Abstract
BACKGROUND
Transfusion‐associated circulatory overload (TACO) is the leading cause of transfusion‐related major morbidity and mortality. Diagnosing TACO is difficult because there are no pathognomonic signs and symptoms. TACO biomarkers may aid in diagnosis, decrease time to treatment, and differentiate from other causes of posttransfusion dyspnea such a transfusion‐related acute lung injury.
STUDY DESIGN AND METHODS
A systematic review of literature was performed in EMBASE, PubMed, the TRIP Database, and the Cochrane Library, from inception to June 2018. All articles discussing diagnostic markers for TACO were included. Non‐English articles or conference abstracts were excluded.
RESULTS
Twenty articles discussing biomarkers for TACO were included. The majority investigated B‐type natriuretic peptide (BNP) and the N‐terminal prohormone cleavage fragment of BNP (NT‐proBNP), markers of hydrostatic pressure that can be determined within 1 hour. The data indicate that a post/pretransfusion NT‐proBNP ratio > 1.5 can aid in the diagnosis of TACO. Posttransfusion levels of BNP less than 300 or NT‐proBNP less than 2000 pg/mL, drawn within 24 hours of the reaction, make TACO unlikely. Cut‐off levels that exclude TACO are currently unclear. In critically ill patients, the specificity of natriuretic peptides for circulatory overload is poor. Other biomarkers, such as cytokine profiles, cannot discriminate between TACO and transfusion‐related acute lung injury.
CONCLUSION
Currently, BNP and NT‐proBNP are the primary diagnostic biomarkers researched for TACO. An NT‐proBNP ratio greater than 1.5 is supportive of TACO, and low levels of BNP or NT‐proBNP can exclude TACO. However, they are unreliable in critically ill patients. Other biomarkers, including cytokines and pulmonary edema fluid‐to‐serum protein ratio have not yet been sufficiently investigated for clinical use.
ABBREVIATIONS
- AHF
acute heart failure
- ARDS
acute respiratory distress syndrome
- BALF
bronchoalveolar lavage fluid
- BNP
B‐type natriuretic peptide
- ICU
intensive care unit
- IL
interleukin
- NPs
natriuretic peptides
- NT‐proBNP
N‐terminal prohormone of BNP
- TACO
transfusion‐associated circulatory overload
- TNCs
transfused negative controls
- TRALI
transfusion‐related acute lung injury.
Transfusion‐associated circulatory overload (TACO) is characterized by acute respiratory distress and pulmonary edema with signs of volume overload following transfusion.1 It is the foremost cause of transfusion‐related mortality in Europe,2, 3, 4 Canada5 and the United States.6 TACO is currently underrecognized,7, 8 as it is challenging to diagnose due to lack of pathognomonic signs and symptoms. Therefore, biomarkers have the potential to more objectively diagnose TACO and differentiate it from respiratory syndromes with similar presentations.
This is especially important in the case of TACO and transfusion‐related acute lung injury (TRALI), which are similarly characterized by pulmonary edema and respiratory distress but have distinct underlying pathophysiologies. TACO is caused by increased hydrostatic pulmonary pressure,1 and TRALI occurs through immunologic pathways in which priming and activation of neutrophils in the lungs result in increased vascular permeability.9 Biomarkers might allow for identification of at‐risk patients and early recognition of TACO, allowing early initiation of treatment, which would likely limit the extent of pulmonary edema and improve patient outcomes. While initial supportive care is similar for both syndromes, including stopping the transfusion, oxygen supplementation, and if required (noninvasive) positive pressure ventilation,10 there are critical differences in their optimal management. Patients with TRALI often present with hypotension9 and are likely to require fluid therapy, in contrast to TACO, in which fluid restriction as well as diuretics are key.1
Considering the high morbidity and mortality, using biomarkers to accurately diagnose TACO and instigate appropriate therapy is highly relevant. The goal of this review is to provide an overview of the current evidence relating to the diagnostic value of biomarkers of TACO, as well as the consideration of potential novel markers for TACO.
STUDY DESIGN AND METHODS
A systematic review of the literature was performed. A broad search strategy was utilized, identifying articles mentioning TACO or transfusion‐associated cardiac overload. The search strategy is detailed in Appendix S1, available as supporting information in the online version of this paper. EMBASE, PubMed, the TRIP Database, and the Cochrane Library were searched from inception to the date of the initial search performed on September 1, 2017, and updated in June 2018. Articles were screened through title and abstract independently by two researchers. The full text of any article relevant to TACO was assessed by two authors, and all articles discussing diagnostic tools were included. A snowball approach was used to identify additional publications. The only search results excluded were articles not written in English and/or conference abstracts.
Statistical analysis
All results are reported as described in the original papers. No statistical analysis was performed due to the low number of included articles.
RESULTS
A total of 481 articles were screened. Twenty articles mention, discuss, or investigate diagnostic markers for TACO (Fig. 1). To date, only seven studies have investigated biomarkers for TACO (Table S1, available as supporting information in the online version of this paper). Biomarkers fall into the following categories, which will be discussed separately: 1) serum biomarkers of fluid overload, 2) pulmonary edema fluid protein, 3) biomarkers of inflammation, and 4) cardiac biomarkers.
Figure 1.
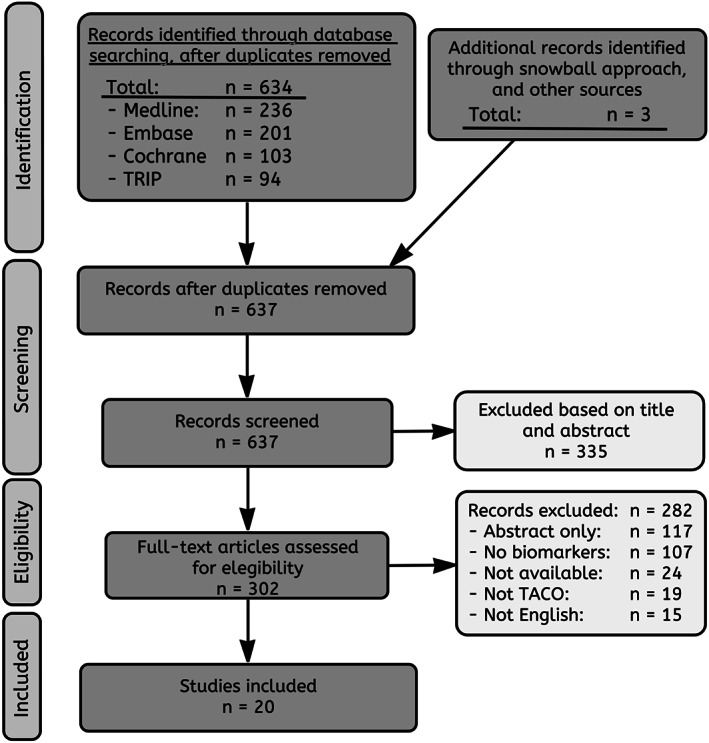
PRISMA flow diagram.
Serum biomarkers of fluid overload
Natriuretic peptides
The function of natriuretic peptides (NPs) is through receptor‐activated arterial and venous vasodilation, reduction of sodium reabsorption by the kidneys, and increased diuresis.11 There are two relevant types: A‐type natriuretic peptide (ANP), which is primarily released by the cardiac atria, and B‐type natriuretic peptide (BNP), primarily released by ventricular myocardium. Both are released as a response to stretch caused by increased pressure exerted on the atria and ventricles, respectively.12 Increased hydrostatic pressure in the pulmonary vascular compartment in TACO is likely a backward failure problem, resulting from increased pressure of both the left atrium and ventricle of the heart. Therefore, elevated NPs provide evidence of hydrostatic pulmonary edema in TACO and help to differentiate it from vascular permeability edema.
NPs have been extensively researched in the field of cardiology in patients with acute heart failure (AHF). The American College of Cardiology guidelines currently include measurement of BNP, or its nonbiologically active N‐terminal prohormone cleavage fragment of BNP (NT‐proBNP), in patients with dyspnea of uncertain origin.13 There is no evidence for whether it is better to use BNP or NT‐proBNP; however, there are differences (Table S2, available as supporting information in the online version of this paper). While both accumulate with renal insufficiency, BNP appears less affected. Alternatively, the biologic half‐life of NT‐proBNP is longer (120 vs. 20 minutes for BNP).11 For ANP there is far less evidence available, and it is infrequently used in clinical practice.
B‐type natriuretic peptide
Current National Healthcare Safety Network Biovigilance Criteria from the Centers for Disease Control and Prevention guidelines14 and draft‐revised International Society of Blood Transfusion criteria for TACO15 advises either BNP or NT‐proBNP as a biomarker to diagnose TACO. No absolute cutoff value is set for either parameter; however, the latter definition includes a NT‐proBNP or a BNP post‐to‐pretransfusion ratio greater 1.5 (Table S3, available as supporting information in the online version of this paper). Well‐equipped clinical laboratories can measure NT‐proBNP in 1 hour; moreover, point‐of‐care testing has been developed that allows for bedside testing of NT‐proBNP with results within 12 minutes.16 It should be noted that BNP measurements are subject to large variations between tests due to test dependent cross‐reactivity with different compounds16, 17, 18; therefore, results and cutoffs cannot be directly translated between hospitals.19 This problem is far less pronounced in NT‐proBNP assays.20
Six articles have looked specifically at NP levels and their value in diagnosing TACO (Table 1), including a total of 793 patients, of which 209 had TACO. Studies include both prospective and retrospective cohorts. Two major categories of TACO patients have been investigated: 1) patients with respiratory distress following transfusion of in‐hospital patients (n = 678) and 2) critically ill patients with pulmonary edema after transfusion (n = 115).
Table 1.
NP values at various time points
| Study | Type | Population | Size | Testing platform | Pre‐Trx (48–0 h) | Post‐Trx (immediately) | Post‐Trx (0–48 h)‡ | p value | |
|---|---|---|---|---|---|---|---|---|---|
|
BNP ref: <150 pg/mL |
Zhou, 200524 | Case–control | Respiratory distress aftertransfusion |
‐ 21 TACO ‐ 19 STR/TNC |
Triage | 216 (110–600) | … | 389 (300–1400) | … |
| 181 (100–250)* | … | 124 (100–300)* | |||||||
| Roubinian, 201723 | Prospective cohort | Hypoxemia after transfusion in mixed cohort | ‐ 22 TACO | Unknown | 1091 (668–2030) | … | 1934 (1552–3000) | <0.01 | |
| ‐ 26 pTRALI | 298 (101–786) | … | 947 (392–2455) | ||||||
| ‐ 47 TRALI | 70 (16–278) | … | 252 (133–593) | ||||||
| Li, 200929 | Prospective cohort | ICU patients |
‐ 50 TACO ‐ 65 TRALI |
Triage | 521.5 (143–2180.3) | … | 559 (287.8–1347.8) | … | |
| 170.5 (41–407.3) | … | 375 (122.5–780.5) | |||||||
| Lieberman, 201365 | Retrospective | Consecutive 100 TACO | ‐ 3‐11 TACO§ | Unknown | 880 (234–880) | … | 762 (281–5628) | … | |
|
NT‐proBNP ref: <900 pg/mL |
Tobian, 200822 | Case–control | Transfusion reactions in mixed cohort | ‐ 16 TACO | Elecsys 2010 | 12603 ± 15329 | … | 12238 ± 17270 | … |
| ‐ 24 STR/TNC | 708 ± 991 | … | 618 ± 809 | ||||||
| Andrzejewski, 201235 | Retrospective observational cohort | Reported RBC transfusion reactions mixed cohort† | ‐ 97 TACO | Unknown | 9350 ± 33858 | 11510 ± 29604 | 14804 ± 37485 | … | |
| ‐ 242 STR | 3016 ± 3772 | 3052 ± 2767 | 4970 ± 4808 | ||||||
| ‐ 161 TNC | … | … | … | ||||||
| Li, 200929 | Prospective cohort | ICU patients | ‐ 50 TACO | Elecsys 2010 | 3410 (686–11951.5) | … | 5197 (1695–15714) | … | |
| ‐ 65 TRALI | 664 (138.5–2402) | … | 1558.5 (628.5–5114) |
Data are reported as median (interquartile range) or mean ± standard deviation.
Estimated from figures.
Andrzejewski: 79% non–critically ill patients.
Post‐Trx (0–48 hours): Sample collection within 48 hours after transfusion.
Lieberman: 100 TACO patients included in study, BNP measured in only a minority of patients.
BNP = B‐type natriuretic peptide; ICU = intensive care unit; NT‐proBNP = N‐terminal prohormone of BNP; STR = suspected transfusion reaction (not otherwise specified); TACO = transfusion‐associated circulatory overload; TNC = transfused negative control; TRALI = transfusion‐related acute lung injury; pTRALI = possible TRALI; Trx = transfusion. Triage = Testing platform by Biosite, Inc; Elecsys 2010 = Testing platform by Roche Diagnostics.
Timing of blood draw
The timing of blood draws has an effect on NP levels. Andrzejewski et al.35 investigated this with respect to the post‐to‐pretransfusion NT‐proBNP ratio. They found that posttransfusion NT‐proBNP continues to rise in the 24 hours following onset of TACO. A confounder is that values also increased in the control group, possibly through iatrogenic fluid therapy in both groups. The continued rise of NT‐proBNP peaks after 24 to 36 hours, which is shown by a study of new‐onset atrial fibrillation.21 These patients have an acute increase in left atrial hydrostatic pressure, lacking a left atrial kick, with a rapid rise in NT‐proBNP. Measurement of NPs at the time of the occurrence of posttransfusion dyspnea is likely to show the clearest picture of the patient's status and should be measured at least less than 24 hours after infusion of the suspected transfusion product.
Diagnosing TACO in a general hospital population versus critically ill
Overall, BNP and NT‐proBNP showed good accuracy diagnosing TACO in an overall hospital population. Two studies investigating transfusion reactions in a mixed cohort of ambulatory, in‐hospital, and intensive care unit (ICU) patients22, 23 showed that a posttransfusion BNP greater than 1000 pg/mL and NT‐proBNP greater than 1000 pg/mL had a ±90% specificity (Table 2). Strikingly, these cutoff levels to diagnose TACO are many times higher compared patients with AHF.22, 23, 24 To reach a sensitivity and specificity of ±90%, BNP cutoffs in TACO were greater than 1000 pg/mL versus greater than 150 pg/mL for AHF; posttransfusion NT‐proBNP cutoffs were more closely related (>1000 pg/mL vs. >900 pg/mL, respectively).25, 26, 27, 28 We are hesitant to suggest an upper cutoff limit for NPs because the range measured is very broad and the cutoff levels are many times higher than those known for this marker. Moreover, a major limitation is that the majority of studies lack a control group of patients transfused but that did not develop a transfusion reaction—transfused negative controls (TNCs)—that show this biomarker rises only in TACO. Based on absolute NT‐proBNP and BNP values of TACO patients, we suggest a lower cutoff limit below which to rule out TACO of BNP less than 300 pg/mL or NT‐proBNP less than 2000 pg/mL. Herein, BNP levels are based on a fluorescence immunoassay (Triage test, Biosite Inc.), which comparatively overestimates.18 The blood draw timing should ideally be at the time of the occurrence of symptoms but can be up to 24 hours after the suspected transfusion. It should be noted that the suggested cutoff levels are twice as high compared to commonly used AHF rule‐out values.
Table 2.
Sensitivity and specificity of natriuretic peptides
| Study | Type | Population | Size | Timing of blood draw* | Cutoff (pg/mL): | OR | Sensitivity (%) | Specificity (%) | AUC (95% CI) | |
|---|---|---|---|---|---|---|---|---|---|---|
|
BNP ref: <150 pg/mL |
Zhou, 200524 | Case–control | Respiratory distress after transfusion | ‐ 21 TACO | ±2 h | Ratio > 1.5† | 26 | 81% | 89% | … |
| ‐ 19 STR/TNC | ||||||||||
| Roubinian, 201723 | Prospective cohort | Hypoxemia mixed cohort | ‐ 22 TACO | ±16 h | >1.000 | 40 | 86% | >81%‡ | … | |
| ‐ 47 TRALI | >750 | … | 88% | 81% | 0.88 | |||||
| Li, 200929 | Prospective cohort | ICU patients | ‐ 50 TACO | <48 h | >1.000 | 37% | 89% | 0.63 (0.51–0.74) | ||
| ‐ 65 TRALI | >550 | 45% | 64% | |||||||
|
NT‐proBNP ref: <900 pg/mL |
Tobian, 200822 | Case–control | Transfusion reactions in mixed cohort | ‐ 16 TACO | <24 h | >1.000 | 59 | 94% | 83% | … |
| ‐ 24 STR/TNC | ||||||||||
| Li, 200929 | Prospective cohort | ICU patients | ‐ 50 TACO | <48 h | >10.000 | … | 40% | 90% | 0.70 (0.59–0.80) | |
| ‐ 65 TRALI | >3.000 | … | 60% | 60% |
Timing of blood draw presented as approximate time to sample collection (±) or all samples collected within period (<).
Ratio: calculated as posttransfusion BNP/baseline BNP.
Specificity >81, not specified.
AUC = area under the curve; BNP = B‐type natriuretic peptide; CI = confidence interval; ICU = intensive care unit; NT‐proBNP = N‐terminal prohormone of BNP; OR = Odds‐ratio; STR = suspected transfusion reaction (not otherwise specified); TACO = transfusion‐associated circulatory overload; TRALI = transfusion‐related acute lung injury; Trx = transfusion; TNC = transfused negative control.
When looking specifically at critically ill patients, the predictive value of NPs is poor, with sensitivity and specificity less than 60% at cutoff values over threefold higher than needed to diagnose AHF29 (Table 2). In previous studies, critical illness has been associated with elevated circulating NPs30 and is correlated with severity of disease and various acute‐phase reactants.31 NPs are elevated even in the absence of AHF, and they correlate poorly with pulmonary capillary wedge pressure in the critically ill.32 Therefore, the diagnostic potential of NPs in the ICU seems limited.
Differentiating TACO and TRALI
Differentiating TACO and TRALI based on NP levels might be possible if the patient is not critically ill and if the severity of the reaction is only mild to moderate. Whereas NPs were found to be a good discriminator in a general hospital population, this effect is lost in critically ill patients,29 which is possibly explained by the severity of TACO and TRALI. Severe TRALI manifests as acute respiratory distress syndrome (ARDS), and previous studies show that BNP is also elevated in these patients, likely caused by hypoxic vasoconstriction resulting in an increased right ventricular afterload.33
Natriuretic peptide ratios
The use of an NT‐proBNP‐to‐BNP ratio has been described before; however, this had no additional value (specifically in prognostication) over use of these biomarkers individually.34 Two studies report NT‐proBNP ratios, comparing post‐to‐pretransfusion values (Table 3). The use of a ratio for this marker is a novel approach to assessing hydrostatic pressure changes; and to our knowledge there is no evidence for this in the field of AHF. ANP ratios have never been investigated, and a BNP ratio was measured in only one study.24 Most studies focus on NT‐proBNP because time from blood draw to measurement is important to consider. BNP and NT‐proBNP remain stable for 24 and 72 hours, respectively, after which they deteriorate (Table S2).7
Table 3.
NP post/pretransfusion ratios
| Study | Type | Population | Size | Directly post‐Trx/pre‐Trx ratio | 24 hrs post‐Trx/pre‐Trx ratio: | p value: | |
|---|---|---|---|---|---|---|---|
| BNP‐Ratio | Zhou, 200524 | Case–control | Respiratory distress posttransfusion | ‐ 21 TACO | 2.1 (1.5–2.7) | … | 0.093 |
| ‐ 10 TNC | 1.0 (0.8–1.2) | ||||||
|
NT‐proBNP Ratio |
Andrzejewski, 201235 | Retrospective observational cohort | Reported RBC transfusion reactions mixed cohort* | ‐ 97 TACO | 2.6 ± 5.1 | 3.8 ± 6.5 |
Directly post‐trx/pre‐trx ratio: 0.008 24 h post‐trx/pre‐trx: 0.017 |
| ‐ 242 STR | 1.0 ± 0.3 | 1.6 ± 1.4 | |||||
| ‐ 161 Trx controls | … | … | |||||
| Li, 200929 | Prospective cohort | ICU patients | ‐ 50 TACO | … | 1.3 (1.0–3.8) | ||
| ‐ 65 TRALI | … | 2.0 (1.3–5.9) |
Data are reported as mean ± standard deviation or median (interquartile range).
Andrzejewski: 79% non–critically ill patients.
Directly post‐Trx = immediately posttransfusion; 24 hrs Post‐Trx = 24 hours after transfusion; Pre‐Trx = pretransfusion; STR = suspected transfusion reaction (not otherwise specified); TACO = transfusion‐associated circulatory overload; TRALI = transfusion‐related acute lung injury; Trx = transfusion; TNC = transfused negative control.
In the mixed‐ambulatory, ICU, and in‐hospital cohort by Andrzejewski et al.,35 a significant increase in NT‐proBNP ratio in TACO patients was observed and not in the group with suspected transfusion reactions other than TACO but further unspecified.35 The study has limitations; namely, the pretransfusion values were high—possibly a positive fluid balance or a circulatory overload–prone population. Additionally, a heterogenous control group was used (all transfusion reactions other than TACO), and values in the TNC group were not measured.
In critically ill patients, only one study investigated the NT‐proBNP‐ratio in 50 TACO patients versus 65 patients with TRALI.29 While increasing in TACO patients, the ratio increased more in the TRALI (1.3 [interquartile range, 1.0–3.8] vs. 2.0 [interquartile range, 1.3–5.9], respectively). The study is primarily limited by the lack of a proper NT‐proBNP baseline sample and the posttransfusion sampling time point (up to 48 h posttransfusion). Interestingly, baseline levels of NPs already appear highly elevated in TACO patients (Table 1). To date, there is no evidence to suggest use of a BNP ratio, as the study by Zhou et al.24 did not show differences between groups. There is limited evidence to support use NT‐proBNP ratios to diagnose TACO outside of the ICU population; however, a ratio cannot differentiate TACO from TRALI. For accurate measurements the in vivo and in vitro characteristics of NT‐proBNP should be considered, and all measurements should be performed using the same test modality.
Serial measurement of NPs
Diuretics are used as empiric therapy to treat TACO, and draft‐revised International Society of Blood Transfusion criteria use response to diuretics as evidence of TACO.36 The use of serial measurement of NPs to guide therapy during hospital admission and treatment have been investigated in the setting of goal‐directed management of AHF, and nonrandomized trials show a survival benefit. Additionally, elevated NPs at hospital discharge have been associated with a poor prognosis. The American College of Cardiology currently advises serial measurements in AHF37; however, it is unclear whether these results can be extrapolated to TACO patients.
A‐type (atrial) natriuretic peptide
ANP is a protein with identical effects to that of BNP, acting on the same receptor.11 A single case report was found describing a patient in which ANP levels showed a rapid increase following transfusion and development of TACO, with subsequent rapid decrease after furosemide therapy was instigated.38 ANP is secreted from preformed vesicles located in the atria, which is released as a result of myocardial stretch,11 and unlike BNP, it does not need to be synthesized. With a half‐life that is 10 times shorter than that of BNP, only 2 minutes, it reflects direct changes in left atrial pressure and volume. ANP might prove a more direct marker for acute overload and acute (flash) pulmonary edema following hemotherapy; though currently there is no literature on use of ANP in this setting. Future studies are needed to measure ANP levels directly before and after transfusion in TACO patients, TNCs, and TRALI patients to prove whether this biomarker has the potential to show acute‐onset pulmonary edema.
Pulmonary edema fluid protein
Analysis of bronchoalveolar lavage fluid (BALF) can be a valuable tool in assessing pulmonary vascular barrier function.39 It can be collected through direct lavage of lung segments through bronchoscopy. Protein quantification in BALF can be used to differentiate between hydrostatic pressure pulmonary edema, which is protein poor (transudate), and vascular permeability edema, which is protein rich (exudate).40 A study in TRALI patients showed a marked total protein increase compared to transfused controls.41 To determine transudate versus exudate, most commonly BALF‐to‐serum‐protein ratio is cited. A value less than 0.65 is suggestive of an intact vascular barrier, as with hydrostatic edema and thus a TACO diagnosis40; conversely, a value greater than 0.75 may be indicative of permeability pulmonary edema.42 There are limitations to this measurement; namely, patients of interest, characterized by respiratory distress, often require intubation to perform a lavage. Furthermore, the timing of lavage is important, as reabsorption of pulmonary edema can skew results. While being a logically sound parameter, BALF‐to‐serum‐protein ratio has never been validated in TACO patients and is therefore currently not evidence based.43
Biomarkers of inflammation
Cytokines
TACO by definition has no immunologic basis, as opposed to TRALI; however, differentiating these based on serum cytokine expression has proven difficult. Roubinian et al.44 compared TACO (n = 29) and TRALI patients (n = 70) to a TNC group (n = 148). Interestingly, only interleukin (IL)‐10 was significantly higher in TACO, elevated prior to transfusion and further rising posttransfusion. In TRALI patients, IL‐6 was already elevated at baseline, following the pattern of neutrophil priming, similar to results of a previous studies.45 In both TRALI and TACO, levels of IL‐6 rose significantly above transfused controls. Based on this study, posttransfusion patients with TACO were not distinguishable from TRALI by serologic cytokine profile alone.
Transfusion product–related biomarkers
A study by Kanai et al.46 prospectively investigated the effect of biologic modifiers in transfused products on pulmonary transfusion reactions in cardiopulmonary and major vascular surgery patients. Anti‐HLA Class II antibodies were identified as a significant independent predictor of TACO. The implications of this association suggest an immunologic tie‐in; however, this is unconfirmed, and its relevance is unknown. A current multicenter randomized controlled trial in the United States (NCT02094118)47 will likely shed more light on biologic modifiers in TACO, investigating the effect of washed allogeneic RBC transfusions in cardiac surgery patients. Washing of RBCs reduces soluble molecules, which include cytokines, antibodies, microparticles, and other biologic modifiers that might trigger TACO. They combine hemodynamic data with biomarkers, including ARDS markers such as PAI‐1, inflammatory cytokines IL‐6 and IL‐8, and other markers of immune activation such as sCD40L. This will allow more insight into TACO as it develops, sampling biomarkers prior to transfusion and over the course of hours and days thereafter.
Cardiac biomarkers
Troponins
One of the primary markers associated with cardiac damage are troponins, and while there is no original data from TACO patients, two articles mention its potential use.48, 49 This complex of proteins is essential in myocytes being directly involved with cell contraction; cardiac‐specific isoforms of troponins including troponin C and cardiac troponin‐1 are excellent markers of cardiomyocyte damage.50 Troponin levels can also be mildly elevated in AHF, and a number of mechanisms are postulated: 1) forward failure, resulting in subendocardial ischemia; 2) increased wall stress as a direct cause of myocyte damage; and/or 3) increased wall stress, resulting in demand ischemia.51 All of these mechanisms are exacerbated by preexisting coronary artery disease limiting oxygen delivery. Troponins are a confounder, however, as a myocardial infarction can also trigger acute heart failure and likely also TACO; therefore, elevated troponins should (until more evidence is available) be considered a risk factor as opposed to a diagnostic marker. The presence of troponins and pulmonary edema would suggest hydrostatic edema over TRALI, but there is no evidence to support this.
Novel cardiac biomarkers
The most promising biomarker for TACO is from the field of cardiology: soluble suppression of tumorgenicity‐2 (sST2). As a decoy receptor for IL‐1 and IL‐33, sST2 regulates the inflammatory processes in chronic heart failure, and like NPs, it is also released acutely in response to myocardial stretch.52 In acute dyspneic patients, it appears to outperform NT‐proBNP as a diagnostic marker for acute heart failure.53 It is a predictor of short‐ and long‐term prognosis54 and is independent of patient weight, renal function, and cardiac ejection fraction.55 No data are currently available on how it functions in the setting of flash pulmonary edema.
Other novel cardiac biomarkers have been mentioned in relation to TACO; however, based on biological characteristics these appear less directly responsive during TACO. These include cystatin C, a kidney damage marker used to signal cardiorenal disease, which is useful in chronic cardiovascular disease but not in AHF56; and growth differentiation factor 15, a cardiac growth hormone that appears elevated in AHF. While it is correlated to dyspnea, troponins, NP levels, and overall cardiovascular mortality,57 it has not been tested as a marker in flash pulmonary edema or as a response marker after treatment. Finally, galectin‐3 is a marker excreted by macrophages and is specifically involved in remodeling and fibrosis in heart disease. It is elevated in patients with acute heart failure58 but is better in predicting long‐term outcomes than conveying severity of dyspnea.59
DISCUSSION
Biomarkers have the potential to be a valuable tool in the diagnosis of TACO, as pathognomonic signs and symptoms are lacking.60 TACO is underrecognized and underreported,7, 8 and biomarkers can influence therapeutic decisions as well as more robustly define TACO patients for research purposes.
Serologic biomarkers including BNP and NT‐proBNP have been investigated. Measurement of these markers takes approximately 1 hour, and point‐of‐care testing can measure NT‐proBNP within a few minutes.16 For BNP, however, there is a large interassay variation that limits translatability of results between hospitals.17, 18 The current review of studies shows that NPs should not be used in a critically ill population, as they are elevated at baseline and do not significantly increase in TACO patients alone. Very low levels of NPs are likely to exclude TACO. Interestingly, pretransfusion levels of NPs in most studies were already highly elevated, which can be a result of a number of factors: 1) known risk factors for TACO include heart failure and kidney dysfunction, both of which increase this marker; 2) selection bias thereby including only a subset of patients; 3) fluid administration is one of the most common interventions in hospitalized patients, and currently no studies have investigated hydrostatic biomarkers in a general in‐hospital population; or 4) elevated NPs may be an independent risk factor that can be used to identify at‐risk patients and should be further investigated.
A post/pretransfusion ratio should be used only if posttransfusion levels are above the suggested cutoffs. In this case, an elevated ratio supports the diagnosis of TACO; however, it does not differentiate TACO from TRALI. Posttransfusion NPs are likely dependable only if samples are drawn within the first 24 hours after transfusion. Pretransfusion levels, if not determined before transfusion, can still be measured from residual blood samples. NT‐proBNP is preferred due to its in vitro stability after a blood draw; prolonged storage will degrade the pretransfusion NPs and falsely increase the ratio. In situations in which NPs are elevated before transfusion but do not increase after transfusion, they should be considered nondiagnostic, and TACO cannot be ruled out. While the overall level of evidence for the use of NPs is poor, based on these test characteristics we propose the following approach detailed in Fig. 2. It should be noted that following this algorithm can guide clinicians toward the diagnosis, but the precise test sensitivity and specificity for this approach remain uncertain.
Figure 2.
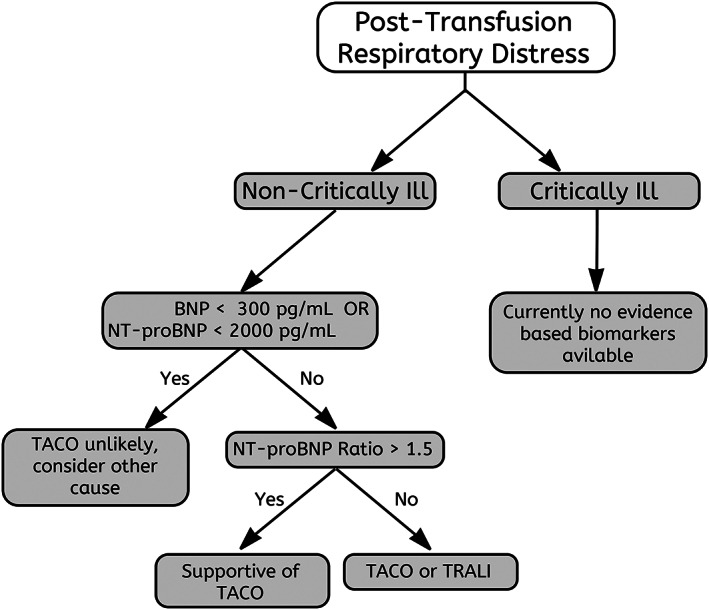
Diagnostic algorithm for TACO. Using diagnostic biomarkers in the diagnosis of TACO. Pretransfusion measurements can be determined from residual blood samples if in vitro stability is taken into consideration. Posttransfusion blood sampling should be performed less than 24 hours after suspected transfusion reaction.
Other diagnostic tests for TACO include BALF analysis, frequently mentioned as a principal discriminator between TACO and other forms of pulmonary edema,40 but to date there are no data to support this. Serologic cytokine profiles have been investigated but currently lack a clear diagnostic capacity to differentiate TACO and TRALI. There are a number of promising cardiac biomarkers that elevate acutely in cardiogenic pulmonary edema and should be reevaluated as more data become available. We have provided key recommendations for the use of biomarkers in the diagnosis of TACO in Table 4.
Table 4.
Key recommendations
| Recommendations for clinicians | |
| 1. | Natriuretic peptides cannot be used to rule in TACO, as cut off values are not clear. |
| 2. | A BNP <300 pg/mL or NT‐proBNP <2000 pg/mL posttransfusion measurement likely rules out TACO. |
| 3. | In critically ill patients, BNP and NT‐proBNP cannot be used to diagnose TACO or differentiate it from TRALI. |
| 4. | NT‐proBNP appears preferable to BNP for calculating post‐to‐pretransfusion ratios. NT‐proBNP has a longer in‐vitro stability and residual pretransfusion blood samples can be used in suspected TACO patients. |
| 5. | Post‐to‐pretransfusion ratio of NT‐proBNP ≥1.5 can aid in the diagnosis of TACO; a ratio should be used only if posttransfusion values are elevated, and a ratio <1.5 does not rule out TACO. |
| 6. | BALF‐protein‐to‐serum‐protein ratio needs to be validated before clinical use. |
| Recommendations for research | |
| 1. | When investigating serologic biomarkers, positive as well as negative controls should be included to better characterize development of TACO. Patients transfused but without adverse reactions should be included, as well as TRALI patients. |
| 2. | Future studies should focus on prevention of TACO through identification of at‐risk patients. Biomarker measurement before transfusion, such as natriuretic peptides, may be valuable in personalizing transfusion speed, number of units, or other interventions such as prophylactic diuretics. |
| 3. | Finding diagnostic biomarkers should be a high priority in critically ill patients. BALF analysis, namely, protein and cytokine quantification, are potential targets. |
| 4. | Cooperation in biomarker research specialties is likely to accelerate understanding, proper diagnosis, and monitoring response to treatment of TACO. Disciplines should include transfusion medicine, cardiology, nephrology, intensive care medicine, and anesthesiology. |
BALF = bronchoalveolar lavage fluid; BNP = B‐type natriuretic peptide; NT‐proBNP = N‐terminal prohormone of BNP; TACO = transfusion‐associated circulatory overload; TRALI = transfusion‐related acute lung injury.
Our review has limitations: The biomarker test characteristics have been analyzed as stand‐alone biomarkers for disease. However, TACO is a clinical diagnosis, and the diagnostic value, when combined with clinical signs and symptoms, has proven better sensitivity and specificity in a number of the studies.22, 24, 61 An ideal biomarker for TACO should be present only in disease and correlate with severity of pulmonary edema. Future research on biomarkers is needed to validate tools such as BALF‐protein‐to‐serum‐protein ratio. Considering the invasive nature of the test, this test is likely a good diagnostic tool specifically in a critically ill population. Additionally, a biomarker such as ANP has the characteristics to potentially be a direct serologic biomarker that appears after development of TACO and be a measure of disease resolution after instigation of treatment.
Other assays such as BALF cytokines have been investigated in ARDS but are currently not used outside of a research setting. Pulmonary damage in ARDS has been widely associated with IL‐6 levels in alveolar fluid, which is associated with systemic inflammation and immune cell influx in the lungs. In an ICU study of 74 patients, BALF cytokine measurements were compared between patients with pneumonia, ARDS, and cardiogenic pulmonary edema.62 In patients with ARDS, IL‐6 is highly elevated while only marginally elevated in cardiogenic pulmonary edema. This marker might prove a more specific measure than serum cytokines following transfusion.
With continued research into biomarkers of TACO, we anticipate a number of areas in which TACO research is likely to progress. The rise in both interleukins and an association with HLA Class II antibodies in TACO suggests an inflammatory component. This is underscored by studies documenting a decrease of TACO since universal leukoreduction of blood products63 and fever in up to one third of patients.35, 64 Speculation about this is beyond the scope of this review; however, if an inflammatory component is involved, then additional avenues for biomarkers are open. Additionally, with more insight into risk factors, it is possible to move toward a patient‐tailored hemotherapy in high‐risk patients. Pretransfusion NPs measured parallel to crossmatching (both taking approximately 1 hour) is likely to influence transfusion practice with interventions such as lowering infusion speed, use of prophylactic furosemide (currently not evidence based), or abstaining from transfusion all together. We have included our recommendations for focusing initial research into TACO biomarkers (Table 4).
CONCLUSION
This systematic review suggests that TACO is unlikely if BNP is less than 300 pg/mL or NT‐proBNP is less than 2000 pg/mL. An NT‐proBNP ratio greater than 1.5 in non‐ICU populations supports the diagnosis of TACO; however, a lower ratio does not exclude this. Herein, NT‐proBNP as an analyte has superior in vitro stability in residual blood samples compared to BNP, being more a pragmatic marker aside from having decreased variability between testing methods. Diagnosing TACO in a critically ill population is more difficult because NPs and other serological biomarkers such as cytokine assays are likely to be skewed; hence, other markers such as BALF analysis may prove more valuable for this patient group. The only other biomarker specifically researched is the measurement of serum cytokines, which show no clear pattern in differentiating TACO and TRALI.
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
Supporting information
Appendix S1. Search Strategy.
Table S1. Included TACO biomarker studies.
Table S2. N‐terminal proBNP versus BNP measurement and cutoffs.
Table S3. Diagnostic criteria of TACO versus TRALI
REFERENCES
- 1. Andrzejewski C, Casey MA, Popovsky MA. How we view and approach transfusion‐associated circulatory overload: pathogenesis, diagnosis, management, mitigation, and prevention. Transfusion 2013;53:3037‐47. [DOI] [PubMed] [Google Scholar]
- 2. Bolton‐Maggs, PH , Thomas D, Watt A. Annual SHOT report 2016. [cited 2017 Nov]. Available from: https://www.shotuk.org/wp-content/uploads/myimages/SHOT-Report-2016_web_11th-July.pdf.
- 3. Transfusie‐ en Transplantatiereacties in Patienten . TRIP rapport hemovigilantie 2015. [cited 2017 Nov]. Available from: https://www.tripnet.nl/wp-content/uploads/2017/12/Trip.HEMO‐uitgebreid‐def‐met‐links.3.pdf.
- 4. Agence Nationale de Sécurité du Médicament et des Produits de Santé . Rapport dactivité hémovigilance 2015. [cited 2017 Nov]. Available from: http://ansm.sante.fr/var/ansm_site/storage/original/application/27ce3d0739821882c0cd87041b8050a7.pdf.
- 5. National Healthcare Safety Network—Centre for Communicable Diseases and Infection Control . Transfusion transmitted injuries surveillance system (TTISS): 2009‐2013 Summary Results. [cited 2018 Aug]. Available from: https://www.canada.ca/content/dam/hc-sc/healthy-canadians/migration/publications/drugs-products-medicaments-produits/blood-transfusion-2013-transfusionnels/alt/transfusion-eng.pdf.
- 6. Food and Drug Administration . Fatalities reported to FDA following blood collection and transfusion: annual summary for FY2016. [cited 2018 Aug]. Available from: http://www.fda.gov/downloads/BiologicsBloodVaccines/SafetyAvailability/ReportaProblem/TTransfusionDonationFatalities/UCM459461.pdf.
- 7. Raval JS, Mazepa MA, Russel SL, et al. Passive reporting greatly underestimates the rate of transfusion‐associated circulatory overload after platelet transfusion. Vox Sang 2015;108:387‐92. [DOI] [PubMed] [Google Scholar]
- 8. Bosboom JJ, Klanderman RB, Peters AL, et al. The practice of diagnosing and reporting transfusion‐associated circulatory overload: a national survey among physicians and haemovigilance officers. Transfus Med 2018;28:363‐70. [DOI] [PubMed] [Google Scholar]
- 9. Vlaar AP, Juffermans NP. Transfusion‐related acute lung injury: a clinical review. Lancet 2013;382:984‐94. [DOI] [PubMed] [Google Scholar]
- 10. Tinegate H, Birchall J, Gray A, et al. Guideline on the investigation and management of acute transfusion reactions. Prepared by the BCSH blood transfusion task force. Br J Haematol 2012;159:143‐53. [DOI] [PubMed] [Google Scholar]
- 11. Fu S, Ping P, Wang F, et al. Synthesis, secretion, function, metabolism and application of natriuretic peptides in heart failure. J Biol Eng 2018;12:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Iwanaga Y, Nishi I, Furuichi S, et al. B‐type natriuretic peptide strongly reflects diastolic wall stress in patients with chronic heart failure. J Am Coll Cardiol 2006;47:742‐8. [DOI] [PubMed] [Google Scholar]
- 13. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J Am Coll Cardiol 2013;62:e147‐239. [DOI] [PubMed] [Google Scholar]
- 14. Center for Disease Control—Division of Healthcare Quality Promotion . National Healthcare Safety Network biovigilance component hemovigilance module surveillance Protocol. [cited 2017 Dec]. Available from: https://www.cdc.gov/nhsn/pdfs/biovigilance/bv-hv-protocol-current.pdf.
- 15. International Society of Blood Transfusion—Working Party on Haemovigilance . Proposed standard definitions for surveillance of non‐infectious adverse transfusion reactions. [cited: 2017 Nov]. Available from: http://www.isbtweb.org/fileadmin/user_upload/Proposed_definitions_2011_surveillance_non_infectious_adverse_reactions_haemovigilance_incl_TRALI_correction_2013.pdf.
- 16. Collin‐Chavagnac D, Dehoux M, Schellenberg F, et al. Head‐to‐head comparison of 10 natriuretic peptide assays. Clin Chem Lab Med 2015;53:1825‐37. [DOI] [PubMed] [Google Scholar]
- 17. Sykes E, Karcher RE, Eisenstadt J, et al. Analytical relationships among Biosite, Bayer, and Roche methods for BNP and NT‐proBNP. Am J Clin Pathol 2005;123:584‐90. [DOI] [PubMed] [Google Scholar]
- 18. Franzini M, Masotti S, Prontera C, et al. Systematic differences between BNP immunoassays: comparison of methods using standard protocols and quality control materials. Clin Chim Acta 2013;424:287‐91. [DOI] [PubMed] [Google Scholar]
- 19. Clerico A, Franzini M, Masotti S, et al. State of the art of immunoassay methods for B‐type natriuretic peptides: an update. Crit Rev Clin Lab Sci 2015;52:56‐69. [DOI] [PubMed] [Google Scholar]
- 20. Mueller T, Gegenhuber A, Poelz W, et al. Comparison of the Biomedica NT‐proBNP enzyme immunoassay and the Roche NT‐proBNP chemiluminescence immunoassay: implications for the prediction of symptomatic and asymptomatic structural heart disease. Clin Chem 2003;49:976‐9. [DOI] [PubMed] [Google Scholar]
- 21. Deftereos S, Giannopoulos G, Kossycakis C, et al. Short‐term fluctuations of plasma NT‐proBNP levels in patients with new‐onset atrial fibrillation: a way to assess time of onset? Heart 2010;96:1033‐6. [DOI] [PubMed] [Google Scholar]
- 22. Tobian AA, Sokoll LJ, Tisch DJ, et al. N‐terminal pro‐brain natriuretic peptide is a useful diagnostic marker for transfusion‐associated circulatory overload. Transfusion 2008;48:1143‐50. [DOI] [PubMed] [Google Scholar]
- 23. Roubinian NH, Looney MR, Keating S, et al. Differentiating pulmonary transfusion reactions using recipient and transfusion factors. Transfusion 2017;57:1684‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhou L, Giacherio D, Cooling L, et al. Use of B‐natriuretic peptide as a diagnostic marker in the differential diagnosis of transfusion‐associated circulatory overload. Transfusion 2005;45:1056‐63. [DOI] [PubMed] [Google Scholar]
- 25. Dao Q, Krishnaswamy P, Kazanegra R, et al. Utility of B‐type natriuretic peptide in the diagnosis of congestive heart failure in an urgent‐care setting. J Am Coll Cardiol 2001;37:379‐85. [DOI] [PubMed] [Google Scholar]
- 26. Maisel AS, Krishnaswamy P, Nowak R, et al. Rapid measurement of B‐type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med 2002;347:161‐7. [DOI] [PubMed] [Google Scholar]
- 27. Januzzi JL, Camargo CA, Anwaruddin S, et al. The N‐terminal Pro‐BNP Investigation of Dyspnea in the Emergency Department (PRIDE) study. Am J Cardiol 2005;95:948‐54. [DOI] [PubMed] [Google Scholar]
- 28. Januzzi JL, van Kimmenade R, Lainchbury J, et al. NT‐proBNP testing for diagnosis and short‐term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients. Eur Heart J 2006;27:330‐7. [DOI] [PubMed] [Google Scholar]
- 29. Li G, Daniels CE, Kojicic M, et al. The accuracy of natriuretic peptides (brain natriuretic peptide and N‐terminal pro‐brain natriuretic) in the differentiation between transfusion‐related acute lung injury and transfusion‐related circulatory overload in the critically ill. Transfusion 2009;49:13‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cuthbertson BH, Patel RR, Croal BL, et al. B‐type natriuretic peptide and the prediction of outcome in patients admitted to intensive care. Anaesthesia 2005;60:16‐21. [DOI] [PubMed] [Google Scholar]
- 31. Varpula M, Pulkki K, Karlsson S, et al. Predictive value of N‐terminal pro–brain natriuretic peptide in severe sepsis and septic shock. Crit Care Med 2007;35:1277‐83. [DOI] [PubMed] [Google Scholar]
- 32. Forfia PR, Watkins SP, Rame JE, et al. Relationship between B‐type natriuretic peptides and pulmonary capillary wedge pressure in the intensive care unit. J Am Coll Cardiol 2005;45:1667‐71. [DOI] [PubMed] [Google Scholar]
- 33. Karmpaliotis D, Kirtane A, Ruisi C, et al. Diagnostic and prognostic utility of brain natriuretic peptide in subjects admitted to the ICU with hypoxic respiratory failure due to noncardiogenic and cardiogenic pulmonary edema. Chest 2007;131:964‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jensen J, Ma LP, Bjurman C, et al. Prognostic values of NTpro BNP/BNP ratio in comparison with NT proBNP or BNP alone in elderly patients with chronic heart failure in a 2‐year follow up. Int J Cardiol 2012;155:1‐5. [DOI] [PubMed] [Google Scholar]
- 35. Andrzejewski C, Popovsky MA, Stec TC, et al. Hemotherapy bedside biovigilance involving vital sign values and characteristics of patients with suspected transfusion reactions associated with fluid challenges: can some cases of transfusion‐associated circulatory overload have proinflammatory aspects? Transfusion 2012;52:2310‐20. [DOI] [PubMed] [Google Scholar]
- 36. International Society of Blood Transfusion—Working Party on Haemovigilance . Transfusion‐associated circulatory overload (TACO)—draft revised reporting criteria. [cited 2017 Nov]. Available from: https://www.aabb.org/research/hemovigilance/Documents/TACO-reporting-criteria-draft-Nov-2016.pdf.
- 37. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America. Circulation 2017;136:e137‐61. [DOI] [PubMed] [Google Scholar]
- 38. Fiebig EW, Wu AH, Krombach J, et al. Transfusion‐related acute lung injury and transfusion‐associated circulatory overload: mutually exclusive or coexisting entities? Transfusion 2007;47:171‐2. [DOI] [PubMed] [Google Scholar]
- 39. Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med 2001;163:1376‐83. [DOI] [PubMed] [Google Scholar]
- 40. Gajic O, Gropper MA, Hubmayr RD. Pulmonary edema after transfusion: how to differentiate transfusion‐associated circulatory overload from transfusion‐related acute lung injury. Crit Care Med 2006;34:S109‐13. [DOI] [PubMed] [Google Scholar]
- 41. Vlaar AP, Hofstra JJ, Determann RM, et al. Transfusion‐related acute lung injury in cardiac surgery patients is characterized by pulmonary inflammation and coagulopathy: a prospective nested case‐control study. Crit Care Med 2012;40:2813‐20. [DOI] [PubMed] [Google Scholar]
- 42. Church GD, Price C, Sanchez R, et al. Transfusion‐related acute lung injury in the paediatric patient: two case reports and a review of the literature. Transfus Med 2006;16:343‐8. [DOI] [PubMed] [Google Scholar]
- 43. Skeate RC, Eastlund T. Distinguishing between transfusion related acute lung injury and transfusion associated circulatory overload. Curr Opin Hematol 2007;14:682‐7. [DOI] [PubMed] [Google Scholar]
- 44. Roubinian NH, Looney MR, Kor DJ, et al. Cytokines and clinical predictors in distinguishing pulmonary transfusion reactions. Transfusion 2015;55:1838‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Silliman CC, Boshkov LK, Mehdizadehkashi Z, et al. Transfusion‐related acute lung injury: epidemiology and a prospective analysis of etiologic factors. Blood 2003;101:454‐62. [DOI] [PubMed] [Google Scholar]
- 46. Kanai R, Iijima T, Hashimoto S, et al. Impact of immunoreactive substances contained in apheresis platelet concentrate on postoperative respiratory function in surgical patients receiving platelet transfusion: a prospective cohort study. Transfus Med 2015;23:344‐50. [DOI] [PubMed] [Google Scholar]
- 47. Ben‐Tal O, Zwang E, Eichel R, et al. Vitamin K‐dependent coagulation factors and fibrinogen levels in FFP remain stable upon repeated freezing and thawing. Transfusion 2003;43:873‐7. [DOI] [PubMed] [Google Scholar]
- 48. Roubinian NH, Murphy EL. Transfusion‐associated circulatory overload (TACO): prevention, management, and patient outcomes. Int J Clin Transfus Med 2015;3:17‐28. [Google Scholar]
- 49. Gilliss BM, Looney MR, Gropper MA, et al. Reducing noninfectious risks of blood transfusion. J Am Soc Anesthesiol 2011;115:635‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Eur Heart J 2012;33:2551‐67. [DOI] [PubMed] [Google Scholar]
- 51. Wettersten N, Maisel AS. Role of cardiac troponin levels in acute heart failure. Card Failure Rev 2015;1:102‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Villacorta H, Maisel AS. Soluble ST2 testing: a promising biomarker in the management of heart failure. Arq Bras Cardiol 2016;106:145‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Huang A, Qi X, Hou W, et al. Prognostic value of sST2 and NT‐proBNP at admission in heart failure with preserved, mid‐ranged and reduced ejection fraction. Acta Cardiol 2018;73:41‐8. [DOI] [PubMed] [Google Scholar]
- 54. Rehman SU, Mueller T, Januzzi JL. Characteristics of the novel interleukin family biomarker ST2 in patients with acute heart failure. J Am Coll Cardiol 2008;52:1458‐65. [DOI] [PubMed] [Google Scholar]
- 55. Januzzi JL, Peacock WF, Maisel AA, et al. Measurement of the interleukin family member ST2 in patients with acute dyspnea. J Am Coll Cardiol 2007;50:607‐13. [DOI] [PubMed] [Google Scholar]
- 56. Ix JH, Shlipak MG, Chertow GM, et al. Association of cystatin C with mortality, cardiovascular events, and incident heart failure among persons with coronary heart disease: data from the heart and soul study. Circulation 2007;115:173‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cotter G, Voors AA, Prescott MF, et al. Growth differentiation factor 15 (GDF‐15) in patients admitted for acute heart failure: results from the RELAX‐AHF study. Eur J Heart Fail 2015;17:1133‐43. [DOI] [PubMed] [Google Scholar]
- 58. Fermann GJ, Lindsell CJ, Storrow AB, et al. Galectin 3 complements BNP in risk stratification in acute heart failure. Biomarkers 2012;17:706‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sygitowicz G, Tomaniak M, Filipiak KJ, et al. Galectin‐3 in patients with acute heart failure: preliminary report on first polish experience. Adv Clin Exp Med 2016;25:617‐23. [DOI] [PubMed] [Google Scholar]
- 60. Bux J, Sachs U. Pulmonary transfusion reactions. Transfus Med Hemother 2008;35:337‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Roubinian NH, Hendrickson JE, Triulzi DJ, et al. Incidence and clinical characteristics of transfusion‐associated circulatory overload using an active surveillance algorithm. Vox Sang 2017;112:56‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Schütte H, Lohmeyer J, Rosseau S, et al. Bronchoalveolar and systemic cytokine profiles in patients with ARDS, severe pneumonia and cardiogenic pulmonary oedema. Eur Respir J 1996;9:1858‐67. [DOI] [PubMed] [Google Scholar]
- 63. Blumberg N, Heal JM, Gettings KF, et al. An association between decreased cardiopulmonary complications (transfusion‐related acute lung injury and transfusion‐associated circulatory overload) and implementation of universal leukoreduction of blood transfusions. Transfusion 2010;50:2738‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Parmar N, Pendergrast J, Lieberman L, et al. The association of fever with transfusion‐associated circulatory overload. Vox Sang 2018;112:70‐8. [DOI] [PubMed] [Google Scholar]
- 65. Lieberman L, Maskens C, Cserti‐Gazdewich C, et al. A retrospective review of patient factors, transfusion practices, and outcomes in patients with transfusion‐associated circulatory overload. Transfus Med Rev 2013;27:206‐12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Search Strategy.
Table S1. Included TACO biomarker studies.
Table S2. N‐terminal proBNP versus BNP measurement and cutoffs.
Table S3. Diagnostic criteria of TACO versus TRALI


