Abstract
Background and Aim
Emerging evidence indicates that psychological stress is involved in the pathogenesis of irritable bowel syndrome, which is characterized by visceral hypersensitivity and may be accompanied by gut dysbiosis. However, how such stress contributes to the development of visceral hypersensitivity is incompletely understood. Here, we aimed to investigate the influence that stress‐induced microbial changes exert on visceral sensitivity, as well as the possible underlying mechanisms associated with this effect.
Methods
Male Sprague–Dawley rats underwent chronic water avoidance stress (WAS) to induce visceral hypersensitivity. Visceral sensitivity, colonic tight junction protein expression, and short‐chain fatty acids of cecal contents were measured. Fecal samples were collected to characterize microbiota profiles. In a separate study, oral gavage of Roseburia in WAS rats was conducted to verify its potential role in the effectiveness on visceral hypersensitivity.
Results
Repeated WAS caused visceral hypersensitivity, altered fecal microbiota composition and function, and decreased occludin expression in the colon. Stressed rats exhibited reduced representation of pathways involved in the metabolism of butyrate and reduced abundance of several operational taxonomic units associated with butyrate‐producing bacteria, such as Lachnospiraceae. Consistently, supplementation with Roseburia hominis, a species belonging to Lachnospiraceae, significantly increased cecal butyrate content. Moreover, Roseburia supplementation alleviated visceral hypersensitivity and prevented the decreased expression of occludin.
Conclusions
Reduction in the abundance of butyrate‐producing Lachnospiraceae, which is beneficial for the intestinal barrier, was involved in the formation of visceral hypersensitivity. R. hominis is a potential probiotic for treating stress‐induced visceral hypersensitivity.
Keywords: butyrate, intestinal barrier, microbiota, stress, visceral hypersensitivity
Introduction
Perturbations in gut microbiota contribute to the development of intestinal disorders, including irritable bowel syndrome (IBS). IBS patients harbor altered gut microbiota in comparison with those of healthy controls.1, 2 The absence or disruption of gut microbes can significantly influence visceral pain perception, leading to visceral hypersensitivity. In particular, germ‐free mice exhibited increased visceral pain responses when exposed to colorectal distension (CRD) stimuli, and visceral hypersensitivity was normalized after microbial colonization.3 Furthermore, rats receiving early‐life antibiotic treatment also displayed visceral hypersensitivity in adulthood and altered microbiota profiles.4 Moreover, supplementation with probiotics/prebiotics or non‐absorbed antibiotics can reverse IBS symptoms.5, 6 However, the bacteria that are critical for IBS pathogenesis have not been identified, and recent results have been inconsistent.
Psychological stress is involved in the initiation, maintenance, and aggravation of IBS. Several lines of evidence have demonstrated the impact of stress on the gut microbiota,7, 8, 9, 10 but the precise mechanisms remain incompletely understood. Interestingly, mice exposed to chronic social defeat stress exhibited reduced representation of pathways involved in the synthesis and metabolism of short‐chain fatty acids (SCFAs).8 SCFAs are metabolites fermented by specific anaerobic bacteria from non‐digestible dietary fiber and resistant starches, and they have extensive regulatory functions in the interaction between microbes and hosts.11 A few studies have shown that SCFAs are associated with IBS; however, whether they are beneficial or harmful remains controversial.12, 13
In this study, to further investigate the possible role of gut microbiota in stress‐induced visceral hypersensitivity, we examined the influence of chronic water avoidance stress (WAS) in rats on the intestinal barrier and bacterial community and function. We also identified stress‐associated microbial species and further verified the beneficial effect of Roseburia, a butyrate‐producing genus that was significantly reduced in WAS rats, on stress‐induced visceral hypersensitivity.
Methods
Animals
Male Sprague–Dawley rats (6–7 weeks old) were obtained from the Department of Laboratory Animal Science, Peking University Health Science Center, Beijing, China. All protocols were approved by the Laboratory Animal Welfare Ethics branch of the Biomedical Ethics Committee of Peking University (LA2016230). Rats were housed in a specific pathogen‐free facility and maintained under a 12‐h light/dark cycle (6 am to 6 pm) at a constant temperature (23 ± 2°C) and humidity (63 ± 2%). Food and water were provided ad libitum. All animals were acclimatized to the facility for 7 days before the experiment began.
Water avoidance stress treatment
Repeated WAS procedures were conducted as described previously.14 Rats were placed on a block (10 × 8 × 8 cm) affixed to the center of the floor in a Plexiglas tank (45 × 25 × 25 cm) filled with 25°C fresh water to a level 1 cm below the top of the block. Rats were kept on the block for 1 h each day (5–6 pm) for 10 consecutive days. In the sham group, rats were placed on an identical platform in containers without water for 1 h each day for 10 days. Any fecal pellets in the containers were counted at the end of each 1‐h WAS or sham session. These were used to estimate the autonomic regulation of distal colonic motility as previously described.15
Assessment of visceral sensitivity to colorectal distension
Colorectal distension tests were performed the day after the last WAS session using a well‐established and validated method.15 Briefly, under light isoflurane anesthesia, a flexible balloon made of a polyethylene plastic bag (4–5 cm) was inserted into the distal colon, with its end 1 cm proximal to the anus. Then, the rats were placed in a Plexiglas cage for 20 min before the CRD test was initiated. Five series of CRDs were performed with pressures in different orders using a Distender Series IIR (G&J Electronics, Ontario, Canada) and resulting in constant pressure values of 20, 40, 60, and 80 mmHg (series 1); 40, 60, 80, and 20 mmHg (series 2); 60, 80, 20, and 40 mmHg (series 3); 80, 20, 40, and 60 mmHg (series 4); and 20, 40, 60, and 80 mmHg (series 5). Each distension lasted for a duration of 20 s, with a 4‐min inter‐stimulus interval. During each distention, abdominal withdrawal reflex (AWR) was scored by two blinded observers following a previously reported scale16: 0, no behavioral response to CRD; 1, brief head movement followed by immobility; 2, contraction of abdominal muscles; 3, lifting of abdomen; 4, body arching and lifting of pelvic structures.
Microbiota 16S rRNA sequencing and analysis
Fecal samples were collected directly from the anus by massaging the distal rectum before CRD testing, and samples were frozen at −80°C until use. Microbial DNA was extracted using a QIAamp Fast Stool Mini Kit (Qiagen, Valencia, CA, USA). The V3–V4 region of the 16S rRNA gene was amplified with barcode‐indexed primers (338F and 806R), and paired‐end sequencing of the amplicons was performed using the Illumina MiSeq PE300 platform (Illumina, San Diego, CA, USA). The difference in the relative abundance of each taxon at different taxonomic levels was analyzed using the linear discriminant analysis effect size method, and predictions regarding the functions of the microbiome were based on 16S rRNA‐derived operational taxonomic units (OTUs) using a computational approach called phylogenetic investigation of communities using reconstruction of unobserved states.17
Determination of cecal short‐chain fatty acids
Cecal SCFAs were determined using ultra‐performance liquid chromatography coupled to tandem mass spectrometry.18 Approximately 100‐mg cecal content was homogenized in 1 mL 50% aqueous acetonitrile and centrifuged. For derivatization, 40‐μL supernatant was mixed with 20 μL of 200‐mM 3‐nitrophenylhydrazine and 20 μL of 120‐mM N‐(3‐dimethylaminopropyl)‐N′‐ethylcarbodiimide·HCl‐6% pyridine solution. The mixture was reacted at 40°C for 30 min, and the solution was diluted to 800 μL with 10% aqueous acetonitrile, of which 10 μL was injected for measurement. An Ultimate 3000 RSLC system (Dionex Inc., Amsterdam, The Netherlands) coupled to a TSQ Quantiva Ultra triple‐quadrupole mass spectrometer (Thermo Fisher, Waltham, MA, USA) was used for SCFA detection, and the final data were processed using Xcalibur 3.0.63 software (Thermo Fisher). The data are expressed as mg SCFA/g cecal content.
Bacterial preparation
Strain A2‐183 was isolated from a healthy human fecal sample and was identified to be a representative strain of butyrate‐producing Roseburia hominis. Strain A2‐183 was routinely grown with synthetic YCFA medium. After cultivation at 37°C for 16 h, strain A2‐183 cells were harvested by centrifugation at 5000 rpm for 5 min and were resuspended in sterile phosphate‐buffered saline. Fresh bacterial cell suspension was prepared daily, and rats were administrated orally at a concentration of 2 × 109 CFU/day. In the experimental group with R. hominis, rats were administrated with the bacterial cell suspension for four consecutive days and were subsequently subjected WAS treatment from day 5 till day 14.
Measurement of serum corticotropin‐releasing hormone levels
Upon euthanasia, blood was withdrawn from the apex cordis and placed in a sterile microtube. After centrifugation at 4°C and 3000 rpm for 15 min, the supernatant was extracted and stored at −80°C until analysis. A radioimmunoarray kit (HY‐10175, Beijing, China) was used to determine corticotropin‐releasing hormone concentrations according to the manufacturer's instructions.
Quantitative reverse transcription–polymerase chain reaction for tight junction protein expression
Total RNA was extracted from distal colon tissues using TRIzol reagent (Life Technologies) according to the manufacturer's instructions. Complementary DNA was synthesized using a reverse transcription kit (KR116‐02, Tiangen, Beijing, China). Quantitative polymerase chain reaction (qPCR) for the typical tight junction protein occludin and β‐actin was performed with a Real‐Time PCR Detection System (QuantStudio5, Thermo Fisher Scientific) using SYBR Green detection. Primer sequences used for qPCR were as follows: forward occludin, 5′‐TCGTGATGTGCATCGCTGTATTCG‐3′; reverse occludin, 5′‐CGTAACCGTAGCCGTAACCGTAAC‐3′; forward β‐actin, 5′‐GGGAAATCGTGCGTGACATT‐3′; reverse β‐actin, 5′‐GCGGCAGTGGCCATCTC‐3′. The qPCR conditions were as follows: one cycle at 95°C for 3 min, followed by 40 two‐temperature cycles at 95°C for 5 s and 60°C for 15 s. PCR amplifications were performed in a total volume of 20 μL containing Talent qPCR PreMix (FP209, Tiangen, Beijing, China). Occludin transcript levels were normalized to that of β‐actin, and relative gene expression was calculated as the fold change (2−ΔΔCt) relative to expression in the control samples.
Assessment of tight junction protein expression
The distal colon tissues were homogenized in RIPA extraction buffer mixed with a protease inhibitor cocktail and then centrifuged at 12 000 rpm for 10 min. The protein concentration of the supernatant was quantified by a BCA protein assay kit. Equal amounts of protein (90 μg) were loaded onto 10% sodium dodecyl sulfate polyacrylamide gels and electrophoresed under a constant voltage of 100 V for 120 min. Proteins were transferred onto nitrocellulose membranes under a constant current of 250 mA for 100 min. The membranes were blocked in 5% (w/v) dried skim milk at 25°C for 1 h and then incubated overnight at 4°C with primary antibodies against occludin (1:10 000, ab167161, Abcam). Glyceraldehyde phosphate dehydrogenase was used as an internal reference protein. After washing three times, the membranes were incubated with antirabbit or mouse secondary antibodies for 1 h at 25°C. Finally, after repeatedly washing three times, they were scanned on an infrared imaging system (LI‐COR Biosciences, Lincoln, NE, USA). Expression levels were determined as the band intensity relative to that of glyceraldehyde phosphate dehydrogenase, and then the ratio values were normalized by that of the control group.
Statistical analysis
Abdominal withdrawal reflex scores was analyzed using two‐way anova, with CRD pressure and treatment as factors followed by Least Significant Difference (LSD) post‐hoc test. The data for fecal pellet output numbers, occludin expression, and SCFA content were analyzed using one‐way anova followed by LSD post‐hoc test or unpaired Student's t‐tests if only two groups were applied. Bacterial relative abundances were analyzed by Wilcoxon test, and the relationships between disease phenotype parameters and alterations in microbial OTUs were analyzed by Pearson's correlation test. Results are expressed as mean ± SEM. Differences between groups were considered significant at P < 0.05. Statistical analyses were performed with SPSS version 20.0 (SPSS, Chicago, IL, USA).
Results
Effect of chronic water avoidance stress on visceral sensitivity and intestinal barrier
Compared with sham controls, AWR scores were higher in WAS rats at pressures of 20 mmHg (1.89 ± 0.16 vs 1.43 ± 0.15, P < 0.05) and 60 mmHg (3.47 ± 0.13 vs 3.06 ± 0.12, P < 0.05) (Fig. 1a). Fecal pellet output numbers were significantly higher in stressed rats than in the sham group (Fig. 1b). Moreover, the relative levels of colonic occludin mRNA (P < 0.05) (Fig. 1c) and protein (P < 0.01) (Fig. 1d,e) decreased significantly in stressed rats.
Figure 1.
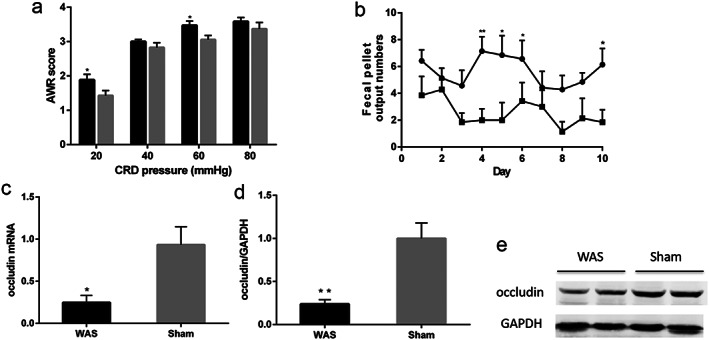
Effect of chronic WAS on visceral sensitivity and intestinal tight junctions. (a) Abdominal withdrawal reflex scores in response to CRD;  , WAS;
, WAS;  , Sham. (b) Fecal pellet output numbers found in the containers after each 1‐h WAS;
, Sham. (b) Fecal pellet output numbers found in the containers after each 1‐h WAS;  , WAS;
, WAS;  , Sham. (c) Real‐time quantitative RT–PCR of colonic occludin mRNA normalized to expression of β‐actin. (d) Quantitative analysis of colonic occludin protein normalized to expression of GAPDH. (e) Representative image of occludin bands in western blotting experiments. *P < 0.05, **P < 0.01, compared with sham controls; n = 7 rats per group. WAS, water avoidance stress; GAPDH, glyceraldehyde phosphate dehydrogenase; CRD, colorectal distension.
, Sham. (c) Real‐time quantitative RT–PCR of colonic occludin mRNA normalized to expression of β‐actin. (d) Quantitative analysis of colonic occludin protein normalized to expression of GAPDH. (e) Representative image of occludin bands in western blotting experiments. *P < 0.05, **P < 0.01, compared with sham controls; n = 7 rats per group. WAS, water avoidance stress; GAPDH, glyceraldehyde phosphate dehydrogenase; CRD, colorectal distension.
Effect of chronic water avoidance stress on fecal microbiota composition and function
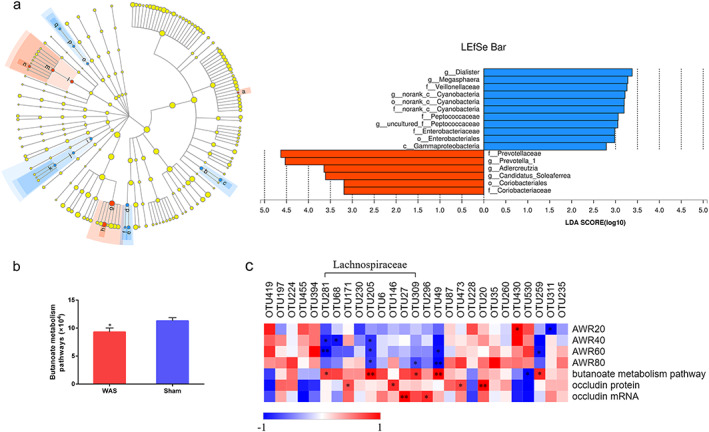
Linear discriminant analysis effect size analysis showed that the relative abundances of the families Prevotellaceae and Coriobacteriaceae and the genus Prevotella_1 increased significantly after WAS treatment, while those of Peptococcaceae and Veillonellaceae were significantly reduced in WAS rats (Fig. 2a). Accordingly, 27 OTUs were found to be significantly altered after stress. Six OTUs were enriched following WAS and belonged to the Acidaminococcaceae (n = 1), Coriobacteriaceae (n = 2), Prevotellaceae (n = 1), and Ruminococcaceae (n = 2). Another 21 OTUs were reduced or eliminated by WAS, and most of these belonged to the Lachnospiraceae (n = 9), Lactobacillaceae (n = 2), Peptococcaceae (n = 1), and Veillonellaceae (n = 2) (Table 1).
Figure 2.
Effect of WAS on microbial composition and function. (a) LEfSe analysis shows significantly different taxa between the two groups (left panel;  , a: g_Candidatus_Soleaferrea;
, a: g_Candidatus_Soleaferrea;  , b: f_Peptococcaceaae;
, b: f_Peptococcaceaae;  , c: g_uncultured_f_Peptococcaceaae;
, c: g_uncultured_f_Peptococcaceaae;  , d: f_Veillonellaceae;
, d: f_Veillonellaceae;  , e: g_Megasphaera;
, e: g_Megasphaera;  , f: g_Diallister;
, f: g_Diallister;  , g: f_Prevotellaceae;
, g: f_Prevotellaceae;  , h: g_Prevotella_1;
, h: g_Prevotella_1;  , i: c_Gammaproteobacteria;
, i: c_Gammaproteobacteria;  , j: o_Enterobacteriales;
, j: o_Enterobacteriales;  , k: f_Enterobacteriaceae;
, k: f_Enterobacteriaceae;  , l: o_Coriobacteriales;
, l: o_Coriobacteriales;  , m: o_Coriobacteriaceae;
, m: o_Coriobacteriaceae;  , n: g_Adlercreutzia;
, n: g_Adlercreutzia;  , o: o_norank_c_Cyanobacteria;
, o: o_norank_c_Cyanobacteria;  , p: f_norank_c_Cyanobacteria;
, p: f_norank_c_Cyanobacteria;  , q: g_norank_c_Cyanobacteria) and bacterial taxa with significantly different abundances (LDA scores > 2) (right panel;
, q: g_norank_c_Cyanobacteria) and bacterial taxa with significantly different abundances (LDA scores > 2) (right panel;  , Sham;
, Sham;  , WAS). Labels beginning with c_ indicate class; o_, order; f_, family; g_, genus. WAS, water avoidance stress. LDA, linear discriminant analysis; LEfSe, LDA effect size. (b) Phylogenetic investigation of communities using reconstruction of unobserved states functional prediction shows significantly decreased butanoate metabolism pathways in the WAS group. *P < 0.05, compared with sham controls. (c) Heatmap showing correlations between altered microbial OTUs and disease phenotype parameters. Color intensity indicates the degree of correlation. Red, positive correlation; blue, negative correlation. n = 7 rats per group. *P < 0.05, **P < 0.01. [Color figure can be viewed at wileyonlinelibrary.com]
, WAS). Labels beginning with c_ indicate class; o_, order; f_, family; g_, genus. WAS, water avoidance stress. LDA, linear discriminant analysis; LEfSe, LDA effect size. (b) Phylogenetic investigation of communities using reconstruction of unobserved states functional prediction shows significantly decreased butanoate metabolism pathways in the WAS group. *P < 0.05, compared with sham controls. (c) Heatmap showing correlations between altered microbial OTUs and disease phenotype parameters. Color intensity indicates the degree of correlation. Red, positive correlation; blue, negative correlation. n = 7 rats per group. *P < 0.05, **P < 0.01. [Color figure can be viewed at wileyonlinelibrary.com]
Table 1.
Significantly different OTUs between WAS and sham control group (%, means ± SEM)
| OTU | Family | Genus | WAS | Sham | P‐value |
|---|---|---|---|---|---|
| OTU419 | f__Acidaminococcaceae | g__Phascolarctobacterium | 0.010 ± 0.003 | 0.001 ± 0.001 | 0.014 |
| OTU197 | f__Bacteroidales_S24‐7_group | g__norank_f__Bacteroidales_S24‐7_group | 0.133 ± 0.025 | 0.382 ± 0.096 | 0.041 |
| OTU224 | f__Christensenellaceae | g__Uncultured Christensenellaceae | 0.001 ± 0.001 | 0.008 ± 0.002 | 0.029 |
| OTU455 | f__Coriobacteriaceae | g__Adlercreutzia | 0.069 ± 0.030 | 0.016 ± 0.002 | 0.041 |
| OTU394 | f__Coriobacteriaceae | g__Enterorhabdus | 0.008 ± 0.003 | 0.001 ± 0.001 | 0.041 |
| OTU281 | f__Lachnospiraceae | g__Lachnospiraceae_NK4A136_group | 0.340 ± 0.122 | 1.629 ± 0.534 | 0.021 |
| OTU68 | f__Lachnospiraceae | g__unclassified_f__Lachnospiraceae | 0.000 ± 0.000 | 0.254 ± 0.199 | 0.031 |
| OTU171 | f__Lachnospiraceae | g__uncultured_f__Lachnospiraceae | 0.029 ± 0.013 | 0.094 ± 0.021 | 0.030 |
| OTU230 | f__Lachnospiraceae | g__unclassified_f__Lachnospiraceae | 0.004 ± 0.002 | 0.032 ± 0.014 | 0.026 |
| OTU205 | f__Lachnospiraceae | g__Roseburia | 0.001 ± 0.001 | 0.029 ± 0.012 | 0.004 |
| OTU6 | f__Lachnospiraceae | g__Lachnospiraceae_NK4A136_group | 0.003 ± 0.003 | 0.028 ± 0.008 | 0.014 |
| OTU146 | f__Lachnospiraceae | g__unclassified_f__Lachnospiraceae | 0.000 ± 0.000 | 0.026 ± 0.016 | 0.031 |
| OTU27 | f__Lachnospiraceae | g__unclassified_f__Lachnospiraceae | 0.005 ± 0.003 | 0.024 ± 0.008 | 0.050 |
| OTU309 | f__Lachnospiraceae | g__unclassified_f__Lachnospiraceae | 0.000 ± 0.000 | 0.005 ± 0.002 | 0.031 |
| OTU296 | f__Lactobacillaceae | g__Lactobacillus | 0.000 ± 0.000 | 0.121 ± 0.075 | 0.031 |
| OTU49 | f__Lactobacillaceae | g__Lactobacillus | 0.000 ± 0.000 | 0.007 ± 0.003 | 0.004 |
| OTU87 | f__norank_c__Cyanobacteria | g__norank_c__Cyanobacteria | 0.001 ± 0.001 | 0.038 ± 0.023 | 0.010 |
| OTU473 | f__Peptococcaceae | g__uncultured_f__Peptococcaceae | 0.041 ± 0.012 | 0.229 ± 0.075 | 0.025 |
| OTU228 | f__Prevotellaceae | g__Prevotella_1 | 9.906 ± 2.323 | 2.898 ± 0.742 | 0.041 |
| OTU20 | f__Ruminococcaceae | g__Ruminococcaceae_UCG‐014 | 0.001 ± 0.001 | 0.020 ± 0.007 | 0.034 |
| OTU35 | f__Ruminococcaceae | g__Oscillibacter | 0.005 ± 0.004 | 0.017 ± 0.005 | 0.019 |
| OTU260 | f__Ruminococcaceae | g__unclassified_f__Ruminococcaceae | 0.001 ± 0.001 | 0.006 ± 0.002 | 0.048 |
| OTU430 | f__Ruminococcaceae | g__Candidatus_Soleaferrea | 0.010 ± 0.003 | 0.004 ± 0.002 | 0.041 |
| OTU530 | f__Ruminococcaceae | g__Ruminiclostridium_5 | 0.004 ± 0.002 | 0.000 ± 0.000 | 0.031 |
| OTU259 | f__Streptococcaceae | g__Streptococcus | 0.000 ± 0.000 | 0.013 ± 0.006 | 0.031 |
| OTU311 | f__Veillonellaceae | g__Megasphaera | 0.000 ± 0.000 | 0.247 ± 0.115 | 0.004 |
| OTU235 | f__Veillonellaceae | g__Dialister | 0.000 ± 0.000 | 0.021 ± 0.010 | 0.011 |
OTUs, operational taxonomic units; SEM, standard error of the mean; WAS, water avoidance stress.
As for the microbial function, phylogenetic investigation of communities using reconstruction of unobserved states functional analysis of the 16S rRNA data revealed that the representation of pathways involved in the metabolism of butanoate, the conjugate base of the SCFA butyrate, was significantly reduced in the WAS group (Fig. 2b).
Correlation between altered microbiota and disease phenotype parameters
As illustrated in the correlation heatmap in Figure 2c, the abundances of OTUs that belonged to the Lachnospiraceae, Lactobacillaceae, and Veillonellaceae were negatively correlated with AWR scores. Several Lachnospiraceae, Lactobacillaceae, and Peptococcaceae‐related OTUs were positively correlated with the expression of occludin. Moreover, OTU281, OTU205, and OTU309 (Lachnospiraceae) and OTU49 (Lactobacillaceae) were positively associated with the representation of butanoate metabolism functional pathways.
Effect of Roseburia hominis on visceral sensitivity
As the numbers of butanoate metabolism functional pathways and OTUs belonging to Lachnospiraceae, a major butyrate‐producing family, were both significantly decreased in the stressed rats (Fig. 2b and Table 1), we examined the effect of supplementation with R. hominis A2‐183, a representative butyrate‐producing strain belonging to Lachnospiraceae, on stress‐induced visceral hypersensitivity (Fig. 3a). Administration of R. hominis significantly attenuated the increase in AWR scores in response to CRD at 40 and 60 mmHg (both P < 0.05) (Fig. 3b). Oral gavage of R. hominis also significantly decreased fecal pellet output numbers compared with those in the WAS group (P < 0.05) (Fig. 3c).
Figure 3.
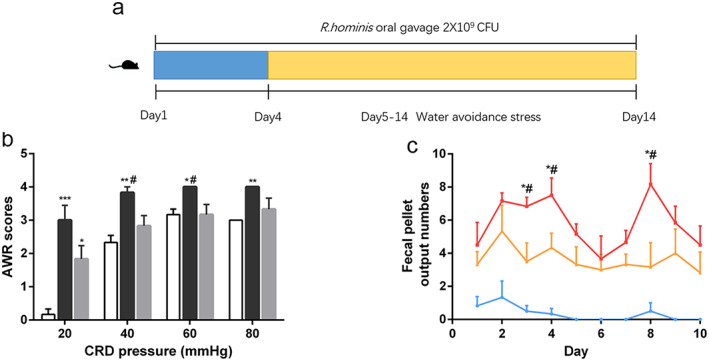
Effect of Roseburia hominis oral gavage on visceral sensitivity. (a) Schematic diagram of the experiment. (b) Abdominal withdrawal reflex scores in response to CRD;  , Sham + PBS;
, Sham + PBS;  , WAS + PBS;
, WAS + PBS;  , WAS + Rh. (c) Fecal pellet output numbers found in the containers after each 1‐h WAS;
, WAS + Rh. (c) Fecal pellet output numbers found in the containers after each 1‐h WAS;  , Sham + PBS;
, Sham + PBS;  , WAS + PBS;
, WAS + PBS;  , WAS + Rh. *P < 0.05, **P < 0.01, *** P < 0.001, compared with sham controls; #P < 0.05, compared with WAS + Rh group. n = 6 rats per group. PBS, phosphate‐buffered saline. Rh, R. hominis; WAS, water avoidance stress; CRD, colorectal distension. [Color figure can be viewed at wileyonlinelibrary.com]
, WAS + Rh. *P < 0.05, **P < 0.01, *** P < 0.001, compared with sham controls; #P < 0.05, compared with WAS + Rh group. n = 6 rats per group. PBS, phosphate‐buffered saline. Rh, R. hominis; WAS, water avoidance stress; CRD, colorectal distension. [Color figure can be viewed at wileyonlinelibrary.com]
Effect of Roseburia hominis on hypothalamic–pituitary–adrenal axis and intestinal barrier
Roseburia hominis prevented the WAS‐induced increase in serum corticotropin‐releasing hormone levels (Fig. 4b), while there was no significant alteration in the relative weight of adrenal glands after R. hominis administration (Fig. 4a). In addition, quantitative RT–PCR analysis showed that supplementation with R. hominis prevented the reduction in occludin mRNA and protein expression induced by WAS (Fig. 5a–c).
Figure 4.
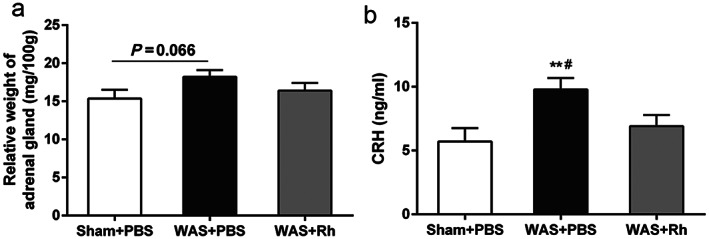
Effect of Roseburia hominis on HPA axis activity. (a) Relative weight of adrenal gland of rats. (b) Serum corticotropin‐releasing hormone level. **P < 0.01, compared with sham controls; #P < 0.05, compared with WAS + Rh group. n = 6 rats per group. HPA, hypothalamic–pituitary–adrenal; PBS, phosphate‐buffered saline; Rh, R. hominis; WAS, water avoidance stress.
Figure 5.
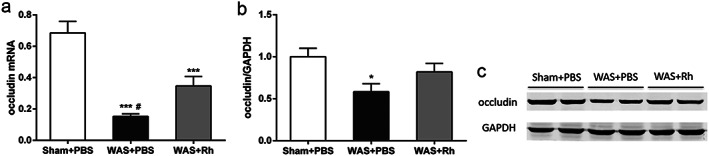
Effect of Roseburia hominis oral gavage on intestinal barrier. (a) Real‐time quantitative RT–PCR of colonic occludin mRNA normalized to expression of β‐actin. (b) Quantitative analysis of colonic occludin protein normalized to expression of GAPDH. (c) Representative image of occludin bands in western blotting experiments. *P < 0.05, ***P < 0.001, compared with sham controls; #P < 0.05, compared with WAS +Rh group. n = 6 rats per group. PBS, phosphate buffered saline; GAPDH, glyceraldehyde phosphate dehydrogenase; Rh, R. hominis; WAS, water avoidance stress.
Effect of Roseburia hominis on short‐chain fatty acids in cecal contents
As R. hominis is a typical butyrate‐producer, we measured SCFA concentrations in cecal contents. The results indicated that R. hominis administration significantly increased the cecal concentration of butyrate (P < 0.01) (Fig. 6c). In addition, propionate concentration tended to increase (P = 0.053) (Fig. 6b), while other SCFAs did not vary upon R. hominis treatment (Fig. 6a,d and Table S1).
Figure 6.
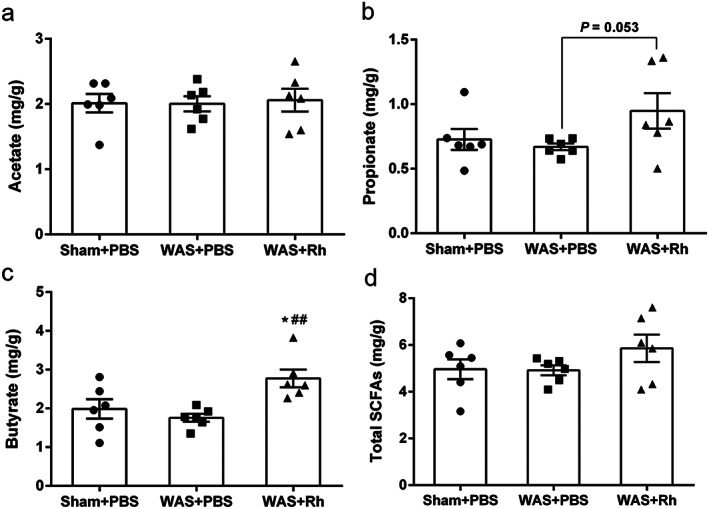
Effect of Roseburia hominis oral gavage on acetate (a), propionate (b), butyrate (c), and total SCFA concentration in cecal contents. *P < 0.05, compared with sham controls. ##P < 0.01, compared with WAS group. n = 6 rats per group. PBS, phosphate buffered saline; Rh, R. hominis; WAS, water avoidance stress; SCFAs, short‐chain fatty acids.
Discussion
We found that psychological stress increased visceral hypersensitivity and decreased the expression of colonic tight junction protein occludin, accompanied by alterations to the composition and function of the gut microbiota in rats. In addition, by verifying the beneficial effect of butyrate‐producing R. hominis on WAS rats, our results suggest that specific bacterial taxa or their metabolites are associated with visceral sensitivity and colonic barrier function.
Exposure to social stressors can affect the gut microbiota, manifesting as a reduction in microbial diversity and richness.7, 8 As a model to mimic IBS symptoms, WAS can also lead to gut dysbiosis. At the family level, the relative abundances of Prevotellaceae and Coriobacteriaceae increased, whereas that of Peptococcaceae decreased significantly after stress treatment. Prevotellaceae is reportedly involved in several intestinal diseases. For example, Prevotellaceae is more abundant in IBS patients than in controls.19 In a mouse model of chemical‐induced colitis, NLRP6‐deficient mice exhibited altered fecal microbiota, characterized by increased Prevotellaceae, which was a key mediator of phenotype transferring from NLRP6‐deficient mice to wild‐type mice.20 Similarly, the abundance of Prevotella, a major genus belonging to the Prevotellaceae, was also elevated in WAS rats. A previous clinical study1 classified all enrolled subjects into three enterotypes, and IBS patients were assigned to Prevotella‐dominant (25%) and Bacteroides‐dominant (60%) types. These results support the potentially important role of Prevotellaceae and Prevotella in IBS morbidity. Additionally, Coriobacteriaceae is among a group of bacterial taxa found to be related to colorectal cancer,21 ulcerative colitis, and Crohn's disease.22 In another mouse model of stress, Coriobacteriaceae levels increased in Balb/c mice after exposure to a grid floor stimulation.23 These results indicate the importance of these bacterial groups to stress‐induced visceral hypersensitivity, which warrants further investigations to understand in what way they interact with their hosts.
A majority of the OTUs that were reduced in the WAS group belonged to Lachnospiraceae. Members of the Lachnospiraceae are able to utilize lactate and acetate to produce butyrate via the butyryl‐CoA or acetate CoA transferase pathways or the butyrate kinase pathway.24 In our study, microbial functional prediction analysis showed the reduced representation of the pathways involved in butanoate metabolism in the stressed rats, and the abundances of several Lachnospiraceae‐related OTUs were significantly correlated with butanoate metabolism function. Among these was Roseburia, one of the core genera in human gut microbiota worldwide.25 Roseburia has been considered to be a potential indicator of intestinal health,26 and links between Roseburia and intestinal diseases, including inflammatory bowel disease,27, 28 IBS,29 and colon cancer,30 have been reported. For example, the microbial composition in ulcerative colitis patients differs from that in healthy volunteers by showing a reduction in R. hominis.28 Moreover, Roseburia inulinivorans is significantly reduced in Crohn's disease patients compared with levels in healthy individuals.31 Similarly, Chassard et al.29 reported that Roseburia was significantly reduced in constipation‐predominant IBS patients compared with levels in healthy subjects. In our study, gavage with the chosen species, R. hominis, prevented the stressed rats from developing visceral hypersensitivity. This further demonstrated the possibly important role of Roseburia in IBS pathogenesis.
Butyrate is the preferred metabolic substrate for colonocytes and provides about 60–70% of the energy necessary for their proliferation, apoptosis, and differentiation.11 Several studies have indicated the association between reduced butyrate levels and gastrointestinal diseases. Specially, patients with IBS had significantly lower serum level of butyrate, which reflects the level of butyrate in cecum and the proximal colon, following lactulose ingestion than healthy controls.32 Increasing evidence demonstrate the beneficial effect of supplementation with butyrate on IBS symptoms. One study showed that administration of microencapsulated sodium butyrate significantly reduced the frequency of abdominal pain in IBS patients.33 Similarly, healthy volunteers who were given intrarectal enemas containing a physiologically relevant dose of butyrate exhibited reductions in pain perception and discomfort.34 Consistent with these, our data indicated alleviation of visceral hypersensitivity and increased cecal butyrate concentration after Roseburia administration. It is reported that butyrate might cause a decrease in visceral pain via modulation of 5‐hydroxytryptamine, leading to an increased colonic compliance. Transient receptor potential vanilloid 1 in the colonic mucosa can be activated by butyrate, which may in turn lead to 5‐hydroxytryptamine release in the colonic lumens. Another mechanism by which butyrate might alleviate visceral hypersensitivity is via inhibition of histone deacetylase, thus induces microglial apoptosis and reduces inflammation‐induced neurotoxicity in rat tissue.34 However, the studies in this area have generated conflicting results on butyrate. For example, one previous study reported that IBS‐D patients had higher n‐butyrate concentration,35 and other studies found that rectal instillation of butyrate to Sprague–Dawley rats resulted in a sustained decrease in their pain threshold.36, 37 A number of reasons have been proposed for these conflicts, including reduced absorption of butyrate due to increased motility and shorter transit times,38 the amount of butyrate administered, the source of butyrate, the timing of butyrate administration, and the differences between the in vivo and in vitro environments.12
An impaired intestinal barrier may allow for pathogen invasion, and luminal commensal bacteria can also affect the barrier in various ways. Our data revealed a positive correlation between four Lachnospiraceae‐related OTUs and occludin expression, and supplementation with R. hominis prevented the decreases in occludin mRNA and protein expression, accompanied by an increase in butyrate production in the intestinal contents. This is consistent with the finding that R. hominis colonization of germ‐free mice can affect intestinal barrier function genes.39 These results suggest the potential role of specific butyrate‐producing bacteria in preventing gut leakage through their metabolites. Moreover, it is reported that butyrate could induce MUC2 mRNA expression and MUC2 secretion in goblet‐like cell,40 as well as promote the assembly of occludin dependent on AMPK pathway.41
Based on these results, we speculate that stress‐induced gut dysbiosis, especially a decrease in the abundance of butyrate‐producing bacteria such as Lachnospiraceae, may contribute to the pathogenesis of stress‐induced visceral hypersensitivity. Moreover, supplementation with butyrate‐producing bacteria may be beneficial for alleviating the visceral hypersensitivity. Thus, R. hominis as a potential probiotic in treating stress‐induced visceral hypersensitivity warrants further confirmation in the future.
Supporting information
Table S1. Cecal short chain fatty acids difference between sham, WAS and WAS + R. hominis groups (means ± SEM).
Acknowledgments
We are greatly thankful for the support of Institute of Acupuncture and Moxibustion China Academy of Chinese Medical Sciences, as well as Metabolomics Facility at Technology Center for Protein Sciences in Tsinghua University.
Zhang, J. , Song, L. , Wang, Y. , Liu, C. , Zhang, L. , Zhu, S. , Liu, S. , and Duan, L. (2019) Beneficial effect of butyrate‐producing Lachnospiraceae on stress‐induced visceral hypersensitivity in rats. Journal of Gastroenterology and Hepatology, 34: 1368–1376. 10.1111/jgh.14536.
Declaration of conflict of interest: All authors disclosed no financial relationships relevant to this publication.
Financial support: This work was supported by the National Natural Science Foundation of China (grant number 81670491) and Capital's Funds for Health Improvement and Research (grant number 2016‐2‐4093).
References
- 1. Liu Y, Zhang L, Wang X et al Similar fecal microbiota signatures in patients with diarrhea‐predominant irritable bowel syndrome and patients with depression. Clin. Gastroenterol. Hepatol. 2016; 14: 1602–1611 e5. [DOI] [PubMed] [Google Scholar]
- 2. Tap J, Derrien M, Tornblom H, Brazeilles R, Cools‐Portier S, Dore J, et al. Identification of an intestinal microbiota signature associated with severity of irritable bowel syndrome. Gastroenterology. 2016. [DOI] [PubMed]
- 3. Luczynski P, Tramullas M, Viola M et al Microbiota regulates visceral pain in the mouse. Elife 2017; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. O'Mahony SM, Felice VD, Nally K et al Disturbance of the gut microbiota in early‐life selectively affects visceral pain in adulthood without impacting cognitive or anxiety‐related behaviors in male rats. Neuroscience 2014; 277: 885–901. [DOI] [PubMed] [Google Scholar]
- 5. Sisson G, Ayis S, Sherwood RA, Bjarnason I. Randomised clinical trial: a liquid multi‐strain probiotic vs. placebo in the irritable bowel syndrome—a 12 week double‐blind study. Aliment Pharmacol. Ther. 2014; 40: 51–62. [DOI] [PubMed] [Google Scholar]
- 6. Acosta A, Camilleri M, Shin A et al Effects of rifaximin on transit, permeability, fecal microbiome, and organic acid excretion in irritable bowel syndrome. Clin. Transl. Gastroenterol. 2016; 7: e173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor‐induced immunomodulation. Brain Behav. Immun. 2011; 25: 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bharwani A, Mian MF, Foster JA, Surette MG, Bienenstock J, Forsythe P. Structural & functional consequences of chronic psychosocial stress on the microbiome & host. Psychoneuroendocrinology 2016; 63: 217–227. [DOI] [PubMed] [Google Scholar]
- 9. Szyszkowicz JK, Wong A, Anisman H, Merali Z, Audet MC. Implications of the gut microbiota in vulnerability to the social avoidance effects of chronic social defeat in male mice. Brain Behav. Immun. 2017; 66: 45–55. [DOI] [PubMed] [Google Scholar]
- 10. de Theije CG, Wopereis H, Ramadan M et al Altered gut microbiota and activity in a murine model of autism spectrum disorders. Brain Behav. Immun. 2014; 37: 197–206. [DOI] [PubMed] [Google Scholar]
- 11. Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short‐chain fatty acids in health and disease. Adv. Immunol. 2014; 121: 91–119. [DOI] [PubMed] [Google Scholar]
- 12. Kannampalli P, Shaker R, Sengupta JN. Colonic butyrate‐ algesic or analgesic? Neurogastroenterol. Motil. 2011; 23: 975–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Farup PG, Rudi K, Hestad K. Fecal short‐chain fatty acids—a diagnostic biomarker for irritable bowel syndrome? BMC Gastroenterol 2016; 16: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gil DW, Wang J, Gu C, Donello JE, Cabrera S, Al‐Chaer ED. Role of sympathetic nervous system in rat model of chronic visceral pain. Neurogastroenterol. Motil. 2016; 28: 423–431. [DOI] [PubMed] [Google Scholar]
- 15. Hong S, Fan J, Kemmerer ES, Evans S, Li Y, Wiley JW. Reciprocal changes in vanilloid (TRPV1) and endocannabinoid (CB1) receptors contribute to visceral hyperalgesia in the water avoidance stressed rat. Gut 2009; 58: 202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Al‐Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology 2000; 119: 1276–1285. [DOI] [PubMed] [Google Scholar]
- 17. Langille MGI, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013; 31: 814–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Han J, Lin K, Sequeira C, Borchers CH. An isotope‐labeled chemical derivatization method for the quantitation of short‐chain fatty acids in human feces by liquid chromatography‐tandem mass spectrometry. Anal. Chim. Acta. 2015; 854: 86–94. [DOI] [PubMed] [Google Scholar]
- 19. Chung CS, Chang PF, Liao CH et al Differences of microbiota in small bowel and faeces between irritable bowel syndrome patients and healthy subjects. Scand. J. Gastroenterol. 2016; 51: 410–419. [DOI] [PubMed] [Google Scholar]
- 20. Elinav E, Strowig T, Kau AL et al NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 2011; 145: 745–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen W, Liu F, Ling Z, Tong X, Xiang C. Human intestinal lumen and mucosa‐associated microbiota in patients with colorectal cancer. PLoS One 2012; 7: e39743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harmsen HJM, Wildeboer‐Veloo ACM, Grijpstra J, Knol J, Degener JE, Welling GW. Development of 16S rRNA‐based probes for the Coriobacterium group and the Atopobium cluster and their application for enumeration of Coriobacteriaceae in human feces from volunteers of different age groups. Appl. Environ. Microbiol. 2000; 66: 4523–4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bangsgaard Bendtsen KM, Krych L, Sorensen DB et al Gut microbiota composition is correlated to grid floor induced stress and behavior in the BALB/c mouse. PLoS One 2012; 7: e46231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Flint HJ, Duncan SH, Scott KP, Louis P. Links between diet, gut microbiota composition and gut metabolism. Proc Nutr Soc 2015; 74: 13–22. [DOI] [PubMed] [Google Scholar]
- 25. Dehingia M, Devi KT, Talukdar NC et al Gut bacterial diversity of the tribes of India and comparison with the worldwide data. Sci. Rep. 2015; 5: 18563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tamanai‐Shacoori Z, Smida I, Bousarghin L et al Roseburia spp.: a marker of health? Future Microbiol. 2017; 12: 157–170. [DOI] [PubMed] [Google Scholar]
- 27. Kumari R, Ahuja V, Paul J. Fluctuations in butyrate‐producing bacteria in ulcerative colitis patients of North India. World J. Gastroenterol. 2013; 19: 3404–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Machiels K, Joossens M, Sabino J et al A decrease of the butyrate‐producing species Roseburia hominis and Fecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2014; 63: 1275–1283. [DOI] [PubMed] [Google Scholar]
- 29. Chassard C, Dapoigny M, Scott KP et al Functional dysbiosis within the gut microbiota of patients with constipated‐irritable bowel syndrome. Aliment Pharm. Ther. 2012; 35: 828–838. [DOI] [PubMed] [Google Scholar]
- 30. Wang TT, Cai GX, Qiu YP et al Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. ISME J 2012; 6: 320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Takahashi K, Nishida A, Fujimoto T et al Reduced abundance of butyrate‐producing bacteria species in the fecal microbial community in Crohn's disease. Digestion 2016; 93: 59–65. [DOI] [PubMed] [Google Scholar]
- 32. Undseth R, Jakobsdottir G, Nyman M, Berstad A, Valeur J. Low serum levels of short‐chain fatty acids after lactulose ingestion may indicate impaired colonic fermentation in patients with irritable bowel syndrome. Clin. Exp. Gastroenterol. 2015; 8: 303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Banasiewicz T, Krokowicz L, Stojcev Z et al Microencapsulated sodium butyrate reduces the frequency of abdominal pain in patients with irritable bowel syndrome. Colorectal Dis. 2013; 15: 204–209. [DOI] [PubMed] [Google Scholar]
- 34. Vanhoutvin SA, Troost FJ, Kilkens TO et al The effects of butyrate enemas on visceral perception in healthy volunteers. Neurogastroenterol. Motil. 2009; 21: 952–e76. [DOI] [PubMed] [Google Scholar]
- 35. Treem WR, Ahsan N, Kastoff G, Hyams JS. Fecal short‐chain fatty acids in patients with diarrhea‐predominant irritable bowel syndrome: in vitro studies of carbohydrate fermentation. J. Pediatr. Gastroenterol. Nutr. 1996; 23: 280–286. [DOI] [PubMed] [Google Scholar]
- 36. Bourdu S, Dapoigny M, Chapuy E et al Rectal instillation of butyrate provides a novel clinically relevant model of noninflammatory colonic hypersensitivity in rats. Gastroenterology 2005; 128: 1996–2008. [DOI] [PubMed] [Google Scholar]
- 37. Long X, Li M, Li LX et al Butyrate promotes visceral hypersensitivity in an IBS‐like model via enteric glial cell‐derived nerve growth factor. Neurogastroenterol. Motil. 2018; 30: e13227. [DOI] [PubMed] [Google Scholar]
- 38. Ringel‐Kulka T, Choi CH, Temas D et al Altered colonic bacterial fermentation as a potential pathophysiological factor in irritable bowel syndrome. Am. J. Gastroenterol. 2015; 110: 1339–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Patterson AM, Mulder IE, Travis AJ et al Human gut symbiont Roseburia hominis promotes and regulates innate immunity. Front. Immunol. 2017; 8: 1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Burger‐van Paassen N, Vincent A, Puiman PJ et al The regulation of intestinal mucin MUC2 expression by short‐chain fatty acids: implications for epithelial protection. Biochem. J. 2009; 420: 211–219. [DOI] [PubMed] [Google Scholar]
- 41. Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP‐activated protein kinase in Caco‐2 cell monolayers. J. Nutr. 2009; 139: 1619–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Cecal short chain fatty acids difference between sham, WAS and WAS + R. hominis groups (means ± SEM).


