Abstract
Aim
This study aimed to examine reported medication error trends in an Australian paediatric hospital over a 5‐year period and to determine the effects of person‐related, environment‐related and communication‐related factors on the severity of medication outcomes. In particular, the focus was on the influence of changes to a hospital site and structure on the severity of medication errors.
Methods
A retrospective clinical audit was undertaken over a 5‐year period of paediatric medication errors submitted to an online voluntary reporting system of an Australian, tertiary, public teaching paediatric hospital. All medication errors submitted to the online system between 1 July 2010 and 30 June 2015 were included.
Results
A total of 3340 medication errors was reported, which corresponded to 0.56% medication errors per combined admissions and presentations or 5.73 medication errors per 1000 bed days. The most common patient outcomes related to errors requiring monitoring or an intervention to ensure no harm occurred (n = 1631, 48.8%). A new hospital site and structure had 0.354 reduced odds of producing medication errors causing possible or probable harm (95% confidence interval 0.298–0.421, P < 0.0001). Patient and family involvement had 1.270 increased odds of identifying medication errors associated with possible or probable harm compared with those causing no harm (95% confidence interval 1.028–1.568, P = 0.027). Interrupted time series analyses showed that moving to a new hospital site and structure was associated with a reduction in reported medication errors.
Conclusion
Encouraging child and family involvement, facilitating hospital redesign and improving communication could help to reduce the harm associated with medication errors.
Keywords: family involvement, hospitalised child, interdisciplinary communication, medication error, patient involvement
What is already known on this topic
Children are at high risk of experiencing medication errors.
Past research has been conducted on identifying the prevalence of medication errors in children; however, little is known about the causes, human factors, contributing factors and patient outcomes of medication errors, as well as the involvement of patients and family members in detecting medication errors.
Past research shows that common types of medication errors include overdose or under‐dose, dose omission, mistakes in administration techniques, wrong medication, wrong person and inattention to children's changing needs, including alterations in body weight.
What this paper adds
Hospitalised children or family members alerted health professionals about the occurrence of medication errors in 15% of occasions.
Patient and family involvement was associated with increased odds of identifying medication errors causing possible or probable harm, while a new hospital site and structure was associated with reduced odds of medication errors causing possible or probable harm.
Interrupted time series results showed that a new hospital site and structure was associated with a reduced percentage of medication errors per combined admissions and presentations and reduced medication errors per 1000 bed days.
Children are at high risk of experiencing medication errors. They are vulnerable due to their limited ability to communicate, especially those who are preverbal and have difficulties in expressing their needs. Insufficient availability of paediatric formulations contributes to the need for increased medication compounding, and a lack of information from manufacturers about preparing these medications can also lead to medication errors.1
Extensive research has been conducted on identifying the prevalence of medication errors in children. Medication error rates differ considerably due to varying contexts of care and methods used for analysis. Variations have ranged between 0.51 per 1000 bed days2 and 82.9 medication errors per 1000 bed days.3
In attempting to reduce medication errors, increased focus has been placed on person‐related factors, such as addressing how nurses, doctors and pharmacists contribute to these errors. While past research has shown that health professionals can influence medication errors,4 less is known about how child and family involvement may prevent and detect medication errors. A retrospective clinical audit conducted in Australia demonstrated that, in 8.0% of reported medication errors (n = 219), children and family members had alerted health professionals that a medication error had occurred.5 Further work is needed to examine child and family involvement in influencing medication errors.
Major causes of medication errors involve communication‐related factors,5, 6 with research showing that communication problems account for over half of all causes associated with medication errors.7 Difficulties with written communication feature predominantly, involving illegible and misinterpreted medication orders. Conversely, details about how communication processes, such as ward rounds and handovers, affect medication errors are largely missing.
Environment‐related factors can have an enormous impact on patient safety. A case study approach was undertaken in the USA8 where extensive consultation was sought from architects, patients, family members, managers and clinicians about what they felt was important in designing a new hospital. Through these consultations, a comprehensive checklist was created to guide the development of the new hospital and enable a culture of patient safety. In this study, there was no information about how environment‐related factors affected medication errors. The hospital targeted was an acute care facility with no details about specific provisions for paediatric care.
Examining the complexities of diverse factors associated with medication errors can help identify how these factors influence the severity of harm and ascertain possible strategies for improved patient safety. The aims of the study were to examine reported medication error trends in an Australian paediatric hospital over a 5‐year period and to determine the effects of person‐related, environment‐related and communication‐related factors on the severity of medication outcomes. In particular, the key intent was to focus on the influence of changes to a hospital site and structure on the severity of medication errors.
Methods
A retrospective clinical audit was undertaken over a 5‐year period of paediatric medication errors submitted to an online voluntary reporting system of an Australian, tertiary, public, teaching paediatric hospital. The hospital, comprising 334 beds, provides a comprehensive range of clinical services, tertiary care, health promotion and prevention programmes. It contains an emergency department, an intensive care unit and a state‐wide trauma service. The human research ethics committee of the hospital approved the conduct of the study. A medication error was defined as any preventable event that may have caused or led to inappropriate medication use or patient harm while the medication was in the control of the health professional, patient or family member.9
On 26 October 2011, during the study period, the hospital had moved from an old site to a new building complex. On that day, all patients were moved from the old hospital site to the new hospital site, including all health professionals involved in providing their care. Alongside the move, there were structural changes associated with the new hospital complex. In the new hospital, most hospitalised children were situated in single rooms, with dedicated spaces for family members. These dedicated spaces were created to facilitate family‐centred care and engagement with clinicians. Dedicated medication rooms were developed for all wards in the new hospital (Fig. 1). In the old hospital, only one medical ward had a dedicated medication room, whereas in all other wards, the medication room was combined with the patient treatment room (Fig. 2). In the old hospital, when the medication room was combined with the patient treatment room, there was extensive activity, with children undergoing procedures such as peripheral intravenous cannula insertion, venepuncture and bladder catheterisation, which sometimes led to distractions from excessive noise and interruptions. In both forms of the medication room, whether combined with the treatment room in the old hospital or as a stand‐alone facility in the new hospital, pharmacists dispensed medications, physicians ordered medications and nurses prepared and administered medications. In the new hospital, the nurse–patient ratio was the same, each unit had the same staff, and guidelines and policies were the same.
Figure 1.
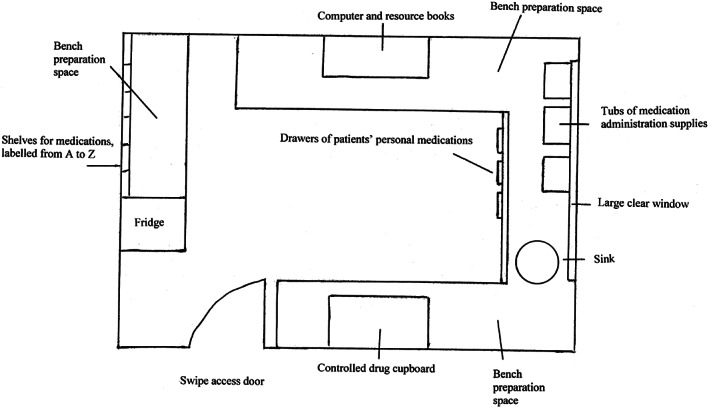
Medication room in the new hospital.
Figure 2.
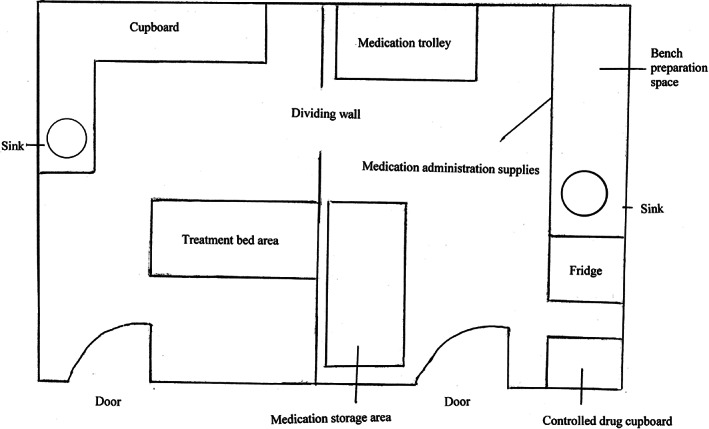
Medication room combined with the patient treatment room in the old hospital.
Data collection and procedure
All medication errors submitted to the online system between 1 July 2010 and 30 June 2015 were included. Children's medical records were accessed. For many years, hospital management adopted a no‐blame policy, actively encouraging clinicians to submit medication errors as they occurred. As a result, reported medication errors contained detailed descriptions of medications errors in the form of free text as well as comprehensive responses to close‐ended questions. A medication safety committee developed the summary data of medication errors, and those with severe outcomes were discussed and analysed as they occurred. Feedback was incorporated in online medication error reports on patient outcomes and in strategies adopted to address recurring problems and causes.
The National Coordinating Council for Medication Error Reporting and Prevention tool was used as the data collection instrument.9 This tool provides a structured and standardised approach in categorising medication errors. The tool enabled person‐, environment‐ and communication‐related factors to be systematically classified.
Data analysis
Descriptive and inferential data analysis was undertaken using SPSS, version 23 (IBM Corp., Chicago, IL, USA). Frequency counts and percentage counts were calculated for all variables of interest. Calculations of medication errors per combined admissions and presentations and medication errors per 1000 bed days were obtained from resourcing the statistical data of the hospital's annual reports. These annual reports contained data on the number of patient admissions and presentations and the number of bed days over the 5‐year period and were used for data collection and analysis.
Univariate associations with severity of medication errors were examined for all person‐related, environment‐related and communication‐related factors using cross tabulations and chi square tests. The outcome or dependent variable was the patient outcome measure (scores of 1–3 relating to no patient harm and scores 4–8 relating to possible or probable patient harm).9 Person‐related factors included the individual responsible for the error (nurse/doctor/pharmacist/patient/family member) and patient and family involvement in identifying a medication error (yes/no). Environment‐related factors included: the hospital site and structure (new/old) and clinical site of the medication errors. Communication‐related factors included: informal bedside communication problem (yes/no), clinical handover problem (yes/no) and medical record documentation problem (yes/no). These communication‐related factors were selected because they were the most commonly occurring data. Use of the new hospital site and structure commenced on 26 October 2011. Thus, medication errors taking place before 26 October 2011 occurred in the old hospital site and structure, while those taking place from 26 October 2011 onwards occurred in the new hospital site and structure. Binary multiple logistic regression modelling was subsequently undertaken, with factors demonstrating univariate associations of less than or equal to P = 0.25. Use of a less stringent P value of 0.25, compared with a P value of 0.05, was arbitrarily chosen to safeguard against excluding potentially important factors at the univariate level when determining what factors to test in the binary logistic modelling level.10 Binary logistic regression modelling involved calculating the ability of various explanatory variables to increase or decrease the odds of a binary categorical outcome, which can only have two values. In this case, the values of the binary outcome were the presence of medication errors causing possible or probable harm and those causing no harm. The level of significance used for logistic modelling was α = 0.05.
The impact of the new hospital site and structure was initially explored through an interrupted time series analysis that included both the step‐change (hospital site, old = 0; new = 1) and slope‐change (time) components and the interaction term. However, because the contribution of the slope‐change and interaction components was statistically non‐significant, our final model retained only the step‐change component.11 Due to over‐dispersion, all analyses were conducted using the quasi‐Poisson model. There was no clear sign of dependencies (i.e. autocorrelation) or seasonality; thus, no further adjustments were made.
Results
During the study period, a total of 3340 medication errors was reported, which corresponded to 0.56% per combined admissions and presentations or 5.73 medication errors per 1000 bed days. In addition, there were 6680 human factors and 4008 causes identified in the database during the study period. The median age of children was 4.3 years (interquartile range 4 months, 13.5 years), and 30.4% were children aged 12 months or younger. Of the reported medication errors, 43.5% occurred in female children. Table 1 shows the clinical settings in which medication errors occurred.
Table 1.
Clinical settings in which medication errors occurred (n = 3340)
| Clinical setting | n | % |
|---|---|---|
| Intensive care unit | 462 | 13.8 |
| Medical unit | 441 | 13.2 |
| Surgical unit | 362 | 10.8 |
| Neonatal intensive care unit | 354 | 10.6 |
| Cardiac unit | 269 | 8.1 |
| Psychiatry unit | 255 | 7.6 |
| Oncology unit | 192 | 5.7 |
| Neuroscience unit | 158 | 4.7 |
| Adolescent unit | 142 | 4.3 |
| Pharmacy department | 139 | 4.2 |
| Emergency department | 128 | 3.8 |
| Perioperative area | 124 | 3.7 |
| Immunology department | 106 | 3.2 |
| Hospital‐in‐the‐home | 79 | 2.4 |
| Medical short stay | 70 | 2.1 |
| Surgical short stay | 44 | 1.3 |
| Radiology | 10 | 0.3 |
| Outpatient units | 5 | 0.1 |
Most medication errors occurred during the daytime between 0700 and 1530 (n = 1736, 52.0%). However, considerable medication errors also occurred after business hours and overnight. Medication errors took place predominantly during weekdays, but 20.6% of medication errors occurred during weekend days. The most common patient outcomes related to errors requiring monitoring or an intervention to ensure that no harm occurred (n = 1631, 48.8%), followed by errors reaching patients that did not cause patient harm (n = 979, 29.3%) (Table 2).
Table 2.
Patient outcomes of medication errors relating to eight categories of harm (n = 3340 medication errors)
| Patient outcome | n | % | Examples of patient outcome |
|---|---|---|---|
| 1 Circumstances had the capacity to cause error | 14 | 0.4 | Warfarin dose not signed on drug chart |
| 2 Error occurred, but the error did not reach the patient | 618 | 18.5 | 600 mcg of naloxone was prescribed on the medication chart instead of 60 mcg – 10× overdose. Patient did not receive the dose of naloxone |
| 3 Error occurred that reached the patient but did not cause patient harm | 979 | 29.3 | Weight recorded as 23 kg when weighed in day surgery admission; on inspection, the patient obviously weighed less than this, but issue was not detected until patient was anaesthetised and after premedication given comprising midazolam and paracetamol (acetaminophen) |
| 4 Error occurred that reached the patient and required monitoring or intervention to confirm no harm | 1631 | 48.8 | Intravenous order for intravenous ticarcillin + clavulanic acid in emergency department but not handed over. Intravenous antibiotics commenced about 14 h late |
| 5 Error occurred that resulted in temporary harm and required intervention | 89 | 2.7 | Noradrenaline (norepinephrine) found to be disconnected following severe hypotension and need of volume and increase of inotrope requirements following a bed turn |
| 6 Error occurred that resulted in temporary harm and required prolonged hospitalisation | 8 | 0.2 | Patient's intravenous antibiotic, flucloxacillin, changed to oral antibiotic but continued to be given as intravenous form, delaying patient discharge |
| 7 Error occurred that resulted in permanent patient harm | 0 | 0 | Not applicable |
| 8 Error occurred that required intervention necessary to sustain life | 1 | 0.1 | Patient given overdose of arginine. Patient was transferred to paediatric intensive care unit for hemofiltration and treatment of the overdose |
Table 3 shows communication‐related factors of medication errors, details about generic and trade name confusion and concerns with labelling and reference materials. A total of 4008 causes were attributed to medication errors. The most common communication‐related factor was problems with medical record documentation (n = 717, 17.9%), which included health professionals not checking for documented allergies and confusion about children's actual recorded weight. Labels of dispensed products sometimes had incorrect details documented, but the contents of the products were correct (e.g. identification of vehicles on intravenous fluid bags with additives). At other times, the labels and the contents were incorrect. Problems with reference materials included limitations associated with available medication resources, leading to delays in administration as clinicians attempted to obtain further information (n = 142, 3.5%). Examples included availability of injectable guidelines for powder volumes of methylprednisolone for established brands but not for new brands and the lack of administration instructions for cyclophosphamide and mesna.
Table 3.
Causes associated with medication errors (n = 4008 causes)
| Cause of medication error | n | % |
|---|---|---|
| Communication‐related factors, n = 3340 (83.3%) | ||
| Medical record documentation | 717 | 17.9 |
| Clinical handover | 571 | 14.2 |
| Informal bedside communication | 542 | 13.5 |
| Misinterpretation of order | 518 | 12.9 |
| Misread or unread order | 481 | 12.0 |
| Illegible handwriting | 235 | 5.9 |
| Decimal point | 107 | 2.7 |
| Units of measurement | 91 | 2.3 |
| Telephone communication | 56 | 1.4 |
| Ward round | 22 | 0.5 |
| Confusion with medication name, n = 229 (5.7%) | ||
| Generic name confusion | 203 | 5.1 |
| Brand name confusion | 26 | 0.6 |
| Confusion with labelling and reference material, n = 439 (11.0%) | ||
| Label of dispensed product is wrong | 161 | 4.0 |
| Problems with reference material | 142 | 3.5 |
| Container of manufacturer similar or confusing | 136 | 3.4 |
Commonly occurring human factors included performance deficits (n = 1436, 21.5%) and knowledge deficits (n = 504, 7.5%). Contributing factors that frequently occurred included clinicians not adequately following policies and procedures (n = 1619, 24%). Problems with patients' movements across transitions, such as transfers involving emergency department and operating rooms, were also evident (n = 461, 6.9%) (Table 4).
Table 4.
Human factors and contributing factors (n = 6680)
| Type of factors | n | % |
|---|---|---|
| Human factors, n = 3340 | ||
| Performance deficit | 1436 | 21.5 |
| Knowledge deficit | 504 | 7.5 |
| Inadequate screening of patient | 445 | 6.7 |
| Miscalculation of dose or infusion rate | 341 | 5.1 |
| Failure to activate delivery system properly | 173 | 2.6 |
| Wrong amount of active medication used | 126 | 1.9 |
| Error in stocking | 108 | 1.6 |
| Wrong diluent used for infusion | 69 | 1.0 |
| Stress | 55 | 0.8 |
| Wrong amount of diluent used | 48 | 0.7 |
| Wrong medication added to infusion | 27 | 0.4 |
| Intimidating behaviour | 8 | 0.1 |
| Contributing factors, n = 3340 | ||
| Policies and procedures | 1619 | 24.2 |
| Frequent interruptions and distractions | 639 | 9.6 |
| Communication relating to patient movements | 461 | 6.9 |
| Lack of available trained health professionals | 216 | 3.2 |
| Inadequate training provided | 155 | 2.3 |
| Insufficient or incorrect counselling offered to patients or parents | 126 | 1.9 |
| Floor stock | 124 | 1.9 |
Bivariate logistic regression modelling identified 10 factors that significantly predicted associations with children experiencing possible or probable harm versus no harm from medication errors (Table 5). In situations where doctors or pharmacists were responsible for the medication error, there were reduced odds of a harmful medication error occurring compared to when nurses were responsible. Involvement of children and families in identifying medication errors had 1.270 increased odds of harmful medication errors occurring compared with those who were not involved. Problems with clinical handover, informal bedside communication and medical record documentation all increased the odds of harmful medication errors. The new hospital site and structure showed 0.354 reduced odds in being associated with harmful medication errors compared with the old hospital site and structure. The intensive care unit, oncology unit and emergency department all demonstrated increased odds of having harmful medication errors compared to medical units.
Table 5.
Binary logistic regression model for explanatory factors associated with medication errors causing possible or probable harm (n = 3340)
| Variable | Reference level | Comparator level | Odds ratio | 95% confidence intervals | P value |
|---|---|---|---|---|---|
| Person‐related factors | |||||
| Person responsible for the medication error | Nurse | Doctor | 0.571 | 0.483–0.674 | <0.0001 |
| Person responsible for the medication error | Nurse | Pharmacist | 0.410 | 0.285–0.591 | <0.0001 |
| Patient and family involvement in detecting an incident | No | Yes | 1.270 | 1.028–1.568 | 0.027 |
| Environment‐related factors | |||||
| New hospital site and structure | Old | New | 0.354 | 0.298–0.421 | <0.0001 |
| Clinical setting where error occurred | Medical ward | Intensive care unit | 1.516 | 1.143–2.010 | 0.004 |
| Clinical setting where error occurred | Medical ward | Oncology unit | 1.794 | 1.250–2.573 | 0.002 |
| Clinical setting where error occurred | Medical ward | Emergency department | 1.728 | 1.131–2.640 | 0.011 |
| Communication‐related factors | |||||
| Problem with informal bedside communication | No | Yes | 1.360 | 1.099–1.685 | 0.005 |
| Problem with medical record documentation | No | Yes | 1.327 | 1.083–1.625 | 0.006 |
| Problem with clinical handover | No | Yes | 2.042 | 1.652–2.523 | <0.0001 |
Figure 3 shows the interrupted time series trends for the effect of the new hospital site and structure on reported medication errors. The risk of percentage medication errors per combined admission and presentation was reduced by 35.4% (relative risk (RR) = 0.598, 95% confidence interval (0.464–0.770)) under the new hospital site and structure and by 36.7% (RR = 0.633 (0.492–0.813)) for the number of medication errors per 1000 bed days.
Figure 3.
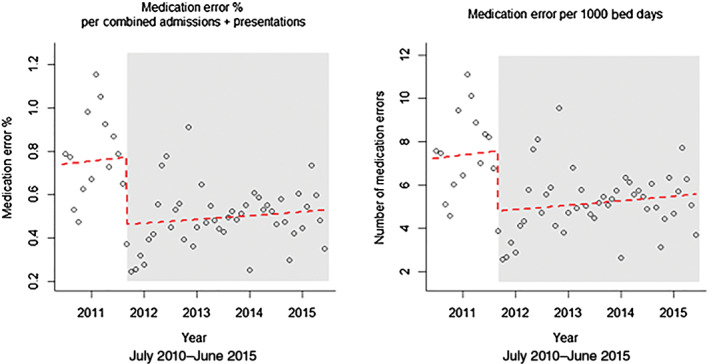
Interrupted time series analysis demonstrating the effect of the new hospital site and structure on medication errors.
Discussion
The clinical audit demonstrated how person‐, environment‐ and communication‐related factors affected medication errors in a paediatric hospital. Implementation of a new hospital site and structure was associated with reduced odds of medication errors producing possible or probable harm and with a reduction in reported medication errors. Patient and family involvement was important in identifying medication errors.
Doctors and pharmacists had reduced odds in been associated with medication errors producing possible or probable harm compared to nurses. In examining the contributing factors affecting health disciplines, nurses were subjected to frequent interruptions and distractions, especially in relation to errors leading to harm. There were also situations where nurses lacked appropriate training in the administration of high‐risk medications in children, and there were reports of nurses having only basic information to help them safely mix and administer intravenous medications. Nurses were also involved in medication errors causing harm where patients moved from one clinical setting to another, which was often compounded by nurses' busyness in the work area and having to compete with multiple priorities. Past research has demonstrated that nurses' lack of familiarity with medications, the environment, procedures and equipment are common causes of medication errors.7
Children and family members alerted health professionals about medication errors in 15% of cases, particularly about those errors associated with possible or probable harm. These alerts from children and family members comprised diverse types of medications, including analgesics, antipsychotics, neurological agents, blood and electrolytes and anti‐infective agents. Hence, this detection occurred regardless of whether the child required medication for treatment of an acute or chronic condition. Family presence possibly contributed to astute awareness of the safety checks conducted by nurses in monitoring and administering medications. As a result, family members acted as watchful observers to identify deviations from expected routines.
The new hospital site and structure was associated with reduced odds of medication errors causing possible or probable harm and a reduction of reported medication errors. Past work has shown that spatial designs can increase proximity between health professionals, patients and family members, thereby enhancing communication.12 It is possible that the new spatial design was conducive for improved communication as family members may have been more likely to speak up if they suspected an actual or possible medication error.13 The presence of dedicated medication rooms, where health professionals discussed medication decisions with minimal distractions, may have also impacted the severity and number of medication errors occurring. Compared with the old hospital, there was greater availability of space to enable collaborative decision‐making. These decisions related to nurses independently checking dose calculations, preparing parenteral medications for administration and discussing options for providing pro re nata ‘as required’ medications. Decisions also comprised physicians communicating about preferences for medication prescription and specialty personnel, such as diabetic educators, and acute pain clinic nurses and physicians interacting about treatment options. Pharmacists also used these rooms to clarify medication orders with physicians, to identify medication supply needs and to organise admission and discharge counselling sessions.
Clinical settings associated with medication errors leading to possible or probable harm included intensive care, emergency care and oncology care. High‐risk medications such as intravenous electrolytes, inotropes, opioid analgesics, anticoagulants and cytotoxic medications are commonly prescribed in these environments, which are more likely to cause catastrophic harm compared with other types of medications.14 In these settings, health professionals encountered problems with patient movements. With patient mobility between these specialty settings and other environments, such as the operating room and radiology department, hospitalised children were at increased risk of experiencing a medication error with each transfer.
Limitations
The study comprised a retrospective audit undertaken in one health service. More medication errors may have occurred than the number documented on the online reporting site. However, because the hospital had a no‐blame reporting policy, and there were detailed explanations accompanying many entries, it is likely that the prevalence is an accurate reflection of what happened in actual practice. At the time of data collection, only 16 months of data were available prior to the hospital move, while 44 months of data were available following the hospital move.
Conclusion
The study showed that children and family members play a significant role in identifying medication errors in clinical practice. Communication‐related factors contribute to the occurrence of medication errors. Implementation of a new hospital site and structure can have an impact on reducing the severity of medication errors, as well as enhancing patient and family contribution in identifying medication errors. Future research should focus on evaluating strategies for patient and family involvement in reducing errors before causing harm. Interventional work involving family‐centred configurations of hospital spaces needs to be tested for their impact on medication errors.
Acknowledgement
Funding was provided by the Australian Research Council, Discovery Grant Scheme (DP130100221).
Conflict of interest: None declared.
References
- 1. Wong ICK, Ghaleb MA, Franklin BD, Barber N. Incidence and nature of dosing errors in paediatric medications – A systematic review. Drug Saf. 2004; 27: 661–70. [DOI] [PubMed] [Google Scholar]
- 2. Ross LM, Wallace J, Paton JY. Medication errors in a paediatric teaching hospital in the UK: Five years operational experience. Arch. Dis. Child. 2000; 83: 492–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wilson DG, McArtney RG, Newcombe RG et al Medication errors in paediatric practice: Insights from a continuous quality improvement approach. Eur. J. Pediatr. 1998; 157: 769–74. [DOI] [PubMed] [Google Scholar]
- 4. Ghaleb MA, Barber N, Franklin BD, Wong ICK. The incidence and nature of prescribing and medication administration errors in paediatric inpatients. Arch. Dis. Child. 2010; 95: 113–8. [DOI] [PubMed] [Google Scholar]
- 5. Manias E, Kinney S, Cranswick N, Williams A. Medication errors in hospitalised children. J. Paediatr. Child Health 2014; 50: 71–7. [DOI] [PubMed] [Google Scholar]
- 6. Sears K, O'Brien‐Pallas L, Stevens B, Murphy GT. The relationship between nursing experience and education and the occurrence of reported pediatric medication administration errors. J. Pediatr. Nurs. 2016; 31: e283–90. [DOI] [PubMed] [Google Scholar]
- 7. Keers RN, Williams SD, Cooke J, Ashcroft DM. Causes of medication administration errors in hospitals: A systematic review of quantitative and qualitative evidence. Drug Saf. 2013; 36: 1045–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reiling J. Creating a culture of patient safety through innovative hospital design In: Henriksen K, Battles J, Marks E, Lewin D, eds. Advances in Patient Safety: From Research to Implementation, vol. 2. Rockville, MD: Agency for Healthcare Research and Quality; 2005; 1–16. [PubMed] [Google Scholar]
- 9. National Coordinating Council for Medication Error Reporting and Prevention . About Medication Errors. Rockville, MD: The Council; 2017. Available from: http://www.nccmerp.org/about-medication-errors [accessed 1 March 2017].
- 10. Stoltzfus JC. Logistic regression: A brief primer. Acad. Emerg. Med. 2011; 18: 1099–104. [DOI] [PubMed] [Google Scholar]
- 11. Lopez Bernal J, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: A tutorial. Int. J. Epidemiol. 2017; 46: 348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu W, Manias E, Gerdtz M. The effects of physical environments in medical wards on medication communication processes affecting patient safety. Health Place 2014; 26: 188–98. [DOI] [PubMed] [Google Scholar]
- 13. Harris N, Badr LK, Saab R, Khalidi A. Caregivers' perception of drug administration safety for pediatric oncology patients. J. Pediatr. Oncol. Nurs. 2014; 31: 95–103. [DOI] [PubMed] [Google Scholar]
- 14. Grissinger M. Your high‐alert medication list is relatively useless without associated risk‐reduction strategies. Pharm. Therap. 2016; 41: 598–600. [PMC free article] [PubMed] [Google Scholar]


