Abstract
Metachromatic leukodystrophy (MLD) is a rare progressive neurological disorder, often accompanied by motor impairments that are challenging to treat. In this case series, we report the course of treatment with intrathecal baclofen (ITB), aimed at improving daily care and comfort in children and young adults with MLD. All patients with MLD in our centre on ITB treatment for a minimum of 6 months were included (n=10; 4 males, 6 females; mean age 10y 8mo [range 6–24y]). Eight patients had MLD with a predominant spastic movement disorder (sMLD) and two were mainly dyskinetic. Patients with sMLD were compared with matched patients with spastic cerebral palsy (CP). Complication rates related to ITB treatment were similar in both groups. ITB treatment course in the first 6 months after pump implantation appears to show more dose increase in most patients MLD, compared to patients with spastic CP. This may be due to the progressive disease in MLD. ITB is a feasible therapy to improve daily care and comfort in patients with MLD and should therefore be considered early.
What this paper adds
Intrathecal baclofen (ITB) is a feasible therapy to improve comfort and daily care in children and young people with metachromatic leukodystrophy (MLD).
In the first 6 months of ITB treatment, MLD seems to show more dose increase compared to spastic cerebral palsy.
What this paper adds
Intrathecal baclofen (ITB) is a feasible therapy to improve comfort and daily care in children and young people with metachromatic leukodystrophy (MLD).
In the first 6 months of ITB treatment, MLD seems to show more dose increase compared to spastic cerebral palsy.
This article's abstract has been translated into Spanish and Portuguese.
Follow the links from the abstract to view the translations.
Resumen
Baclofen intratecal en la leucodistrofia metacromática
La leucodistrofia metacromática (LDM) es una rara enfermedad neurológica progresiva, habitualmente acompañada de déficit motor de muy difícil abordaje. En esta serie de casos reportamos el curso del tratamiento con baclofen intratecal (BIT), con el fin de mejorar el cuidado diario y el bienestar en niños y adultos jóvenes con esta afectación. Todos los pacientes de nuestro centro portadores de LDM y en tratamiento con BIT fueron incluidos (n=10; 4 varones, 6 mujeres, edad medias 10 años 8 meses [rango 6‐24 años]). Ocho pacientes tenían una LDM con un desorden del movimiento predominantemente espástico (LDMesp) y dos eran predominantemente diskinética. Los pacientes con LDMesp fueron comparados con pacientes portadores de Parálisis Cerebral Infantil (PCI). Las ratios de complicaciones relacionados con el tratamiento con BIT fueron similares en ambos grupos. El curso del tratamiento con BIT en los primeros 6 meses luego de la implantación de la bomba aparentemente muestra un mayor aumento de la dosis en gran parte de los pacientes con LDM, comparado con los pacientes con PCI espástica. Esto podría deberse a las características progresivas de la LDM. El BIT es una terapia factible y mejora el bienestar y cuidado diario de los pacientes con LDM, y, debería por ello, ser considerada tempranamente como tratamiento.
Resumo
Baclofeno intratecal em leucodistrofia metacromática
A leucodistrofia metacromática (LDM) é uma desordem neurológica rara e progressiva, frequentemente acompanhada por deficiências motoras que são difíceis de tratar. Nesta série de casos, reportamos o curso do tratamento com baclofeno intratecal (BIT), com o objetivo de melhorar o cuidado diário e conforto em crianças e jovens com LDM. Todos os pacientes com LDM em nosso centro para tratamento com BIT por no mínimo 6 meses foram incluídos (n=10; 4 do sexo masculino, 6 do sexo feminino; média de idade 10a 8m [variação 6–24a]). Oito pacientes tinham LDM com desordem do movimento predominantemente espástica (eLDM) e dois eram predominantemente discinéticos. Pacientes com eLDM foram pareados com pacientes com paralisia cerebra (PC) espástica. As taxas de complicação relacionadas ao tratamento com BIT foram similares em ambos os grupos. O curso do tratamento com BIT nos primeiros 6 meses após implantação da bomba parece mostrar maior aumento de dose nos pacientes com LDM, comparados aos com PC espástica. Isso pode ser devido à doença progressiva em LDM. BIT é uma terapia viável para melhorar o cuidado diário e conforto em pacientes com LDM, e deve portanto ser considerado precocemente.
ABBREVIATIONS
- dMLD
Metachromatic leukodystrophy with a predominant dyskinetic movement disorder
- ITB
Intrathecal baclofen
- MLD
Metachromatic leukodystrophy
- sMLD
Metachromatic leukodystrophy with a predominant spastic movement disorder
Metachromatic leukodystrophy (MLD, OMIM 250100) is an autosomal recessive lysosomal disorder leading to progressive neurological decline in a previously healthy child or adult. Increasing motor impairments such as spasticity and dyskinesia are challenging to treat and often hamper daily care and comfort.1, 2
MLD is divided into three clinical subtypes, based on the age at onset: the late‐infantile form starts before 30 months of age, the juvenile form before 16 years, and the adult form thereafter. First symptoms and signs in younger patients consist of motor deterioration, older patients usually present with cognitive and psychiatric symptoms.3
Currently, there is no curative treatment for MLD. Allogenic hematopoietic cell transplantation has led to encouraging results, if performed in an early stage of the disease, especially for juvenile and adult patients.4 First results for a combined autologous hematopoietic cell transplantation with gene‐therapy approach are promising.5
However, in the many patients for whom the diagnosis comes too late and who are no longer eligible for hematopoietic cell transplantation, relentless disease progression is inevitable. All juvenile patients with MLD develop a spastic and often also dyskinetic movement disorder; young patients rapidly, older patients more slowly. Spasticity, dyskinesia, and pain can limit optimal daily care, transfers, and sitting.
When side effects occur and problems persist with oral spasmolytic medication like baclofen, intrathecal baclofen (ITB) therapy is an alternative treatment. To reach an adequate effect, the dose is increased in a stepwise manner by continuous delivery or, when vanishing of effect occurs repeatedly, by a flexible programme with periodic bolus administration.6, 7
During the last decades, experience with ITB has increased, and it is reported to be an effective therapy to treat spasticity related problems in daily life for spastic cerebral palsy (CP).8, 9 Positive effects in dyskinetic CP have also been reported, but current scientific evidence is limited. ITB has recently been described to have beneficial effects in treatment of spasticity in progressive neurological diseases like MLD.9, 10
As very little is known about the clinical course of ITB treatment in MLD, our aim is to describe the characteristics of juvenile patients with MLD who were treated with ITB, and the course of ITB treatment in this group. To ascertain whether the progressive nature of the disease required other dose adaptations than normally applied in static encephalopathies in children and young adults, we contrasted the course of ITB treatment in MLD with a predominant spastic movement disorder (sMLD) with patients with spastic CP.
Case series
All patients with juvenile MLD (diagnosis established by typical clinical and magnetic resonance imaging findings, arylsulfatase A activity and ARSA mutation analysis) who were treated with ITB in our centre between February 2002 and December 2016, and had a baclofen pump for at least 6 months, were included. Ten patients with MLD were treated with ITB in this period. Eight patients had sMLD and two patients had MLD with a predominant dyskinetic movement disorder (dMLD) (Table 1). At the start of the therapy, all children were non‐walking, described by the Gross Motor Function Classification for MLD (GMFC‐MLD) level III to VI.11 This classification system is adapted from the Gross Motor Function Classification System (GMFCS) which was created for use with children with CP.12
Table 1.
Patient characteristics
| Patient characteristics | sMLD | dMLD | Spastic CP |
|---|---|---|---|
| Sex (n) | |||
| Male | 4 | 4 | |
| Female | 4 | 2 | 4 |
| Clinical subtype (n) | |||
| Late infantile | 0 | 0 | |
| Early juvenile | 3 | 0 | |
| Late juvenile | 5 | 2 | |
| Adult | 0 | 0 | |
| Type of predominant movement disorder (n) | |||
| Spastic | 8 | 0 | 8 |
| Dyskinetic | 0 | 2 | 0 |
| Age at diagnosis | |||
| Mean (range) | 8y (5–14y) | 8y (7–9y) | |
| Age when wheelchair dependent | |||
| Mean (range) | 9y (6–17y) | 8y 6mo (8–9y) | |
| Age at pump implantation | |||
| Mean (range) | 11y (6–24y) | 9y 6mo (9–10y) | 11y (5–26y) |
| GMFC‐MLD/GMFCS levela (n) | |||
| III | 1 | 0 | 0 |
| IV | 2 | 0 | 3 |
| V | 4 | 2 | 5 |
| VI | 1 | 0 | |
| Position of catheter tip (n) | |||
| C4 | 0 | 2 | 0 |
| Th4 | 5 | 0 | 3 |
| Th10 | 3 | 0 | 5 |
| Purpose of ITB (multiple goals possible) (n) | |||
| Improve care | 7 | 0 | 8 |
| Decrease pain | 1 | 2 | 2 |
| Improve transfers | 1 | 0 | 0 |
| Improve sitting position | 3 | 1 | 1 |
| Prevent contractures | 0 | 0 | 1 |
| Complications (n) | |||
| Pump infectionb | 0 | 0 | 1 |
| Catheter disconnectionc | 1 | 0 | 1 |
aScore at pump implantation. bAt 21 days: negative cultures, removal of the pump. Second implantation included in the study. cOne patient with sMLD at 20 days: revision of the catheter; one patient with spastic CP at 1mo: revision of the catheter. sMLD, metachromatic leukodystrophy with a predominant spastic movement disorder; dMLD, metachromatic leukodystrophy with a predominant dyskinetic movement disorder; CP, cerebral palsy; GMFC‐MLD, Gross Motor Function Classification in metachromatic leukodystrophy; GMFCS, Gross Motor Function Classification System; ITB, intrathecal baclofen.
For patients with sMLD (n=8), a matched control group of patients with spastic CP treated with ITB was composed, out of all patients with a baclofen pump in our centre. This spastic CP group was matched with the sMLD group for age, sex, and mobility level (non‐walking, GMFCS levels IV and V). Only the patients with sMLD were matched with patients with spastic CP, as patients with spasticity appear to require different dosing than patients with dyskinesia,13 and the group of patients with a predominant dMLD (n=2) was too small to match.
Patient characteristics are summarized in Table 1. We reported the course of treatment during the first 6 months after implantation. Dose pump settings at 6 months after implantation, including dose of ITB in micrograms (μg) per day and ITB dosing mode (simple continuous or flexible programme with bolus administration), were used as outcome parameters as we hypothesized that in a progressive disease such as MLD, other adaptations of total dose of ITB and dosing mode might be necessary because of its progressive nature. The starting dose after pump implantation was used as first measurement (t=0).
In one patient with MLD and one patient with spastic CP, catheter disconnection occurred as a complication, necessitating surgical revision. In one patient with spastic CP, the baclofen pump became infected within 1 month after implantation, leading to pump removal. The patient was included in the study after reimplantation 2 years later.
All patients with MLD were wheelchair dependent within 3 years after diagnosis (mean 8y 10mo, range 6–17y). Six out of eight patients with sMLD (aged 6–8y) were started on ITB therapy within 2 years after wheelchair dependency. In two older children (13y and 17y at the time of wheelchair dependency), ITB treatment started 7 years later. In both patients with dMLD, ITB therapy started 1 year after wheelchair dependency.
In the patients with sMLD, the mean starting dose was 68μg per day (standard deviation [SD] 23.60, range 40–100μg). At 6 months, five out of eight patients with sMLD had a flexible programme with bolus administration. The mean daily dose at 6 months, including the boluses when a flexible programme was used, was 210μg (SD 119.97, range 70–428μg) (Fig. 1).
Figure 1.
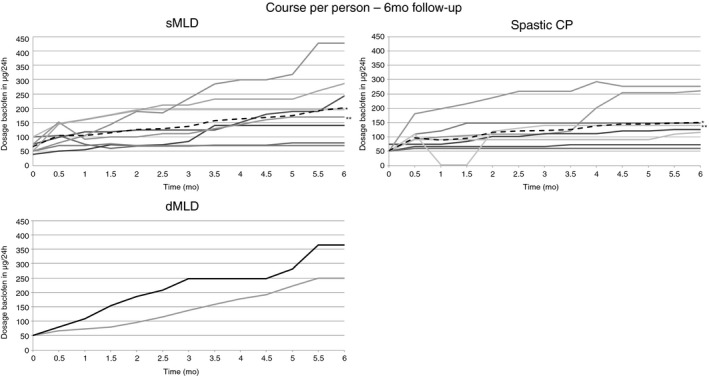
Course per patient in sMLD, spastic CP, and dMLD group: 6 months follow‐up. aMean of the group. bCatheter disconnection: one patient with sMLD at 20 days after pump implantation; one patient with spastic CP at 1 month after pump implantation. sMLD, metachromatic leukodystrophy with predominant spastic movement disorder; CP, cerebral palsy; dMLD, metachromatic leukodystrophy with predominant dyskinetic movement disorder.
In patients who had a flexible programme, mean dose of ITB given by boluses was 112μg per day (SD 128.29, range 10–335μg) administered in a median of 2 (range 2–3) boluses.
In patients with spastic CP, the starting dose was 67μg per day (SD 19.64, range 50–96μg). At 6 months, four had a simple continuous programme and four had a flexible programme with boluses added. Mean dose of baclofen was then 150μg per day (SD 79.37, range 60–277μg) (Fig. 1). In patients with a flexible programme, mean dose of baclofen given by boluses was 35μg per day (SD 33.91, range 10–85μg), administered in a median of 1 (range 1–3).
Both patients with dMLD started at 50μg per day. At 6 months, both patients had a flexible programme with bolus administration. The daily dose at 6 months was 250μg and 365μg per day. The mean dose of ITB given by boluses was 80μg in 2 boluses and 200μg in 3 boluses.
Discussion
In this report, we show that ITB is a feasible therapy in patients with MLD with either predominant spastic or dyskinetic movement disorder. The complication rate of ITB treatment in MLD in the first 6 months of treatment was similar to that of patients with spastic CP and similar to rates reported in previous studies.14, 15
With regards to comparing the treatment course between sMLD and spastic CP, the small numbers in the current study do not permit meaningful statistical analysis. The starting dose of ITB and the number of patients with a flexible programme are comparable in both groups. However, while in most patients with spastic CP a stable dosing is reached within 6 months, in most patients with sMLD the dose continues to be adjusted to higher daily doses during the first 6 months of ITB treatment. Given the progressive nature of the disease, this is not surprising. Also, the number of boluses and the dose of the ITB boluses tends to be higher in sMLD. Regarding the low number of cases, the patients with dMLD similarly appear to require higher daily doses, more boluses, and a higher dose of ITB given by boluses after 6 months of ITB treatment.
We evaluated whether goals were reached by interview at follow‐up visits to the clinic, but we did not use standardized questionnaires. Families reported clear improvements in comfort and daily care. A previous study on the effect of ITB on activities of daily life in spastic CP, dyskinetic CP, and progressive neurological disorders showed that caregivers were generally satisfied with the improvement in comfort and daily care. In this study, the group with progressive neurological disorders showed less improvement in comfort during ITB treatment than the two CP groups, possibly related to the progressive nature of the disease. This study comprised only two patients with MLD.9
As a serious spastic movement disorder associated with pain and discomfort usually occurs rather rapidly after wheelchair dependency in MLD (especially in younger patients), ITB should be considered at the moment of wheelchair dependency in the disease course.
To obtain more evidence on optimal treatment programmes and efficacy of ITB in MLD, future research should address treatment course and outcomes in larger cohorts. This could likely be achieved by studying larger clinical cohorts with systematic gathering of data in multicentre studies; for example, by centres of expertise participating in the European Reference Network for Rare Neurological Diseases. It is important to address outcomes at the level of body structure and function (e.g. pain, contractures, and deformities) as well as activities and participation, using goal attainment measures, and environmental factors, like ease of care. At the same time it should be taken into account that MLD is a progressive disorder with inherent decline of function despite treatment.
Acknowledgements
The authors have stated that they had no interests which might be perceived as posting a conflict of bias.
References
- 1. Biffi A, Lucchini G, Rovelli A, Sessa M. Metachromatic leukodystrophy: an overview of current and prospective treatments. Bone Marrow Transplant 2008; 42(Suppl. 2): S2–6. [DOI] [PubMed] [Google Scholar]
- 2. Patil SA, Maegawa GH. Developing therapeutic approaches for metachromatic leukodystrophy. Drug Des Devel Ther 2013; 7: 729–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Rappard DF, Boelens JJ, Wolf NI. Metachromatic leukodystrophy: disease spectrum and approaches for treatment. Best Pract Res Clin Endocrinol Metab 2015; 29: 261–73. [DOI] [PubMed] [Google Scholar]
- 4. van Rappard DF, Boelens JJ, van Egmond ME, et al. Efficacy of hematopoietic cell transplantation in metachromatic leukodystrophy: the Dutch experience. Blood 2016; 127: 3098–101. [DOI] [PubMed] [Google Scholar]
- 5. Sessa M, Lorioli L, Fumagalli F, et al. Lentiviral haemopoietic stem‐cell gene therapy in early‐onset metachromatic leukodystrophy: an ad‐hoc analysis of a non‐randomised, open‐label, phase 1/2 trial. Lancet 2016; 388: 476–87. [DOI] [PubMed] [Google Scholar]
- 6. Albright AL, Barry MJ, Shafton DH, Ferson SS. Intrathecal baclofen for generalized dystonia. Dev Med Child Neurol 2001; 43: 652–7. [DOI] [PubMed] [Google Scholar]
- 7. Dralle D, Müller H, Zierski J, Klug N. Intrathecal baclofen for spasticity. Lancet 1985; 2: 1003. [DOI] [PubMed] [Google Scholar]
- 8. Albright AL, Gilmartin R, Swift D, Krach LE, Ivanhoe CB, McLaughlin JF. Long‐term intrathecal baclofen therapy for severe spasticity of cerebral origin. J Neurosurg 2003; 98: 291–5. [DOI] [PubMed] [Google Scholar]
- 9. Bonouvrié L, Becher J, Soudant D, et al. The effect of intrathecal baclofen treatment on activities of daily life in children and young adults with cerebral palsy and progressive neurological disorders. Eur J Paediatr Neurol 2016; 20: 538–44. [DOI] [PubMed] [Google Scholar]
- 10. Bonouvrié LA, van Schie PE, Becher JG, van Ouwerkerk WJ, Vermeulen RJ. Intrathecal baclofen for progressive neurological disease in childhood: a systematic review of literature. Eur J Paediatr Neurol 2012; 16: 279–84. [DOI] [PubMed] [Google Scholar]
- 11. Kehrer C, Blumenstock G, Raabe C, Krägeloh‐Mann I. Development and reliability of a classification system for gross motor function in children with metachromatic leucodystrophy. Dev Med Child Neurol 2011; 53: 156–60. [DOI] [PubMed] [Google Scholar]
- 12. Towns M, Rosenbaum P, Palisano R, Wright FV. Should the Gross Motor Function Classification System be used for children who do not have cerebral palsy? Dev Med Child Neurol 2018; 60: 147–54. [DOI] [PubMed] [Google Scholar]
- 13. Albright AL, Barry MJ, Fasick P, Barron W, Shultz B. Continuous intrathecal baclofen infusion for symptomatic generalized dystonia. Neurosurgery 1996; 38: 934–8. [DOI] [PubMed] [Google Scholar]
- 14. van Hulst BM, Tel PA, de Groot V, et al. Complicaties bij kinderen met intrathecale baclofentherapie en (gerelateerde) verzorgertevredenheid.[In Dutch]. Tijdschrift voor kindergeneeskunde 2009; 77: 191–7. [Google Scholar]
- 15. Ward A, Hayden S, Dexter M, Scheinberg A. Continuous intrathecal baclofen for children with spasticity and/or dystonia: Goal attainment and complications associated with treatment. J Paediatr Child Health 2009; 45: 720–6. [DOI] [PubMed] [Google Scholar]


