Summary
Background
Current guidelines recommend echinocandins as first‐line therapy for candidemia. However, several non‐Candida yeast are non‐susceptible to echinocandins (echinocandin non‐susceptible yeast, ENSY), including Cryptococcus, Geotrichum, Malassezia, Pseudozyma, Rhodotorula, Saprochaete, Sporobolomyces and Trichosporon. In laboratories that are not equipped with rapid diagnostic tools, it often takes several days to identify yeast, and this may lead to inappropriate presumptive use of echinocandins in patients with ENSY fungemia. The aim of this study was to determine the distribution of ENSY species during a 1‐year, laboratory surveillance programme in Asia.
Methods
Non‐duplicate yeast isolated from blood or bone marrow cultures at 25 hospitals in China, Hong Kong, India, Singapore, Taiwan and Thailand were analysed. Isolates were considered to be duplicative if they were obtained within 7 days from the same patient.
Results
Of 2155 yeast isolates evaluated, 175 (8.1%) were non‐Candida yeast. The majority of non‐Candida yeast were ENSY (146/175, 83.4%). These included Cryptococcus (109 isolates), Trichosporon (23), Rhodotorula (10) and Malassezia (4). The proportion of ENSY isolates (146/2155, 6.7%) differed between tropical (India, Thailand and Singapore; 51/593, 8.6%) and non‐tropical countries/regions (China, Hong Kong and Taiwan; 95/1562, 6.1%, P = 0.038). ENSY was common in outpatient clinics (25.0%) and emergency departments (17.8%) but rare in intensive care units (4.7%) and in haematology‐oncology units (2.9%). Cryptococcus accounted for the majority of the non‐Candida species in emergency departments (21/24, 87.5%) and outpatient clinics (4/5, 80.0%).
Conclusions
Isolation of non‐Candida yeast from blood cultures was not rare, and the frequency varied among medical units and countries.
Keywords: candidemia, echinocandin, fungemia, presumptive therapy, yeast
1. INTRODUCTION
The epidemiology of yeast infections and fungemia continues to evolve throughout the world, in parallel with advances in medical care for critically ill and immunocompromised patients and the extensive use of antifungal agents.1, 2, 3, 4, 5 Among human fungal pathogens, Candida species are the most common yeast that cause bloodstream infections. In the majority of guidelines, echinocandins are the recommended first‐line therapy for candidemia, due to their clinical efficacy, fungicidal activity, favourable safety profile, and limited drug interactions, and concerns about fluconazole resistance.6, 7, 8, 9, 10, 11
Among non‐Candida yeast, Cryptococcus is the most common fungal pathogen that causes community‐acquired invasive fungal disease and is intrinsically resistant to echinocandins.4, 12, 13 In Taiwan, the proportion of Cryptococcus neoformans from blood or bone marrow increased from 14% (8/59) between 1957 and 1972 to 33% (29/87) between 1982 and 1997.14 In addition, the emergence of rare yeast species such as Trichosporon and Rhodotorula poses a major threat because of their low susceptibility and potential to develop resistance to one or more antifungal agents.4, 12, 13, 15 Overall, common human yeast pathogens known to be intrinsically resistant or non‐susceptible to echinocandins (echinocandin non‐susceptible yeast, ENSY), including Cryptococcus, Geotrichum, Malassezia, Pseudozyma, Rhodotorula, Saprochaete, Sporobolomyces and Trichosporon.4, 12, 15, 16, 17
Species identification is therefore important in order to target echinocandin therapy only at patients with susceptible yeast infections. However, it usually takes an additional 1‐3 days to identify yeast to species level using either manual methods or commercially available API ID32C, AuxaColor and Vitek 2 systems.18, 19 This delay in identification means that, in some cases, inappropriate echinocandin therapy may be initiated in patients with reported yeast isolation from blood. This underlines the limits of presumptive treatment for fungemia and stresses the necessity to introduce rapid identification methods for yeast species identification.19
However, it is concerning that in a recent survey of mycology laboratory practice across seven Asian countries, rapid identification methods such as matrix‐assisted laser desorption ionisation‐time of flight mass spectrometry, and molecular identification methods such as PCR and sequencing were available only in 27 laboratories (12.3%) and 37 (16.9%) among 219 respondents, respectively.20 Most laboratories that perform identification with MALDI still require a subculture, which delays identification by 1 to 3 days (especially for slower growing basidiomycetous yeast). The potential impact of presumptive treatment with echinocandins for fungemia remains uncertain.
The aim of the current collaborative study was to determine the frequency of isolation from blood of yeast species that are intrinsically resistant or non‐susceptible to echinocandins. The study was based on laboratory‐based surveillance at 25 hospitals located in six Asian countries/regions.21 We also reviewed and compared published data around the world.
2. MATERIALS AND METHODS
2.1. Study design and mycology data collection
This was a 1‐year, cross‐sectional, laboratory‐based surveillance study conducted between July 1, 2010 and June 30, 2011. It was designed by the Asia Fungal Working Group (AFWG) under the auspices of the International Society for Human and Animal Mycology. A total of 25 hospitals participated in the study, located in China (10 hospitals), Hong Kong (1), India (4), Singapore (1), Taiwan (6) and Thailand (3). Details of the background of these hospitals, the capacity and practice of their mycology laboratories, and the incidence and distribution of candidemia have been published elsewhere.21 The study was approved by the Institutional Review Board or Research Ethics Committee at 21 of the hospitals; approval for research was waived at the other four centres.
Fungi were identified by the local microbiology or mycology laboratories at each study site. Blood culture systems and methods for fungal identification were as previously described.21 Microbiology laboratories in the participating hospitals identify the yeast by morphology (17 of 25 hospitals), CHROMagar (15), API20C of ID32C (17), manual assimilation/fermentation tests (6) and automatic methods (such as Vitek) (12). Only four hospitals provided the molecular identification methods such as PCR and sequencing in routine practice during the study period.
The data recorded for each isolate included the date of collection, hospital service, genus and species, whenever available. Duplicates were removed from the analysis. Yeast isolates were considered to be duplicate if they came from the same source type in the same patient, within 7 days of each other, and the final identifications were the same.22 The frequencies of ENSY among non‐duplicate yeast isolated from blood cultures were analysed; these included Cryptococcus, Geotrichum, Malassezia, Pseudozyma, Rhodotorula, Saprochaete, Sporobolomyces and Trichosporon.4, 12, 15, 16, 17
2.2. Statistical analysis
Statistical analyses were performed using SPSS v19 (SPSS Inc., Chicago, IL, USA). Categorical variables were analysed using the chi‐square test, and continuous variables were compared by Student's t‐test. A P‐value < 0.05 was considered to be statistically significant.
3. RESULTS
3.1. Fungal isolates
From 51 254 clinical isolates submitted across the 25 participating hospitals, 2155 non‐duplicate yeast isolates from blood, and bone marrow specimens were included in the present analysis. Of these, 1980 (91.9%) isolates were Candida species and 175 (8.1%) were other, non‐Candida yeast (Table 1). Among the 175 non‐Candida yeast isolates, the majority were ENSY (146/175, 83.4%) which included Cryptococcus (109), Trichosporon (23), Rhodotorula (10) and Malassezia (4). In this study cohort, there were no Geotrichum, Pseudozyma, Saprochaete or Sporobolomyces which are intrinsically resistant to echinocandins.
Table 1.
Distribution of non‐duplicate yeast isolates in blood or bone marrow specimens
| Fungus | Number of isolates | (%) |
|---|---|---|
| Total yeast isolates | 2155 | 100 |
| Candida species | 1980 | 91.9 |
| Non‐Candida spp. | 175 | 8.1 |
| Cryptococcus speciesa , b | 109 | 5.1 |
| Trichosporon speciesa , c | 23 | 1.1 |
| Rhodotorula speciesa | 10 | 0.5 |
| Kodamaea (Pichia) ohmeri d | 7 | 0.3 |
| Malassezia speciesa , d | 4 | 0.2 |
| Hansenula anomala (Pichia anomala)d | 4 | 0.2 |
| Hansenula polymorpha d | 2 | 0.1 |
| Yarrowia lipolytica d | 2 | 0.1 |
| Other non‐Candida yeaste | 14 | 0.6 |
Yeast that are intrinsically resistant or with high probability of non‐susceptibility to echinocandins and ESCMID, and ECMM support a recommendation against use of echinocandins.12
Cryptococcus neoformans (102 isolates), Cryptococcus laurentii (2) and Cryptococcus spp. (5).
Trichosporon asahii accounted for 9 of these isolates.
Kodamaea (Pichia) ohmeri (7 isolates; 4 from Taiwan and 3 from China), Hansenula anomala (Pichia anomala) (4 isolates; 2 from Taiwan and 2 from India), Hansenula polymorpha (2 isolates; both from China), Malassezia species (4 isolates; 2 from Taiwan, 1 from Hong Kong and 1 from Thailand), Yarrowia lipolytica (2 isolates; both from Taiwan).
Fourteen isolates were not reported to a genus level.
3.2. Genus distribution by country/region
The proportions of ENSY among yeast‐in‐blood isolates (overall, 146/2155, 6.7%) from each country/region are shown in Figure 1. ENSY proportions in most country/region are below 10%, except for Thailand, which ENSY proportions >20% were observed. The proportion of ENSY isolates in tropical countries/regions (India, Thailand and Singapore) is higher than that in non‐tropical countries/regions (China, Hong Kong and Taiwan) (8.6% [51/593] vs 6.1% [95/1562], P = 0.038). However, the higher rate of Cryptococcus spp. in Thailand when compared with the rates in other countries is the main reason for the above‐mentioned difference because, after excluding Cryptococcus spp., no significant difference was observed (1.6% [9/551] vs 1.9% [28/1495], P = 0.852).
Figure 1.
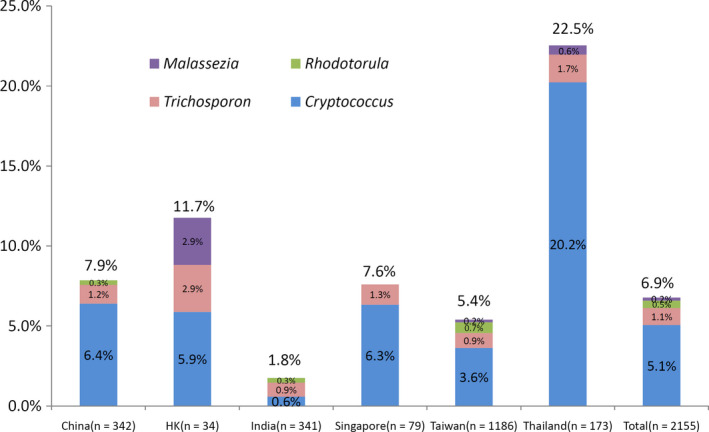
Distribution within each country/region of 146 yeast isolated from blood and bone marrow specimens that were intrinsically resistant or had a high probability of non‐susceptibility to echinocandins. HK, Hong Kong
Cryptococcus spp. were most frequently observed in Thailand (20.2% of 173 yeast‐in‐blood isolates) but were rare in India (0.6% of 341 isolates) (Figure 1). A total of 23 Trichosporon isolates were reported, of which 11 were from Taiwan and four were from China. Among 10 Rhodotorula isolates, eight were reported from Taiwan.
3.3. Species distribution according to medical services
Among the 2155 yeast‐in‐blood isolates included in this analysis, information on the type of medical service from which they came were available for 2144 isolates. Of the 11 isolates for which medical service information was not available, three were ENSY. Thus, 143 ENSY were analysed according to medical service.
Overall, almost two‐thirds (59.4%) of ENSY came from general wards other than haematology‐oncology units (Figure 2A). However, this was largely because most yeast isolates overall came from these wards. When analysed according to each different type of medical service, the proportion of total isolates that were ENSY varied substantially, ranging from 2.9% in haematology‐oncology wards to 25.0% in outpatient clinics (Figure 2B). The proportions of ENSY in outpatient clinics is higher than haematology‐oncology wards and intensive care units (ICUs) (25.0% [5/20] vs 2.9% [6/206], P = 0.001; 25.0% [5/20] vs 4.8% [23/482], P = 0.003). The proportions of ENSY in emergency rooms (ERs) are higher than haematology‐oncology wards and ICUs (17.8% [24/135] vs 2.9% [6/206], P = 0.001; 17.8% [24/135] vs 4.8% [23/482], P = 0.003).
Figure 2.

Proportion of the total number of 143 yeast isolated from blood and bone marrow specimens that were intrinsically resistant or had a high probability of non‐susceptibility to echinocandins coming from each hospital service (A). Distribution within each medical service of yeast that were intrinsically resistant or had a high probability of non‐susceptibility to echinocandins (B). “Other wards” included general wards other than those specialising in haematology‐oncology. ER, emergency rooms; Hema, haematology‐oncology ward; ICU, intensive care unit; OPD, outpatient clinic
Cryptococcus accounted for 87.5% (21/24) of non‐Candida species isolated from ERs and 80.0% (4/5) from outpatient clinics. The proportion of Cryptococcus among non‐Candida species in ERs was significantly higher than in ICUs (52.1% [12/23], P = 0.008) and haematology‐oncology wards (33.3% [2/6], P = 0.016).
3.4. Species distribution by season
Based on the date provided, the season of collection could be assigned to each isolate. The proportion of ENSY varied from 4.2% in autumn to 8.2% in winter, but these differences were not statistically significant. The proportions of individual species (Cryptococcus, Trichosporon and Rhodotorula) also did not vary substantially according to season, which was either due to small sample sizes or variations between countries (Figure S1).
4. DISCUSSION
In this 1‐year surveillance study conducted in 25 hospitals across Asia, yeast that were intrinsically resistant or non‐susceptible to echinocandins accounted for 6.7% of 2155 non‐duplicate isolates from the blood. The frequency varied from 2.9% in haematology‐oncology wards to 25.0% in outpatient clinics.
Most non‐Candida yeast are known to be resistant to echinocandins;4 and indeed, this study showed that 83.4% of such isolates were ENSY. Hence, it is clinically significant that non‐Candida yeast accounted for 8.1% of all yeast‐in‐blood isolates (Table 1). Moreover, proportions varied substantially between the six countries/regions included in the survey, with non‐Candida species making up 24.9% of isolates from Thailand but only 2.3% of those from India. This wide variation in the proportion of non‐Candida species accords with other reports from around the world (summarised in Table 2). Overall, frequencies were higher in tropical countries, such as Mexico, Brazil and Thailand.23, 24, 25 Our study also showed similar results. (Figure 2)
Table 2.
The proportions of non‐Candida yeast in blood specimens by region/country or patient populationa
| Region/country | Setting/population | Study period | Patients (inpatients, outpatients or both) | Number of yeast isolates | Proportion of non‐Candida yeast | Proportion of Cryptococcus spp. in non‐Candida yeast | Remark | References |
|---|---|---|---|---|---|---|---|---|
| Global | ||||||||
| Artemis study | Multinational, multicentre, general | 1997‐2007 | both | 11 240, non‐Candida yeast | 32.2% | Saccharomyces (11.7%), Trichosporon (10.6%), and Rhodotorula (4.1%) were the leading three non‐Candida fungi. | 22 | |
| Americas | ||||||||
| US | Single hospital | 1998‐2010 | both | 2984 | 3.1% | NA | Rhodotorula spp. (21 isolates), Saccharomyces (8) and Trichosporon (8) were the leading three non‐Candida fungi. | 31 |
| Mexico | Single hospital | 2005‐2014 | both | 91 | 34.1% | 93.9% | Underlying disease was HIV/AIDS in 63% of cases | 23 |
| Argentina | Multicentre | 2007‐2008 | inpatients | 461 | 8.9% | 78.0% | C. neoformans was mainly associated with HIV/AIDS | 28 |
| Paediatric patients (<15 y) | 177 | 3.4% | 0% | 2 isolates (33.3%) were Trichosporon spp. | ||||
| Brazil | Single hospital | 1996‐2004 | both | 1195 | 14.6% | 45.4% | Pichia anomala (18.4%) and Rhodotorula spp (16.1%) were also common. | 24 |
| Brazil | Single hospital | 2001‐2003 | both | 229 | 2.6% | 100% | 25 | |
| 2011‐2013 | 288 | 5.9% | 100% | |||||
| Europe | ||||||||
| Europe | Multicentre, cancer patients | 2005‐2009 | both | 279 | 7.5% | 19.0% | 8 Trichosporon spp. (38.1%) were the most common isolates in non‐Candida yeast | 32 |
| Sweden | Nationwide | 2005‐2006 | both | 403 | 1.0% | 0% | 33 | |
| Belgium | Multicentre | 2005‐2006 | both | 412 | 7.8% | 12.5% | 21 Saccharomyces cerevisiae (65.6%) were the most common isolates in non‐Candida yeast | 26 |
| Denmark | Nationwide | 2004‐2009 | both | 3982 | 1.1% | 31.8% | 22 S. cerevisiae (50%) were the most common isolates in non‐Candida yeast | 27 |
| France | Regional, Paris | NA | 3668 | 5.1% | 73.3% | 19 Geotrichum (10.1%) were the second common non‐Candida yeast | 12 | |
| Italy | Single hospital | 2005‐2013 | both | 1250 | 1.9% | 29.2% | Non‐Candida yeast were dominated by Rhodotorula spp. (n = 9) and C. neoformans, which together accounted for 1.3% of all bloodstream isolates and 66.6% of all non‐Candida yeast | 34 |
| Portugal | Multicentre | 2011‐2012 | inpatients | 240 | 3.8% | 88.9% | C. neoformans was the most common isolate in non‐Candida yeast | 29 |
| Spain | Multicentre | 2009‐2010 | both | 1374 | 1.9% | 38.5% | C. neoformans was the most common isolate in non‐Candida yeast | 35 |
| Spain | Multicentre paediatric patients (<15 y) | 2009‐2010 | inpatients | 203 | 1.0% | 0% | Only two isolates were non‐Candida yeast; one was Trichosporon asahii, the other was Rhodotorula glutinis | 36 |
| Spain | Multicentre | 2010‐2011 | both | 781 | 2.4% | 26.3% | C. neoformans was the most common non‐Candida yeast isolate, followed by Trichosporon asahii (15.8%) | 37 |
| Asia | ||||||||
| Asia | 25 hospitals | 2010‐2011 | both | 2071 | 7.8% | 67.7% | This study | |
| China | 10 hospitals | 2010‐2011 | both | 325 | 9.8% | 68.8% | This study | |
| China | Single hospital | 2010‐2012 | inpatients | 141 | 7.8% | 45.5% | C. neoformans was the most common isolate in non‐Candida yeast | 38 |
| China | 4 hospitals | 2012‐2013 | both | 137 | 8.0% | 9% | Pichia spp. were the most common isolates in non‐Candida yeast (45%) | 39 |
| Hong Kong | Single hospital | 2010‐2011 | both | 34 | 11.8% | 50% | This study | |
| India | Single hospital, paediatric patients | 2007 | inpatients | 102 | 21% | 0% | P. anomala was the most common isolate (85.7%), followed by Trichosporon asahii (14.3%) | 40 |
| India | 4 hospitals | 2010‐2011 | inpatients | 305 | 2.6% | 25% | This study | |
| Singapore | Single hospital | 2010‐2011 | inpatients | 78 | 7.7% | 83.3% | This study | |
| Taiwan | 6 hospitals | 2010‐2011 | both | 1160 | 6.2% | 59.7% | This study | |
| Thailand | 3 hospitals | 2010‐2011 | both | 169 | 23.1% | 89.7% | This study | |
Pubmed search using the following phrase: (fungemia [Title/Abstract]) OR yeast in blood cultures [Title/Abstract])) AND (“2007/1/1”[Date ‐ Publication]: “2015/11/31”[Date ‐ Publication]), accessed on 30 November 2015, limited to English literature. The publications that were most representative of the country/region or the most up‐to‐date were selected when more than one was found.
In the present study, Cryptococcus was the predominant ENSY species. Cryptococcus is the leading ENSY pathogen in many countries worldwide (Table 2), although there are exceptions. For example, in Belgium and Denmark, Saccharomyces cerevisiae were the predominant non‐Candida isolates.26, 27 Higher proportions of Cryptococcus in blood are closely related to human immunodeficiency diseases.23, 28, 29 The proportions of non‐Candida yeast varied from 1.0% to 34% in different countries, patient papulations and medical settings (Table 2). In our study, almost a quarter of non‐Candida yeast occurred in the outpatient clinics or ERs, which may explain the difference between hospital‐based studies and inpatient‐only studies (Table 2).
Not all yeast are susceptible to echinocandins.4, 12, 13, 15, 17 Furthermore, there are currently no guidelines or recommendations that explicitly describe how to select appropriate presumptive therapy for fungemia in regions where rapid identification systems are not available and non‐Candida yeast‐in‐blood is common. Physicians in these countries should be familiar with common presentations and risk factors associated with Cryptococcus and other non‐Candida infections (Table S1). For example, in Asia, cryptococcemia may be a possibility in a cirrhotic patient presenting at an ER, with altered mental status and community‐acquired sepsis of unknown source. Rapid microbiological evidence can be obtained by examining spinal fluid with India ink and detecting antigens in serum. These additional clues and tests may help physicians to select the most appropriate antifungal agent.12
This study has several limitations. First, this laboratory‐based surveillance study is limited by the overall lack of clinical, outcome, and epidemiologic data, and was unable to assess the impact among patients with ENSY fungemia treated with echinocandins. Second, although patient isolates were deduplicated if within 7 days, this study did not provide the numbers of patients with these 2155 yeast isolates represent. Third, we did not collect isolates for identification at a central reference laboratory. Among 175 non‐Candida isolates, fourteen isolates were not identified to a genus level. Therefore, we probably underestimated the frequency and impact of rare and emerging yeast.4, 12, 16 Forth, antifungal susceptibility testing for these ENSY were not performed during this surveillance; and hence, we are unable to comment on the emergence of resistance to echinocandins.
The main strength of this study is an Asian multicentre laboratory‐based data analysis that the frequency of isolation from the blood of yeast species that are ENSY. The ENSY identified in this study were mainly belonged to Basidiomycetes (Cryptococcus, Trichosporon and Rhodotorula), a group well known intrinsically resistant to echinocandins. On the other hand, the non‐Candida Ascomycetes (such as Pichia, Hansenula and Saccharomyces) were rarely identified. A recent large‐scale study involving 1698 yeast isolates showed that Basidiomycetes are less susceptible all antifungal drugs tested compared to Ascomycetes.17
In conclusion, this study revealed that yeast that are intrinsically resistant or non‐susceptibility to echinocandins are not uncommonly isolated from blood cultures in representative countries in Asia. These data suggest that an operational algorithm for management of patients when yeast are detected in blood specimens is warranted in areas where non‐Candida yeast‐in‐blood is common and in hospitals that do not possess the latest diagnostic technology. Improved communication among physicians and laboratories, as well as the acquisition of modern, rapid laboratory equipment that can provide results to the species level (and/or in vitro susceptibilities) are needed to guide antifungal therapy for yeast isolated from blood cultures.30
AUTHOR CONTRIBUTIONS
This study was coordinated by the principal investigators, YC Chen and BH Tan. YC Chen initiated the project and core AFWG members (YC Chen, BH Tan, A Chakrabarti, RY Li, A Chindamporn, AL Tan, Z Liu, AK Patel, SP Watcharananan and PL Sun) designed the protocol together. YC Chen supervised SY Lin, LY Hsu and UI Wu for data analyses. SY Lin, PL Lu, JH Yang and YC Chen prepared the manuscript. The authors had full access to all the data in the study, critical review and comments during preparation, and had final responsibility for the decision to submit for publication.
TRANSPARENCY DECLARATIONS
YC Chen has received research grants from Pfizer and Gilead, those grants have nothing to do with this study. All authors declared no conflict of interests.
Supporting information
ACKNOWLEDGMENTS
We are grateful to the mycology staff at each hospital for their sustained efforts to support the clinical staff and for their commitment to improving practice in mycology laboratories. Additionally, the authors are grateful to Professor Calvin Kunin for critical review of the manuscript.
APPENDIX 1.
Other members of the study network
Additional members of the study network include Ying‐Chun Xu, Department of Clinical Laboratory, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing, China; Hui Wang, Department of Clinical Laboratory, Peking University People's Hospital Peking University, Beijing, China; Zi‐Yong Sun, Department of Clinical Laboratory, Tongji Hospital, Huazhong University of Science and Technology, Wuhan, China; Lan‐Lan Wang, Department of Clinical Laboratory, West China Hospital, Sichuan University, Chengdu, China; Juan Lu, Department of Clinical Laboratory, The First Clinical College of Harbin Medical University, Harbin, China; Qing Yang, Department of Clinical Laboratory, The First Affiliated Hospital of College of Medicine, Zhejiang University, Hangzhou, China; Qiang‐Qiang Zhang, Department of Dermatology, Huashan Hospital, Fudan University, Shanghai, China; Hai‐Feng Shao, Department of Clinical Laboratory, Nanjing General Hospital of Nanjing Military Commend, Nanjing, China; Kang Liao, Department of Clinical Laboratory, The First Affiliated Hospital of Sun Yat‐Sen University, Guangzhou, China; Patrick CY Woo, Department of Microbiology, The University of Hong Kong, Hong Kong; Rungmei SK Marak, Department of Microbiology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India; Anupma Jyoti Kindo, Department of Microbiology, Sri Ram Chandra Medical College and Research Institute, Chennai, India; Chieh‐Liang Wu, Department of Internal Medicine Veteran General Hospital, Taichung, Taiwan; Mao‐Wang Ho, Division of Infectious Diseases, China Medical University Hospital, Taichung, Taiwan; Lih‐Shinn Wang, Department of Internal Medicine, Buddhist Tzu Chi General Hospital, Hualien, Taiwan; and Pattaya Riengchan, Bhumibol Adulyadej Hospital, Bangkok, Thailand.
Lin S‐Y, Lu P‐L, Tan BH, et al.; on behalf of the Asia Fungal Working Group (AFWG) . The epidemiology of non‐Candida yeast isolated from blood: The Asia Surveillance Study. Mycoses. 2019;62:112–120. 10.1111/myc.12852
Funding information
This study was supported by a grant from Pfizer (201108019RC) and the Ministry of Science and Technology, Taiwan (NSC 102‐2314‐B‐002 ‐158 ‐MY3) to YC Chen. The sponsors had no role in the study design or conduct, preparation, review or approval of the manuscript or in the decision to submit the manuscript for publication.
Contributor Information
Yee‐Chun Chen, Email: yeechunchen@gmail.com.
the Asia Fungal Working Group (AFWG):
Ying‐Chun Xu, Hui Wang, Zi‐Yong Sun, Lan‐Lan Wang, Juan Lu, Qing Yang, Qiang‐Qiang Zhang, Hai‐Feng Shao, Kang Liao, Patrick CY Woo, Rungmei SK Marak, Anupma Jyoti Kindo, Chieh‐Liang Wu, Mao‐Wang Ho, Lih‐Shinn Wang, and Pattaya Riengchan
REFERENCES
- 1. Patterson TF. Advances and challenges in management of invasive mycoses. Lancet. 2005;366:1013‐1015. [DOI] [PubMed] [Google Scholar]
- 2. Maschmeyer G. The changing epidemiology of invasive fungal infections: new threats. Int J Antimicrob Agents. 2006;27(Suppl 1):3‐6. [DOI] [PubMed] [Google Scholar]
- 3. Pappas PG, Alexander BD, Andes DR, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant‐Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis. 2010;50:1101‐1111. [DOI] [PubMed] [Google Scholar]
- 4. Miceli MH, Diaz JA, Lee SA. Emerging opportunistic yeast infections. Lancet Infect Dis. 2011;11:142‐151. [DOI] [PubMed] [Google Scholar]
- 5. Chen PY, Chuang YC, Wang JT, et al. Comparison of epidemiology and treatment outcome of patients with candidemia at a teaching hospital in Northern Taiwan, in 2002 and 2010. J Microbiol Immunol Infect. 2014;47:95‐103. [DOI] [PubMed] [Google Scholar]
- 6. Mora‐Duarte J, Betts R, Rotstein C, et al. Comparison of caspofungin and amphotericin B for invasive candidiasis. N Engl J Med. 2002;347:2020‐2029. [DOI] [PubMed] [Google Scholar]
- 7. Kuse ER, Chetchotisakd P, da Cunha CA, et al. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidiasis: a phase III randomised double‐blind trial. Lancet. 2007;369:1519‐1527. [DOI] [PubMed] [Google Scholar]
- 8. Reboli AC, Rotstein C, Pappas PG, et al. Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med. 2007;356:2472‐2482. [DOI] [PubMed] [Google Scholar]
- 9. Andes DR, Safdar N, Baddley JW, et al. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient‐level quantitative review of randomized trials. Clin Infect Dis. 2012;54:1110‐1122. [DOI] [PubMed] [Google Scholar]
- 10. Cornely OA, Bassetti M, Calandra T, et al. ESCMID guideline for the diagnosis and management of Candida diseases 2012: non‐neutropenic adult patients. Clin Microbiol Infect. 2012;18(Suppl 7):19‐37. [DOI] [PubMed] [Google Scholar]
- 11. Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1‐e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arendrup MC, Boekhout T, Akova M, et al. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of rare invasive yeast infections. Clin Microbiol Infect. 2014;20(Suppl 3):76‐98. [DOI] [PubMed] [Google Scholar]
- 13. Castanheira M, Messer SA, Jones RN, et al. Activity of echinocandins and triazoles against a contemporary (2012) worldwide collection of yeast and moulds collected from invasive infections. Int J Antimicrob Agents. 2014;44:320‐326. [DOI] [PubMed] [Google Scholar]
- 14. Chen YC, Chang SC, Shih CC, et al. Clinical features and in vitro susceptibilities of two varieties of Cryptococcus neoformans in Taiwan. Diagn Microbiol Infect Dis. 2000;36:175‐183. [DOI] [PubMed] [Google Scholar]
- 15. Colombo AL, Padovan AC, Chaves GM. Current knowledge of Trichosporon spp. and trichosporonosis. Clin Microbiol Rev. 2011;24:682‐700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Farmakiotis D, Kontoyiannis DP. Epidemiology of antifungal resistance in human pathogenic yeasts: current viewpoint and practical recommendations for management. Int J Antimicrob Agents. 2017;50:318‐324. [DOI] [PubMed] [Google Scholar]
- 17. Desnos‐Ollivier M, Robert V, Raoux‐Barbot D, et al. Antifungal susceptibility profiles of 1698 yeast reference strains revealing potential emerging human pathogens. PLoS ONE. 2012;7:e32278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Paugam A, Ancelle T, Lortholary O, et al. Longer incubation times for yeast fungemia: importance for presumptive treatment. Diagn Microbiol Infect Dis. 2014;80:119‐121. [DOI] [PubMed] [Google Scholar]
- 19. Posteraro B, Efremov L, Leoncini E, et al. Are the conventional commercial yeast identification methods still helpful in the era of new clinical microbiology diagnostics? a meta‐analysis of their accuracy. J Clin Microbiol. 2015;53:2439‐2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chindamporn A, Chakrabarti A, Li RY, et al. Survey of laboratory practices for diagnosis of fungal infection in seven Asian countries – an Asia Fungal Working Group (AFWG) initiative. Med Mycol. 2017;56(4):416‐425. [DOI] [PubMed] [Google Scholar]
- 21. Tan BH, Chakrabarti A, Li RY, et al. Incidence and species distribution of candidaemia in Asia: a laboratory‐based surveillance study. Clin Microbiol Infect. 2015;21:946‐953. [DOI] [PubMed] [Google Scholar]
- 22. Pfaller MA, Diekema DJ, Gibbs DL, et al. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: 10.5‐year analysis of susceptibilities of noncandidal yeast species to fluconazole and voriconazole determined by CLSI standardized disk diffusion testing. J Clin Microbiol. 2009;47:117‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gaona‐Flores VA, Campos‐Navarro LA, Cervantes‐Tovar RM, et al. The epidemiology of fungemia in an infectious diseases hospital in Mexico city: a 10‐year retrospective review. Med Mycol. 2016;54:600‐604. [DOI] [PubMed] [Google Scholar]
- 24. De Almeida GM, Costa SF, Melhem M, et al. Rhodotorula spp. isolated from blood cultures: clinical and microbiological aspects. Med Mycol. 2008;46:547‐556. [DOI] [PubMed] [Google Scholar]
- 25. Castro LL, Schutze M, Bucker DH, et al. Prevalence of fungemia in a tertiary hospital: analysis of the last decade. Rev Assoc Med Bras. 1992;2016(62):315‐319. [DOI] [PubMed] [Google Scholar]
- 26. Costa‐de‐Oliveira S, Pina‐Vaz C, Mendonca D, et al. A first Portuguese epidemiological survey of fungaemia in a university hospital. Eur J Clin Microbiol Infect Dis. 2008;27:365‐374. [DOI] [PubMed] [Google Scholar]
- 27. Arendrup MC, Bruun B, Christensen JJ, et al. National surveillance of fungemia in Denmark (2004 to 2009). J Clin Microbiol. 2011;49:325‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cordoba S, Vivot W, Bosco‐Borgeat ME, et al. Species distribution and susceptibility profile of yeasts isolated from blood cultures: results of a multicenter active laboratory‐based surveillance study in Argentina. Rev Argent Microbiol. 2011;43:176‐185. [DOI] [PubMed] [Google Scholar]
- 29. Faria‐Ramos I, Neves‐Maia J, Ricardo E, et al. Species distribution and in vitro antifungal susceptibility profiles of yeast isolates from invasive infections during a Portuguese multicenter survey. Eur J Clin Microbiol Infect Dis. 2014;33:2241‐2247. [DOI] [PubMed] [Google Scholar]
- 30. Kothari A, Morgan M, Haake DA. Emerging technologies for rapid identification of bloodstream pathogens. Clin Infect Dis. 2014;59:272‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chitasombat MN, Kofteridis DP, Jiang Y, et al. Rare opportunistic (non‐Candida, non‐Cryptococcus) yeast bloodstream infections in patients with cancer. J Infect. 2012;64:68‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cornely OA, Gachot B, Akan H, et al. Epidemiology and outcome of fungemia in a cancer cohort of the Infectious Diseases Group (IDG) of the European Organization for Research and Treatment of Cancer (EORTC 65031). Clin Infect Dis. 2015;61:324‐331. [DOI] [PubMed] [Google Scholar]
- 33. Ericsson J, Chryssanthou E, Klingspor L, et al. Candidaemia in Sweden: a nationwide prospective observational survey. Clin Microbiol Infect. 2013;19:E218‐E221. [DOI] [PubMed] [Google Scholar]
- 34. Posteraro B, Spanu T, Fiori B, et al. Antifungal susceptibility profiles of bloodstream yeast isolates by Sensititre YeastOne over nine years at a large Italian teaching hospital. Antimicrob Agents Chemother. 2015;59:3944‐3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Peman J, Canton E, Quindos G, et al. Epidemiology, species distribution and in vitro antifungal susceptibility of fungaemia in a Spanish multicentre prospective survey. J Antimicrob Chemother. 2012;67:1181‐1187. [DOI] [PubMed] [Google Scholar]
- 36. Peman J, Canton E, Linares‐Sicilia MJ, et al. Epidemiology and antifungal susceptibility of bloodstream fungal isolates in pediatric patients: a Spanish multicenter prospective survey. J Clin Microbiol. 2011;49:4158‐4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Guinea J, Zaragoza O, Escribano P, et al. Molecular identification and antifungal susceptibility of yeast isolates causing fungemia collected in a population‐based study in Spain in 2010 and 2011. Antimicrob Agents Chemother. 2014;58:1529‐1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li W, Hu YA, Li FQ, et al. Distribution of yeast isolates from invasive infections and their in vitro susceptibility to antifungal agents: evidence from 299 cases in a 3‐year (2010 to 2012) surveillance study. Mycopathologia. 2015;179:397‐405. [DOI] [PubMed] [Google Scholar]
- 39. Dong D, Li Z, Zhang L, et al. Clinical and microbiological investigation of fungemia from four hospitals in China. Mycopathologia. 2015;179:407‐414. [DOI] [PubMed] [Google Scholar]
- 40. Chakrabarti A, Chatterjee SS, Rao KL, et al. Recent experience with fungaemia: change in species distribution and azole resistance. Scand J Infect Dis. 2009;41:275‐284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


