Sir: In non‐small‐cell lung cancer, higher programmed death‐ligand 1 (PD‐L1) expression by tumour cells is associated with a better response to immunotherapy.1 In routine clinical practice, a substantial number of thoracic oncologists tend to use cytological techniques such as fine needle aspiration (FNA) more often than biopsies. Cytopathological analysis of pleural fluid, trans‐oesophageal or bronchial FNA specimens and bronchial washes is frequently performed, allowing tumour typing and a concise evaluation for treatment targets. Up to now, a limited number of studies have focused on histological versus cytological correlation of PD‐L1 immunohistochemistry. PD‐L1 immunocytochemistry is performed on formalin‐fixed cytological material, and Ilie et al. demonstrated, for example, a correlation coefficient of 0.898 on the Ventana Ultra platform.2, 3 Routinely used fixatives in cytology are usually based on methanol instead of formalin, which might negatively affect PD‐L1 staining intensity. To date, it remains unclear whether a methanol‐based fixative without formalin fixation is suitable for predictive immunocytochemical PD‐L1 tests.
The use of FNA on a lobectomy sent for histological examination allows fixation and processing of tumour cytology concurrently with histology and staining of PD‐L1 of the same site. Paired FNA and standard histological processing was performed on 64 lobectomies with two routine immunohistochemical PD‐L1 staining methods [22C3 (Agilent, Amstelveen, the Netherlands) laboratory‐developed test (LDT) and SP263 CE‐IVD assay (Roche, Almere, the Netherlands), both on Ventana Benchmark Ultra (Roche, Almere, the Netherlands].4 The 22C3 LDT using the Ventana platform was validated against the Dako Pharm Dx assay using the Dako Link 48 platform. During validation, the 22C3 LDT showed stronger staining than the SP263 assay, which is in contrast to the Blueprint results of Tsao et al.5 These differences in staining intensities are probably attributable to the platform used. In the Blueprint studies, 22C3 was stained on the appropriate Dako platform, and showed, just like SP263, a slightly less bright result than that obtained with 22C3 on a Ventana immunostainer. A further evaluation of the effects of different fixatives on cytology for PD‐L1 staining can be performed with commercially available cell lines with a gradual increase in staining intensity (Histocyte, Newcastle upon Tyne, UK). Cell lines that are grown and fixed for extended time periods with different fixatives allow the detection of slight differences by fixing of cells for longer and beyond routinely used time periods.
PD‐L1 expression was scored by two trained pathologists in three tumour proportion categories: <1%, 1–50% and >50% membranous staining of tumour cells. After consensus between both pathologists had been reached, the results were used to evaluate agreements between cytology and histology. Weighted Cohen's kappa on the PD‐L1 scores for histology and cytology of 64 lobectomy specimens was calculated. For the 22C3 method, Cohen's kappa showed moderate agreement between PD‐L1 staining scores of histology and cytology (0.53), whereas the SP263 method showed substantial agreement (0.67). A striking difference was found between a subgroup consisting of agar cell blocks fixed in 10% buffered formalin and a subgroup consisting of Cellient cell blocks (Hologic, Zaventem, Belgium) fixed in methanol‐based CytoLyt/PreservCyt (Hologic) (Figure 1). PD‐L1 staining in Cellient cell blocks showed fair to moderate agreement between cytology and histology, with weighted kappas of 0.36 for 22C3 (n = 38) and 0.60 for SP263 (n = 37). PD‐L1 staining in agar cell blocks showed substantial agreement between cytology and histology [weighted kappa for 22C3 of 0.77 (n = 26), and weighted kappa for SP263 of 0.76 (n = 26)]. Further evaluation of fixation effects was performed with cell lines. A Cellient cell block containing cells that were fixed for Σ2 h in CytoLyt showed lower staining intensity than formalin‐fixed cells. Longer fixation times resulted in similar findings, with a further decrease in staining intensity after 48–72 h when methanol fixation was used instead of formalin (Figure 2).
Figure 1.
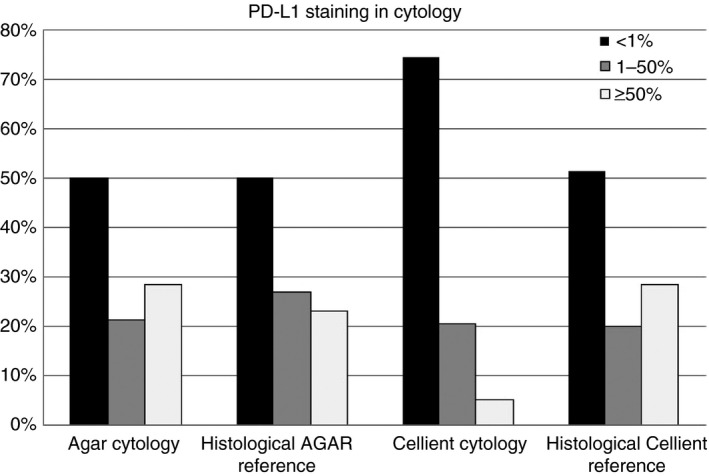
Programmed death‐ligand 1 (22C3 laboratory‐developed test on Ventana Benchmark Ultra) staining, categorised in three bins comparing agar cell blocks (n = 28) and Cellient cell blocks (n = 39) with their histological counterparts.
Figure 2.
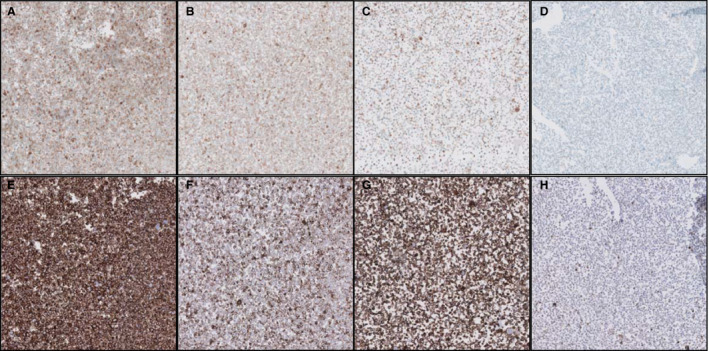
Decreasing Programmed death‐ligand 1 expression with increasing fixation time in a cell line: 2 h (A,E) or 24 h (C,G) of fixation in formalin, and 2 h (B,F) or 24 h (D,H) of fixation in CytoLyt. A–D, SP263 CE‐IVD assay. E–H, 22C3 laboratory‐developed test.
In conclusion, our results indicate that cytology can be used reliably for determination of PD‐L1 expression when formalin fixation is used. Moreover, PD‐L1‐expressing cell lines confirmed the deleterious effect of methanol fixation, making the routinely used CytoLyt/PreservCyt fixatives in the Cellient technique less appropriate for predictive testing for immunotherapy.
Acknowledgements
This study was supported by an unrestricted research grant from Roche.
References
- 1. Herbst RS, Baas P, Dong‐Wan K et al Pembrolizumab versus Docetaxel for previously treated, PD‐L1 positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): a randomized controlled trial. Lancet 2016; 387; 1540–1550. [DOI] [PubMed] [Google Scholar]
- 2. Ilie M, Juco J, Huang L et al Use of the 22C3 anti‐programmed death‐ligand 1 antibody to determine programmed death‐ligand 1 expression in cytology samples obtained from non‐small cell lung cancer patients. Cancer Cytopathol. 2018; 126; 264–274. [DOI] [PubMed] [Google Scholar]
- 3. Wang H, Agulnik J, Kasymjanova G et al Cytology cell blocks are suitable for immunohistochemical testing for PD‐L1 in lung cancer. Ann. Oncol. 2018; 29; 1417–1422. [DOI] [PubMed] [Google Scholar]
- 4. Adam J, Le Stang N, Rouquette I et al Multicenter harmonization study for PD‐L1 IHC testing in non‐small‐cell lung cancer. Ann. Oncol. 2018; 29; 953–958. [DOI] [PubMed] [Google Scholar]
- 5. Tsao MS, Kerr KM, Kockx M et al PD‐L1 immunohistochemistry comparability study in real‐life clinical samples: results of Blueprint phase 2 project. J. Thorac. Oncol. 2018; 13; 1302–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]


