanovic, I. , Dahl‐Jensen, D. , Johnsen, S. J. , Fischer, H. , Gkinis, V. , Guillevic, M. , Hoek, W. Z. , Lowe, J. J. , Pedro, J. B. , Popp, T. , et al. (2014). A stratigraphic framework for abrupt climatic changes during the Last Glacial period based on three synchronized Greenland ice‐core records: refining and extending the INTIMATE event stratigraphy. Quaternary Science Reviews 106, 14–28. [Google Scholar]
- Rasmussen, T. L. , Thomsen, E. & Moros, M. (2016). North Atlantic warming during Dansgaard–Oeschger events synchronous with Antarctic warming and out‐of‐phase with Greenland climate. Scientific Reports 6, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raupach, M. , Kirby, J. , Barrett, D. & Briggs, P. (2001). Balances of Water, Carbon, Nitrogen and Phosphorus in Australian Landscapes: (1) Project Description and Results. CSIRO Land and Water, Canberra. [Google Scholar]
- Rick, T. C. , Kirch, P. V. , Erlandson, J. M. & Fitzpatrick, S. M. (2013). Archeology, deep history, and the human transformation of island ecosystems. Anthropocene 4, 33–45. [Google Scholar]
- Ripple, W. J. & Van Valkenburgh, B. (2010). Linking top‐down forces to the Pleistocene megafaunal extinctions. BioScience 60, 516–526. [Google Scholar]
- Ritchie, J. C. & Cwynar, L. C. (1982). The late Quaternary vegetation of the north Yukon In Paleoecology of Beringia (eds Hopkins D. M., Matthews J. V., Schweger C. E. and Young S. B.), pp. 113–126. Academic Press, New York. [Google Scholar]
- Rivadeneira, M. M. , Hunt, G. & Roy, K. (2009). The use of sighting records to infer species extinctions: an evaluation of different methods. Ecology 90, 1291–1300. [DOI] [PubMed] [Google Scholar]
- Rogers, R. L. & Slatkin, M. (2017). Excess of genomic defects in a woolly mammoth on Wrangel island. PLoS genetics 13, e1006601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohling, E. J. , Grant, K. , Hemleben, C. , Siddall, M. , Hoogakker, B. A. A. , Bolshaw, M. & Kucera, M. (2008). High rates of sea‐level rise during the last interglacial period. Nature Geoscience 1, 38–42. [Google Scholar]
- Romer, A. (1933). Man and the Vertebrates. Penguin, London. [Google Scholar]
- Roy, K. , Valentine, J. W. , Jablonski, D. & Kidwell, S. M. (1996). Scales of climatic variability and time averaging in Pleistocene biotas: implications for ecology and evolution. Trends in Ecology & Evolution 11, 458–463. [DOI] [PubMed] [Google Scholar]
- Ruddiman, W. , Fuller, D. , Kutzbach, J. , Tzedakis, P. , Kaplan, J. , Ellis, E. , Vavrus, S. , Roberts, C. , Fyfe, R. & He, F. (2016). Late Holocene climate: natural or anthropogenic? Reviews of Geophysics 54, 93–118. [Google Scholar]
- Ruddiman, W. F. (2003). The anthropogenic greenhouse era began thousands of years ago. Climatic Change 61, 261–293. [Google Scholar]
- Ruddiman, W. F. (2007). The early anthropogenic hypothesis: challenges and responses. Reviews of Geophysics 45, 1–37. [Google Scholar]
- Ruddiman, W. F. (2014). Earth Transformed. WH Freeman, New York. [Google Scholar]
- Rupp, T. S. , Olson, M. , Adams, L. G. , Dale, B. W. , Joly, K. , Henkelman, J. , Collins, W. B. & Starfield, A. M. (2006). Simulating the influences of various fire regimes on caribou winter habitat. Ecological Applications 16, 1730–1743. [DOI] [PubMed] [Google Scholar]
- Saltré, F. , Rodríguez‐Rey, M. , Brook, B. W. , Johnson, C. N. , Turney, C. S. , Alroy, J. , Cooper, A. , Beeton, N. , Bird, M. I. & Fordham, D. A. (2016). Climate change not to blame for late Quaternary megafauna extinctions in Australia. Nature Communications 7, 10511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandom, C. , Faurby, S. , Sandel, B. & Svenning, J.‐C. (2014). Global late Quaternary megafauna extinctions linked to humans, not climate change. Proceedings of the Royal Society B: Biological Sciences 281, 20133254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt‐Nielsen, K. (1984). Scaling: Why is Animal Size so Important? Cambridge University Press, London. [Google Scholar]
- Schreve, D. C. (2001). Differentiation of the British late Middle Pleistocene interglacials: the evidence from mammalian biostratigraphy. Quaternary Science Reviews 20, 1693–1705. [Google Scholar]
- Seierstad, I. K. , Abbott, P. M. , Bigler, M. , Blunier, T. , Bourne, A. J. , Brook, E. , Buchardt, S. L. , Buizert, C. , Clausen, H. B. , Cook, E. , Dahl‐Jensen, D. , Davies, S. M. , Guillevic, M. , Johnsen, S. J. , Pedersen, D. S. , Popp, T. J. , et al. (2014). Consistently dated records from the Greenland GRIP, GISP2 and NGRIP ice cores for the past 104 ka reveal regional millennial‐scale δ18O gradients with possible Heinrich event imprint. Quaternary Science Reviews 106, 29–46. [Google Scholar]
- Shakun, J. D. , Burns, S. J. , Fleitmann, D. , Kramers, J. , Matter, A. & Al‐Subary, A. (2007). A high‐resolution, absolute‐dated deglacial speleothem record of Indian Ocean climate from Socotra Island, Yemen. Earth and Planetary Science Letters 259, 442–456. [Google Scholar]
- Shakun, J. D. , Lea, D. W. , Lisiecki, L. E. & Raymo, M. E. (2015). An 800‐kyr record of global surface ocean δ18O and implications for ice volume‐temperature coupling. Earth and Planetary Science Letters 426, 58–68. [Google Scholar]
- Shapiro, B. , Drummond, A. J. , Rambaut, A. , Wilson, M. C. , Matheus, P. E. , Sher, A. V. , Pybus, O. G. , Gilbert, M. T. P. , Barnes, I. , Binladen, J. , Willerslev, E. , Hansen, A. J. , Baryshnikov, G. F. , Burns, J. A. , Davydov, S. , et al. (2004). Rise and fall of the Beringian steppe bison. Science 306, 1561–1565. [DOI] [PubMed] [Google Scholar]
- Signor, P. W. & Lipps, J. H. (1982). Sampling bias, gradual extinction and catastrophies in the fossil record. Geological Society of America Special Paper 190, 190–296. [Google Scholar]
- Sinclair, A. , Mduma, S. A. , Hopcraft, J. G. C. , Fryxell, J. M. , Hilborn, R. & Thirgood, S. (2007). Long‐term ecosystem dynamics in the Serengeti: lessons for conservation. Conservation Biology 21, 580–590. [DOI] [PubMed] [Google Scholar]
- Singh, N. , Grachev, I. A. , Bekenov, A. & Milner‐Gulland, E. (2010). Tracking greenery across a latitudinal gradient in central Asia–the migration of the saiga antelope. Diversity and Distributions 16, 663–675. [Google Scholar]
- Solow, A. R. (1993). Inferring extinction from sighting data. Ecology 74, 962–964. [Google Scholar]
- Solow, A. R. (2003). Estimation of stratigraphic ranges when fossil finds are not randomly distributed. Paleobiology 29, 181–185. [Google Scholar]
- Soulé, M. E. (1983). What do we really know about extinction? In Genetics and Conservation; A Reference for Managing Wild Animals and Plant Populations (eds Schonewald‐Cox C. M., Chambers S. M., MacBryde B. and Thomas W. L.), pp. 111–124. Benjamin‐Cummings, London. [Google Scholar]
- Steadman, D. W. & Martin, P. S. (2003). The late Quaternary extinction and future resurrection of birds on Pacific islands. Earth‐Science Reviews 61, 133–147. [Google Scholar]
- Stewart, J. R. , Lister, A. M. , Barnes, I. & Dalén, L. (2010). Refugia revisited: individualistic responses of species in space and time. Proceedings of the Royal Society of London B: Biological Sciences 277, 661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockhecke, M. , Timmermann, A. , Kipfer, R. , Haug, G. H. , Kwiecien, O. , Friedrich, T. , Menviel, L. , Litt, T. , Pickarski, N. & Anselmetti, F. S. (2016). Millennial to orbital‐scale variations of drought intensity in the Eastern Mediterranean. Quaternary Science Reviews 133, 77–95. [Google Scholar]
- Stuart, A. J. (1991). Mammalian extinctions in the late Pleistocne of northern Eurasia and North America. Biological Reviews 66, 453–562. [DOI] [PubMed] [Google Scholar]
- Stuart, A. J. (2015). Late Quaternary megafaunal extinctions on the continents: a short review. Geological Journal 50, 338–363. [Google Scholar]
- Stuart, A. J. , Kosintsev, P. A. , Higham, T. F. G. & Lister, A. M. (2004). Pleistocene to Holocene extinction dynamics in giant deer and woolly mammoth. Nature 431, 684–689. [DOI] [PubMed] [Google Scholar]
- Surovell, T. A. , Pelton, S. R. , Anderson‐Sprecher, R. & Myers, A. D. (2016). Test of Martin's overkill hypothesis using radiocarbon dates on extinct megafauna. Proceedings of the National Academy of Sciences 113, 886–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland, G. D. , Harestad, A. S. , Price, K. & Lertzman, K. P. (2000). Scaling of natal dispersal distances in terrestrial birds and mammals. Conservation Ecology 4, 16. [Google Scholar]
- Svenning, J.‐C. , Eiserhardt, W. L. , Normand, S. , Ordonez, A. & Sandel, B. (2015). The influence of paleoclimate on present‐day patterns in biodiversity and ecosystems. Annual Review of Ecology, Evolution, and Systematics 46, 551–572. [Google Scholar]
- Svenning, J.‐C. & Sandel, B. (2013). Disequilibrium vegetation dynamics under future climate change. American Journal of Botany 100, 1266–1286. [DOI] [PubMed] [Google Scholar]
- Svenning, J.‐C. & Skov, F. (2007). Ice age legacies in the geographical distribution of tree species richness in Europe. Global Ecology and Biogeography 16, 234–245. [Google Scholar]
- Swihart, R. K. , Slade, N. A. & Bergstrom, B. J. (1988). Relating body size to the rate of home range use in mammals. Ecology 69, 393–399. [Google Scholar]
- Taylor, C. R. & Heglund, N. C. (1982). Energetics and mechanics of terrestrial locomotion. Annual Review of Physiology 44, 97–107. [DOI] [PubMed] [Google Scholar]
- Thompson, W. G. , Allen Curran, H. , Wilson, M. A. & White, B. (2011). Sea‐level oscillations during the last interglacial highstand recorded by Bahamas corals. Nature Geoscience 4, 684–687. [Google Scholar]
- Tierney, J. E. (2013). Abrupt shifts in Horn of Africa hydroclimate since the Last Glacial Maximum. Science 342, 843–846. [DOI] [PubMed] [Google Scholar]
- Tierney, J. E. , Russell, J. M. , Huang, Y. , Damsté, J. S. S. , Hopmans, E. C. & Cohen, A. S. (2008). Northern hemisphere controls on tropical southeast African climate during the past 60,000 years. Science 322, 252–255. [DOI] [PubMed] [Google Scholar]
- Tinner, W. & Kaltenrieder, P. (2005). Rapid responses of high‐mountain vegetation to early Holocene environmental changes in the Swiss Alps. Journal of Ecology 93, 936–947. [Google Scholar]
- Tollefsrud, M. M. , Kissling, R. O. Y. , Gugerli, F. , Johnsen, Ø. , SkrØPpa, T. , Cheddadi, R. , Van Der Knaap, W. O. , LataŁOwa, M. , TerhÜRne‐Berson, R. , Litt, T. , Geburek, T. , Brochmann, C. & Sperisen, C. (2008). Genetic consequences of glacial survival and postglacial colonization in Norway spruce: combined analysis of mitochondrial DNA and fossil pollen. Molecular Ecology 17, 4134–4150. [DOI] [PubMed] [Google Scholar]
- Torrence, C. & Compo, G. P. (1998). A practical guide to wavelet analysis. Bulletin of the American Meteorological Society 79, 61–78. [Google Scholar]
- Turvey, S. T. (2009). Holocene Extinctions. Oxford University Press, New York. [Google Scholar]
- Tzedakis, P. C. (2010). The MIS 11‐MIS 1 analogy, southern European vegetation, atmospheric methane and the "early anthropogenic hypothesis". Climate of the Past 6, 131–144. [Google Scholar]
- Tzedakis, P. C. , Raynaud, D. , McManus, J. F. , Berger, A. , Brovkin, V. & Kiefer, T. (2009). Interglacial diversity. Nature Geoscience 2, 751–755. [Google Scholar]
- Tzedakis, P. C. , Wolff, E. W. , Skinner, L. C. , Brovkin, V. , Hodell, D. A. , McManus, J. F. & Raynaud, D. (2012). Can we predict the duration of an interglacial? Climate of the Past 8, 1473–1485. [Google Scholar]
- van Aarde, R. J. & Jackson, T. P. (2007). Megaparks for metapopulations: addressing the causes of locally high elephant numbers in southern Africa. Biological Conservation 134, 289–297. [Google Scholar]
- Vartanyan, S. L. , Arslanov, K. A. , Karhu, J. A. , Possnert, G. & Sulerzhitsky, L. D. (2008). Collection of radiocarbon dates on the mammoths (Mammuthus primigenius) and other genera of Wrangel Island, northeast Siberia, Russia. Quaternary Research 70, 51–59. [Google Scholar]
- Vartanyan, S. L. , Arslanov, K. A. , Tertychnaya, T. V. & Chernov, S. B. (1995). Radiocarbon dating evidence for mammoths on Wrangel Isand, Arctic Ocean until 2000 BC. Radiocarbon 37, 1–6. [Google Scholar]
- Vavrus, S. , Philippon‐Berthier, G. , Kutzbach, J. E. & Ruddiman, W. F. (2011). The role of GCM resolution in simulating glacial inception. The Holocene 21, 819–830. [Google Scholar]
- Vavrus, S. , Ruddiman, W. & Kutzbach, J. (2008). Climate model tests of the anthropogenic influence on greenhouse‐induced climate change: the role of early human agriculture, industrialization, and vegetation feedbacks. Quaternary Science Reviews 27, 1410–1425. [Google Scholar]
- Walker, M. J. C. , Coope, G. R. & Lowe, J. J. (1993). The Devensian (Weichselian) Lateglacial palaeoenvironmental record from Gransmoor, East Yorkshire, England: a contribution to the ‘North Atlantic seaboard programme’ of IGCP‐253, ‘Termination of the Pleistocene’. Quaternary Science Reviews 12, 659–680. [Google Scholar]
- Wallace, A. (1876). The Geographical Distribution of Animals, with a Study of the Relations of Living and Extinct Faunas as Elucidating the Past Changes of the Earth's Surface. Macmillan, London. [Google Scholar]
- Wang, X. , Auler, A. S. , Edwards, R. L. , Cheng, H. , Ito, E. , Wang, Y. , Kong, X. & Solheid, M. (2007). Millennial‐scale precipitation changes in southern Brazil over the past 90,000 years. Geophysical Research Letters 34, L23701. [Google Scholar]
- Wang, Y. J. , Cheng, H. , Edwards, R. L. , An, Z. S. , Wu, J. Y. , Shen, C. C. & Dorale, J. A. (2001). A high‐resolution absolute‐dated late Pleistocene monsoon record from Hulu Cave, China. Science 294, 2345–2348. [DOI] [PubMed] [Google Scholar]
- Watrin, J. , Lézine, A.‐M. & Hély, C. (2009). Plant migration and plant communities at the time of the “green Sahara”. Comptes Rendus Geoscience 341, 656–670. [Google Scholar]
- Werdelin, L. & Lewis, M. E. (2013). Temporal change in functional richness and evenness in the eastern African Plio‐Pleistocene carnivoran guild. PLoS One 8, e57944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werdelin, L. & Sanders, W. J. (2010). Cenozoic Mammals of Africa. University of California Press, Los Angeles. [Google Scholar]
- West, G. B. , Brown, J. H. & Enquist, B. J. (1999). The fourth dimension of life: fractal geometry and allometric scaling of organisms. Science 284, 1677–1679. [DOI] [PubMed] [Google Scholar]
- White, C. R. & Seymour, R. S. (2005). Allometric scaling of mammalian metabolism. Journal of Experimental Biology 208, 1611–1619. [DOI] [PubMed] [Google Scholar]
- Whittaker, R. H. (1970). Communities and Ecosystems. Macmillan, New York. [Google Scholar]
- Whittaker, R. J. , Triantis, K. A. & Ladle, R. J. (2008). A general dynamic theory of oceanic island biogeography. Journal of Biogeography 35, 977–994. [Google Scholar]
- Williams, J. W. , Shuman, B. N. , Webb, T. , Bartlein, P. J. & Leduc, P. L. (2004). Late‐quaternary vegetation dynamics in North America: scaling from taxa to biomes. Ecological Monographs 74, 309–334. [Google Scholar]
- Williams, M. (2001). Morphoclimatic maps at 18 ka, 9 ka & 0 ka In Atlas of Billion‐Year Earth History of Australia and Neighbors in Gondwanaland (ed. Veevers J.), pp. 45–48. GEOMOC Press, Syndey. [Google Scholar]
- Willis, K. , Bennett, K. D. , Burrough, S. , Macias‐Fauria, M. & Tovar, C. (2013). Determining the response of African biota to climate change: using the past to model the future. Philosophical Transactions of the Royal Society B: Biological Sciences 368, , 20120491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmshurst, J. M. , Anderson, A. J. , Higham, T. F. & Worthy, T. H. (2008). Dating the late prehistoric dispersal of Polynesians to New Zealand using the commensal Pacific rat. Proceedings of the National Academy of Sciences 105, 7676–7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfarth, B. , Veres, D. , Ampel, L. , Lacourse, T. , Blaauw, M. , Preusser, F. , Andrieu‐Ponel, V. , Kéravis, D. , Lallier‐Vergès, E. , Björck, S. , Davies, S. M. , de Beaulieu, J.‐L. , Risberg, J. , Hormes, A. , Kasper, H. U. , et al. (2008). Rapid ecosystem response to abrupt climate changes during the last glacial period in western Europe, 40–16 ka. Geology 36, 407–410. [Google Scholar]
- Wolff, E. W. , Chappellaz, J. , Blunier, T. , Rasmussen, S. O. & Svensson, A. (2010). Millennial‐scale variability during the last glacial: the ice core record. Quaternary Science Reviews 29, 2828–2838. [Google Scholar]
- Wood, J. R. , Alcover, J. A. , Blackburn, T. M. , Bover, P. , Duncan, R. P. , Hume, J. P. , Louys, J. , Meijer, H. J. , Rando, J. C. & Wilmshurst, J. M. (2017). Island extinctions: processes, patterns, and potential for ecosystem restoration. Environmental Conservation 44, 348–358. [Google Scholar]
- Wroe, S. & Field, J. (2006). A review of the evidence for a human role in the extinction of Australian megafauna and an alternative interpretation. Quaternary Science Reviews 25, 2692–2703. [Google Scholar]
- Wroe, S. , Field, J. H. , Archer, M. , Grayson, D. K. , Price, G. J. , Louys, J. , Faith, J. T. , Webb, G. E. , Davidson, I. & Mooney, S. D. (2013). Climate change frames debate over the extinction of megafauna in Sahul (Pleistocene Australia–New Guinea). Proceedings of the National Academy of Sciences 110, 8777–8781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, M. , Schurgers, G. , Rummukainen, M. , Smith, B. , Samuelsson, P. , Jansson, C. , Siltberg, J. & May, W. (2016). Vegetation–climate feedbacks modulate rainfall patterns in Africa under future climate change. Earth System Dynamics 7, 627–647. [Google Scholar]
- Yin, Q. & Berger, A. (2015). Interglacial analogues of the Holocene and its natural near future. Quaternary Science Reviews 120, 28–46. [Google Scholar]
- Zazula, G. D. , Froese, D. G. , Elias, S. A. , Kuzmina, S. & Mathewes, R. W. (2007). Arctic ground squirrels of the mammoth‐steppe: paleoecology of Late Pleistocene middens (∼24000‐29450 14 C yr BP), Yukon Territory, Canada. Quaternary Science Reviews 26, 979–1003. [Google Scholar]
- Zazula, G. D. , MacPhee, R. D. , Southon, J. , Nalawade‐Chavan, S. , Reyes, A. V. , Hewitson, S. & Hall, E. (2017). A case of early Wisconsinan “over‐chill”: new radiocarbon evidence for early extirpation of western camel (Camelops hesternus) in eastern Beringia. Quaternary Science Reviews 171, 48–57. [Google Scholar]
- Zazula, G. D. , MacPhee, R. D. E. , Metcalfe, J. Z. , Reyes, A. V. , Brock, F. , Druckenmiller, P. S. , Groves, P. , Harington, C. R. , Hodgins, G. W. L. , Kunz, M. L. , Longstaffe, F. J. , Mann, D. H. , McDonald, H. G. , Nalawade‐Chavan, S. & Southon, J. R. (2014). American mastodon extirpation in the Arctic and Subarctic predates human colonization and terminal Pleistocene climate change. Proceedings of the National Academy of Sciences 111, 18460–18465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zazula, G. D. , Schweger, C. E. , Beaudoin, A. B. & McCourt, G. H. (2006). Macrofossil and pollen evidence for full‐glacial steppe within an ecological mosaic along the Bluefish River, eastern Beringia. Quaternary International 142, 2–19. [Google Scholar]
- Zimov, S. A. , Zimov, N. S. , Tikhonov, A. N. & Chapin, F. S. (2012). Mammoth steppe: a high‐productivity phenomenon. Quaternary Science Reviews 57, 26–45. [Google Scholar]


