Summary
Essentials.
Recombinant von Willebrand factor (rVWF) is effective in von Willebrand disease (VWD).
A phase 3 study of rVWF, with/without recombinant factor VIII (rFVIII) before surgery in VWD.
Overall rVWF's efficacy was rated excellent/good; rVWF was administered alone in most patients.
rVWF was well‐tolerated and hemostasis was achieved in patients with severe VWD undergoing surgery.
Summary
Background
Recombinant von Willebrand factor (rVWF) has demonstrated efficacy for on‐demand treatment of bleeding in severe von Willebrand disease (VWD), warranting evaluation in the surgical setting.
Objectives
This study (NCT02283268) evaluated the hemostatic efficacy/safety profile of rVWF, with/without recombinant factor VIII (rFVIII), in patients with severe VWD undergoing surgery.
Patients/Methods
Patients received rVWF 40–60 IU kg−1, VWF ristocetin cofactor activity was measured 12–24 h before surgery. If endogenous FVIII activity (FVIII:C) target levels were achieved 3 h before surgery, rVWF was administered alone 1 h before surgery; rVWF was co‐administered with rFVIII if target endogenous FVIII levels were not achieved. rVWF was infused postoperatively to maintain target trough levels. Overall and intraoperative hemostatic efficacy, the pharmacodynamics of rVWF administration and the incidence of adverse events (AEs) were assessed.
Results
All patients treated with rVWF for major (n = 10), minor (n = 4) and oral (n = 1) surgery had overall and intraoperative hemostatic efficacy ratings of excellent (73.3% and 86.7%) or good (26.7% and 13.3%). Most rVWF infusions (89.4%) were administered alone, resulting in hemostatically effective levels of endogenous FVIII within 6 h, which were sustained for 72–96 h; 70% (n = 7/10) of major surgeries were performed without rFVIII co‐administration. Six patients reported 12 treatment‐emergent AEs. Two patients each had one serious AE: diverticulitis (not treatment related) and deep vein thrombosis (sponsor‐assessed as possibly treatment related). No severe allergic reactions or inhibitory antibodies were reported.
Conclusions
These data support the efficacy and safety profile of rVWF in patients with severe VWD undergoing elective surgery.
Keywords: clinical trial, general surgery, pharmacodynamics, von Willebrand disease, von Willebrand factor
Introduction
Von Willebrand disease (VWD), the most common inherited bleeding disorder, is caused by quantitative and qualitative defects in von Willebrand factor (VWF) 1, 2, a large multimeric plasma glycoprotein that mediates the initial adhesion and aggregation of platelets at sites of vascular injury 1, 3, 4. VWF also binds to and stabilizes coagulation factor VIII (FVIII), thus increasing its half‐life in circulation 1, 3, 4, 5.
The goal of VWD therapy is to normalize VWF and, in some cases, FVIII levels 1, 2, 6. Approved therapies for the treatment of VWD include desmopressin, plasma‐derived (pd) VWF/FVIII concentrates and recombinant VWF (rVWF, VONVENDI® [US]/VEYVONDI™ [Europe]; Baxalta, part of Shire, Lexington, MA, USA). As desmopressin is only effective in some patients with VWD (e.g. less severe type 1 VWD with a baseline VWF level of > 10 IU dL−1), and repeated closely spaced administrations may cause tachyphylaxis, replacement therapy is often used to provide hemostatic efficacy in the surgical setting 7.
Most pdVWF concentrates contain both VWF and FVIII, and the ratio between VWF and FVIII in these products can vary significantly. After repeated dosing of pdVWF/FVIII concentrates, FVIII accumulation of > 150% may occur, which has been associated with an increased risk of thromboembolic events in the surgical setting 7, 8, 9. Additionally, there are batch‐to‐batch variations in VWF ristocetin cofactor activity (VWF:RCo), and all lack hemostatically effective ultra‐large multimers (ULMs) 3, 6, 10, 11.
rVWF was developed to address some of these limitations. Unlike other concentrates, rVWF replaces missing or defective VWF, stabilizing endogenous FVIII. Dosing can be optimized to VWF levels alone, without the concern of FVIII accumulation, and VWF:RCo activity does not vary between batches. Additionally, during manufacture, rVWF is not exposed to ADAMTS‐13 and, therefore, contains a full multimeric profile 11, including the ULMs typically seen only in platelets and endothelial cells 12.
The efficacy and safety profile of rVWF in on‐demand management of bleeding episodes 13, along with a trend towards a longer half‐life of rVWF compared with another available concentrate 14, supported further evaluation of rVWF in perioperative management of bleeding in VWD. The goal of this study was to evaluate the hemostatic efficacy and safety of rVWF (vonicog alfa) with/without rFVIII (ADVATE® [Antihemophilic Factor (Recombinant); Baxalta, part of Shire, Lexington, MA, USA], hereafter referred to as rFVIII) in patients with severe VWD undergoing elective surgery. To better understand the effects of rVWF administration on endogenous FVIII activity (FVIII:C) levels a baseline pharmacokinetics (PK) and pharmacodynamics (PD) analysis was conducted.
Methods
Study design and patient population
This was a phase 3, prospective, open‐label, uncontrolled, non‐randomized study at 14 sites in 10 countries in patients undergoing major, minor or oral surgery (ClinicalTrials.gov Identifier: NCT02283268) (Fig. S1). Patients scheduled for major surgery had an initial PK/PD evaluation over a 72‐h period within 42 days of surgery, and results were used to guide preoperative dosing. The study was conducted in accordance with the standards of Good Clinical Practice. The final protocol was approved by the relevant ethics committees or institutional review boards, and written informed consent was provided by all participants.
Patients aged ≥ 18 years with severe VWD of all types who had planned elective surgery were eligible for the study. Severe VWD was defined as follows: type 1 (VWF:antigen [Ag] and VWF:RCo < 20 IU dL−1), type 2A (lack of high‐molecular‐weight multimers and VWF:RCo/VWF:Ag < 0.6), type 2B (identification of specific genotype), type 2M (presence of all multimers and VWF:RCo/VWF:Ag < 0.6), type 2N (FVIII:C levels < 10% with documented genetics) and type 3 (VWF:Ag ≤ 3 IU dL−1). Patients with type 3 VWD had to have a history of ≥ 20 exposure days to VWF/FVIII concentrates (including cryoprecipitate or fresh frozen plasma). For patients with type 1 or type 2 VWD, a minimum of 5 exposure days or a past major surgery requiring VWF/FVIII‐containing products (including cryoprecipitate or fresh frozen plasma) was required. Patients were excluded if they tested positive for VWF or FVIII inhibitors or had a history of a thromboembolic event, hypersensitivity to VWF, or any immunologic disorder.
At 12–24 h before surgery, rVWF 40–60 IU kg−1 VWF:RCo was given intravenously to allow endogenous FVIII:C levels to rise to ≥ 30 IU dL−1 (minor/oral surgery) or ≥ 60 IU dL−1 (major surgery), which were to be assessed within 3 h of initiation of the surgery. If target FVIII:C levels were achieved, rVWF alone was administered within 1–2 h before surgery to achieve the peak levels described in Table 1. If target FVIII:C levels were not achieved, rVWF was co‐administered with rFVIII (ADVATE®, Antihemophilic Factor [Recombinant]) within 1–2 h before surgery to meet recommended peak levels. Intraoperative and postoperative dosing were individualized to maintain target trough levels according to PK/PD results, as well as the intensity and duration of the hemostatic challenge. Post‐surgery, patients were monitored for 14 days and target trough plasma levels of VWF:RCo and FVIII:C were maintained according to the type of surgery the patient received (Table 2).
Table 1.
VWF:RCo and FVIII:C target levels: recommendations for the prevention of excessive bleeding during and after surgery
| Type of surgery | VWF:RCo target peak plasma level, IU dL−1 | FVIII:C target peak plasma level*, IU dL−1 | Calculation of rVWF dose, IU VWF:RCo received† |
|---|---|---|---|
| Minor/oral | 50–60 | 40–50 | ΔVWF:RCo × BW (kg)/IR‡ |
| Major | 100 | 80–100 | ΔVWF:RCo × BW (kg)/IR‡ |
ΔVWF:RCo = target peak plasma VWF:RCo – baseline plasma VWF:RCo; BW, body weight; FVIII:C, factor VIII activity; IR, incremental recovery; rFVIII, recombinant factor VIII; rVWF, recombinant von Willebrand factor; VWF:RCo, von Willebrand factor ristocetin cofactor activity. *Additional rFVIII may be administered to attain the recommended FVIII:C target peak plasma levels. These levels are in accordance with those outlined in the guideline on the core SPC for human plasma derived von Willebrand factor (CPMP/BPWG/278/02) 24. †Administered within 1–2 h before surgery. ‡If the IR is not available, assume an IR of 2.0 IU dL−1 per IU kg−1.
Table 2.
Target VWF:RCo and FVIII:C trough plasma level and minimum duration of treatment recommendations for subsequent maintenance doses for the prevention of excessive bleeding during and after surgery
| Type of surgery | VWF:RCo trough plasma level | FVIII:C trough plasma level | Minimum duration of treatment | Frequency of dosing | ||
|---|---|---|---|---|---|---|
| Up to 72 h post‐surgery | After 72 h post‐surgery | Up to 72 h post‐surgery | After 72 h post‐surgery | |||
| Major | > 50 IU dL−1 | > 30 IU dL−1 | > 50 IU dL−1 | > 30 IU dL−1 | 72 h | Every 12–24 h to every other day |
| Minor | ≥ 30 IU dL−1 | – | > 30 IU dL−1 | – | 48 h | Every 12–24 h to every other day |
| Oral | ≥ 30 IU dL−1 | – | > 30 IU dL−1 | – | 8–12 h | Every 12–24 h to every other day |
Major surgeries were defined as those that carried a significant risk of loss of a large volume of blood or blood loss into a confined anatomical space, such as major orthopedic, abdominal, gynecologic, head and neck, intracranial, cardiovascular or spinal surgery, and extraction of impacted third molars. Minor surgical procedures included placement of intravenous access devices, removal of small skin lesions, arthroscopy, gastroscopy, colonoscopy or conization. Oral surgeries included extractions of < 3 non‐molar teeth with no bony involvement. Antithrombotic prophylaxis was left to the discretion of the physician.
PK/PD analysis
In 11 patients, the baseline evaluation was completed using rVWF at a dose of 50 ± 5 IU kg−1 VWF:RCo administered within 42 days before the planned surgery. PK/PD was assessed before infusion of rVWF and at 0.5, 1, 6, 12, 24, 48 and 72 h post‐infusion. PK parameters (including area under the curve, peak concentration, time to peak concentration, terminal half‐life and incremental recovery at peak concentration) for VWF:RCo were calculated and analyzed using standard methods. Mean VWF:RCo and endogenous FVIII:C levels were calculated over time.
Efficacy evaluations
The primary outcome measure included overall investigator‐assessed hemostatic efficacy of rVWF at 24 h after the last perioperative infusion or at completion of the study, whichever occurred earlier, using a four‐point rating scale (‘excellent’, ‘good’, ‘moderate’ or ‘none’) based on hemostasis relative to a hemostatically normal subject without VWD (Table S1). Intraoperative hemostatic efficacy was assessed by the surgeon using the four‐point efficacy rating scale (Table S1). Intraoperative actual versus predicted blood loss was also surgeon evaluated.
Safety evaluations
Safety evaluations included the incidence/severity of adverse events (AEs), thromboembolic events and severe allergic reactions. Additional diagnostic procedures were conducted according to the local institution's medical standard of care and individual patient risk factors.
Safety evaluations were based on the criteria outlined in the guideline on the clinical investigation of human plasma‐derived von Willebrand factor products 15. The Ristocetin cofactor (VWF:RCo) assay and the FVIII binding (VWF:FVIIIB) assay were used to test for the presence of inhibitory anti‐VWF antibodies. Neutralizing antibodies to VWF:RCo and VWF:FVIIIB activities were assessed by the Nijmegen modification of the Bethesda assay. Plasma was assayed for the presence of antibodies against CHO protein (total Ig), murine IgG and human Furin (total Ig) using proprietary enzyme immunoassays.
Plasma samples were analyzed for binding antibodies to VWF, CHO protein, murine protein and Furin protein at the Charité Universitätmedizin in Berlin, Germany. Tests to confirm the presence of inhibitory antibodies to FVIII and VWF were performed in the Department of Medical and Chemical Laboratory Diagnostics at the Medical University of Vienna, Austria.
Statistics
It was planned to have a minimum of 15 patients with severe VWD who were undergoing surgery, with ≥ 10 major surgical procedures evaluated. Descriptive statistical analyses included point estimates and 90% confidence intervals (CIs) for the incidence of individuals with hemostatic efficacy rated ‘excellent/good’ and were determined using a Clopper Pearson test and SAS v9.4. PK/PD and safety were summarized using descriptive statistics.
Results
Patient disposition and baseline characteristics
A flow chart of patient selection is shown in Fig. S2. Patient demographics and baseline clinical characteristics are summarized in Table 3. Major surgeries were performed in 10 patients (66.7%), minor surgeries in four patients (26.7%) and oral surgery in one patient (6.7%) (Table 4). The types of surgical procedures are shown in Table 4. Although thromboprophylaxis was allowed at the discretion of the investigator, only one patient received such treatment (described in the Safety section).
Table 3.
Patient demographics and baseline clinical characteristics
| Characteristic | Total (N = 15) |
|---|---|
| Sex, n (%) | |
| Male | 7 (46.7) |
| Female | 8 (53.3) |
| Age, median (range), years | 40 (20–70) |
| Weight, median (range), kg | 73.5 (52–127.2) |
| BMI, median (range), kg m−2 | 25.6 (17.1–38) |
| VWD type, n (%) | |
| 1 | 3 (20) |
| 2A | 2 (13.3) |
| 2B | 1 (6.7) |
| 2M | 1 (6.7) |
| 3 | 8 (53.3) |
| FVIII:C, mean (SD), IU dL−1 | |
| All VWD types (N = 15) | 16.4 (19.9) |
| Type 1 VWD (n = 3) | 17 (4) |
| Type 2A VWD (n = 2) | 34.5 (23.3) |
| Type 2B VWD (n = 1) | 36 |
| Type 2M VWD (n = 1) | 66 |
| Type 3 VWD (n = 8)* | 3.0 (1.5) |
| Mean (SD) VWF:RCo, IU dL−1 | |
| All VWD types (N = 14) | 10.6 (13.3) |
| Type 1 VWD (n = 3) | 14.3 (3.1) |
| Type 2A VWD (n = 2) | 29.0 (26.9) |
| Type 2B VWD (n = 1) | 23 |
| Type 2M VWD (n = 1) | 13 |
| Type 3 VWD (n = 7)* | 1.7 (4.5) |
BMI, body mass index; FVIII:C, factor VIII activity; SD, standard deviation; VWD, von Willebrand disease; VWF:RCo, von Willebrand factor ristocetin cofactor activity. *One patient with type 3 VWD had screening assessments performed with an inadequate washout period. Therefore, the respective VWF:RCo result was excluded. For FVIII:C, the test was repeated as an unscheduled visit to confirm eligibility; the FVIII:C result from the unscheduled visit is included in this table.
Table 4.
Summary of FVIII:C and doses of rWVF with or without rFVIII for each surgical procedure
| Patient number | VWD type | Surgical procedure | FVIII:C at screening, IU dL−1 | 12–24 h before surgery, rVWF dose, IU kg−1 | FVIII:C within 3 h before surgery, IU dL−1 | 1 h pre‐op rVWF dose*, IU kg−1 | 1 h pre‐op rFVIII dose*, IU kg−1 | Total post‐op rVWF dose, IU kg−1 | Total post‐op rFVIII dose, IU kg−1 |
|---|---|---|---|---|---|---|---|---|---|
| Major surgery | |||||||||
| 1 | 3 | Total knee replacement | 6 | 57.6 | 68 | 57.6 | N/A | 533.3 | N/A |
| 4 | 2A | Total knee replacement | 18 | 41.7 | 73 | 35.8 | N/A | 47.7 | N/A |
| 5 | 3 | Total hip replacement | 3 | 57.1 | 88 | 32.6 | 14.5 | 244.7 | N/A |
| 7 | 3 | Complex dental extraction | 3 | 46.3 | 100 | 34.8 | N/A | 231.7 | N/A |
| 8 | 3 | Molar extraction | 4 | 51.4 | 107 | 50.8 | 22.8 | 237.1 | 60.7† |
| 9 | 1 | ACL surgery | 17 | 47.1 | 108 | 15.7 | N/A | 70.6 | N/A |
| 10 | 2A | Laparoscopic cystectomy | 51 | 55.1 | 90 | 39.4 | N/A | 126.0 | N/A |
| 11 | 2M | Laparoscopic cholecystectomy | 66 | 37.4 | 114 | 23.5 | N/A | 197.9 | N/A |
| 13 | 2B | Meniscectomy | 36 | 56.9 | 85 | 55.7 | N/A | 189.8 | N/A |
| 14 | 3 | Prosthesis left ankle | 1 | 47.2 | 36 | 82.7 | 42.3 | 236.2 | N/A |
| Minor surgery | |||||||||
| 2 | 3 | Nasopharyngoscopy‐laryngoscopy | 3 | 55.0 | 79 | 46.4 | N/A | 115.9 | N/A |
| 3 | 3 | Nasopharyngoscopy‐laryngoscopy | 2 | 55.8 | 130 | 8.0 | N/A | N/A | N/A |
| 12 | 1 | Colonoscopy | 21 | 59.9 | 99 | 34.2 | N/A | N/A | N/A |
| 15 | 3 | Radioisotope synovectomy | 2‡ | 58.5 | 55 | 44.5 | N/A | 42.8 | 17.4 |
| Oral surgery | |||||||||
| 6 | 1 | Tooth extraction§ | 13 | 36.1 | 62 | 18.1 | N/A | 36.1 | N/A |
ACL, anterior cruciate ligament; FVIII:C, factor VIII activity; N/A, not applicable; rFVIII, recombinant factor VIII; rVWF, recombinant von Willebrand factor; VWD, von Willebrand disease. *Dosing occurred within 1–2 h before surgery. †Patient received a total of six doses (7.6 IU kg−1 × 4, 15.2 IU kg−1 × 2). ‡Two screening assessments: first result of 29 IU dL−1 (inadequate washout period); second result of 2 IU dL−1. §Only patient to receive intraoperative dosing (18.1 IU kg−1 rVWF + 8.1 IU kg−1 rFVIII).
PK/PD analysis
Eleven patients were included in the PK/PD analysis: major surgery (n = 10); minor surgery (n = 1). The PK parameters for VWF:RCo are shown in Table S2. As expected for an IV bolus administration, median pre‐dose corrected VWF:RCo activity and VWF:Ag levels had their peak shortly after administration (i.e. Cmax corresponded with the first sampling time‐point) and then declined in an exponential manner (Fig. 1). Administration of rVWF alone stabilized endogenous FVIII, with a gradual increase in FVIII:C levels as the infused rVWF bound to the endogenous FVIII (Fig. 1A, B). After administration of a single dose of rVWF, mean FVIII:C levels showed substantial increases, from 20.6 IU dL−1 pre‐infusion to 34.1 IU dL−1 at 1 h post‐infusion, 67.5 IU dL−1 at 6 h post‐infusion and 86.9 IU dL−1 at 12 h post‐infusion for all patients (n = 11). For patients with type 3 VWD (n = 5), FVIII:C levels increased from 1.8 IU dL−1 pre‐infusion to 15.6 IU dL−1 at 1 h post‐infusion, 51.8 IU dL−1 at 6 h post‐infusion and 76 IU dL−1 at 12 h post‐infusion. Peak FVIII:C levels were observed at 24 h post‐infusion and gradually declined over the next 48 h, but were still around 50 IU dL−1 on average at 72 h post‐infusion (Fig. 1A, B). The mean hourly rise in endogenous FVIII:C levels from pre‐infusion to 6 h post‐infusion was 8.44 IU dL−1 (range, 4.43–11.57) for all patients and 9.01 IU dL−1 (range, 7.54–10.88) for patients with type 3 VWD. For patients with type 3 VWD, one reached > 60 IU dL−1 FVIII within 6 h post‐infusion, whereas the other four patients reached 46–52 IU dL−1 FVIII during the same time period.
Figure 1.
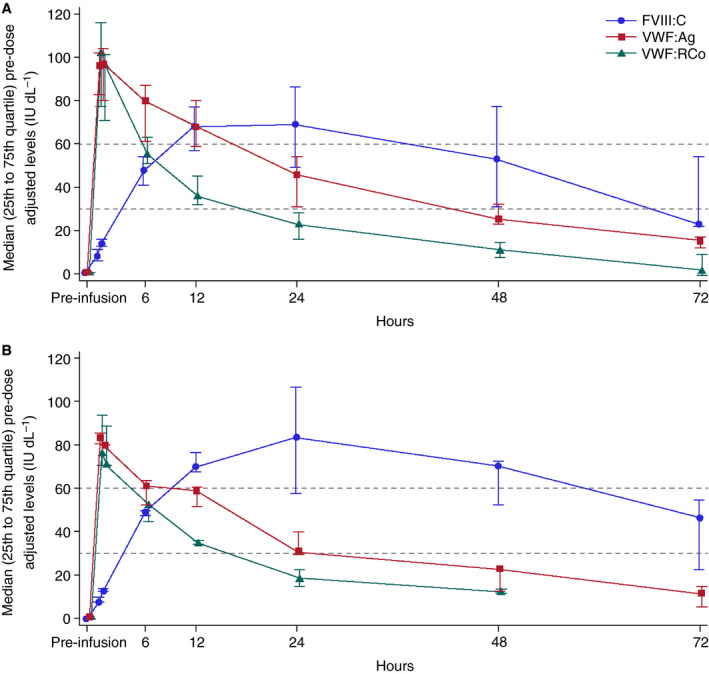
Median pre‐dose adjusted von Willebrand factor ristocetin cofactor activity (VWF:RCo), von Willebrand factor antigen (VWF:Ag) and endogenous factor VIII activity (FVIII:C) levels. (A) All patients with pharmacokinetic data available (n = 11). (B) Patients with type 3 von Willebrand disease (n = 5).
Hemostatic efficacy in all patients
Overall hemostatic efficacy, the primary endpoint, was rated as ‘excellent’ or ‘good’ in 100% (n = 15/15) of patients (90% CI, 81.9–100). Intraoperative hemostatic efficacy was also rated as ‘excellent’ or ‘good’ in 100% (n = 15/15) of patients (90% CI, 81.9–100). For patients with type 3 VWD (n = 8), both the overall and intraoperative hemostatic efficacy were rated as ‘excellent’ for seven patients and ‘good’ in one patient.
Excluding infusions for the PK/PD analysis and those given to treat bleeds and maintain hemostasis, patients received a total of 104 surgical infusions of rVWF: 93 infusions (89.4%) of rVWF alone and 11 infusions (10.6%) administered with rFVIII in five patients. Results are summarized for the various time periods in Table 5 and presented by patient in Table 4. Of the five patients who received 11 concomitant rVWF and rFVIII infusions, three patients with type 3 VWD received a single co‐administration 1 h before undergoing major surgery; two of the three patients received rFVIII despite having FVIII:C levels ≥ 60 IU dL−1 (Table 4). The patient undergoing molar extraction received six co‐administrations postoperatively; the postoperative pre‐infusion FVIII:C level ranged from 110 to 152 IU dL−1 in five cases. This patient's peak FVIII:C level during this time was 168 IU dL−1. A patient with type 1 VWD received a single co‐administration intraoperatively for tooth extraction, although the intraoperative pre‐infusion FVIII:C level was 72 IU dL−1. Lastly, a patient with type 3 VWD received a single co‐administration postoperatively for radioisotope synovectomy, although the postoperative pre‐infusion FVIII:C level was 73 IU dL−1. This patient also received a postoperative dose of rVWF alone. Two patients received no additional rVWF postoperatively.
Table 5.
Surgical infusions of rVWF and rFVIII
| Time of infusion | Number of infusions with rVWF alone | Number of infusions with rVWF and rFVIII | Number of total infusions |
|---|---|---|---|
| 12–24 h before surgery | 15 | 0 | 15 |
| 1 h before surgery | 12 | 3 | 15 |
| Intraoperative | 0 | 1 | 1 |
| Postoperative (0–14 days) | 66 | 7 | 73 |
| Total infusions, n (%) | 93 (89.4) | 11* (10.6) | 104 (100.0) |
rFVIII, recombinant factor VIII; rVWF, recombinant von Willebrand factor. *Only two of the 11 infusions were co‐administered with rFVIII because the target FVIII:C level was not met: one patient with 36 IU dL−1 within 3 h before surgery (prosthesis left ankle) and one patient with 23 IU dL−1 postoperatively (molar extraction) (see text for details).
Most patients (n = 12) received rVWF infusions no more than once daily and, of these, 11 patients were not dosed every day, with some infusions separated by 2–9 days. Three patients undergoing major surgery (laparoscopic cholecystectomy in a type 2M patient, molar extraction in a type 3 patient and prosthesis left ankle in a type 3 patient) were treated twice daily on ≥ 1 day, with most infusions given ~12 h apart.
The overall median surgical dose of rVWF was 220.4 IU kg−1 (range, 63.8–648.4 IU kg−1) (Table 6. The mean actual intraoperative blood loss relative to predicted blood loss was 70% (Table S3), rated ‘excellent’ for 13 patients and ‘good’ for two patients.
Table 6.
Median rVWF exposure overall and by surgery classification
| Dose | Surgery classification | Overall (N = 15) | ||
|---|---|---|---|---|
| Minor (n = 4) | Major (n = 10) | Oral (n = 1) | ||
| Total infusions*, median (range), n | 3 (2–4) | 7.5 (4–15) | 5 | 6 (2–15) |
| Exposure days, n (range) | 3 (2–4) | 6.5 (4–15) | 4 | 6 (2–15) |
| Dose at 12–24 h before surgery, median (range), IU kg−1 | 57.2 (55.0–59.9) | 49.3 (37.4–57.6) | 36.1 | 55.0 (36.1–59.9) |
| Dose within 1–2 h before surgery, median (range), IU kg−1 | 39.3 (8.0–46.4) | 37.6 (15.7–82.7) | 18.1 | 35.8 (8.0–82.7) |
| Intraoperative dose, median (range), IU kg−1 | 0 | 0 | 18.1 | 18.1 |
| Postoperative doses, median (range), IU kg−1 | 79.3 (42.8–115.9) | 214.8 (47.7–533.3) | 36.1 | 189.8 (36.1–533.3) |
| Total surgical dose, median (range), IU kg−1 | 119.9 (63.8–217.3) | 307.6 (125.2–648.4) | 108.4 | 220.4 (63.8–648.4) |
rVWF, recombinant von Willebrand factor. *Total number of preoperative doses (12–24 h before surgery), preoperative doses (within 1–2 h before surgery), intraoperative doses and postoperative doses.
Hemostatic efficacy by surgery classification
Major surgery
Overall hemostatic efficacy was rated ‘excellent’ or ‘good’ in 100% (n = 10/10) of major surgeries: ‘excellent’ (n = 7/10); ‘good’ (n = 3/10). Intraoperative hemostatic efficacy was also rated ‘excellent’ or ‘good’ in 100% (n = 10/10) of major surgeries: ‘excellent’ (n = 8/10); good (n = 2/10).
Seventy percent of patients (n = 7/10) undergoing major surgery did not receive rFVIII co‐administration. Patients who underwent major surgery received a total of 87 surgical infusions of rVWF: 78 infusions (89.7%) of rVWF alone and nine (10.3%) administered with rFVIII. At ~1 h before surgery, seven rVWF infusions were administered alone and three were co‐administered with rFVIII (all in type 3 VWD); doses are shown in Table 4. No intraoperative doses of rVWF were administered. During the postoperative period, 61 rVWF infusions were administered alone and six were with rFVIII.
A median surgical rVWF dose of 307.6 IU kg−1 (range, 125.2–648.4 IU kg−1) was used in the 10 patients undergoing major surgery (Table 6). The mean actual intraoperative blood loss relative to predicted blood loss was 69% (Table S3): rated ‘excellent’ for eight patients and ‘good’ for two patients.
Minor/Oral surgery
Overall and intraoperative hemostatic efficacy were rated as ‘excellent’ in 100% (n = 4/4) of patients who underwent minor surgery and were rated as ‘good’ and ‘excellent’, respectively, in the patient who underwent oral surgery.
Patients who underwent minor and oral surgery received a total of 17 surgical infusions of rVWF: 15 infusions (88.2%) of rVWF alone and two (11.8%) administered with rFVIII. Doses of rVWF given to the patients who underwent minor surgery and the one patient who underwent oral surgery are shown in Table 4. One patient with type 1 VWD who underwent oral surgery received intraoperative co‐administration of rVWF and rFVIII. Postoperatively, three infusions of rVWF were administered alone and one was administered with rFVIII for the minor surgeries and two infusions of rVWF were administered alone for the oral surgery.
A median surgical rVWF dose of 119.9 IU kg−1 (range, 63.8–217.3 IU kg−1) was used in the four patients undergoing minor surgery and 108.4 IU kg−1 was used in the patient who underwent oral surgery (Table 6). The actual intraoperative blood loss relative to predicted blood loss is shown in Table S3, and was rated ‘excellent’ for all five patients.
Safety
A total of 12 treatment‐emergent AEs were reported in six patients, including acne, anemia, deep vein thrombosis (DVT), diverticulitis, dizziness, dry skin, headache, joint swelling, nasopharyngitis, pelvic pain and peripheral swelling. Among them, two patients had serious AEs (one patient with diverticulitis and one patient with DVT). None of these events were considered treatment related by the investigator. The patient with the DVT was a 42‐year‐old female with type 3 VWD and body mass index of 24.6 kg m−2 who underwent major total hip replacement surgery and had overall hemostatic efficacy rated as ‘excellent’. A Duplex scan of lower extremity veins at the start of surgery did not reveal any signs of thrombosis. At the discretion of the investigator, the patient began thromboprophylaxis with dabigatran 220 mg daily on the day of surgery. A Duplex scan was performed according to the standard of care at the local hospital on postoperative day 4, although the patient was non‐symptomatic. This revealed a non‐serious, asymptomatic left non‐occlusive thrombosis of common and deep femoral veins. The patient's ADAMTS‐13 level was 37% on this day. Thromboprophylaxis was changed from dabigatran to enoxaparin 80 mg twice daily (with treatment continuing until discharge on day 17 when she was placed on rivaroxaban 20 mg daily).
Continuation of the DVT event as a floating thrombus of the left common femoral vein was seen via Duplex scan on postoperative day 8, was considered a serious AE, and resulted in placement of a retrievable filter in the inferior vena cava without complications. The event subsequently resolved (the filter was removed 2 months later) and was considered by the investigator to be related to the numerous risk factors (i.e. obesity, surgical procedure and immobilization) of the patient rather than to rVWF or rFVIII (peak level, 158 IU dL−1 on postoperative day 3). Despite the existence of these confounding factors, the continued administration of treatment in the postoperative period led the study sponsor to reassess this event as ‘possibly related’ to treatment.
No deaths or severe allergic reactions were reported. No neutralizing antibodies to VWF, FVIII or Chinese hamster ovary proteins, Furin or murine IgG were detected. One patient with type 3 VWD who underwent a total knee replacement and received an intraoperative transfusion of packed red blood cells (500 mL volume) had a positive result for binding antibodies to VWF on postoperative days 7, 8, 10 and 11; these antibodies were non‐inhibitory and had no effect on VWF:RCo.
Discussion
This is the first study evaluating a novel rVWF factor concentrate in patients with severe VWD undergoing surgery. The overall and intraoperative hemostatic efficacy of rVWF (with/without co‐administration of rFVIII) was assessed relative to the expected level of bleeding in people without VWD undergoing similar surgery. The primary endpoint was achieved, as overall hemostatic efficacy was rated as ‘excellent’ or ‘good’ for all 15 patients treated with rVWF, and intraoperative hemostatic efficacy was rated as ‘excellent’ or ‘good’ for all surgeries.
During the study, 80% (12/15) of patients did not receive any preoperative FVIII and 67% (10/15) of patients and 70% (7/10) of the major surgeries performed were treated with rVWF alone. Five patients received 11 infusions that included both rVWF and rFVIII. Only two of these infusions were performed when the FVIII:C level was < 60 IU dL−1; the remaining nine were performed despite the FVIII:C level being above the target level. The doses of rFVIII used during co‐administration were small (four infusions of 7.6 IU kg−1, two infusions of 15.2 IU kg−1, and one infusion each of 8.1, 14.5, 17.4, 22.8 and 42.3 IU kg−1) to maintain a specific FVIII:C level, rather than to achieve a large increase. These results can be attributed to the PK/PD findings, which showed substantial increases in endogenous FVIII:C levels after administration of rVWF alone, with all patients achieving mean FVIII:C > 60 IU dL−1 by 6 h post‐infusion. Mean endogenous FVIII:C levels were 28.5 IU dL−1 as early as 30 min post‐infusion, 67.5 IU dL−1 6 h post‐infusion and 90.6 IU dL−1 24 h post‐infusion, with corresponding levels of 10.8, 51.8 and 83.4 IU dL−1 in patients with type 3 VWD. Despite having mean FVIII:C levels < 2 IU dL−1 at baseline, administration of rVWF alone allowed patients to achieve target VWF:RCo and FVIII: C levels quickly. Compared with type 3 patients, those with type 1 and 2 started with higher baseline levels of FVIII:C, and therefore achieved target levels more rapidly.
Our findings are consistent with previous studies showing that replacement of VWF with a plasma‐derived factor concentrate with very low levels of FVIII immediately stabilizes endogenous FVIII and that there is a gradual increase in FVIII:C levels as the infused VWF binds newly synthesized protein, thus leading to hemostatic levels within several hours post‐infusion 1, 16. Considering some type 1 and 2 patients will already be at, or nearly at, target FVIII:C levels preoperatively, our results suggest they may only need the rVWF infusion at 1 h before surgery. These data also suggest that the majority of postoperative infusions of once‐daily rVWF alone sustained FVIII:C at hemostatically effective levels.
The lack of FVIII in rVWF may be especially important in the surgical setting, during long‐term prophylaxis, in the elderly and in the obstetric setting 12, 17. Physicians have the flexibility to choose whether or not to co‐administer FVIII. In this study, because of the rapid and sustained increases in endogenous FVIII:C levels after infusion of rVWF alone, the vast majority of infusions did not include any exogenous FVIII. As the administration of exogenous FVIII was at the discretion of the physician, lack of rapid access to the measurement of FVIII:C levels at some centers, as well as the length and intensity of individual surgical procedures, could have factored into the decision to administer the drug.
The acceptable safety profile of rVWF in this study was consistent with that seen in the Phase 3 on‐demand trial 13. In the current study, 12 treatment‐emergent AEs were reported in six patients who received 104 surgical infusions of rVWF. There were no reports of severe allergic reactions, neutralizing or inhibitory antibody development, or deaths. There was a single report of DVT in one patient. The patient received thromboprophylaxis to reduce the risk of DVT. However, obesity 18, 19 and gender (female vs male) 19, 20, 21, 22 are independent risk factors for DVT development in patients undergoing orthopedic surgery. This patient also had a low ADAMTS‐13 level around the time of the event. The individual patient risk factors, in addition to post‐surgery administration of rVWF to maintain hemostasis, confound the situation. Recombinant von Willebrand factor contains a greater proportion of ULMs versus pdVWFs, during initial infusion 3, 4, 11, 23. However, they undergo rapid proteolysis, indicating appropriate susceptibility of rVWF to physiologic regulation by ADAMTS‐13 14. Therefore, in the presence of physiologic levels of ADAMTS‐13, the prothrombotic potential of ULMs would be expected to be low. Furthermore, the absence of both VWF and ADAMTS‐13 in a single patient is thought to be extremely rare. Therefore, the event was considered ‘possibly related’ to treatment by the study sponsor.
The primary limitation of this study was the small sample size, limiting the generalizability of the results. However, similar results were observed across study centers, potentially indicating the robustness of rVWF for treating surgical bleeding in VWD. Therefore, these findings should be confirmed in a larger study or with real‐world data.
Clinical implications and conclusions
These data support the efficacy and safety profile of rVWF (with/without co‐administration of rFVIII) in achieving hemostasis in patients with severe VWD who were undergoing elective surgery. In this study, infusion of rVWF alone resulted in hemostatically effective levels of endogenous FVIII after 6 h, that were sustained for 72–96 h. These data allow physicians to consider the risk factors for each patient and to decide whether rVWF should be administered alone or with rFVIII during and after surgery. Many clinicians avoid the use of plasma‐derived VWF/FVIII concentrates; therefore, a recombinant product may be especially important to them. In most cases, infusions of rVWF alone no more than once daily were sufficient to maintain stable endogenous FVIII:C levels within the target range. This was likely to be the result of either reduced clearance or the extended half‐life of rVWF. Patient management can therefore focus on achieving optimal efficacy without concern over unnecessary exposure to excessive FVIII levels and its associated risks. This may be of particular benefit in patients with high baseline FVIII levels or malignancies, the elderly, and those undergoing major surgeries. Based on our experience with elective surgery, it is recommended that during emergency surgery baseline VWF:RCo and FVIII:C levels should be assessed prior to surgery and intraoperative peak plasma levels, as indicated for elective surgery, should be targeted by infusion of adequate doses of rVWF and, as needed, rFVIII. The presence of ULMs in rVWF are likely to contribute to the rapid stabilization of endogenous FVIII:C levels. This could have important clinical implications, potentially reducing the need to administer exogenous FVIII and delaying admission until the day of surgery. It remains to be seen whether current guidelines 7 for the use of VWF concentrates will apply to rVWF, given the efficacy and extended normalization of FVIII:C levels observed in this small study population.
Addendum
F. Peyvandi, A. Mamaev, J.‐D. WANG, O. Stasyshyn, M. Timofeeva, N. Curry, A. R. Cid, T. T. Yee, K. Kavakli and G. Castaman were principal investigators for the study. A. Sytkowski was the medical director for the study. F. Peyvandi, A. Mamaev, O. Stasyshyn, M. Timofeeva, A. R. Cid, T. T. Yee, K. Kavakli, G. Castaman and A. Sytkowski made substantial contributions to the conception and design of the work. A. Sytkowski made substantial contributions to the statistical analysis and the interpretation of data for the work. F. Peyvandi and G. Castaman made substantial contributions to the acquisition, statistical analysis, and interpretation of data for the work. A. Mamaev, J.‐D. Wang, A. R. Cid, O. Stasyshyn, M. Timofeeva, N. Curry, T. T. Yee and K. Kavakli made substantial contributions to the acquisition and interpretation of data for the work. All authors critically revised the manuscript for important intellectual content, approved the final version submitted and agreed to be accountable for all aspects of the work.
Disclosure of Conflict of Interest
F. Peyvandi has worked as a consultant for Kedrion and Octapharma, received speaker fees for educational meetings at Ablynx and Shire, and is a member of advisory boards at Ablynx and F. Hoffmann‐La Roche. A. Mamaev is the principal investigator on clinical trials at Baxalta, Octapharma, Generium, Hoffmann la Roche and Green Cross and has worked as lector providing educational background for physicians at Octapharma, Baxter, Hoffmann la Roche, CSL Behring and Generium. J.‐D. Wang has received research funding from Shire and honoraria from Shire, Bayer and Novo Nordisk. O. Stasyshyn has worked as a consultant for Novo Nordisk and Pfizer. N. Curry has worked as a consultant for LFB and Bayer, has received research funding from CSL Behring, and serves as a member of Sobi's board of directors, speaker's bureau and advisory committee. R. Cid has received honoraria from Novo Nordisk, Shire and LFB. K. Kavakli has received honoraria from Shire, and is a member of an advisory board at Shire. G. Castaman has received research funding (directly to the institution) from Pfizer and CSL Behring, and has served as a member on the board of directors, speaker's bureau or advisory committee of CSL Behring, Shire, Bayer, Sobi, Novo Nordisk, Kedrion, Genzyme and Pfizer. A. Sytkowski was an employee of Shire US Inc. at the time of the study. M. Timofeeva and T.T. Yee declare that they have no conflict of interest.
Supporting information
Fig. S1. Study design.
Fig. S2. Patient disposition.
Table S1. Overall and intraoperative hemostatic efficacy rating scale
Table S2. PK parameters for VWF:RCo
Table S3. Intraoperative blood loss volume
Acknowledgments
Research was funded by the Sponsor, Baxalta (part of Shire, Lexington, MA, USA). The authors would like to thank the patients for their participation in the study, C. von Auer (Ill. Med. Klinik der Johannes Gutenberg‐Universität, Universitätsmedizin Mainz, Mainz, Germany) for her contributions as a principal investigator for the study, A. Grigorian (Shire, Westlake Village, CA, USA) for writing the clinical study report (CSR), B. Ploder (Shire, Vienna, Austria) for statistical analysis of the data and ensuring its accuracy within the CSR and manuscript, as well as E. Biguzzi and S. Siboni (Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy) for their contributions to the conduct of the study. Under the direction of the authors, writing/editorial support for this manuscript was provided by C. Wing, PhD, employee of Excel Medical Affairs (Southport, CT, USA), and was funded by Baxalta (part of Shire, Lexington, MA, USA). Initial support for this manuscript was provided by C4 MedSolutions (Yardley, PA, USA), a CHC Group Company, funded by Baxalta (part of Shire, Lexington, MA, USA). All authors read the scientific content of this manuscript and critically revised it. Although employees of the sponsor were involved in the design, collection, analysis, interpretation, fact‐checking of information, and coordination and collation of comments, the development of content of this manuscript, the interpretation of the data, and the decision to submit the manuscript for publication in JTH was made by the authors independently. All authors had access to the clinical study data.
Peyvandi F, Mamaev A, Wang J‐D, Stasyshyn O, Timofeeva M, Curry N, Cid AR, Yee TT, Kavakli K, Castaman G, Sytkowski A. Phase 3 study of recombinant von Willebrand factor in patients with severe von Willebrand disease who are undergoing elective surgery. J Thromb Haemost 2019; 17: 52–62.
Manuscript handled by: D. DiMichele
Final decision: P.H. Reitsma, 17 September 2018
References
- 1. Leebeek FW, Eikenboom JC. von Willebrand's disease. N Engl J Med 2016; 375: 2067–80. [DOI] [PubMed] [Google Scholar]
- 2. Mannucci PM. Treatment of von Willebrand's disease. N Engl J Med 2004; 351: 683–94. [DOI] [PubMed] [Google Scholar]
- 3. Budde U, Metzner HJ, Muller HG. Comparative analysis and classification of von Willebrand factor/factor VIII concentrates: impact on treatment of patients with von Willebrand disease. Semin Thromb Hemost 2006; 32: 626–35. [DOI] [PubMed] [Google Scholar]
- 4. Reininger AJ. The function of ultra‐large von Willebrand factor multimers in high shear flow controlled by ADAMTS13. Hamostaseologie 2015; 35: 225–33. [DOI] [PubMed] [Google Scholar]
- 5. Furlan M. von Willebrand factor: molecular size and functional activity. Ann Hematol 1996; 72: 341–8. [DOI] [PubMed] [Google Scholar]
- 6. Castaman G, Goodeve A, Eikenboom J; European Group on von Willebrand Disease . Principles of care for the diagnosis and treatment of von Willebrand disease. Haematologica 2013; 98: 667–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miesbach W, Berntorp E. von Willebrand disease ‐ the ‘Dos’ and ‘Don'ts’ in surgery. Eur J Haematol 2017; 98: 121–7. [DOI] [PubMed] [Google Scholar]
- 8. Gill JC, Mannucci PM. Thromboembolic incidence with transiently elevated levels of coagulation factors in patients with von Willebrand disease treated with VWF:FVIII concentrate during surgery. Haemophilia 2014; 20: e404–6. [DOI] [PubMed] [Google Scholar]
- 9. Mannucci PM, Franchini M. Laboratory monitoring of replacement therapy for major surgery in von Willebrand disease. Haemophilia 2017; 23: 182–7. [DOI] [PubMed] [Google Scholar]
- 10. Batlle J, Lopez‐Fernandez MF, Fraga EL, Trillo AR, Perez‐Rodriguez MA. von Willebrand factor/factor VIII concentrates in the treatment of von Willebrand disease. Blood Coagul Fibrinolysis 2009; 20: 89–100. [DOI] [PubMed] [Google Scholar]
- 11. Turecek PL, Mitterer A, Matthiessen HP, Gritsch H, Varadi K, Siekmann J, Schnecker K, Plaimauer B, Kaliwoda M, Purtscher M, Woehrer W, Mundt W, Muchitsch EM, Suiter T, Ewenstein B, Ehrlich HJ, Schwarz HP. Development of a plasma‐ and albumin‐free recombinant von Willebrand factor. Hamostaseologie 2009; 29(Suppl 1): S32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Franchini M, Mannucci PM. von Willebrand factor (Vonvendi(R)): the first recombinant product licensed for the treatment of von Willebrand disease. Expert Rev Hematol 2016; 9: 825–30. [DOI] [PubMed] [Google Scholar]
- 13. Gill JC, Castaman G, Windyga J, Kouides P, Ragni M, Leebeek FW, Obermann‐Slupetzky O, Chapman M, Fritsch S, Pavlova BG, Presch I, Ewenstein B. Hemostatic efficacy, safety, and pharmacokinetics of a recombinant von Willebrand factor in severe von Willebrand disease. Blood 2015; 126: 2038–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mannucci PM, Kempton C, Millar C, Romond E, Shapiro A, Birschmann I, Ragni MV, Gill JC, Yee TT, Klamroth R, Wong WY, Chapman M, Engl W, Turecek PL, Suiter TM, Ewenstein BM. Pharmacokinetics and safety of a novel recombinant human von Willebrand factor manufactured with a plasma‐free method: a prospective clinical trial. Blood 2013; 122: 648–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. European Medicines Agency . Guideline on the clinical investigation of human plasma derived von Willebrand factor products (CPMP/BPWG/220/02). Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/01/WC500067126.pdf. Last accessed May 2018.
- 16. Borel‐Derlon A, Federici AB, Roussel‐Robert V, Goudemand J, Lee CA, Scharrer I, Rothschild C, Berntorp E, Henriet C, Tellier Z, Bridey F, Mannucci PM. Treatment of severe von Willebrand disease with a high‐purity von Willebrand factor concentrate (Wilfactin): a prospective study of 50 patients. J Thromb Haemost 2007; 5: 1115–24. [DOI] [PubMed] [Google Scholar]
- 17. Singal M, Kouides PA. Recombinant von Willebrand factor: a first‐of‐its‐kind product for von Willebrand disease. Drugs Today (Barc) 2016; 52: 653–64. [DOI] [PubMed] [Google Scholar]
- 18. Mantilla CBHT, Schroeder DR, Berry DJ, Brown DL. Risk factors for clinically relevant pulmonary embolism and deep venous thrombosis in patients undergoing primary hip or knee arthroplasty. Anesthesiology 2003; 99: 552–60. [DOI] [PubMed] [Google Scholar]
- 19. Zhang ZH, Shen B, Yang J, Zhou ZK, Kang PD, Pei FX. Risk factors for venous thromboembolism of total hip arthroplasty and total knee arthroplasty: a systematic review of evidences in ten years. BMC Musculoskelet Disord 2015; 16: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Piovella F, Wang CJ, Lu H, Lee K, Lee LH, Lee WC, Turpie AG, Gallus AS, Planes A, Passera R, Rouillon A. Deep‐vein thrombosis rates after major orthopedic surgery in Asia. An epidemiological study based on postoperative screening with centrally adjudicated bilateral venography. J Thromb Haemost 2005; 3: 2664–70. [DOI] [PubMed] [Google Scholar]
- 21. Motohashi M, Adachi A, Takigami K, Yasuda K, Inoue M, Sasaki S, Matsui Y. Deep vein thrombosis in orthopedic surgery of the lower extremities. Ann Vasc Dis 2012; 5: 328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Singh R, Muzzafar K, Haseeb M, Bhat SA, Ghani A. Epidemiology of deep vein thrombosis in patients undergoing major lower extremity orthopedic surgery: a two‐year study from tertiary care hospital of north India. Int J Sci Study 2017; 5: 41–4. [Google Scholar]
- 23. Stockschlaeder M, Schneppenheim R, Budde U. Update on von Willebrand factor multimers: focus on high‐molecular‐weight multimers and their role in hemostasis. Blood Coagul Fibrinolysis 2014; 25: 206–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guideline on the core SPC for human plasma derived von willebrand factor (CPMP/BPWG/278/02). Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003423.pdf. Last accessed June 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Study design.
Fig. S2. Patient disposition.
Table S1. Overall and intraoperative hemostatic efficacy rating scale
Table S2. PK parameters for VWF:RCo
Table S3. Intraoperative blood loss volume


