Abstract
Objectives
To describe the feasibility of a novel cell‐based endoscopic technique using buccal epithelium, expanded and encapsulated in a thermoreversible gelation polymer scaffold for the treatment of urethral stricture.
Methods
Six male patients with bulbar urethral stricture ranging from 2.0 to 3.5 cm in length were included in this pilot study. Autologous buccal epithelial cells from a small buccal mucosal biopsy were isolated, cultured and encapsulated in thermoreversible gelation polymer scaffold, and were implanted at the stricture site after a wide endoscopic urethrotomy.
Results
All the patients voided well, with a mean peak flow rate of 24 mL/s. Urethroscopy carried out at 6 months showed healthy mucosa at the urethrotomy site. However, two of the six patients had recurrence at 18 and 24 months, respectively.
Conclusions
This endoscopic‐based Buccal epithelium Expanded and Encapsulated in Scaffold‐Hybrid Approach to Urethral Stricture (BEES‐HAUS) technique is a promising alternative for the open substitution buccal graft urethroplasty. It is possible to achieve the benefits of open substitution buccal urethroplasty with this endoscopic technique.
Keywords: buccal mucosa, cell therapy, scaffold, urethral stenosis, urethral stricture
Abbreviations & Acronyms
- AUA
American Urological Association
- BEES‐HAUS
Buccal epithelium Expanded and Encapsulated in Scaffold‐Hybrid Approach to Urethral Stricture
- DMEM
Dulbecco's modified Eagle's medium
- LCST
lower critical solution temperature
- NIPAAm‐co‐BMA
N‐isopropylacrylamide‐co‐n‐butyl methacrylate
- PB
peripheral blood
- PBS
phosphate‐buffered saline
- RGU
retrograde urethrogram
- TE
tissue‐engineered
- TGP
thermoreversible gelation polymer
Introduction
The definitive treatment of urethral strictures involves reconstruction with additional tissue in the form of substitution urethroplasty or an end‐to‐end anastomotic urethroplasty. Buccal mucosal urethroplasty is an open surgical substitution urethral reconstruction. It is a major procedure with morbidity of graft harvesting and urethral reconstruction. Although recurrence is the most worrisome complication, the procedure is associated with other complications, such as bleeding, wound infection, prolonged operative times and ejaculatory disturbances. The long‐term success rates of urethroplasty surgery range from 62% to 100%, with the best results seen at high‐volume centers and in the hands of experienced surgeons.1, 2 The complications associated with buccal mucosal urethroplasty warrant the advent of novel techniques.3, 4
Tissue engineering technology using cell‐seeded or unseeded matrices to reconstruct the urethra has been used in various studies with mixed results.5, 6, 7, 8 The Buccal epithelium Expanded and Encapsulated in Scaffold‐Hybrid Approach to Urethral Stricture (BEES‐HAUS) is a tissue engineering‐based cell therapy procedure that is completely endoscopic and is a much less morbid procedure. In this procedure, autologous cultured buccal epithelial cells expanded and encapsulated in TGP scaffold are implanted at the stricture site after a wide endoscopic urethrotomy to form an epithelial layer.
Methods
Patient details
The present prospective longitudinal experimental pilot study included six male patients with a mean age of 41 years (range 28–70 years) diagnosed with penobulbar urethral stricture. The study was approved by ethics committee of the hospital within which the work was undertaken, and it was carried out conforming to the provisions of the Declaration of Helsinki (as revised in Fortaleza, Brazil, October 2013). Written informed consent was obtained from all the patients. In all the patients, a detailed history was taken including the AUA symptom score. Three patients had prior optical urethrotomy, one underwent prior buccal mucosal graft urethroplasty and two patients had no prior interventions. The AUA symptom score ranged from 16 to 22, with a mean of 19. There was no history of trauma, and the etiology was idiopathic in all patients. On physical examination, external genitalia were normal with no signs of lichen sclerosis. Patients were investigated by complete urine examination, urine culture and sensitivity, ultrasound of abdomen and pelvis, uroflowmetry, and RGU. The mean peak flow rate was 6 mL/s, with a range of 4.2 to 7.0 mL/s. RGU showed stricture involving the bulbar and penobulbar region, and stricture length ranged from 2.0 to 3.5 cm in all the patients. All patients were initially subjected to small buccal mucosal biopsy under local anesthesia. The buccal epithelial cells from the biopsy were isolated and cultured in the cell culture laboratory for 10–12 days. Later, the patients were admitted for an endoscopic urethrotomy and implantation of cultured buccal cells embedded in scaffold.
About the TGP scaffold
The TGP used in the present study is a co‐polymer composed of thermoresponsive polymer blocks (poly [N‐isopropyl acrylamide‐co‐n‐butyl methacrylate] poly [NIPAAm‐co‐BMA]) and hydrophilic blocks (polyethylene glycol).9 These thermoresponsive polymer blocks are hydrophilic at temperatures below the sol‐gel transition temperature (35°C, which is close to the LCST of an aqueous solution of PNIPAAm [32°C]) and hydrophobic at temperatures above the sol‐gel transition temperature. The sol‐gel transition temperature can be altered by changing the composition of NIPAAm‐co‐BMA and polyethylene glycol.10 This thermoresponsive property is advantageous, because the cells to be cultured can be added in the solution at lower temperatures and when the temperature rises to 37°C, the cells become embedded in a three‐dimensional scaffold that can be cultured in the incubator at 37°C.9 TGP has been shown to support the growth of different cell types, including corneal limbal stem cells,11, 12 hepatocytes,13 chondrocytes14 and continuous cell lines, such as the Vero, HEp‐2, HeLa, BHK‐21, McCoy and CHO cell lines,15 For use in the present study, the lyophilized TGP (1 g) vial was obtained from Nichi In Biosciences, Chennai, India.
Buccal mucosal biopsy
A small buccal mucosal graft is harvested under local anesthesia from each patient. The graft is divided into four tissue bits of 3 mm3 each, two of the buccal mucosal tissue bits were placed in PBS and two in the TGP‐based transportation cocktail with antibiotics contained in tissue transport vials.
For use of serum in culture, 30 mL of the patient's own PB was collected in each case and was transported to the laboratory in PBS at 4°C. Cells were cultured for 10–12 days in the laboratory.
Buccal epithelial cell culturing
Dispase II (4 mg/mL) enzyme was added to the dishes containing tissue pieces and the dishes were incubated at 37°C for 1 h. Enzyme activity was then stopped by washing with DMEM (Invitrogen, Waltham, MA, USA) containing 10% of the patient's serum. The tissues were then treated with trypsin–ethylenediaminetetraacetic acid (0.25% solution) for 15 min. The cell pellet was collected after centrifugation and resuspended in DMEM culture medium. The cell viability was analyzed by the trypan blue dye exclusion method. The cells were then cultured using a TGP‐based method.16 Medium composed of DMEM with 10% autologous serum, insulin (5 μg/mL), human recombinant epidermal growth factor (5 ng/mL), penicillin–streptomycin (50 IU/mL) and amphotericin B (5 μg/mL; all reagents from Invitrogen) was used for culture of cells. The cells were cultured for 10–12 days. The cells proliferated and had a cobblestone appearance characteristic of epithelial cells (Fig. 1). Cells were counted by the trypan blue exclusion method. Before cell harvest, a small portion of the culture medium containing cells was subjected to testing for bacteria and fungi. Culture for the presence of bacteria or fungi was negative in all our cases. Cells were harvested from the culture dishes and a cell pellet was obtained by centrifugation. The final increased cell counts after culture expansion are shown in Table 1. Cells were suspended in TGP reconstituted with saline and used for implantation in the operation theater.
Figure 1.
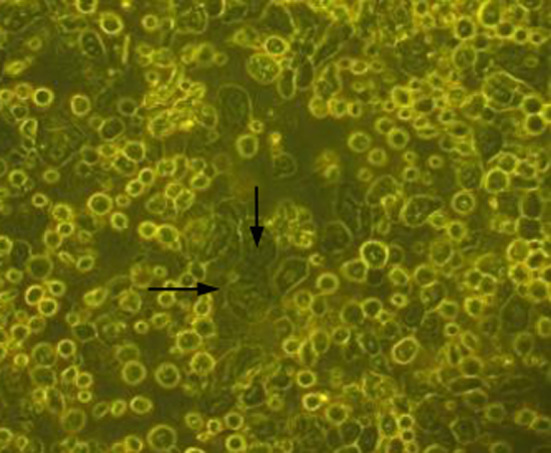
Cells in culture in the TGP‐based method (magnification: ×20). The arrows indicate the cells with cobblestone morphology.
Table 1.
Patient characteristics and procedure details
| Sl No. | Etiology | Age (years) | Prior interventions | Graft size (cm) | Initial cell count (million) | Final cell count (million) | Stricture length (cm) | Follow‐up (months) | Auxiliary procedure |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Idiopathic | 38 |
Endoscopic urethrotomy (twice) |
0.5 × 0.5 | 0.3 | 1.4 | 2.5 | 18 |
Buccal substitution urethroplasty |
| 2 | Idiopathic | 28 | – | 2 × 1 | 1.57 | 11.6 | 2 | 40 | – |
| 3 | Idiopathic | 70 | Buccal substitution | 2.5 × 1.5 | 0.76 | 10 | 2 | 39 | – |
| 4 | Idiopathic | 37 | 2 × 1.5 | 2.24 | 9.2 | 2.5 | 36 | – | |
| 5 | Idiopathic | 45 | Endoscopic urethrotomy | 2 × 1.5 | 1.2 | 8.06 | 3.5 | 36 | – |
| 6 | Idiopathic | 32 | – | 2 × 1.5 | 2.2 | 18.5 | 2 | 10 |
Buccal substitution urethroplasty |
BEES‐HAUS procedure
Cystourethroscopy was carried out under spinal anesthesia. A 0.032‐inch guide wire was passed across the stricture, and using 21‐Fr urethrotomy sheath and a straight cold knife, a wide dorsal urethrotomy at the stricture site was carried out from a 9 o'clock to a 3 o'clock position until petechial hemorrhages are seen. Stricture involved 2.0–3.5 cm length of bulbar/penobulbar urethra with 1 cm of unhealthy pale mucosa on both the proximal and distal ends. Urethrotomy was extended into pale unhealthy mucosa on both the proximal and distal ends. A 14‐Fr silicon Foley catheter was placed, balloon inflated and the balloon was pulled toward the bladder neck. Later, urethroscopy was carried out with a 17‐Fr compact cystourethroscope by the side of the Foley catheter to the urethrotomy site and using a 5‐Fr ureteric catheter through the cystoscope, the cultured buccal epithelial cells suspended in TGP were instilled at the urethrotomy site so as to cover the entire urethrotomy site. The TGP, which was liquid at low temperature, immediately solidified at body temperature, thus fixing the cells. The catheter was left in situ for 3 weeks.
Post‐treatment follow up
The urethral catheter was removed after 3 weeks. Follow up was carried out every 6 months by symptom assessment and uroflowmetry. A check urethroscopy was carried out at 6 months. Urethrogram was carried out at 1‐year follow up. None of the patients were advised to carry out clean intermittent self‐catheterization. Urethral calibration was carried out when the patients experienced poor urinary flow or when the peak flow rate was <12 mL/s.
Results
After urethral catheter removal, all patients voided well with good stream. There was symptomatic improvement in all patients, and uroflow improved with a mean flow of 24 mL/s. The check urethroscopy carried out at 6 months, with a 19‐Fr scope revealing healthy, pinkish epithelium at the urethrotomy site with good urethral lumen (Fig. 2). In one patient, biopsy from the urethrotomy site showed buccal epithelial cells, providing evidence of healing of the implanted area by buccal epithelium (Fig. 3). Retrograde urethrogram carried out at the end of 1 year showed a patent urethra with wide lumen at the urethrotomy and implantation site (Fig. 4). Four patients were voiding well and did not require any auxiliary procedure at 3 years of follow up. Recurrence was seen in two patients – at 18 months in one patient and at 2 years in the other. One patient who underwent two prior endoscopic internal urethrotomies had narrowing at the same site. Other patient, who had a dense stricture at urethrotomy, also had recurrence. Both the patients underwent buccal mucosal graft urethroplasty at 18 months and 2 years after this procedure. The remaining four patients were voiding with a peak flow rate of >20 mL/s at 3‐year follow up.
Figure 2.
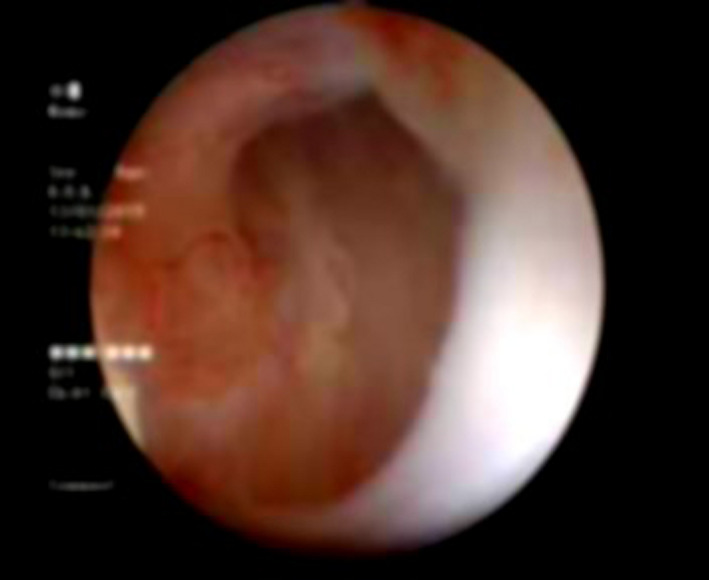
Follow‐up urethroscopy view at the implantation site: pink granulating tissue covering the urethrotomy site.
Figure 3.
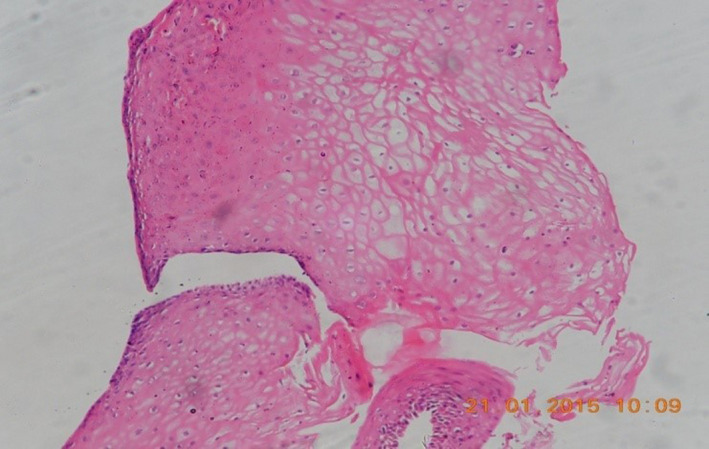
Photomicrograph of tissue from the implanted site of the urethra showing stratified squamous epithelium (magnification: ×400).
Figure 4.
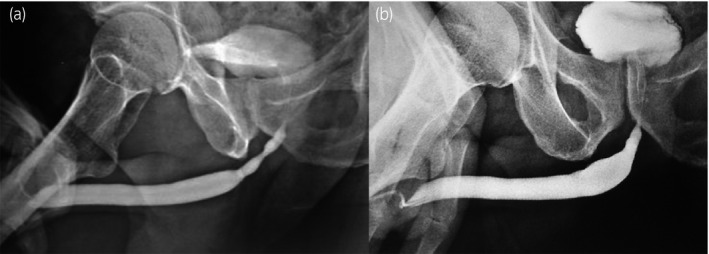
(a) Pre‐BEES‐HAUS procedure urethrogram. (b) Post‐BEES‐HAUS procedure urethrogram.
Discussion
The definitive treatment for urethral stricture remains one of the most challenging problems in urology. It often involves major reconstruction with additional tissue. Penile skin, scrotal skin, bladder mucosa and, recently, buccal mucosa have been used for repair of urethral defects (substitution urethroplasty).5 These materials are often sufficient to restore to which they are being grafted, but they are effective for only a short period. On long‐term follow up, recurrence of stricture can occur and requires an auxiliary procedure, such as dilatation urethrotomy or regrafting.17
A great deal of research has been carried out to overcome this, and tissue engineering techniques could play a significant role in the reconstruction of the urethra. The evolution has begun with acellular grafts and then progressed to cellular matrix grafts.18, 19, 20 A major challenge in developing tissue‐engineered materials for urethral reconstruction is to find a suitable scaffold biomaterial. This biomaterial must be biocompatible to minimize any inflammatory or foreign body response once implanted; it should also encourage appropriate cell adhesion, proliferation, migration, and differentiation to promote and facilitate development of new tissue.21 Vascularization of TE materials can also pose a significant challenge, because an adequate blood supply is required for supporting both the growth and proliferation of seeded cells.
The TGP scaffold used in the present study is biologically inert, antifibrinogenic, and has been used earlier in the liver and cornea. Nagaya et al. reported the use of TGP for regeneration of focal liver injury.22 In their study, TGP induced efficient regeneration of hepatocytes by reducing the fibrosis and enhancing proliferation. Sudha et al. showed the growth of human cadaver corneal limbal epithelial cells cultured in TGP, and they showed that the cells expressed both the limbal and corneal phenotype.11 Sitalakshmi et al. showed that transplantation of limbal epithelial cells grown in TGP could reconstruct the surgically damaged corneal epithelium in a rabbit model without inducing fibrosis.12 Earlier, TGP was applied for tissue engineering. TGP has been shown to support the tissue engineering of articular cartilage, with the cells growing in culture without dedifferentiation for a longer period of time in vitro 14 and the TE cartilage has been successfully transplanted to rabbits with follow‐up biopsy showing native hyaline cartilage regenerated in the rabbit.23 Also, bone marrow mononuclear cells have been transplanted clinically embedded in the TGP scaffold for treating human patients with alveolar bone defects.24 Hence, TGP was considered for the present study to serve as a scaffold for urethral tissue engineering. The fibrosis‐inhibiting nature of TGP could be of great advantage in the BEES‐HAUS procedure. We presume that the stricture recurrence is delayed or prevented by the implanted buccal cells forming an epithelial layer along with the antifibrotic nature of TGP. Hishikawa et al. compared collagen scaffolds with TGP and reported that collagen gel altered the gene expression profile of mesenchymal stem cells but that TGP did not.25 TGP has been subjected to toxicity assessment studies, including tests for cumulative skin irritation, reverse mutation, in vitro chromosomal aberrations, antigenicity, eye irritation and single administration toxicity, and has been established to be safe (unpubl. data).
Bhargava et al. made a TE buccal mucosa using separated keratinocytes and fibroblasts from the epidermal and dermal layers of the oral mucosa.8 The mucosa was implanted into five patients to repair urethral stricture. Three patients were cured; one required total patch excision, and the other required partial patch excision owing to patch hyperplasia and fibrosis.
Although the authors could reduce the morbidity of graft harvesting in this novel tissue engineering technique, the procedure involved open surgery for graft substitution with its associated morbidity. In our procedure, buccal epithelial cells in a liquid scaffold facilitated implantation of cells by an endoscopic technique.
Autologous buccal mucosa has been used in substitution urethroplasty for many years with acceptable results.5, 6, 7, 8 We used autologous cultured buccal epithelial cells in the present study, thus obviating the risks of antigenicity and infections associated with allogenic tissue transfer. The success of urethroplasty mainly depends on the caliber of the native urethral lumen and severity of underlying spongiofibrosis The stricture recurrence rates after buccal substitution urethroplasty at 5, 10 and 15 years are 21, 31 and 58%, respectively.17 The results of BEES‐HAUS are comparable with open substitution; however, long‐term follow up with a large number of patients is required. Barbagli and Lazzeri26 in a comment on a study of TE autologous urethra27 suggested that long‐term follow up is the main factor affecting the validity of the efficacy and safety of such innovative tissue engineering treatments.
In the present study, two patients had recurrence after 18 and 24 months of follow up, respectively. The recurrence in the first patient could be attributed to the severity of spongiofibrosis, prior two interventions and low final cell count (1.4 million). Recurrence in the other patient could be due to severe fibrosis identified at endoscopic urethrotomy. Both the patients underwent standard buccal mucosal graft urethroplasty. One of our patients had stricture recurrence after formal buccal graft urethroplasty, which was managed by the BEES‐HAUS procedure. We believe that the procedure can also be used as a salvage procedure after failed buccal graft urethroplasty, as redo open surgery has significant morbidity. At 3‐year follow up, four out of six patients were voiding well and the urethral lumen accommodated a 16‐Fr catheter. We have documented the successful buccal epithelial implantation by doing biopsy after 6 months from the implantation site.
In long strictures and redo procedures, the donor area available is a major limitation in open substitution procedures. In a retrospective study of oral mucosal graft harvesting, Barbagli et al. found that bilateral graft harvesting in long strictures was a significant predictor of patient dissatisfaction.28 BEES‐HAUS, an endoscopic tissue engineering‐based cell therapy, has tremendous potential even in recurrent and long strictures. In contrast to earlier published studies, where TE grafts are sutured to native urethra by an open surgical method, our BEES‐HAUS technique is entirely endoscopic with minimal morbidity. Based on the present study, we consider the use of buccal mucosal epithelial cells to be more advantageous to address the stricture of the male urethra. Instead of buccal mucosal epithelial cells used in this study, which are mature adult cells, should stem cells or progenitor cells be considered as an alternative cell source, their teratogenic and/or mutagenic potentials should be ruled out first. Then their differentiation to a urothelial epithelium and long‐term outcome of safety should be confirmed before clinical translation. The limitations of the present study were the small cohort with a short follow‐up period. The procedure is carried out in short segment strictures with moderate‐to‐severe spongiofibrosis. A multicentric study with a large number of patients and long‐term follow up is required to establish this novel procedure.
Our initial few cases show that novel cell‐based therapy using BEES‐HAUS is a promising alternative for open substitution buccal graft urethroplasty. It is possible to achieve the benefits of open substitution buccal urethroplasty with this endoscopic technique without donor site morbidity.
Conflict of interest
Samuel JK Abraham is an applicant to several patents on the usage of the TGP.
Acknowledgments
The authors acknowledge Loyola ICAM College of Engineering Technology (LICET) and Loyola Institute of Frontier Energy (LIFE) for their support ot our research work.
References
- 1. Markiewicz MR, Lukose MA, Margarone JE et al The oral mucosa graft: a systematic review. J. Urol. 2007; 178: 387–94. [DOI] [PubMed] [Google Scholar]
- 2. Barbagli G, Guazzoni G, Lazzeri M. One‐stage bulbar urethroplasty: retrospective analysis of the results in 375 patients. Eur. Urol. 2008; 53: 828–33. [DOI] [PubMed] [Google Scholar]
- 3. Meeks JJ, Erickson BA, Granieri MA, Gonzalez CM. Stricture recurrence after urethroplasty: a systematic review. J. Urol. 2009; 182: 1266–70. [DOI] [PubMed] [Google Scholar]
- 4. Zimmerman WB, Santucci RA. Buccal mucosa urethroplasty for adult urethral strictures. Indian J. Urol. 2011; 27: 364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Kemp V, de Graaf P, Fledderus JO, Ruud Bosch JL, de Kort LM. Tissue engineering for human urethral reconstruction: systematic review of recent literature. PLoS ONE 2015; 10: e0118653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vasyutin IA, Lyundup AV, Viranov AZ, Butnaru DV, Kuznetsov SL. Urethra reconstruction with tissue‐engineering technology. Vestn. Ross. Akad. Med. Nauk 2017; 72: 17–25. [DOI] [PubMed] [Google Scholar]
- 7. Mangera A. Chapple CR Tissue engineering in urethral reconstruction–an update. Asian J. Androl. 2013; 15: 89–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bhargava S, Patterson JM, Inman RD, MacNeil S, Chapple CR. Tissue‐engineered buccal mucosa urethroplasty‐clinical outcomes. Eur. Urol. 2008; 53: 1263–9. [DOI] [PubMed] [Google Scholar]
- 9. Yoshioka H, Mikami M, Mori Y, Tsuchida E. A synthetic hydrogel with thermoreversible gelation. I. preparation and rheological properties. J. Macromol. Science. Part A. 1994; 31: 113–20. [Google Scholar]
- 10. Yoshioka H, Mori Y, Cushman JA. A synthetic hydrogel with thermoreversible gelation, III: an NMR study of the sol–gel transition. Polym. Adv. Technol. 1994; 5: 122–7. [Google Scholar]
- 11. Sudha B, Madhavan HN, Sitalakshmi G et al Cultivation of human corneal limbal stem cells in Mebiol gel–A thermo‐reversible gelation polymer. Indian J. Med. Res. 2006; 124: 655–64. [PubMed] [Google Scholar]
- 12. Sitalakshmi G, Sudha B, Madhavan HN et al Ex vivo cultivation of corneal limbal epithelial cells in a thermoreversible polymer (Mebiol Gel) and their transplantation in rabbits: an animal model. Tissue Eng. Part A 2009; 15: 407–15. [DOI] [PubMed] [Google Scholar]
- 13. Parveen N, Khan AA, Baskar S et al Intraperitoneal transplantation of hepatocytes embedded in thermoreversible gelation polymer (Mebiol Gel) in acute liver failure rat model. Hepat. Mon. 2008; 8: 275–80. [Google Scholar]
- 14. Arumugam S, Manjunath S, Senthilkumar R et al In vitro expansion and characterization of human chondrocytes using a novel thermoreversible gelation polymer (TGP). J. Orthopaedics 2011; 8: e5. [Google Scholar]
- 15. Madhavan HN, Malathi J, Joseph PR, Mori Y, Abraham S, Yoshioka H. A study on the growth of continuous culture cell lines embedded in Mebiol Gel. Curr. Sci. 2004; 87: 1275–7. [Google Scholar]
- 16. Rao SK, Sudhakar J, Parikumar P et al Successful transportation of human corneal endothelial tissues without cool preservation in varying Indian tropical climatic conditions and in vitro cell expansion using a novel polymer. Indian J. Ophthal. 2014; 62: 130–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Andrich DE, Dunglison N, Greenwell TJ, Mundy AR. The long‐term results of urethroplasty. J. Urol. 2003; 170: 90–2. [DOI] [PubMed] [Google Scholar]
- 18. Atala A, Guzman L, Retik AB. A novel inert collagen matrix for hypospadias repair. J. Urol. 1999; 162: 1148–51. [DOI] [PubMed] [Google Scholar]
- 19. Sievert KD, Bakircioglu ME, Nunes L, Tu R, Dahiya R, Tanagho EA. Homologous acellular matrix graft for urethral reconstruction in the rabbit: histological and functional evaluation. J. Urol. 2000; 163: 1958–65. [PubMed] [Google Scholar]
- 20. Fu Q, Deng CL, Liu W, Cao YL. Urethral replacement using epidermal cell‐seeded tubular acellular bladder collagen matrix. BJU Int. 2007; 99: 1162–5. [DOI] [PubMed] [Google Scholar]
- 21. Atala A, Terlecki RP. Tissue engineering of urethra: the Basics, current concept, and the Future In: Brandes SB. (ed). Advanced male urethral and Genital reconstructive surgery 2nd edn Humana Press, New York 2014; 507–18. [Google Scholar]
- 22. Nagaya M, Kubota S, Suzuki N, Akashi K, Mitaka T. Thermoreversible gelation polymer induces the emergence of hepatic stem cells in the partially injured rat liver. Hepatology 2006; 43: 1053–62. [DOI] [PubMed] [Google Scholar]
- 23. Arumugam S, Bhupesh Karthik B, Rajeswar C et al Transplantation of autologous chondrocytes ex‐vivo expanded using thermoreversible gelation polymer in a rabbit model of articular cartilage defect. J. Orthop. 2017; 14: 223–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sankaranarayanan S, Jetty N, Gadagi JS, Preethy S, Abraham SJ. Periodontal regeneration by autologous bone marrow mononuclear cells embedded in a novel thermo reversible gelation polymer. J. Stem Cells. 2013; 8: 99–103. [PubMed] [Google Scholar]
- 25. Hishikawa K, Miura S, Marumo T et al Gene expression profile of human mesenchymal stem cells during osteogenesis in three‐dimensional thermoreversible gelation polymer. Biochem. Biophys. Res. Commun. 2004; 317: 1103–7. [DOI] [PubMed] [Google Scholar]
- 26. Barbagli G, Lazzeri M. Re: tissue‐engineered autologous urethras for patients who need reconstruction: an observational study. Eur. Urol. 2011; 60: 1303–4. [DOI] [PubMed] [Google Scholar]
- 27. Raya‐Rivera A, Esquiliano DR, Yoo JJ, Lopez‐Bayghen E, Soker S, Atala A. Tissue‐engineered autologous urethras for patients who need reconstruction: an observational study. Lancet. 2011; 377: 1175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Barbagli G, Fossati N, Sansalone S et al Prediction of early and late complications after oral mucosal graft harvesting: multivariable analysis from a cohort of 553 consecutive patients. J. Urol. 2014; 191: 688–93. [DOI] [PubMed] [Google Scholar]


