Abstract
The aim of this study was to provide evidence for the hypothesis that estimated glomerular filtration rate from serum Cystatin C (eGFRcys) is better to be determined for all elderly type 2 diabetes mellitus (T2DM) patients based on eGFRcys upward and downward reclassification rate for hypothetical metformin dose reduction by eGFRcys at the GFR decision point of 45 mL/min/1.73 m2. A total of 265 consecutive T2DM elderly patients (age range 65‐91 years) from outpatient diabetic clinic were included in the study. The Kidney Disease Improving Global Outcomes (KDIGO) guidelines for metformin dosing were strictly followed. Estimated glomerular filtration rate from serum creatinine (eGFRcrea) led to results of metformin eligibility. Each of the results of eGFRcrea‐based eligibility was further compared to eGFRcys‐based eligibility. Creatinine was measured by enzymatic method standardized against international reference material SRM 967. Cystatin C was determined by method traceable to DA ERM 471 international standard. eGFRcrea and eGFRcys were calculated according to Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) equations. A downward reclassification rate was higher than upward reclassification rate (31 vs 3, respectively; P < 0.0001). The median (IQR) eGFRcrea was higher than eGFRcys (73 (58‐85) vs 63 (50‐75) mL/min/1.73 m2, respectively; P < 0.0001). eGFRcys reclassified significant proportion of patients with T2DM from metformin eligible CKD stages to less or non‐eligible stages. The downward reclassification was more frequent in patients older than 80 years (P < 0.01). Cystatin C‐based eGFR selects more complicated patients, where lower doses of metformin are possibly advisable. We recommend calculating both eGFRcrea and eGFRcys for metformin dosing in elderly patients with T2DM.
Keywords: creatinine, cystatin C, estimated glomerular filtration rate, glomerular filtration, metformin
1. INTRODUCTION
Metformin belongs to oral antidiabetic glucose‐lowering biguanide agents. Metformin is the first line therapy for T2DM. The mechanism of action includes reduction of hepatic glucose production and increased glucose utilization by the gut. The most common side effects are gastrointestinal problems such as abdominal discomfort and diarrhoea. Lactic acidosis is a less frequent complication but can have serious consequences. This drug is renally excreted from the body as the active compound.1
KDIGO guidelines define CKD and classify it into six stages according to eGFR. The reduction of metformin dose is recommended when eGFR is 30‐44 mL/min/1.73 m2 (stage 3b) and discontinuation when eGFR is <30 mL/min/1.73 m2 (stages 4 and 5). eGFR can be calculated from serum creatinine and/or cystatin C. eGFRcrea is the most common test for estimation of GFR in clinical practice.2 KDIGO guidelines recommend confirming eGFRcrea decline below 45 mL/min/1.73 m2 by determination of eGFRcys or direct GFR measurement for metformin dosing.3 Real‐world practice of monitoring diabetic patients does not include the gold standard of GFR measurement.
U.S. Food and Drug Administration (FDA) guidance, which is included in American Diabetes Association Standards of medical care in diabetes 2018, states that metformin is contraindicated in patients with eGFR < 30 mL/min/1.73 m2, eGFR should be monitored while taking metformin, the benefits and risks of continuing treatment should be reassessed when eGFR falls to <45 mL/min/1.73 m2, metformin should not be initiated for patients with an eGFR <45 mL/min/1.73 m2, and metformin should be temporarily discontinued at the time of or before iodinated contrast imaging procedures in patients with an eGFR of 30‐60 mL/min/1.73 m2.4
The European population is becoming older. People over 65 years of age frequently have reduced muscle mass due to chronic diseases and lower physical activity. Also, people with CKD have reduced muscle mass and cut‐off for metformin dose reduction in such patients is identical with that of 3b stage of CKD.
The common trend in muscle mass wasting with ageing led us to perform this study which determines the confirmation rate, upward reclassification rate to higher GFR and downward reclassification rate to lower GFR for metformin dose reduction by eGFRcys in elderly T2DM patients at the GFR decision point of 45 mL/min/1.73 m2.
2. MATERIALS AND METHODS
2.1. Subjects
This was a retrospective, descriptive, observational study. A total of 265 consecutive T2DM elderly patients (age range 65‐91 years) from outpatient diabetic clinic were included in the study. There were 30 patients older than 80 years. The data were collected from January 2013 to December 2017.
We strictly followed the KDIGO guidelines for metformin dosing. This led to results of metformin eligibility. We further compared each of the results to cystatin C‐based eligibility.
2.2. Laboratory methods
Creatinine was measured by spectrophotometric sarcosine‐based enzymatic method standardized against international reference material SRM 967. Cystatin C was determined by particle‐enhanced turbidimetric immunoassay (PETIA) method traceable to DA ERM 471 international standard. eGFRcrea and eGFRcys were calculated according to CKD‐EPI equations developed in 2009 and 2012, respectively.5
2.3. Statistical analysis
D'Agostino‐Pearson test was used for normal distribution testing. eGFRs were expressed as median and IQR. The Spearman's rank correlation coefficient was used for association analysis. Wilcoxon paired samples test was employed for comparison of medians. Mann‐Whitney test for independent samples was used for comparison of medians between males and females and between different age groups. Two‐sided chi‐squared test was employed for comparison of proportions. Bland‐Altman plot displayed paired eGFR results and the bias between GFR estimations at different concentration ranges.
The level of significance was defined as a P value below 0.05.
Data analysis was performed using MedCalc statistical software version 17.4 (MedCalc Software bvba, Ostend, Belgium).
ETHICAL APPROVAL
The study was approved by the ethics committee of the Tomas Bata hospital.
The study was conducted in accordance with the Basic & Clinical Pharmacology & Toxicology policy for experimental and clinical studies. (Basic Clin Pharmacol Toxicol 2018;123:233‐35).6
3. RESULTS
A total of 265 T2DM patients were included in the study (125 females and 140 males).
The median (range) age was 72 (65‐91) years.
The median (IQR) of male age was 72 (68‐76) years.
The median (IQR) of female age was 73 (70‐76) years.
Normal distribution of eGFRcrea and age was rejected by D'Agostino‐Pearson test (P = 0.0001, P < 0.0001, respectively). That is why non‐parametric statistical tests were employed.
The median (IQR) eGFRcrea was higher than eGFRcys (73 (58‐85) vs 63 (50‐75) mL/min/1.73 m2, respectively; P < 0.0001).
eGFRcrea and eGFRcys were positively associated (r = 0.705, P < 0.0001).
The downward reclassification rate was higher than upward reclassification rate (31 vs 3, respectively; P < 0.0001) at the decision point of 45 mL/min/1.73 m2. In 26 cases, patients were reclassified from completely metformin eligible stages to stage 3b. In five cases, eGFRcys reclassified GFR to the G4 stage. It included four patients who were reclassified from the G3b stage to the G4 stage.
GFR classification stages according to eGFRs and reclassification rates by eGFRcys are shown in Table 1.
Table 1.
GFR classification stages according to eGFRs and reclassification rates by eGFRcys
| Stage G1 | Stage G2 | Stage G3a | Stage G3b | Stage G4 | Stage G5 | |
|---|---|---|---|---|---|---|
| eGFRcreaa | 36 | 158 | 52 | 17 | 2 | 0 |
| eGFRcysb | 27 | 124 | 71 | 36 | 7 | 0 |
| eGFRcys upwardc | 13 | 7 | 2 | 0 | 0 | 0 |
| eGFRcys samed | 14 | 99 | 23 | 10 | 2 | 0 |
| eGFR downwarde | 0 | 18 | 46 | 26 | 5 | 0 |
Estimated glomerular filtration rate from serum creatinine.
Estimated glomerular filtration rate from serum cystatin C.
Upward reclassification rates by eGFRcys.
Same classification by both estimations.
Downward reclassification rates by eGFRcys.
All paired data of eGFRcrea and eGFRcys are displayed in Bland‐Altman difference plot (Figure 1). It shows bias between methods at different concentration ranges.
Figure 1.
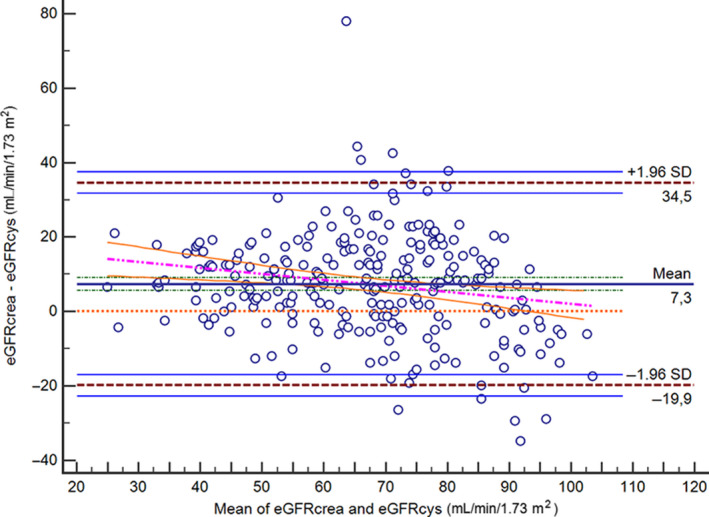
The figure shows the Bland‐Altman difference plot between eGFRcrea and eGFRcys in elderly patients with type 2 diabetes. eGFRcrea, estimated glomerular filtration rate from serum creatinine; eGFRcys, estimated glomerular filtration rate from serum cystatin C; SD, standard deviation
The median (IQR) eGFRcrea in patients older than 80 years was lower than eGFRcrea in younger patients (54 (46‐64) vs 74 (61‐86) mL/min/1.73 m2, respectively; P < 0.0001).
The median (IQR) eGFRcys in patients older than 80 years was lower than eGFRcys in younger ones (46 (36‐54) vs 66 (53‐77) mL/min/1.73 m2, respectively; P < 0.0001).
The median (IQR) eGFRcrea in males was higher than eGFRcrea in females (76 (62‐86) vs 70 (55‐83) mL/min/1.73 m2, respectively; P < 0.05).
The median (IQR) eGFRcys in males was higher than eGFRcys in females (66 (54‐77) vs 61 (47‐74) mL/min/1.73 m2, respectively; P < 0.05).
In the subgroup of patients older than 80 years, there were nine downward reclassification cases to 3b GFR stage. The rate is higher compared to younger patients P < 0.01).
4. DISCUSSION
We determined the confirmation rate, upward reclassification rate to higher GFR and downward reclassification rate to lower GFR for hypothetical metformin dose reduction by eGFRcys in elderly diabetic patients at the GFR cut‐off point of 45 mL/min/1.73 m2.
Downward reclassification rate was more frequent than upward reclassification.
In five cases, eGFRcys reclassified GFR to the G4 stage, which would result in metformin withdrawal. The downward reclassification was more frequent in patients older than 80 years.
The downward reclassification ability of eGFRcys was shown by Tuot et al7 in a study which included 550 consecutive Veterans with diabetes. The downward reclassification trend found in Tuot's study supports our results.
Pottel et al8 reported that GFR measured by gold standard method had decreasing trend in GFR with increasing age. He also reported lower GFR in older females compared to matched males. It supports our results of lower eGFR in patients older than 80 years and in females.
The reclassification of GFR to more advanced CKD stages by eGFRcys was also demonstrated in a primary care cohort study by Shardlow et al9, but creatinine was measured by Jaffe compensated method in their study. This is consistent with our results.
The impact of analytical performance characteristics of a creatinine method on eGFRcrea was demonstrated in the work by Klee et al10 The same is true for cystatin C. Our study used creatinine and cystatin C tests traceable to international reference materials, which leads to low bias of both methods.
A different study by Šálek et al performed on 565 diabetic patients that also used standardized creatinine and cystatin C assays and estimated GFR according to CKD‐EPI equations reported similar results: eGFRcrea results were higher than eGFRcys in the GFR range below 60 mL/min/1.73 m2 11 Jabor et al extended the study to 1515 diabetic patients. The study extension confirmed higher eGFRcrea in the GFR range below 60 mL/min/1.73 m2 12
A study by Fan et al13 showed positive bias of eGFRcrea in diabetic patients in the GFR range below 60 mL/min/1.73 m2 It supports our results.
On the contrary, it was shown in a study by Delanaye et al that eGFRcrea classified 4.3% more patients as CKD than eGFRcys in a group of diabetic patients. Their study used the same CKD‐EPI equations, but creatinine was measured by Jaffé compensated method and cystatin C measurement was not standardized (only recalculated according to manufacturer recommendation).14 These results are not in accordance with our results. Only harmonized methods with traceability of measurement can produce reliable results and are safe for patients.
The prognostic value of eGFRcys is an important aspect for decision making. The data reported in The Atherosclerosis Risk in Communities Study showed greater prognostic importance of eGFRcys.15
The major limitation of our study was the absence of the gold standard method for measurement of GFR but our algorithm was in accordance with the KDIGO guidelines. General limitations of cystatin C should be also emphasized. A case study by Brown et al16 demonstrated falsely high concentration of cystatin C in a patient with acquired immunodeficiency syndrome with opportunistic infection of Pneumocystis jiroveci pneumonia and taking prednisone. Stewens described factors other than glomerular filtration rate that affect both serum cystatin C and creatinine levels such as diabetes, C‐reactive protein, white blood count and serum albumin.17Mosbin stated that the accumulation of metformin in the setting of renal insufficiency might be expected to precipitate lactic acidosis in some patients who are at risk.18 It is consistent with our approach to find patients at risk by eGFRcys.
In summary, eGFRcys reclassified some patients with T2DM from metformin eligible stages to 3b and 4 stage of CKD. Cystatin C‐based eGFR selects more complicated patients, where lower doses of metformin are possibly advisable.
We recommend calculating both eGFRcrea and eGFRcys for metformin dosing in elderly patients with T2DM.
CONFLICT OF INTEREST
None declared.
ACKNOWLEDGEMENT
No funding received.
Šálek T, Adamíková A. Cystatin C measurement leads to lower metformin dosage in elderly type 2 diabetic patients. Basic Clin Pharmacol Toxicol. 2019;124:298–302. 10.1111/bcpt.13132
REFERENCES
- 1. Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60:1577‐1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fuček M, Dika Ž, Karanović S, et al. Reliability of CKD‐EPI predictive equation in estimating chronic kidney disease prevalence in the Croatian endemic nephropathy area. Biochem Med (Zagreb). 2018;28:010701 10.11613/BM.2018.010701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO . clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2012;2013(3):1‐163. [Google Scholar]
- 4. American Diabetes Association . 10. Microvascular complications and foot care. Diabetes Care. 2018;41:S105‐S118. [DOI] [PubMed] [Google Scholar]
- 5. Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and Cystatin C. N Engl J Med. 2012;367:20‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nyborg PT, Bergmann TK, Lykkesfeldt J. Basic & clinical pharmacology & toxicology policy for experimental and clinical studies. Basic Clin Pharmacol Toxicol. 2018;123:233‐235. [DOI] [PubMed] [Google Scholar]
- 7. Tuot DS, Scherzer R, Leong H, Hung AM, Grunfeld C, Shlipak MG. Use of Cystatin C to inform metformin eligibility among adult veterans with diabetes. J Clin Transl Endocrinol. 2016;3:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pottel H, Delanaye P, Weekers L, et al. Age‐dependent reference intervals for estimated and measured glomerular filtration rate. Clin Kidney J. 2017;10:545‐551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shardlow A, McIntyre NJ, Fraser SDS, et al. The clinical utility and cost impact of cystatin C measurement in the diagnosis and management of chronic kidney disease: a primary care cohort study. PLoS Med. 2017;14:e1002400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Klee GG, Schryver PG, Saenger AK, Larson TS. Effects of analytic variations in creatinine measurements on the classification of renal disease using estimated glomerular filtration rate (eGFR). Clin Chem Lab Med. 2007;45:737‐741. [DOI] [PubMed] [Google Scholar]
- 11. Šálek T, Ponížil P. Estimated glomerular filtration rate in diabetic patients. Klin Biochem Metab. 2014;22:4‐7. [Google Scholar]
- 12. Jabor A, Franeková J, Kubíček Z, Šálek T. Estimated glomerular filtration rate and problems in the interpretation of CKD‐EPI equations (Short communication – data expansion and comments to the article “Estimated glomerular filtration rate in diabetic patients” published by Šálek T. and Ponížil P.). Klin Biochem Metab 2014;22:8‐10. [Google Scholar]
- 13. Fan L, Inker LA, Rossert J, et al. Glomerular filtration rate estimation using cystatin C alone or combined with creatinine as a confirmatory test. Nephrol Dial Transplant. 2014;29:1195‐1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Delanaye P, Cavalier E, Moranne O, Lutteri L, Krzesinski J‐M, Bruyère O. Creatinine‐or cystatin C‐based equations to estimate glomerular filtration in the general population: impact on the epidemiology of chronic kidney disease. BMC Nephrol. 2013;14:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Waheed S, Matsushita K, Sang Y, et al. Combined association of albuminuria and cystatin C‐based estimated GFR with mortality, coronary heart disease, and heart failure outcomes: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2012;60:207‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brown CS, Kashani KB, Clain JM, Frazee EN. Cystatin C falsely underestimated GFR in a critically ill patient with a new diagnosis of AIDS. Case Rep Nephrol. 2016;2016:9349280 10.1155/2016/9349280. Available from: http://downloads.hindawi.com/journals/crin/2016/9349280.pdf. Accessed Sep 5, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stevens LA, Schmid CH, Greene T, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75:652‐660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Misbin RI. The phantom of lactic acidosis due to metformin in patients with diabetes. Diabetes Care. 2004;27:1791‐1793. [DOI] [PubMed] [Google Scholar]


