Abstract
Aims
Localised‐ and diffuse‐type tenosynovial giant cell tumours (TGCT) are regarded as different clinical and radiological TGCT types. However, genetically and histopathologically they seem indistinguishable. We aimed to correlate CSF1 expression and CSF1 rearrangement with the biological behaviour of different TGCT‐types with clinical outcome (recurrence).
Methods and results
Along a continuum of extremes, therapy‐naive knee TGCT patients with >3‐year follow‐up, mean age 43 (range = 6–71) years and 56% females were selected. Nine localised (two recurrences), 16 diffuse‐type (nine recurrences) and four synovitis as control were included. Rearrangement of the CSF1 locus was evaluated with split‐apart fluorescence in‐situ hybridisation (FISH) probes. Regions were selected to score after identifying CSF1‐expressing regions, using mRNA ISH with the help of digital correlative microscopy. CSF1 rearrangement was considered positive in samples containing >2 split signals/100 nuclei. Irrespective of TGCT‐subtype, all cases showed CSF1 expression and in 76% CSF1 rearrangement was detected. Quantification of CSF1‐expressing cells was not informative, due to the extensive intratumour heterogeneity. Of the four synovitis cases, two also showed CSF1 expression without CSF1 rearrangement. No correlation between CSF1 expression or rearrangement with clinical subtype and local recurrence was detected. Both localised and diffuse TGCT cases showed a scattered distribution in the tissue of CSF1‐expressing cells.
Conclusion
In diagnosing TGCT,CSF1 mRNA‐ISH, in combination with CSF1 split‐apart FISH using digital correlative microscopy, is an auxiliary diagnostic tool to identify rarely occurring neoplastic cells. This combined approach allowed us to detect CSF1 rearrangement in 76% of the TGCT cases. Neither CSF1 expression nor presence of CSF1 rearrangement could be associated with the difference in biological behaviour of TGCT.
Keywords: colony stimulating factor 1, FISH, giant cell tumour of tendon sheath, mRNA ISH, pigmented villonodular, rare diseases, synovitis, tenosynovial giant cell tumour
Introduction
Tenosynovial giant cell tumour (TGCT), previously known as pigmented villonodular synovitis (PVNS) and giant cell tumour of tendon sheath, is a rare, neoplastic lesion arising from the synovial lining of joints, bursae or tendon sheaths in predominantly young adults. Excluding digits, this mono‐articular disease is diagnosed most commonly around the knee or other weight‐bearing joints.1, 2, 3
Initially, TGCT was believed to be an inflammatory disease.4 After genomic aberrations were discovered, TGCT was evidently considered neoplastic.5, 6, 7, 8, 9, 10 Chromosomal aberrations include trisomy for chromosomes 5 and 7 and translocations involving the short arm of chromosome 1p11‐13, most commonly translocated to the chromosome 2q37 region. At the 1p13 breakpoint, the colony stimulating factor 1 (CSF1) gene is located. The translocation leads to a classical promoter fusion event in which the collagen 6A3 (COL6A3) promoter element is fused to CSF1. As a result, the fusion leads to deregulated expression of CSF1.11 The excessive CSF1 secretion attracts inflammatory cells that express the CSF1 receptor (CSF1R) (i.e. monocytes and macrophages). Consequently, in TGCT tissue, only a small percentage of cells (2–16%) are neoplastic, carrying the t(1;2) translocation. This phenomenon is coined ‘the landscape effect’.11, 12 Based on CSF1 rearrangements (translocation), two groups are described. The first group is defined by both CSF1 overexpression and CSF1 translocation, whereas the second group lacks the classical translocation. The latter group probably carries other rearrangements altering CSF1 regulation, leading to high CSF1 mRNA and CSF1 protein levels.12
According to the 2013 World Health Organisation (WHO) classification, TGCT is subdivided into a lobulated well circumscribed lesion (localised type) and a more locally aggressive lesion, involving a large part or all the synovial lining (diffuse type) (Figure 1).1, 2, 13 The standard choice of treatment was surgical resection of the lesional tissue, either arthroscopically or with an open resection.14, 15, 16, 17 The localised‐type TGCT is known to have a favourable course after resection (average recurrence rates <6%), while the diffuse‐type TGCT generally causes significant morbidity due to the high risk of local recurrence (>50% depending on surgical procedure and follow‐up time).15, 18, 19 Therefore, at present diffuse‐type TGCT is also treated with CSF1 inhibitors such as nilotinib, imatinib, pexidartinib, emactuzumab, cabrilazimab and MSC110.20 Long‐term efficacy data have not yet been reported with these newer agents.
Figure 1.
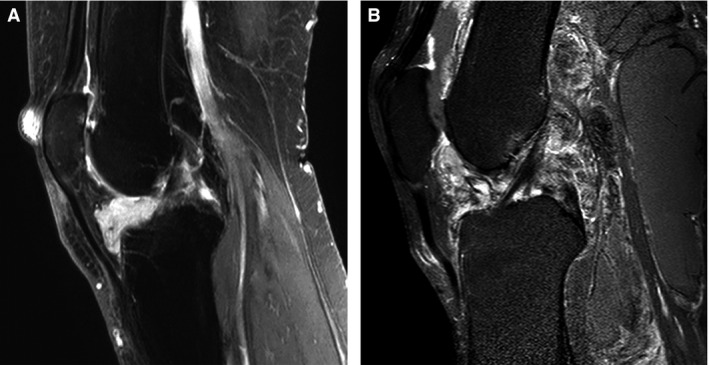
Localised and diffuse tenosynovial giant cell tumours (TGCT) sagittal T1‐weighted magnetic resonance (MR) image after intravenous contrast injection with fat suppression. Tumour region enhances by contrast injection. A, A localised‐TGCT involving Hoffa's fat pad in the anterior part of the left knee in a 55‐year‐old female patient (L4835). B, Left knee in a 61‐year‐old male patient with extensive recurrent diffuse TGCT located intra‐ and extra‐articular with an additional posterior large Baker's cyst including tumour (L3496).
Recurrent TGCT is rarely lethal, but is a chronic illness with substantial morbidity to the joint leading to functional and quality of life impairment, caused by the course of the disease itself and multiple treatments.21 Clinically, localised and diffuse TGCT are clearly two very different diseases. However, histopathologically they seem indistinguishable, with both subtypes containing an admixture of mononuclear cells (histiocyte‐like and larger cells) and multinucleated giant cells, lipid‐laden foamy macrophages (also known as xanthoma cells), siderophages (macrophages including haemosiderin depositions), stroma with lymphocytic infiltrate and some degree of collagenisation.1, 2
It remains unclear why localised and diffuse TGCT are microscopically and genetically identical but clinically distinct. Moreover, predictors for progressive disease or local recurrence are lacking. In this study, we investigate whether CSF1 overexpression and rearrangement are correlated with tumour characteristics (localised/diffuse TGCT) and clinical outcome (recurrence). We hypothesise that diffuse‐type TGCT, compared with localised‐type TGCT, would have a higher load of neoplastic cells. We expect that a higher tumour load is associated with recurrent disease.
Methods
Case Acquisition and Study Design
Subtypes of TGCT (localised or diffuse) were defined based on clinical features and radiological imaging according to the WHO 2013 classification.1, 2 Along a continuum of extremes, 25 patients with TGCT affecting the knee were selected carefully: patients with small or very large localised or diffuse lesions, with and without recurrent disease. All cases showed all the characteristic histological features of TGCT (mononuclear cells, giant cells, macrophages, siderophages, foam cells or lymphocyte clusters). Included patients were therapy‐naive (one diagnostic arthroscopy elsewhere was allowed) and treated with open synovectomy at the Leiden University Medical Centre (LUMC). A clinical follow‐up of at least 3 years was required for inclusion. For comparison, we used tissue specimens of four patients with non‐TGCT synovitis. Written informed consent was obtained from all patients. This study was performed in accordance with the Code of Conduct for responsible use in the Netherlands (Dutch Federation of Medical Scientific Societies) and approved by the local medical ethical committee (P13.029).
Inclusion of Selected Cases and Tissue Specimens
Nine localised‐ and 16 diffuse‐type TGCT patients were included, mean age at surgery 43 (range = 6–71) years, mean follow‐up 57 (range = 36–121) months (Table 1), with a slight female predominance (56%). Two localised‐ and nine diffuse‐type TGCT patients had recurrent disease, after mean 26 (range = 14–53) months. The mean age at surgery of the four patients with non‐TGCT synovitis was 53 (range = 44–65) years, including two (50%) females.
Table 1.
Descriptives of study population
| Localised | Localized recurrence | Diffuse | Diffuse recurrence | No TGCT | |
|---|---|---|---|---|---|
| Total number | 7 | 2 | 7 | 9 | 4 |
| Mean age at surgery (R), years | 33 (6–55) | 41 (20–62) | 54 (33–71) | 42 (17–63) | 53 (44–65) |
| Male:female | 5:2 | 0:2 | 2:5 | 4:5 | 2:2 |
| Mean time to recurrence (R), m | NA | 31 (18–44) | NA | 24 (14–53) | NA |
| Mean follow up (R), m | 61 (39–100) | 81 (40–121) | 54 (39–97) | 51 (36–70) | NA |
Localised, localised tenosynovial giant cell tumours (TGCT); Diffuse, diffuse‐TGCT; R, range; m, months; NA, not applicable.
For each patient, multiple formalin‐fixed paraffin‐embedded (FFPE) tissue blocks and corresponding haematoxylin and eosin (H&E)‐stained 4‐μm slides of the primary resected specimen were reviewed by an expert bone and soft tissue pathologist (J.V.M.G.B.) to confirm TGCT diagnosis and to select representative areas of the tumour with the highest proportion of suspected neoplastic cells.
A large tissue heterogeneity was observed between the different blocks. As a control for the landscaped CSF1 mRNA expression, multiple blocks were selected for three cases (L4046, L3496 and L4954), representing various tissue compositions.
CSF1 mRNA Expression
The RNAscope 2.5 high definition (HD)‐RED assay (322350; Advanced Cell Diagnostics, Newark, CA, USA) was used to detect CSF1 mRNA expression. This assay visualises single RNA molecules per cell by a novel method of in‐situ hybridisation (ISH). The double Z probe design allowed simultaneous signal amplification and background suppression.22 Positive [PPIB (cyclophilin B)] and negative controls (bacillus subtilis strain SMY) ensured reliable results. mRNA hybridisation was performed according to the manufacturer's protocols.
CSF1 Rearrangement
To identify the presence of CSF1 rearrangements at region 1p13, DNA fluorescence in‐situ hybridisation (FISH) analysis was performed on all tissue specimens using bacterial artificial chromosome (BAC) clones: RP11‐354C7 (centromeric to CSF1) and RP11‐96F24 (telomeric to CSF1) bracketing CSF1 locus, to identify both translocation and inversion. Probe labelling and hybridisation were performed according to previously described protocols.23 An index case outside the study population (L4018) was included with a COBRA–FISH molecular karyotyping‐proven inv(1)(p13;q23) as reference for the detection of the chromosome inversion in tissue section.24 Detailed descriptions of mRNA ISH and FISH procedures are presented in the Supporting information Appendix S1.
Scoring and Correlative Analysis
All slides were scanned in brightfield and/or fluorescence on a Pannoramic P250 or MIDI digital scanner (3DHistech, Budapest, Hungary). Scanned images were visualised using the Pannoramic Viewer (V2.1; 3DHistech). Interpretation was performed manually by a senior FISH expert (K.S.), blinded towards TGCT‐type and clinical outcome.
Because CSF1‐expressing regions were expected to contain neoplastic cells, three of these regions were selected. With the use of digital correlative microscopy, regions with CSF1 mRNA expressing (supposed neoplastic) cells were identified and the same areas were scored after FISH analysis. If the distance between the two signals was larger than the size of a single hybridisation signal, cells were recorded as CSF1 split‐positive. All nuclei within the selected area with a complete set of signals were evaluated. Nuclei with an incomplete set of signals were excluded from counting. Samples containing >2/100 nuclei with a CSF1 split were considered CSF1 split‐positive.
Results
CSF1 mRNA Expression
Specimens of all localised and diffuse TGCT cases showed a scattered, tissue‐infiltrating distribution of CSF1‐expressing cells (Figure 2). Corresponding to the landscape effect, heterogeneous distribution of CSF1‐expressing cells was observed when sections from multiple bock were analysed, meaning that regions completely devoid of CSF1‐expressing cells were seen in regions containing a large proportion of foam cells or regions with lymphocytic infiltrates. The CSF1 mRNA pattern expression was not observed in multinucleate giant cells, siderophages or foam cells. Consequently, due to the great heterogeneity between different blocks derived from one tumour and within regions in one section, quantification of CSF1‐expressing cells, meaning the expression of the proportion of CSF1‐positive cells, was not informative and was not analysed further (Supporting information, Figure S1). Selecting the block with the highest possible neoplastic cell component, we did not observe a clear difference in distribution of CSF1 between different TGCT cases. Cells with CSF1 mRNA expression were distributed diffusely and showed an infiltrating scattered pattern throughout the sections, with some clustering at various regions within a tissue element (Figure 2, Supporting information, Figures S2 and S3).
Figure 2.
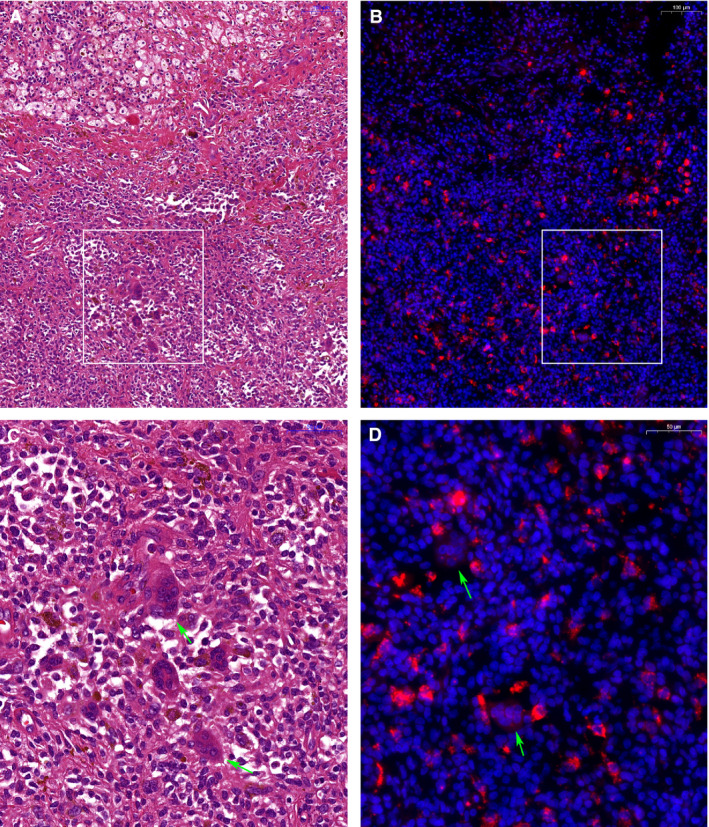
Conventional histology and mRNA in‐situ hybridisation (ISH) from a 61‐year‐old male patient (L3496), with extensive recurrent diffuse tenosynovial giant cell tumours (TGCT). This is the same patient as Figure 1 (right). Left panel, haematoxylin and eosin (H&E)‐stained section (A,C) with matching CSF1 mRNA ISH (B,D) in the right panel. White boxes in (A) and (B) show regions at higher resolution in (C) and (D). Heterogeneous cellular composition of TGCT is visible including foam cells, inflammatory cells, synovial‐like cells, siderophages and characteristic giant cells (A,C). mRNA ISH shows a scattered distribution of CSF1‐expressing cells with granular cytoplasmic signals (red signal), identifying CSF1 expressing cell‐nuclei [blue signal after 4′,6‐diamidino‐2‐phenylindole (DAPI) staining]. Green arrowheads show giant cells without CSF1 expression. Scale bars are in the top right corner, 100 μm for (A) and (B) and 50 μm for (C) and (D).
For the control cases, two of the four cases with synovitis showed expression of CSF1 (L5619, L5620). However, in these two cases CSF1 expression was restricted to cells localised in the synovial lining, which was different from the scattered distribution seen in TGCT (Figure 3). The other two cases with synovitis showed no expression of CSF1 (L3715, L5622).
Figure 3.
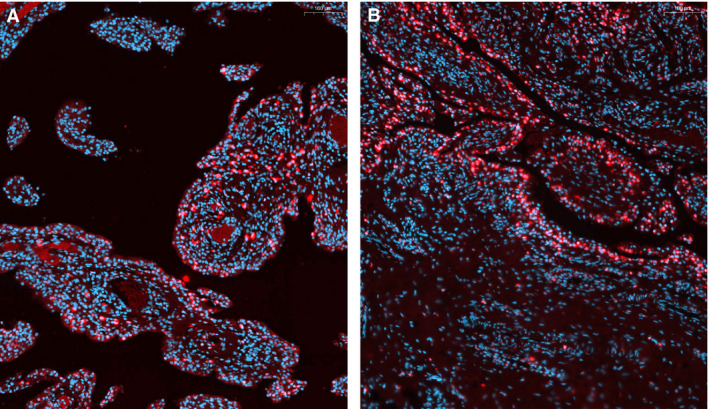
Distribution of synovial lining CSF1 mRNA in‐situ hybridisation (ISH)‐positive cells in tenosynovial giant cell tumours (TGCT) and reactive synovitis. A, 61‐year‐old male patient (L3496) with diffuse‐type TGCT. Cells with red cytoplasmic staining after mRNA ISH show a deep infiltrating pattern in synovial villi with rare occurrence at the synovial lining parts. This is the same patient as Figure 1 (right) and Figure 2. B, 45‐year‐old female patient (L5620) with synovitis, showing CSF1 expressing cells (red cytoplasmic signal) restricted to cells localised in the synovial lining. Nuclei are displayed in blue after 4′,6‐diamidino‐2‐phenylindole (DAPI) staining, scale bars are in the right top corner (100 μm).
CSF1 Rearrangement
The CSF1 probe set showed a clear split‐apart signal, even for detection of chromosome inversion using our molecular karyotyping proven index case with an inv(1)(p13;q23), indicating that cases with no split signal are unlikely to have similar inversion. Due to great heterogeneity, CSF1 split scoring was performed on selected areas based on the presence of CSF1‐expressing cells identified by mRNA ISH using correlative digital microscopy. Using this approach, CSF1 gene rearrangement was detected in 76% of all TGCT cases: 77% in localised type and 75% in diffuse type (Figure 4, Supporting information, Figure S2). For further stratification of positive cases, rearrangement of the CSF1 locus was present in 78% of localised TGCT without recurrence, 100% of localised TGCT with recurrent disease, 86% of diffuse TGCT without recurrence and 67% of diffuse TGCT including recurrent disease (Table 2, Supporting information, Table S1 patient and tumour characteristics). There was no CSF1 gene rearrangement in all four synovitis control cases.
Figure 4.
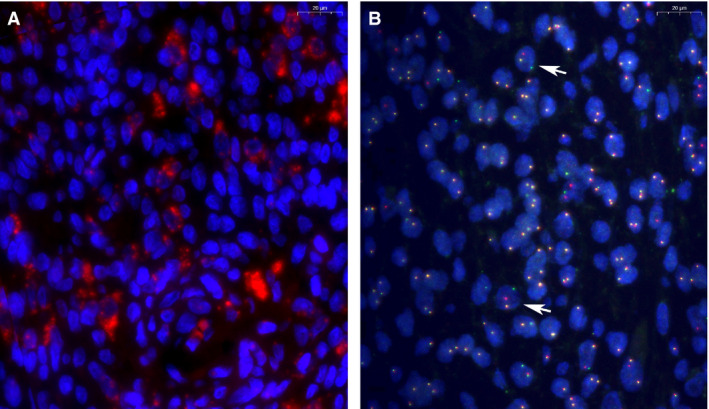
Correlative microscopy used to identify neoplastic cells. A, mRNA in‐situ hybridisation (ISH) helps to identify regions with cells overexpressing CSF1 mRNA (red signal), blue nuclei after 4′,6‐diamidino‐2‐phenylindole (DAPI) staining. B, CSF1 locus specific split‐apart probe set using BAC probes: centromeric (red) and telomeric (green) probes. Yellow signal represents co‐localisation of the signal, meaning no rearrangement. White arrowheads indicate cells with split‐apart signal, indicating rearrangement of the CSF1 gene. Samples are from a 61‐year‐old male patient (L3496), with extensive recurrent diffuse tenosynovial giant cell tumours (TGCT), the same patient as Figures 1A, 2 and 3A. Scale bars are in the top right corner (20 μm).
Table 2.
Proportion of cases with CSF1 mRNA expression and CSF1 gene rearrangementa
| n | CSF1 overexpression | CSF1 gene rearrangement | |
|---|---|---|---|
| Localised | 7 | 7 (100%) | 5 (78%) |
| Localised recurrence | 2 | 2 (100%) | 2 (100%) |
| Diffuse | 7 | 7 (100%) | 6 (86%) |
| Diffuse recurrence | 9 | 9 (100%) | 6 (67%) |
| Synovitis | 4 | 2 (50%) | 0 (0%) |
Localised, localised tenosynovial giant cell tumours (TGCT); Diffuse, diffuse‐TGCT.
Comprehensive patient and tumour characteristics are shown in Supporting information, Table S1.
Discussion
Localised‐ and diffuse‐type TGCT are histopathologically identical and carry the same chromosomal translocation, leading to uncontrolled overexpression of CSF1 due to a gene fusion between COL6A3 and CSF1 genes. Undeniably, localised‐ and diffuse‐type TGCT are clinically different diseases. In a well‐defined TGCT population with >3 years’ follow‐up, molecular differences in primary resected tissue between both subtypes and clinical outcome (recurrence) were evaluated. We were unable to find a clear association between CSF1 overexpression or CSF1 rearrangement and the biological behaviour in TGCT of the knee.
In this study, 76% CSF1 rearrangement was detected when lumping all our 25 cases together, compared with 61% of the evaluated cases by Cupp et al.12 Further subdivided, our study revealed no difference in CSF1 rearrangement for localised TGCT (77%) and diffuse TGCT (75%). Conversely, West et al. reported a large difference between these two types; 87% rearrangement in localised and 35% in diffuse TGCT.11 The relatively high percentage of rearrangement in our study could be attributed to our scoring on preselected areas, based on high CSF1 expression. In addition, our DNA FISH analysis, using bacterial artificial chromosome (BAC) clones (RP11‐354C7 and RP11‐96F24) bracketing the CSF1 locus, identifies not only a translocation, but also an inversion for CSF1 rearrangements. Panagopoulous et al. revealed a CSF1‐S100A10 fusion gene, with translocation t(1;1)(q21;p11) as the sole karyotypic abnormality.25 Nilsson et al. found that 30% of the TGCT specimens did not have a rearrangement involving the 1p13 locus, where CSF1 is located using the split‐apart interphase FISH approach, similar to ours.8 Next to the translocation, Panagopoulos et al. reported the replacement of the 3′‐UTR of CSF1, resulting in overexpression or a longer lifetime of CSF1 mRNA due to loss of the 3‐UTR controlling region.25 Similar cryptic changes leading to loss of the smaller gene region involving the 3′‐UTR segment of CSF1 are beyond the detection level of our FISH probes. Next to this, other as‐yet unidentified alterations leading to deregulated CSF1 expression cannot be ruled out in cases with CSF1 mRNA expression without CSF1 rearrangement of the CSF1 locus.
To date, clinically reliable antibodies working on FFPE tissue sections to detect CSF1 or CSF1R are lacking. Therefore, mRNA ISH was the best‐regarded option to identify CSF1 overexpressing cells. Consistent with previous reports, all 25 evaluated cases showed CSF1 up‐regulation.11 Exact determination of the proportion of CSF1‐expressing cells was considered not meaningful, as in all tumours considerable intratumoural heterogeneity was observed between selected blocks and with individual tissue sections, reflecting the ‘landscape effect’.11 This heterogeneity prevents any conclusion regarding the true neoplastic cell load in the tumour and a possible correlation to clinical outcome.
Deregulated CSF1 expression is believed to be the central mechanism of tumorigenesis for TGCT. CSF1, also called macrophage colony‐stimulating factor, is a cytokine produced by many different cell types, including macrophages, fibroblasts, endothelial cells and osteoblasts (and other cancer types, especially in bone metastasis).26 CSF1 is expressed in neoplastic cells infiltrating throughout the lesion. Secreted CSF1 recruits non‐neoplastic macrophages into the tumour. By binding to its receptor CSF1R (type III receptor tyrosine kinase), CSF1 promotes survival, proliferation and differentiation of cells of the mononuclear phagocyte lineage (e.g. monocytes, macrophages and osteoclasts).27, 28 Besides its general biological function, CSF1 is involved in inflammatory or reactive synovitis (rheumatoid arthritis, chronic arthritis) and cancer (breast, endometrial, ovarian, lung, kidney).12, 27 When CSF1 is expressed in reactive synovitis, its expression is restricted to cells in the synovial lining,12, 29 as was confirmed in our synovitis control cases.
Inhibition of signalling between CSF1 and CSF1R targets the underlying cause of the disease.29, 30 The involvement of this pathway contributed to the introduction of systemic therapies for extensive diffuse TGCT.20 Primarily, imatinib31 or related drugs such as nilotinib32 showed efficacy in the treatment. Recently, new CSF1R blockers were developed and are being investigated in clinical trials: emactuzumab and cabiralizumab (FPA008), both monoclonal antibodies directed against CSF1R,33, 34, 35 pexidartinib (PLX3397; retains CSF1R in inactive state)29 and MSC110 (an antagonist of the CSF1 ligand).35 Emactuzumab (n = 29) showed an overall response rate of 86% (two patients with a complete response) and a rate of disease control of 96%, including a significant functional and symptomatic improvement (median follow‐up 12 months).33 The preliminary results for cabiralizumab (n = 22) are consistent with radiographic response and improvement in pain and function in five of 11 patients (45%).34 In a randomised, placebo‐controlled Phase III study, pexidartinib showed an improved overall response rate by RECIST: 39% in the pexidartinib group (n = 61) and 0% of the placebo group (n = 59), after a median 6‐month follow‐up.36 However, long‐term results still need to be evaluated with these newer agents.
Within our well‐defined patient cohort, all patients had a minimum follow‐up of 3 years. However, patients without recurrent disease at the time of analysis could still develop this in due course, as it is known that local recurrence might develop years after initial surgery.1, 2, 15, 19, 37 Verspoor et al. calculated an overall recurrence rate of 72% in 75 patients with diffuse TGCT of the knee with a mean follow‐up from index treatment of 13.9 years. They suggested a trend towards the longer the follow‐up, the greater the number of recurrences.19
In conclusion, DNA FISH analysis, using bacterial artificial chromosome (BAC) clones (RP11‐354C7 and RP11‐96F24) bracketing the CSF1 locus, can identify both chromosomal rearrangement‐caused translocation or inversion of the CSF1 locus. Figure 5 summarises the workflow in the current study and the proposed workflow for molecular pathology work‐up of TGCT cases. The use of CSF1 mRNA ISH, in combination with CSF1 split‐apart FISH, is an auxiliary diagnostic tool to confirm the diagnosis of TGCT. This combined approach allowed us to detect CSF1 gene rearrangement in 76% of the TGCT cases. At the molecular level, localised‐ and diffuse‐type TGCT are indistinguishable when evaluating CSF1 expression and the presence of the pathognomonic translocation involving the CSF1 gene.
Figure 5.
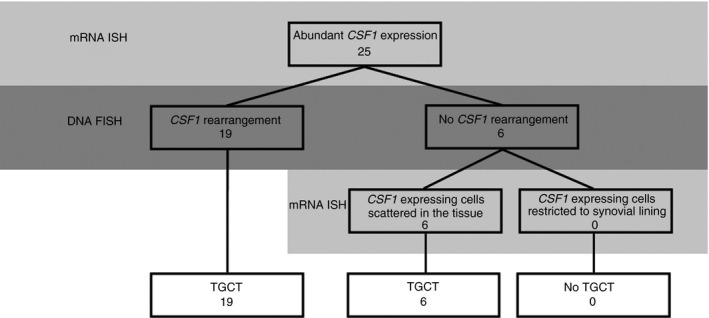
Proposed workflow for molecular pathology work‐up of tenosynovial giant cell tumours (TGCT) cases. Numbers present TGCT cases in this study. CSF1, colony stimulating factor 1; mRNA ISH, mRNA in‐situ hybridisation; DNA FISH, DNA fluorescence in‐situ hybridisation.
Conflicts of interest
There are no conflicts of interest by any of the authors regarding this manuscript.
Supporting information
Figure S1. Low power magnification overview of TGCT case from a 61‐year‐old male patient (L3496), the same patient as Figures 1A, 2, 3A, 4.
Figure S2. Overview of TGCT localised case without recurrence from a 55‐year‐old female patient (L4385), presented in Figure 1A.
Figure S3. Correlative microscope image comparing sections of a diffuse, non‐recurrent TGCT case.
Table S1. Patient and tumour characteristics.
Appendix S1. Detailed description of mRNA ISH and FISH porcedures.
Acknowledgements
The authors thank I. Briaire‐de Bruijn for her technical assistance. The department of orthopaedics of the Leiden University Medical Center (LUMC) receives research funding by Daiichi Sankyo.
Mastboom M J L, Hoek D M, Bovée J V M G, van de Sande M A J & Szuhai K (2019) Histopathology 74, 332–340. 10.1111/his.13744 Does CSF1 overexpression or rearrangement influence biological behaviour in tenosynovial giant cell tumours of the knee?
References
- 1. de St Aubain S, van de Rijn M. Tenosynovial giant cell tumour, localized type In Fletcher CDM, Hogendoorn PCW, Mertens F. eds. WHO classification of tumours of soft tissue and bone. Lyon: IARC, 2013; 100–101. [Google Scholar]
- 2. de St Aubain S, van de Rijn M. Tenosynovial giant cell tumour, diffuse type In Fletcher CDM, Hogendoorn PCW, Mertens F. eds. WHO classification of tumours of soft tissue and bone. Lyon: IARC, 2013; 102–103. [Google Scholar]
- 3. Mastboom MJL, Verspoor FGM, Verschoor AJ et al Higher incidence rates than previously known in tenosynovial giant cell tumors. Acta Orthop. 2017; 88; 688–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jaffe HLLL, Sutro CJ. Pigmented villonodular synovitis, bursitis and tenosynovitis. Arch. Pathol. 1941; 31; 731–765. [Google Scholar]
- 5. Fletcher JA, Henkle C, Atkins L, Rosenberg AE, Morton CC. Trisomy 5 and trisomy 7 are nonrandom aberrations in pigmented villonodular synovitis: confirmation of trisomy 7 in uncultured cells. Genes Chromosom. Cancer 1992; 4; 264–266. [DOI] [PubMed] [Google Scholar]
- 6. Mertens F, Orndal C, Mandahl N et al Chromosome aberrations in tenosynovial giant cell tumors and nontumorous synovial tissue. Genes Chromosom. Cancer 1993; 6; 212–217. [DOI] [PubMed] [Google Scholar]
- 7. Ohjimi Y, Iwasaki H, Ishiguro M et al Short arm of chromosome 1 aberration recurrently found in pigmented villonodular synovitis. Cancer Genet. Cytogenet. 1996; 90; 80–85. [DOI] [PubMed] [Google Scholar]
- 8. Nilsson M, Hoglund M, Panagopoulos I et al Molecular cytogenetic mapping of recurrent chromosomal breakpoints in tenosynovial giant cell tumors. Virchows Arch. 2002; 441; 475–480. [DOI] [PubMed] [Google Scholar]
- 9. Sciot R, Rosai J, Dal Cin P et al Analysis of 35 cases of localized and diffuse tenosynovial giant cell tumor: a report from the chromosomes and morphology (CHAMP) study group. Mod. Pathol. 1999; 12; 576–579. [PubMed] [Google Scholar]
- 10. Brandal P, Bjerkehagen B, Heim S. Molecular cytogenetic characterization of tenosynovial giant cell tumors. Neoplasia 2004; 6; 578–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. West RB, Rubin BP, Miller MA et al A landscape effect in tenosynovial giant‐cell tumor from activation of csf1 expression by a translocation in a minority of tumor cells. Proc. Natl Acad. Sci. USA 2006; 103; 690–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cupp JS, Miller MA, Montgomery KD et al Translocation and expression of csf1 in pigmented villonodular synovitis, tenosynovial giant cell tumor, rheumatoid arthritis and other reactive synovitides. Am. J. Surg. Pathol. 2007; 31; 970–976. [DOI] [PubMed] [Google Scholar]
- 13. Mastboom MJL, Verspoor FGM, Hanff DF et al Severity classification of tenosynovial giant cell tumours on mr imaging. Surg. Oncol. 2018; 27; 544–550. 10.1016/j.suronc.2018.07.002 [DOI] [PubMed] [Google Scholar]
- 14. Stephan SR, Shallop B, Lackman R, Kim TW, Mulcahey MK. Pigmented villonodular synovitis: a comprehensive review and proposed treatment algorithm. J. Bone Joint Surg. Rev. 2016; 4; pii: 01874474‐201607000‐00005. [DOI] [PubMed] [Google Scholar]
- 15. Palmerini E, Staals EL, Maki RG et al Tenosynovial giant cell tumour/pigmented villonodular synovitis: outcome of 294 patients before the era of kinase inhibitors. Eur. J. Cancer 2015; 51; 210–217. [DOI] [PubMed] [Google Scholar]
- 16. Patel KH, Gikas PD, Pollock RC et al Pigmented villonodular synovitis of the knee: a retrospective analysis of 214 cases at a UK tertiary referral centre. Knee 2017; 24; 808–815. [DOI] [PubMed] [Google Scholar]
- 17. Griffin AM, Ferguson PC, Catton CN et al Long‐term outcome of the treatment of high‐risk tenosynovial giant cell tumor/pigmented villonodular synovitis with radiotherapy and surgery. Cancer 2012; 118; 4901–4909. [DOI] [PubMed] [Google Scholar]
- 18. van der Heijden L, Gibbons CL, Hassan AB et al A multidisciplinary approach to giant cell tumors of tendon sheath and synovium – a critical appraisal of literature and treatment proposal. J. Surg. Oncol. 2013; 107; 433–445. [DOI] [PubMed] [Google Scholar]
- 19. Verspoor FG, Zee AA, Hannink G, van der Geest IC, Veth RP, Schreuder HW. Long‐term follow‐up results of primary and recurrent pigmented villonodular synovitis. Rheumatology (Oxf.) 2014; 53; 2063–2070. [DOI] [PubMed] [Google Scholar]
- 20. Brahmi M, Vinceneux A, Cassier PA. Current systemic treatment options for tenosynovial giant cell tumor/pigmented villonodular synovitis: targeting the CSF1/CSF1R axis. Curr. Treat. Options Oncol. 2016; 17; 10. [DOI] [PubMed] [Google Scholar]
- 21. van der Heijden L, Mastboom MJ, Dijkstra PD, van de Sande MA. Functional outcome and quality of life after the surgical treatment for diffuse‐type giant‐cell tumour around the knee: a retrospective analysis of 30 patients. Bone Joint J. 2014; 96‐B; 1111–1118. [DOI] [PubMed] [Google Scholar]
- 22. Wang F, Flanagan J, Su N et al RNAscope: a novel in situ RNA analysis platform for formalin‐fixed, paraffin‐embedded tissues. J. Mol. Diagn. 2012; 14; 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Szuhai K, Bezrookove V, Wiegant J et al Simultaneous molecular karyotyping and mapping of viral DNA integration sites by 25‐color cobra‐fish. Genes Chromosom. Cancer 2000; 28; 92–97. [DOI] [PubMed] [Google Scholar]
- 24. Szuhai K, Tanke HJ. Cobra: combined binary ratio labeling of nucleic‐acid probes for multi‐color fluorescence in situ hybridization karyotyping. Nat. Protoc. 2006; 1; 264–275. [DOI] [PubMed] [Google Scholar]
- 25. Panagopoulos I, Brandal P, Gorunova L, Bjerkehagen B, Heim S. Novel CSF1‐s100a10 fusion gene and CSF1 transcript identified by rna sequencing in tenosynovial giant cell tumors. Int. J. Oncol. 2014; 44; 1425–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hung JY, Horn D, Woodruff K et al Colony‐stimulating factor 1 potentiates lung cancer bone metastasis. Lab. Invest. 2014; 94; 371–381. [DOI] [PubMed] [Google Scholar]
- 27. Achkova D, Maher J. Role of the colony‐stimulating factor (CSF)/CSF‐1 receptor axis in cancer. Biochem. Soc. Trans. 2016; 44; 333–341. [DOI] [PubMed] [Google Scholar]
- 28. Barreda DR, Hanington PC, Belosevic M. Regulation of myeloid development and function by colony stimulating factors. Dev. Comp. Immunol. 2004; 28; 509–554. [DOI] [PubMed] [Google Scholar]
- 29. Tap WD, Wainberg ZA, Anthony SP et al Structure‐guided blockade of CSF1R kinase in tenosynovial giant‐cell tumor. N. Engl. J. Med. 2015; 373; 428–437. [DOI] [PubMed] [Google Scholar]
- 30. Peyraud F, Cousin S, Italiano A. CSF‐1R inhibitor development: current clinical status. Curr. Oncol. Rep. 2017; 19; 70. [DOI] [PubMed] [Google Scholar]
- 31. Cassier PA, Gelderblom H, Stacchiotti S et al Efficacy of imatinib mesylate for the treatment of locally advanced and/or metastatic tenosynovial giant cell tumor/pigmented villonodular synovitis. Cancer 2012; 118; 1649–1655. [DOI] [PubMed] [Google Scholar]
- 32. Gelderblom H, Cropet C, Chevreau C et al Nilotinib in locally advanced pigmented villonodular synovitis: a multicentre, open‐label, single‐arm, phase 2 trial. Lancet Oncol. 2018; 19; 639–648. [DOI] [PubMed] [Google Scholar]
- 33. Cassier PA, Italiano A, Gomez‐Roca CA et al CSF1R inhibition with emactuzumab in locally advanced diffuse‐type tenosynovial giant cell tumours of the soft tissue: a dose‐escalation and dose‐expansion phase 1 study. Lancet Oncol. 2015; 16; 949–956. [DOI] [PubMed] [Google Scholar]
- 34. Sankhala KK, Blay JY, Ganjoo KN et al A phase I/II dose escalation and expansion study of cabiralizumab (Cabira; FPA‐008), an anti‐CSF1R antibody, in tenosynovial giant cell tumor (TGCT, diffuse pigmented villonodular synovitis D‐PVNS). 2017 Annual Meeting ASCO. J Clin Oncol. 2017; 35(Suppl.); 11078. [Google Scholar]
- 35. Clinical Trials . Database of privately and publicly funded clinical studies conducted around the world. Available at: https://clinicaltrials.gov. (accessed 6 June 2018).
- 36. Tap WD, Gelderblom H, Stacchiotti S et al Final results of enliven: a global, double‐blind, randomized, placebo‐controlled, phase 3 study of pexidartinib in advanced tenosynovial giant cell tumor (tgct). ASCO Conference, Chicago, 2018.
- 37. Mastboom MJL, Verspoor FGM, Gelderblom H, van de Sande MAJ. Limb amputation after multiple treatments of tenosynovial giant cell tumour: series of 4 dutch cases. Case Rep. Orthop. 2017; 2017; 7402570. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Low power magnification overview of TGCT case from a 61‐year‐old male patient (L3496), the same patient as Figures 1A, 2, 3A, 4.
Figure S2. Overview of TGCT localised case without recurrence from a 55‐year‐old female patient (L4385), presented in Figure 1A.
Figure S3. Correlative microscope image comparing sections of a diffuse, non‐recurrent TGCT case.
Table S1. Patient and tumour characteristics.
Appendix S1. Detailed description of mRNA ISH and FISH porcedures.


