Abstract
Chronic non‐healing wounds are a burden in the Long‐Term Care (LTC) sector, increasing costs, morbidity, and mortality and causing pain and suffering. The objective of this LTC Innovation pilot was to test the value of a promising new neuromuscular stimulation device in elevating the experience and satisfaction of the residents, engaging and empowering the nursing staff, and improving healing and/or reducing costs. Small, wireless, and worn at the knee, this muscle pump activator is self‐contained, wearable, and battery‐powered to increase lower‐leg blood circulation (up to 60% of that achieved by walking). It has no wires, weighs just 10 g, and is easy to use. Nurses in four LTC homes identified residents with non‐healing lower leg wounds. Consent was obtained, and on‐site training was delivered. Eleven residents were recruited. Only seven met the inclusion criteria for venous/mixed or diabetic foot ulcers. Of the seven who met the criteria and were adherent with best practices and the muscle pump activator, four healed 100%, and one healed 90%. Two patients with other aetiologies, who were also adherent, healed. All adherent residents had an average weekly decrease in wound size of 9.75% and were extremely happy with the results. Three residents who were non‐adherent had a 9.25% increase in wound size per week. One patient with diabetic foot ulcers developed skin changes at the end of life and passed away. Nursing staff and cognisant residents can easily adjust the pulse of muscle pump activator, and application and removal are simple. Most residents feel engaged with the therapy “because they feel it working”. The LTC corporation feels that it is a great adjunctive solution for many types of lower‐leg wounds (venous, mixed, diabetic, pressure) in addition to best practices in the LTC and Retirement home sectors.
Keywords: blood flow, geko, long‐term care, muscle pump activator, wounds
1. INTRODUCTION
In an aged population already living with multiple morbidities and polypharmacy, chronic non‐healing wounds are a burden in the Long‐Term Care (LTC) sector.1 In addition to other age‐related diseases such as osteoporosis, metabolic disorders, and dementia, impaired wound healing in the elderly results in significant disability, morbidity, and mortality, causing pain and suffering and a poor quality of life.1, 2 Chronic wounds are at risk of infection or deterioration, requiring hospitalisation or surgical intervention or leading to death. In two studies, 27% to 70% of individuals in LTC with a chronic wound died within 6 months of initial care.2, 3 The Canadian Institute for Health Information's (CIHI) last report on compromised wounds in Canada was for 2011 to 2012, where 10% of LTC residents were identified as having compromised wounds (n = 13 298).4 Some authors consider this an under‐estimate of the true numbers.5, 6 A summary of the types of wounds and prevalence is shown in Table 1.4
Table 1.
Type of compromised wounds in long‐term care in Canada
| Type of compromised wound | Long‐term care |
|---|---|
| Venous and arterial leg ulcer | 2033 (1.5%) |
| Pressure ulcers (injuries) | 9338 (6.7%) |
| Any chronic wounds | 10 922 (7.9%) |
| Skin barrier breaches | 1270 (0.9%) |
| Iatrogenic wounds | 1818 (1.3%) |
| Any compromised wound | 13 298 (9.6%) |
| Health care setting total | 138 994 individual residents |
Adapted from Canadian Institute for Health Information (CIHI) compromised wounds in four health settings (2011‐2012), used with permission.4
The length of time to heal wounds in geriatric residents in LTC is not well documented. A study in 2007 in the USA identified that, at 6 months, 66% of wounds, including pressure ulcers, venous, mixed aetiology, ischaemic, or neuropathic ulcers, were not healed.7 Residents with chronic wounds, such as venous, arterial, and diabetic ulcers, often have impaired blood flow in the lower legs. As many as 60% of people over the age of 80 have gait disturbances,8 including a shuffling walk because of pain, limited ankle mobility, or neurological disorders. If they do not have a full flexion/dorsiflexion action when walking, it reduces their calf muscle pump function, increasing venous stasis and venous hypertension, and negatively impacts the severity of venous ulcerations. Residents in LTC who are still able to walk may spend much of their waking hours either lying or sitting, further limiting the benefit of exercise in preventing or healing leg ulcers. In addition to the negative impact on the resident's quality of life, morbidity, and mortality, chronic wounds also increase overall health care costs.9 These increased costs of managing chronic wounds in LTC include nursing time for care of the wound, cost of dressings and supplies, and increased laundry costs because of uncontained wound exudate. Recent Canadian estimates for cost of wound care in LTC include nursing care required for wounds consuming 25% to 50% of total nursing time, while dressing supplies and equipment account for 11% to 21% of total costs in LTC, depending on the severity of the wound.10 In a 2014 multi‐method study on LTC in Ontario, Canada, using telemedicine, Advanced Practice Nurses, and a multidisciplinary team to care for residents with pressure injuries (ulcers), the interventions resulted in a reduced average cost of $650.00 per resident but did NOT show a significant improvement in the rate of healing.11 It is desirable on many levels to shorten the duration of these chronic wounds if they develop in spite of preventative measures.
A muscle pump‐activating (MPA) medical device (geko device FirstKind, High Wycombe, UK) is a wrist watch‐sized, wearable device that weighs 10 g and is self‐contained, wireless, and internally powered. It is applied externally to the leg at the fibular head (Figure 1).
Figure 1.
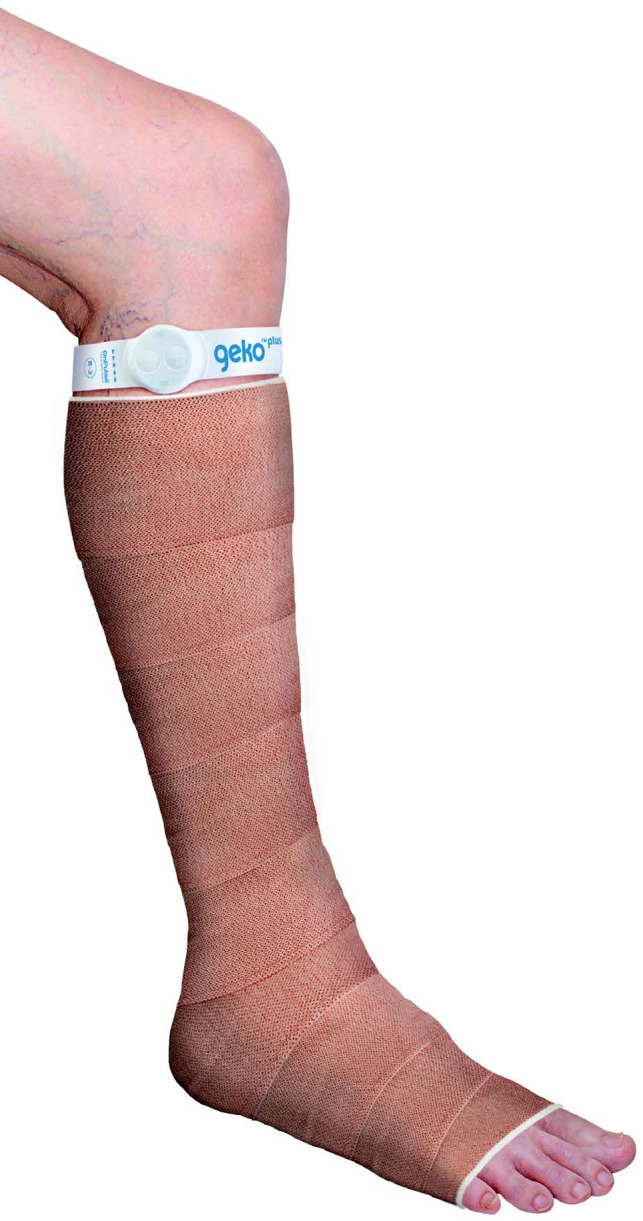
The MPA device applied at the fibular head
Firing once per second, it stimulates the common peroneal nerve proximal to the posterior/anterior bifurcation, causing simultaneous activation and isometric contraction of the tibialis, peroneus longus and medial and lateral gastrocnemius muscles,12, 13 peroneus brevis muscle groups, extensor hallucis longus, extensor digitorum longus, peroneus tertius, and extensor digitorum brevis muscle groups. This response acts as a calf muscle pump while compressing the venous valve system in the legs.12, 13
Initially developed to prevent deep vein thrombosis (DVT) during long‐haul flights, it has many positive effects on venous, arterial, and microcirculation in the legs, even in individuals with peripheral vascular disease (PVD), which affects 5% of residents in LTC facilities in Ontario.14 Also of importance in LTC, where 11% of residents have congestive heart failure and 12% have atherosclerotic heart disease,14 use of the MPA device increases the coronary blood flow in unstenosed vessels,15 without increasing either heart rate12 or affecting systemic blood pressure.12, 16
In four Canadian evaluations to determine the effect of the MPA device as an adjunctive therapy to usual best practices with non‐healing venous leg ulcers (VLUs),17, 18 24 patients had a combined 140+‐year history of wounds. Seventeen patients who were adherent to best practice treatment and use of the MPA device had a reduction of surface area (SA) of 8.3%/week, which would be considered a “Normal” healing trajectory over 4 weeks in newly admitted patients.19 In two evaluations, where the length of stay and initial wound measurements were available, the healing rate prior to the MPA device implementation was a 0.06% reduction in SA per week, compared with a 9.35% reduction per week with the MPA device (P < 0.01).18
At the same time, Revera, an LTC provider in Canada committed to helping seniors' live life to the fullest, created an Innovators in Aging programme20 as a platform to invest in and scale innovations to improve the aging experience. By establishing pilot programmes in one or more homes, they could determine the role a product would have in:
Elevating the experience and satisfaction of their residents,
Engaging and empowering their staff,
Improving efficiency or reducing costs, and
Healing chronic, intractable wounds.
Following the pilot, the company determines if it wants to incorporate the product throughout its LTC wound programmes. In preparation for the programme launch, in late 2015, they selected the MPA device to evaluate the effectiveness on leg and foot ulcers in their LTC population. This pilot evaluation of the MPA device in LTC provided the basis for this observational case series paper.
2. MATERIALS AND METHODS
The MPA R‐2 devices were provided in kind by Perfuse Medtec Inc. (London, Ontario, Canada) as an adjunct to the resident's usual standard‐of‐care wound treatment. The devices were used 5 days per week for 6 hours each day on both legs. On weekends, all residents continued with standard of care but did not receive MPA therapy. The wound care nurses in each home were trained to use the MPA device and used a train‐the‐trainer model for additional nurses to support them.
2.1. Participants and procedures
Five pilot sites were selected by Revera. The target was to be a minimum of 3 residents per site to a maximum of 25. It was to include ALL residents at pilot sites with diabetic foot ulcers (DFUs), VLUs and/or mixed VLUs/arterial leg ulcers (ALUs).
In addition, participating residents had to:
Be 19 years of age or over
Have a lower leg assessment performed, including ABPI if able and/or vascular lab studies (APBI of ≥0.6)
Have a history of adherence to prescribed plan of care
Be able to provide consent or have their substitute decision maker (SDM) provide consent to participate
Have received best practice for at least 30 days
Have experienced ulcer reduction <30% following best practice for last 30 days.
For residents with DFUs, additional criteria included:
Plantar or heel DFUs, which had been off‐loaded with appropriate, effective offloading devices (ie, not orthotics in shoes)
Requirements that the ulcer may have started as a result of trauma or pressure or resident may have neuropathy or arterial ischaemic disease, or a combination
Residents with VLUs or mixed VLUs/ALUs may also have had one or more of the following:
The ability to tolerate compression (the MPA device is an adjunct to compression therapy)
A fixed ankle joint or other indicator that caused delayed healing.
Ethics review was obtained from the Regional Centre for Excellence in Ethics, Homewood Health Centre, 150 Delhi Street, Guelph, Ontario, N1E 6K9 (REB# 17‐10). Residents or their SDM signed an informed consent form to participate in the pilot. Figure 2 shows the documentation responsibilities and schedule for site visits planned for the pilot. Wherever possible, the LTC used its own documentation practices to collect data. Electronic tablets were provided by Perfuse Medtec Inc. to allow for consistent photography of the wounds, and with resident consent, data and photography were shared.
Figure 2.
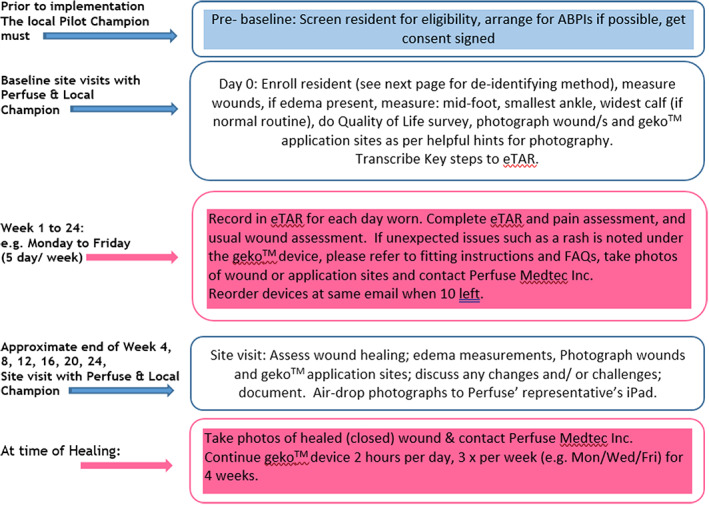
Documentation responsibilities and site visit schedules
2.2. Statistical analysis
The total number of wounds present was to be compared with the number of wounds healed or changes in SA at the end of the pilot. Box 1 shows the method used to calculate the percentage change in ulcer SA over time.21 The weekly ulcer healing rate would be calculated by dividing these data by the number of weeks between the initial ulcer at the time of admission to the point of MPA implementation (baseline) and from then with the MPA device until complete reepithelialisation (wound closure), patient discharge, or at the last point of pilot. Ulcers not healed at the final assessment were determined to be improving or deteriorating. A comparison t‐test was to be used to compare the difference in healing rate per week between the two stages of the study before and after the MPA device was applied. As each resident's wound was to be self‐controlled (pair‐wise comparison between before MPA and with MPA) to show effect, only those with the initial wound measurements could be analysed.
BOX 1. Percentage change in surface area from initial to current.
*SA = Surface area calculated as longest length × perpendicular widest width.
3. RESULTS
The number of residents with eligible wounds was lower than expected. One home had no residents with these types of wounds, so it was not included in the evaluation. Eleven residents with a minimum of one ulcer each in four LTC homes in Ontario and Manitoba started the pilot using the MPA device in 2016. All of these residents had multiple comorbidities, which included amputation of lower leg because of gangrene, anaemia, chronic cellulitis of lower leg, cerebral vascular accident, dementia, type 2 diabetes, dyslipidaemia, hypertension, osteomyelitis shoulder, pruritus, PVD, fracture with open reduction internal fixation of femur, crush injury forefoot, bilateral foot and ankle deformities, fixed ankles, atrial fibrillation, congestive heart failure, vascular dementia, ethyl alcohol abuse, gout, ischaemic heart disease, paranoid delusions, remote embolectomy, recurrent VLUs, bipolar affective disorder, chronic renal failure with haemodialysis, coronary bypass grafts, oesophagitis, depression, hypothyroidism, bladder cancer and rectum, lymphoedema, hyponatremia, and obesity.
At the baseline visit, it was discovered that four of the residents who had already consented to participate had types of lower‐leg wounds that were not included in the eligibility criteria. As the residents, families, and nurses were eager to try the MPA device, a decision was made to expand the criteria to include them. All wounds were considered to be non‐healing, with a combined duration of 13.7 years and a mean of 1.2 years per resident. See Table 2 for wound aetiologies and comments.
Table 2.
Numbers of residents per wound aetiology
| Numbers of residents | Aetiology of wounds |
|---|---|
| 3 | Diabetic foot ulcers; one resident also had abrasions on one shin |
| 4 | VLUs; two had atypical appearance |
| 2 | Pressure ulcers on heels |
| 1 | Non‐healing surgical incision below‐knee amputation of 3 months’ duration; infected with ++ pain |
| 1 | Initially called a DFU; rapidly developed multiple areas of breakdown thought to be skin changes at end of life |
DFU, diabetic foot ulcers; VLU, venous leg ulcers.
Two residents were quickly excluded from the study, and their wounds were not included in the data analysis, one because of pending and above‐knee amputation and the second because of his or her palliative condition.
One resident with areas of multiple wounds did not have his or her wounds measured at baseline, although photographs were taken to document wound appearance. With the MPA added and oral antibiotic treatment for infection, the nurses estimated a 90% decrease in size over 27 weeks, with most lesions closed at that time. A second resident with dementia, hypertension, and coronary artery disease, for whom the initial wound measurements were not available, had two unstageable pressure injuries on one heel and foot of 2 months duration. These healed over 17 weeks with pressure redistribution devices in place. Neither resident could be included in the data analysis.
Of the remaining seven residents, the initial wound measurements and date of occurrence were available for 10 wounds with a pre‐MPA weekly change in SA (SA = Length × width = cm2) of −7.35%, an increase in size. With the addition of the MPA device to current wound care treatments, there was a statistically significant 3.2% weekly decrease in SA (P = 0.004) (Figure 3), whether or not the resident was adherent to best practices such as compression therapy or offloading DFUs. One of these residents also had multiple abrasions on one leg that healed in 8 weeks but had not been measured and so were not included in the data analysis.
Figure 3.
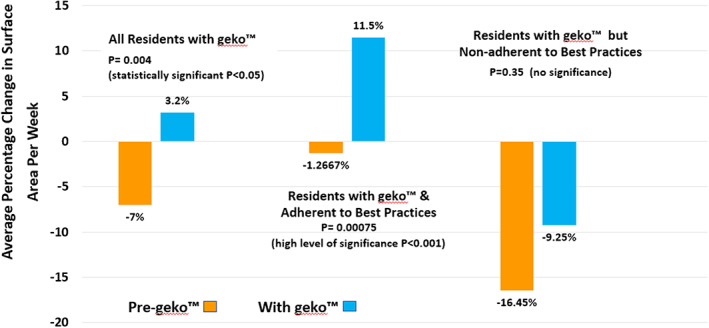
Healing rate of all measured wounds pre‐MPA versus with MPA
Furthermore, the four residents with six wounds who were adherent to available best practices and use of the MPA device showed an even higher significance of 11.5% weekly average decrease in SA, compared with their pre‐MPA weekly average increase in size of −1.26% (P = 0.00075) (Figure 3). These healed with an average time to healing of 10 weeks (range of 8‐17 weeks) (Figure 4).
Figure 4.
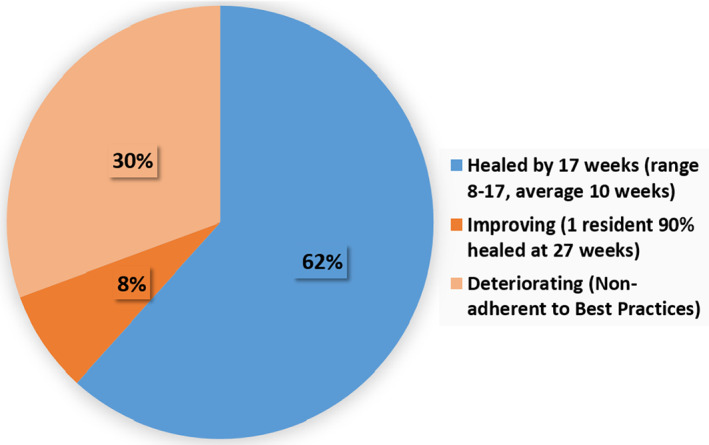
Ulcer healing status with MPA through 27 weeks (all residents)
This contrasts sharply with the three residents with four measured wounds who were non‐adherent with best practices with an average −9.25% (increase) in wound size per week over 27 weeks. Two residents declined any compression therapy, and the third continued to weight bear on his plantar surface DFU when transferring or self‐propelling his wheelchair and, as a result, wore holes through two different foam pressure redistribution devices. The deterioration was less than their pre‐MPA average weekly increase of −16.45% but was not statistically significant (P = 0.35) (Figure 3).
The following case study highlights a resident with unexpectedly positive results. A 92‐year‐old female resident with atrial fibrillation, type 2 diabetes mellitus, benign hypertension, arthritis, glaucoma, and dementia developed bilateral heel pressure injuries 4.5 months prior to the MPA pilot. Although there were no initial measurements available, the wound nurse stated that there had been no change in size since consent. Her SDM for health care signed the consent form to participate. Wound measurements at baseline for the pilot were: right heel 0.9 × 0.6 cm and left heel 2.1 × 1.7 cm. The wound bases were dry; the left contained dried exudate and debris (Figure 5).
Figure 5.
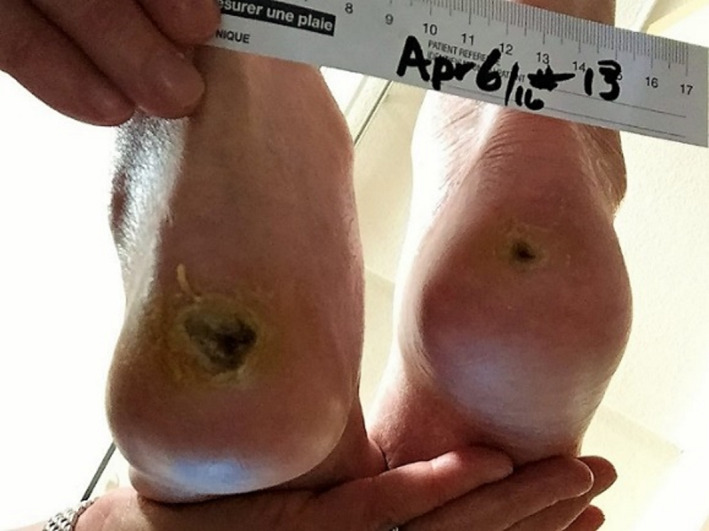
Baseline MPA pilot wound appearance
The current treatment was to clean with a colloidal iodine solution twice weekly, leaving the wounds open to air and using an off‐loading heel boot. The resident was to be turned and repositioned every 2 hours. She sat in a geriatric chair during the day, with the heel boots in place. It was determined that the wounds would benefit from conservative sharp wound debridement, and this was performed following the baseline visit. At 1 month, the left heel wound continued to have thick hyperkeratotic skin surrounding it and a dry base, while the right heel wound was almost closed (Figure 6).
Figure 6.
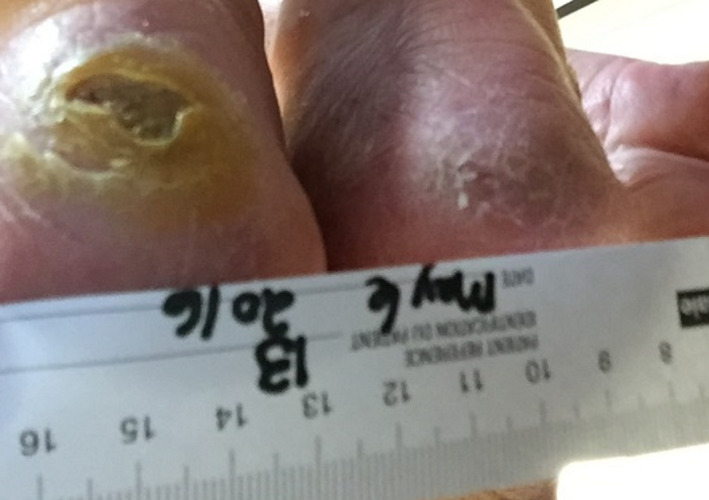
Wound appearance at 1 month
Because of the resident's dementia, the nurses thought that she might not keep the MPA device in place, but she did and healed both pressure injuries quite quickly: the right was closed by 8 weeks, the left in 12 weeks (Figure 7), and the MPA devices were discontinued.
Figure 7.
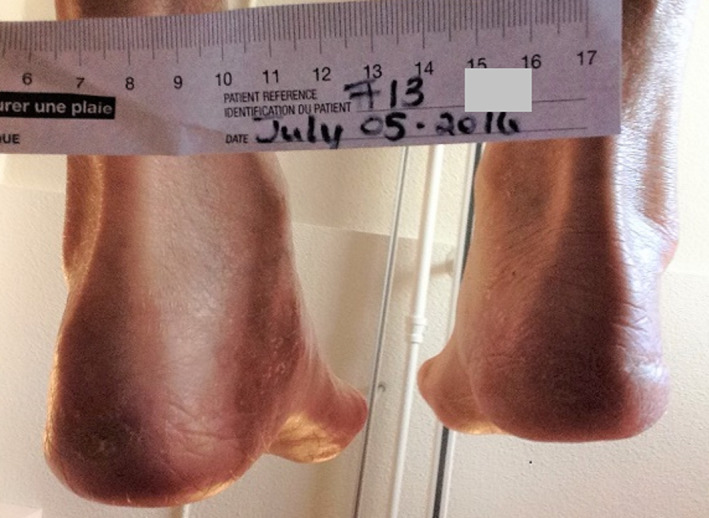
Both wounds resolved in 8 to 12 weeks
The SDM was happy that the resident was chosen for the pilot. In a follow‐up message sent 16 months after healing, the nurse let us know that this resident had recently passed away and that the MPA device had contributed to her last year of life being free of pain and suffering from the pressure injuries. There was no recurrence of the wounds.
3.1. The residents' experience
In preparing the residents and their families/SDMs for the pilot, the MPA device was described as a tapping sensation with possible movement of the foot and toes because of the motor response. Awareness of the sensation caused by the device should diminish over a short period of time. The nurses documented any feedback that they received from the resident or their families/SDMs, shown in Box 2.
BOX 2. Resident and family on the MPA pilot.
One resident whose wounds closed was extremely thrilled with the progress of the healing process and, on numerous occasions, sought the nurse out to say how much more enjoyable life was without the discomfort and knowledge of the wound.
The son of another resident who healed was very pleased with the outcome of the pilot. On several occasions, the resident’s son was present during the application of the device. During a care conference, he was noted as “singing his praises re: the device” and how it had helped his mother.
Another resident was comfortable with the initial application of the device and was quite happy throughout the process. He observed “marked improvement” in his wound healing and stated “I know it’s the machine.”
One resident commented that he liked how the device felt on his feet. His son was very receptive to the pilot, and the resident was glad to be healed and no longer needed to have dressings performed.
Negative feedback came from one resident who found the “strong beat” annoying and did not like to have the devices showing below her skirts.
4.
3.2. The nurse's perspective
Six nurses participated in a post‐pilot online survey consisting of questions created by Revera about the MPA device, the process, and the support received. While the pilot process took extra time for documentation and photographs, it does not appear to have negatively influenced their experience with the device. The responses are outlined in Table 3.
Table 3.
Online survey for nurses (number of responses in brackets)
| Question | Strongly disagree | Disagree | Neutral | Agree | Strongly agree |
|---|---|---|---|---|---|
| I believe geko is a great adjunct treatment for management of wounds | 50% (3) | 50% (3) | |||
| I believe geko significantly enhanced clinical outcomes for the residents that use it | 16.67% (1) | 16.67% (1) | 66.6% (4) | ||
| I believe geko elevated the experience and quality of life for our residents | 33.3% (2) | 16.6% (1) | 50% (3) | ||
| geko was easy to use and helped me do my job better | 33.3% (2) | 66.6% (4) | |||
| The amount of training and support provided by the program was adequate | 16.6% (1) | 16.6% (1) | 66.6% (4) | ||
| Our staff thought that geko was a big hit!!! | 33.3% (2) | 33.3% (2) | 33.3% (2) | ||
| The residents/families thought that this pilot was a big hit!!! | 16.6% (1) | 33.3% (2) | 50% (3) | ||
| The staff at my home were excited and engaged about the pilot | 66.6% (4) | 33.3% (2) | |||
| I feel that I got adequate amount of support from my executive Director to champion this product | 33.3% (2) | 66.6% (4) | |||
| I feel that I got adequate amount of support from my Regional director to champion this product | 33.3% (2) | 66.6% (4) | |||
| I feel that I got adequate amount of support from my team at the home to champion this product | 50% (3) | 50% (3) | |||
| I feel that I got adequate amount of support from the Revera innovation support team to champion this product | 66.6% (4) | 33.3% (2) | |||
| Overall, I recommend the using geko across Revera communities (where appropriate) | 33.3% (2) | 66.6% (4) | |||
| I have a good understanding of the Revera innovation challenge | 83.3% (5) | 16.6% (1) | |||
| I have a good understanding of Revera's innovators in Aging program www.reveraliving.com/innovation | 16.6% (1) | 83.3% (5) | |||
| I like that Revera is focusing on innovation to enhance the experience of its residents and staff | 33.3% (2) | 66.6% (4) |
Other comments from the nurses included the following: “Overall, this pilot project was a great success and I have been very vocal about it. Thank you for allowing us to participate with you in this endeavour.” Nurses found the MPA devices easy to apply and use, and monitoring the use of the device for any complications or issues was not overly time consuming.
4. DISCUSSION
This was a real‐world situation, where wound measurements are not always taken or documented on the initial assessment. This meant that two residents were not part of the data analysis, and it so happened that these two took longer to heal than the group of four who healed and did have all of the required measurements. Statistically, there is no problem with small numbers if the effect size is sufficient to show significance. In this case, with very small P values (e.g. P = 0.00075), there is a near‐zero chance that the differences we observe are because of random chance – we are 99.925% sure the effect is real. Larger numbers with the same P value would (by definition) not increase our level of confidence in the outcome. It may be, of course, that larger N (with the same effect size) would have given us an even smaller P value. However, it is the P value, and not N, that gives us confidence in the outcome. As the data is paired by patient, the groups are therefore perfectly matched so that there is no concern about non‐homogeneity between groups, sometimes encountered with small numbers.
Another concern with small numbers is that they do not allow the data to be tested for normality on the supposition that normality is a prerequisite for the validity of the t‐test. The t‐test does not require normality in the sampled data, or indeed normality of the population from which the data is sampled, but rather the normality of the distribution of sample means drawn from that population. This is supported by the Central Limit Theorem, even where the population is non‐normal; therefore, no normality test is required.
Achieving this highly statistically significant healing response in residents who were adherent with available best practices, such as compression therapy and pressure redistribution, and use of the MPA device was a very positive experience for staff and residents alike. Although it was not within the scope of the project to factor in the residents' ages, comorbidities, mobility, or nutritional status, all factors that can negatively impact healing, it is noted that only one resident had <5 comorbidities, and three had more than 10 each. All of the wounds were considered to be non‐healing and had not responded to previous local wound treatments, which were continued as “standard of care” during the pilot. As a result of this pilot, Revera, the LTC organisation, made a commitment to use the MPA device in its homes and has incorporated criteria for its use into their wound care algorithms.
The experience of implementing the MPA device for wound care proved to have a much broader impact than just evaluating another product. The Ankle Brachial Pressure Index (ABPI), an indicator of peripheral arterial circulation status, was not available for these residents, although it was intended as part of the eligibility criteria. It became evident that this test was difficult to obtain in this setting without investment in the technology and training of staff or paying for an Enterostomal Therapy Nurse to come in to perform the ABPI. As a result, the nurses became much more aware of the need for comprehensive lower‐leg wound assessments, including the need to capture and perform an ABPI. In future, this will allow them to aid in assessing the individual resident's ability to heal and to wear compression bandaging if indicated. In addition, an internal review has recognised the need for off‐loading devices to reduce pressure for residents with leg/foot wounds. As such, changes to the initial wound assessment has been made for residents with lower‐leg and foot wounds so that off‐loading interventions can be implemented early on.
Another unexpected result of this project was that the Ontario Long Term Care Association chose the geko device as the Best New Long‐Term Care Product of Service of the Year in 2016.22
4.1. Limitations
This is an observational case study series based on an Innovative Pilot for new innovations in care. It is pragmatic in that the wound care received in conjunction with the MPA device is not proscribed by a specific protocol, and the application of best practices depended on the knowledge, ability, and resources of the individual home and staff, and the resident's willingness to comply. The numbers of residents, wounds, and nurses participating are quite small, yet the statistical significance is extremely high. Further evaluations with the MPA device are planned in this complex population to determine if these results are reproducible.
5. CONCLUSIONS
Revera's Innovators in Aging programme continues to review submissions on a quarterly basis, and successful applicants are invited to partner with Revera as an Innovators in Aging portfolio company, building on the positive experience with the MPA device and eight other Innovation partnerships https://www.reveraliving.com/about-revera/innovation-at-revera. This population of residents in LTC living with non‐healing wounds have advanced age and multiple comorbidities, limited mobility, and other factors that create barriers to healing. The MPA device may not be required for all wounds encountered in the LTC sector, but if utilised when wounds are first identified as not healing at the expected rate, in spite of best practices (eg, less than 30% reduction in SA at 30 days), it could prevent chronic, non‐healing wounds and the associated burdens to quality of life, morbidity, and mortality, and subsequently reduce costs.
CONFLICTS OF INTEREST
Connie Harris is a Clinical, Education, and Research Consultant; Dorace Ramage is a Clinical and Education Consultant with Perfuse Medtec Inc. Both worked in this capacity for this pilot. Subsequent to the pilot, Revera has made a financial investment in Sky Medical, the developer of the geko device.
ACKNOWLEDGEMENTS
The geko devices were provided in kind for the duration of this pilot by Perfuse Medtec Inc.
Harris C, Ramage D, Boloorchi A, Vaughan L, Kuilder G, Rakas S. Using a muscle pump activator device to stimulate healing for non‐healing lower leg wounds in long‐term care residents. Int Wound J. 2019;16:266–274. 10.1111/iwj.13027
REFERENCES
- 1. Makrantonaki E, Wlaschek M, Scharffetter‐Kochanek K. Pathogenesis of wound healing disorders in the elderly. J Dtsch Dermatol Ges. March 2017;15(3):255‐275. [DOI] [PubMed] [Google Scholar]
- 2. Takahashi PY, Cha SS, Kiemele LJ. Six‐month mortality risks in long term care residents with chronic ulcers. Int Wound J. December 2008;5(5):625‐631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brown G. Long‐term outcomes of full‐thickness pressure ulcers: healing and mortality. Ostomy Wound Manage. 2003;49:42‐50. Available at. http://www.o-wm.com/content/long-term-outcomes-full-thickness-pressure-ulcers-healing-and-mortality Accessed April 17, 2017. [PubMed] [Google Scholar]
- 4. Canadian Institute for Health Information (CIHI) . Compromised wounds in canada executive summary. https://secure.cihi.ca/free_products/AiB_Compromised_Wounds_EN.pdf. Published August 2013. Accessed April 11, 2018.
- 5. Stacey M, Waters N, Houghton P. How many wounds? Wound Care Canada. 2017;15(1):38‐39. [Google Scholar]
- 6. Comtois‐Spitman CM, Knight M, Pitcher ND, Truax A‐E M. Poster: Prevalence of chronic wounds in Canada. Wounds Canada Meeting 2017. https://www.woundscanada.ca/docman/public/leaders-and-change-makers/posters-2017/1108-prevalence-of-chronic-wounds-in-canada-compared-to-other/file. Accessed April 11, 2018.
- 7. Takahashi PY, Kiemele LJ, Chandra A, Cha SS, Targonski PV. A retrospective cohort study of factors that affect healing in long‐term care residents with chronic wounds. Ostomy Wound Manage. January 2009;55(1):32‐37. [PubMed] [Google Scholar]
- 8. Salzman B. Gait and balance disorders in older adults. Am Fam Physician. 2010;82(1):61‐68. [PubMed] [Google Scholar]
- 9. Spilsbury K, Nelson A, Cullum N, Iglesias C, Nixon J, Mason S. Pressure ulcers and their treatment and effects on quality of life: hospital inpatient perspectives. J Adv Nurs. 2007;57(5):494‐504. [DOI] [PubMed] [Google Scholar]
- 10. Basinski A, McIsaac C, Cheng T, Woo K, Neves P. Profiling and case costing of wound care in long‐term care homes. Ontario Association of Long Term Care Applied Research Day. February 25‐16, 2014. Toronto, Ontario, Canada. Content provided by McIsaac C. Abstract available at: https://www.eiseverywhee.com/file_uploads/828f058a830d5720eda82740ccbba6f0_0368‐000117‐AntoniBasinskiAM2.pdf. Accessed March 01, 2018.
- 11. Stern A, Mitsakakis N, Paulden M, et al. Pressure ulcer multidisciplinary teams via telemedicine: a pragmatic cluster randomized stepped wedge trial in long term care. BMC Health Serv Res. February 24, 2014;14:83. 10.1186/1472-6963-14-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tucker AT, Maass A, Bain DS, et al. Augmentation of venous, arterial and microvascular blood supply in the leg by isometric neuromuscular stimulation via the peroneal nerve. Int J Angiol. 2010;19:e31‐e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang Q, Styf J, Ekström L, Holm AK. Effects of electrical nerve stimulation on force generation, oxygenation and blood volume in muscles of the immobilized human leg. Scand J Clin Lab Invest. August 2014;74(5):369‐377. [DOI] [PubMed] [Google Scholar]
- 14. Daneman N, Gruneir A, Newman A, et al. Antibiotic use in long‐term care facilities. J Antimicrob Chemother. 2011;66(12):2856‐2863. https://academic.oup.com/jac/article/66/12/2856/699109 Accessed February 28, 2018. [DOI] [PubMed] [Google Scholar]
- 15. Camuglia AC, Alemayehu M, McLellan A, Wall S, Abu‐Romeeh N, Lavi S. The impact of peripheral nerve stimulation on coronary blood flow and endothelial function. Cardiovasc Drugs Ther. 2015;29:527‐533. [DOI] [PubMed] [Google Scholar]
- 16. Jawad H. The Effectiveness of the geko™ Medical Device versus Intermittent Pneumatic Compression: A Comparative Study. In: Jawad H, ed. The effect of a novel electrical stimulation method for improving lower limb blood flow in healthy volunteers [PhD dissertation]. Queen Mary University of London/St. Bart's. 2012. https://qmro.qmul.ac.uk/jspui/handle/123456789/3120. Accessed April 11, 2018.
- 17. Harris C, Loney A, Brooke J, et al. Refractory venous leg ulcers: observational pilot of innovative new technology. Int Wound J. 2017;14(6):1100‐1107. Available at. http://onlinelibrary.wiley.com/doi/10.1111/iwj.12766/epdf. Accessed December 11, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harris C, Duong R, Vanderheyden G, et al. Pilot of a neuromuscular electrical stimulation device for non‐healing venous leg ulcers. Int Wound J. 2017;14(6):1189‐1198. Available at. http://onlinelibrary.wiley.com/doi/10.1111/iwj.12784/epdf. Accessed December 11, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kantor J, Margolis DJ. A Multicenter Study of Percentage Change in Venous Leg Ulcer Area as a Prognostic Index of Healing at 24 Weeks. Br J Dermatol. 2000;142:960‐964. [DOI] [PubMed] [Google Scholar]
- 20. Revera . Revera innovators in aging. 2018. http://www.reveraliving.com/about-revera/innovation-at-revera. Accessed April 11, 2018.
- 21. Sussman C. Wound measurements and prediction of healing. In: Sussman C, Bates‐Jensen BM, eds. Wound care: a collaborative practice manual. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:134. [Google Scholar]
- 22. Quality and Innovation Awards 2016. This is long term care. Long Term Care Today. 2017;28(1):25. https://view.imirus.com/1163/document/12519/page/24 Accessed March 1, 2018. [Google Scholar]


