Summary
Currently, there are no tools to predict postsurgery outcome after kidney transplantation. This study assesses whether frailty influence 30‐day postoperative complications after kidney transplantation. One‐hundred and fifty kidney transplantations were prospectively included. Frailty was assessed using a frailty indicator, consisting of 15 questions, covering most domains of functioning. Postoperative complications were measured by the Comprehensive Complication Index (CCI). Using a linear regression model, 30‐day postoperative complications and frailty correlation were adjusted for confounders, including sex, age, ASA Score, Charlson Comorbidity Index, hypertension, BMI, smoking, dialysis, duration of dialysis, type of transplantation, and retransplantation. The mean frailty score was 2.07(±1.6) and 23 patients were classified as frail (GFI ≥4). The mean CCI‐score was 18(±15.6), the mean CCI‐score for “frail” patients 30.1(±17.2) compared to 15.5 (±14.2) for “non‐frail” patients (N = 116). In a regression analysis, a significant relationship between CCI‐score and frailty (β = 13.3; 95% CI 5.7–20.9; P = 0.0007) and transplantation type (β = 4.9; 95% CI: 0.72–9.16; P = 0.02) was found, independent of confounders. In conclusion, frailty and type of transplantation are independent factors associated with an increased risk of postoperative complications.
Keywords: elderly, frailty, kidney transplantation, postoperative complications
Introduction
Currently, there is a lack of reliable tools that can help to predict 30‐day outcome after a kidney transplantation. Even models that are based on relatively large databases have poor predictive values of 67% and 64% for 1‐ and 3‐year adult graft survival, respectively 1. This means that there is no risk prediction tool available that is materially better than a risk prediction by chance.
Recently, frailty has emerged as a significant risk factor for adverse postoperative outcomes. Frailty is the clinically recognizable medical condition, also called syndrome or phenotype, that is, the result of processes leading to an increased vulnerability for serious deteriorations in health and the diminished ability to cope with physical stressors. It is related to declines in energy, strength, and function as well as to an increased inflammatory state, including elevated levels of interleukin 6 (IL‐6), C‐reactive protein (CRP), and an elevated white blood cell count 2, 3, 4. Frailty, as defined in the landmark paper by Fried 2, included at least three of five of the following criteria: unintentional loss of weight, low physical activity, low energy, low grip strength, and a slowed walking speed. Since then, various frailty scoring lists have been designed to optimize the predictive value. One of these is the Groningen Frailty Indicator (GFI), an instrument that includes aspects that have not been covered by previous methods, proved independent of age and comprises both a professional and a self‐assessed version 5, 6. Furthermore, the GFI can be assessed quickly, using only a simple questionnaire which usually does not require more than five minutes to be completed and which covers most domains of functioning, making it easy and fast to get a reliable impression of the degree of frailty. Frailty has previously been proven to be an independent predictor for adverse outcome after major surgery in which frail patients had 2.5 times higher odds for postoperative complications within 30 days after surgery 7.
Additionally, the GFI proved to have a positive predictive value for postoperative delirium after vascular surgery 8.
The aim of this study was to determine which factors, with emphasis on frailty, significantly influence postoperative outcome after kidney transplantation as measured by the Comprehensive Complication Index (CCI) 9.
Patients and methods
Study design and participants
From January 2015 to October 2016, 150 consecutive living and deceased kidney transplant recipients were prospectively included at the University Medical Center Groningen, the Netherlands (UMCG). Eleven patients were excluded for reasons such as combined kidney–pancreas transplantation or procedures that got cancelled due to insufficient health of the donor or recipient, allowing 139 patients to be included for the final analysis in the study. Every patient that agreed on measuring the GFI and that did not get excluded for one of the previously mentioned reasons got subsequently included.
Follow‐up, clinical and laboratory data were prospectively collected and complemented by reviewing the digital medical records. For this study, the Medical Ethical Committee granted dispensation for the Dutch law regarding patient‐based medical research (WMO) obligation (registration no METc2017/070). Patient data were anonymously processed and electronically stored. All procedures were conducted in accordance with the Declarations of Helsinki and Istanbul.
Frailty
At admission, frailty was assessed by a nurse or doctor not involved in this study for each patient using the GFI 6 (Table 1). The GFI is classified in eight separate groups of in total 15 questions that are consistent with the domains of functioning: mobility, visual functioning, auditory functioning, nutritional status, comorbidity, cognition, psychosocial aspects and fitness, resulting in a minimal score of 0 and a maximal score of 15. Based on previous publications, frailty was defined as a GFI score ≥4 6, 8, 10.
Table 1.
The Groningen Frailty Indicator (GFI)
| Yes | No | |
|---|---|---|
|
Mobility Can the patient perform this task without any help? (using tools like walking sticks, wheelchairs or walker is regarded as independent) | ||
| 1. Go shopping | 0 | 1 |
| 2. Walk around outside (around the house or to neighbors) | 0 | 1 |
| 3. Dressing and undressing | 0 | 1 |
| 4. Toilet visit | 0 | 1 |
| Vision | ||
| 5. Does the patient experience problems in daily life by poor vision? | 1 | 0 |
| Hearing | ||
| 6. Does the patient experience problems in daily life by poor hearing? | 1 | 0 |
| Nutrition | ||
| 7. Has the patient involuntarily lost weight (≥6 kg) in the past 6 months (or ≥3 kg in 1 month) | 1 | 0 |
| Comorbidity | ||
| 8. Does the patient currently use four or more different types of medication? | 1 | 0 |
| Yes | No | Sometimes | |
|---|---|---|---|
| Cognition | |||
| 9. Does the patient currently has complaints about his memory (or has a history of dementia) | 1 | 0 | 0 |
| Psychosocial | |||
| 10. Does the patient sometimes experience emptiness around him? | 1 | 0 | 1 |
| 11. Does the patient sometimes miss people around him? | 1 | 0 | 1 |
| 12. Does the patient sometimes feel abandoned? | 1 | 0 | 1 |
| 13. Has the patient recently felt sad or depressed? | 1 | 0 | 1 |
| 14. Has the patient recently felt nervous or anxious? | 1 | 0 | 1 |
| Physical fitness | |||
| 15. Which grade would the patient give its physical fitness (0–10, ranging from very bad to good) 0–6 = 1 7–10 = 0 | 1 | 0 | |
| Total score GFI | |||
A score of four or more indicates a higher risk for frailty.
Complications
Postoperative complications were registered and analyzed using the CCI, which is a tool that summarizes all postoperative complications with respect to their severity according to the Clavien–Dindo classification of surgical complications, consisting of five complication grades including four subgrades 9. In short, grade one consists of any deviation from the normal postoperative course, without the need of surgical, endoscopic, radiological, or pharmacological treatment besides antiemetics, antipyretics, analgetics, diuretics, and electrolytes, and physical therapy. The second grade includes all other pharmacological treatments, blood transfusions, and parenteral nutrition. Third grade complications require surgical, endoscopic, or radiological treatment. Grade four includes life‐threatening complications requiring intensive care unit (ICU) management, whereas grade five concerns the death of the patient. The CCI takes the quantity of appearance of each complication into account, using a specific calculation that yields a score from 0 to 100, thereby giving a very detailed assessment for every patient. The kidney transplantation procedure, as performed at our hospital, has been published previously by our group 11. Our primary outcome measure was 30‐day postoperative complications according to the CCI.
Clinical data selection
Collected data included age (years), sex, American Society of Anesthesiologists physical status classification system (ASA) score, hypertension, body mass index (BMI), smoking (y/n), dialysis (y/n), duration of dialysis (months), type of transplantation (living or deceased), and retransplantation (y/n). Comorbidity was determined by the age‐adjusted Charlson Comorbidity Index, based on the previous medical history. The Charlson Comorbidity Index is a widely used method for predicting mortality. It is composed from a total of 22 differently weighted comorbidities (myocardial infarct, congestive heart failure, peripheral vascular disease, dementia, cerebrovascular disease, chronic lung disease, connective tissue disease, ulcer, chronic liver disease, diabetes, hemiplegia, moderate or severe kidney disease, diabetes with end organ damage, tumor, leukemia, lymphoma, moderate or severe liver disease, malignant tumor, metastasis, AIDS) which can be adjusted for age and results in a prediction of the 1‐year mortality and is widely used 12.
Statistical analysis
Summary statistics were obtained using conventional methods, normally distributed data are expressed as mean and standard deviation (SD) and skewed data as medians and interquartile range (IQR). Frequencies and proportions are reported for categorical data.
The analyses of the effect of GFI on 30‐day outcome as measured by the CCI were adjusted for potentially important confounders (sex, age (years), ASA score, Charlson Comorbidity Index, hypertension, body mass index (BMI), smoking (y/n), dialysis (y/n), duration of dialysis (months), type of transplantation (living or deceased), and retransplantation) by using a preselection, that is, starting with a univariate analysis of all mentioned variables with the 30‐day CCI as dependent variable and then using the most significant variables with a P‐value <0.2 for the multivariable analysis including all remaining variables. Variables that were known to affect both frailty and complications (duration of dialysis and retransplantation) were, independent of statistical significance, added to the adjusted model. Due to its overlap with the Charlson Comorbidity Index and in order not to overfit the model, we excluded the ASA score out of the adjusted model 13, 14. Estimates of the effects were reported with corresponding 95% confidence intervals. A linear regression was carried out using the Statistical Package for the Social Sciences (Released 2015. IBM SPSS Statistics for Windows, Version 23.0. IBM Corp., Armonk, NY, USA).
Results
Baseline characteristics
Baseline characteristics are presented in Table 2. Mean age was 51.8 (±14.5 SD) years (18–81) and 62.9% were male. Mean age for males was 52.7 (±14.6) years and 50.4 (±14.3) years for women. Mean age for frail patients was 50.1 years and 52.2 for nonfrail patients. Mean BMI was 26 (±4.5) kg/m2. Eighteen percent of the patients (N = 25) were smoking at the time of transplantation, 61.9% had hypertension, and the mean Charlson Comorbidity Index was 3.92 (±1.9) points.
Table 2.
Baseline characteristics
| Parameters | Number, mean ± SDa, or median with IQRb | Percentage or range |
|---|---|---|
| Number of patients | 139 | |
| Recipient gender | ||
| Male | 87 | 62.6% |
| Female | 52 | 37.4% |
| Age (years) | Mean 51.81 ± SD 14.5 | 18–81 years |
| ASA scorec | Median 3 IQR 0 | 1–4 |
| Comorbidity (Charlson)d | Median 3 IQR 3 | 2–11 |
| Hypertension | 86 | 61.9% |
| BMIe recipient | Median 25.5 IQR 5.4 | 18.0–42.5 |
| Smoking | 25 | 18% |
| Pretransplant dialysis | 81 | 58.3% |
| Pre‐emptive | 58 | 41.7% |
| Duration of dialysis | Median 7 IQR 32 | 0–87 months |
| Transplantation type | ||
| Deceased | 26 | 18.7% |
| Living | 113 | 81.3% |
| Retransplantation | 25 | 18% |
| Pre‐emptive retransplantation | 5 | 11.6% |
Standard deviation.
Interquartile range.
American Society of Anesthesiologists score (classification system for assessing the fitness of patients prior to surgery; range 1–5).
Charlson Comorbidity Index (predicts 1‐year mortality based on age and the patients’ comorbidities; (0–10).
Body Mass Index (body mass (kg)/(height (m)2)).
Eighty‐two patients (58.3%) were on dialysis prior to the transplantation with a median duration of 7 (IQR 32) months. Nineteen percent of the transplantations were deceased kidney transplantations, 81.3% were performed with a living donor, and 18% were retransplantations.
Frailty and postoperative complications
The mean GFI score for the entire population was 2.07 (±1.6, range 0–8). Twenty‐three patients were considered frail with a GFI score ≥4 (Fig. 1).
Figure 1.
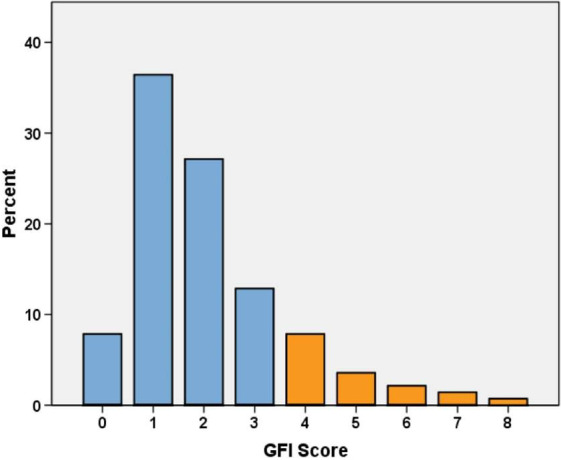
Distribution of GFI score at admission. GFI, Groningen Frailty Indicator, Orange bars = GFI ≥4 (frail).
The mean CCI for all kidney transplant recipients was 18 (±15.6, range 0–91.1), whereas the mean score for patients who were classified as frail (GFI ≥4) was 30.1(±17.2, range 8.7–91.1) (N = 23), compared to a mean score of 15.5 (±14.2, range 0–62.9) for the nonfrail (GFI <4) patients (N = 116) (Fig. 2). Delayed graft function (DGF) occurred in 8% (N = 11) of all patients, with 36% (N = 4) of these being retransplantations. DGF occurred in 55% (N = 6/11) of the nonheart‐beating transplantations, in 20% (N = 3/15) of the heart‐beating transplantations, and in 1.8% (N = 2/113) of the total living donations. 7.8% (N = 9/116) of the nonfrail and 8.7% (N = 2/23) of the frail patients had a delayed graft function.
Figure 2.
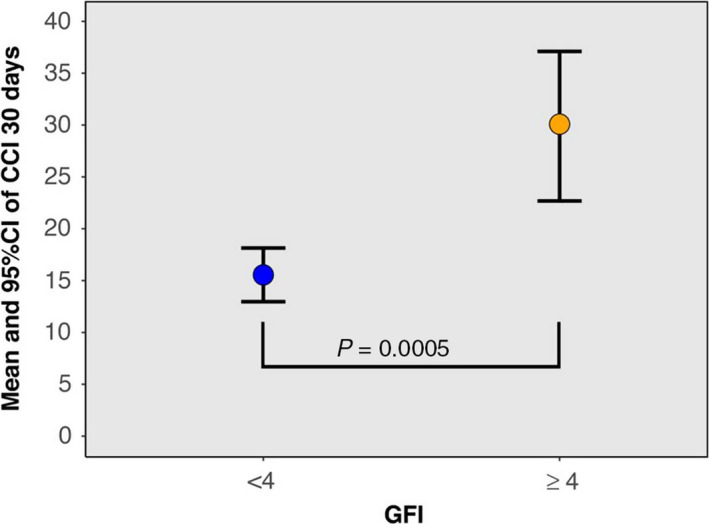
Relation between frailty and CCI. Relation between Groningen Frailty Indicator Score (GFI; ≥4 considered as frail) and Comprehensive Complication Score (CCI) within 30‐day postsurgery.
The number of major complications was low, with most complications being graded as grade one or two within the Clavien–Dindo classification (i.e., candida infections, supraventricular tachycardia, metabolic acidosis, and clostridium infections). All grade two to five complications were broken down into the following events: minor cardiovascular (CV) events [Atrial fibrillation (AF) de novo, arrhythmia not treated with medication or cardioversion, stable angina pectoris complaints], major CV events (myocardial infarction, arrhythmia treated with medication or cardioversion, ICU admission because of CV events), pulmonary events, diabetic events (impaired glucose regulation with symptoms and intensified treatment), surgical interventions (redo surgery, abscess/wound drainages, applying vacuum‐assisted closure devices, and endoscopic procedures), and death of a patient (Table 3). There were no statistically significant differences in these events between frail and nonfrail patients. The mean 30‐day postsurgery eGFR for all patients was 54.2 ml/min*173 m2 with no significant difference between the frail and nonfrail patients.
Table 3.
Distribution of major complications between frail and nonfrail patients
| GFI <4 | GFIa ≥4 | P‐value | |
|---|---|---|---|
| Minor CV events | 6% (N = 7) | 4.3% (N = 1) | 0.36 |
| Major CV events | 0.8% (N = 1) | 4.3% (N = 1) | 0.42 |
| Pulmonary events | 1.7% (N = 2) | 4.3% (N = 1) | 0.75 |
| Diabetic eventsa | 4.3% (N = 5) | 13% (N = 3) | 0.42 |
| Surgical interventionsb | 9.5% (N = 11) | 8.7% (N = 2) | 0.34 |
| Death of a patient | 0% (N = 0) | 0% (N = 0) | NA |
CV, cardiovascular; GFI, Groningen Frailty Indicator.
Impaired glucose regulation with symptoms.
Redo surgery, abscess/wound drainages, applying vacuum‐assisted closure devices and endoscopic procedures.
Thirty‐day outcome
Univariate analyses for variables potentially associated with the CCI score are shown in Table 4. For the adjusted analysis, 8 (GFI, age, Charlson Comorbidity Index, smoking, dialysis, duration of dialysis, type of transplantation, retransplantation) of the initial 12 variables (GFI, sex, age, ASA, Charlson Comorbidity Index, hypertension, BMI, smoking, dialysis, duration of dialysis, type of transplantation, retransplantation) were added to the multivariable model (Table 5) and backward selection was applied.
Table 4.
Univariate analysis with the 30‐day Comprehensive Complication Index as dependent variable
| Variable | B | 95% CI | P‐value |
|---|---|---|---|
| GFI ≥4 | 14.54 | 7.90–21.18 | <0.01 |
| Sex | 0.54 | −4.90 to 5.98 | 0.84 |
| Age | 0.19 | 0.01–0.37 | 0.04 |
| ASA scorea | 4.54 | −1.98 to 11.05 | 0.17 |
| Charlson Comorbidity Indexb | 2.36 | 0.95–3.78 | <0.01 |
| Hypertension | 0.79 | −4.77 to 6.35 | 0.78 |
| BMIc | −0.18 | −0.76 to 0.41 | 0.55 |
| Smoking | 4.67 | −2.02 to 11.37 | 0.17 |
| Preemptive | −3.83 | −9.33 to 1.67 | 0.17 |
| Duration dialysis | 0.07 | −0.05 to 0.19 | 0.24 |
| Kidney transplantation type | 11.22 | 4.72–17.73 | <0.01 |
| Retransplantation | 4.06 | −2.80 to 10.92 | 0.24 |
American Society of Anesthesiologists score (classification system for assessing the fitness of patients prior to surgery; range 1–5).
Charlson Comorbidity Index (predicts 1‐year mortality based on age and the patients’ comorbidities.
Body Mass Index (body mass (kg)/(height (m)2)).
Table 5.
Multivariable model on the association of frailty with the 30‐day Comprehensive Complication Index
| Variable | B | 95% CI | P‐value |
|---|---|---|---|
| GFI ≥4 | 13.31 | 5.72–20.89 | <0.01 |
| Age | 0.001 | −0.28 to 0.28 | 0.99 |
| Charlson Comorbidity Indexa | 1.19 | −0.94 to 3.32 | 0.27 |
| Smoking | 4.41 | −2.63 to 11.45 | 0.22 |
| Preemptive | 1.59 | −5.86 to 9.04 | 0.67 |
| Duration dialysis | −0.08 | −0.25 to 0.09 | 0.35 |
| Type of transplantation | 4.94 | 0.72–9.16 | 0.02 |
| Retransplantation | 3.56 | −4.27 to 11.38 | 0.37 |
Charlson Comorbidity Index (predicts 1‐year mortality based on age and the patients’ comorbidities.
The adjusted analysis showed that frailty (β = 13.3; 95% CI: 5.72–20.89; P = 0.0007) and type of transplantation (β = 4.9; 95% CI: 0.7–9.2, P = 0.02) were statistically significant factors associated with an increase in CCI (Table 5). Being frail and type of transplantation resulted in an average of 13.3‐point and 4.9‐point increase in the CCI score, respectively (Fig. 2).
Discussion
This study shows that frailty, the Charlson Comorbidity Indicator, and the transplantation type proved to be independent risks factors for the occurrence of postoperative complications after kidney transplantation. Out of these three, frailty has been shown to be the most influential factor. Identifying frail patients, especially when receiving a deceased donor kidney, can be an important step in managing postoperative complications. In recent years, frailty has gained an increased interest as a predictive tool for the outcome after (major) surgical procedures by accurately and easily measuring the patient's physiologic reserves and the ability to cope with surgical stressors. Previous research has shown the predictive power of frailty in various medical contexts but an investigation of the prognostic capacities of frailty for complications after kidney transplantation, measured by a simple questionnaire, is still difficult to be efficiently implemented. Frailty is a complex status consisting of several components and domains. Usually, these domains are separately tested in which the sum of these tests determine the degree of frailty. This approach may be time‐consuming, affecting the clinical applicability and usability. The GFI is certainly not the holy grail when it comes to measuring frailty but covers most areas and appears to be strongly correlated to postoperative outcomes. This, combined with the user's convenience, enables the clinician to determine frailty relatively simple and be informed on the postoperative risks. Regarding kidney transplantations, there is a 61% higher risk (P = 0.002) of early hospital readmission (≥1 hospitalization within 30 days after post‐transplantation hospital discharge) for frail patients compared to nonfrail recipients 15 as well as a more than twofold increased risk of mortality 16. Also, there appears to be a relationship between delayed graft function in kidney transplant recipients and frailty, which could be related to the chronic inflammatory processes seen in frail patients 17. Additionally, frailty status has been shown to support the mortality prediction for patients with advanced kidney disease and the shared decision‐making about commencing dialysis in these patients 18.
The phenotype of the frail patient seems related to the increased inflammatory state 3 and a decreased immune function 19 including elevated cortisol levels 20, which is a likely explanation for a delayed recovery and increased risk for postoperative complications.
Because of a continuing shortage of donors, there is a growing pressure on the waiting list for suitable organs. Additionally, the demand for kidney transplantations is constantly growing due to the demographic development of our society. More elderly people are therefore considered more prone to chronic and end‐stage kidney disease requiring transplantation. Having a tool that helps to quickly and efficiently assess the postoperative risks after kidney transplantation is essential for improving the optimal treatment of the patient as well as optimizing time management and therefore effectiveness and hospital capacities.
Frail patients can be supported by preventive measures in order to reduce the occurrence and severity of the expected complications, thereby improving the medical outcome. These measures can include for example preoperative conditioning, consisting of exercise intervention programs that improve functional outcomes, also known as the concept of prehabilitation 21, 22. Optimization of nutrition prior to surgery, combined with early mobilization after surgery 23, might also help to reduce the risks of frail patients, which are often anemic, malnourished, and hypoalbuminemic 24. Implementation of such measures, based on the result of frailty assessment as part of the preoperative process and a more individualized and adjusted healthcare system, could significantly help to improve the short‐term outcome after kidney transplantation and increase its efficiency, by for example optimizing the expected length of stay. Interestingly, in our cohort, frailty did not lead to significantly more CV or pulmonary complications, redo surgery, or death. The increase in CCI‐score was mainly determined by Clavien–Dindo grade 1 complications, which consist of any deviation from the normal postoperative course without the need for pharmacological treatment or surgical, endoscopic and radiological interventions, except for antiemetics, antipyretics, analgesics, diuretics drugs, electrolyte supplementation, blood transfusion, total parental nutrition, and physiotherapy. Most likely this is caused by the high number of living donor recipients and it is expected that this number will be higher among frail deceased donor recipients.
With the previously demonstrated effect of frailty on mortality and early hospital readmission after kidney transplantation 15, 16 and our results of the increased surgical complication risk, frailty should play an important role in patient evaluation and preparation.
Our study has several limitations that need to be addressed. First, only the 30‐day outcome has been analyzed, whereas a long‐term observation might be more conclusive. Second, quality of life of the patient has not been taken into consideration, even though it might have more impact on the patient than surgical complications alone. Third, with the GFI, we made a reliable estimate of the degree of frailty but cannot rule out that we have missed certain components. However, we believe that the usability outweighs the risk of over‐ or underestimating frailty. Also, even though the specificity is relatively low, the profit that can be achieved is very high. Fourth and final, although we prospectively and consecutively included our patients, we missed a number of (complete) GFI forms, of mostly deceased transplantation recipients. This appears to be due to the variable, unplannable and often nightly times on which patients were admitted to the hospital and the reduced staff capacity prior to surgery. Because of the risk of bias, we decided to refrain from determining the GFI after surgery at the time this was noticed. Unfortunately, this has also led to a skewed distribution between living and deceased kidney transplantations. In general, living donor recipients are in a better state of health with less need for dialysis. This will in all likelihood have led to an underestimation of frailty in our population and we expect an even greater effect of frailty in the deceased kidney transplant program. Our team is continuing to work on this project by increasing the number of patients and future studies will have focus on long‐term outcome and an even more detailed approach on frailty (bioimpedance, nutritional status, grip strength) in kidney transplant recipients.
Conclusion
Frailty is an independent predictor for the 30‐day postsurgery outcome after kidney transplantation, causing a 13.4‐point Comprehensive Complication Index increase even after adjusting for important confounders and risk factors. Frailty should be considered an important prognostic preoperative tool for kidney transplantations and be part of patient evaluation and preparation.
Authorship
LS: acquired the data and was involved in data analysis, interpretation, writing the manuscript and contributed to the final adjustments after critically revising it for intellectual content. MEM: was involved in data analysis, interpretation, and contributed to the final adjustments to the manuscript after critically revising it for intellectual content. GJN‐M: contributed to the final adjustments to the manuscript after critically revising it for intellectual content. SPB: contributed to the final adjustments to the manuscript after critically revising it for intellectual content. SJLB: contributed to the final adjustments to the manuscript after critically revising it for intellectual content. RAP: conceived the study and its design, acquired the data, and was involved in data analysis, interpretation, and writing the manuscript.
Funding
The authors have declared no funding.
Conflict of interest
The authors have declared no conflicts of interest.
References
- 1. Schold JD, Srinivas TR, Howard RJ, Jamieson IR, Meier‐Kriesche HU. The association of candidate mortality rates with kidney transplant outcomes and center performance evaluations. Transplantation 2008; 85: 1. [DOI] [PubMed] [Google Scholar]
- 2. Fried L. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: 146. [DOI] [PubMed] [Google Scholar]
- 3. De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflammation markers predicting frailty and mortality in the elderly. Exp Mol Pathol 2006; 80: 219. [DOI] [PubMed] [Google Scholar]
- 4. Leng SX, Xue QL, Tian J, et al Inflammation and frailty in older women. J Am Geriatr Soc 2007; 55: 864. [DOI] [PubMed] [Google Scholar]
- 5. Peters LL, Boter H, Buskens E, Slaets JP. Measurement properties of the Groningen Frailty Indicator in home‐dwelling and institutionalized elderly people. J Am Med Dir Assoc 2012; 7: 546. [DOI] [PubMed] [Google Scholar]
- 6. Steverink N, Slaets J, Schuurmans H, Van Lis M. Measuring frailty: developing and testing the GFI (Groningen Frailty Indicator). Gerontologist 2001; 10: 236. [Google Scholar]
- 7. Makary MA, Segev DL, Pronovost PJ, et al Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg 2010; 6: 901. [DOI] [PubMed] [Google Scholar]
- 8. Pol RA, van Leeuwen BL, Visser L, et al Standardised frailty indicator as predictor for postoperative delirium after vascular surgery: a prospective cohort study. Eur J Vasc Endovasc Surg 2011; 12: 824. [DOI] [PubMed] [Google Scholar]
- 9. Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg 2013; 7: 1. [DOI] [PubMed] [Google Scholar]
- 10. Meulendijks FG, Hamaker ME, Boereboom FT, Kalf A, Vögtlander NP, van Munster BC. Groningen frailty indicator in older patients with end‐stage renal disease. Ren Fail 2015; 37: 1419. [DOI] [PubMed] [Google Scholar]
- 11. Kuipers TG, Hellegering J, El Moumni M, et al Kidney temperature course during living organ procurement and transplantation. Transpl Int 2017; 30: 162. [DOI] [PubMed] [Google Scholar]
- 12. Charlson ME, Pompei P, Ales KL, et al A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373. [DOI] [PubMed] [Google Scholar]
- 13. Heinze G, Dunkler D. Five myths about variable selection. Transpl Int 2017; 30: 6. [DOI] [PubMed] [Google Scholar]
- 14. Heinze G, Wallisch C, Dunkler D. Variable selection – a review and recommendations for the practicing statistician. Biom J 2018; 60: 431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McAdams‐DeMarco MA, Law A, Salter ML, et al Frailty and early hospital readmission after kidney transplantation. Am J Transplant 2013; 8: 2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McAdams‐DeMarco MA, Law A, King E, et al Frailty and mortality in kidney transplant recipients. Am J Transplant 2015; 15: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garonzik‐Wang JM, Govindan P, Grinnan JW, et al Frailty and delayed graft function in kidney transplant recipients. Arch Surg 2012; 147: 190. [DOI] [PubMed] [Google Scholar]
- 18. Pugh JJ, Aggett J, Goodland A, Prichard A. Frailty and comorbidity are independent predictors of outcome in patients referred for pre‐dialysis education. Clin Kidney J 2016; 4: 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leng SX, Cappola AR, Andersen RE, et al Serum levels of insulin‐like growth factor‐I (IGF‐I) and dehydroepiandrosterone sulfate (DHEA‐S), and their relationships with serum interleukin‐6, in the geriatric syndrome of frailty. Aging Clin Exp Res 2004; 16: 153. [DOI] [PubMed] [Google Scholar]
- 20. Varadhan R, Walston J, Cappola AR, et al Higher levels and glunted diurnal variation of cortisol in frail older women. J Gerontol A Biol Sci Med Sci 2008; 63: 190. [DOI] [PubMed] [Google Scholar]
- 21. Arthur HM, Daniels C, McKelvie R, Hirsh J, Rush B. Effect of a preoperative intervention on preoperative and postoperative outcomes in low‐risk patients awaiting elective coronary artery bypass graft surgery. A randomized, controlled trial. Ann Intern Med 2000; 133: 253. [DOI] [PubMed] [Google Scholar]
- 22. Revenig LM, Ogan K, Guzzo TJ, Canter DJ. The use of frailty as a surgical risk assessment tool in elderly patients. Curr Geriatr Rep 2014; 3: 1. [Google Scholar]
- 23. Pashikanti L, von Ah D. Impact of early mobilization protocol on the medical‐surgical inpatient population: an integrated review of literature. Clin Nurse Spec 2012; 26: 87. [DOI] [PubMed] [Google Scholar]
- 24. Waters DL, Baumgartner RN, Garry PJ, Vellas B. Advantages of dietary, exercise‐related, and therapeutic interventions to prevent and treat sarcopenia in adult patients: an update. Clin Interv Aging 2010; 5: 259. [DOI] [PMC free article] [PubMed] [Google Scholar]


