Summary
What is known and objective
Febuxostat and allopurinol are xanthine oxidase inhibitors for urate‐lowering therapy. The efficacy and safety of febuxostat and allopurinol have been mostly reported in hyperuricemia patients with normal renal function. Here, we aimed to compare the effects of these two drugs in early post‐renal transplant recipients, focusing on evaluating the urate‐lowering effect and recovery of allograft renal function.
Methods
A retrospective cohort study was performed in early post‐renal transplant recipients with new onset of hyperuricemia receiving febuxostat or allopurinol therapy. Serum uric acid (UA) and estimated glomerular filtration rate (eGFR) were detected on days 3, 7 and 15 and months 1, 3 and 6 after therapy initiation. Liver and blood functions were monitored and other adverse events were recorded.
Results and discussion
A total of 48 and 33 patients were enrolled in the febuxostat and allopurinol groups, respectively. Significant UA‐lowering effects were observed on day 3 in both groups. Febuxostat caused a more rapid UA decline, starting on day 3 and lasting for 1 month. The most apparent contrast was found in UA level (267.25 ± 93.66 vs 334.18 ± 96.56 μmol/L, P = 0.003) on day 7; 62.5% and 30.3% of patients achieved target UA level in febuxostat and allopurinol groups respectively on day 3 (P = 0.004), but there was no significant difference between two groups from days 15 to months 6. The median times to achieve target UA level were 3 and 5 days in febuxostat and allopurinol groups respectively (P = 0.002). The eGFR levels and recovering rates were gradually upregulated but no significant differences were found between two groups. No abnormities related to febuxostat or allopurinol were observed.
What is new and conclusion
This is the first comprehensive evaluation of UA‐lowering effects of febuxostat and allopurinol in early post‐renal transplant recipients. Febuxostat caused a marginally quicker serum UA‐lowering effect than allopurinol, but there was no advantage for long‐term use of febuxostat. The drugs had no significant differences in impacting renal allograft function recovery, and both were well tolerated.
Keywords: allopurinol, efficacy, febuxostat, hyperuricemia, renal allograft function, renal transplantation, safety
1. WHAT IS NEW AND OBJECTIVE
Hyperuricemia is a metabolic disorder that causes the level of serum uric acid (UA) to exceed the upper limit of the normal reference level. Hyperuricemia is one of the common complications in renal transplant recipients, with an incidence rate from 25.1% at 3 months to 44.2% at 5 years after transplantation.1 Risk factors for hyperuricemia include reduced renal allograft function and drugs (particularly calcineurin inhibitors and diuretics).2, 3, 4
Studies have suggested a positive association between hyperuricemia and progression of disease in individuals with renal insufficiency, including chronic kidney disease (CKD) and unrecovered allograft kidney.5, 6, 7, 8, 9, 10 Elevated serum UA is associated with abnormal endothelial function, mitochondria dysfunction and glomerular arteriolopathy and also could result in the formation of urate crystals in the nephron collecting ducts with tubular obstruction and then cause the damage of kidney structure progressively.11
Available UA‐lowering agents include the xanthine oxidase inhibitors such as febuxostat, allopurinol and benzbromarone. Benzbromarone increases the urinary excretion of uric acid, but the use of benzbromarone is limited due to reduced effects in patients with renal insufficiency and the risk of severe hepatotoxicity.12 Febuxostat and allopurinol have both been shown to be efficacious in hyperuricemia and gout and are well tolerated, thus recommended for UA‐lowering therapy, especially in patients with renal insufficiency.13 While a more potent UA‐lowering effect has been shown with febuxostat, there was no evidence that febuxostat is superior to allopurinol for clinically relevant outcomes and, given its higher cost, febuxostat should not be routinely used for chronic gout.14
For patients with CKD, many studies have been reported the beneficial effects of febuxostat and allopurinol on renal function. A randomized controlled trial demonstrated that allopurinol reduced the rate of decline in GFR in patients with CKD with eGFR <60 mL/min/1.73 m2.15 Febuxostat showed a renoprotective effect and retarded the progression of kidney disease in patients compared with allopurinol.16, 17, 18, 19 However, the impact of these two drugs on allograft renal function is conflicting. A retrospective analysis20 of 54 renal transplant recipients using allopurinol demonstrated that allopurinol use was associated with preservation of eGFR in renal transplant recipients. Other studies found no apparent effect on eGFR for febuxostat21 and did not find a difference in change of eGFR from baseline between febuxostat and allopurinol in stable renal transplant recipients.22 Moreover, renal function can change over time in post‐operative renal transplant recipients,23 and it is plausible to hypothesize an effect of promoting renal function recovery of febuxostat or allopurinol based on the protective effect in renal insufficiency patients with CKD. It is therefore useful to study the time‐varying effects of febuxostat and allopurinol on serum UA levels in post‐renal transplant recipients with new onset of hyperuricemia. Data about the safety of febuxostat and allopurinol in post‐renal transplant recipients also need to be replenished.
Thus, we performed a retrospective cohort study to compare the effects on reducing serum UA level, the influence on renal allograft function recovery and the safety between febuxostat and allopurinol in post‐renal transplant recipients with hyperuricemia.
2. METHODS
2.1. Study patients
We reviewed medical records of all post‐renal transplant recipients hospitalized between January 2015 and September 2017 at the First Affiliated Hospital of Sun Yat‐sen University in Guangzhou, China. The inclusion criteria were as follows: (a) serum UA level ≥420 μmol/L for male and post‐menopausal women, serum UA level ≥360 μmol/L for non‐menopausal women; (b) receiving febuxostat or allopurinol for lowering serum UA level. The exclusion criteria were as follows: (a) age <18 years or >70 years; (b) concomitant gout or hyperuricemia before receiving renal transplantation; (c) discontinued treatment of immunosuppressive agents because of medical reasons; (d) death; (e) use allopurinol or febuxostat before receiving renal transplantation to prevent the occurrence of hyperuricemia; (f) discontinued serum UA‐lowering agents, or the treatment of hyperuricemia for patients was changed from febuxostat to allopurinol or benzbromarone, or changed from allopurinol to febuxostat or benzbromarone during the follow‐up period.
2.2. Study design
This was a single‐center, retrospective observational cohort study designed to compare the effects of febuxostat and allopurinol on lowering serum UA level, renal allograft function recovery and safety in early post‐operative renal transplantation patients with hyperuricemia. The study was performed in accordance with the Declaration of Helsinki and the Principles of Good Clinical Practice. The Institutional Research Ethics Committee approved the study.
The patient enrolment flow chart is shown in Figure 1. There were 6 months follow‐up after beginning the treatment of hyperuricemia. Patients in the febuxostat group were given 40 mg once daily of febuxostat tablets (Jiangsu Wanbang Pharmacy Ltd., Xuzhou, China), and the frequency was adjusted according to the serum UA level. Patients in allopurinol group were given allopurinol tablet (Shanghai Xinyi Wanxiang Pharmacy Ltd., Shanghai, China) 50‐100 mg per dosage or sustained release capsules (Heilongjiang Aolida Ned Pharmaceutical Co. Ltd., Harbin, China) 250 mg per dosage, and frequency was adjusted according the serum UA level.
Figure 1.
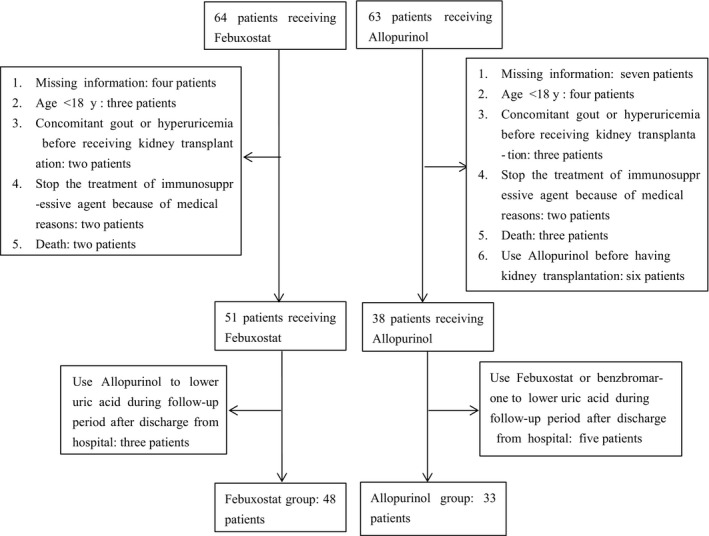
Flow of participants included in the study
All of the recipients were given a triple immunosuppressive regimen of tacrolimus (Prograf, Astellas, Killorglin, Ireland) or cyclosporin A (Huadong medicine Co. Ltd., Zhejiang, China), mycophenolate mofetil (Cellcept, Roche, Basel, Switzerland) and prednisone (Guangdong Huanan Pharmacy Ltd, Dongguan, China). Initial doses of tacrolimus or cyclosporin A were administered according to body weight and preoperatively started on the day of transplantation and continued with these doses, until the first measurement of tacrolimus C 0 was performed in the morning of day 3 and cyclosporin A C 0 was performed in the morning of day 7. Subsequent tacrolimus or cyclosporin A doses were adjusted basing on the results of C 0 monitoring, target C 0 range at 6‐8 ng/mL was maintained for tacrolimus and 150‐200 ng/mL for cyclosporin A.
2.3. Data collection
Patient demographic features (age, sex, BMI, baseline blood pressure, baseline lipid levels, cause of end‐stage renal disease, donor type, delayed graft function rate, the time post‐transplantation when UA‐lowering drugs initiated) and medication data (scheme of immunosuppressive treatment, trough level of tacrolimus and cyclosporine when beginning UA‐lowering treatment, concomitant drugs, the dosage of febuxostat and allopurinol) were collected at the time of entry. The dosages of immunosuppressive agents and other concomitant drugs were continued and adjusted according to each individual patient's clinical condition. For evaluating the UA‐lowering effect and impact on renal function recovery, serum UA and creatinine levels were detected on days 3, 7 and 15 and months 1, 3 and 6 after initiation of febuxostat and allopurinol treatment. Adverse events were evaluated by medical records and laboratory data including white blood cell count (WBC), haemoglobin (Hb), platelet (PLT), alanine aminotransferase (ALT) and aspartic transaminase (AST) were also collected for safety evaluation.
The IDMS‐traceable MDRD equation24 was used to calculated estimated glomerular filtration rate (eGFR) for all included patients, eGFR[mL/min/1.73 m2] = 175 × Scr−1.154 × age−0.203 (0.742 if female). (Scr = serum creatinine (mg/dL), Scr: 1 mg/dL = 88.402 μmol/L, 1 μmol/L = 0.0113 mg/dL).
2.4. Statistical analysis
Statistical analyses and calculations were performed using SPSS software (version 22; SPSS/IBM, Armonk, NY) and Prism 6 (GraphPad Software, La Jolla, CA). Quantitative variables were described with mean ± standard deviation (SD) if normally distributed or median ± interquartile range (IQR) if not normally distributed. Qualitative variables are given as counts and ratios (%). Baseline variables were compared between febuxostat group and allopurinol group to detect any variability at baseline. Between‐group comparison of numeric parametric data was done by unpaired t test or Wilcoxon rank sum test. To determine differences between groups of categorical data (eg proportion of patients who achieve target serum uric acid), chi‐square test or Fisher's exact test were used as applicable. P < 0.05 was considered statistically significant.
3. RESULTS
3.1. Patient baseline characteristics
The flow chart in Figure 1 showed that 64 patients receiving febuxostat and 63 patients receiving allopurinol were screened for initial enrolment. With further exclusion, 48 patients in the febuxostat group and 33 patients in the allopurinol group were available for analysis. Baseline characteristics are presented in Table 1. There were no significant differences in the febuxostat and allopurinol groups with respect to baseline variables, apart from the level of high‐density lipoprotein (HDL), which was higher in the febuxostat group at baseline. In the febuxostat group, three patients switched from 40 mg twice daily to 40 mg once daily according to the level of serum UA, and four patients increased the frequency to 40 mg once daily due to uncontrolled UA level at initial 40 mg once two days; the other 41 patients maintained 40 mg once daily throughout the study. In the allopurinol group, eight patients were given allopurinol sustained release capsules with 250 mg twice daily, five patients adjusted the sustained release capsules from 250 mg once 2 days to once daily based on the serum UA level, five patients using 50 mg allopurinol tablet changed the frequencies between once daily and twice daily, and the other 15 patients kept the tablet at 50 mg once daily throughout the study.
Table 1.
Baseline demographic and clinical characteristics
|
Febuxostat N = 48 |
Allopurinol N = 33 |
P‐value | |
|---|---|---|---|
| Female, N (%) | 32 (66.67) | 21 (63.64) | 0.778 |
| Age, year | 35.50 ± 22.00 | 35.00 ± 14.00 | 0.532 |
| BMI, kg/m2 | 21.05 ± 2.97 | 22.03 ± 3.17 | 0.161 |
| Cause of ESRD, no. of patients (%) | |||
| Hypertension | 28 (58.33) | 17 (51.52) | 0.168 |
| Diabetes mellitus | 2 (4.17) | 0 (0) | |
| Glomerulonephritis | 8 (6.67) | 4 (12.12) | |
| IgA nephropathy | 2 (4.17) | 4 (12.12) | |
| Polycystic kidney diseases | 2 (4.17) | 0 (0) | |
| Unknown | 6 (12.50) | 8 (24.24) | |
| Donor type, N (%) | |||
| Deceased donor | 39 (81.25) | 22 (66.67) | 0.135 |
| Living donor | 9 (18.75) | 11 (33.33) | |
| 2nd kidney transplants, N (%) | 2 (4.17) | 0 (0) | 0.646 |
| Time post‐transplant that the uric acid lowering drugs were initiated, days | 12 ± 9 | 12 ± 10 | 0.494 |
| Delayed graft function, no. of patient (%) | 7 (14.58) | 4 (12.90) | 0.749 |
| Initial scheme of immunosuppressive treatment, N (%) | |||
| Tacrolimus + mycophenolate +glucocorticoid | 46 (95.83) | 31 (93.94) | 0.701 |
| Cyclosporine + mycophenolate + glucocorticoid | 2 (4.17) | 2 (6.06) | |
| Trough level of tacrolimus when the uric acid lowering drugs were initiated, ng/mL | 6.85 ± 4.7 | 6.2 ± 3.9 | 0.262 |
| Trough level of cyclosporine when the uric acid lowering drugs were initiated, ng/mL | 137.5 ± 17.68 | 137.5 ± 88.39 | 1.000 |
| Systolic blood pressure, mm Hg | 151.04 ± 15.80 | 147.45 ± 18.29 | 0.349 |
| Diastolic blood pressure, mm Hg | 93.42 ± 11.36 | 94.21 ± 10.65 | 0.752 |
| TC, mmol/L | 4.78 ± 1.38 | 4.68 ± 1.22 | 0.734 |
| TG, mmol/L | 1.38 ± 1.14 | 1.69 ± 1.66 | 0.408 |
| LDL, mmol/L | 2.91 ± 0.99 | 2.88 ± 2.70 | 0.891 |
| HDL, mmol/L | 1.17 ± 0.28 | 0.99 ± 0.39 | 0.007 |
| Systolic blood pressure, mm Hg | 151.04 ± 15.80 | 147.45 ± 18.29 | 0.349 |
| Diuretic | 38 (79.17) | 26 (78.79) | 0.967 |
| PPIs | 48 (100) | 31 (93.94) | 0.318 |
| Aspirin | 3 (6.25) | 5 (15.15) | 0.347 |
Values for categorical variables are given as number (percentage); for continuous variables, as mean ± standard deviation or median ± interquartile range.
ESRD, end‐stage renal disease; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; PPIs, proton‐pump inhibitor; TC, total cholesterol; TG, triglyceride.
3.2. Efficacy in lowering serum uric acid
The mean serum UA levels of febuxostat and allopurinol groups were 572.23 ± 117.80 μmol/L and 587.06 ± 106.11 μmol/L before the treatment (P > 0.05). As shown in Figure 2A and Table 2, there was a decrease in UA levels in febuxostat and allopurinol groups after initiation of treatment, whereas the UA levels were significantly lower in febuxostat group than in allopurinol group on day 3, 7 and 15 and month 1 (P < 0.05). The most apparent difference was found on day 7, with UA levels 267.25 ± 93.66 μmol/L for febuxostat group and 334.18 ± 96.56 μmol/L for allopurinol group (P = 0.003). The significant differences between the two groups disappeared on months 3 and 6. Similar results were found in the serum UA decline proportion (Figure 2B and Table 2). On day 7, the decline proportions were 52.92% ± 14.82% in the febuxostat group and 41.79% ± 15.28% in the allopurinol group (P = 0.002).
Figure 2.
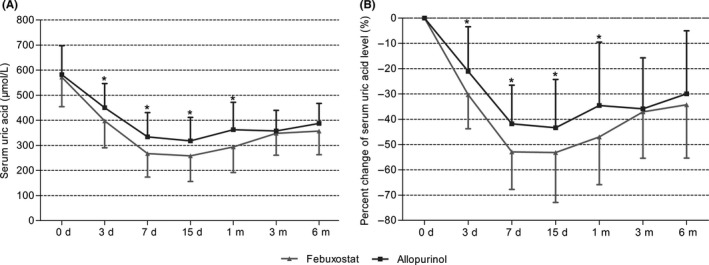
Effects of febuxostat and allopurinol on A, serum uric acid levels and B, percentage changes of serum uric acid levels for early post‐renal transplant recipients after initial treatment. Values are given as mean ± standard deviation, *P < 0.05
Table 2.
The serum uric acid levels, percentage changes of serum uric acid levels and percentage of patients who achieved target serum uric acid levels in febuxostat group and allopurinol group in different time
| Serum uric acid | Percentage changes of serum uric acid levels | Percentage of patients who achieved target serum uric acid levels | |
|---|---|---|---|
| Febuxostat | |||
| Pretreatment | 572.23 ± 117.80 | 0.00 | 0.00 (48/48) |
| Day 3 | 397.58 ± 106.98* | −30.31 ± 13.45* | 62.50 (30/48)* |
| Day 7 | 267.25 ± 93.66* | −52.92 ± 14.82* | 93.75 (45/48)* |
| Day 15 | 258.61 ± 102.50* | −53.16 ± 19.77* | 89.13 (41/46) |
| Month 1 | 294.22 ± 102.59* | −46.97 ± 18.93* | 82.61 (38/46) |
| Month 3 | 347.89 ± 86.82 | −37.10 ± 18.33 | 75.56 (34/45) |
| Month 6 | 357.32 ± 94.37 | −34.25 ± 21.14 | 73.53 (25/34) |
| Allopurinol | |||
| Pretreatment | 587.06 ± 106.11 | 0.00 | 0.00 (33/33) |
| Day 3 | 450.39 ± 96.21 | −21.06 ± 17.66 | 30.30 (10/33) |
| Day 7 | 334.18 ± 96.56 | −41.79 ± 15.28 | 75.76 (25/33) |
| Day 15 | 317.91 ± 93.70 | −43.38 ± 19.12 | 81.82 (27/33) |
| Month 1 | 362.73 ± 109.00 | −34.56 ± 25.10 | 66.67 (22/33) |
| Month 3 | 357.77 ± 82.29 | −35.89 ± 20.19 | 70.00 (21/30) |
| Month 6 | 387.52 ± 79.70 | −29.93 ± 24.92 | 58.62 (17/29) |
Serum uric acid is expressed in μmol/L, percentage change of serum uric acid level is expressed in %, and values are given as mean ± standard deviation. Percentage of patients who achieve target serum uric acid is expressed in %, and values are given as percentage (number of patients/total number).
P < 0.05 vs allopurinol in different time.
We investigated the effects of the two drugs on the percentage of patients achieving target serum UA level (Figure 3 and Table 2) and observed that 62.50% and 30.30% of patients achieved target UA level in febuxostat and allopurinol group on day 3 respectively (P = 0.004), and the proportions were 93.75% and 75.76% on day 7 (P = 0.046). However, no significant differences were observed from day 15. Furthermore, Figure 4 showed that the median times to achieve target UA level was 3 days and 5 days in the febuxostat group and allopurinol group, respectively (P = 0.002).
Figure 3.
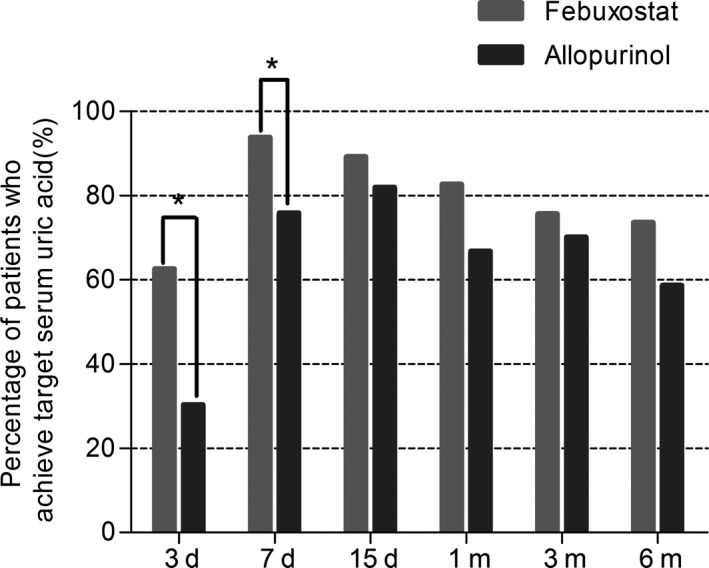
Percentage of patients who achieved target serum uric acid levels in febuxostat group and allopurinol group in different time. *P < 0.05
Figure 4.
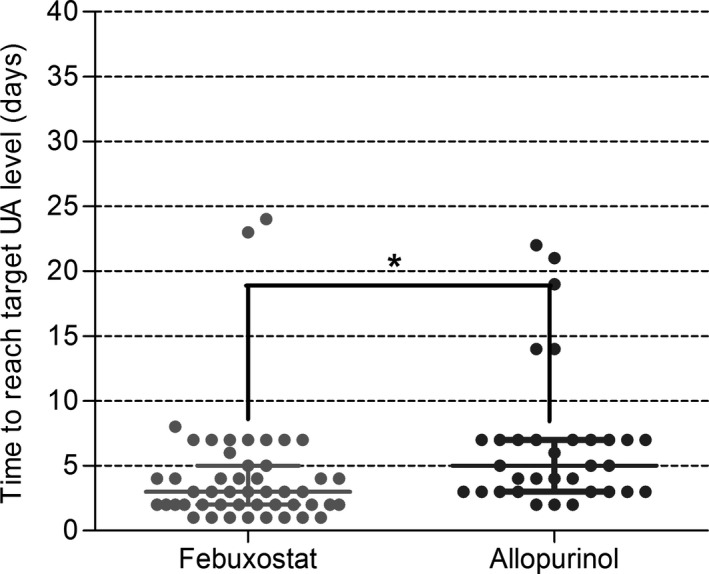
Time to reach target serum uric acid levels in febuxostat group and allopurinol group of early post‐renal transplant recipients with new onset of hyperuricemia. *P <0.05. Spots stand for the time to reach target serum uric acid level for every patient. Straight horizontal lines stand for median and interquartile range
Overall, the above data suggest that, compared to allopurinol, febuxostat may cause a slightly quicker serum UA‐lowering effect at the beginning of treatment, but the advantage disappeared relatively quickly with the extension of treatment.
3.3. Impacts on renal allograft function recovery
eGFR was adopted for evaluation of renal allograft function recovery. As shown in Figure 5A and Table 3, the eGFRs in baseline were 13.40 ± 30.66 and 18.52 ± 21.69 mL/min/1.73 m2 in the febuxostat and allopurinol groups, respectively (P > 0.05); they then increased gradually to 60.21 ± 19.85 and 51.00 ± 18.50 mL/min/1.73 m2. However, no significant differences were found between the two groups in all of the monitoring time. Likewise, similar results were also found in the increasing rate of eGFR (Figure 5B and Table 3).
Figure 5.
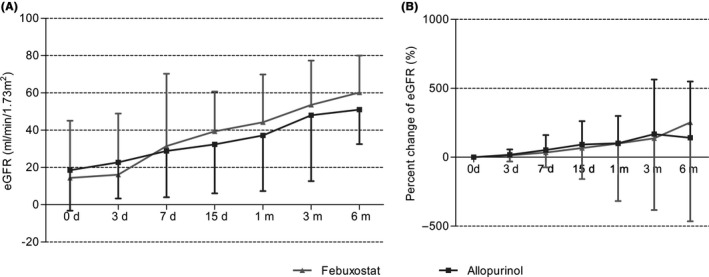
Effects of febuxostat and allopurinol on A, estimated glomerular filtration rates and B, percentage changes of eGFR for early post‐renal transplant recipients after initial treatment. eGFR is expressed in mL/min/1.73 m2, values are given as median ±interquartile range except values of febuxosta group and allopurinol group in month 6 are given as mean ± standard deviation. Values in figure (B) are given as mean ± standard deviation
Table 3.
Estimated glomerular filtration rate and percentage changes of eGFR in febuxostat group and allopurinol group of early post‐renal transplant recipients after initial treatment
| eGFR | Percentage changes of eGFR | |
|---|---|---|
| Febuxostat | ||
| Pretreatment | 13.40 ± 30.66 | 0.00 |
| Day 3 | 16.13 ± 32.75 | 13.33 ± 43.95 |
| Day 7 | 31.43 ± 38.83 | 33.83 ± 106.70 |
| Day 15 | 39.33 ± 21.40 | 67.02 ± 225.72 |
| Month 1 | 44.25 ± 25.67 | 99.69 ± 417.76 |
| Month 3 | 53.58 ± 23.76 | 132.27 ± 520.89 |
| Month 6 | 60.21 ± 19.85a | 252.33 ± 716.80 |
| Allopurinol | ||
| Pretreatment | 18.52 ± 21.69 | 0.00 |
| Day 3 | 22.72 ± 19.39 | 18.33 ± 38.36 |
| Day 7 | 28.87 ± 24.85 | 52.54 ± 108.37 |
| Day 15 | 32.38 ± 26.22 | 92.18 ± 169.72 |
| Month 1 | 37.15 ± 29.84 | 101.03 ± 198.85 |
| Month 3 | 48.03 ± 35.35 | 167.37 ± 396.55 |
| Month 6 | 51.00 ± 18.50a | 141.27 ± 408.56 |
eGFR is expressed in mL/min/1.73 m2, and values are given as median ± interquartile range except value a are given as mean ± standard deviation. Percentage changes of eGFR are expressed in %, and values are given as median ± interquartile range.
3.4. Safety
Common adverse drug reactions related to febuxostat and allopurinol include abnormalities of hematologic and hepatic functions. Thus, laboratory data including WBC, PLT, Hb, ALT and AST were analyzed throughout the study, and no abnormities related to the two drugs were observed (Table 4). Meanwhile, none of the patients in both groups experienced other adverse reactions related to febuxostat or allopurinol. During the study period, there happened one patient in the febuxostat group with ALT and AST levels rising from 11 U/L to 12 U/L in the beginning to 172 U/L and 95 U/L at month 1, which might be explained by the use of tacrolimus, because ALT and AST recovered to normal levels after switching tacrolimus to cyclosporine A. Besides, in the febuxostat group, one patient had leucopenia and another one had hyperkalemia. In the allopurinol group, one patient had hyperkalemia and another one had mild diarrhea. However, all of the above adverse reactions were not considered to be related to febuxostat or allopurinol, since these reactions improved after discontinuation of other suspected drugs (such as mycophenolate mofetil induced diarrhoea and tacrolimus induced hyperkalemia) while continuing use of febuxostat or allopurinol.
Table 4.
Safety of febuxostat and allopurinol in patients who used febuxostat or allopurinol to lower serum uric acid after having renal transplantation
| WBC × 109/L | Hb, g/L | PLT × 109/L | ALT, U/L | AST, U/L | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | Median ±IQR | Range | Median ± IQR | Range | |
| Febuxostat | ||||||||||
| Pretreatment | 10.57 ± 4.45 | 3.14‐22.53 | 97.19 ± 15.86 | 69‐139 | 149.23 ± 45.86 | 58‐185 | 14.5 ± 12.75 | 5‐151 | 17.50 ± 10.75 | 6‐299 |
| Month 1 | 7.02 ± 2.72 | 3.00‐16.00 | 108.13 ± 18.59 | 77‐150 | 204.76 ± 75.91 | 39‐407 | 18.00 ± 16.00 | 5‐172 | 17.00 ± 13.00 | 11‐95 |
| Month 3 | 5.93 ± 2.32 | 2.00‐12.00 | 122.96 ± 16.95 | 85‐152 | 213.36 ± 50.19 | 133‐328 | 16.00 ± 15.00 | 2‐62 | 21.00 ± 8.50 | 10‐52 |
| Month 6 | 6.91 ± 1.82 | 4.00‐11.00 | 130.50 ± 17.71 | 90‐157 | 224.00 ± 57.93 | 99‐324 | 13.00 ± 8.50 | 5‐92 | 18.50 ± 6.25 | 11‐70 |
| Allopurinol | ||||||||||
| Pretreatment | 10.03 ± 3.48 | 4.00‐18.00 | 94.97 ± 17.12 | 64‐134 | 136.70 ± 47.09 | 69‐302 | 16.00 ± 20.00 | 4‐63 | 15.00 ± 12.50 | 6‐120 |
| Month 1 | 7.79 ± 2.98 | 3.00‐17.00 | 93.21 ± 16.51 | 68‐134 | 212.85 ± 78.07 | 109‐461 | 18.00 ± 15.50 | 6‐47 | 17.00 ± 10.00 | 8‐31 |
| Month 3 | 5.73 ± 2.60 | 2.00‐13.00 | 113.27 ± 16.96 | 78‐150 | 207.27 ± 48.49 | 107‐325 | 15.50 ± 10.25 | 4‐29 | 19.00 ± 8.25 | 11‐31 |
| Month 6 | 6.41 ± 2.10 | 3.00‐12.00 | 114.38 ± 23.20 | 70‐154 | 208.86 ± 51.42 | 103‐340 | 18.00 ± 16.00 | 5‐85 | 21.00 ± 6.50 | 13‐45 |
ALT, alanine aminotransferase; AST, aspartate transaminase; Hb, haemoglobin; PLT, platelet; WBC, white blood cell.
4. DISCUSSION
In our present study, febuxostat was demonstrated to cause a slightly quicker serum UA lowering effect than allopurinol at the beginning of treatment, while the advantage disappeared with the extension of treatment. No significant differences were found between the two drugs in promoting the renal allograft function recovery. The therapy for hyperuricemia with febuxostat and allopurinol in post‐renal transplant recipients were both well tolerated, and no severe adverse effects were observed.
Serum UA levels and decline proportions between febuxostat and allopurinol groups showed significant variances on days 3, 7 and 15 and month 1, but disappeared on months 3 and 6 in our study. A similar result was observed in another retrospective study25; stable renal transplant recipients with hyperuricemia prescribed febuxostat, allopurinol and benzbromarone had no significant difference in serum UA levels on the months 1, 3, 6 and 12 (P > 0.05). This phenomenon may owe to the reason that both drugs had to be adjusted with lower dosage or reduced dosing frequency for better controlling the UA level at appropriate concentration.
Median time to achieve target serum UA level was marginally shorter in febuxostat group than in allopurinol group in renal transplant recipients (3 vs 5 days, P = 0.02). A recent retrospective study26 also indicated that febuxostat led to faster achievement of target serum UA levels than allopurinol in patients diagnosed with gout (346 vs 397 days and 431 vs 478 days for reaching serum UA levels <6 mg/dL and <5 mg/dL, respectively, P < 0.001 for both comparisons). As compared to gout, shorter time to reach target serum UA level was observed in post‐renal transplant recipients with new onset of hyperuricemia, probably because UA excretion disorder was caused by renal tubule injury of renal allograft,2 and serum UA levels may be easily controlled for functional recovery of renal allograft.
Although hyperuricemia has been known to negatively be correlated with eGFR and associated with the worsening of renal allograft function,10 whether UA‐lowering therapy using febuxostat or allopurinol plays a role in promoting renal allograft function recovery is still unclear. In our study, gradual recoveries of renal allograft function in both febuxostat and allopurinol group were demonstrated by increasing eGFR, and we found no significant variances between the two drugs in impacting eGFR increase. Febuxostat and allopurinol show a renoprotective effect and prevent the progression of renal disease in CKD patients.15, 27 Febuxostat and allopurinol may have a promotion effect on renal allograft function recovery in early post‐renal transplant recipients, but this needs further research.
No severe adverse effect was found in our study, which was confirmed by previous studies related to the safety of febuxostat or allopurinol in renal transplantation recipients. However, the safety of these two drugs in renal transplantation recipients still need to be verified by enlarging the number of patients and extending the follow‐up period.
5. WHAT IS NEW AND CONCLUSION
As we know, few studies have been reported on the effects of febuxostat and allopurinol in renal transplant recipients with hyperuricemia. A retrospective case‐control study performed in renal transplant recipients suggested that allopurinol reduced serum UA levels and is associated with preservation of eGFR.20 Besides, UA‐lowering effects of febuxostat in renal transplant patients were also investigated.21, 24 As compared to the reported studies, we compared the efficacy and safety of febuxostat and allopurinol.
In conclusion, febuxostat caused a slightly quicker serum UA‐lowering effect than allopurinol at the beginning of the treatment in early post‐renal transplant recipients. There was no significant difference between the two drugs in impacting the renal allograft function recovery. Both drugs were well tolerated, but long‐term safety needs to be further assessed.
CONFLICT OF INTEREST
The authors declare no conflict of interest that could appear to have influenced the submitted work.
AUTHOR CONTRIBUTIONS
Pan Chen and Changxi Wang involved in the conception and design of the study. Changxi Wang, Longshan Liu and Qian Fu acquired the data. Xiaoju Shen, Jingjie Li, Xiang Gao and Xiao Chen analysed and interpreted the data. Xiaoju Shen and Jingjie Li drafted the article. Pan Chen, Jingjie Li and Xiang Gao critically revised the article for important intellectual content.
ACKNOWLEDGEMENTS
This study was financially supported by National Natural Science Foundation of China [Grant: 81503156, 81601347], Natural Science Foundation of Guangdong Province (No. 2014A030310096) and Public Welfare Research and Capacity Building Fund of Guangdong (No. 2016A020218006).
Shen X, Li J, Fu Q, et al. Comparison of efficacy and safety between febuxostat and allopurinol in early post‐renal transplant recipients with new onset of hyperuricemia. J Clin Pharm Ther. 2019;44:318–326. 10.1111/jcpt.12794
Shen and Li equally contributed to the study.
Contributor Information
Pan Chen, Email: chenp73@mail.sysu.edu.cn.
Changxi Wang, Email: 13600450862@163.com.
REFERENCES
- 1. Han M, Lee JP, Park S, et al. Early onset hyperuricemia is a prognostic marker for kidney graft failure: Propensity score matching analysis in a Korean multicenter cohort. PLoS ONE. 2017;12(5):e0176786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mazzali M. Uric acid and transplantation. Semin Nephrol. 2005;25(1):50‐55. [DOI] [PubMed] [Google Scholar]
- 3. Ben Salem C, Slim R, Fathallah N, Hmouda H. Drug‐induced hyperuricaemia and gout. Rheumatology (Oxford, England). 2017;56(5):679‐688. [DOI] [PubMed] [Google Scholar]
- 4. Clive DM. Renal transplant‐associated hyperuricemia and gout. J Am Soc Nephrol. 2000;11(5):974‐979. [DOI] [PubMed] [Google Scholar]
- 5. Srivastava A, Kaze AD, McMullan CJ, Isakova T, Waikar SS. Uric acid and the risks of kidney failure and death in individuals with CKD. Am J Kidney Dis. 2018;71(3):362‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rincon‐Choles H, Jolly SE, Arrigain S, et al. Impact of uric acid levels on kidney disease progression. Am J Nephrol. 2017;46(4):315‐322. [DOI] [PubMed] [Google Scholar]
- 7. Akalin E, Ganeshan SV, Winston J, Muntner P. Hyperuricemia is associated with the development of the composite outcomes of new cardiovascular events and chronic allograft nephropathy. Transplantation. 2008;86(5):652‐658. [DOI] [PubMed] [Google Scholar]
- 8. Haririan A, Metireddy M, Cangro C, et al. Association of serum uric acid with graft survival after kidney transplantation: a time‐varying analysis. Am J Transplant. 2011;11(9):1943‐1950. [DOI] [PubMed] [Google Scholar]
- 9. Haririan A, Nogueira JM, Zandi‐Nejad K, et al. The independent association between serum uric acid and graft outcomes after kidney transplantation. Transplantation. 2010;89(5):573‐579. [DOI] [PubMed] [Google Scholar]
- 10. Bandukwala F, Huang M, Zaltzman JS, Nash MM, Prasad GV. Association of uric acid with inflammation, progressive renal allograft dysfunction and post‐transplant cardiovascular risk. Am J Cardiol. 2009;103(6):867‐871. [DOI] [PubMed] [Google Scholar]
- 11. Johnson RJ, Nakagawa T, Jalal D, Sanchez‐Lozada LG, Kang DH, Ritz E. Uric acid and chronic kidney disease: which is chasing which? Nephrol Dial Transplant. 2013;28(9):2221‐2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yoshida M, Cho N, Akita H, Kobayashi K. Association of a reactive intermediate derived from 1',6‐dihydroxy metabolite with benzbromarone‐induced hepatotoxicity. J Biochem Mol Toxicol. 2017;31(10). 10.1002/jbt.21946 [DOI] [PubMed] [Google Scholar]
- 13. Richette P, Doherty M, Pascual E, et al. 2016 updated EULAR evidence‐based recommendations for the management of gout. Ann Rheum Dis. 2017;76(1):29‐42. [DOI] [PubMed] [Google Scholar]
- 14. Faruque LI, Ehteshami‐Afshar A, Wiebe N, Tjosvold L, Homik J, Tonelli M. A systematic review and meta‐analysis on the safety and efficacy of febuxostat versus allopurinol in chronic gout. Semin Arthritis Rheum. 2013;43(3):367‐375. [DOI] [PubMed] [Google Scholar]
- 15. Goicoechea M, Garcia de Vinuesa S, Verdalles U, et al. Allopurinol and progression of CKD and cardiovascular events: long‐term follow‐up of a randomized clinical trial. Am J Kidney Dis. 2015;65(4):543‐549. [DOI] [PubMed] [Google Scholar]
- 16. Tsuruta Y, Mochizuki T, Moriyama T, et al. Switching from allopurinol to febuxostat for the treatment of hyperuricemia and renal function in patients with chronic kidney disease. Clin Rheumatol. 2014;33(11):1643‐1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tanaka K, Nakayama M, Kanno M, et al. Renoprotective effects of febuxostat in hyperuricemic patients with chronic kidney disease: a parallel‐group, randomized, controlled trial. Clin Exp Nephrol. 2015;19(6):1044‐1053. [DOI] [PubMed] [Google Scholar]
- 18. Kim HA, Seo YI, Song YW. Four‐week effects of allopurinol and febuxostat treatments on blood pressure and serum creatinine level in gouty men. J Korean Med Sci. 2014;29(8):1077‐1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sezai A, Soma M, Nakata K, et al. Comparison of febuxostat and allopurinol for hyperuricemia in cardiac surgery patients with chronic kidney disease (NU‐FLASH trial for CKD). J Cardiol. 2015;66(4):298‐303. [DOI] [PubMed] [Google Scholar]
- 20. Osadchuk L, Bashir MH, Tangirala B, et al. Effect of allopurinol on slowing allograft functional decline in kidney transplant recipients. Exp Clin Transplant. 2014;12(3):190‐194. [PubMed] [Google Scholar]
- 21. Tojimbara T, Nakajima I, Yashima J, Fuchinoue S, Teraoka S. Efficacy and safety of febuxostat, a novel nonpurine selective inhibitor of xanthine oxidase for the treatment of hyperuricemia in kidney transplant recipients. Transpl Proc. 2014;46(2):511‐513. [DOI] [PubMed] [Google Scholar]
- 22. Baek CH, Kim H, Yang WS, Han DJ, Park SK. Efficacy and safety of febuxostat in kidney transplant patients. Exp Clin Transplant. 2018;16(4):401‐406. [DOI] [PubMed] [Google Scholar]
- 23. Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function–measured and estimated glomerular filtration rate. N Engl J Med. 2006;354(23):2473‐2483. [DOI] [PubMed] [Google Scholar]
- 24. Sofue T, Inui M, Hara T, et al. Efficacy and safety of febuxostat in the treatment of hyperuricemia in stable kidney transplant recipients. Drug Des Devel Ther. 2014;8:245‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Singh JA, Akhras KS, Shiozawa A. Comparative effectiveness of urate lowering with febuxostat versus allopurinol in gout: analyses from large U.S. managed care cohort. Arthritis Res Ther. 2015;17:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sircar D, Chatterjee S, Waikhom R, et al. Efficacy of febuxostat for slowing the GFR decline in patients with CKD and asymptomatic hyperuricemia: a 6‐month, double‐blind, randomized, placebo‐controlled trial. Am J Kidney Dis. 2015;66(6):945‐950. [DOI] [PubMed] [Google Scholar]
- 27. Yeo Y, Han DJ, Moon DH, et al. Suitability of the IDMS‐traceable MDRD equation method to estimate GFR in early postoperative renal transplant recipients. Nephron Clin Pract. 2010;114(2):c108‐117. [DOI] [PubMed] [Google Scholar]


