Abstract
Background
This systematic review summarises association between short interpregnancy intervals and adverse perinatal health outcomes in high‐resource settings to inform recommendations for healthy birth spacing for the United States.
Methods
Five databases and a previous systematic review were searched for relevant articles published between 1966 and 1 May 2017. We included studies meeting the following criteria: (a) reporting of perinatal health outcomes after a short interpregnancy interval since last livebirth; (b) conducted within a high‐resource setting; and (c) estimates were adjusted for maternal age and at least one socio‐economic factor.
Results
Nine good‐quality and 18 fair‐quality studies were identified. Interpregnancy intervals <6 months were associated with a clinically and statistically significant increased risk of adverse outcomes in studies of preterm birth (eg, aOR ≥ 1.20 in 10 of 14 studies); spontaneous preterm birth (eg, aOR ≥ 1.20 in one of two studies); small‐for‐gestational age (eg, aOR ≥ 1.20 in 5 of 11 studies); and infant mortality (eg, aOR ≥ 1.20 in four of four studies), while four studies of perinatal death showed no association. Interpregnancy intervals of 6‐11 and 12‐17 months generally had smaller point estimates and confidence intervals that included the null. Most studies were population‐based and few included adjustment for detailed measures of key confounders.
Conclusions
In high‐resource settings, there is some evidence showing interpregnancy intervals <6 months since last livebirth are associated with increased risks for preterm birth, small‐for‐gestational age and infant death; however, results were inconsistent. Additional research controlling for confounding would further inform recommendations for healthy birth spacing for the United States.
Keywords: birth spacing, birth‐to‐conception interval, interpregnancy interval, perinatal, preterm, review
1. BACKGROUND
A short interpregnancy interval since last livebirth—the time between one birth and the start of the next pregnancy—may increase the risk of adverse perinatal outcomes in the subsequent pregnancy. This increased risk could be due to inadequate maternal repletion of nutritional status following the delivery of a live infant, increased cervical insufficiency or vertical transmission of infections following a short interpregnancy interval.1 A previous systematic review and meta‐analysis found that an interpregnancy interval <6 months is associated with 40% higher odds of preterm birth, 61% higher odds of low birthweight and 26% higher odds of small‐for‐gestational age in the subsequent pregnancy.2 Short interpregnancy intervals up to 17 months were also associated with greater risks for these outcomes.2 These findings, along with other supportive evidence, informed the 2005 World Health Organization (WHO) recommendation that women should wait for a minimum of 24 months between livebirth and conception of the next child in order to reduce the risk of adverse maternal, perinatal and infant outcomes.3
The applicability of the WHO recommendations to the United States is unclear because breast feeding, nutrition, age at first birth and parity differ between the United States and the lower‐resource countries upon which most of the evidence reviewed for the WHO recommendation is based.4, 5, 6, 7 Further, the evidence for the WHO recommendations does not include the findings of research conducted since 2006. Recent studies using maternally linked birth records and employing matched study design have found mostly null associations between short interpregnancy interval and adverse outcomes,8, 9, 10, 11 prompting renewed concern that previously observed associations may be due to confounding.12
The purpose of this systematic review is to summarise research on the associations between short interpregnancy intervals and adverse perinatal outcomes in high‐resource settings. The association between short interpregnancy intervals and adverse maternal outcomes in high‐resource settings is reported separately in this journal supplement.13 Findings from this review can be used to inform evidence‐based recommendations for healthy birth spacing for the United States.14 At present, although short interpregnancy interval is a recognised risk factor for preterm birth and low birthweight,15, 16 and the American College of Obstetricians and Gynecologists recommends women be advised to avoid interpregnancy intervals shorter than 6 months,16 there are no federal recommendations on healthy birth spacing for the United States.
2. METHODS
This systematic review adhered to established methodological standards.17, 18 Investigators developed an analytic framework outlining the target population and relationships between interpregnancy intervals and outcomes (Figure S1). The key question guiding this systematic review was “In postpartum women in the United States, what is the effect of short interpregnancy intervals (any interval <24 months) versus a longer interval on short‐term perinatal health outcomes: low birthweight, preterm birth, small‐for‐gestational age, intrauterine growth restriction, APGAR score, neonatal intensive care unit admission, stillbirth, neonatal mortality, infant mortality, and congenital anomaly?” In this study, we synthesise findings for the most commonly examined perinatal outcomes, including preterm birth, spontaneous preterm birth, small‐for‐gestational age, perinatal death and infant mortality. Although low birthweight was a commonly studied outcome, we did not synthesise the evidence for this outcome because its value as a health indicator is limited primarily to lower‐resource settings where accurate estimates of gestational age are unavailable.19 However, all relevant perinatal findings are presented in a supplemental table (Table S1).
The protocol (available upon request) is based on a previous systematic review published in 2006 on the effects of birth spacing on adverse perinatal outcomes.2 The 2006 review included studies published between 1966 and January 2006 in PubMed/MEDLINE; between 1980 and January 2006 in EMBASE, POPLINE and ECLA; and between 1982 and January 2006 in CINAHL and LILACS using a combination of medical subject headings and keyword terms to identify relevant studies.
In contrast to the 2006 review, the updated review includes only studies published in English and conducted in the United States, Canada, Australia, New Zealand and the European countries categorised as “very high” on the United Nations Human Development Index20 to identify studies most applicable to women in the United States. In addition, the updated review concerns potential consequences of short rather than long interpregnancy intervals because the former are more amenable to prevention, such as through the provision of postpartum contraception services.
The 2006 review included 67 studies with statistical adjustment for maternal age and at least one socio‐economic position measure (52 cohort or cross‐sectional studies and 15 case‐control studies). Twenty‐nine studies were conducted on study populations from the United States and other high‐resource countries, of which 19 examined interpregnancy intervals (as opposed to birth‐to‐birth intervals).21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39
2.1. Literature search
Using the same search terms as the 2006 review,2 we conducted electronic searches of PubMed/Medline, POPLINE, EMBASE, CINAHL and the Cochrane Database of Systematic Reviews for relevant articles published between 1 January 2006 and 1 May 2017. In addition to search terms for specific outcomes, we also included general terms, such as “perinatal outcome,” “perinatal morbidity,” “pregnancy outcome,” “adverse outcome,” “obstetric outcome” and “infant outcome.” Specific search terms and publication date ranges are listed in Table S2.
2.2. Inclusion/exclusion criteria
Inclusion criteria for studies were developed a priori using the PICOTSS (population, intervention/exposure, comparison group, outcome, time, setting and study design) framework40 and independently applied to the search results by two study authors (KAA and JAH) in a two‐stage review process (Table S3).41 Studies from the 2006 review meeting new, more restrictive inclusion criteria were also included. Included studies met the following criteria:
The study population consisted of women of reproductive age with at least one livebirth who became pregnant again. Women whose last delivery was a stillbirth were also included, as long as they comprised <5% of the study population (ie, the cohort was not specifically drawn from women with a prior stillbirth).
The study measured interpregnancy interval since last livebirth—defined as the interval between delivery of a birth (liveborn or stillborn) and start of the subsequent pregnancy (also known as birth‐to‐conception interval)—rather than other types of intervals (eg, post‐abortion or post‐pregnancy loss interpregnancy interval, birth‐to‐birth interval). This definition was imposed because there are separate recommendations for interpregnancy interval following pregnancy losses.3 Further, birth‐to‐birth intervals are the sum of the interpregnancy interval and the duration of the subsequent pregnancy; therefore, women with adverse pregnancy outcomes associated with shorter pregnancy duration (such as stillbirth or preterm birth) will have systematically shorter birth‐to‐birth intervals than women without these outcomes. This systematic difference creates the potential for bias due to reverse causation (ie, a short birth‐to‐birth interval being the result of, rather than the cause of, an adverse outcome).
The study compared a short interpregnancy interval, defined as any interval shorter than 24 months, to a longer interpregnancy interval (the reference interval). The reference interval had to have clearly defined lower and upper boundaries (ie, “18‐23 months” rather than “>18 months”). Clearly defined boundaries were required because of the reverse J‐shaped relationship between interpregnancy interval and many adverse perinatal outcomes.36 Reference categories without an upper boundary can represent a heterogeneous risk group. For similar reasons, studies that modelled interpregnancy interval assuming a continuous, linear association with the adverse perinatal outcome were also excluded.
The study examined at least one of the following outcomes: preterm birth, small‐for‐gestational age, foetal death, perinatal death, neonatal death, infant death, low birthweight, intrauterine growth restriction, Apgar score, neonatal intensive care unit or congenital anomaly.
The study was published between 1 January 2006 and 1 May 2017.
The study was designed as a randomised controlled trial, cohort, cross‐sectional or case‐control study and could use unmatched (between‐woman) or matched (within‐sibling) designs. The study adjusted for maternal age and at a least one measure of socio‐economic position.
The study included at least 100 individuals.
In addition, included studies were available as full‐text English‐language publications (ie, not an abstract from a conference presentation) and presented the relevant findings and estimates of precision numerically (eg, 95% confidence interval [CI] or standard error).
2.3. Data abstraction, study quality assessment, data synthesis
A structured Excel‐based abstraction form was developed for data abstraction (available on request). Two study authors independently abstracted relevant data from full‐text articles of included studies; discrepancies were resolved through discussion.41 Data included study design, source, setting, numbers and characteristics of participants, interpregnancy intervals, comparisons, adjustment for confounders, perinatal outcomes and results.
Included studies were assessed for internal and external study quality using criteria outlined by the U.S. Preventive Services Task Force and rated as good, fair or poor.42, 43 Two reviewers independently assessed quality and discrepancies were resolved through consensus. In a few instances, we contacted study authors to discuss study details needed for completing the quality assessment.
Internal validity was determined by evaluating sources of potential information bias (misclassification), confounding and selection bias. Assessments were guided by the key study design considerations identified by a recent Office of Population Affairs’ expert work group reviewing the evidence on short birth spacing and adverse pregnancy outcomes.44 These included the extent to which the study incorporated a detailed measure of socio‐economic position, accounted for pregnancy intention, identified early pregnancy losses occurring between the last birth and the subsequent pregnancy being evaluated (which could result in differential misclassification of interpregnancy interval) and accounted for perinatal death (stillbirth or neonatal death) in the previous pregnancy.44
External validity (generalisability) was determined by comparing the study population to either the general obstetric population in the United States or, for studies of women with specific obstetric history, a population with similar history in the United States.
Study design classification was based on when interpregnancy interval information was most likely documented in relation to when perinatal outcomes were assessed. As many of the studies were population‐based samples of birth records, information on interpregnancy interval was assumed to originate from the prenatal medical record, which would have therefore been captured prior to the pregnancy outcome being known.45 Consequently, population‐based record samples were considered cohort studies.
Results of studies rated as having good or fair internal validity were qualitatively synthesised, taking into account both the magnitude and precision of relative risk estimates.
3. RESULTS
Figure 1 shows a literature flow diagram of inclusions and exclusions. The literature search identified 490 unique references of which 416 were excluded after reviewing the title and abstract, most commonly because the studies were conducted outside the United States or other high‐resource settings or did not describe original research. A total of 74 articles were identified for full‐text review for perinatal outcomes, of which 21 studies met eligibility criteria in addition to 11 studies from the 2006 review. The most common reasons for exclusion at the full‐text review stage were that the studies did not measure interpregnancy intervals, did not have a well‐defined comparison group or did not adjust for maternal age and at least one measure of socio‐economic position. Multiple reasons for exclusion were possible at each stage of review, but all exclusion reasons were not documented because once a study met one exclusion criterion it was then not further reviewed.
Figure 1.

Literature flow diagram. *947 records include eight unique records identified from a targeted review conducted on September 22, 2017 to find articles on interpregnancy intervals and uterine rupture, placental abruption and placenta previa, which were outcomes relevant to the maternal outcomes systematic review
The 32 included studies are described in Table 1.8, 9, 10, 11, 21, 22, 25, 27, 28, 29, 34, 35, 36, 37, 38, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62 The majority of studies were conducted in the United States (n = 18),11, 21, 25, 27, 28, 29, 36, 37, 38, 49, 51, 54, 55, 56, 57, 58, 59, 62 78% of which used U.S. birth certificate data (n = 14).11, 21, 25, 36, 37, 38, 49, 51, 54, 55, 57, 58, 59, 62 Other study settings included Canada (n = 4),9, 46, 47, 50 the Netherlands (n = 3),10, 48, 52 Scotland (n = 2),34, 53 Sweden (n = 2),35, 60 Australia (n = 1),8 Denmark (n = 1)22 and Poland (n = 1).61 Variations in birth registration practices across study settings (and thus, what is documented as a life birth) could have limited the validity of comparison across studies.63 Most studies had large sample sizes (only two studies had sample size <300010, 62 and the largest study was 847 618 individuals47), and over half of studies (n = 17) used 18‐23 months as the interpregnancy interval reference group for analyses (Table 1). One study was a case‐control study,54 and the rest were cohort studies, which included four studies with interview data.27, 28, 29, 52 No studies evaluated the effects of an intervention designed to reduce short interpregnancy intervals on subsequent perinatal outcomes. Most studies evaluated more than one perinatal outcome.
Table 1.
Characteristics and quality of 32 included studies
| Author Year | Location | Design and data source | Study population (N) | Inter pregnancy intervals (mo) | Outcomesa | Covariates in adjusted analysis | Subgroup/stratified analyses | Quality ratings |
|---|---|---|---|---|---|---|---|---|
| Klebanoff et al (1988)27, b | USA | Cohort; interviews and medical record data |
Women with two consecutive singleton pregnancies resulting in livebirth during 1959‐1966 (N = 5938) |
3‐5.9 6‐8.9 9‐11.9 12‐14.9 15‐17.9 18‐20.9 21‐23.9 ≥24 |
Birthweight; low birthweight; small‐for‐gestational age | Assessed at first pregnancy: maternal age (number of levels not stated), education (number levels not stated), socio‐economic index (number of levels not stated), smoking, birthweight of first child, weight at start of first pregnancy, maternal race/ethnicity. | None |
Fair internal validity Strengths: measure of interpregnancy interval accounted for intervening miscarriages; adjusted for time‐varying covariates at the time of the first rather than the second pregnancy. Weaknesses: did not account for pregnancy intention or past neonatal death. Fair external validity: based on women who agreed to participate in a prospective cohort study; drop in participation between eligible first and second pregnancy. |
| Lieberman et al (1989)29, b | Boston, MA, USA | Cohort; interviews and medical record data |
Women with full‐term live born infants delivered during 1977‐1980 whose last pregnancy was a full‐term livebirth (N = 4467) |
≤3 3‐6 6‐12 12‐18 18‐24 24‐36c 36‐48 48‐60 60‐72 72‐96 ≥96 |
Small‐for‐gestational age | Assessed at time of subsequent pregnancy: maternal age (2 level), welfare recipient (2 level), race, education (2 level), smoking, alcohol use, prepregnancy weight, weight gain, maternal height, infant sex, chronic hypertension. | None |
Fair internal validity Strengths: data obtained from medical record abstraction and interview, increased accuracy compared with birth certificate records; accounted for prior stillbirth. Weaknesses: adjusted for confounders at subsequent pregnancy rather than initial pregnancy, which may be an overadjustment; did not adjust for pregnancy intention, past neonatal death, or intervening pregnancy losses. Fair external validity: single‐centre hospital in Boston. |
| Lang et al (1990)28, b | Boston, MA, USA | Cohort; interviews and medical record data |
Women with appropriate‐for‐gestational age liveborn infants delivered during 1977‐1980 whose last pregnancy was a full‐term livebirth (N = 4489) |
≤3 4‐6 7‐12 13‐18 29‐24 25‐36c 37‐48 ≥49 |
Spontaneous preterm | Assessed at time of subsequent pregnancy: maternal age (number of levels not stated), insurance status (number of levels not stated), race, education (number of levels not stated), marital status, prenatal care, planned pregnancy, smoking, prepregnancy weight, parity, among several others. | None |
Good internal validity Strengths: controlled for multiple potential confounders including pregnancy intention, prior stillbirth or neonatal death and multiple measures of socio‐economic position. Weaknesses: adjusted for confounders at the time of the subsequent pregnancy rather than the initial pregnancy, which may be an overadjustment. Fair external validity: single‐centre hospital in Boston. |
| Adams et al (1997)21, b | Georgia, USA | Cohort; birth certificate data maternally linked to foetal death data | Low‐risk women with a second consecutive pregnancy of singleton livebirth during 1989‐1992 (N = 23 388 white; N = 4885 black) |
0‐5 6‐8 9‐1 12‐17 18‐23 24‐35c 36‐47 ≥48 |
Preterm | Assessed at time of second delivery: maternal age (4 level), education (3 level), prenatal care initiation in first trimester, paternity same as first pregnancy. | Maternal race (white, black) |
Fair internal validity Strengths: large, population‐based study; restricted to low‐risk women reduces some potential for confounding. Weaknesses: accuracy of many birth certificate variables is poor; did not control for pregnancy intention, prior neonatal death or intervening miscarriages. Good external validity: population‐based sample from Georgia. |
| Basso et al (1998)22, b | Denmark | Cohort; random sample from population‐based registry of pregnancies maternally linked with education, employment, and income |
First two consecutive live singleton births during 1980‐1992, (N = 10 187) |
≤4 4‐≤8 8‐≤12 12‐≤24 24‐≤36c >36 |
Preterm; low birthweight | Assessed at time of first delivery: maternal age (5 levels), parity, social status (3 levels); Assessed at time of second delivery: change in social status, gestational age (for low birthweight outcome only). | Gestational age of the first pregnancy (<37, 37‐38, 39‐40, ≥41 wk) |
Good internal validity Strengths: large population‐based sample with detailed information from database linkages; socio‐economic position adequately measured; excluded births after past stillbirth or infant death. Weaknesses: did not account for pregnancy intention. Good external validity: national population‐based sample from Denmark. |
| Zhu et al (1999)36, b | Utah, USA | Cohort; birth certificate data |
Singleton infants born alive during 1989‐1996 to women who had previously delivered at least one live infant (N = 173 205) |
0‐5 6‐11 12‐17 18‐23c 24‐59 60‐119 ≥120 |
Low birthweight; preterm birth; small‐for‐gestational age | Assessed at time of subsequent pregnancy: maternal age at delivery (4 levels), marital status, education (2 levels), outcome of most recent recognised pregnancy, previous living children, previous liveborn children who died, previous spontaneous or induced abortions, among others. | Age, education, weight, previous stillbirths or abortion, and rural or urban residence. pregnancy. |
Fair internal validity Strengths: large, population‐based cohort. Weaknesses: accuracy of many birth certificate variables is poor; did not control for pregnancy intention; limited measure of socio‐economic position; did not account for intervening early pregnancy losses. Good external validity: population‐based sample from Utah. |
| Fuentes‐Afflick et al (2000)25, b | USA | Cohort; national birth certificate data; Hispanic and non‐Hispanics matched on birth county | Singleton infants born during 1991 to women with a previous livebirth (N = 246 726) |
<6 6‐11 12‐17 18‐59c >59 |
Preterm (23‐32; 33‐37 wk) | Assessed at time of subsequent pregnancy: maternal race/ethnicity, maternal age (5 level), education (5 level), country of birth, parity, previous preterm or small‐for‐gestational age infant, prenatal care and infant sex. | None |
Fair internal validity rating Strengths: large, population‐based cohort. Weaknesses: accuracy of many birth certificate variables is poor; did not control for pregnancy intention, prior neonatal death, or intervening miscarriages. Fair external validity: study population limited to Mexican‐origin Hispanic and non‐Hispanic white women in the United States. |
| Zhu et al (2001)37, b | Michigan, USA | Cohort; birth certificate data |
Singleton infants born alive during 1993‐1998 to women with previous livebirth (N = 346 250 white; N = 89 077 black) |
0‐5 6‐11 12‐17 18‐23c 24‐59 60‐119 ≥120 |
Low birthweight; preterm birth; small‐for‐gestational age | Assessed at time of subsequent pregnancy: maternal age at delivery (6 levels), education (2 levels), marital status, outcome of most recent pregnancy, number of previous pregnancies, adequacy of prenatal care, smoking, alcohol. | Race, education, other covariates |
Fair internal validity Strengths: large, population‐based cohort. Weaknesses: accuracy of many birth certificate variables is poor; did not control for pregnancy intention; limited measure of socio‐economic status; did not account for early pregnancy losses. Good external validity: population‐based sample from Michigan. |
| Smith et al (2003)34, b | Scotland | Cohort; hospital discharge data linked to registry of infant and foetal death data |
Women with second birth during 1992‐1998 whose first infant was full term and liveborn (N = 69 055) |
1‐5 6‐11 12‐17 18‐23c 24‐59 |
Low birthweight (<5th percentile); preterm (24‐32; 33‐36 wk); foetal abnormality; stillbirth; other neonatal deaths | Assessed at time of subsequent pregnancy: maternal age (3 levels), marital status, height, socio‐economic deprivation category (5 levels), smoking, previous birthweight, previous caesarean section. | Married, non‐smokers, age ≥25 |
Good internal validity Strengths: use of hospital discharge records increased accuracy of diagnoses; controlled for detailed individual‐level measure of socio‐economic position, prior stillbirth or neonatal death and intervening pregnancy losses; adjusted for covariates at time of first, not second pregnancy. Weaknesses: did not account for pregnancy intention. Good external validity: population‐based sample from Scotland, UK. |
| Stephansson et al (2003)35, b | Sweden | Cohort; population‐based birth registry linked to cause of death registry, education registry, and immigration registry, maternally linked |
Women who delivered consecutive first and second singletons during 1983‐1997 (N = 362 368) |
0‐3 4‐7 8‐11 12‐35c 36‐71 ≥72 |
Stillbirth; early neonatal death | Assessed at time of subsequent pregnancy: smoking status, maternal age (4 levels), education (2 levels), cohabitating with father, maternal country of birth, diabetes, hypertensive disease, year of delivery, outcome of first pregnancy. | None |
Good internal validity Strengths: data obtained from high‐quality population birth registry linked with individual records on educational achievement and immigration status; accounted for prior stillbirth or neonatal death. Weaknesses: did not account for pregnancy intention or intervening losses; adjustment for covariates at time of second pregnancy such as maternal age may be an overadjustment. Good external validity: national population‐based sample from Sweden. |
| Zhu et al (2003)38, b | Michigan, USA | Cohort; birth certificate data, maternally linked |
Singleton infants born alive during 1989‐2000 to women with previous live infant (N = 565 911) |
0‐5 6‐11 12‐17 18‐23c 24‐59 60‐95 96‐136 |
Low birthweight |
Assessed at time of subsequent pregnancy: Preceding infant's birthweight, paternal acknowledgement, maternal age at delivery (6 levels), race, education (2 levels), adequacy of prenatal care utilisation, outcome of most recent pregnancy, smoking, alcohol. |
Factors included as covariates and birth‐order pair |
Poor internal validity Strengths: large, population‐based cohort. Weaknesses: control for socio‐economic position limited; did not control for pregnancy intention, prior neonatal death, or account for losses between pregnancies. Good external validity: population‐based sample from Michigan. |
| Smith et al (2007)53 | Scotland | Cohort; hospital discharge data linked to registry of infant and foetal death data |
Women with second birth during 1992‐2001 whose first infant was liveborn (N = 133 163) |
<6 6‐11 12‐23c 24‐5 y 6‐10 y >10 y |
Antepartum unexplained stillbirth |
Assessed at time of first delivery: preterm, small‐for‐gestational age/preeclampsia and caesarean section. Assessed at time of subsequent delivery: maternal age (6 levels), social deprivation category (7 levels), height, smoking, marital status. |
Size for age at birth (small vs appropriate‐for‐gestational age) |
Fair internal validity Strengths: large population‐based sample; hospital discharge records increased accuracy; individual‐level measure of socio‐economic position. Weaknesses: no adjustment for pregnancy intention or prior neonatal death; does not account for intervening miscarriages. Good external validity: population‐based study from Scotland, UK. |
| van Eijsden et al (2008)52 | Amsterdam, the Netherlands | Cohort; prospective community‐based pregnancy study |
Multiparae giving birth to a viable full‐term infant during 2003‐2004 (N = 3153) |
<6 6‐11 12‐17 18‐23c 24‐59 ≥60 |
Birthweight; small‐for‐gestational age | Assessed at time of subsequent delivery: maternal age (3 levels), prepregnancy BMI, infant sex, height, parity, gestational age, smoking, alcohol, psychosocial stress, pregnancy intention, cohabitant status, education (3 levels), country of birth. | Folic acid supplementation |
Fair internal validity Strengths: controlled for pregnancy intention, psychosocial stress and multiple measures of socio‐economic position. Weaknesses: did not account for prior neonatal death or stillbirth; does not account for intervening miscarriages. Fair external validity: limited to 67% who agreed to participate; folic acid supplementation lower than in the United States. |
| Villamor et al (2008)60 | Sweden | Cohort; population‐based birth registry, maternally linked |
Women with first two consecutive births during 1992‐2004, whose first‐born did not have oral cleft malformation (N = 219 983) |
12‐23 24‐35 36‐47 ≥48 |
Isolated cleft palate; all cleft palate |
Maternal height, country of origin; Assessed at time of first pregnancy: BMI, maternal age (4 levels), paternal age, maternal education (6 levels), preeclampsia, gestational diabetes, preterm delivery, small‐ or large‐for‐gestational age, stillbirth or infant death; Assessed at time of subsequent pregnancy: year of delivery, smoking, pregestational diabetes. |
None |
Fair internal validity Strengths: high‐quality, validated database (Swedish Medical Birth Register). Weaknesses: did not control for pregnancy intention; does not account for intervening miscarriages. Good external validity: national population‐based sample from Sweden. |
| de Weger et al (2011)48 | The Netherlands | Cohort; population‐based registry of pregnancies, deliveries, and readmissions |
Women with singleton delivery by gynaecologist in a hospital during 2000‐2007 with one previous delivery (N = 263 142) |
<6 6‐11 12‐17 18‐23c ≥24 |
Preterm; low birthweight; small‐for‐gestational age | Assessed at time of subsequent delivery: maternal age at first delivery (6 levels), mean household income of neighbourhood (5 levels), use of artificial reproductive techniques, ethnic origin, year of birth. | None |
Fair internal validity Strengths: large, population‐based registry, excluded women with losses <20 wk. Weaknesses: limited measure of socio‐economic position; did not control for pregnancy intention or prior stillbirth or neonatal death. Poor external validity: only women delivered by a gynaecologist (~65%); health behaviours and risks likely differ by caregiver. |
| Salihu et al (2012)55 | Hillsborough County, Florida, USA | Cohort; birth certificate data linked to Healthy Start Program data |
Women with consecutive singleton first and second pregnancies during 2002‐2009 (N = 36 718) |
<6 6‐<18 18‐<24c ≥24 |
Low birthweight; preterm; small‐for‐gestational age; any feto‐infant morbidity | Assessed at time of subsequent delivery: maternal age (2 level), parity, race/ethnicity, smoking, maternal education (2 level), marital status, adequacy of prenatal care, conditions during pregnancy. | Programme participation status |
Poor internal validity Strengths: stratified analyses by zip code to examine effects in neighbourhoods served by Healthy Start Program. Weaknesses: control for socio‐economic position limited to a binary variable; did not control for pregnancy intention, prior neonatal death or stillbirth, or account for early pregnancy losses. Fair external validity: limited to a population‐based sample from a single county in Florida. |
| Howard et al (2013)49 |
Louisiana, USA |
Cohort; birth certificate data |
Women with a singleton birth during 1995‐2007 with single previous pregnancy that ended in livebirth or foetal death (>20 wk or ≥350 g) (N = 96 387) |
<9 9‐23c |
Preterm | Assessed at time of subsequent delivery: maternal age (y) and race, smoking, number of prenatal visits, timing of first prenatal visit, low birthweight, marital status, education (3 levels), number of terminations, previous foetal death and previous caesarean section. | None |
Fair internal validity Strengths: large, population‐based sample; propensity score analysis to control for confounding. Weaknesses: accuracy of many birth certificate variables is poor; did not account for early pregnancy losses, prior neonatal death or pregnancy intention. Good external validity: population‐based sample from Louisiana. |
| Hussaini et al (2013)54 |
Arizona, USA |
Case‐control: birth certificate data linked to infant mortality data |
Non‐first‐born singleton infant deaths during 2003‐2007 and a random sample of non‐first‐born singleton survivors (N = 1466 cases; N = 2000 controls) |
<6 6‐11 12‐17 18‐23c 24‐59 ≥60 |
Infant deaths; neonatal deaths; post‐neonatal deaths | Assessed at time of subsequent delivery: preterm birth, low birthweight, small‐for‐gestational age, maternal medical risk factor, infant sex, smoking, history of preterm birth, number of living children, maternal race/ethnicity, weight gained during pregnancy, no prenatal care, marital status, maternal age (3 levels), education (2 levels), insurance status (3 levels), geographic area of residence. | None |
Fair internal validity Strengths: large, population‐based sample. Weaknesses: accuracy of many birth certificate variables is poor; did not account for early pregnancy losses, prior neonatal death or pregnancy intention. Good external validity: cases included all infant deaths in Arizona during study period, controls drawn from random sampling of birth certificates in the state during same period. |
| Ball et al (2014)8 | Perth, Western Australia | Cohort (sibling comparison); population‐wide database of births, maternally linked |
Women with their first three births as liveborn singletons during 1980‐2010 (N = 40 441) |
0‐5 6‐11 12‐17 18‐23c 24‐59 60‐119 ≥120 |
Preterm; small‐for‐gestational age; low birthweight | Assessed at time of second and third birth: maternal age (6 levels), parity, birth year, socio‐economic disadvantage index (5 levels). | None |
Good internal validity Strengths: use of sibling comparison design to control for confounding by time‐invariant characteristics. Weaknesses: no adjustment for potentially time‐varying confounding such as smoking or pre‐pregnancy BMI. Poor external validity: limited to women with three or more births with discordant perinatal outcomes representing a small subset of the target population. |
| Hinkle et al (2014)56 | Utah, USA | Cohort; maternal and infant hospital electronic medical records supplemented with ICD9 discharge codes, maternally linked |
Women with two singleton deliveries >20 wks’ gestation in their first and second pregnancy during 2002‐2010 (N = 25 241) |
<12 12‐≤18 18‐23c >23 |
Incident small‐for‐gestational age in second pregnancy; recurrent small‐for‐gestational age in second pregnancy | Assessed at time of subsequent delivery: maternal age (5 levels), race/ethnicity, marital status, insurance (2 levels), smoking, alcohol, prepregnancy weight, gestational weight gain, diabetes, hypertension, asthma, thyroid disease, depression or other mental health condition. | Small‐for‐gestational age birth in first pregnancy |
Poor internal validity Strengths: data based on medical record abstraction increased accuracy of diagnoses and gestational age estimation. Weaknesses: control for socio‐economic position limited to a binary variable; did not control for pregnancy intention, prior neonatal death or stillbirth, or account for intervening early pregnancy losses. Good external validity: sample of multiparae from 20 Utah hospitals. |
| Naimi et al (2014)47 | Quebec, Canada | Cohort; birth certificate records linked with area‐level measures of social and material deprivation |
Singleton livebirths during 1989‐2010 to women with at least one previous birth (N = 847 618) |
0‐18 18‐23c 24‐<60 ≥60 |
Preterm | Assessed at time of subsequent delivery: maternal education (4 levels) and birth year; maternal age (y), paternal ages, countries of birth, native languages; area‐level measures of material and social deprivation (levels not stated). | None |
Fair internal validity Strengths: large population‐based study, controlled for multiple measures of socio‐economic position. Weaknesses: no control for pregnancy intention or prior neonatal death; does not account for intervening stillbirths or miscarriages; material and social deprivation measures from the neighbourhood rather than individual level. Good external validity: population‐based sample from the province of Quebec, Canada. |
| Chen et al (2015)50 | Northern Alberta, Canada | Cohort; province‐wide delivery records from hospital and midwife attended deliveries, linked to maternal demographic database |
Women with two consecutive singleton deliveries in northern Alberta during 1999‐2007 (N = 46 243) |
0‐5 6‐11 12‐17c 18‐23 24‐35 ≥36 |
Preterm (<28; <34 wk); low birthweight (<1500; <1000 g); small‐for‐gestational age (<3rd percentile); perinatal death; Apgar scores; NICU admission | Assessed at time of subsequent delivery: maternal age (3 levels), smoking, social assistance (3 levels), parity, diabetes, maternal/gestational hypertension, previous stillbirth, previous small‐for‐gestational age, infant sex, congenital anomalies | None |
Fair internal validity Strengths: high‐quality data from population‐based clinical records. Weaknesses: no adjustments for previous neonatal death or pregnancy intention; early pregnancy losses during interpregnancy interval not identified. Good external validity: population‐based study from Northern Alberta, Canada. |
| Jelliffe‐Pawlowski et al (2015)57 |
California, USA |
Cohort; birth certificate data linked to hospital discharge record and prenatal screening data |
Singleton livebirths with expected dates of delivery during 2009‐2010, with 1st and 2nd trimester prenatal aneuploidy serum screening (N = 125 202) |
<6 6‐23 24‐59c ≥60 |
Preterm (<32; 32‐36 wk); medically indicated (<32; 32‐36 wk) | Assessed at time of subsequent delivery: maternal race/ethnicity, maternal age (3 levels), insurance (4 levels), education (3 levels), nativity, BMI at onset of pregnancy, pre‐existing hypertension, preeclampsia, preexisting diabetes, gestational diabetes, primiparity, previous caesarean sections and preterm births, mid‐pregnancy serum biomarkers. | None |
Fair internal validity Strengths: linkage of birth certificate records with hospital discharge data improves data quality for medical diagnoses. Weaknesses: accuracy of many birth certificate variables is poor; did not control for pregnancy intention, prior stillbirth or neonatal death, or intervening miscarriages. Good external validity: population‐based sample from California. |
| Mburia‐Mwalili et al. (2015)58 | Nevada, USA | Cohort; population‐based birth defects surveillance system, linked to birth certificate data |
Singleton livebirths during 2006‐2011 to women with at least one previous livebirth (N = 124 341) |
0‐5 6‐11 12‐17 18‐23c 24‐35 ≥36 |
At least one birth defect | Assessed at time of subsequent delivery: infant sex, maternal age (5 levels), race/ethnicity, education (3 levels), number of previous births, smoking and/or alcohol use, prescription drug use. | None |
Fair internal validity Strengths: linkage with state birth defects surveillance system ensures higher data quality for outcome assessment. Weaknesses: accuracy of many birth certificate variables is poor; did not control for pregnancy intention, prior stillbirth or neonatal death or intervening miscarriages. Good external validity: population‐based sample from Nevada. |
| Merklinger‐Gruchala et al (2015)61 | Krakow, Poland | Cohort; birth registry records |
Singleton livebirths during 1995‐2009 to women with at least one previous livebirth (N = 39 968) |
0‐5 6‐11 12‐17 18‐23c 24‐59 60‐119 ≥120 |
Low birthweight | Assessed at time of subsequent delivery: marital status, maternal employment and education indicator (4 levels), parity, infant sex, maternal age (y), gestational age. | Parity |
Fair internal validity Strengths: population‐based sample includes maternal employment and education. Weaknesses: did not control for pregnancy intention, prior stillbirth or neonatal death, or intervening miscarriages; no information on day of birth. Fair external validity: population‐based study from Krakow, Poland. |
| Appareddy et al (2016)51 | Tennessee, USA | Cohort; birth certificate data linked to infant mortality data |
Women with a previous livebirth, who gave birth during 2012‐2014 and had IPI <5 y (N = 101 912) |
<6 6‐12 12‐18 18 ≤ 60c |
Low birthweight; preterm birth (<34 wk); NICU admission; infant mortality | Assessed at time of subsequent delivery: maternal age (y), marital status, education (2 levels), race; WIC use during pregnancy (2 levels), pre‐pregnancy BMI, number of previous pregnancies, timing of prenatal care initiation, smoking. | WIC use during pregnancy |
Fair internal validity Strengths: large, population‐based sample. Weaknesses accuracy of many birth certificate variables is poor. Good external validity: based on all vital statistics records from Tennessee. |
| Shachar et al (2016)11 | California, USA | Cohort (sibling comparison); birth certificate data linked to hospital discharge records, infant death, and foetal death data, maternally linked |
Women with three consecutive livebirths during 1991‐2010 (N = 302 706) |
<6 6‐11 12‐17 18‐23c 24‐59 60‐119 ≥120 |
Preterm | Parity, education (4 levels), maternal age (y), year of birth, previous preterm birth. | None |
Good internal validity Strengths: use of sibling comparison design to control for confounding by time‐invariant characteristics. Weaknesses: did not control for risk factors such as smoking and pre‐pregnancy BMI that vary between a woman's pregnancies. Poor external validity: limited to women with three or more births with discordant perinatal outcomes. |
| Coo et al (2017)46 | Manitoba, Canada | Cohort; province‐wide hospital discharge data linked with 8 other provincial datasets |
Sibling pairs representing two consecutive singleton livebirths in Manitoba during 1985‐2014 (N = 171 688) |
<6 6‐11 12‐17 18‐23c 24‐59 ≥60 |
Preterm (<34; 34‐36; 37‐38 wk); low birthweight; small‐for‐gestational age; medically indicated preterm; spontaneous preterm |
Assessed at time of subsequent delivery: birth year, child's sex, maternal age at delivery (6 levels); parity, adequacy of prenatal care, high school graduate (3 levels); received income assistance (3 levels); socio‐economic index (6 levels), smoking, alcohol, substance use, chronic hypertension, maternal/gestational diabetes, previous pregnancy losses or stillbirths, perinatal outcome of previous birth. | None |
Good internal validity Strengths: detailed measures of socio‐economic position and multiple other confounders; accounted for prior stillbirth and early pregnancy losses during the interpregnancy interval. Weaknesses: did not account for pregnancy intention or previous neonatal loss. Good external validity: population‐based study from the Canadian province of Manitoba. |
| Hanley et al (2017)9 | British Columbia, Canada | Cohort (sibling comparison); database of obstetric and neonatal medical charts, British Columbia Perinatal Data Registry, with deliveries linked maternally |
Women with at least three singleton deliveries during 2000‐2015 delivered at 20‐44 wks’ gestation (N = 38 178) |
0‐5 6‐11 12‐17 18‐23c 24‐59 ≥60 |
Preterm; small‐for‐gestational age; NICU; low birthweight | Maternal age at each delivery (6 levels); delivery year, maternal diabetes, maternal hypertension, smoking, history of perinatal death. | None |
Good internal validity Strengths: use of sibling comparison design to control for confounding by time‐invariant characteristics. Weaknesses: adjustment for maternal age at pregnancy following IPI an over‐adjustment. Poor external validity: Limited to women with three or more births with discordant perinatal outcomes. |
| Koullali et al (2017)10 | The Netherlands | Cohort; population‐based registry of pregnancies, deliveries, and readmissions, maternally linked |
Women with three sequential singleton pregnancies during 1999‐2009, with the first pregnancy resulting in spontaneous preterm birth (N = 2361) |
0‐5 6‐11 12‐17 18‐23c 24‐59 ≥60 |
Preterm (<32 wk); low birthweight; small‐for‐gestational age | Assessed at time of previous delivery: maternal age (3 levels), race/ethnicity, socio‐economic position (3 levels), artificial reproductive techniques, year of birth. | None |
Good internal validity Strengths: use of sibling comparison design to control for confounding by time‐invariant characteristics. Weaknesses: did not adjust for potential time‐varying confounders such as smoking or pre‐pregnancy BMI. Poor external validity: limited to women with three or more births with discordant perinatal outcomes; women with prior spontaneous preterm birth in first pregnancy; delivered by a gynaecologist. |
| Goyal et al (2017) | Ohio, USA | Cohort; birth certificate data for home‐visiting programme participants and matched controls |
Women with consecutive singleton first birth (>23 wks’ gestation and no neonatal death) during 2007‐2009 and second births during 3‐y follow‐up (N = 854) |
≤6 7‐<36c |
Preterm | Assessed at time of second birth: prior preterm birth; race/ethnicity; education level (3 level), insurance paid for delivery (4 level), maternal age (2 level), breast‐feeding status, marital status, pre‐ or during pregnancy hypertension, diabetes, and obesity (separately); sexually transmitted infection during pregnancy; and (assessed at time of first birth): enrolment in Healthy Start Program, delivery method, smoking, year of birth. | Programme participants |
Poor internal validity Strengths: excluded prior neonatal deaths. Weaknesses: small sample size; did not account for pregnancy intention or intervening early pregnancy losses; propensity score matching performed for primary analysis was not intended to balance covariates for IPI comparison; adjustment for covariates at time of second pregnancy such as maternal age may be an overadjustment. Fair external validity: limited to first time mothers participating in home‐visiting programme and their matched controls from seven counties in Ohio. |
| McKinney et al (2017)59 | Ohio, USA | Cohort; birth certificate data linked to infant mortality data |
Livebirths during 2007‐2014 to multiparae (N = 604 217) |
0‐5 6‐<12 12‐<24c 24‐<60 ≥60 |
Infant mortality | Assessed at time of subsequent delivery: marital status, Medicaid (2 levels), smoking, maternal age (y), race/ethnicity. | Maternal race |
Poor internal validity Strengths: large, population‐based cohort. Weaknesses: socio‐economic position limited to a binary variable; did not control for pregnancy intention, prior neonatal death, stillbirth, early pregnancy losses; selection bias from missing IPI information among those with high infant mortality rates. Good external validity: population‐based sample from Ohio. |
BMI, body mass index; IPI, interpregnancy interval; NICU, Neonatal Intensive Care Unit; WIC, Special Supplemental Nutrition Program for Women, Infants, and Children.
Unless otherwise specified, preterm was defined as livebirth/delivery <37 wks’ gestational age, low birthweight as livebirth/delivery and <2500 g, small‐for‐gestational age as livebirth/delivery and <10th percentile by gestational age in weeks (or by sex and gestational age in weeks), perinatal death as foetal death plus neonatal death within the first week of life, infant death as death within the first year of life, neonatal death as death within the first 28 d of life and post‐neonatal death as death within the first 39 d to 1 y of life.
Identified from previous review.
Reference group.
For calculating relevant odds ratios, estimates for <3 mo were inverted so that reference group was 18‐20.9 mo.
For calculating relevant odds ratios, estimates for <12 mo were inverted so that reference group was 12‐23 months.
Nine of the 32 studies met criteria for good internal validity,8, 9, 10, 11, 22, 28, 34, 35, 46 18 fair21, 25, 27, 29, 36, 37, 47, 48, 49, 50, 51, 52, 53, 54, 57, 58, 60, 61 and five poor38, 55, 56, 59, 62 (Table 1). Most studies included a limited set of covariates for adjustment, and no study accounted for all the study design considerations outlined above. However, previous pregnancy stillbirth or neonatal death was accounted for in eight studies,9, 35, 36, 37, 38, 46, 50, 60 pregnancy intention was measured in two studies,28, 52 and intervening pregnancy loss was accounted for—usually in the study cohort definition—in seven studies.10, 21, 28, 29, 49, 55, 56
Generally, studies rated as good‐quality accounted for a measure of socio‐economic position beyond maternal education (the primary socio‐economic measure on the U.S. birth certificate), in addition to at least one of the key study design considerations listed above. These higher quality studies included four studies that used a matched (within‐sibling comparison) design,8, 9, 10, 11 which controls for time‐fixed confounders by using a woman as her own control. Studies rated as poor‐quality generally adjusted for only a single binary measure of socio‐economic position, usually maternal education. All studies, except one,27 included adjustment for covariates measured during or after the end of the interpregnancy interval, which can introduce overadjustment bias if these covariates operate as causal intermediates.64 Most studies found attenuated estimates after adjustment for covariates, but the magnitude of that attenuation varied by perinatal outcome, length of interpregnancy interval and covariate adjustment set; generally, the shortest interpregnancy intervals evaluated showed the greatest attenuation after adjustment (Table S1). In the light of the potential for residual confounding and overadjustment bias, statistical meta‐analysis was not performed and results were synthesised qualitatively.
Most studies (n = 19) were rated as having good external validity, reflecting the common use of population‐based data (including birth certificate records and population perinatal registries). Eight studies met criteria for fair‐quality25, 27, 28, 29, 52, 55, 61, 62 and five for poor.8, 9, 10, 11, 48 The eight studies meeting criteria for fair‐quality25, 27, 28, 29, 52, 55, 61, 62 were limited by including populations from only a single hospital or county;28, 29, 55 including Healthy Start Program participants and matched controls from selected counties;62 excluding certain race/ethnicity groups25; having low participation or follow‐up rates27, 52 or taking place in settings with markedly different access to reproductive health services compared to the United States.61 The five poor‐quality studies8, 9, 10, 11, 48 included four matched studies, which, by design, were restricted to women with three or more pregnancies and discordant pregnancy outcomes.8, 9, 10, 11 The other poor‐quality study included only hospital births delivered by gynaecologists in the Netherlands,48 which represents higher risk pregnancies compared to those among women delivered in other settings in that country.
3.1. Preterm birth
Preterm birth (defined as <37 weeks’ gestation) was assessed in 14 cohort studies (Figure 2A,B). Among the six good‐quality studies (Figure 2A),8, 9, 10, 11, 22, 46 four reported statistically significant adjusted odds ratios (aORs) for interpregnancy intervals of approximately <6 months10, 11, 22, 46 of which all had effect sizes ≥1.20 (ranging from 1.2 [95% CI 1.13, 1.27] for <6 vs 18‐23 months11 to 3.6 [95% CI 2.04, 6.35] for ≤4 vs 24‐36 months).22 One study reported an aOR = 1.26 (95% CI 1.16, 1.36) for 6‐11 vs 18‐23 months.46 The remaining aOR estimates from these studies were smaller in magnitude or not statistically significant.
Figure 2.

A, Adjusted odds ratios and 95% confidence intervals for the association between interpregnancy interval and preterm birth among studies rated as having “good” internal validity from high‐resource settings. Black solid circles indicate the reference category, and red solid circles indicate studies using a sibling comparison design; B, Adjusted odds ratios and 95% confidence intervals for the association between interpregnancy interval and preterm birth among studies rated as having “fair” internal validity from high‐resource settings. Black solid circles indicate the reference category. Confidence intervals are not discernible for some studies because they fell within the range covered by the point estimate symbol (black hollow circle); C, Adjusted odds ratios and 95% confidence intervals for the association between interpregnancy interval and spontaneous preterm birth among studies from high‐resource settings. Black solid circles indicate the reference category
Among the eight fair‐quality studies (Figure 2B), all reported statistically significant aORs for the shortest interpregnancy interval examined in each study.21, 36, 37, 47, 48, 49, 50, 51 Six of these studies found significant associations for <6‐month interpregnancy intervals (estimates ranged in magnitude from an aOR = 1.2 (95% CI 1.1, 1.3)37 for <6 vs 18‐23 months to an aOR = 1.92 (95% CI 1.79, 2.07)48 for <6 vs 18‐23 months). One study reported an aOR = 1.2 (95% CI 1.1, 1.2) for interpregnancy intervals 6‐11 months,37 and the remaining studies that reported statistically significant estimates were small in magnitude (eg, aOR<1.20). The good‐quality studies usually reported lower estimates for a given interpregnancy interval compared with fair‐quality studies. Generally, for all studies, the shorter the interpregnancy interval, the higher the reported estimate.
3.2. Spontaneous preterm birth
Spontaneous preterm birth was assessed in two good‐quality cohort studies (Figure 2C).28, 46 Odds ratios were significantly higher with shorter intervals in one study (aOR = 1.83 [95% CI 1.65, 2.03] for <6 vs 18‐23 months; aOR = 1.26 [95% CI 1.14, 1.38] for 6‐11 vs 18‐23 months).46
3.3. Small‐for‐gestational age
Small‐for‐gestational age (defined as <10th percentile) was assessed in 11 cohort studies; eight studies used external weight‐for‐gestational age charts to define small‐for‐gestational age, one used an internally derived chart, and in two studies, the choice of charts was not stated (Figures 3 and S1). Among the four good‐quality studies,8, 9, 10, 46 interpregnancy intervals <6, 6‐11 or 12‐17 months were associated with increased risks in one study, although the magnitude of increased risk was small (eg, aOR = 1.13 [95% CI 1.04, 1.23] for <6 months vs 18‐23 months).46
Figure 3.
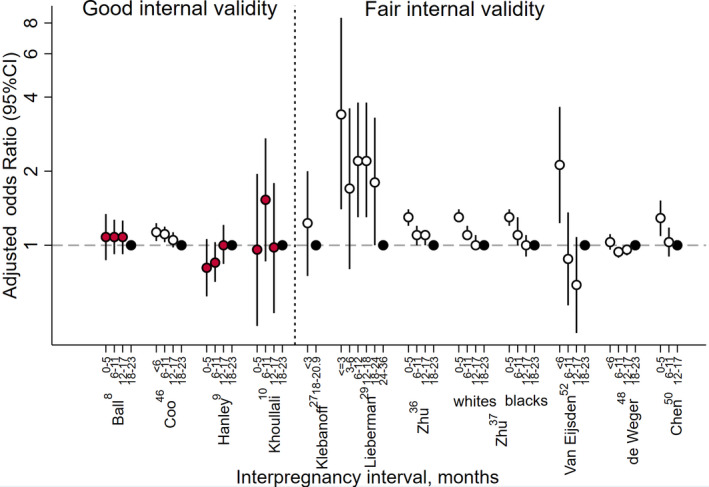
Adjusted odds ratios and 95% confidence intervals for the association between interpregnancy interval and small for gestational age birth among studies from high‐resource settings. Black solid circles indicate the reference category, red solid circles indicate studies using a sibling comparison design, and vertical dashed line separates studies with good internal validity from those with fair internal validity. Confidence intervals are not discernible for some studies because they fell within the range covered by the point estimate symbol (black hollow circle)
Among the seven fair‐quality studies,27, 29, 36, 37, 48, 52, 65 five reported statistically significant aORs for interpregnancy intervals approximately <6 months.29, 36, 37, 52, 65 Of these, all had aORs ≥1.20. One study also reported statistically significant aORs ≥1.20 for interpregnancy intervals 6‐12, 12‐18 and 18‐24 months vs 24‐36 months.29 The remaining aOR estimates were small in magnitude or not statistically significant. As with preterm birth, generally the good‐quality studies reported lower estimates than the fair‐quality studies for interpregnancy intervals <6 months.
3.4. Perinatal death
Perinatal death was assessed in four cohort studies (Figure 4A).34, 35, 50, 53 The two good‐quality studies34, 35 reported increased risks for interpregnancy intervals <6 months (stillbirth ≥28 weeks aOR = 1.3 [95% CI 0.8, 2.1]35 for 0‐3 vs 12‐35 months and “other stillbirth” aOR = 2.3 [95% CI 0.7, 7.2]34 for 1‐5 vs 18‐23 months); however, neither estimate was statistically significant. The two fair‐quality studies50, 53 also did not find significantly increased risks for interpregnancy intervals <6 months (unexplained antepartum stillbirth aOR = 0.88 [95% CI 0.45, 1.70]53 for <6 vs 24‐60 months and perinatal death aOR = 0.90 [95% CI 0.51, 1.59]50 for 0‐5 vs 12‐17 months).
Figure 4.

A, Adjusted odds ratios and 95% confidence intervals for the association between interpregnancy interval and perinatal death among studies from high‐resource settings. Black solid circles indicate the reference category, and vertical dashed line separates studies with good internal validity from those with fair internal validity; B, Adjusted odds ratios and 95% confidence intervals for the association between interpregnancy interval and infant death among studies from high‐resource settings. Black solid circles indicate the reference category, and vertical dashed line separates studies with good internal validity from those with fair internal validity
3.5. Infant mortality
Infant mortality was assessed in four studies (three cohort studies and one case‐control study) (Figure 4B).34, 50, 51, 54 A good‐quality study reported an aOR of 3.6 (95% CI 1.2, 10.7) for neonatal death (death within the first four weeks of life) for interpregnancy intervals <6 vs 18‐23 months.34
In the three fair‐quality studies, interpregnancy intervals of <6 months were consistently associated with significantly increased risks of infant mortality and neonatal mortality,50, 51, 54 with point estimates ranging from aOR = 1.44 (95% CI 1.06, 1.95)51 to aOR = 2.23 (95% CI 1.19, 4.16).50 There were significantly increased risks in one study for interpregnancy intervals of 6‐11 months for infant mortality (aOR = 1.68) and neonatal mortality (aOR = 1.62);54 and in the same study for interpregnancy intervals of 12‐17 months for infant mortality (aOR = 1.48) and neonatal mortality (aOR = 1.49).54
3.6. Other perinatal outcomes
Additional good or fair quality studies examined the following outcomes: alternative preterm birth definitions (n = 6);25, 34, 46, 50, 51, 57 birthweight (n = 2);27, 52 low birthweight (including alternative definitions such as very low birthweight) (n = 13);8, 9, 10, 22, 27, 34, 36, 37, 46, 48, 50, 51, 61 alternative small‐for‐gestational age definitions (n = 1);50 neonatal intensive care unit (n = 3);9, 50, 51 Apgar score (n = 1);50 and congenital anomalies (n = 3).34, 58, 60 Estimates reported from each study are presented in a supplemental table (Table S1). As with the adverse perinatal outcomes already described, the highest risks (mostly aOR<2.0) were found for the shortest interpregnancy intervals, and there was attenuation of estimates after adjustment for confounders. Several studies found statistically significant increased risks for interpregnancy intervals <6 months10, 36, 37, 46, 48, 50, 51 and 6‐11 months37, 46, 50 for low birthweight and for interpregnancy intervals <6 months,25, 34, 46, 51 6‐11 months25, 46 or 12‐17 months25, 46 for alternative preterm birth definitions. No significant associations were found between interpregnancy interval and alternative small‐for‐gestational age definitions (<3rd percentile), neonatal intensive care unit admission, Apgar scores or congenital anomalies.9, 34, 50, 51, 58, 60
4. DISCUSSION
Among births in high‐resource settings, clinically relevant and statistically significant associations between short interpregnancy intervals since last livebirth and perinatal health were supported by some studies, but not all studies. The most consistent evidence of an association was seen for intervals <6 months vs a longer interval (most commonly 18‐23 months) and in studies of preterm birth and infant death, less consistent evidence was found for small‐for‐gestational age, while studies of perinatal death showed no relationship. However, the number of studies, precision of estimates and consistency of results varied (Table 2). Often, lower quality studies reported higher estimates for short interpregnancy intervals compared with higher quality studies, estimates were attenuated after covariate adjustment, and, within each study, estimates were highest for the shortest interpregnancy interval examined. Most studies examined population‐based samples of births, most commonly using U.S. vital records, and many accounted for a similar set of covariates. Generally the highest quality studies, in terms of internal validity, had limited external generalisability.
Table 2.
Summary of evidence
| Outcome | Studies (k) Study designs Observations (n) | Summary of findings | Consistency and precision | Other limitations | Strength of evidence | Applicability |
|---|---|---|---|---|---|---|
| Preterm birth | 14 studies (cohort); N = 2 557 668 | Risk was significantly higher with shorter IPI in 10 studies (aOR ≥ 1.20 for ~<6 mo in 10 studies, but only four of six good‐quality studies; point estimates decreased with increasing IPI). | Inconsistent in good quality studies, precise | Limited adjustment for confounders and validity of US vital statistics‐based data sources. | Moderate | High |
| Spontaneous preterm birth | 2 studies (cohort); N = 176 177 | Risk was significantly higher with shorter IPI in 1 study (aOR = 1.83 for <6 mo; aOR = 1.26 for 6‐11). | Inconsistent, imprecise | Few studies; limited adjustment for confounders and validity of US vital statistics‐based data sources. | Low | Moderate |
| Small‐for‐gestational age | 11 studies (cohort); N = 1 184 143 | Risk was significantly higher with shorter IPI in 5 studies (aOR ≥ 1.20 for <6 mo in five studies, but none were good‐quality studies). | Inconsistent, precise | Limited adjustment for confounders and validity of US vital statistics‐based data sources. | Low | High |
| Perinatal death | 4 studies (cohort); N = 610 829 | There were non‐significant increased risks with shorter IPI in two studies for <6 mo. | Inconsistent, imprecise | Few studies; variation in outcome definition, limited adjustment for confounders and validity of US vital statistics‐based data sources. | Low | Moderate |
| Infant mortality | 4 studies (3 cohort and 1 case‐control); N = 220 676 | Risk was significantly higher with shorter IPI in 4 studies (aOR ≥ 1.20 for <6 mo in 4 studies; 6‐11 mo in 1 study; 12‐17 mo in 1 study). | Consistent, precise | Few studies; variation in outcome definition, limited adjustment for confounders, and validity of US vital statistics‐based data sources. | Moderate | Moderate |
IPI, interpregnancy interval; aOR, adjusted odds ratio.
Our findings are generally consistent with a previously published 2006 review2 upon which our systematic review protocol was based. The previous review also observed an inverse relationship between shorter interpregnancy intervals and adverse perinatal outcomes and an attenuation of estimates after covariate adjustment. The previous review also reported increased adjusted odds of preterm birth and small‐for‐gestational age birth for interpregnancy intervals <6 months, but found significantly greater risks for these adverse outcomes for interpregnancy intervals up to 17 months. The reviews differ by study aims, methods of study inclusion and data synthesis. While the 2006 review used statistical meta‐analysis to determine combined estimates, we opted against producing a single summary measure due to concerns about study quality and heterogeneity. In addition, we tiered our qualitative synthesis of studies based on our assessment of internal validity and considered both magnitude and statistical significance when synthesising the evidence.
While results of our review support associations between an interpregnancy interval <6 months and some adverse perinatal outcomes, our findings provide less support for intervals of 6‐11 months and 12‐17 months, particularly for small‐for‐gestational age, compared with the previous review, and a more recent review of studies from low‐ and middle‐income countries.66 Our conclusions are generally consistent with a recent systematic review and meta‐analysis67 of high‐quality studies from largely high‐ and moderate‐income countries showing that risks were mostly confined to interpregnancy intervals <6 months. Together, this suggests that adverse associations from short interpregnancy intervals for high‐resource settings may be limited to very short interpregnancy intervals (<6 months or possibly 6‐11 months) as opposed to up to 18 or 24 months for lower‐resource settings.
Future research using additional data sources and methods and with more rigorous control for confounding would be valuable for informing the development of recommendations for healthy birth spacing for U.S. women. Although we synthesised evidence only from studies rated as having “Good” or “Fair” internal validity, no study included in our review accounted for all the factors we identified as being important for ruling out major concerns of bias: detailed information on maternal socio‐economic position, pregnancy intention and history of perinatal losses to reduce confounding, and accounting for intervening pregnancy losses in order to reduce exposure misclassification, which could potentially be differential by perinatal outcome. Most (78%) of the studies from the United States used information on interpregnancy interval and covariates from U.S. birth certificates, essentially replicating the same study design in different geographic areas across the country. Now that interpregnancy interval is available on national files from states that have adopted the 2003 revised U.S. birth certificate (all states as of 2016), new analyses of national data can be expected, with presumably similar findings, albeit greater precision.
Further research conducted among populations at high risk of adverse perinatal outcomes is also needed, as most of the studies conducted to date have been among population‐based samples, which can obscure important differences among subgroups. In addition, research is lacking on the effects of interventions aimed at reducing short interpregnancy intervals on subsequent pregnancy outcomes in high‐resource settings, although a recently published intervention study from Bangladesh suggests these types of interventions may lead to decreased risk of preterm birth.68 These types of studies in the U.S. would be useful in targeting health care services.
Limitations of this review include using only English‐language articles and restricting the focus to the more commonly studied adverse perinatal health outcomes. Also, our systematic review protocol may have excluded some potentially germane studies, because of our strict inclusion and exclusion criteria. For example, several studies were excluded because they did not adjust for socio‐economic position and/or maternal age, but did investigate the influence of these factors in exploratory analysis65, 69, 70, 71, 72, 73 or restricted their analysis to certain age groups, thereby controlling for socio‐economic position to some extent.74 We opted against inclusion of such studies in order to maintain consistency with the previous review. We also excluded otherwise eligible studies that did not provide precision estimates for their measures of effect24, 26 and those that modelled interpregnancy interval as a continuous, linear variable.75, 76, 77 In addition, since the publication end date, several relevant studies of interpregnancy intervals and adverse perinatal outcomes from high‐resource settings have been published using data from Missouri, Canada and Denmark,78, 79, 80 including a matched analysis study among births in Sweden81 and a study using linked birth certificate and assisted reproductive technology surveillance data from the United States;82 findings were generally in line with the evidence we present in this review.
This is the first systematic review of interpregnancy interval and adverse perinatal outcomes restricted to studies from high‐resource settings, which enhances the applicability of our findings to women in the United States. We present evidence tiered by assessed internal validity quality, helping to highlight the potential role of bias in our current understanding of the evidence base. Finally, our systematic review is unique, because it includes a number of recently published studies that used maternally linked births to conduct matched sibling comparison analyses, which provide a novel approach to control for confounding by difficult‐to‐measure characteristics, such as socio‐economic position. Inclusion of the findings from these new study designs is critical for enhancing the overall internal validity of the evidence for this topic, though the limited external generalisability of these study cohorts is a concern.
In conclusion, we found that among higher quality studies conducted in high‐resource settings, short interpregnancy intervals (<6 or <12 months) are associated with increased risks for preterm birth, small‐for‐gestational age and infant death, although associations were less consistent in the highest quality studies. It remains unclear whether these associations represent causal effects given the limited study designs and data sources used, inconsistency of findings and comparison groups, similar confounder adjustments and limited generalisability of the highest quality studies. Additional research targeting high‐risk populations and controlling for confounding would further inform recommendations for healthy birth spacing in the United States.
FINANCIAL DISCLOSURE
No financial or other disclosures of conflict of interests were reported by the authors of this study.
DISCLAIMER
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Office of Population Affairs, Office of the Secretary for Health, Department of Health and Human Services.
Supporting information
ACKNOWLEDGEMENT
The authors thank Julia Rollison and Stephen Tregear, from Atlas Research LLC, for conducting the preliminary systematic review and drafting the evidence report. The authors also acknowledge the critical feedback they received during the expert workgroup meeting Birth Spacing and Adverse Pregnancy Outcomes, in Washington, DC, on 14‐15 September 2017.
Ahrens KA, Nelson H, Stidd RL, Moskosky S, Hutcheon JA. Short interpregnancy intervals and adverse perinatal outcomes in high‐resource settings: An updated systematic review. Paediatr Perinat Epidemiol. 2019;33:O25–O47. 10.1111/ppe.12503
Funding information This project was supported, in part, by contracts between the Office of Population Affairs and Atlas Research LLC (# HHSP233201450040A).
REFERENCES
- 1. Conde‐Agudelo A, Rosas‐Bermudez A, Castano F, Norton MH. Effects of birth spacing on maternal, perinatal, infant, and child health: a systematic review of causal mechanisms. Stud Fam Plann. 2012;43:93‐114. [DOI] [PubMed] [Google Scholar]
- 2. Conde‐Agudelo A, Rosas‐Bermudez A, Kafury‐Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta‐analysis. J Am Med Assoc. 2006;295:1809‐1823. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization . Report of a WHO Technical Consultation on Birth Spacing: Geneva, Switzerland, 13–15 June 2005. Department of Reproductive Health and Research (RHR). Geneva, Switzerland: World Health Organization; 2006. http://apps.who.int/iris/bitstream/10665/69855/1/WHO_RHR_07.1_eng.pdf. Accessed March 15, 2018.
- 4. World Health Organization . World Health Assembly, 65. Nutrition of women in the preconception period, during pregnancy and the breastfeeding period. Report by the Secretariat. Geneva, Switzerland: World Health Organization; 2012. http://apps.who.int/gb/ebwha/pdf_files/EB130/B130_11-en.pdf. Accessed March 15, 2018.
- 5. Total Fertility Rate. The World Factbook. Washington, DC: The Central Intelligence Agency, Office of Public Affairs; 2017. https://www.cia.gov/library/publications/the-world-factbook/rankorder/2127rank.html. Accessed March 15, 2018.
- 6. UNICEF . Breastfeeding: A mother's gift, for every child. Nutrition Section, Programme Division, Data and Analytics Section, Division of Data, Research and Policy, and Division of Communication. 3 United Nations Plaza, New York, NY 10017, USA: Nutrition Section, Programme Division, UNICEF; 2018. https://www.unicef.org/publications/files/UNICEF_Breastfeeding_A_Mothers_Gift_for_Every_Child.pdf. Accessed May 10, 2018.
- 7. Martin JA, Hamilton BE, Osterman MJ, Driscoll AK, Mathews TJ. Births: Final Data for 2015. Natl Vital Stat Rep. 2017;66:1. [PubMed] [Google Scholar]
- 8. Ball SJ, Pereira G, Jacoby P, de Klerk N, Stanley FJ. Re‐evaluation of link between interpregnancy interval and adverse birth outcomes: retrospective cohort study matching two intervals per mother. BMJ. 2014;349:g4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hanley GE, Hutcheon JA, Kinniburgh BA, Lee L. Interpregnancy interval and adverse pregnancy outcomes: an analysis of successive pregnancies. Obstet Gynecol. 2017;129:408‐415. [DOI] [PubMed] [Google Scholar]
- 10. Koullali B, Kamphuis EI, Hof MH, et al. The effect of interpregnancy interval on the recurrence rate of spontaneous preterm birth: a retrospective cohort study. Am J Perinatol. 2017;34:174‐182. [DOI] [PubMed] [Google Scholar]
- 11. Shachar B, Mayo J, Lyell D, et al. Interpregnancy interval after live birth or pregnancy termination and estimated risk of preterm birth: a retrospective cohort study. Br J Obstet Gynaecol. 2016;123:2009‐2017. [DOI] [PubMed] [Google Scholar]
- 12. Klebanoff MA. Interpregnancy interval and pregnancy outcomes: causal or not? Obstet Gynecol. 2017;129:405‐407. [DOI] [PubMed] [Google Scholar]
- 13. Hutcheon JA, Nelson HD, Stidd R, Moskosky S, Ahrens KA. Short interpregnancy intervals and adverse maternal outcomes in high resource settings: an updated systematic review. Paediatr Perinat Epidemiol. 2018, In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Institute of Medicine . Clinical Practice Guidelines We Can Trust. The National Academies Press; 2011. www.nap.edu/catalog.php?record_id=13058. [PubMed] [Google Scholar]
- 15. Division of Health Promotion and Disease Prevention, Institute of Medicine . Committee to Study the Prevention of Low Birthweight In: Preventing Low Birthweight. Washington, DC: National Academies Press; (US) Copyright (c) National Academy of Sciences; 1985. [Google Scholar]
- 16. ACOG Committee Opinion No. 736: Optimizing postpartum care. Obstet Gynecol. 2018; 131:e140‐e150. [DOI] [PubMed] [Google Scholar]
- 17. Procedure manual. U.S. Preventive Services Task Force. November 2017. https://www.uspreventiveservicestaskforce.org/Page/Name/procedure-manual. Accessed May 29, 2018.
- 18. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arnold CC, Kramer MS, Hobbs CA, McLean FH, Usher RH. Very low birth weight: a problematic cohort for epidemiologic studies of very small or immature neonates. Am J Epidemiol. 1991;134:604‐613. [DOI] [PubMed] [Google Scholar]
- 20. United Nations Human Development Index, 2015. New York, NY: United Nations; 2015. http://hdr.undp.org/en/composite/HDI. Accessed March 21, 2018.
- 21. Adams MM, Delaney KM, Stupp PW, McCarthy BJ, Rawlings JS. The relationship of interpregnancy interval to infant birthweight and length of gestation among low‐risk women, Georgia. Paediatr Perinat Epidemiol. 1997;11(Suppl. 1):48‐62. [DOI] [PubMed] [Google Scholar]
- 22. Basso O, Olsen J, Knudsen LB, Christensen K. Low birth weight and preterm birth after short interpregnancy intervals. Am J Obstet Gynecol. 1998;178:259‐263. [DOI] [PubMed] [Google Scholar]
- 23. Ekwo EE, Moawad A. The relationship of interpregnancy interval to the risk of preterm births to black and white women. Int J Epidemiol. 1998;27:68‐73. [DOI] [PubMed] [Google Scholar]
- 24. Fedrick J, Adelstein P. Influence of pregnancy spacing on outcome of pregnancy. BMJ. 1973;4:753‐756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fuentes‐Afflick E, Hessol NA. Interpregnancy interval and the risk of premature infants. Obstet Gynecol. 2000;95:383‐390. [DOI] [PubMed] [Google Scholar]
- 26. Kallan JE. Reexamination of interpregnancy intervals and subsequent birth outcomes: evidence from U.S. linked birth/infant death records. Soc Biol. 1997;44:205‐212. [PubMed] [Google Scholar]
- 27. Klebanoff MA. Short interpregnancy interval and the risk of low birthweight. Am J Public Health. 1988;78:667‐670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lang JM, Lieberman E, Ryan KJ, Monson RR. Interpregnancy interval and risk of preterm labor. Am J Epidemiol. 1990;132:304‐309. [DOI] [PubMed] [Google Scholar]
- 29. Lieberman E, Lang JM, Ryan KJ, Monson RR, Schoenbaum SC. The association of inter‐pregnancy interval with small for gestational age births. Obstet Gynecol. 1989;74:1‐5. [PubMed] [Google Scholar]
- 30. Miller JE. Determinants of intrauterine growth retardation: evidence against maternal depletion. J Biosoc Sci. 1989;21:235‐243. [DOI] [PubMed] [Google Scholar]
- 31. Miller JE. Birth intervals and perinatal health: an investigation of three hypotheses. Fam Plann Perspect. 1991;23:62‐70. [PubMed] [Google Scholar]
- 32. Ochoa Sangrador C, Luque Benlloch C, Carrascal Tejado A. [Prematurity, low birth weight and the interval between pregnancies]. Anales Espanoles de Pediatria. 1996;45:67‐70. [PubMed] [Google Scholar]
- 33. Shults RA, Arndt V, Olshan AF, Martin CF, Royce RA. Effects of short interpregnancy intervals on small‐for‐gestational age and preterm births. Epidemiology. 1999;10:250‐254. [PubMed] [Google Scholar]
- 34. Smith GC, Pell JP, Dobbie R. Interpregnancy interval and risk of preterm birth and neonatal death: retrospective cohort study. BMJ. 2003;327:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stephansson O, Dickman PW, Cnattingius S. The influence of interpregnancy interval on the subsequent risk of stillbirth and early neonatal death. Obstet Gynecol. 2003;102:101‐108. [DOI] [PubMed] [Google Scholar]
- 36. Zhu BP, Rolfs RT, Nangle BE, Horan JM. Effect of the interval between pregnancies on perinatal outcomes. N Engl J Med. 1999;340:589‐594. [DOI] [PubMed] [Google Scholar]
- 37. Zhu BP, Haines KM, Le T, McGrath‐Miller K, Boulton ML. Effect of the interval between pregnancies on perinatal outcomes among white and black women. Am J Obstet Gynecol. 2001;185:1403‐1410. [DOI] [PubMed] [Google Scholar]
- 38. Zhu BP, Le T. Effect of interpregnancy interval on infant low birth weight: a retrospective cohort study using the Michigan Maternally Linked Birth Database. Matern Child Health J. 2003;7:169‐178. [DOI] [PubMed] [Google Scholar]
- 39. Kleijer ME, Dekker GA, Heard AR. Risk factors for intrauterine growth restriction in a socio‐economically disadvantaged region. J Matern Fetal Neonatal Med. 2005;18:23‐30. [DOI] [PubMed] [Google Scholar]
- 40. Thompson M, Tiwari A, Fu R, Moe E, Buckley DI. AHRQ methods for effective health care In: A Framework to Facilitate the Use of Systematic Reviews and Meta‐Analyses in the Design of Primary Research Studies. Rockville, MD: Agency for Healthcare Research and Quality (US); 2012. [PubMed] [Google Scholar]
- 41. Nelson H. Systematic Reviews to Answer Health Care Questions. Philadelphia, PA: Lippincott Williams & Wilkins; 2014. [Google Scholar]
- 42. US Preventive Services Task Force . Appendix VI. Criteria for assessing internal validity of individual studies. https://www.uspreventiveservicestaskforce.org/Page/Name/appendix-vi-criteria-for-assessing-internal-validity-of-individual-studies. Accessed May 24, 2018.
- 43. US Preventive Services Task Force . Appendix VII. Criteria for assessing external validity (generalizability) of individual studies. https://www.uspreventiveservicestaskforce.org/Page/Name/appendix-vii-criteria-for-assessing-external-validity-generalizability-of-individual-studies. Accessed May 24, 2018.
- 44. Hutcheon J, Moskosky S, Ananth C, et al. Good practices for the design, analysis, and interpretation of observational studies on birth spacing and perinatal health outcomes. Paediatr Perinat Epidemiol. 2018, In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Guide to Completing the Facility Worksheets for the Certificate of Live Birth and Report of Fetal Death. National Vital Statistics System. http://www.cdc.gov/nchs/data/dvs/GuidetoCompleteFacilityWks.pdf. Published 2016. Accessed June 28, 2016.
- 46. Coo H, Brownell MD, Ruth C, Flavin M, Au W, Day AG. Interpregnancy interval and adverse perinatal outcomes: A record‐linkage study using the Manitoba population research data repository. J Obstet Gynaecol Can. 2017;39:420‐433. [DOI] [PubMed] [Google Scholar]
- 47. Naimi AI, Moodie EE, Auger N, Kaufman JS. Stochastic mediation contrasts in epidemiologic research: interpregnancy interval and the educational disparity in preterm delivery. Am J Epidemiol. 2014;180:436‐445. [DOI] [PubMed] [Google Scholar]
- 48. de Weger FJ, Hukkelhoven CW, Serroyen J, te Velde ER, Smits LJ. Advanced maternal age, short interpregnancy interval, and perinatal outcome. Am J Obstet Gynecol. 2011;204:421.e421‐429. [DOI] [PubMed] [Google Scholar]
- 49. Howard EJ, Harville E, Kissinger P, Xiong X. The association between short interpregnancy interval and preterm birth in Louisiana: a comparison of methods. Matern Child Health J. 2013;17:933‐939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chen I, Jhangri GS, Lacasse M, Kumar M, Chandra S. Relationship between interpregnancy interval and adverse perinatal and neonatal outcomes in Northern Alberta. J Obstet Gynaecol Can. 2015;37:598‐605. [DOI] [PubMed] [Google Scholar]
- 51. Appareddy S, Pryor J, Bailey B. Inter‐pregnancy interval and adverse outcomes: Evidence for an additional risk in health disparate populations. J Matern Fetal Neonatal Med. 2017;30:2640‐2644. [DOI] [PubMed] [Google Scholar]
- 52. van Eijsden M, Smits LJ, van der Wal MF, Bonsel GJ. Association between short interpregnancy intervals and term birth weight: the role of folate depletion. Am J Clin Nutr. 2008;88:147‐153. [DOI] [PubMed] [Google Scholar]
- 53. Smith GC, Shah I, White IR, Pell JP, Dobbie R. Previous preeclampsia, preterm delivery, and delivery of a small for gestational age infant and the risk of unexplained stillbirth in the second pregnancy: a retrospective cohort study, Scotland, 1992‐2001. Am J Epidemiol. 2007;165:194‐202. [DOI] [PubMed] [Google Scholar]
- 54. Hussaini KS, Ritenour D, Coonrod DV. Interpregnancy intervals and the risk for infant mortality: a case control study of Arizona infants 2003‐2007. Matern Child Health J. 2013;17:646‐653. [DOI] [PubMed] [Google Scholar]
- 55. Salihu HM, August EM, Mbah AK, et al. The impact of birth spacing on subsequent feto‐infant outcomes among community enrollees of a federal Healthy Start project. J Community Health. 2012;37:137‐142. [DOI] [PubMed] [Google Scholar]
- 56. Hinkle SN, Albert PS, Mendola P, et al. Differences in risk factors for incident and recurrent small‐for‐gestational‐age birthweight: a hospital‐based cohort study. BJOG. 2014;121:1080‐1088; discussion 1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jelliffe‐Pawlowski LL, Baer RJ, Blumenfeld YJ, et al. Maternal characteristics and mid‐pregnancy serum biomarkers as risk factors for subtypes of preterm birth. BJOG. 2015;122:1484‐1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mburia‐Mwalili A, Yang W. Interpregnancy interval and birth defects. Birth Defects Res A Clin Mol Teratol. 2015;103:904‐912. [DOI] [PubMed] [Google Scholar]
- 59. McKinney D, House M, Chen A, Muglia L, DeFranco E. The influence of interpregnancy interval on infant mortality. Am J Obstet Gynecol. 2017;216:316.e311‐316.e319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Villamor E, Sparen P, Cnattingius S. Risk of oral clefts in relation to prepregnancy weight change and interpregnancy interval. Am J Epidemiol. 2008;167:1305‐1311. [DOI] [PubMed] [Google Scholar]
- 61. Merklinger‐Gruchala A, Jasienska G, Kapiszewska M. Short interpregnancy interval and low birth weight: a role of parity. Am J Hum Biol. 2015;27:660‐666. [DOI] [PubMed] [Google Scholar]
- 62. Goyal NK, Folger AT, Hall ES, Greenberg JM, Van Ginkel JB, Ammerman RT. Home visiting for first‐time mothers and subsequent pregnancy spacing. J Perinatol. 2017;37:144‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Joseph KS, Liu S, Rouleau J, et al. Influence of definition based versus pragmatic birth registration on international comparisons of perinatal and infant mortality: population based retrospective study. BMJ (Clinical Research ed). 2012;344:e746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ananth CV, Schisterman EF. Confounding, causality, and confusion: the role of intermediate variables in interpreting observational studies in obstetrics. Am J Obstet Gynecol. 2017;217:167‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Smits LJ, Elzenga HM, Gemke RJ, Hornstra G, van Eijsden M. The association between interpregnancy interval and birth weight: what is the role of maternal polyunsaturated fatty acid status? BMC Pregnancy Childbirth. 2013;13:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kozuki N, Lee AC, Silveira MF, et al. The associations of birth intervals with small‐for‐gestational‐age, preterm, and neonatal and infant mortality: a meta‐analysis. BMC Public Health. 2013;13(Suppl. 3):S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wendt A, Gibbs CM, Peters S, Hogue CJ. Impact of increasing inter‐pregnancy interval on maternal and infant health. Paediatr Perinat Epidemiol. 2012;26:239‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Baqui AH, Ahmed S, Begum N, et al. Impact of integrating a postpartum family planning program into a community‐based maternal and newborn health program on birth spacing and preterm birth in rural Bangladesh. J Glob Health. 2018;8:020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Atreya MR, Muglia LJ, Greenberg JM, DeFranco EA. Racial differences in the influence of interpregnancy interval on fetal growth. Matern Child Health J. 2017;21:562‐570. [DOI] [PubMed] [Google Scholar]
- 70. Chen I, Jhangri GS, Chandra S. Relationship between interpregnancy interval and congenital anomalies. Am J Obstet Gynecol. 2014;210:564.e561‐568. [DOI] [PubMed] [Google Scholar]
- 71. DeFranco EA, Seske LM, Greenberg JM, Muglia LJ. Influence of interpregnancy interval on neonatal morbidity. Am J Obstet Gynecol. 2015;212:386.e381‐389. [DOI] [PubMed] [Google Scholar]
- 72. Getz KD, Anderka MT, Werler MM, Case AP. Short interpregnancy interval and gastroschisis risk in the National Birth Defects Prevention Study. Birth Defects Res A Clin Mol Teratol. 2012;94:714‐720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kwon S, Lazo‐Escalante M, Villaran MV, Li CI. Relationship between interpregnancy interval and birth defects in Washington State. J Perinatol. 2012;32:45‐50. [DOI] [PubMed] [Google Scholar]
- 74. Nerlander LM, Callaghan WM, Smith RA, Barfield WD. Short interpregnancy interval associated with preterm birth in U S adolescents. Matern Child Health J. 2015;19:850‐858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Guardino CM, Schetter CD, Saxbe DE, Adam EK, Ramey SL, Shalowitz MU. Diurnal salivary cortisol patterns prior to pregnancy predict infant birth weight. Health Psychol. 2016;35:625‐633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Manzanares S, Maroto‐Martin MT, Naveiro M, Sanchez‐Gila M, Lopez‐Criado S, Puertas A. Risk of recurrence of small‐for‐gestational‐age foetus after first pregnancy. J Obstet Gynaecol. 2017;37:723‐726. [DOI] [PubMed] [Google Scholar]
- 77. Subramaniam A, Wetta LL, Owen J. Relationship between interpregnancy interval and cervical length in high‐risk women. J Matern Fetal Neonatal Med. 2016;29:1205‐1208. [DOI] [PubMed] [Google Scholar]
- 78. Shree R, Caughey AB, Chandrasekaran S. Short interpregnancy interval increases the risk of preterm premature rupture of membranes and early delivery. J Matern Fetal Neonatal Med. 2018;31:3014‐3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Coo H, Brownell MD, Ruth C, Flavin M, Au W, Day AG. Interpregnancy interval and congenital anomalies: a record‐linkage study using the manitoba population research data repository. J Obstet Gynaecol Can. 2017;39:996‐1007. [DOI] [PubMed] [Google Scholar]
- 80. Hegelund ER, Urhoj SK, Andersen AN, Mortensen LH. Interpregnancy interval and risk of adverse pregnancy outcomes: a register‐based study of 328,577 pregnancies in Denmark 1994‐2010. Matern Child Health J. 2018. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 81. Class QA, Rickert ME, Oberg AS, et al. Within‐family analysis of interpregnancy interval and adverse birth outcomes. Obstet Gynecol. 2017;130:1304‐1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Palmsten K, Homer MV, Zhang Y, et al. In vitro fertilization, interpregnancy interval, and risk of adverse perinatal outcomes. Fertil Steril. 2018;109(840–848):e841. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


