Summary
Background
Costunolide (COS), a naturally occurring sesquiterpene lactone, is known to exert anti‐inflammatory, antioxidant, and anticancer effects. This study was undertaken to investigate the effects of costunolide on the promotion of hair growth.
Methods
Real‐time cell analyzer (RTCA), measurement of 5α‐reductase activity, mRNA expression, and Western blotting were adopted to address whether COS can stimulate the proliferation of human hair follicle dermal papilla cells (hHFDPCs). The effect of COS on in vivo hair growth was examined by reconstitution assay and shaven dorsal skin in C57BL/6 mice.
Results
Costunolide significantly promoted the proliferation of hHFDPCs, which is comparable to that of tofacitinib. COS also inhibited the 5α‐reductase activity in hHFDPCs. While COS increased the level of β‐catenin and Gli1 mRNA and proteins, it suppressed transforming growth factor (TGF)‐β1–induced phosphorylation of Smad‐1/5 in hHFDPCs. COS increased the number of cultured hHFDPCs to induce hair follicles from mouse epidermal cells in Spheres formation of reconstitution assay. Topical application of COS on the shaven back of C57BL/6 mice significantly improved the hair growth.
Conclusions
Our results illustrate that COS promotes hair growth in vitro and in vivo by regulating the amount of growth factors and/or the activity of cellular responses through coordination of the WNT‐β‐catenin, hedgehog‐Gli, and TGF‐β1–Smad pathways.
Keywords: BMP/Smad, costunolide, human hair follicle dermal papilla cells, mouse hair growth, Shh/Gli, TGF, WNT/ β‐catenin
1. INTRODUCTION
Alopecia, a generic term for hair loss, frequently occurs in men and imparts a significant impact on social interaction and psychological welfare. Besides genetic predisposition, various life style–related and environmental factors, such as lack of nutritional balance, intake of certain medications, and surgery, may often lead to hormonal imbalance, thereby contributing to excessive hair loss.1 Current treatment for alopecia includes direct implantation of hair follicles or therapeutic intervention with finasteride or minoxidil.2 In spite of their clinical efficacy, these hair growth–promoting agents exhibit potential adverse effects. The use of finasteride has been reported to be associated with erectile dysfunction, self‐harm and depression, while minoxidil rash and dermatitis‐like reactions, thereby compromising quality of life.3 Moreover, alopecia is caused mostly by aberrant cell cycles of hair follicles, which are not specifically targeted by these drugs. Thus, there is a continuous quest for searching novel hair growth–promoting agents with specific molecular targets.
Hair follicles undergo four cell cycle phases: growth (anagen), regression (catagen), rest (telogen), and shedding (exogen).4 In particular, the onset of anagen phase requires the activation of multipotent epithelial stem cells located in the bulge region, a specialized part of the hair follicle. Adjacent mesenchymal cells (dermal papilla) transmit activation signals and direct multipotent epithelial stem cells to migrate and differentiate into the hair bulb, which ultimately transforms into new hair.5 Multiple signaling molecules, including Wnts, Sonic hedgehog (Shh), and TGF‐β emanating from adjacent mesenchymal cells, contribute to the anagen initiation of multipotent epithelial stem cells. However, the detailed mechanisms how these signaling molecules coordinate to activate the cell cycle of hair follicles are largely unknown.6 Targeting these multicomponent signaling of hair growth regulation would be a rational approach for developing novel therapeutics for the treatment of alopecia. Thus, this study has been designed to examine the effects of costunolide (COS), a naturally occurring sesquiterpene lactone, on the proliferation of human hair follicle dermal papilla cells (hHFDPC) as well as the in vivo growth of hair in mice. COS is conventionally used in herbal medicine. Previous studies have demonstrated that COS exhibits anti‐inflammatory,7 antioxidant,8 and antitumorigenic activities.9 Our study revealed that COS can stimulate hair growth via modulation of TGF‐β, Shh, and WNT/β‐catenin signaling pathways and stimulating proliferation of human hair follicle dermal papilla cells (hHFDPC).
2. MATERIALS AND METHODS
2.1. Cell culture, chemicals, and antibodies
Human hair follicle dermal papilla cells were purchased from Abm Inc (Richmond, British Columbia, Canada). DMEM and fetal bovine serum (FBS) were acquired from Invitrogen (Carlsbad, CA, USA). HDPCs were cultured in DMEM supplemented with 10% fetal bovine serum (FBS) and 100 U/mL penicillin and 100 U/ml streptomycin. Cells were maintained in 5% CO2 incubator within a humidified atmosphere of at 37°C. Costunolide (COS) was purchased from AK Scientific, Inc (Union City, CA, USA). CellTiter‐Glo® Lumine Cell Viability Assay Kit was purchased from Promega (Madison, WI, USA). Recombinant TGF‐β1 and Shh were purchased from R&D systems (Minneapolis, MN, USA). β‐Actin antibody was obtained from Sigma‐Aldrich (St. Louis, MO, USA). Polyclonal antibodies against total β‐catenin, phospho‐specific β‐catenin (Thr41/Ser45), Gli1, Shh, Smad1, phospho‐specific Smad1/5 (Ser463/465), cyclin D1, CDK2, CDK4, phospho‐specific Rb, Rb, p21, and p27 were purchased from Cell Signaling Technology (Beverly, MA, USA). All other chemicals used in our experiments fall in the molecular biology grade.
2.2. Real‐time cell analyzer (RTCA) system
The xCELLigence System (ACEA Biosciences, San Diego, California, USA) allows label‐free and real‐time monitoring of cellular processes, such as cell proliferation, cytotoxicity, adhesion, viability, invasion, and migration using electronic cell sensor array technology. The electrode impedance, displayed as cell index (CI) values, provides quantitative information about the biological status of cells, including the number, viability, and morphology. In brief, 150 μL of cell culture media at room temperature was added into each well of E‐plate 8 of xCELLigence System. The E‐plate 8 was then connected and checked in the cell culture incubator for proper electrical contacts, and the background impedance was measured during 24 hours. Meanwhile, the HDPCs were resuspended in cell culture medium and adjusted to 20 000 cells/well. Cell suspension (50 μL) was added to 150 μL medium containing wells on E‐plate 8, in order to determine the optimum cell concentration. After 30‐minute incubation at room temperature, E‐plate 8 was placed into the cell culture incubator. And then, adhesion, growth, and proliferation of the cells were monitored every 1 hour for a period of up to 24 hours via the incorporated sensor electrode arrays of the E‐Plate 8. After 24 hours, 0‐3 μmol/L of COS in 200 μL cell culture medium were added, and live cells were monitored every 15 minutes for a period of up to 72 hours. The electrical impedance was measured by the RTCA‐integrated software of the xCELLigence system as a dimensionless parameter termed CI.
2.3. CellTiter‐Glo® luminescent cell growth assay
Cell proliferation and cytotoxicity were assessed using a CellTiter‐Glo® Luminescent Cell Viability Assay Kit (Promega, Madison, WI, USA). Briefly, HDPC cells were seeded in 96‐well plate (7000 cells/well) for 24 hours. After attachment, cells were exposed to COS (0‐3 μmol/L) in serum‐free medium for 72 hours. An equal volume of CellTiter‐Glo® Reagent to cell culture media was added and incubated at room temperature for 10 minutes. The amount of ATP was determined by LuBi microplate luminometer (Micro Digital Ltd., Seoul, South Korea).
2.4. Measurement of endogenous 5α‐reductase enzyme activity
Cells were exposed to COS (3 μmol/L) alone or in combination with testosterone (0.4 μmol/L) for 24 hours. After collection of cell lysates, endogenous 5α‐reductase 2 activity was determined using human 3‐oxo‐5‐alpha‐steroid 4‐dehydrogenase 2(SRD5A2) ELISA Kit (Cusabio Biotech, Wuhan, China).
2.5. RNA isolation and quantitative real‐time PCR
After an exposure to COS (0‐3 μmol/L) for 48 hours, total RNA from HDPCs was extracted using NucleoSpin® RNA Kit (Macherey‐Nagel Gmbh & Co., Düren, Germany). Reverse transcription of total RNA was performed using iScript™ cDNA Synthesis Kit (Bio‐Rad, Hercules, CA, USA). Briefly, 2 μg of total RNA was used for cDNA preparation. Synthesized cDNA was further amplified by PCR using specific primers against β‐catenin, Smad2, Gli2, and GAPDH. PCR products were analyzed by 1% agarose gel using 1X TAE buffer, and relative mRNA levels were quantified using myECL imager analysis software (Thermo Fisher Scientific, Waltham, MA, USA). Quantitative real‐time PCR was performed using the iQ™ SYBR® Green Supermix (Bio‐Rad, Hercules, CA, USA), in which the expression of target genes was normalized to that of GAPDH. The primers for individual quantitative real‐time PCR are as follows: β‐catenin forward 5′‐CCCACTAATGTCCAGCGTTT‐3′, reverse 5′‐AACCAAGCATTTTCACCAGG‐3′; glycogen synthase kinase (GSK)‐3β forward 5′‐AACTGCCCGACTAACAACAC‐3′, reverse 5′‐ATTGGTCTGTCCACGGTC TC‐3′; lymphoid enhancer factor (Lef)‐1/T Cell factor (TCF) forward 5′‐AATCATCCCGGC CAGCA‐3′, reverse 5′‐TGTCGTGGTAGGGCTCCTC‐3′; VEGF forward 5′‐GGAGAGAT GAGCTTCCTACAG‐3′, reverse 5′‐TCACC GCCTTGGCTTGTCACA‐3′; CDK4 forward 5′‐ACCTGAGATGGAGGAGTC‐3′, reverse 5′‐AAGGCAGAGATTCGC TTG‐3′; BMP1 forward 5′‐CAGTCCTTTGAGATTGAGCGC‐3′, reverse 5′‐TGCTGCTCTCACTGT GCCC‐3′; BMP2 forward 5′‐CAACACTGTCGCAGCTTC‐3′, reverse 5′‐GAA GAATCTCC GGGTTGTTTTC‐3′; BMP4 forward 5′‐CACTGGCTGACCACCTCAAC‐3′, reverse 5′‐GGCACCCACATCCCTCTACT ‐3′; BMP6 forward 5′‐AACCAACCACGCGATTGTG‐3′, reverse 5′‐AAGTCTCATCGTCCCACCTC‐3′; Gli1 forward 5′‐TACTCACGCCTCGAAA ACCT‐3′, reverse 5′‐GTCTGCTTTCCTCCCTGATG‐3′; GAPDH forward 5′‐TGGCAAA TTCCATGCAC‐3′, reverse 5′‐CCATGGTGGTGAAGACGC‐3′.
2.6. Western blotting
After HDPC cells were exposed to COS (0‐3 μmol/L) for 48 hours, cells were lysed with a cell lysis buffer (100 mmol/L Tris pH 7.5, 150 mmol/L NaCl, 5 mmol/L EDTA, 1% Triton X‐100, 5 mmol/L DTT, 0.1 mmol/L PMSF, 10% glycerol, protease inhibitor) for 2‐hour incubation on ice. After collection of cell lysates, an equal amount of proteins (20 μg) was separated on a SDS/8%‐15%‐polyacrylamide gel and transferred to polyvinylidene difluoride (PVDF) membrane (Thermo Scientific, Pittsburgh, PA, USA). After blocking for 1 hour at room temperature with 5% (w/v) nonfat dried milk in Tris‐Buffered Saline Tween‐20 [TBST: 10 mmol/L Tris (pH 8.0) and 150 mmol/L NaCl solution containing 0.05% Tween‐20], membranes were hybridized with specific primary antibodies. Blots were washed three times with 1× TBST and incubated with horseradish peroxidase (HRP)‐conjugated secondary antibodies. The resulting image was captured by an enhanced chemiluminescence Western blotting detection system (myECL imager, Thermo Scientific, Pittsburgh, PA, USA).
2.7. Measurement of hair growth in mice
Seven‐week‐old female C57BL6 mice were purchased from Orient Bio Co (Seoul, Korea). After 7‐day acclimation in the facility, mice were divided into 2 randomized groups (n = 4) and dorsal hair was shaven by clippers. One group of mice was treated topically with 200 μL of COS (3 μmol/L) twice daily for 15 days, while the other group served as control and was treated with the vehicle (50% ethanol) in the same manner. The hair growth activity was monitored. All animals were taken care according to protocols approved by the Institutional Animal Care and use Committee (IACUCs).
2.8. Reconstitution assay
The hair‐inductive capacity of the human DP was assessed in accordance with the established reconstitution assay.10 Implantation was performed as described previously.11 Briefly, COS‐treated mouse dermal cells or COS‐treated DP spheres were combined with freshly isolated neonatal mouse epidermal cells and cotransplanted subcutaneously into the skin on the backs of nude mice. Three weeks later, skin samples were excised from the mice and examined to verify hair induction.
3. RESULTS
3.1. COS promotes the growth of hHFDPCs and inhibits the 5α‐reductase activity
In this study, we examined the effects of COS on the growth of hHFDPCs. Tofacitinib was used as a positive control. Our results show that COS promoted proliferation of hHFDPCs in a time‐ (Figure 1B) and concentration‐dependent (Figure 1C) manner. We also examined the inhibitory effect of COS on the activity of 5α‐reductase enzyme, which catalyzes the conversion of testosterone to the more potent androgen metabolite dihydrotestosterone. COS exhibited 50 ± 6.3% inhibition of 5α‐reductase activity when cotreated with testosterone as compared to testosterone control (Figure 1D).
Figure 1.
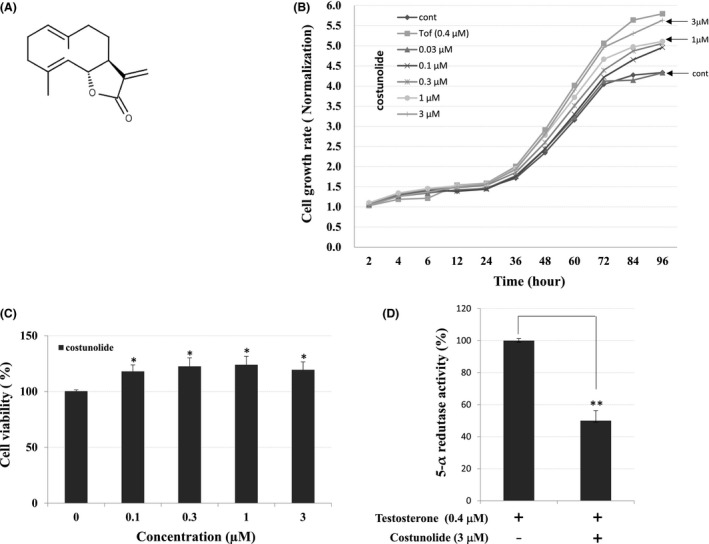
Effects of COS on hHFDPCs. A, Chemical structures of COS. B, Real‐time xCELLigence system and C, CellTiter‐Glo® luminescent cell growth assay show that COS promotes proliferation of hHFDPCs. D, Inhibitory effects of COS on the 5α‐reductase in hHFDPCs. Each values represent mean ± SE in triplicates. Inhibitory activities of COS on 5α‐reductase; inhibition (%) = {1‐(sample/control)} × 100. The asterisk(s) indicate a significant statistical significance (*P < .05, **P < .01)
3.2. COS affects the expression of genes involved in the hair growth
To investigate the effect of COS on regulatory factors of hair growth, we analyzed transcriptional changes of various genes, using both conventional and real‐time quantitative reverse transcriptase‐PCR (RT‐PCR). Treatment of hHFDPCs with COS resulted in the transcriptional activation of β‐catenin, cdk4, and Gli1 but decreased the mRNA levels of BMP1, BMP2, and BMP6 (Figure 2A). This finding was further confirmed by conventional RT‐PCR analysis, where tofacitinib was included as a reference control. As shown in Figure 2B, conventional RT‐PCR analysis showed exactly same gene expression pattern observed in real‐time PCR analysis. The reference compound Tofacitinib also elicited expression of β‐catenin, cdk4, Gli1, and Smad2 gene expression in hHFDPCs similar to that exhibited by COS (Figure 2B).
Figure 2.
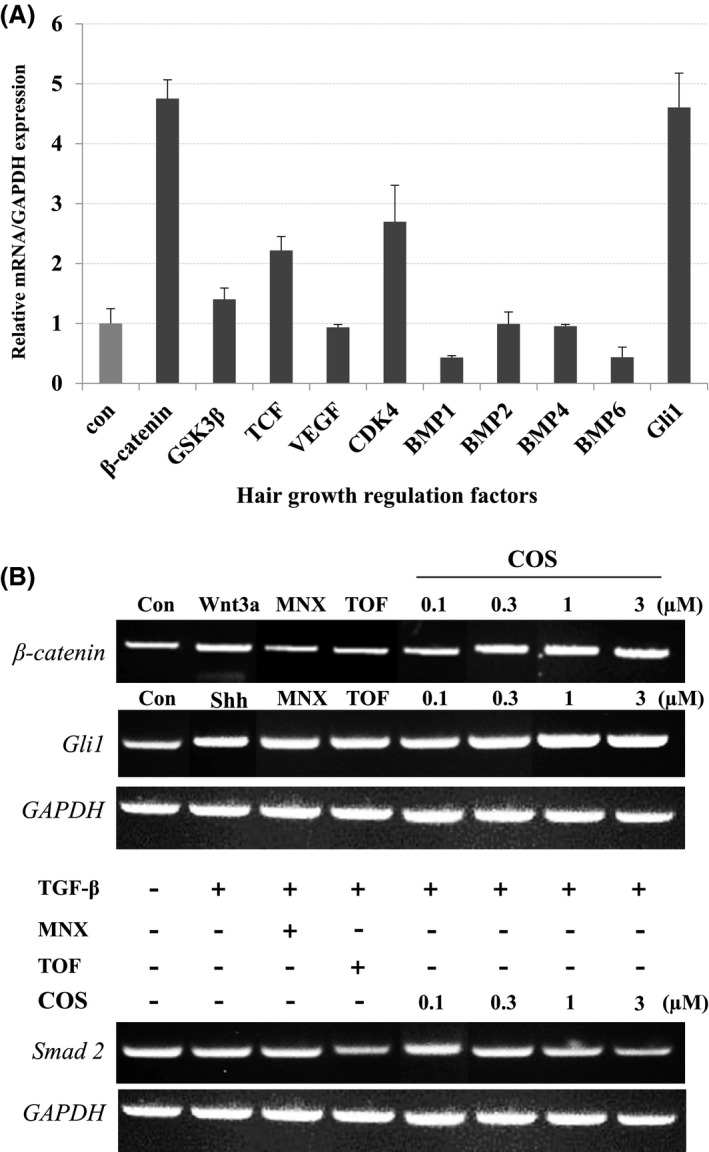
Effects of COS on Transcriptional Expression of Hair Growth Regulatory Genes. A, The gene expression of regulatory factors implicated in the hair growth was monitored by real‐time qPCR in hHFDPCs. B, The level of hair growth regulating factors was detected by conventional RT‐PCR. GAPDH was used as an internal control. The asterisk(s) indicate a significant statistical significance (*P < .05, **P < .01). MNX, Minoxidil; TOF, Tofacitinib
3.3. Effects of COS on the activation of β‐Catenin, Gli, and Smads in hHFDPCs
Based on the effects of COS on mRNA expression of hair growth regulatory genes, we examined whether COS could modulate the phosphorylation of several key regulators of hair growth. As shown in Figure 3A, COS decreased the constitutive phosphorylation of β‐catenin (Thr41 and Ser45). Treatment of hHFDPCs with recombinant WNT3a also abolished a constitutive β‐catenin phosphorylation. A similar inhibition of β‐catenin phosphorylation was also observed when hHFDPCs were exposed to tofacitinib and minoxidil. To examine the effects of COS on the phosphorylation of Smads, hHFDPCs were first stimulated with recombinant TGF‐β1 as Smads are not constitutively phosphorylated in hHFDPCs. Treatment with COS markedly inhibited TGF‐β1–induced Smad1/5 phosphorylation at Ser463/465 residues in hHFDPCs. However, COS attenuated Gli1 activation in a concentration‐dependent manner comparable to Shh (Figure 3A). Immunoblot analysis of cell proliferation markers revealed that COS increased the expression of cyclin D1 and CDK4 but reduced the expression of p27. Moreover, COS elevated the expression of phosphorylated retinoblastoma (p‐Rb) as compared to untreated cells, while the expression level of Rb remained unchanged (Figure 3B). These results suggest that the transcriptional upregulation of cyclin D and downregulation of p27 by COS might contribute to cell cycle progression, thereby resulting in hHFDPC proliferation .
Figure 3.
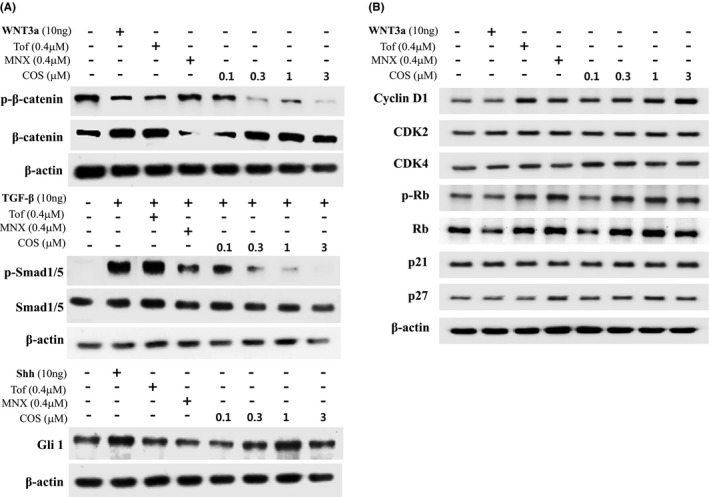
Effect of COS on the Level or Activity of Proteins Implicated in Hair Growth. A, The level of p‐β‐catenin, β‐catenin, p‐Smad1/5, Smad1, and Gli1 was detected by Western blotting in hHFDPCs. B, The level of cell cycle–related proteins was detected by Western blotting in hHFDPCs. β‐Actin protein was used as an internal control. Each blot is representative for 3 experiments
3.4. Effects of COS on the hair growth in mice in vivo
To address the role of COS in hair follicle regeneration in vivo, we employed primary mouse dermal cells. Freshly isolated mouse dermal cells were plated and treated with COS for 2 days, and then implanted in mice. Impairment of hair follicle regeneration was observed when implanting COS‐treated mouse dermal cells (106 cells) combined with mouse epidermal cells (3 × 105 cells) (Figure 4A). Quantitatively, the induction of 128±56 hair follicles was observed in the control groups, while induction of 246 ± 8 hair follicles was observed in the COS‐treated groups (Figure 4B,C). Finally, we attempted to examine the effects of COS on the hair growth in mice. The back of C57BL/6 mice was shaved and topically treated with vehicle (ethanol) or COS for 15 days. Compared to the vehicle control, COS promoted significant hair growth in mice (Figure 5A). Histopathological analysis of mouse skins including the follicular and dermal layers at autopsy showed that the diameter and depth of hair follicles were remarkably higher when mice were administered with COS.
Figure 4.
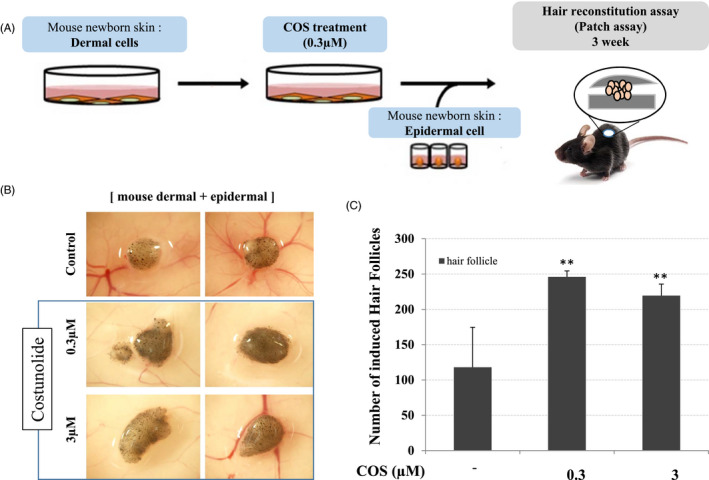
Effect of COS on the Hair Growth in C57BL/6 Mice. COS‐treated mouse epidermal cells were impaired in hair follicle neogenesis when combined with human DP spheres. A, A schematic illustration of the experimental procedure is shown. B, Hair follicles were ordinarily induced when control and COS‐treated mouse epidermal cells (106 cells) were implanted together with human DP spheres (106 cells; 100 DP spheres). C, Total induced hair follicles were counted at each injection site, and data are shown as means ± SD of quadruplicate per experiment from three independent experiments (*P < .05, **P < .01)
Figure 5.
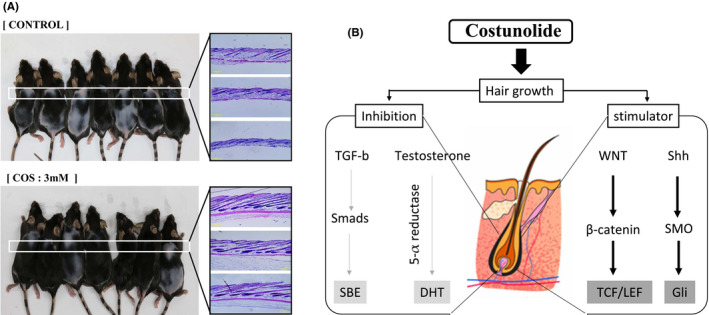
Scheme of COS regulation in hair growth. Back of C57BL/6 mice was shaved and topically treated with vehicle (ethanol) or COS for 15 days. A, The gross image of dorsal skin (Upper Panel) and histopathological results are provided (Lower Panel). B, A representative scheme how COS regulates the WNT/ β‐catenin and Shh pathway in human hair dermal follicle papilla cells
4. DISCUSSION
A wide variety of sesquiterpene lactones, including COS, have been previously shown to exhibit antioxidant, anti‐infective, antineoplastic, and anti‐inflammatory properties with potential therapeutic applications.7, 8, 9, 12 The present study shows, for the first time, that COS promotes hair growth in vivo, likely by activating intracellular signaling pathways associated with cell cycle regulation in hair follicular cells including the Wnt/β‐catenin, Shh/Gli, and TGF‐β/Smad signaling pathways.
During postnatal development, hair follicles cycle between relative resting (telogen), active growth and hair shaft production (anagen), and apoptosis‐driven regression stages.1 For resting postnatal hair follicles to either undergo morphogenesis or initiate a new growth phase, must be activated to stimulate differentiation, and thereby the generation of a fiber‐producing hair bulb.13 Wnt signaling in this context (comprising epidermal Wnt ligands) mediates the activation of hair follicular stem cells by stabilizing and regulating dermal β‐catenin signaling, which is itself indispensable for fibroblast proliferation and hair follicle induction.14 Indeed, transgenic mice that express a stabilized form of β‐catenin have been shown to exhibit de novo hair follicle formation increased follicle density and precocious re‐entry into the regenerative phase of hair growth.15, 16 Similarly, Hedgehog(Shh) signaling also mediates β‐catenin activity to direct hair follicle morphogenesis and hair cycle initiation, such that constitutive Shh deletion results in the arrest of hair follicle morphogenesis at the bud. Interestingly, the activation of Shh signaling in the hair follicle mediates, at least in part, the anagen‐inducing effect exerted by noggin signaling. Accordingly, Shh signaling activity has been shown to be upregulated in hair follicles after noggin treatment, and conversely, noggin anagen‐inducing activity has been shown to be abrogated in situ by treatment with anti‐Shh antibodies. In contrast, BMP4 signaling activity downregulates Shh signaling in the hair follicle and thus represents an important upstream effector of Shh during the initiation of the hair growth phase in telogen hair follicles.17, 18 The results of the current study suggested that COS treatment increased both the level of β‐catenin and Gli1 mRNA and proteins levels in the analyzed hHFDPCs, and it is likely that these effects on Wnt and Shh signaling activation contributed to the observed increase in hair follicular cell proliferation (Figure 2 and 3).
Dermal papillae (DPs) isolated from balding scalp tissue exhibit increased expression of the androgen receptor (AR), as well as type II 5‐alpha reductase, which converts testosterone to dihydrotestosterone (DHT). DHT can induce DPs to secrete factors that inhibit keratinocyte growth, including TGF‐β1 and DKK‐1; thus, localized high levels of DHT and ARs likely cause the patterned distribution of alopecia characteristic of balding scalp DPs, while decreased 5‐α reductase activity levels likely reflect an increased proportion of inactive hair follicles.19 In the present study, COS treatment reduced 5‐alpha reductase levels by 50% and also inhibited DHT‐stimulated activation of TGF‐β/BMP signaling. TGF‐β/BMPs play pivotal roles in the control of many developmental programs and act by binding specific BMP receptors to trigger a signal that is transduced to the nucleus via the recruitment of either Smad1/5 transcriptional regulators or components of the mitogen‐activated protein kinase (MAPK) pathway including morphogenesis of the skin.18, 20 While the role of COS in modulating various components of the TGF‐β/BMP signaling pathway and also their impact on hair growth, requires further investigation, the results of the present study strongly suggest that COS treatment inhibited TGF‐beta/BMP/Smad signaling in the analyzed hHFDPCs. Furthermore, the histopathological analysis of the murine dermis conducted by the present study revealed that COS treatment increased the diameter and depth of dermally implanted hair follicles, and likewise, that human DP spheres also promoted hair follicle growth when transplanted in combination with COS‐treated mouse epidermal cells.
Overall, the present study demonstrates that COS treatment promotes DP proliferation and stimulates hair growth by both activating Wnt/β‐catenin and Shh signaling, and inhibiting TGF‐β/BMP/Smad signaling in hair follicular cells (Figure 5B). While additional studies are required to investigate the molecular mechanisms underlying these effects, the results of the present study strongly support COS as a novel candidate therapeutic target for the treatment of alopecia.
CONFLICT OF INTEREST
Authors declare no competing financial interests.
ACKNOWLEDGMENTS
This research was financially supported by the Ministry of Trade, Industry and Energy (MOTIE, R0004663) and Korea Institute for Advancement of Technology (KIAT) through the Promoting Regional specialized Industry.
Kim YE, Choi HC, Nam G, Choi BY. Costunolide promotes the proliferation of human hair follicle dermal papilla cells and induces hair growth in C57BL/6 mice. J Cosmet Dermatol. 2019;18:414–421. 10.1111/jocd.12674
REFERENCES
- 1. Cotsarelis G, Millar SE. Towards a molecular understanding of hair loss and its treatment. Trends Mol Med. 2001;7:293‐301. [DOI] [PubMed] [Google Scholar]
- 2. Avram M, Rogers N. Contemporary hair transplantation. Dermatol Surg. 2009;35:1705‐1719. [DOI] [PubMed] [Google Scholar]
- 3. Jain R, De‐Eknamkul W. Potential targets in the discovery of new hair growth promoters for androgenic alopecia. Expert Opin Ther Targets. 2014;18:787‐806. [DOI] [PubMed] [Google Scholar]
- 4. Cotsarelis G. Epithelial stem cells: a folliculocentric view. J Invest Dermatol. 2006;126:1459‐1468. [DOI] [PubMed] [Google Scholar]
- 5. Alonso L, Fuchs E. Stem cells in the skin: waste not, Wnt not. Genes Dev. 2003;17:1189‐1200. [DOI] [PubMed] [Google Scholar]
- 6. Millar SE. Molecular mechanisms regulating hair follicle development. J Invest Dermatol. 2002;118:216‐225. [DOI] [PubMed] [Google Scholar]
- 7. Taniguchi M, Kataoka T, Suzuki H, et al. Costunolide and dehydrocostus lactone as inhibitors of killing function of cytotoxic T lymphocytes. Biosci Biotechnol Biochem. 1995;59:2064‐2067. [DOI] [PubMed] [Google Scholar]
- 8. Eliza J, Daisy P, Ignacimuthu S. Antioxidant activity of costunolide and eremanthin isolated from Costus speciosus (Koen ex. Retz) Sm. Chem Biol Interact. 2010;188:467‐472. [DOI] [PubMed] [Google Scholar]
- 9. Robinson A, Kumar TV, Sreedhar E, et al. A new sesquiterpene lactone from the roots of Saussurea lappa: structure‐anticancer activity study. Bioorg Med Chem Lett. 2008;18:4015‐4017. [DOI] [PubMed] [Google Scholar]
- 10. Zheng Y, Du X, Wang W, et al. Organogenesis from dissociated cells: generation of mature cycling hair follicles from skin‐derived cells. J Invest Dermatol. 2005;124:867‐876. [DOI] [PubMed] [Google Scholar]
- 11. Kang BM, Kwack MH, Kim MK, et al. Sphere formation increases the ability of cultured human dermal papilla cells to induce hair follicles from mouse epidermal cells in a reconstitution assay. J Invest Dermatol. 2012;132:237‐239. [DOI] [PubMed] [Google Scholar]
- 12. Jeon WJ, Kim KM, Kim EJ, et al. Costunolide increases osteoblast differentiation via ATF4‐dependent HO‐1 expression in C3H10T1/2 cells. Life Sci. 2017;178:94‐99. [DOI] [PubMed] [Google Scholar]
- 13. Fuchs E, Merrill BJ, Jamora C, et al. At the roots of a never‐ending cycle. Dev Cell. 2001;1:13‐25. [DOI] [PubMed] [Google Scholar]
- 14. Yang Y, Li Y, Wang Y, et al. Versican gene: regulation by the beta‐catenin signaling pathway plays a significant role in dermal papilla cell aggregative growth. J Dermatol Sci. 2012;68:157‐163. [DOI] [PubMed] [Google Scholar]
- 15. DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557‐4568. [DOI] [PubMed] [Google Scholar]
- 16. Andl T, Reddy ST, Gaddapara T, et al. WNT signals are required for the initiation of hair follicle development. Dev Cell. 2002;2:643‐653. [DOI] [PubMed] [Google Scholar]
- 17. Botchkarev VA, Botchkareva NV, Nakamura M, et al. Noggin is required for induction of the hair follicle growth phase in postnatal skin. FASEB J. 2001;15:2205‐2214. [DOI] [PubMed] [Google Scholar]
- 18. Botchkarev VA. Bone morphogenetic proteins and their antagonists in skin and hair follicle biology. J Invest Dermatol. 2003;120:36‐47. [DOI] [PubMed] [Google Scholar]
- 19. Chueh SC, Lin SJ, Chen CC, et al. Therapeutic strategy for hair regeneration: hair cycle activation, niche environment modulation, wound‐induced follicle neogenesis, and stem cell engineering. Expert Opin Biol Ther. 2013;13:377‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. ten Dijke P, Hill CS. New insights into TGF‐beta‐Smad signalling. Trends Biochem Sci. 2004;29:265‐273. [DOI] [PubMed] [Google Scholar]


