Abstract
Oomycetes of the genus Saprolegnia are responsible for severe economic losses in freshwater aquaculture. Following the ban of malachite green in food fish production, the demand for new treatments pushes towards the selection of more safe and environment‐friendly products. In the present work, in vitro activity of ten chemicals and three commercial products was tested on different strains of Saprolegnia, using malachite green as reference compound. The compounds were screened in agar and in water to assess the minimum inhibitory concentration (MIC) and the minimum lethal concentration (MLC), respectively. Two strains of Saprolegnia parasitica and one isolate of Saprolegnia delica were tested in triplicate per each concentration. Among tested chemicals, benzoic acid showed the lowest MIC (100 ppm) followed by acetic acid, iodoacetic acid and copper sulphate (250 ppm). Sodium percarbonate was not effective at any tested concentration. Among commercial products, Virkon™S was effective in inhibiting the growth of the mycelium (MIC = MLC = 1,000 ppm). Actidrox® and Detarox® AP showed MIC = 5,000 and 1,000 ppm, respectively, while MLCs were 10‐fold lower than MICs, possibly due to a higher activity of these products in water. Similarly, a higher effectiveness in water was observed also for iodoacetic acid.
Keywords: in vitro test, minimum inhibitory concentration, minimum lethal concentration, Saprolegnia spp., treatment
1. INTRODUCTION
The genus Saprolegnia includes oomycetes that are widely distributed in the aquatic environment; most members of this genus are saprotrophs, however, some species can be pathogenic to fish (Van West, 2006). Saprolegnia infections are an economically relevant aspect of salmonid aquaculture, causing severe losses in the farmed stock, and are characterized by the presence of whitish or greyish cotton‐like tuft on eggs or over the skin and gills of fish, on which it may spread and cover more than 80% of the body surface (Van Den Berg, McLaggan, Diéguez‐Uribeondo, & Van West, 2013). The infection causes the destruction of the epidermis and hyphae can reach the underlying fish tissues (Pickering & Willoughby, 1982) and blood vessels (Hatai & Hoshiai, 1992), leading to impaired osmoregulation. Respiratory failure may also occur as a consequence of gill infection. Behavioural changes include lethargy and loss of equilibrium. If untreated, saprolegniosis may lead to death of the infected fish (Van West, 2006).
The apparent low efficacy of prophylactic measures and the scarcity of registered treatments for the control of saprolegniosis urge the screening of new molecules and products active against Saprolegnia spp.
At present, no alternative treatment with effectiveness comparable to malachite green (MG) has been found for Saprolegnia infections (Sudova, Machova, Svobodova, & Vesely, 2007). MG has long been used in aquaculture (Foster & Woodbury, 1936) as effective fungicide, and for the treatment of protozoan infections (Ichtyophthirius sp., Rahkonen, Koski, Shinn, Wootten, & Sommerville, 2002). Treatments with MG were administered either as monocomponent baths or as multicomponent baths (in combination with formaldehyde and other products) (Sudova et al., 2007). However, particular concern is directed towards its use in the production of food fish: The high affinity and persistence of MG and, particularly, its reduced form (leucomalachite green—LMG) in animal tissues (Alborali, Sangiorgi, Leali, Guadagnini, & Sicura, 1997; Bauer, Dangschat, Knoppler, & Neudegger, 1988; Clifton‐Hadley & Alderman, 1987; Machova et al., 1996; Plakas, El‐Said, Stehly, Gingerich, & Allen, 1996) generates public health issues related to its potential carcinogenicity, teratogenicity and mutagenicity in humans, as suggested by experimental evidence in mammals (Culp et al., 2006; Mayer & Jorgenson, 1983; Werth, 1958). Following a considerable amount of studies demonstrating its toxicity and carcinogenicity in different animal species, the use of MG in the production of fish destined to human consumption is not authorized in the European Union (EU) (European Commission, 2010). However, since residues of MG and LMG have been detected in aquaculture products in monitoring programmes in EU Member States, the European Food Safety Authority (EFSA) established that food contaminated with MG/LMG at or below the reference point for action (RPA) of 2 μg/kg is unlikely to represent a public health concern (EFSA, 2016).
Several other chemicals have been used with different degrees of success for the treatment of Saprolegnia infections.
Among products specifically registered for aquaculture in some European countries, Pyceze® is employed in daily baths as prophylactic/therapeutic measure against fungal and bacterial infections in fish food eggs. The active ingredient of Pyceze® is bronopol (2‐bromo‐2‐nitropropane‐1,3‐diol), a broad‐range biocide used as a preservative in pharmaceutical and cosmetic industry (Bryce, Croshaw, Hall, Holland, & Lessel, 1978; Kumanova, Vassileva, Dobreva, Manova, & Kupenov, 1989; Toler, 1985), which would therefore pose no severe toxicological risks to human health. Nevertheless, its toxicity towards phytoplankton and zooplankton has been demonstrated. Furthermore, bronopol must be diluted prior to disposal, which significantly increases management costs (Nakagawa, Hara, Tokuyama, Takada, & Imamura, 2012).
Formalin, a solution of 37% formaldehyde, has been effectively employed to prevent (Bly, Quiniou, Lawson, & Clem, 1996; Schreier, Rach, & Howe, 1996) and treat (Cline & Post, 1972; Marking, Rach, & Schreier, 1994; Walser & Phelps, 1994) Saprolegnia infection in fish eggs. As a prophylactic measure, it inhibits cyst germination at a concentration of 250 mg/L (Bly et al., 1996). Daily flushes of formalin with 100, 200 and 400 mg/L increased per cent hatch of channel catfish Ictalurus punctatus eggs in comparison with non‐treated eggs (Walser & Phelps, 1994). Although formalin is still included in the list of allowed substances for all the food‐producing species and is marketed as a biocide for the disinfection of equipment and facilities (European Commission, 2010), it is not listed under the Biocidal Products Regulation (BPR) (European Parliament and Council, 2012), which is required for all products marketed as biocides. Moreover, formalin is currently not approved as a veterinary medicine for the treatment of live fish in most of the EU countries. However, in Spain, one formalin‐based product (Aquacen) has a marketing authorization for the control of Philasterides dicentrarchi in the farming of turbot Psetta maxima (Verner‐Jeffreys & Taylor, 2015). In the United States, formalin has been approved by Food and Drug Administration (USFDA, 2018) in three commercial formulations (Formalin‐F™, Formacide‐B and Parasite‐S®) for egg disinfection in aquaculture. From 1 January 2016, formaldehyde has been classified as a category 1B carcinogen (European Commission, 2014), therefore its use should be subjected to certain restrictions. Furthermore, besides the carcinogenic risk, formaldehyde represents a risk for exposed workers since it is a powerful irritant and allergenic substance.
Several other studies were performed to identify suitable compounds for the treatment or disinfection against Saprolegnia.
Ozone has shown effectiveness comparable to formalin in preventing saprolegniosis in brown trout (Salmo trutta fario) eggs (Forneris et al., 2003). The possible use of ozonized water in fish tanks is, however, controversial due to its strong oxidant properties (Fukunaga, Suzuki, & Takama, 1991) and potential toxicity to the branchial epithelium that could negatively affect respiration and osmoregulation.
Copper sulphate has been effectively used in aquaculture to control algal growth (James, Thomas, Gordon, & Frieda, 1998; Kiyoshi & Claude, 1993; Song, Marsh, Voice, & Long, 2011) and parasitic infection (Ling, Sin, & Lam, 1993; Schlenk, Gollon, & Griffin, 1998) proving effective against Saprolegnia both in vitro (Marking et al., 1994) and when administered to fish eggs in flow‐through systems (Straus, Mitchell, Carter, & Steeby, 2012). Sun, Hu, and Yang (2014) showed how copper sulphate inhibits the mycelium growth at concentrations ≥0.5 mg/L and the release of primary zoospores at 1 mg/L. On the other hand, sublethal concentrations can induce stress in rainbow trout Oncorhynchus mykiss which may become more susceptible to Saprolegnia (Carballo, Munoz, Cuellar, & Tarazona, 1995).
The fungicide activity of iodophores was demonstrated for the treatment of eggs in disinfectant baths, allowing to increase the hatching rate (Walser & Phelps, 1994). Despite their effectiveness and suitability for the disinfection of fish eggs, the potential use of iodophores for the treatment of a large number of fish is limited, due to the high concentrations needed (Bruno, van West, & Beakes, 2011) resulting in increased costs and potential toxicity.
Sodium chloride and a mixture of sodium and calcium chloride (26:1) tested as a prophylactic measure permitted to increase the hatching rate in salmonids; however, the required concentrations (20‐30 g/L) are hardly applicable in intensive farms (Edgell, Lawseth, Mclean, & Britton, 1993; Marking et al., 1994; Schreier et al., 1996; Waterstrat & Marking, 1995).
Hydrogen peroxide and boric acid are reported in the literature as promising compounds to control Saprolegnia infections. Hydrogen peroxide is an effective antimicrobial, showing antimycotic, antibacterial and antiviral activity coupled with low environmental impact, producing molecular oxygen and water as a result of its degradation. It was successfully tested against Saprolegnia mainly on fish eggs, resulting in increased hatching rates (Rach, Redman, Bast, & Gaikowski, 2005; Rach, Valentine, Schreier, Gaikowski, & Crawford, 2004; Small & Wolters, 2003; USFDA, 2007). At present, one hydrogen peroxide‐based product (Perox‐Aid®, Syndel, USA) is approved by the U.S. Food and Drug Administration (U.S. Food & Drug Administration (USFDA), 2018) for use in hatcheries.
Boric acid and borates have been used since the 19th century, as bactericides, fungicides and antiseptics (Quarles, 2001) and in several pesticides (EPA, 1993). This compound is safely used for the treatment of animal mycoses and in human medicine (De Seta, Schmidt, Vu, Essmann, & Larsen, 2009; Shi, Li, Qin, & Tian, 2011).
Ali, Thoen, Evensen, and Skaar (2014) tested boric acid in vitro against Saprolegnia parasitica and Saprolegnia diclina strains and in vivo on eggs and larvae of Atlantic salmon (Salmo salar). In vitro results showed inhibition of the mycelium growth at concentrations above 0.6 g/L, while in vivo tests allowed a high survival rate after continuous (0.2‐1.4 g/L) and intermittent (1‐4 g/L) exposure of eggs and larvae. These results suggest that boric acid could be safely used in aquaculture although its environmental impact must be carefully investigated.
The aim of this work was to perform an in vitro screening of promising molecules and commercial products against Saprolegnia spp. in order to provide information for the selection of safer and more environmentally friendly alternative treatment of saprolegniosis in aquaculture. The activity of new molecules is compared to the effectiveness of two compounds (malachite green, copper sulphate) used in the past to control saprolegniosis in aquaculture.
2. MATERIALS AND METHODS
2.1. Strains tested
Tests were carried out on three Saprolegnia strains: one reference strain of S. parasitica (CBS 223.65 provided by CSIC‐RJB Madrid, Spain) isolated in Holland from northern pike (Esox lucius), one field strain of S. parasitica (ITT 320/15/20) isolated in Italy from brown trout (Salmo trutta fario) and one field strain of Saprolegnia delica (ITT 290/15/15) isolated in Italy from rainbow trout (Oncorhynchus mykiss). Each strain has been tested in triplicate per each concentration.
2.2. Inocula
Saprolegnia spp. strains were maintained with periodic subcultures on glucose‐yeast (GY) agar medium (5 g D‐(+)‐glucose, 1 g yeast extract, 12 g agar in 1L deionized water) supplemented with 6 mg/L of penicillin and 10 mg/L of oxolinic acid (GY + P + Ox) (Alderman & Polglase, 1986) and kept at 18°C. For the in vitro trials, subcultures of the strains employed were incubated at 18°C until growth covered the full diameter of the dish (48‐72 hr). Inocula were obtained from the outer 10 mm of the culture, using a sterile 5‐mm‐diameter glass cannula (protocol I) or cutting a 4 × 4 mm piece with a sterile scalpel.
2.3. Products tested
Products under test include ten molecules (acetic acid, benzoic acid, boric acid, copper sulphate, iodoacetic acid, lactic acid, oxalic acid, tartaric acid, hydrogen peroxide and sodium percarbonate) belonging to different chemical classes and three commercial products (Actidrox®, De Marco, Italy; Detarox® AP, Perdomini, Italy; and Virkon™S, Dupont, UK). Malachite green was used as a reference compound, and copper sulphate was added because it is widely used to control saprolegniosis in aquacultured fish.
Each product was dissolved and/or diluted in sterile deionized water (with the exception of benzoic acid which was dissolved in absolute ethanol and subsequently diluted in water) at concentrations of 0.1; 1; 5; 10; 50; 100; 250; 500; 1,000; 5,000 ppm.
2.4. In vitro tests
Tests were performed following protocols I and II according to Alderman (1982).
For protocol I, different concentrations of the products under test were added to sterilized liquid GY agar at a temperature of 49°C. Mixtures were then distributed in six‐well plates (Ø 35 mm), allowing to test five different concentrations and one negative control. Following overnight solidification, a 5‐mm‐diameter well was excised in the centre of the agar using a sterile glass cannula. The well was then filled with a standard 5‐mm inoculum, culture surface uppermost. Plates were incubated at 18°C and checked after 24, 48, 72 hr and 6 days, determining the colony diameter of the growing mycelium as average of two axes measured at 90° from each other. The minimum inhibitory concentration (MIC) was defined as the lowest concentration inhibiting the growth of the mycelium after 6 days of incubation.
For protocol II, polycarbonate filter membranes (diameter 25 mm, pore size 5 μm—Whatman International Ltd., UK) were sterilized by autoclaving and then placed on the surface of 92‐mm petri dish (six per each petri dish). Filters were used as a support for the inoculum, obtained by placing a 4 × 4 mm piece of agar with growing mycelium. The inoculum was placed, inverted, at the centre of the filter. The dishes containing the filters were then incubated for 24 hr, until the resulting mycelial growth had almost reached the edge of the filters. The agar attached to the original inocula was then clipped off using hot forceps tips. The mycelia together with their supporting filters were lifted off the agar surface and placed in sterile 92‐mm petri dishes containing different concentrations of the products under test (Figure 1). Sterile deionized water was used as negative control. Filters were kept in contact with the solution for 1 hr and submitted to periodic agitation in order to achieve a better contact of the mycelium with the product under test. Subsequently, filters were washed twice with sterile water (for 5 and 30 min, respectively) and seeded on fresh dishes containing GY + P + Ox agar medium. Dishes were incubated at 18°C and checked after 24, 48, 72 hr and 6 days, determining the radial growth of the mycelium beyond the filter as average of two axes measured at 90° from each other. The minimum lethal concentration (MLC) was defined as the lowest concentration inhibiting any further growth of the mycelium after 6 days of incubation.
Figure 1.
Protocol II: Filters supporting mycelium immersed in a solution of the product under test [Colour figure can be viewed at wileyonlinelibrary.com]
3. RESULTS
Minimum inhibitory concentrations and MLCs obtained for each tested compound and each strain are listed in Table 1. Triplicates were always consistent among each other.
Table 1.
Minimum inhibitory concentrations (MIC) and minimum lethal concentrations (MLC) of tested compounds for the three Saprolegnia strains considered
| Compound | MIC | MLC | ||||
|---|---|---|---|---|---|---|
| A (ppm) | B (ppm) | C (ppm) | A (ppm) | B (ppm) | C (ppm) | |
| Malachite green | 5 | 5 | 5 | 5 | 5 | 5 |
| Copper sulphate | 250 | 250 | 250 | 1,000 | 5,000 | 5,000 |
| Acetic acid | 250 | 250 | 250 | 250 | 500 | 500 |
| Benzoic acid | 100 | 100 | 100 | 250 | 250 | 250 |
| Boric acid | 1,000 | 1,000 | 1,000 | a | a | a |
| Iodoacetic acid | 250 | 250 | 100 | 50 | 50 | 100 |
| Lactic acid | 500 | 500 | 5,000 | 500 | 500 | 1,000 |
| Oxalic acid | 500 | 500 | 1,000 | 1,000 | 5,000 | 5,000 |
| Tartaric acid | 500 | 500 | 1,000 | a | a | a |
| Sodium percarbonate | a | a | a | a | a | a |
| Hydrogen peroxide | 5,000 | 5,000 | 5,000 | 5,000 | 5,000 | 5,000 |
| Actidrox® | 5,000 | 5,000 | 5,000 | 500 | 500 | 500 |
| Detarox®AP | 1,000 | 1,000 | 1,000 | 100 | 100 | 100 |
| Virkon™S | 1,000 | 1,000 | 1,000 | 1,000 | 1,000 | 1,000 |
A, Saprolegnia parasitica CBS 223.65.
B, Saprolegnia parasitica ITT 320/15/20.
C, Saprolegnia delica ITT 290/15/15.
Minimum inhibitory concentration or MLC not found at tested concentrations.
The effectiveness of malachite green (positive control) was confirmed in both tests (MIC = MLC = 5 ppm; Figure 2a). In agar, 50 ppm of copper sulphate was effective in slowing down the mycelium radial growth and in inhibiting the aerial mycelium at 6 days, but it produced complete inhibition only at 250 ppm (Figure 2b). In water trials, following 1‐hr exposure, copper sulphate had a lethal effect at concentrations of 1,000 ppm on S. parasitica strain CBS 223.65 and 5,000 ppm on the other two strains.
Figure 2.
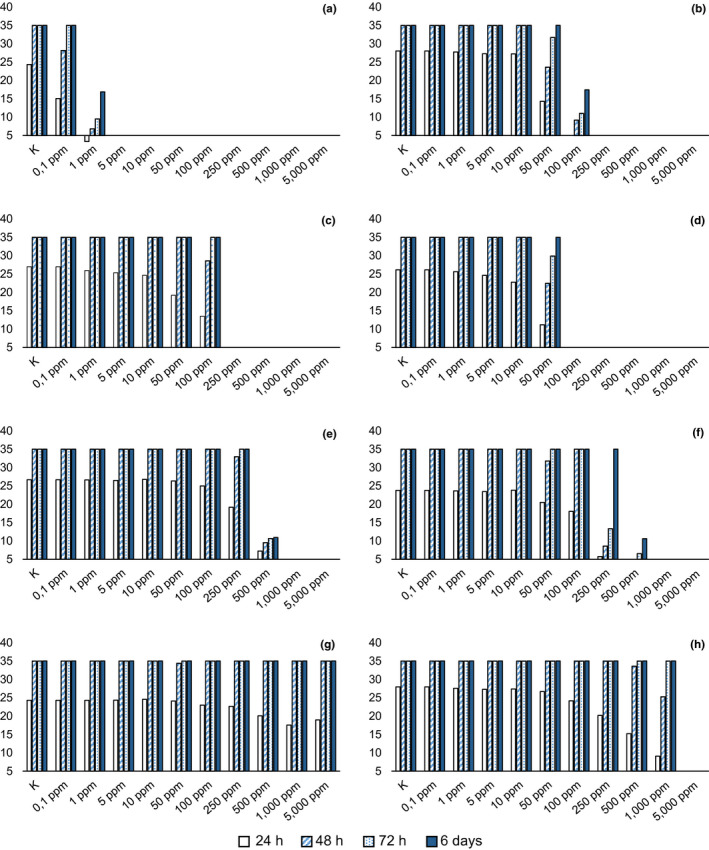
Average diameter (mm) of mycelium with different concentrations of compound in agar. (a) malachite green; (b) copper sulphate; (c) acetic acid; (d) benzoic acid; (e) boric acid; (f) oxalic acid; (g) sodium percarbonate; (h) hydrogen peroxide [Colour figure can be viewed at wileyonlinelibrary.com]
Among tested molecules, benzoic acid showed the lowest MIC (100 ppm), with radial mycelial growth considerably slowed down at 50 ppm (Figure 2d), followed by iodoacetic and acetic acid. With respect to iodoacetic acid, different MICs were observed in S. parasitica and S. delica (Figure 3e,f), but for all tested strains the inhibition of aerial mycelium on agar was observed at concentrations of 10 ppm at 6 days. Moreover, for S. parasitica, the MLC was lower than MIC.
Figure 3.
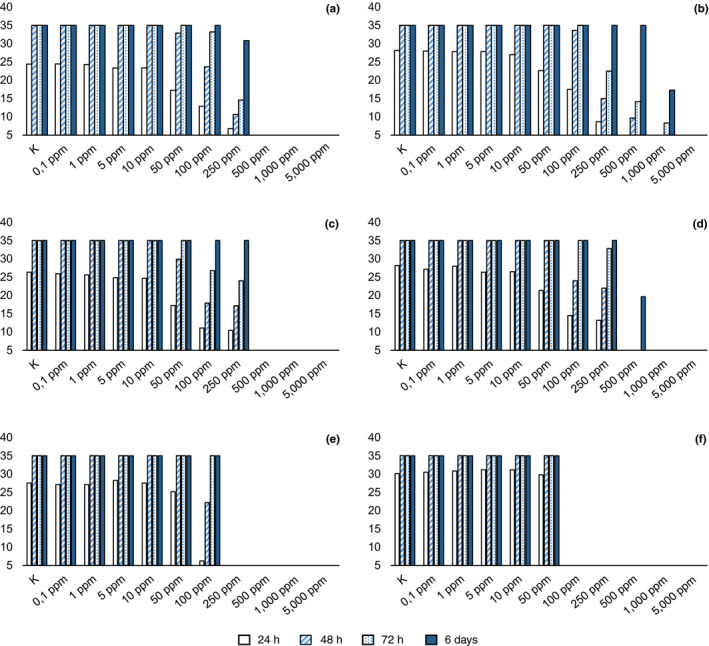
Average diameter (mm) of mycelium with different concentrations of product in agar. (a) Actidrox; (b) Detarox; (c): Virkon [Colour figure can be viewed at wileyonlinelibrary.com]
For acetic acid, the MIC in agar resulted in 250 ppm (Figure 2c), but its activity in water was confirmed at 250 ppm only for S. parasitica strain CBS 223.65, while for the other two strains the MLC was set at 500 ppm.
Boric acid was effective in slowing down the mycelium growth in agar at 500 ppm and produced complete inhibition at 1,000 ppm (Figure 2e); MLC was not determined at tested concentrations.
For S. parasitica, the MIC of lactic acid was set at 500 ppm (Figure 3a) while for S. delica at 5,000 ppm (Figure 3b), but lower concentrations progressively reduced radial growth in agar. At 6 days, reduced growth was still noticeable at 250 ppm. The two Saprolegnia species tested showed a different behaviour with respect to lactic acid also in water trials and, in particular, MLC was lower than MIC for S. delica.
Tartaric acid completely inhibited the radial growth of Saprolegnia spp. for 72 hr at a concentration of 500 ppm, but some mycelial growth was observed for S. delica at 6 days; therefore, the MIC resulted in 1,000 ppm (Figure 3d), while for S. parasitica the MIC was confirmed at 500 ppm (Figure 3c). The same MICs were observed for oxalic acid, but a concentration of 250 ppm was effective in slowing down the mycelium growth for 72 hr, while a concentration of 500 ppm completely inhibited Saprolegnia growth for 48 hr (Figure 2f).
Minimum lethal concentration of tartaric acid was not determined at tested concentrations, whereas oxalic acid in water was lethal at 1,000 ppm for one S. parasitica strain (CBS 223.65) only, while the other two tested strains showed a MLC = 5,000 ppm.
Sodium percarbonate was effective only in slowing down the mycelium growth at the highest tested concentrations (5,000 ppm), but at 48 hr the diameter of mycelium was equal to the control (Figure 2g); therefore, MIC and also MLC were not determined at tested concentrations.
Hydrogen peroxide showed equal values of MIC and MLC (5,000 ppm) for all tested strains, but slower growth was observed at 250 ppm after 24 hr (Figure 2h; Figure 4).
Figure 4.

Mycelial growth after 6 days with different concentrations of hydrogen peroxide in agar, showing minimum inhibitory concentration at 5,000 ppm [Colour figure can be viewed at wileyonlinelibrary.com]
As for commercial products, in agar, Actidrox® progressively slowed down mycelium growth with increasing concentrations of the product after 24 hr (Figure 5a); the MIC was set at 5,000 ppm. In water, this product showed a different effectiveness, resulting lethal at 500 ppm. Similarly, Detarox was 10‐fold more effective in water than in agar (MIC = 1,000 ppm ‐ Figure 5b; MLC = 100 ppm).
Figure 5.
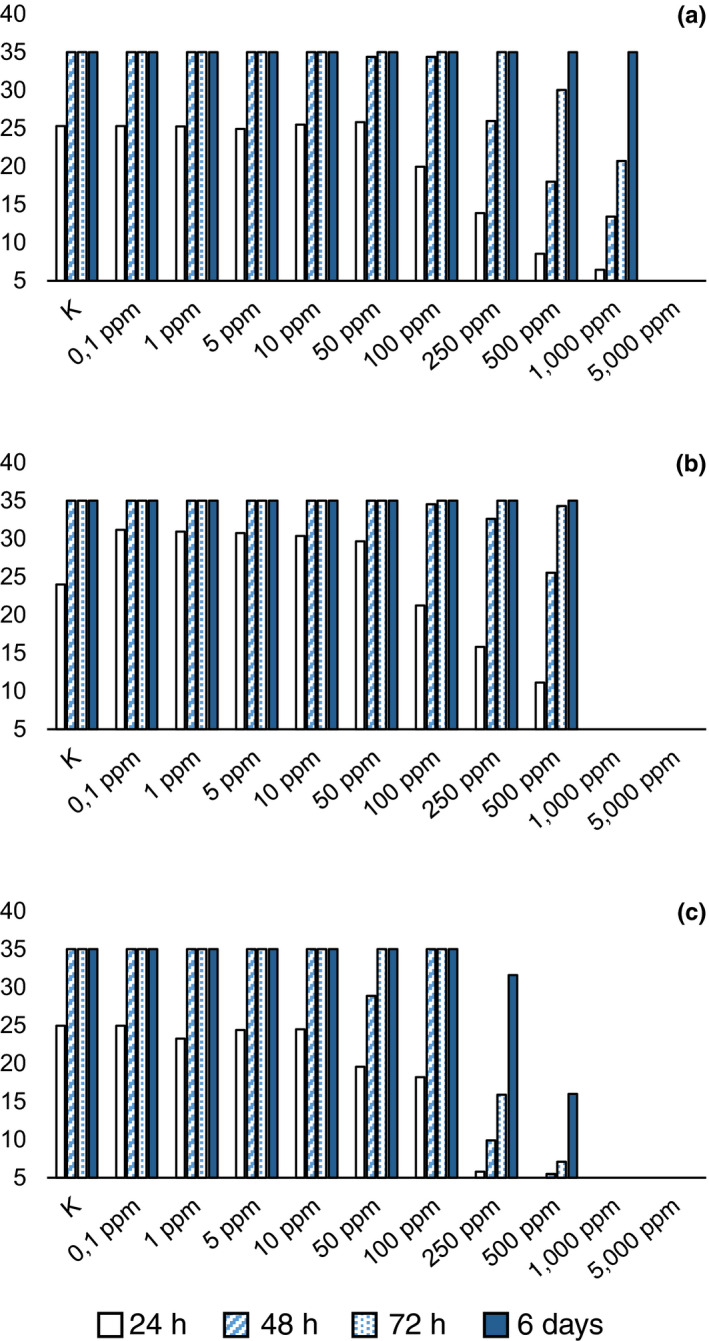
Average diameter (mm) of Saprolegnia parasitica and Saprolegnia delica mycelium with different concentrations of compound in agar. (a): S. parasitica, lactic acid; (b) S. delica, lactic acid; (c) S. parasitica, tartaric acid; (d) S. delica, tartaric acid; (e) S. parasitica, iodoacetic acid; (f) S. delica, iodoacetic acid [Colour figure can be viewed at wileyonlinelibrary.com]
Finally, Virkon™S showed a MIC = MLC = 1,000 ppm (Figure 5c).
4. DISCUSSION
In our study, the activity of malachite green and its suitability as positive reference for the screening of antifungal compounds were confirmed (Bailey & Jeffrey, 1989; Marking et al., 1994). Although copper sulphate has been used in the past in fish culture for the control of parasitic and Saprolegnia infections (Straus et al., 2012; Sun et al., 2014), currently it is not approved for therapeutic use in aquaculture. According to recent research, the activity of this compound may vary according to different stages of Saprolegnia development: Sun et al. (2014) showed how copper sulphate prohibited the release of primary zoospores at concentrations ≥ 1 mg/L and inhibited mycelium growth at concentrations ≥ 0.5 mg/L for 24 hr. In our study, we evaluated the effect of copper sulphate on hyphal growth, showing that after 6 days of continuous exposure in agar, a complete inhibition was achieved only at 250 ppm (MIC); however, concentrations of 50 and 100 ppm inhibited the aerial mycelium. Also for iodoacetic acid, an inhibition of aerial mycelium on agar was observed at concentrations lower than MIC. The mechanisms that determine this would require further investigation, but chemically induced morphological hyphae changes with inhibition of aerial mycelium of Saprolegnia were already hypothesized in a previous work (Kaminskyj & Heath, 1992).
Sodium percarbonate is an environmentally safe compound that has been successfully tested in vitro against developmental stages of Ichthyophthirius multifiliis (Buchmann, Jensen, & Kruse, 2003; Heinecke & Buchmann, 2009); dosages of 12.5 mg/L for 180 min and 62.5 mg/L for 90 min were effective in killing I. multifiliis theronts (Buchmann et al., 2003), while tomont stage appears considerably more tolerant to the chemical. In our study, it was not possible to identify a MIC and MLC values of sodium percarbonate for Saprolegnia mycelium. However, a possible higher activity of this compound against other developmental stages of Saprolegnia (i.e., zoospores) cannot be excluded.
The compounds tested in the present study, with few exceptions (hydrogen peroxide, malachite green, Virkon™S), performed differently in protocols I and II (Table 1). As a general rule, the MLC was higher than the MIC, and for some of the tested molecules (boric and tartaric acid, sodium percarbonate) it was not possible to determine a MLC at tested concentrations. In water trials, Saprolegnia strains were kept in contact with the tested compounds for 1 hr only, while in agar such contact was continuous. Our results would therefore suggest that for some compounds, a more prolonged bath would possibly be required in order to achieve a lethal effect at lower concentrations.
However, one molecule (iodoacetic acid) and two commercial products (Actidrox®, Detarox®AP) resulted considerably more effective in water.
For Actidrox®, the different performances observed in the two protocols are possibly linked to the characteristics of the product itself, which releases peracetic acid (effective against a wide range of microorganisms) when solubilized in water. A similar behaviour was observed for Detarox®AP, an acidic sanitizer with oxidant properties, formulated with stabilized peracetic acid and hydrogen peroxide, that is widely used in the food industry for the disinfection of production equipment. Also for Detarox®AP, active compounds showed greater effectiveness in the presence of water. Both peracetic acid and hydrogen peroxide show a wide‐range antimicrobial activity (Baldry, 1983; Jussila, Makkonen, & Kokko, 2011; Kitis, 2004) and low environmental impact, and are considered suitable alternative sanitizers (Pedersen, Meinelt, & Straus, 2013). Particularly, results of in vitro assessments (Jaafar, Kuhn, Chettri, & Buchmann, 2013; Jussila et al., 2011; Picón‐Camacho, Marcos‐Lopez, Beljean, Debeaume, & Shinn, 2012; Picón‐Camacho, Marcos‐Lopez, Bron, & Shinn, 2012) highlight a promising role of peracetic acid‐based products for the control of parasitic and “oomycotic” infections (i.e., white spot disease, crayfish plague) in aquaculture. In water, Detarox®AP is degraded quickly, leaving residues of acetic acid and its salts; this would suggest a possible low environmental impact of the product but represent a challenge in controlling the effectiveness of the product in the farm. Peracetic acid decay can be significantly affected by organic matter content (Pedersen et al., 2013) and possibly influenced by other water properties (hardness, ion composition). Similarly, information available in the literature (Barnes, Gabel, Durben, Hightower, & Berger, 2004) suggests that possible differences in the activity of hydrogen peroxide may occur, depending on physical and chemical properties of the water.
Hydrogen peroxide alone is regarded as one of the most promising antibacterial (Wagner, Oplinger, Arndt, Forest, & Bartley, 2010), antiparasitic (Grave, Horsberg, Lunestad, & Litleskare, 2004; Picón‐Camacho, Marcos‐Lopez, Bron et al., 2012) and antifungal (Marking et al., 1994) compounds to be used in fish culture. MIC and MLC values obtained in the present study for this compound (5,000 ppm) would possibly limit its applicability in the field due to its toxicity on eggs at concentration > 1,000 μl/L (Gaikowski, Rach, Olson, Ramsay, & Wolgamood, 1998; Gaikowski, Rach, & Ramsay, 1999); however, after 24 hr at 500 ppm hyphal growth was considerably slowed down. Daily administration of the compound would therefore allow to effectively control the infection at a concentration lower than the observed MIC/MLC. These results confirm previous in vivo experiments conducted on eggs of rainbow trout (Marking et al., 1994; Schreier et al., 1996) and of chinook salmon Oncorhynchus tshawytscha (Waterstrat & Marking, 1995) that showed how concentrations of hydrogen peroxide ranging from 500 to 1,000 ppm are effective in controlling S. parasitica and S. ferax infection. Rach, Gaikowski, Howe, and Schreier (1998) and Rach et al. (2005) demonstrated that the toxicity of hydrogen peroxide to fish eggs varies according to different species, but was always above 1,000 μl/L; their results are in accordance with previous studies (Marking et al., 1994; Schreier et al., 1996) in documenting increased hatching rates in fish eggs treated with 1,000 ppm hydrogen peroxide.
Iodoacetic acid performed differently for S. parasitica and S. delica in the two protocols, being more effective at inhibiting S. delica in agar, but more lethal to S. parasitica in water. This product is toxic and corrosive but not considered hazardous to the environment (iodoacetic acid Sigma‐Aldrich Safety Data Sheet, https://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=IT&language=EN-generic&productNumber=I4386&brand=-SIAL&PageToGoToURL=https%3A%2F%2Fwww.sigmaaldrich.com%2Fcatalog%2Fproduct%2Fsial%2Fi4386%3Flang%3Dit)). It is included among the disinfection by‐products (DBPs) that could be produced in raw water after disinfection process. Furthermore, studies are in progress to evaluate whether subtoxic doses in water could represent a carcinogenic risk for humans (Marsà, Cortés, Hernándeza, & Marcosa, 2018).
Benzoic acid is an antifungal compound naturally produced in fruit to fight fungal infections (Brown & Swinburne, 1971); this molecule and its derivatives have long been used as antimicrobial preservatives in the food industry and as antifungal agents in topical preparations for the treatment of human infections (Rowe, Sheskey, & Quinn, 2009) and show activity at different levels of fungal development (Amborabé, Fleurat‐Lessard, Chollet, & Roblin, 2002). In our screening, benzoic acid has shown the lowest MIC (100 ppm). This compound is slightly soluble in water (<1 mg/ml at 20°C); therefore, in order to obtain the desired concentrations, it was first dissolved in absolute ethanol and then diluted with deionized water. Ethanol used for the solubilization of benzoic acid was tested in parallel, showing no effectiveness. This result is consistent with other evidence in the literature (Hoskonen, Heikkinen, Eskelinen, & Pirhonen, 2015).
With respect to protocol I, boric acid considerably slowed down the mycelium growth at 500 ppm but complete inhibition was achieved at 1,000 ppm, suggesting that the MIC of boric acid could be set in between the two concentrations (500 and 1,000 ppm), and be possibly close to values reported in previous investigations (600 ppm, Ali et al., 2014).
Virkon™S is a mixture of peroxygens, surfactants, organic acids and inorganic salts. Used in the farming industry for the disinfection of equipment and facilities, the product is described as effective against a wide range of viruses, bacteria and fungi (Virkon™S product container label). In aquaculture, Virkon™S is used at a concentration of 1% w/v for the disinfection of ponds and farm equipment (Sudova et al., 2007). Particularly, in salmonids, the experimental exposure to 1% Virkon™S for 15 min was effective in controlling Gyrodactylus salaris infection (Koski, Anttila, & Kuusela, 2016). However, to the best of our knowledge, the effectiveness of this product against Saprolegnia has never been tested. Our results suggest a possible use of Virkon™S at concentrations lower than 1% w/v for the control of saprolegniosis.
For some compounds (lactic acid, oxalic acid, tartaric acid), remarkably different behaviours were observed between the two Saprolegnia species here tested (S. delica and S. parasitica) during in vitro trials, in which higher concentrations were needed to inhibit S. delica. This oomycete species is widely distributed in natural freshwater systems (Sarowar, Van Den Berg, McLaggan, Young, & Van West, 2014), while S. parasitica is considered more primarily pathogenic (Van West, 2006). Information about the selective activity of antifungal compounds towards different Saprolegnia species would allow for the identification of compounds that inhibit the growth of the pathogenic S. parasitica but result harmless or less harmful to the many saprophytic species of Saprolegnia naturally occurring in freshwater ecosystems, thus helping to preserve the structure and diversity of natural oomycete communities.
In conclusion, the in vitro tests performed here show that, with the exception of sodium percarbonate, the compounds/products tested are effective against Saprolegnia spp., although at different concentrations. Among these, benzoic acid and iodoacetic acid showed the lowest MIC/MLC, respectively; however, acetic acid and peracetic acid‐based products, particularly in combination with hydrogen peroxide, represent promising candidates for controlling saprolegniosis in aquaculture, due to their effectiveness associated with low environmental impact.
In order to assess the possible field application of these most promising compounds, further tests will be necessary to evaluate their efficacy on different developmental stages of Saprolegnia (i.e., zoospores), their possible cytotoxic effects, and ultimately their safety and efficacy in vivo.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
ACKNOWLEDGEMENTS
This project has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No. 634429. This publication reflects the views only of the author, and the European Commission cannot be held responsible for any use which may be made of the information contained therein. The authors would also like to thank CSIC‐RJB Madrid, Spain, for providing S. parasitica reference strain CBS 223.65.
Tedesco P, Fioravanti ML, Galuppi R. In vitro activity of molecules and commercial products against Saprolegnia parasitica and Saprolegnia delica strains. J Fish Dis. 2019;42:237–248. 10.1111/jfd.12923
REFERENCES
- Alborali, L. , Sangiorgi, E. , Leali, M. , Guadagnini, P. F. , & Sicura, S. (1997). The persistence of malachite green in the edible tissue of rainbow trout (Oncorhynchus mykiss). Rivista Italiana di Acquacoltura, 32, 45–60. [Google Scholar]
- Alderman, D. J. (1982). In vitro testing of fisheries chemotherapeutants. Journal of Fish Diseases, 8, 289–298. 10.1111/j.1365-2761.1982.tb00464.x [DOI] [Google Scholar]
- Alderman, D. J. , & Polglase, J. L. (1986). Aphanomyces astaci: Isolation and culture. Journal of Fish Diseases, 9, 367–379. 10.1111/j.1365-2761.1986.tb01030.x [DOI] [Google Scholar]
- Ali, S. E. , Thoen, E. , Evensen, Ø. , & Skaar, I. (2014). Boric acid inhibits germination and colonization of Saprolegnia spores in vitro and in vivo. PLoS ONE, 9, e91878 10.1371/journal.pone.0091878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amborabé, B. E. , Fleurat‐Lessard, P. , Chollet, J. F. , & Roblin, G. (2002). Antifungal effects of salicylic acid and other benzoic acid derivatives towards Eutypa lata: Structure–activity relationship. Plant Physiology and Biochemistry, 40, 1051–1060. 10.1016/S0981-9428(02)01470-5 [DOI] [Google Scholar]
- Bailey, T. A. , & Jeffrey, S. M. (1989). Evaluation of 215 candidate fungicides for use in fish culture. In Investigations in Fish Control (No. 99). US Fish and Wildlife Service. pp. 1–9.
- Baldry, M. G. C. (1983). The bactericidal, fungicidal and sporicidal properties of hydrogen peroxide and peracetic acid. Journal of Applied Microbiology, 54, 417–423. 10.1111/j.1365-2672.1983.tb02637.x [DOI] [PubMed] [Google Scholar]
- Barnes, M. E. , Gabel, A. C. , Durben, D. J. , Hightower, T. R. , & Berger, T. J. (2004). Changes in water hardness influence colonization of Saprolegnia diclina . North American Journal of Aquaculture, 66, 222–227. 10.1577/A03-058.1 [DOI] [Google Scholar]
- Bauer, K. , Dangschat, H. , Knoppler, H. O. , & Neudegger, J. (1988). Uptake and elimination of malachite green in rainbow trout (in German). Archiv für Lebensmittelhygiene, 39, 97–102. [Google Scholar]
- Bly, J. E. , Quiniou, S. M. A. , Lawson, L. A. , & Clem, L. W. (1996). Therapeutic and prophylactic measures for winter saprolegniosis in channel catfish. Diseases of Aquatic Organisms, 24, 25–33. 10.3354/dao024025 [DOI] [Google Scholar]
- Brown, A. E. , & Swinburne, T. R. (1971). Benzoic acid: An antifungal compound formed in Bramley's Seedling apple fruits following infection by Nectria galligena Bres. Physiological Plant Pathology, 1, 469–475. 10.1016/0048-4059(71)90009-9 [DOI] [Google Scholar]
- Bruno, D. W. , van West, P. , & Beakes, G. W. (2011). Saprolegnia and other oomycetes In Woo P. T. K., & Bruno D. W. (Eds.), Fish diseases and disorders. Volume 3: Viral, bacterial and fungal infections (2nd edn, pp. 669–720). Oxfordshire, UK: CABI International; 10.1079/9781845935542.0669 [DOI] [Google Scholar]
- Bryce, D. M. , Croshaw, B. , Hall, J. E. , Holland, V. R. , & Lessel, B. (1978). The activity and safety of the antimicrobial agent bronopol (2‐bromo‐2‐nitropropan‐1, 3‐diol). Journal of the Society of Cosmetic Chemists, 29, 3–24. [Google Scholar]
- Buchmann, K. , Jensen, P. B. , & Kruse, K. D. (2003). Effects of sodium percarbonate and garlic extract on Ichthyophthirius multifiliis theronts and tomocysts: In vitro experiments. North American Journal of Aquaculture, 65, 21–24. [DOI] [Google Scholar]
- Carballo, M. , Munoz, M. J. , Cuellar, M. , & Tarazona, J. V. (1995). Effects of waterborne copper, cyanide, ammonia, and nitrite on stress parameters and changes in susceptibility to saprolegniosis in rainbow trout (Oncorhynchus mykiss). Applied and Environmental Microbiology, 61, 2108–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton‐Hadley, R. S. , & Alderman, D. J. (1987). The effects of malachite green upon proliferative kidney disease. Journal of Fish Diseases, 10, 101–107. 10.1111/j.1365-2761.1987.tb00725.x [DOI] [Google Scholar]
- Cline, T. F. , & Post, G. (1972). Therapy for trout eggs infected with Saprolegnia . The Progressive Fish‐Culturist, 34, 148–151. 10.1577/1548-8640(1972)34[148:TFTEIW]2.0.CO;2 [DOI] [Google Scholar]
- Culp, S. J. , Mellick, P. W. , Trotter, R. W. , Greenlees, K. J. , Kodell, R. L. , & Beland, F. A. (2006). Carcinogenicity of malachite green chloride and leucomalachite green in B6C3F(1) mice and F344 rats. Food and Chemical Toxicology, 44, 1204–1212. 10.1016/j.fct.2006.01.016 [DOI] [PubMed] [Google Scholar]
- De Seta, F. , Schmidt, M. , Vu, B. , Essmann, M. , & Larsen, B. (2009). Antifungal mechanisms supporting boric acid therapy of Candida vaginitis. Journal of Antimicrobial Chemotherapy, 63, 325–336. 10.1093/jac/dkn486 [DOI] [PubMed] [Google Scholar]
- Edgell, P. , Lawseth, D. , Mclean, W. E. , & Britton, E. W. (1993). The use of salt‐solutions to control Fungus (Saprolegnia) infestations on salmon eggs. The Progressive Fish‐Culturist, 55, 48–52. [DOI] [Google Scholar]
- Environmental Protection Agency (EPA) (1993). Boric acid and its sodium salts. Washington, D.C: EPA 738‐R‐93‐017; U.S. Environmental Protection Agency; Office of Prevention, Pesticides and Toxic Substances; U.S. Government Printing Office; https://nepis.epa.gov/Exe/ZyPDF.cgi?Dockey=20000AHB.PDF [Google Scholar]
- European Commission (2010). Commission Regulation (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Official Journal of the European Union, 15, 1–72. [Google Scholar]
- European Commission (2014). Commission Regulation (EU) No. 605/2014 of 5 June 2014 amending, for the purposes of introducing hazard and precautionary statements in the Croatian language and its adaptation to technical and scientific progress, Regulation (EC) No 1272/2008 of the European Parliament and of the Council on classification, labelling and packaging of substances and mixtures. Official Journal of the European Union, 167, 36–49. [Google Scholar]
- European Food Safety Authority (EFSA) (2016). Malachite green in food. EFSA Panel on Contaminants in the Food Chain (CONTAM). EFSA Journal, 14(7), 4530 pp 80. 10.2903/j.efsa.2016.4530 [DOI] [Google Scholar]
- European Parliament and Council (2012). Regulation (EU) No. 528/2012 of the European Parliament and of the Council of 22 May 2012 concerning the making available on the market and use of biocidal products. Official Journal of the European Union, 167, 1–123. [Google Scholar]
- Forneris, G. , Bellardi, S. , Palmegiano, G. , Saroglia, M. , Sicuro, B. , Gasco, L. , & Zoccarato, I. (2003). The use of ozone in trout hatchery to reduce saprolegniasis incidence. Aquaculture, 221, 157–166. 10.1016/S0044-8486(02)00518-5 [DOI] [Google Scholar]
- Foster, F. J. , & Woodbury, L. (1936). The use of malachite green as a fish fungicide and antiseptic. The Progressive Fish‐Culturist, 3, 7–9. 10.1577/1548-8640(1936)318[7:TUOMGA]2.0.CO;2 [DOI] [Google Scholar]
- Fukunaga, K. , Suzuki, T. , & Takama, K. (1991). Effect of ozone on the compositions of gill and erythrocyte membrane lipids and proteins of Japanese charr (Salvelinus leucomaenis). Comparative Biochemistry & Physiology – Part B: Biochemistry & Molecular Biology, 100, 481–487. 10.1016/0305-0491(91)90208-u [DOI] [Google Scholar]
- Gaikowski, M. P. , Rach, J. J. , Olson, J. J. , Ramsay, R. T. , & Wolgamood, M. (1998). Toxicity of hydrogen peroxide treatments to rainbow trout eggs. Journal of Aquatic Animal Health, 10, 241–251. [DOI] [Google Scholar]
- Gaikowski, M. P. , Rach, J. J. , & Ramsay, R. T. (1999). Acute toxicity of hydrogen peroxide treatments to selected lifestages of cold‐, cool‐, and warmwater fish. Aquaculture, 178, 191–207. 10.1016/S0044-8486(99)00123-4 [DOI] [Google Scholar]
- Grave, K. , Horsberg, T. E. , Lunestad, B. T. , & Litleskare, I. (2004). Consumption of drugs for sea lice infestations in Norwegian fish farms: Methods for assessment of treatment patterns and treatment rate. Diseases of Aquatic Organisms, 60, 123–131. 10.3354/dao060123 [DOI] [PubMed] [Google Scholar]
- Hatai, K. , & Hoshiai, G. (1992). Mass mortality in cultured coho salmon (Oncorhynchus kisutch) due to Saprolegnia parasitica Coker. Journal of Wildlife Diseases, 28, 532–536. 10.7589/0090-3558-28.4.532 [DOI] [PubMed] [Google Scholar]
- Heinecke, R. D. , & Buchmann, K. (2009). Control of Ichthyophthirius multifiliis using a combination of water filtration and sodium percarbonate: Dose‐response studies. Aquaculture, 288, 32–35. 10.1016/j.aquaculture.2008.11.017 [DOI] [Google Scholar]
- Hoskonen, P. , Heikkinen, J. , Eskelinen, P. , & Pirhonen, J. (2015). Efficacy of clove oil and ethanol against Saprolegnia sp. and usability as antifungal agents during incubation of rainbow trout Oncorhynchus mykiss (Walbaum) eggs. Aquaculture Research, 46, 581–589. 10.1111/are.12200 [DOI] [Google Scholar]
- Jaafar, R. M. , Kuhn, J. A. , Chettri, J. K. , & Buchmann, K. (2013). Comparative efficacies of sodium percarbonate, peracetic acid, and formaldehyde for control of Ichthyobodo necator‐an ectoparasitic flagellate from rainbow trout. Acta Ichthyologica et Piscatoria, 43, 139–143. 10.3750/AIP2013.43.2.06 [DOI] [Google Scholar]
- James, P. M. , Thomas, H. S. , Gordon, L. S. , & Frieda, B. T. (1998). Copper tolerance by the freshwater algal species Oocystis pusilla and its ability to alter free‐ion copper. Aquatic Toxicology, 44, 69–82. 10.1016/S0166-445X(98)00067-8 [DOI] [Google Scholar]
- Jussila, J. , Makkonen, J. , & Kokko, H. (2011). Peracetic acid (PAA) treatment is an effective disinfectant against crayfish plague (Aphanomyces astaci) spores in aquaculture. Aquaculture, 320, 37–42. 10.1016/j.aquaculture.2011.08.008 [DOI] [Google Scholar]
- Kaminskyj, S. G. , & Heath, I. B. (1992). Age‐dependent differential responses of Saprolegnia hyphal tips to a helical growth‐inducing factor in the agar substitute, gellan. Experimental mycology, 16, 230–239. 10.1016/0147-5975(92)90031-L [DOI] [Google Scholar]
- Kitis, M. (2004). Disinfection of wastewater with peracetic acid: A review. Environment International, 30, 47–55. 10.1016/S0160-4120(03)00147-8 [DOI] [PubMed] [Google Scholar]
- Kiyoshi, M. , & Claude, E. B. (1993). Comparative evaluation of the solubility and algal toxicity of copper sulfate and chelated copper. Aquaculture, 117, 287–302. 10.1016/0044-8486(93)90326-T [DOI] [Google Scholar]
- Koski, P. , Anttila, P. , & Kuusela, J. (2016). Killing of Gyrodactylus salaris by heat and chemical disinfection. Acta Veterinaria Scandinavica, 58, 21 10.1186/s13028-016-0202-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumanova, R. , Vassileva, M. , Dobreva, S. , Manova, S. , & Kupenov, L. (1989). Evaluating bronopol. Manufacturing Chemist, 60, 36–37. [Google Scholar]
- Ling, K. H. , Sin, Y. M. , & Lam, T. J. (1993). Effect of copper sulphate on ichthyophthiriasis (white spot disease) in goldfish (Carassius auratus). Aquaculture, 118, 23–35. 10.1016/0044-8486(93)90277-6 [DOI] [Google Scholar]
- Machova, J. , Svobodova, Z. , Svobodník, J. , Piacka, V. , Vykusova, B. , & Kocova, A. (1996). Persistence of malachite green in tissues of rainbow trout after a long‐term therapeutic bath. Acta Veterinaria Brno, 65, 151–159. 10.2754/avb199665020151 [DOI] [Google Scholar]
- Marking, L. L. , Rach, J. J. , & Schreier, T. M. (1994). Evaluation of antifungal agents for fish culture. The Progressive Fish Culturist, 56, 225–231. [DOI] [Google Scholar]
- Marsà, A. , Cortés, C. , Hernándeza, A. , & Marcosa, R. (2018). Hazard assessment of three haloacetic acids, as byproducts of water disinfection, in human urothelial cells. Toxicology and Applied Pharmacology, 347, 70–78. 10.1016/j.taap.2018.04.004 [DOI] [PubMed] [Google Scholar]
- Mayer, F. P. , & Jorgenson, T. A. (1983). Teratological and other effects of malachite green on development of rainbow trout and rabbits. Transactions of the American Fisheries Society, 112, 818–824. [DOI] [Google Scholar]
- Nakagawa, K. , Hara, C. , Tokuyama, S. , Takada, K. , & Imamura, N. (2012). Saprolmycins A‐E, new angucycline antibiotics active against Saprolegnia parasitica . The Journal of Antibiotics, 65, 599–607. 10.1038/ja.2012.86 [DOI] [PubMed] [Google Scholar]
- Pedersen, L. F. , Meinelt, T. , & Straus, D. L. (2013). Peracetic acid degradation in freshwater aquaculture systems and possible practical implications. Aquacultural Engineering, 53, 65–71. 10.1016/j.aquaeng.2012.11.011 [DOI] [Google Scholar]
- Pickering, A. D. , & Willoughby, L. G. (1982). Saprolegnia infections of salmonid fish In Roberts R. J. (Ed.), Microbial diseases of fish (pp. 271–297). London, UK: Academic Press. [Google Scholar]
- Picón‐Camacho, S. M. , Marcos‐Lopez, M. , Beljean, A. , Debeaume, S. , & Shinn, A. P. (2012). In vitro assessment of the chemotherapeutic action of a specific hydrogen peroxide, peracetic, acetic, and peroctanoic acid‐based formulation against the free‐living stages of Ichthyophthirius multifiliis (Ciliophora). Parasitology Research, 110, 1029–1032. 10.1007/s00436-011-2575-1 [DOI] [PubMed] [Google Scholar]
- Picón‐Camacho, S. M. , Marcos‐Lopez, M. , Bron, J. E. , & Shinn, A. P. (2012). An assessment of the use of drug and non‐drug interventions in the treatment of Ichthyophthirius multifiliis Fouquet, 1876, a protozoan parasite of freshwater fish. Parasitology, 139, 149–190. 10.1017/S0031182011001867 [DOI] [PubMed] [Google Scholar]
- Plakas, S. M. , El‐Said, K. R. , Stehly, G. R. , Gingerich, W. H. , & Allen, J. L. (1996). Uptake, tissue distribution, and metabolism of malachite green in the channel catfish (Ictalurus punctatus). Canadian Journal of Fisheries and Aquatic Sciences, 53, 1427–1433. 10.1139/f96-061 [DOI] [Google Scholar]
- Quarles, W. (2001). Boric acid, borates and household pests. The IPM Practitioner, 23, 1–12. [Google Scholar]
- Rach, J. J. , Gaikowski, M. P. , Howe, G. E. , & Schreier, T. M. (1998). Evaluation of the toxicity and efficacy of hydrogen peroxide treatments on eggs of warm‐and coolwater fishes. Aquaculture, 165, 11–25. 10.1016/S0044-8486(98)00248-8 [DOI] [Google Scholar]
- Rach, J. J. , Redman, S. , Bast, D. , & Gaikowski, M. P. (2005). Efficacy of hydrogen peroxide versus formalin treatments to control mortality associated with saprolegniasis on lake trout eggs. North American Journal of Aquaculture, 67, 148–154. 10.1577/A04-062.1 [DOI] [Google Scholar]
- Rach, J. J. , Valentine, J. J. , Schreier, T. M. , Gaikowski, M. P. , & Crawford, T. G. (2004). Efficacy of hydrogen peroxide to control saprolegniasis on channel catfish (Ictalurus punctatus) eggs. Aquaculture, 238, 135–142. 10.1016/j.aquaculture.2004.06.007 [DOI] [Google Scholar]
- Rahkonen, R. , Koski, P. , Shinn, A. , Wootten, R. , & Sommerville, C. (2002). Post malachite green: Alternative strategies for fungal infections and white spot disease. Bulletin of the European Association of Fish Pathologists, 22, 152–157. [Google Scholar]
- Rowe, R. C. , Sheskey, P. J. , & Quinn, M. E. (2009). Handbook of pharmaceutical excipients, 6th ed London, UK: Pharmaceutical Press. [Google Scholar]
- Sarowar, M. N. , Van Den Berg, A. H. , McLaggan, D. , Young, M. R. , & Van West, P. (2014). Reprint of: Saprolegnia strains isolated from river insects and amphipods are broad spectrum pathogens. Fungal Biology, 118, 579–590. 10.1016/j.funbio.2014.05.005 [DOI] [PubMed] [Google Scholar]
- Schlenk, D. , Gollon, J. L. , & Griffin, B. R. (1998). Efficacy of copper sulfate for the treatment of ichthyophthiriasis in channel catfish. Journal of Aquatic Animal Health, 10, 390–396. [DOI] [Google Scholar]
- Schreier, T. M. , Rach, J. J. , & Howe, G. E. (1996). Efficacy of formalin, hydrogen peroxide, and sodium chloride on fungal‐infected rainbow trout eggs. Aquaculture, 140, 323–331. 10.1016/0044-8486(95)01182-X [DOI] [Google Scholar]
- Shi, X. Q. , Li, B. Q. , Qin, G. Z. , & Tian, S. P. (2011). Antifungal activity and possible mode of action of borate against Colletotrichum gloeosporioides on mango. Plant Disease, 95, 63–69. 10.1094/PDIS-06-10-0437 [DOI] [PubMed] [Google Scholar]
- Small, B. C. , & Wolters, W. R. (2003). Hydrogen peroxide treatment during egg incubation improves channel catfish hatching success. North American Journal of Aquaculture, 65, 314–317. 10.1577/C02-048 [DOI] [Google Scholar]
- Song, L. , Marsh, T. L. , Voice, T. C. , & Long, D. T. (2011). Loss of seasonal variability in a lake resulting from copper sulfate algaecide treatment. Physics and Chemistry of the Earth ‐ Parts A/B/C, 36, 430–435. 10.1016/j.pce.2010.04.016 [DOI] [Google Scholar]
- Straus, D. L. , Mitchell, A. J. , Carter, R. R. , & Steeby, J. A. (2012). Hatch rate of channel catfish Ictalurus punctatus (Rafinesque 1818) eggs treated with 100 mg/L copper sulphate pentahydrate. Aquaculture Research, 43, 14–18. 10.1111/j.1365-2109.2010.02791.x [DOI] [Google Scholar]
- Sudova, E. , Machova, J. , Svobodova, Z. , & Vesely, T. (2007). Negative effects of malachite green and possibilities of its replacement in the treatment of fish eggs and fish: A review. Veterinární Medicina, 52, 527–539. [Google Scholar]
- Sun, Q. , Hu, K. , & Yang, X. L. (2014). The efficacy of copper sulfate in controlling infection of Saprolegnia parasitica . Journal of the World Aquaculture Society, 45, 220–225. 10.1111/jwas.12113 [DOI] [Google Scholar]
- Toler, J. C. (1985). Preservative stability and preservative systems. International Journal of Cosmetic Science, 7, 157–164. 10.1111/j.1467-2494.1985.tb00409.x [DOI] [PubMed] [Google Scholar]
- U.S. Food & Drug Administration (USFDA) (2018). Approved Aquaculture Drugs. Retrieved from https://www.fda.gov/animalveterinary/developmentapprovalprocess/aquaculture/ucm132954.htm last updated 08/08/2018.
- U.S. Food and Drug Administration (USFDA) (2007). Freedom of information summary: Original new animal dug application.NADA 141–255 35% PEROX‐AID hydrogen peroxide liquid solution. (pp. 1–37). Washington, D.C.: USFDA. [Google Scholar]
- Van Den Berg, A. H. , McLaggan, D. , Diéguez‐Uribeondo, J. , & Van West, P. (2013). The impact of the water moulds Saprolegnia diclina and Saprolegnia parasitica on natural ecosystems and the aquaculture industry. Fungal Biology Reviews, 27, 33–42. 10.1016/j.fbr.2013.05.001 [DOI] [Google Scholar]
- Van West, P. (2006). Saprolegnia parasitica, an oomycete pathogen with a fishy appetite: New challenges for an old problem. Mycologist, 20, 99–104. 10.1016/j.mycol.2006.06.004 [DOI] [Google Scholar]
- Verner‐Jeffreys, D. W. , & Taylor, N. J. (2015). Review of freshwater treatments used in the Scottish freshwater rainbow trout aquaculture industry. Scottish Aquaculture Research Forum Report SARF100. 1–51.
- Wagner, E. J. , Oplinger, R. W. , Arndt, R. E. , Forest, A. M. , & Bartley, M. (2010). The safety and effectiveness of various hydrogen peroxide and iodine treatment regimens for rainbow trout egg disinfection. North American Journal of Aquaculture, 72, 34–42. 10.1577/A09-005.1 [DOI] [Google Scholar]
- Walser, C. A. , & Phelps, R. P. (1994). The use of formalin and iodine to control Saprolegnia infections on channel catfish, Ictalurus punctatus, eggs. Journal of Applied Aquaculture, 3, 269–278. 10.1300/J028v03n03_05 [DOI] [Google Scholar]
- Waterstrat, P. R. , & Marking, L. L. (1995). Communications: clinical evaluation of formalin, hydrogen peroxide, and sodium chloride for the treatment of Saprolegnia parasitica on fall chinook salmon eggs. The Progressive Fish‐Culturist, 57, 287–291. [DOI] [Google Scholar]
- Werth, G. (1958). Disturbances of the heredity pattern and production of tumours by experimental tissue anoxia. Arzneimittelforschung ‐ Drug Research, 8, 735–744. [PubMed] [Google Scholar]


