Abstract
BACKGROUND
Antimicrobial stewardship programs have been established in hospitals, but less studied in long‐term care facilities (LTCFs), a setting with unique challenges related to patient populations and available resources. This systematic review sought to provide a comprehensive assessment of antimicrobial stewardship interventions implemented in LTCFs, using meta‐analysis to examine their impact on overall antimicrobial use.
METHODS
Electronic searches of MEDLINE, Embase, and CINAHL (1990 to July 2018) identified any antimicrobial stewardship interventions in LTCFs, with no restriction on patient population, study design, or outcomes. Intervention components were categorized using the Cochrane Effective Practice and Organization of Care taxonomy on implementation strategies. Random‐effects meta‐analysis used ratio of means to facilitate pooling of different metrics of antimicrobial use.
RESULTS
Eighteen studies (one randomized controlled trial [RCT], four cluster RCTs, four controlled pre/post studies, and nine uncontrolled pre/post studies) met inclusion, using 13 different antimicrobial stewardship intervention strategies; 15 studies used multifaceted (maximum, seven; median, four) interventions. The three most commonly implemented strategies were educational materials, educational meetings, and guideline implementation. Intervention labor intensity and resource requirements varied considerably among interventions. Meta‐analysis of 11 studies demonstrated that antimicrobial stewardship strategies were associated with a 14% reduction in overall antimicrobial use (95% confidence interval = −8% to −20%; P < .0001), with similar results by study design but high heterogeneity (I2 = 86%) for the uncontrolled pre/post study subgroup and no heterogeneity (I2 = 0%) for the cluster RCT and controlled pre/post study subgroups. Funnel plot analysis suggested publication bias, with a lack of publication of smaller studies showing increased antibiotic use.
CONCLUSION
Antimicrobial stewardship strategies implemented in long‐term care vary considerably in design and resource intensity, but collectively suggest potential to reduce antimicrobial use in this challenging setting. J Am Geriatr Soc 67:392–399, 2019.
Keywords: antimicrobial stewardship, antimicrobial use, long‐term care
Introduction
The emergence of antimicrobial resistance presents a serious public health threat and is propagated by inappropriate and extensive use of antimicrobials in all healthcare sectors. Residents in long‐term care facilities (LTCFs) are at risk of infections, and are challenging to assess, which prompts the overuse of antibiotics in this setting. Antibiotic use is extensive, and antibiotic resistance is common, in LTCFs.1 The prevalence of residents receiving at least one antibiotic in a single year has been reported to be between 47% and 79%.1, 2 Furthermore, as in the acute‐care and primary care settings, residents in LTCFs are also vulnerable to inappropriate use, with estimated prevalence as high as 75%.3, 4 There is significant variability in antimicrobial use among various LTCFs and LTC prescribers.2 Residents in facilities with higher antimicrobial use are more likely to experience negative outcomes, such as acquiring an antibiotic‐resistant organism.5 A point‐prevalence study showed that up to 43% of long‐term care residents had positive culture results for at least one antimicrobial‐resistant pathogen.6
Guidelines for infection prevention and control in LTCFs encourage the program to include an antimicrobial stewardship component.7 In addition, the US Centers for Medicare and Medicaid Services has mandated the implementation of antimicrobial stewardship programs (ASPs) in LTCFs since 2017.8 Until recently, ASPs have existed mostly in the hospital setting, but they remain less well studied in LTCFs. Although the two different settings share the same antimicrobial stewardship goals, the structure and process differ considerably. LTCFs pose a unique challenge, with multiple factors that affect the quality of antibiotic prescribing decisions. For example, clinicians often have competing priorities and multiple roles, with many decisions being made off site.9 Hospitals have invested considerably in developing ASPs, whereas most LTCFs lack the resources to emulate the ASP frameworks that have succeeded in acute‐care hospitals.
There has not been consensus on specific strategies or resources required to support ASP in LTC settings.10 Implementation requires flexibility to target local needs and can be hindered by the limited resources and evidence available reflecting the effectiveness of ASP overall or the effectiveness of specific ASP strategies.10 This systematic review aimed to provide a comprehensive summary of the characteristics of antimicrobial stewardship interventions in LTC and to use meta‐analysis to evaluate the impact of ASPs on overall antimicrobial use in LTCFs.
Methods
Search Strategy
We conducted literature searches of three databases (CINAHL, Embase, and Ovid MEDLINE) for English‐language articles published between 1990 and July 31, 2018, which examined any antimicrobial stewardship intervention in an LTC setting. The broad search was conducted to ensure all primary research articles were captured and involved no limits by study design, intervention type, or outcome examined. The reference lists of relevant review articles were reviewed to identify studies that may have been missed in the electronic search.
Study Selection and Inclusion and Exclusion Criteria
Titles, abstracts, and full‐text screening were performed in duplicate by two independent reviewers. Any disagreement was resolved by discussion and consensus. There was no restriction on study design or patient population (eg, age and condition). Any type of LTCF was included (eg, long‐term care homes and nursing facilities). Studies involving interventions in an LTC ward within an acute‐care hospital were excluded because this group may not be generalizable to the broader LTCF population. There was no restriction according to the type of intervention or outcome, as long as the study objective was focused on implementation of an antimicrobial stewardship strategy with any outcome(s) reported after the intervention.
Data Extraction
Data extraction for predefined variables was undertaken by one reviewer, with 20% verified by a second reviewer. All outcomes were extracted (ie, patient, prescribing, and use) to demonstrate the variety of outcome types reported. All outcomes were presented narratively, only outcome data on antimicrobial use were extracted further for meta‐analysis (described later), and outcomes were extracted at the furthest time point if there were multiple measures after intervention. Data used for meta‐analyses were extracted in duplicate by two independent reviewers. Any disagreements were resolved by consensus. Intervention components were categorized by the Cochrane Effective Practice and Organization of Care (EPOC) taxonomy on implementation strategies,11 with the addition of financial incentives, which is no longer listed as an implementation strategy.
Quality Appraisal
Quality appraisal was performed in duplicate by two independent reviewers using the quality assessment tool for quantitative studies developed by the Effective Public Health Practice Project (EPHPP), which is designed to be applicable to a variety of public health interventions.12 This tool assesses a range of study designs,13 allowing all included studies in this review to be assessed by the same tool.
Meta‐Analysis
To provide a best estimate of the impact of antimicrobial stewardship on LTCF antimicrobial use, we performed a meta‐analysis to pool the results of any study that examined an outcome of antimicrobial use, regardless of the nature of the antimicrobial stewardship intervention. If a study reported multiple outcomes related to antimicrobial use, we selected the outcome that best approximated total use (rather than class‐ or condition‐specific antibiotic use). These outcomes varied across the studies, ranging from days of antibiotic therapy, to defined daily doses, to number of prescriptions per 1000 resident days. Therefore, we used a ratio of means approach for analyzing continuous outcomes, such that the effects of interventions would be comparable across these different outcome metrics.14, 15 The ratio of means was calculated by dividing the mean antimicrobial use in the antimicrobial stewardship intervention group by the mean antimicrobial use in the nonintervention group, for studies without preintervention data. For controlled studies, in which pre‐post data were available for both the intervention and nonintervention groups, we determined the ratio of the mean antimicrobial use in the post period divided by the pre period for both the intervention and nonintervention groups, and then calculated a ratio of these ratios. For pre/post studies without a control group, the postintervention data were divided by the preintervention data. Variances of each ratio were calculated assuming identical P values as the published P values (or the calculated P values from the published 95% confidence intervals [CIs]) provided for the differences, or differences of differences for each included study. For studies reporting ranges for differences or difference of differences, SDs were estimated from published ranges, as described by Wan et al.16 The studies were weighted by the inverse of the variance to calculate pooled outcomes.14 A random‐effects model was used, which incorporates between‐study heterogeneity and gives wider and more conservative CIs when heterogeneity is present.17 Between‐study heterogeneity was assessed using the I2 measure, the percentage of total variability across studies attributable to heterogeneity rather than chance; and statistical heterogeneity was considered low if I2 was 25% to 49%, moderate if I2 was 50% to 74%, and high if I2 was 75% or greater.18
We performed a stratified meta‐analysis according to study design to examine the pooled effect size of antimicrobial strategies separately among cluster randomized controlled trials (RCTs), controlled pre‐post studies, and uncontrolled pre‐post studies. Last, we performed a sensitivity analysis excluding two studies that measured antimicrobial outcomes that were limited to a subset of infectious syndromes.19, 20 To assess for publication bias, a funnel plot comparing the effect measure with study precision for each study was examined for evidence of asymmetry.
Results
A total of 4667 unique citations were identified; 4666 from the database search strategy and 1 additional study by searching the reference list of the identified reviews. Altogether, 18 articles met the inclusion criteria (see flowchart in Figure 1). The study designs included pre and post (n = 9),21, 22, 23, 24, 25, 26, 27, 28, 29 controlled pre and post (n = 4),19, 30, 31, 32 cluster RCTs (n = 4),20, 33, 34, 35 and patient‐level RCT (n = 1).36 More than half of the studies did not report or target a specific condition (n = 11)20, 21, 22, 23, 24, 27, 29, 31, 32, 33, 35; the number of study sites ranged from 1 to 46. Most studies were conducted in the United States (n = 12),19, 21, 22, 23, 24, 25, 26, 27, 28, 30, 32, 36 followed by one each in Australia,29 Canada,35 Canada joint with United States,34 Netherlands,31 Sweden,20 and the United Kingdom.33 The earliest publication year was 2001.36 Tables 1 and 2 and Supplementary Tables S1 and S2 summarize the details of the included studies and intervention strategies.
Figure 1.
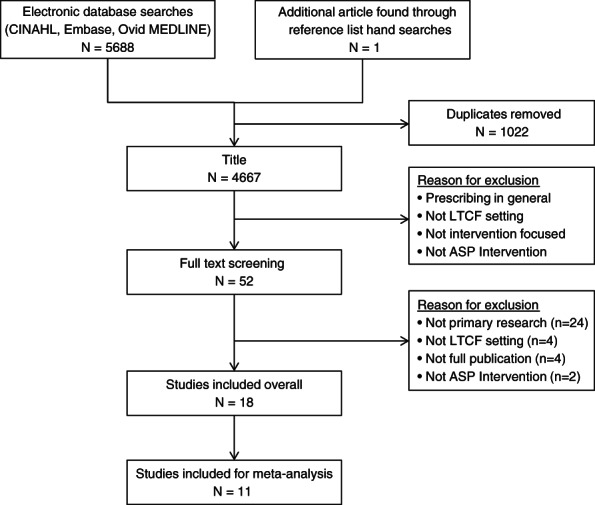
Literature search and screening results. ASP indicates antimicrobial stewardship program; LTCF, long‐term care facility.
Table 1.
Study and intervention characteristics of included studies
| Study first author | Country | Study design | No. of sites | Participant treatment condition |
|---|---|---|---|---|
| Cooper,28 2017 | United States | Pre/post | 1 | UTI |
| Doernberg,26 2015 | United States | Pre/post | 3 | UTI |
| Fleet,33 2014 | United Kingdom | RCT: cluster | 30 | Any |
| Furuno,21 2014 | United States | Pre/post | 1 | NR |
| Gugkaeva,27 2012 | United States | Pre/post | 1 | NR |
| Jump,22 2012 | United States | Pre/post | 1 | NR |
| Linnebur,30 2011 | United States | Controlled pre/post | 16 | NHAP |
| Loeb,34 2005 | Canada and United States | RCT: cluster | 24 | UTI |
| McMaughan,19 2016 | United States | Controlled pre/post | 12 | UTI |
| Monette,35 2007 | Canada | RCT: cluster | 8 | Any |
| Naughton,36 2001 | United States | RCT | 10 | NHAP |
| Pettersson,20 2011 | Sweden | RCT: cluster | 46 | Any |
| Rahme,23 2016 | United States | Pre/post | 1 | Any |
| Stuart,29 2015 | Australia | Pre/post | 1 | NR |
| Sloane,24 2014 | United States | Pre/post | 4 | Any |
| van Buul,31 2015 | Netherlands | Controlled pre/post | 10 | NR |
| Zabarsky,25 2008 | United States | Pre/post | 1 | UTI |
| Zimmerman,32 2014 | United States | Controlled pre/post | 12 | Any |
Abbreviations: NHAP, nursing home–acquired pneumonia; NR, not reported; RCT, randomized controlled trial; UTI, urinary tract infection.
Table 2.
Outcomes reported by included studies
| Study first author | Clinical outcome | Antimicrobial prescribing outcome | Use outcome |
|---|---|---|---|
| Cooper,28 2017 |
|
NR |
|
| Doernberg,26 2015 |
|
|
NR |
| Fleet,33 2014 | NR |
|
NR |
| Furuno,21 2014 | NR |
|
NR |
| Gugkaeva,27 2012 | NR |
|
NR |
| Jump,22 2012 |
|
|
NR |
| Linnebur,30 2011 |
|
|
NR |
| Loeb,34 2005 |
|
|
|
| McMaughan,19 2016 | NR |
|
NR |
| Monette,35 2007 | NR |
|
NR |
| Naughton,36 2001 |
|
|
NR |
| Pettersson,20 2011 |
|
|
|
| Rahme,23 2016 |
|
|
NR |
| Stuart,29 2015 |
|
|
|
| Sloane,24 2014 | NR |
|
NR |
| van Buul,31 2015 | NR |
|
NR |
| Zabarsky,25 2008 | NR |
|
|
| Zimmerman,32 2014 | NR |
|
NR |
Abbreviations: EENTI, ear, eye, nose, and throat infection; NR, not reported; RTI, respiratory tract infection; UTI, urinary tract infection.
Antimicrobial Stewardship Interventions
Most of the studies were multifaceted (n = 15),19, 20, 21, 23, 24, 25, 28, 29, 30, 31, 32, 33, 34, 35, 36 and the number of intervention strategies used per study varied from one to seven, with a median of four (Supplementary Table S2 provides a detailed list of strategies per study). The three most frequently used strategies were educational material (n = 13),19, 20, 21, 23, 24, 25, 28, 29, 30, 32, 33, 34, 36 educational meetings (n = 12),19, 20, 23, 24, 25, 28, 29, 30, 31, 32, 34, 36 and guideline implementation (n = 7).20, 23, 30, 31, 32, 35, 36 Six studies implemented an intervention to shift organizational culture21, 22, 23, 27, 30, 31 (eg, creating a new onsite infectious disease consultation service or implementing an ASP). Other strategies that have been implemented in LTCFs included audit and feedback (n = 5),20, 25, 26, 29, 35 reminders (n = 5),25, 29, 33, 34, 36 local opinion leader (n = 4),28, 29, 30, 34patient‐mediated interventions (n = 3),23, 24, 32 continuous quality improvement (n = 2),24, 32 educational outreach (n = 2),30, 34 financial incentives (n = 2),24, 30 community of practice (n = 1),33 and tailored interventions (n = 1).31 More than half of the studies had an educational component (n = 14),19, 20, 21, 23, 24, 25, 28, 29, 30, 31, 32, 33, 34, 36 and most studies had aspects beyond education (n = 13).20, 21, 23, 24, 25, 28, 29, 30, 31, 32, 33, 34, 36
Intervention Intensity and Resource
There was a vast variability in the level of intervention intensity and resources required. Intervention implementation ranged from a one‐time only session to 72 weekly commitments. Of 16 studies, 12 involved ongoing commitment (eg, use of a guideline or a tool), often in addition to the time allocated to participate in the intervention.19, 21, 23, 24, 27, 28, 29, 30, 32, 33, 34, 35 Interventions also tended to be outside of the routine practice of the daily workflow of the LTCF healthcare providers. Ten studies specified the profession of those delivering the intervention, which could involve pharmacists,26, 27, 30 physicians,22, 23, 25, 26, 27, 30, 32, 36 and nurses,19, 22, 25, 29, 30, 32, 36 and often in multidisciplinary combinations.
Quality Appraisal
Most of the studies had a high risk of bias,19, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36 with one moderate risk of bias20 on methodological quality assessment; therefore, meta‐analysis did not exclude studies on the basis of the methodological quality, and sensitivity analysis was not conducted on the basis of methodological quality.
Meta‐Analysis
The meta‐analysis of the impact of antimicrobial stewardship strategies on overall antibiotic use in LTC included 11 studies that reported this outcome and demonstrated a statistically significant 14% reduction in antimicrobial use (95% CI = −8% to −20% [ratio of means, 0.86, 95% CI = 0.80‐0.92]; P < .0001; see forest plot in Figure 2). The subgroup analyses by study design showed that the reduction was similar regardless of study design (interaction P = .37) and remained statistically significant for cluster RCTs (10% reduction; 95% CI = −5% to −14%; P < .001) and uncontrolled pre and post studies (18% reduction; 95% CI = −6% to −29%; P = .006); controlled pre and post studies followed the trend but did not reach statistical significance (15% reduction; 95% CI = −29% to 2%; P = .07). Collectively, the heterogeneity score was moderate (I2 = 69%), driven primarily by the subgroup of uncontrolled pre and post studies exhibiting high heterogeneity (I2 = 86%), whereas there was no heterogeneity among cluster RCTs (I2 = 0%) and controlled pre and post studies (I2 = 0%), indicating that the variation in the latter two subgroups is due to chance alone and not due to underlying differences. Two studies examined outcomes that were less comparable to the measures of overall antimicrobial use in the other studies; one examined total infections treated with antibiotics,20 and the other assessed prescription counts for asymptomatic bacteriuria as a proportion of total urinary tract treatments.19 Sensitivity analysis, excluding these two studies, yielded a nearly identical estimate to the main analysis (14% reduction; 95% CI = −7% to −21%; P = .0001). Visual inspection of the funnel plot (Figure 3) suggested evidence of asymmetry with a lack of publication of smaller studies showing increased antibiotic use (ie, ratio of means, greater than one).
Figure 2.
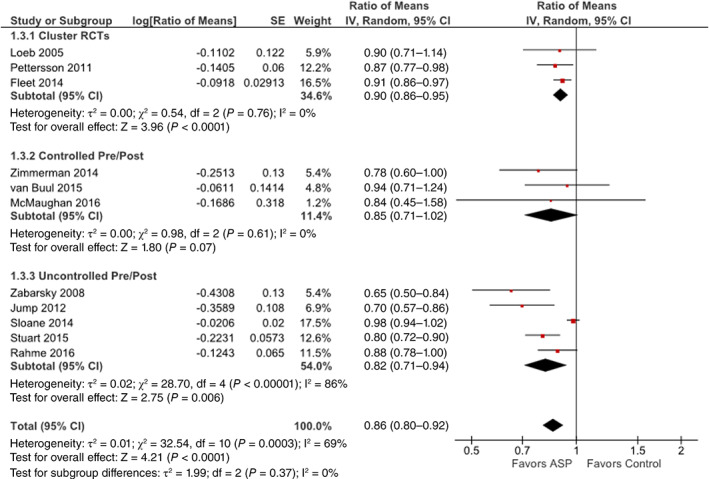
Forest plot showing the impact of antimicrobial stewardship strategies on overall antibiotic use in long‐term care facilities, including the 11 studies that reported this outcome. Individual and pooled ratio of means with 95% confidence intervals (CIs) are shown, and pooled results were calculated using inverse variance weighting and random‐effects models. Pooled ratios of means are also presented separately for the subgroups of cluster randomized controlled trials (RCTs), controlled pre/post observational studies, and uncontrolled pre/post observational studies. ASP indicates antimicrobial stewardship program. [Color figure can be viewed at wileyonlinelibrary.com]
Figure 3.
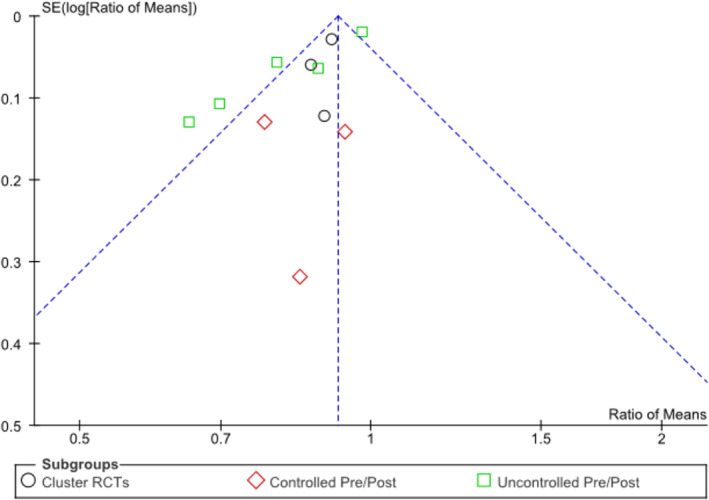
Funnel plot comparing the antimicrobial use effect measure, Ratio of means, for each of the 11 included studies (with different symbols for the cluster randomized controlled trial [RCT] [circles] and controlled [diamonds] and uncontrolled [squares] pre/post study subgroups) on the x‐axis, with each study's effect measure precision, expressed as the SE of the natural logarithm of the ratio of means, SE(log[Ratio of Means]), on the y‐axis demonstrating asymmetry with a lack of publication of smaller (lower precision) studies showing increased antibiotic use (ie, ratio of means, greater than one).
Discussion
The emerging public health threat of AMR requires a collective effort to improve antimicrobial use across the healthcare continuum. The US Centers for Medicare and Medicaid Services has mandated that ASP is required in LTCFs since 2017.8 The growing interest in implementation of antimicrobial stewardship in LTCFs is seen in the number of studies published in recent years. However, there are only a handful of qualitative reviews available, most likely because of the limited primary research conducted. Further to narratively describing ASP in LTCFs, some have also categorized interventions as centralized (programmatic) vs decentralized (nonprogrammatic)9 or by a human factor engineering approach including five work system components that interact and influence one another (eg, specific task, tools, and technology).37
This systematic review is the first to provide quantitative analysis of the impact of antimicrobial stewardship interventions in LTCFs and suggests that antimicrobial stewardship interventions contribute to a significant reduction in antimicrobial use by 14% when pooling across all types of interventions. It was reasonable to expect heterogeneity because of pooling of different interventions and study designs; however, the direction of the effect consistently favored the intervention, and the result was robust across subtypes of studies and sensitivity analysis. Only one study reported multiple time points for outcome measurements,25 and the furthest time point was included for meta‐analysis. It is noteworthy that reductions in antimicrobial use were similar for both time points, suggesting that the decrease was sustained over 2.5 years in this single‐center study. Sensitivity analysis (described in the Figure 2 legend) also showed that the overall effect remained unchanged.
This review also used an implementation approach to describe antimicrobial stewardship interventions in LTCFs using the EPOC taxonomy of health system interventions with additional information on intervention intensity and resource requirements. This framework offers a new approach to understand the current landscape while recognizing that tailored interventions may be required by different LTCFs, depending on various factors (eg, available resources or specific concerns needed to be addressed). Programs described by the included studies are all unique, with no standardization of strategies, evaluation, or reporting. Intervention intensity varied and often required time commitment outside of daily routines. Expertise in antimicrobial stewardship is often necessary because pharmacists and physician specialists are frequently involved in intervention delivery or providing ongoing assistance. Most studied interventions are multifaceted, with different strategies implemented simultaneously; consequently, the benefits of specific strategies are unclear. Multifaceted interventions are not necessarily more effective than single‐component interventions,38 especially in a resource‐limited setting. Nevertheless, it is reasonable to allow flexibility in designing antimicrobial stewardship strategies to be implemented in LTCFs to adequately reflect local needs.
Interventions with an enabling component (eg, audit and feedback) consistently increase the effect of interventions in hospital settings, and interventions that include audit and feedback have been shown to be more effective than those that did not.39 However, LTCFs are unlikely to be able to implement intense and resource‐heavy interventions, such as the audit and feedback strategies used in hospital settings.9 Thus, it is not surprising to see a wide range of antimicrobial stewardship strategies applied in LTCFs. Although knowledge gaps may influence antibiotic prescribing and decision making, education alone is insufficient to generate sustainable improvement in antibiotic prescribing and passive education activities should be combined with other antimicrobial stewardship strategies.9, 40 It is, therefore, encouraging to see that 12 of the 13 included studies with an education component included aspects beyond education. Furthermore, three of those studies also included a patient education component, which may be an essential objective because pressure from patients and family may contribute to prescribing decisions in LTCFs.9, 41
The main challenges of conducting meta‐analysis on the impact of antimicrobial stewardship strategies in LTCFs are the few published studies and the lack of diversity of study designs, interventions, and outcomes. A review found that less than 0.3% of published citations on antibiotic use and resistance focused on LTCFs, indicating a lack of research and development in this area compared with other healthcare settings (eg, primary care).1 Moreover, the review found publications were mostly focused on antimicrobial resistance, resulting in relatively few studies reporting antibiotic use.1 A second limitation of this systematic review is the lack of an appropriate and validated quality appraisal tool for this topic area. The use of the EPHPP quality appraisal tool, which provides assessment based on six individual quality domains, judged most of the studies to be at high risk of bias. The decrease in antibiotic use determined by the pooled analysis may be an overestimate because of potential publication bias as all studies demonstrated effectiveness (ie, decreased antibiotic use), although not all were statistically significant. This may not be surprising because it is less likely for groups to publish ASP implementation studies in which antibiotic use increased. Last, no studies reported cost‐related outcomes. Given the limited resources available in this setting, sustainability and cost are vital factors to consider and should be addressed.
Conclusion
This systematic review provides the current landscape of antimicrobial stewardship strategies implemented in LTCFs and resource‐related factors to consider when designing an ASP in this setting. Antimicrobial stewardship strategies in LTCFs differ considerably in design and resource intensity. Given the variability in interventions used and the presence of multifaceted approaches, there is no one specific strategy that can be recommended at this time to improve antibiotic use in residents of LTCFs. It is also important for ASP researchers to remain cautious about potential publication bias. However, collectively, antimicrobial stewardship strategies appear to be associated with significantly reduced antimicrobial use in this challenging setting.
Supporting information
Supplementary Table S1. Intervention characteristics of included studies
Supplementary Table S2. Intervention implementation strategies used by included studies
Acknowledgments
Conflict of Interest
The authors have no conflicts of interest to report.
Author Contributions
All authors meet the criteria for authorship stated in the Uniform Requirements for Manuscripts Submitted to Biomedical Journals: (1) substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; (2) drafting the article or revising it critically for important intellectual content; and (3) final approval of the version to be published.
Presented at Infection Prevention and Control (IPAC) Canada 2018 National Education Conference.
Correction added February 4, 2019, after first online publication. This article was recategorized from “Clinical Investigation” to “Review Article.”
REFERENCES
- 1. van Buul LW, van der Steen JT, Veenhuizen RB et al. Antibiotic use and resistance in long term care facilities. J Am Med Dir Assoc 2012;13:568.e1–568.13. [DOI] [PubMed] [Google Scholar]
- 2. Daneman N, Gruneir A, Bronskill SE et al. Prolonged antibiotic treatment in long‐term care: role of the prescriber. JAMA Intern Med 2013;173:673–682. [DOI] [PubMed] [Google Scholar]
- 3. Mody L, Crnich C. Effects of excessive antibiotic use in nursing homes. JAMA Intern Med 2015;175:1339–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Daneman N, Gruneir A, Newman A et al. Antibiotic use in long‐term care facilities. J Antimicrob Chemother 2011;66:2856–2863. [DOI] [PubMed] [Google Scholar]
- 5. Daneman N, Bronskill SE, Gruneir A et al. Variability in antibiotic use across nursing homes and the risk of antibiotic‐related adverse outcomes for individual residents. JAMA Intern Med 2015;175:1331–1339. [DOI] [PubMed] [Google Scholar]
- 6. Trick WE, Weinstein RA, DeMarais PL et al. Colonization of skilled‐care facility residents with antimicrobial‐resistant pathogens. J Am Geriatr Soc 2001;49:270–276. [DOI] [PubMed] [Google Scholar]
- 7. Smith PW, Bennett G, Bradley S et al. SHEA/APIC guideline: infection prevention and control in the long‐term care facility, July 2008. Infect Control Hosp Epidemiol 2008;29:785–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. The Centers for Medicare and Medicaid Services . MS finalizes improvements in care, safety, and consumer protections for long‐term care facility residents. Available at https://www.cms.gov/newsroom/press‐releases/cms‐finalizes‐improvements‐care‐safety‐and‐consumer‐protections‐long‐term‐care‐facility‐residents. Accessed September 10, 2018. Updated 2016.
- 9. McElligott M, Welham G, Pop‐Vicas A et al. Antibiotic stewardship in nursing facilities. Infect Dis Clin North Am 2017;31:619–638. [DOI] [PubMed] [Google Scholar]
- 10. Nicolle LE. Antimicrobial stewardship in long term care facilities: what is effective? Antimicrob Resist Infect Control 2014;3:6‐2994‐3‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Effective Practice and Organisation of Care (EPOC) . EPOC taxonomy: 2015. Available at https://epoc.cochrane.org/epoc-taxonomy. Accessed May 7, 2018. Updated 2018.
- 12. National Collaborating Centres for Methods and Tools . Quality assessment tool for quantitative studies. Available at https://www.nccmt.ca/knowledge-repositories/search/14. Accessed September 5, 2018. Updated 2004.
- 13. Harder T, Takla A, Rehfuess E et al. Evidence‐based decision‐making in infectious diseases epidemiology, prevention and control: matching research questions to study designs and quality appraisal tools. BMC Med Res Methodol 2014;14:69 –2288‐14‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Friedrich JO, Adhikari NK, Beyene J. Ratio of means for analyzing continuous outcomes in meta‐analysis performed as well as mean difference methods. J Clin Epidemiol 2011;64:556–564. [DOI] [PubMed] [Google Scholar]
- 15. Friedrich JO, Adhikari NK, Beyene J. The ratio of means method as an alternative to mean differences for analyzing continuous outcome variables in meta‐analysis: a simulation study. BMC Med Res Methodol 2008;8:32–2288‐8‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wan X, Wang W, Liu J et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135 –2288–14‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 18. Higgins JP, Thompson SG, Deeks JJ et al. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McMaughan DK, Nwaiwu O, Zhao H et al. Impact of a decision‐making aid for suspected urinary tract infections on antibiotic overuse in nursing homes. BMC Geriatr 2016;16:81 –016–0255‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pettersson E, Vernby A, Molstad S et al. Can a multifaceted educational intervention targeting both nurses and physicians change the prescribing of antibiotics to nursing home residents? a cluster randomized controlled trial. J Antimicrob Chemother 2011;66:2659–2666. [DOI] [PubMed] [Google Scholar]
- 21. Furuno JP, Comer AC, Johnson JK et al. Using antibiograms to improve antibiotic prescribing in skilled nursing facilities. Infect Control Hosp Epidemiol 2014;35:S56–S61. [DOI] [PubMed] [Google Scholar]
- 22. Jump RL, Olds DM, Seifi N et al. Effective antimicrobial stewardship in a long‐term care facility through an infectious disease consultation service: keeping a LID on antibiotic use. Infect Control Hosp Epidemiol 2012;33:1185–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rahme C, Jacoby H, Avery L. Impact of a hospital's antibiotic stewardship team on fluoroquinolone use at a long‐term care facility. Ann Long‐Term Care 2016;24:13–20. [Google Scholar]
- 24. Sloane PD, Zimmerman S, Reed D et al. Antibiotic prescribing in 4 assisted‐living communities: incidence and potential for improvement. Infect Control Hosp Epidemiol 2014;35:S62–S68. [DOI] [PubMed] [Google Scholar]
- 25. Zabarsky TF, Sethi AK, Donskey CJ. Sustained reduction in inappropriate treatment of asymptomatic bacteriuria in a long‐term care facility through an educational intervention. Am J Infect Control 2008;36:476–480. [DOI] [PubMed] [Google Scholar]
- 26. Doernberg SB, Dudas V, Trivedi KK. Implementation of an antimicrobial stewardship program targeting residents with urinary tract infections in three community long‐term care facilities: a quasi‐experimental study using time‐series analysis. Antimicrob Resist Infect Control 2015;4:54–015‐0095‐y. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gugkaeva Z, Franson M. Pharmacist‐led model of antibiotic stewardship in a long‐term care facility. Ann Long‐Term Care 2012;20:22–26. [Google Scholar]
- 28. Cooper D, Titler M, Struble L et al. A multifaceted, evidence‐based program to reduce inappropriate antibiotic treatment of suspected urinary tract infections. Ann Long‐Term Care 2017;25:36–43. [Google Scholar]
- 29. Stuart RL, Orr E, Kotsanas D et al. A nurse‐led antimicrobial stewardship intervention in two residential aged care facilities. Healthcare Infect 2015;20:4–6. [Google Scholar]
- 30. Linnebur SA, Fish DN, Ruscin JM et al. Impact of a multidisciplinary intervention on antibiotic use for nursing home‐acquired pneumonia. Am J Geriatr Pharmacother 2011;9:442–450.e1. [DOI] [PubMed] [Google Scholar]
- 31. van Buul LW, van der Steen JT, Achterberg WP et al. Effect of tailored antibiotic stewardship programmes on the appropriateness of antibiotic prescribing in nursing homes. J Antimicrob Chemother 2015;70:2153–2162. [DOI] [PubMed] [Google Scholar]
- 32. Zimmerman S, Sloane PD, Bertrand R et al. Successfully reducing antibiotic prescribing in nursing homes. J Am Geriatr Soc 2014;62:907–912. [DOI] [PubMed] [Google Scholar]
- 33. Fleet E, Gopal Rao G, Patel B et al. Impact of implementation of a novel antimicrobial stewardship tool on antibiotic use in nursing homes: a prospective cluster randomized control pilot study. J Antimicrob Chemother 2014;69:2265–2273. [DOI] [PubMed] [Google Scholar]
- 34. Loeb M, Brazil K, Lohfeld L et al. Effect of a multifaceted intervention on number of antimicrobial prescriptions for suspected urinary tract infections in residents of nursing homes: cluster randomised controlled trial. BMJ 2005;331:669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Monette J, Miller MA, Monette M et al. Effect of an educational intervention on optimizing antibiotic prescribing in long‐term care facilities. J Am Geriatr Soc 2007;55:1231–1235. [DOI] [PubMed] [Google Scholar]
- 36. Naughton BJ, Mylotte JM, Ramadan F et al. Antibiotic use, hospital admissions, and mortality before and after implementing guidelines for nursing home‐acquired pneumonia. J Am Geriatr Soc 2001;49:1020–1024. [DOI] [PubMed] [Google Scholar]
- 37. Katz MJ, Gurses AP, Tamma PD et al. Implementing antimicrobial stewardship in long‐term care settings: an integrative review using a human factors approach. Clin Infect Dis 2017;65:1943–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Squires JE, Sullivan K, Eccles MP et al. Are multifaceted interventions more effective than single‐component interventions in changing health‐care professionals' behaviours? an overview of systematic reviews. Implement Sci 2014;9:152 –014–0152‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Davey P, Marwick CA, Scott CL et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev 2017;2:CD003543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Barlam TF, Cosgrove SE, Abbo LM et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016;62:e51–e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dobson EL, Klepser ME, Pogue JM et al. Outpatient antibiotic stewardship: interventions and opportunities. J Am Pharm Assoc 2017;57:464–473. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Intervention characteristics of included studies
Supplementary Table S2. Intervention implementation strategies used by included studies


