Abstract
Macrophages play an important role in a wide variety of physiologic and pathologic processes. Plasticity and functional polarization are hallmarks of macrophages. Macrophages commonly exist in two distinct subsets: classically activated macrophages (M1) and alternatively activated macrophages (M2). M2b, a subtype of M2 macrophages, has attracted increasing attention over the past decade due to its strong immune‐regulated and anti‐inflammatory effects. A wide variety of stimuli and multiple factors modulate M2b macrophage polarization in vitro and in vivo. M2b macrophages possess both protective and pathogenic roles in various diseases. Understanding the mechanisms of M2b macrophage activation and the modulation of their polarization might provide a great perspective for the design of novel therapeutic strategies. The purpose of this review is to discuss current knowledge of M2b macrophage polarization, the roles of M2b macrophages in a variety of diseases and the stimuli to modulate M2b macrophage polarization.
Keywords: Autoimmunity, Chemokines, Disease pathogenesis, Inflammation, Manipulation of immune response, Monocyte/Macrophage
Review outlines the current knowledge of the stimuli of M2b macrophage polarization and the roles of these cells in diseases.

Abbreviations
- A2R
A2 adenosine receptor
- AD
Alzheimer's disease
- ALD‐DNA
activated lymphocyte‐derived DNA
- Aβ
amyloid‐β
- BCL6
B‐cell lymphoma‐6
- BMDMs
bone marrow‐derived macrophages
- Cath
cathelicidin
- CCL1‐ODN
CCL1 antisense oligodeoxynucleotides
- CD
cluster of differentiation
- Chil3
chitinase‐like 3
- cTnI
cardiac troponin I
- DCs
dendritic cells
- DS
Down syndrome
- DSS
dextran sodium sulfate
- GAS5
growth arrest‐specific transcript 5
- H
human
- HCC
hepatocellular carcinoma
- HK‐SA
heat‐killed S. aureus
- HMGB1
high‐mobility group box 1
- I/R
ischemia/reperfusion
- IBD
inflammatory bowel disease
- IC
immune complex
- IGF
insulin‐like growth factor
- IRFs
interferon‐regulatory factors
- LIGHT
homologous to lymphotoxin, inducible expression, competes with herpes simplex virus (HSV) glycoprotein D for binding to HSV entry mediator, a receptor expressed on T lymphocytes
- LN
lupus nephritis
- M
mouse
- M1
classically activated macrophages
- M2
alternatively activated macrophages
- MBL
mannose‐binding lectin
- MerTK
Mer receptor tyrosine kinase
- MLNs
mesenteric lymph nodes
- MRSA
methicillin‐resistant S. aureus
- MSK1/2
mitogen‐ and stress‐activated protein kinase‐1/2
- NMD
nonsense‐mediated RNA decay
- ORM1
orosomucoid 1
- oxLDL
oxidized low‐density lipoprotein
- PRC2
polycomb repressive complex 2
- Q‐PCR
quantitative real time polymerase chain reaction
- SAA
serum amyloid a
- SCI
spinal cord injury
- SLE
systemic lupus erythematosus
- SPHK1
sphingosine kinase 1
- TAMs
tumor‐associated macrophages
- TBSA
total body surface area
- TNFSF14
TNF superfamily member 14
- Tregs
T‐regulatory cells
- UTR
untranslated region
- VEGF
vascular endothelial growth factor
1. INTRODUCTION
Macrophages are widely distributed innate immune cells that play an indispensable role in a variety of physiologic and pathologic processes, including organ development, host defense, acute and chronic inflammation, and tissue homeostasis and remodeling.1, 2 The sources of macrophages include tissue resident macrophages that originate from progenitor cells generated in the yolk sac and monocyte‐derived macrophages that originate from bone marrow hematopoietic stem cells.3 They can be phenotypically polarized by surrounding microenvironmental stimuli and signals to mount specific functional programs.4 Several forms of macrophages have been described in mice and humans based on the production of specific factors, expression of cell surface markers, and biologic activities.2, 4, 5 Polarized macrophages can be broadly classified in two main groups: classically activated macrophages (M1), which steer proinflammatory responses, and alternatively activated macrophages (M2), which drive immune regulation and tissue remodeling. M2 macrophages can be further subdivided in M2a, M2b, M2c and M2d based upon the applied stimuli and the resultant transcriptional changes.1, 2, 4, 6, 7
M1 macrophages, which are typically induced by TLR ligands (bacterial LPS) or by some cytokines, such as IFN‐γ, TNF‐α, and GM‐CSF, are characterized by a high capacity to present antigen, high proinflammatory cytokine production (IL‐1β, IL‐6, IL‐12, and IL‐23), high chemokine (C‐X‐C motif) ligand 9 (CXCL9) production, and low levels of IL‐10.1, 2, 4, 7, 8 Functionally, M1 macrophages can facilitate immunity to remove foreign pathogens and tumor cells, mediate tissue damage induced by ROS, and impair wound healing and tissue regeneration.8, 9, 10
M2 macrophages can be polarized by several stimulatory factors, which include cytokines (IL‐4, IL‐10, and IL‐13), glucocorticoids, immune complexes (IC) and LPS. Different subtypes of M2 macrophages can be induced by different stimulatory factors. M2a macrophages, also named wound‐healing macrophages, are induced by IL‐4 and IL‐13 and express high levels of mannose receptor (MR, also called CD206), decoy IL‐1 receptor (IL‐R) and CCL17, and they secrete pro‐fibrotic factors such as TGF‐β, insulin‐like growth factor (IGF), and fibronectin to contribute to tissue repair.4, 11, 12, 13 M2c subtype of macrophages (acquired deactivation macrophages) are induced by IL‐10 via activating signal transducer and activator of transcription 3 (STAT3) through IL‐10R and strongly exhibit anti‐inflammatory activities by releasing large amounts of IL‐10 and pro‐fibrotic activity by secreting high levels of TGF‐β.4, 13, 14, 15 In addition, M2c macrophages exhibit high expression of Mer receptor tyrosine kinase (MerTK), thus resulting in their efficient phagocytosis of apoptotic cells.8, 16 M2d macrophages, representing a novel M2 subset that are also known as tumor‐associated macrophages (TAMs), are induced by costimulation with TLR ligands and A2 adenosine receptor (A2R) agonists or by IL‐6.8, 17, 18 These cells are mainly characterized by high IL‐10, TGF‐β, and vascular endothelial growth factor (VEGF), and low IL‐12, TNF‐α, and IL‐1β production.1, 8, 17, 18, 19, 20 They constitute the major inflammatory component in neoplastic tissue, contributing to angiogenesis and cancer metastasis.17, 18, 19, 20, 21, 22
As an M2 subtype, M2b macrophages, also known as regulatory macrophages, can be induced upon combined exposure to IC and TLR agonists or by IL‐1R agonists and express high levels of CCL1 and TNF superfamily member 14 (TNFSF14).2, 4, 7, 14, 23, 24 In addition to proinflammatory cytokines (IL‐1β, IL‐6, and TNF‐α), M2b cells also express and secrete substantial amounts of the anti‐inflammatory cytokine IL‐10 and low levels of IL‐12, which is the functional converse of M1 cells.2, 4, 24 Based on the expression profile of cytokines, chemokines, and other secreted factors, M2b macrophages regulate the breadth and depth of the immune response and the inflammatory reaction.2 In cancer and infectious diseases, M2b macrophages promote tumor development and parasite, bacterial, and fungal infections by blunting the immune and inflammatory response.25, 26, 27, 28, 29, 30, 31 Moreover, M2b macrophages can reduce spinal cord injury (SCI) and myocardial ischemia/reperfusion (IR) injury, and contribute to recovery from these injuries.6, 24, 32 Despite various important roles in many diseases, the definite and specific molecular markers of M2b macrophage have not yet been unified and established to date.
In the current review, we will present and discuss the phenotypes and functions of M2b macrophages under normal conditions and during disease pathogenesis and the possible mechanisms underlying M2b activation. Then, we will introduce the stimulatory factors to alleviate diseases by targeting M2b macrophage polarization.
2. PHENOTYPIC MARKERS OF M2B
In 2002, Mosser and Anderson reported a novel phenotype of activated macrophage which was induced by LPS plus anti‐ovalbumin (OVA) IgG/OVA ICs or anti‐sheep erythrocyte IgG/erythrocytes ICs that was clearly distinct from either M1 or M2 cells and termed these cells type II activated macrophage.23 These cells were defined as M2b macrophages by Locati and his colleagues.1, 4 Since then, many markers of M2b have been reported, including IL‐10, CCL1, LIGHT, CD86, SPHK1, TNF‐α, and IL‐6.
2.1. IL‐10
IL‐10 is a potent anti‐inflammatory cytokine that is expressed and secreted by many types of cells, including monocytes/macrophages, dendritic cells (DCs), B cells, and T‐regulatory cells (Tregs).33 As the primary marker of M2b, IL‐10 was reported by Mosser and his colleagues to distinguish M2b from M1 and M2a.23, 34 M2b cells express not only high levels of IL‐10, but also low levels of IL‐12.23, 24 High IL‐10 or/and low IL‐12/IL‐10 ratio is/are widely accepted as the characteristic marker(s) of M2b macrophages.1, 2, 4, 5, 6, 7, 8, 9, 10, 11, 12 IL‐10 can be tested by quantitative real time polymerase chain reaction (Q‐PCR) for mRNA or by Western blotting, ELISA, or flow cytometry assays for protein. However, IL‐10 is not a useful marker to distinguish M2b from M2c and M2d because M2c and M2d macrophages also produce high levels of IL‐10.8, 16 Some specific molecules are needed to more accurately distinguish M2b macrophages from other subpopulations of M2 macrophages.
2.2. CCL1
CCL1 (formerly known as I‐309 and TCA3 in humans and mice, respectively) was the first among a long succession of C‐C motif chemokines to be discovered.35, 36 CCL1 attracts monocytes, natural killer (NK) cells, immature B cells and DCs by interacting with the cell surface CCR8.37 In addition to monocytes/macrophages, human immune cells that produce CCL1 include T cells, mast cells and DCs.38 Moreover, studies have shown that M2b, but not other subtypes of macrophages (M1, M2a, and M2c), express high levels of CCL1.30, 39, 40 Interestingly, CCL1 released from M2b cells is essentially required for maintaining the properties of M2b monocytes/macrophages.40 Growing evidences have shown that CCL1 is the specific molecular identifier of M2b and may serve as a marker for identifying M2b from the other subtypes of macrophages.1, 2, 4, 8, 20, 25, 26, 27, 28, 29, 41, 42, 43
2.3. TNFSF14
TNFSF14, also known as LIGHT (homologous to lymphotoxin, inducible expression, competes with herpes simplex virus [HSV] glycoprotein D for binding to HSV entry mediator, a receptor expressed on T lymphocytes) or CD258, is mainly expressed on T lymphocytes and immature DCs and may function as a costimulatory factor for the activation of lymphoid cells and as a deterrent to infection by HSV.44, 45 In addition, LIGHT has been shown to induce strong anti‐tumor immunity to inhibit the growth of primary tumors and eradicate metastases.46 In 2006, Mosser and his colleagues found that LIGHT was specifically expressed in M2b macrophages.34 Over the last decade, in vitro and in vivo substantial studies have shown that LIGHT is a specific marker for mice M2b monocytes/macrophages.24, 40, 43, 47, 48, 49, 50 Because LIGHT is expressed by other of cell types,45, 51 its combination with other markers is a better strategy to identify mice M2b macrophages.
2.4. CD86
CD86, also known as B7‐2, a type I membrane protein that is a member of the immunoglobulin superfamily, is expressed on antigen‐presenting cells (including macrophages, B cells, and DCs) to provide the costimulatory signals necessary for T cell activation and survival by binding to CD28 or/and cytolytic T cell–associated sequence‐4 (CTLA‐4, also called CD152).52, 53 CD86 works in tandem with CD80 (known as B7‐1) to prime T cells.54 Among the subpopulations of macrophages, CD86 is expressed in M2b macrophages and considered as the marker for this subtype.32 However, abundant studies have shown that CD86 is expressed in and used as a marker to identify M1 macrophages.55, 56, 57, 58, 59, 60, 61 These studies indicate that CD86 should be expressed in both M1 and M2b macrophages. Although many review articles have shown that CD86 is the marker for M1 and M2b,5, 6, 8, 41, 62, 63 CD86 is a suitable marker for discriminating M2b from the other subtypes of M2 macrophages, but not from M1 macrophages.
2.5. SPHK1
Sphingosine kinases (two isoforms, SPHK1 and SPHK2) catalyze the formation of sphingosine 1‐phosphate (S1P), a lipid mediator that functions in mammalian cells as an intracellular second messenger, as well as a ligand for S1P‐specific G‐protein‐coupled receptors.64 SPHK1 can be expressed in many types of cells, including monocytes, macrophages, hepatic stellate cells, and smooth muscle cells.34, 65, 66, 67, 68 Previous studies have shown that SPHK1 is mainly expressed in M2b and suggested that it can be used as a marker for M2b monocytes/macrophages.1, 34, 47, 48 However, Mantovani and his colleagues reported that SPHK1 is expressed at higher levels in M1 than M2 cells.69 In addition, some researchers have found that SPHK1 is expressed in M2c.6, 70, 71 The expression of SPHK1 is not restricted to M2b macrophages66, 67, 69; thus, it should not be used as a sole marker to identify M2b macrophages.
2.6. TNF‐α
TNF‐α, a pleiotropic mediator, is central to host defense and inflammatory responses by binding its receptors (TNFR1 and TNFR2).72 Initially, TNF‐α was considered as a proinflammatory cytokine. However, later preclinical and clinical studies have shown that it also mediates a paradoxical anti‐inflammatory and immune‐modulatory effect.72, 73 Its pleiotropic effects often lead to opposing outcomes during the development of immune‐mediated diseases.74 Although it can be produced by many other cell types such as neutrophils, lymphocytes, NK cells, and mast cells, TNF‐α is chiefly produced by activated macrophages.72, 73 In activated macrophages, M1 and M2b are the main cell types expressing and secreting TNF‐α.75 Accumulating evidences have shown that TNF‐α, in combination with other markers, such as IL‐10, LIGHT, CCL1, or/and IL‐6, can discriminate M2b from the other subtypes of macrophages very well.30, 71, 75, 76, 77, 78, 79, 80 In addition, TNF‐α is considered a marker of M2b macrophages in many review articles.6, 8, 9, 11, 41, 62, 63
2.7. IL‐6
IL‐6 is a prototypic cytokine with redundant and pleiotropic activities on immune and nonimmune cells and often displays hormone‐like characteristics that affect homeostatic processes.81 IL‐6 binds to its receptor IL‐6R, and this complex then binds to a second membrane protein, glycoprotein 130 (gp130), which dimerizes and initiates intracellular signaling.82, 83 Promptly and transiently produced in response to infections and tissue injuries, IL‐6 contributes to host defense through the stimulation of acute phase responses, immune reactions, and hematopoiesis.84, 85 Almost all stromal cells and immune cells are involved in the production of IL‐6.86, 87 It is secreted by monocytes and macrophages after stimulation of TLR ligands.83, 86 In typical methods of inducing macrophage polarization, LPS is a component of the stimuli to induce M1 and M2b. IL‐6 is highly expressed in M1 and M2b macrophages but not in M2a and M2c.71, 78 Growing data show that in combination with other markers (IL‐10, LIGHT, CCL1, or/and TNF‐α), IL‐6 can identify M2b from the other macrophage subsets.30, 71, 77, 78, 79, 80, 88, 89, 90
In addition to the above molecules, other molecules used to identify M2b macrophages include MHCII/HLA‐DR, CD163, CD64, PD‐L1 IL‐1β, and CCL2.26, 27, 30, 75, 91, 92, 93 However, these molecules are not specific markers of M2b, nor even specific markers of macrophages. Excluding CCL1 and LIGHT, none of the above markers are uniquely expressed in M2b and have been considered a sole marker to identify M2b among macrophage subtypes. Due to the expression of CCL1 and LIGHT in other cell types, it is necessary to combine with the above‐mentioned molecules to more accurately distinguish M2b cells from other subtypes of macrophages and from different cell types. The different phenotypes, cell surface markers, and functions of monocytes/macrophages are summarized in Table 1.
Table 1.
Comparative markers and biologic functions of monocyte/macrophage subtypes
| Phenotypes | Stimuli | Markers | Functions | References |
|---|---|---|---|---|
| Human monocytes/macrophages | ||||
| M1 | IFN‐γ, LPS, GM‐CSF, TNF‐α | CXCL9, IL‐12 high/IL‐10 low, iNOS, IL‐6, CD80, CD86, TNF‐α | Pro‐inflammation, microbicidal effect, tumor resistance | 1, 2, 6, 7, 8, 11, 27, 63, 105 |
| M2a | IL‐4, IL‐13 | CCL17, IL‐1R, CD206, Dectin‐1, IL‐10, DC‐SIGN | Anti‐inflammatory, wound healing | 1, 2, 6, 7, 8, 63, 105, 141 |
| M2b | LPS+IC, IL‐1β+IC | CCL1, IL‐10 high/IL‐12 low, TNF‐α, CD86, IL‐6 | Immunoregulation, promoting infection, tumor progression | 1, 2, 6, 7, 8, 11, 27, 39, 41, 63, 75 , 90, 136, 163, 164 |
| M2c | IL‐10, Glucocorticoids | CXCL13, CD206, CD163, IL‐10, TGF‐β, MerTK | Immunosuppression, phagocytosis, tissue remodeling | 1, 6, 7, 8, 63 |
| M2d | LPS+A2R ligands, IL‐6 | VEGF, IL‐10, TGF‐β | Tumor progression, angiogenesis | 1, 8, 17, 19 |
| Mouse monocytes/macrophages | ||||
| M1 | IFN‐γ, LPS, GM‐CSF, TNF‐α | CXCL9, IL‐12 high/IL‐10 low, iNOS, IL‐6, CD80, CD86, TNF‐α | Pro‐inflammation, microbicidal effect, tumor resistance, | 1, 2, 6, 8, 11, 26, 63 |
| M2a | IL‐4, IL‐13 | CCL17, IL‐1R, Dectin‐1, IL‐10, Arg‐1, Chil3, FIZZ1 | Anti‐inflammatory, wound healing, | 1, 2, 6, 8, 31, 63 |
| M2b | LPS+IC, IL‐1β+IC | CCL1, IL‐10 high/IL‐12 low, TNF‐α, CD86, IL‐6, LIGHT | Immunoregulation, promoting infection, tumor progression | 1, 2, 6, 8, 11, 41, 43, 63, 71, 164 |
| M2c | IL‐10, Glucocorticoids | CXCL13, CD206, CD163, IL‐10, TGF‐β, MerTK, Arg‐1 | Immunosuppression, phagocytosis, tissue remodeling | 1, 6, 8, 63 |
| M2d | LPS+A2R ligands, IL‐6 | VEGF, IL‐10, TGF‐β, iNOS | Tumor progression, angiogenesis | 1, 8, 12, 18, 19, 63 |
A2R, A2 adenosine receptor; Arg‐1, arginase‐1; CCL, chemokine (C‐C motif) ligand; CD, cluster of differentiation; Chil3, chitinase‐like 3; CXCL, chemokine (C‐X‐C motif) ligand; DC‐SIGN, dendritic cell‐specific intercellular adhesion molecule‐3‐grabbing non‐integrin; FIZZ1, found in inflammatory zone 1; IC, immune complex; LIGHT, homologous to lymphotoxin, inducible expression, competes with herpes simplex virus (HSV) glycoprotein D for binding to HSV entry mediator, a receptor expressed on T lymphocytes; iNOS, inducible nitric oxide synthase; MerTK, Mer receptor tyrosine kinase; VEGF, vascular endothelial growth factor.
3. MODULATION OF M2B MACROPHAGE POLARIZATION
As mentioned earlier, macrophages can display a continuum of phenotypes illustrated by distinct gene expression profiles with the capacity to switch from one phenotype to another depending on the external stimuli. Several factors, such as posttranscriptional regulators, signaling molecules, transcriptional factors, and physical factors, have been found to play pivotal roles in the control of M2b macrophage polarization (Fig. 1).
Figure 1.
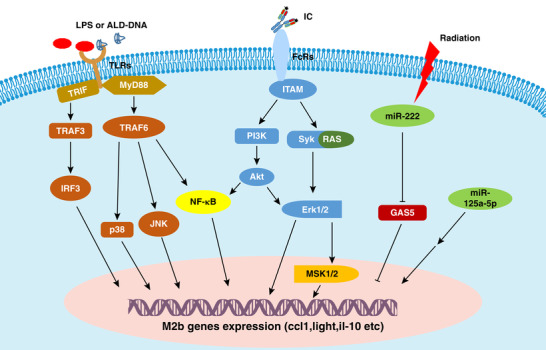
Regulation of macrophage M2b polarization. LPS and IC are known to interact with specific receptors on macrophages, such as TLR4 and FcRs, and subsequently induce M2b polarization. Notably, NF‐κB, MAPK, PI3K/Akt, and IRF3 are activated in M2b macrophages. Moreover, radiation promotes M2b macrophage polarization by regulating the miR‐222/GAS5 signaling pathway
3.1. Modulation by microRNA (miRNA)
miRNAs are short noncoding RNAs of approximately 22 nucleotides (nt), which have been highly conserved during evolution and play crucial roles in immune and inflammatory responses.94, 95 Upon binding to the 3′‐untranslated region (UTR) of target mRNAs, miRNAs negatively regulate gene expression by increasing mRNA degradation or/and by repressing the translation process.96 miRNAs play pivotal roles in the regulation of macrophage development and functions. Recent studies have shown that miR‐21, miR‐125a‐3p, miR‐146a, miR‐147, miR‐214, and miR‐455 are up‐regulated in M1 macrophages, whereas miR‐let7c, miR‐143‐3p, miR‐145‐5p, and miR‐193 are more abundant in M2 macrophages.7, 97, 98, 99 The up‐regulation of miR‐155 in macrophages contributes to the increased production of CCL2 and TNF by directly repressing B‐cell lymphoma‐6 (BCL6) expression and the reduced expression of Arg‐1 and Chil3.100, 101 By contrast, increased miR‐223 promotes M2 polarization.102
miR‐222 plays a crucial role in M2b polarization. miR‐222 is up‐regulated in M2b macrophages induced by radiation.49 Moreover, up‐regulation of miR‐222 can promote M2b polarization by increasing the expression of CCL1.49 The underlying mechanism is that miR‐222 promotes the degradation of long noncoding RNA (lncRNA) growth arrest‐specific transcript 5 (GAS5), which is a CCL1 gene silencer.49, 103 miR‐125a‐5p expression appears to be induced by LPS and inhibited by IFN‐γ and IL‐4.104, 105 Interestingly, inhibition of miR‐125a‐5p contributes to the weak expression of M2b macrophage markers, whereas miR‐125a‐5p overexpression enhances M2b polarization.104 In addition, miR‐27a is increased but miR‐26a‐2 is decreased in M2b macrophages.7 Further studies are needed to investigate the mechanisms of these miRNAs in M2b polarization.
3.2. Modulation by lncRNA
The lncRNAs are a large class of nonprotein‐coding transcripts that are more than 200 nt in length and are involved in various physiologic and pathologic processes.106 lncRNA mediated transcriptional or posttranscriptional regulation is an important mechanism for epigenetic programming.107 Studies have shown that lncRNAs play essential roles in macrophage activation and polarization. Lnc‐MC regulates the differentiation of macrophages through the absorption of miR‐199a‐5p,108 and overexpression of lncRNA E33 induces inflammatory gene expression, activates inflammatory signaling pathways, and increases foam cell formation in macrophages.109 TCONS_00019715 expression is decreased when M1 is converted to M2 but increased when M2 is converted to M1.110 This study indicated that TCONS_00019715 promotes macrophage polarization to the M1 phenotype. LncRNA COX‐2 is more highly expressed in M1 macrophages and increases the expression levels of IL‐12, iNOS, and TNF‐α.111 A recent study has shown that lncRNA GAS5 suppresses IFN regulatory factor 4 (IRF4) transcription by binding polycomb repressive complex 2 (PRC2) to inhibit M2 polarization.112
Importantly, GAS5 is a negative regulator of M2b macrophage polarization. GAS5 is known as a silencer of CCL1, which is an essential chemokine for M2b polarization.113 The RNA level of GAS5 is reduced in M2b macrophages.43 The reduction of GAS5 RNA in M2b is mediated by activation of the nonsense‐mediated RNA decay (NMD) pathway.43 In contrast, overexpression GAS5 reduces the mRNA levels of CCL1 and LIGHT and inhibits M2b polarization in BMDMs under the stimulation with LPS plus IC.43 Moreover, radiation also reduces the expression of GAS5 by up‐regulating the level of miR‐222 and then polarizes monocytes to M2b phenotype.49
3.3. Modulation by signaling factors
After binding of receptors to their ligands, the ligand signals can be transferred into the cells via signaling pathways and regulate the physiologic functions of cells, including gene expression, cytokine secretion, cell proliferation, and differentiation. NF‐κB, MAPKs, PI3K/Akt, and IRFs are involved in M2b macrophage polarization.
3.3.1. NF‐κB
NF‐κB family members, including p50, p52, p65, RelB, and c‐Rel, can form homodimers or/and heterodimers, which regulate the expression of specific target genes. NF‐κBs are the core regulators in the initiation and resolution of inflammation.114 The activation of NF‐κB induced by TLR ligands produces proinflammatory factors, and the activation of NF‐κB p65 is a hallmark of M1 macrophage activation.115, 116 In contrast, the activation of NF‐κB p50 appears to be essential for M2 macrophage polarization in vitro and in vivo.117 Indeed, the activation of NF‐κB plays a pivotal role in M2b macrophage polarization. In lupus nephritis (LN), activated lymphocyte‐derived DNA (ALD‐DNA) induces the macrophage activation and M2b polarization by activating the NF‐κB and the IRF3 signaling pathways via calcium signaling.80 Moreover, the activation of NF‐κB as well as MAPK signaling in macrophages is inhibited by mannose‐binding lectin (MBL), a recognition receptor with binding activity to DNA, resulting in a decrease in M2b macrophage polarization.88 Furthermore, ALD‐DNA‐induced M2b polarization is dependent on the acceleration of NF‐κB p50 translocation into the nucleus mediated by the PI3K and MAPK pathways.118 These observations suggest that NF‐κB p50 may be the crucial modulator for M2b macrophage polarization.
3.3.2. MAPKs
MAPKs are a type of protein kinase specific to serine and threonine. MAPKs are involved in directing cellular responses to various stimuli and regulate many processes, such as proliferation, differentiation, cell survival, and apoptosis.119 The activation of MAPKs (ERK1/2, p38, and JNK) is enhanced in M2b macrophages induced by granulin or ALD‐DNA.79, 118 After dectin‐1 binding to zymosan (a component derived from fungi), kinases ERK1/2 and p38 are activated and induce M2b macrophage polarization by provoking mitogen‐ and stress‐activated protein kinase‐1/2 (MSK1/2).47 Moreover, MBL blunts macrophage M2b polarization by inhibiting MAPK and NF‐κB signaling.88 These observations suggest that the MAPK signal is a crucial positive modulator of M2b macrophage polarization.
3.3.3. PI3K/Akt
The PI3K/Akt pathway not only regulates macrophage survival, migration, and proliferation, but also orchestrates the response to different metabolic and inflammatory signals in macrophages.120, 121 PI3K/Akt signaling is activated by TLR4 and other pathogen recognition receptors, as well as Fc receptors, modulating downstream signals that control cytokine production.122, 123 Activation of the PI3K/Akt pathway plays a critical role in restricting proinflammatory and promoting anti‐inflammatory responses in LPS‐stimulated macrophages.124 A recent study has shown that the activation of Akt is enhanced in influenza virus‐induced M2b cells from alveolar macrophages, and inhibition of PI3K/Akt signaling with LY294002 can cause a dramatic down‐regulation of M2b polarization markers.125 In addition, PI3K/Akt signaling is activated in ALD‐DNA‐induced M2b macrophages.118 These data indicate that the PI3K/Akt pathway is involved in M2b macrophage polarization.
3.3.4. IRFs
IRFs, a family of transcription factors expressed in macrophages, play a pivotal role in the polarization of macrophages, depending on the IRF family member.11 IRF5 acts as a mediator of M1 macrophage polarization in human and murine macrophages.126, 127 By contrast, IRF4 is a modulator of M2 macrophage polarization in response to parasite infection.127, 128 Additionally, IRF3 and NF‐κB are activated during M2b macrophage polarization by ALD‐DNA.80 This observation suggests that IRF3 may play a core role in M2b activation.
NF‐κB, MAPKs, PI3K/Akt, and IRFs are important signaling factors involved in cell survival, proliferation, biosynthesis, cell metabolism, and the inflammatory response. These signaling pathways interact with each other and regulate the macrophage switch toward M2b rather than M1 polarization.
3.4. Modulation by radiation
Acute radiation exposure can cause lethal injuries to the hematopoietic and gastrointestinal systems.129 After 5–9 Gy whole body radiation, the macrophages of mesenteric lymph nodes (MLNs) polarize toward the M2b phenotype.49, 50 This subtype of macrophages is not converted to M1 macrophages in response to stimulation by E. faecalis antigens and inhibits the conversion of macrophages from resident M0 to M1.50 A further study has shown that miR‐222, induced by whole body radiation, reduces the expression of GAS5, resulting in an increase in CCL1 levels and macrophage conversion to M2b.49 Moreover, after treatment with CCL1 antisense oligodeoxynucleotides (CCL1‐ODN, which can decrease the mRNA level of CCL1), M2b macrophages disappear in the MLNs of radiated mice, and M1 is generated in the MLNs of these mice following E. faecalis stimulation.50 However, it is unclear how radiation induces the expression of miR‐222.
In addition, some reports have shown that thoracic radiotherapy increases cardiovascular events and atherosclerosis development in patients with cancer.11, 130, 131 Interestingly, local 14 Gy radiation in ApoE−/− mice results in a larger number of M1 and smaller number of M2 macrophages in atherosclerotic lesions.132 Additionally, radiation reduces the phagocytic capacity of M2 macrophages, likely contributing to the increase in apoptotic cells and to the shift of macrophage polarization toward a proinflammatory M1 phenotype in vitro.132 These observations indicate that during different diseases, different doses of radiation have various effects on the macrophages in different tissues. Due to the extensive application of radiotherapy in cancer patients, it will be important to investigate the effect of radiation on macrophage phenotype to improve the radiotherapy efficacy in further studies.
4. M2B MACROPHAGES AND DISEASES
The immune system is the host's defense against infection, inflammation, tumors, and other diseases. Macrophages are multifunctional leukocytes that recognize and remove invading pathogens, toxins, cellular debris, and apoptotic cells in healthy or inflamed tissues. Due to their formidable immunoregulatory effects, M2b macrophages play various roles in infection, inflammation, tissue repair, and tumor progression. In this section, we focus on the roles of M2b in different diseases.
4.1. M2b macrophages in infectious diseases
Under normal physiologic conditions, monocytes in the blood retain a quiescent state (M0: CCL1−, CD163−, CD14+).27, 29 During infection, monocytes/macrophages acquire the M1 phenotype (IL‐10−, IL‐12+, iNOS+, CXCL9+), which is a major effector cells for the first line of host antibacterial defense.20, 26, 133, 134, 135 Mild burn injury (5% of the total body surface area [TBSA] burn) induces M1 macrophage polarization.26 However, moderate and severe burn injury (>15% TBSA) results in M2b monocyte polarization (CCL1+, IL‐12−, IL‐10+, LIGHT+, CD14+, CD163+) in humans and mice, promoting the infection.26, 27, 40, 136, 137 The possible reason for this phenomenon is an inhibitory effect on the immune response by the substantial factors released after severe burn injury and induction of M2b polarization. In addition, chronic alcohol consumption also leads to the M2b macrophage polarization (CCL1+, CD14+, CD163+, IL‐10+, LIGHT+, CD11b+), increasing opportunities for bacterial infection,29, 135, 138 but the mechanism responsible for M2b polarization by alcohol consumption is unclear.
Bacteria and viruses directly induce M2b macrophage polarization (IL‐10+, IL‐12−, IL‐6+, TNF‐α+, CD11b+, MHCⅡ+).77, 91, 125 Additionally, parasitic infections can increase the number and proportion of M2b macrophages (CCL1+, IL‐10+, TNF‐α+, IL‐1β+, IL‐6+, TGF‐β+, CCL17+) in the peritoneal cavity,30 and M2b polarization (IL‐12low, IL‐10high, LIGHT+, TNF‐α+) promotes the persistence of infection.139 Despite a strong phagocytic capacity, M2b macrophages are not the main cells responsible for killing bacteria.20, 135, 140 Moreover, M2b monocytes/macrophages not only have no antibacterial effect, but also they increase susceptibility to opportunistic pathogens such as S. aureus, methicillin‐resistant S. aureus (MRSA) (M2b: CCL1+, CD163+, CD64−, CD209−), E. faecalis, C. albicans (M2b: IL‐10+, TNF‐α+, MR+, Dectin‐1+), and K. pneumonia.26, 27, 31, 40, 50, 135, 136, 137, 138, 141 Some studies have shown that M1 macrophages are not easily generated in immunosuppressed hosts (such as severely burned patients) with a predominance of M2b.26, 27, 40 The most likely explanation for this phenomenon is that M2b inhibits macrophage conversion from quiescent M0 to M1, resulting in a decrease in anti‐pathogens (Fig. 2).26, 27, 40, 135, 141
Figure 2.
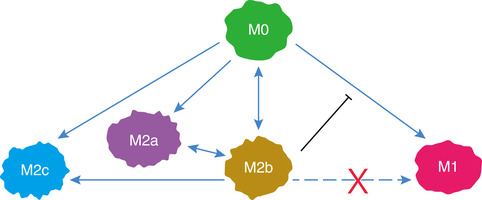
Phenotypic conversion in different types of macrophages. M0 macrophages can polarize to M1 or M2 (M2a, M2b, M2c) in response to different inducers. M2b macrophages can convert to the other subtypes of M2 macrophages in response to different stimuli. Notably, M2b macrophages inhibit the conversion from M0 to M1. Moreover, M2b cannot repolarize to M1 during exposure to M1 inducer
4.2. M2b macrophages in autoimmune diseases
M2b macrophages play an important role in inflammatory diseases, especially in autoimmune or autoinflammatory diseases. Studies have shown that M1 macrophages play an important inflammatory role in the pathogenesis of systemic lupus erythematosus (SLE), and M2b macrophages actually have a direct role in causing SLE.41 In ALD‐DNA‐inducible murine models of LN, infiltrated macrophages in nephritic tissues exhibit M2b functional polarization (IL‐10+, TNF‐α+, IL‐1β+, IL‐6+, MCP‐1+, iNOS+, CD80+, CD86+, MHCⅡ+, CD11b+, PD‐L1+).79, 89, 93, 118 In addition, a recent study has shown that renal M2b macrophages (IL‐10+, LIGHT+, CD80+, CD86+) are markers of disease remission in LN.142 Furthermore, ALD‐DNA and IC can induce macrophage polarization to M2b in vitro (IL‐10+, TNF‐α+, IL‐1β+, IL‐6+, MCP‐1+, iNOS+, CD80+, CD86+, MHCⅡ+).78, 79, 80, 89 The accepted mechanism of LN is that the deposition of IC causes damage in the renal tissue. M2b macrophages induced by ALD‐DNA or/and IC in the renal tissue release many factors that may lead to sustained kidney damage.
In addition, resident macrophages play a crucial role in maintaining intestinal homeostasis and have an important effect on the development of inflammatory bowel disease (IBD).143 Dextran sodium sulfate (DSS), an inducible chemical compound of IBD, can directly induce M2b macrophage polarization (LIGHT+, SPHK1+).48 The probable mechanism for this phenomenon is an inhibitory effect of IL‐10 secretion by M2b macrophages on pathogen clearance, augmenting inflammation, and causing IBD onset.48 Moreover, M2b (CCL1+, IL‐6+) represent a dominant subpopulation of monocytes in patients with active systemic juvenile idiopathic arthritis, an inflammatory disease of childhood.90 In these autoimmune diseases, autoantibodies exist and react with the antigens to form IC, resulting in M2b macrophage polarization. These data suggest that M2b macrophage may be a key mediator of the initiation and progression of autoimmune diseases.
4.3. M2b macrophages in diseases of the nerve system
In mammals, macrophages derived from blood monocytes and activated microglia (brain‐resident macrophages) indefinitely persist at the site of SCI.6, 144 After SCI, M2b macrophages, the key macrophage subtype regulating the proliferative phase of repair, are improperly activated.6 In addition, the age‐dependent decrease in M2b macrophage (CD86+, IL‐10+) is associated with impaired functional recovery and enhanced tissue damage after mild‐moderate SCI in aged mice.32 The probable underlying mechanism is that M2b macrophages release high levels of anti‐inflammatory IL‐10, facilitating neuroprotection through noninflammatory mechanisms including direct neuronal activation of PI3K/Akt.32, 145, 146 These data indicate that M2b macrophages may improve tissue repair after SCI.
Inflammatory responses in the brain, which can be demonstrated by changes in the properties of microglia, are a common feature of human neurodegenerative diseases including Alzheimer's disease (AD).92 M1, M2a, M2b, and M2c microglia are found in the brain of AD patients.147 In the early stage of AD, microglia are apparently polarized to either M1 or M2a macrophages, whereas the markers for M1, M2a, and M2c are elevated at a later stage of AD.147, 148 Interestingly, microglia are polarized to M2b macrophages (CD86+, CD64+) in the brain of AD in Down syndrome (DS) patients with the deposition of high levels of amyloid‐β (Aβ).148 Furthermore, intracranial injection of IVIg, anti‐Aβ antibody or IgG into the brain parenchyma of Aβ‐depositing transgenic mice stimulates M2b polarization (CD86+, CD64+) and promotes the clearance of Aβ deposits.70 M2b macrophages not only inhibit inflammation but also phagocytize and remove Aβ in the AD murine model.70 These data indicate that M2b macrophages may play an important role in improving brain diseases and nerve injury.
4.4. M2b macrophages in glycolipid metabolic disorders
Macrophages are the main component of adipose tissue immune cells (40–60% of all immune cells in fatty tissue) and obesity significantly increases the number of adipose tissue macrophages.149 In the adipose tissue of obese individuals, the percentage of macrophages in the stromal vascular fraction may reach up to 40–50% in the case of the morbidly obese, as compared to approximately 5–10% in lean individuals.150, 151 Under normal physiologic conditions, the resident macrophages of fatty tissue belong to the M2 phenotype, which are important in the homeostasis of adipose tissue.150, 152, 153 However, the number of M1 macrophages increases in the adipose tissue of obese individuals, which contributes to the inflammatory processes and insulin resistance.153 The effects of the glycolipid metabolic disorder on the macrophage phenotypic change may be distinct in different tissues. Macrophages exposed to the diabetic environment are also preferentially polarized toward M1, contributing to multiple pathologies in the target organs.154 M1 cells are the main macrophages in the adipose tissue of obese people.153 Additionally, macrophages in the peritoneal cavity and the cecal tissue from high‐fat fed mice present M2b polarization (IL‐10+, TNF‐α+, MR+, Dectin‐1+) at the site of infection that is associated with an increased susceptibility to gastrointestinal candidiasis.31 Moreover, rosiglitazone (PPAR‐γ ligand), an anti‐diabetic drug, can induce the macrophage phenotypic switch from M2b to M2a in a STAT‐6 dependent manner.31, 155, 156 Additionally, M2a polarization after treatment with rosiglitazone favors gastrointestinal fungal elimination independently of reduced blood glucose.31
4.5. M2b macrophages in cardiovascular diseases
Both pro‐atherosclerotic and anti‐atherosclerotic functions have been demonstrated for macrophages in atherosclerosis.150 M1 macrophages display a proinflammatory profile and are found in rupture‐prone lesions, which suggests that this subtype of macrophage has pro‐atherosclerotic effects and is associated with plaque vulnerability.11, 12 IL‐4‐dependent M2a macrophages promote oxidized low‐density lipoprotein (oxLDL) uptake through CD36 and are abnormally high in patients with symptomatic atherosclerotic carotid plaques.11 Importantly, heat‐killed S. aureus (HK‐SA) reduce the number of inflammatory Ly‐6Chi monocytes in the circulation and attenuate leukocyte recruitment, resulting in significant inhibition of macrophage infiltration in atherosclerotic plaques in vivo.76 Furthermore, HK‐SA induces bone marrow‐derived macrophages (BMDMs) to switch to the M2b phenotype (IL‐10+, TNF‐α+, PD‐L1+) in vitro,76 which suggests that M2b macrophages may have anti‐atherosclerotic effects via inhibition leukocyte infiltration.
In myocardial disease, our recent study indicated that M2b macrophages (IL‐10+, LIGHT+, F4/80+) have cardioprotective effects.24 We induced BMDM polarization to M2b macrophages by exposure to LPS and IC. Then, we transplanted M2b macrophages into the myocardial I/R injury zone and found that the serum cardiac troponin I (cTnI) level, infarct area, and apoptosis index were decreased in the group of transplanted M2b macrophages. The cardioprotective effect of the transplanted M2b macrophages occurs via their reduction of NF‐κB signaling activation and of the up‐regulated expression of A20 in heart caused by I/R injury.
These observations suggest that macrophages play crucial roles in cardiovascular diseases, and interventions in macrophage polarization may provide a novel therapeutic opportunity to combat cardiovascular diseases.
4.6. M2b macrophages in tumors
Within the tumor, macrophages are a major stromal component, in which they are commonly termed TAM, which exhibit functions similar to M2 macrophages and can be characterized as the M2d subtype.20, 21, 22, 157 Among tumor‐infiltrating cells in some cancers, macrophages represent the largest population of cells, contributing to at least one‐third of the total tumor mass.158, 159, 160 Mounting evidences have shown that M2d macrophages can release some factors that directly stimulate tumor cell survival, proliferation, motility, and angiogenesis, and affect the tumor microenvironment via paracrine cytokines, leading to tumor metastasis and invasion.8, 11, 12, 157, 161, 162
In addition to M2d, other subtypes of macrophage are present in tumor tissues and play important roles in tumor survival, metastasis, and invasion. M1 macrophages are proinflammatory, promote Th1 responses, and display tumoricidal activity by producing large amounts of toxic intermediates (NO and ROS).8, 11, 12 Like M1, M2a and M2c macrophages from monocyte polarization possess anti‐tumorigenic effects in hepatocellular carcinoma (HCC), but the underlying mechanisms are unclear.162 A recent study has shown that CD14+ monocytes isolated from advanced HCC patients show the M2b phenotype (CD68+, CCL1+) and do not possess the properties of M2d (CXCL8+).25 Additionally, macrophages in tumors of HCC patients are also identified as the M2b phenotype (IL‐12−, IL‐10+, CCL1+, iNOS−).25 Accumulating data have shown that M2b monocytes/macrophages can promote the growth, invasion, and recurrence of cancers in vitro and in vivo.25, 28, 162, 163 These observations indicate that M2b macrophages may be one important component of TAMs, demonstrating different quantities in different cancers. Further studies are needed to investigate the percentage of M2b in TAMs of different tumors and the molecular mechanism by which M2b macrophages promote tumor development.
5. M2B MACROPHAGE POLARIZATION STIMULATION
Polarization is a hallmark of macrophages in response to stimulation with various agents. Different phenotypes of macrophages can switch to other subtypes in response to different stimulants (Fig. 2). Although the internal environment is complex and various, it can be modified by exogenous stimuli, which induce the activation and polarization of macrophages, thereby improving some diseases. In this section, we will focus on stimuli leading to M2b macrophage polarization, including inducers and inhibitors.
5.1. Inducers of M2b macrophages
LPS plus IC is the classical inducer of M2b macrophage polarization.6, 7, 8, 11, 43, 71, 75, 90 IL‐1β in combination with IC is also used to induce macrophage polarization to M2b in vitro.8, 11, 41, 140, 164 Additionally, DSS, a polyanionic derivative of dextran, which is usually employed as an inducer in the colitis model, can induce increases in LIGTH and IL‐10 (biomarkers of M2b) in mice peritoneal macrophages in vitro.48 In addition, ALD‐DNA, HK‐SA, orosomucoid 1 (ORM1), and serum amyloid‐A (SAA) can switch mouse and human macrophages from other phenotypes to the M2b subtype in vitro.76, 78, 79, 89, 141, 162 There two mechanisms for M2b macrophage polarization induced by ALD‐DNA: (a) ALD‐DNA can bind to TLR9, activating the TLR pathway165, 166; (b) ALD‐DNA induces the production of anti‐DNA antibodies or/ and binds to these antibodies to form ICs that activate the FcR pathway.167, 168 In animal models and in patients, alcohol abuse, acute radiation exposure, and severe burns can stimulate macrophage conversion from a quiescent phenotype to M2b macrophages.26, 49, 50, 135, 136 Some studies have shown that catecholamine levels are increased during the acute phase in the plasma of severely burned patients and can up‐regulate the expression of high‐mobility group box 1 (HMGB1) in macrophages, triggering CCL2 production.136, 169, 170 Additionally, CCL2 polarizes macrophages toward the M2b phenotype.137 These data indicate that some of these inducers may have therapeutic effect for diseases with or caused by excessive inflammation.
5.2. Inhibitors of M2b macrophages
Due to its formidable effects in promoting infection and inhibiting the immune response, strategies to inhibit M2b polarization are of interest to reduce the infection and activate immunity. Recent studies have advanced this possibility. CCL1 is a specific marker of M2b macrophages and is essential for the maintenance of M2b properties.40 In 2012, Kobayashi and his colleagues reported that M2b macrophages disappear after treatment with CCL1‐ODN (binds to CCL1 RNA to modulate its function) in the MLNs of irradiated mice.50 Accumulating evidences have demonstrated that CCL1‐ODN treatment can inhibit M2b polarization by reversing M2b to the quiescent state (M0) in monocytes/macrophages of humans and mice (Fig. 2).25, 26, 27, 29, 40, 49, 135, 141 These data suggest that CCL1‐ODN is a useful inhibitor of M2b in vivo and in vitro. Furthermore, Kobayashi et al have reported that recombinant CCL2 treatment can induce M2b polarization from resident monocytes and that M2b monocytes are not generated from quiescent monocytes after cultivation with burn patient sera pretreated with CCL2 antibody.137 In addition, rosiglitazone, a PPAR‐γ ligand that is approved for glycemic control in people with type 2 diabetes, can switch the M2b subtype of tissue resident macrophages induced by high fat food toward M2a polarization in the peritoneal cavity and cecal tissue, facilitating intestinal Candida elimination (Fig. 2).31 Propranolol, a noncardioselective sympatholytic β‐blocker that is mainly used to treat various cardiovascular diseases, inhibits the production of IL‐10 and CCL1 (markers of M2b) in monocytes caused by severe burn.136 These data indicate that these approved drugs may have therapeutic effects in immunologic/inflammatory diseases caused by M2b macrophages. The unequivocal mechanisms of PPAR‐γ ligand and β‐blocker in inhibiting M2b polarization and the role of these drugs in immunologic/inflammatory diseases remain to be demonstrated.
6. CONCLUDING REMARKS
The concept of macrophage polarization has been increasingly appreciated and gradually accepted. Over the past decade, M2b polarization has attracted increasing attention. As illustrated in this review, many molecules have been used as markers of M2b, but some of them are not specific. In addition, a wide variety of stimuli can induce M2b macrophage activation, and multiple factors coordinate a complex network to drive macrophage M2b polarization. Additionally, M2b macrophages possess both protective and pathogenic roles in various diseases. Nevertheless, the accepted characteristic molecules of M2b macrophages and the roles of M2b in pathophysiology are still unclear. Thus, in future work, it will be important to study the distinguishing characteristics of the different subtypes of macrophages, especially the tissue‐resident macrophages originating from the yolk sac, because this knowledge will facilitate our ability to compare findings between research groups and different macrophages and expand our understanding of the unique contributions of macrophages, especially M2b macrophages, in multiple physiologic and pathologic processes. Furthermore, a uniform nomenclature for macrophages and their subtypes will also become increasingly important given that the research about the subtypes and functions of macrophages will most rapidly increase in the next few years. Another question that need to be answered concerns the precise mechanisms of M2b polarization and depolarization, so that we can choose some small molecules to induce or inhibit M2b polarization for treating some diseases caused by M2b macrophages in the further.
DISCLOSURES
The authors declare no conflicts of interest.
AUTHORSHIP
L.‐X.W., S.‐X.Z., and H.‐J.W. wrote the manuscript and designed the figures. X.‐L.R. edited the manuscript. J.G. wrote and edited the manuscript.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (Nos. 81503313, 81530102 for J.G.), the Ph.D. Start‐up Fund of Natural Science Foundation of Guangdong Province (Nos. 2018A030310403 for L.‐X.W.), and the Medical Scientific Research Foundation of Guangdong Province (No. A2018068 for L.‐X.W.). We thank Prof Zi‐li Lei (Institute of Chinese Medicine Sciences, Guangdong Pharmaceutical University) for editing and reviewing our manuscript.
Wang L‐x, Zhang S‐x, Wu H‐j, Rong X‐l, Guo J. M2b macrophage polarization and its roles in diseases. J Leukoc Biol. 2019;106:345–358. 10.1002/JLB.3RU1018-378RR
REFERENCES
- 1. Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. [DOI] [PubMed] [Google Scholar]
- 2. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Perdiguero EG, Geissmann F. The development and maintenance of resident macrophages. Nat Immunol. 2016;17:2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mantovani AA, Sica S, Sozzani P, et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. [DOI] [PubMed] [Google Scholar]
- 5. Chinetti‐Gbaguidi G, Colin S, Staels B. Macrophage subsets in atherosclerosis. Nat Rev Cardiol. 2015;12:10–17. [DOI] [PubMed] [Google Scholar]
- 6. Gensel JC, Zhang B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 2015;1619:1–11. [DOI] [PubMed] [Google Scholar]
- 7. Graff JW, Dickson AM, Clay G, McCaffrey AP, Wilson ME. Identifying functional microRNAs in macrophages with polarized phenotypes. J Biol Chem. 2012;287:21816–21825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shapouri‐Moghaddam A, Mohammadian S, Vazini H, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233:6425–6440. [DOI] [PubMed] [Google Scholar]
- 9. Bashir S, Sharma Y, Elahi A, Khan F. Macrophage polarization: the link between inflammation and related diseases. Inflamm Res. 2016;65:1–11. [DOI] [PubMed] [Google Scholar]
- 10. Arora S, Dev K, Agarwal B, Das P, Syed MA. Macrophages: their role, activation and polarization in pulmonary diseases. Immunobiology. 2018;223:383–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Colin S, Chinetti‐Gbaguidi G, Staels B. Macrophage phenotypes in atherosclerosis. Immunol Rev. 2014;262:153–166. [DOI] [PubMed] [Google Scholar]
- 12. De Paoli F, Staels B, Chinetti‐Gbaguidi G. Macrophage phenotypes and their modulation in atherosclerosis. Circ J. 2014;78:1775–1781. [DOI] [PubMed] [Google Scholar]
- 13. Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44:450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ambarus CA, Santegoets KC, van Bon L, et al. Soluble immune complexes shift the TLR‐induced cytokine production of distinct polarized human macrophage subsets towards IL‐10. Plos One. 2012;7:e35994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roszer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm. 2015;2015:816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zizzo G, Hilliard BA, Monestier M, Cohen PL. Efficient clearance of early apoptotic cells by human macrophages requires M2c polarization and MerTK induction. J Immunol. 2012;189:3508–3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Duluc D, Delneste Y, Tan F, et al. Tumor‐associated leukemia inhibitory factor and IL‐6 skew monocyte differentiation into tumor‐associated macrophage‐like cells. Blood. 2007;110:4319–4330. [DOI] [PubMed] [Google Scholar]
- 18. Wang Q, Ni H, Lan L, et al. Fra‐1 protooncogene regulates IL‐6 expression in macrophages and promotes the generation of M2d macrophages. Cell Res. 2010;20:701–712. [DOI] [PubMed] [Google Scholar]
- 19. Ferrante CJ, Leibovich SJ. Regulation of macrophage polarization and wound healing. Adv Wound Care (New Rochelle). 2012;1:10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Atri C, Guerfali FZ, Laouini D. Role of human macrophage polarization in inflammation during infectious diseases. Int J Mol Sci. 2018;19:E1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sunakawa Y, Stintzing S, Cao S, et al. Variations in genes regulating tumor‐associated macrophages (TAMs) to predict outcomes of bevacizumab‐based treatment in patients with metastatic colorectal cancer: results from TRIBE and FIRE3 trials. Ann Oncol. 2015;26:2450–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu H, Xu JB, He YL, et al. Tumor‐associated macrophages promote angiogenesis and lymphangiogenesis of gastric cancer. J Surg Oncol. 2012;106:462–468. [DOI] [PubMed] [Google Scholar]
- 23. Anderson CF, Mosser DM. A novel phenotype for an activated macrophage: the type 2 activated macrophage. J Leukoc Biol. 2002;72:101–106. [PubMed] [Google Scholar]
- 24. Yue Y, Yang X, Feng K, et al. M2b macrophages reduce early reperfusion injury after myocardial ischemia in mice: a predominant role of inhibiting apoptosis via A20. Int J Cardiol. 2017;245:228–235. [DOI] [PubMed] [Google Scholar]
- 25. Asai A, Tsuchimoto Y, Ohama H, et al. Host antitumor resistance improved by the macrophage polarization in a chimera model of patients with HCC. Oncoimmunology. 2017;6:e1299301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nishiguchi T, Ito I, Lee JO, et al. Macrophage polarization and MRSA infection in burned mice. Immunol Cell Biol. 2017;95:198–206. [DOI] [PubMed] [Google Scholar]
- 27. Ito I, Bhopale KK, Nishiguchi T, et al. The polarization of M2b monocytes in cultures of burn patient peripheral CD14(+) cells treated with a selected human CCL1 antisense oligodeoxynucleotide. Nucleic Acid Ther. 2016;26:269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu RX, Wei Y, Zeng QH, et al. Chemokine (C‐X‐C motif) receptor 3‐positive B cells link interleukin‐17 inflammation to protumorigenic macrophage polarization in human hepatocellular carcinoma. Hepatology. 2015;62:1779–1790. [DOI] [PubMed] [Google Scholar]
- 29. Tsuchimoto Y, Asai A, Tsuda Y, et al. M2b monocytes provoke bacterial pneumonia and gut bacteria‐associated sepsis in alcoholics. J Immunol. 2015;195:5169–5177. [DOI] [PubMed] [Google Scholar]
- 30. Lefevre L, Lugo‐Villarino G, Meunier E, et al. The C‐type lectin receptors dectin‐1, MR, and SIGNR3 contribute both positively and negatively to the macrophage response to Leishmania infantum. Immunity. 2013;38:1038–1049. [DOI] [PubMed] [Google Scholar]
- 31. Lefevre L, Gales A, Olagnier D, et al. PPARgamma ligands switched high fat diet‐induced macrophage M2b polarization toward M2a thereby improving intestinal Candida elimination. Plos One. 2010;5:e12828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang B, Bailey WM, Braun KJ, Gensel JC. Age decreases macrophage IL‐10 expression: implications for functional recovery and tissue repair in spinal cord injury. Exp Neurol. 2015;273:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hedrich CM, Bream JH. Cell type‐specific regulation of IL‐10 expression in inflammation and disease. Immunol Res. 2010;47:185–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Edwards JP, Zhang X, Frauwirth KA, Mosser DM. Biochemical and functional characterization of three activated macrophage populations. J Leukoc Biol. 2006;80:1298–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Burd PR, Freeman GJ, Wilson SD, et al. Cloning and characterization of a novel T cell activation gene. J Immunol. 1987;139:3126–3131. [PubMed] [Google Scholar]
- 36. Miller MD, Hata S, De Waal Malefyt R, Krangel MS. A novel polypeptide secreted by activated human T lymphocytes. J Immunol. 1989;143:2907–2916. [PubMed] [Google Scholar]
- 37. Tiffany HL, Lautens LL, Gao JL, et al. Identification of CCR8: a human monocyte and thymus receptor for the CC chemokine I‐309. J Exp Med. 1997;186:165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McCully ML, Moser B. The human cutaneous chemokine system. Front Immunol. 2011;2:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sironi M, Martinez FO, D'Ambrosio D, et al. Differential regulation of chemokine production by Fcgamma receptor engagement in human monocytes: association of CCL1 with a distinct form of M2 monocyte activation (M2b, Type 2). J Leukoc Biol. 2006;80:342–349. [DOI] [PubMed] [Google Scholar]
- 40. Asai A, Nakamura K, Kobayashi M, Herndon DN, Suzuki F. CCL1 released from M2b macrophages is essentially required for the maintenance of their properties. J Leukoc Biol. 2012;92:859–867. [DOI] [PubMed] [Google Scholar]
- 41. Orme J, Mohan C. Macrophage subpopulations in systemic lupus erythematosus. Discov Med. 2012;13:151–158. [PubMed] [Google Scholar]
- 42. Jiang Z, Zhu L. Update on the role of alternatively activated macrophages in asthma. J Asthma Allergy. 2016;9:101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ito I, Asai A, Suzuki S, Kobayashi M, Suzuki F. M2b macrophage polarization accompanied with reduction of long noncoding RNA GAS5. Biochem Biophys Res Commun. 2017;493:170–175. [DOI] [PubMed] [Google Scholar]
- 44. Mauri DN, Ebner R, Montgomery RI, et al. LIGHT, a new member of the TNF superfamily, and lymphotoxin alpha are ligands for herpesvirus entry mediator. Immunity. 1998;8:21–30. [DOI] [PubMed] [Google Scholar]
- 45. Zhu M, Fu YX. The role of core TNF/LIGHT family members in lymph node homeostasis and remodeling. Immunol Rev. 2011;244:75–84. [DOI] [PubMed] [Google Scholar]
- 46. Yu P, Fu YX. Targeting tumors with LIGHT to generate metastasis‐clearing immunity. Cytokine Growth Factor Rev. 2008;19:285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Elcombe SE, Naqvi S, Van Den Bosch MW, et al. Dectin‐1 regulates IL‐10 production via a MSK1/2 and CREB dependent pathway and promotes the induction of regulatory macrophage markers. Plos One. 2013;8:e60086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kono Y, Miyoshi S, Fujita T. Dextran sodium sulfate alters cytokine production in macrophages in vitro. Pharmazie. 2016;71:619–624. [DOI] [PubMed] [Google Scholar]
- 49. Suzuki F, Loucas BD, Ito I, et al. Survival of mice with gastrointestinal acute radiation syndrome through control of bacterial translocation. J Immunol. 2018;201:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kobayashi M, Nakamura K, Cornforth M, Suzuki F. Role of M2b macrophages in the acceleration of bacterial translocation and subsequent sepsis in mice exposed to whole body [137Cs] gamma‐irradiation. J Immunol. 2012;189:296–303. [DOI] [PubMed] [Google Scholar]
- 51. del Rio ML, Lucas CL, Buhler L, Rayat G, Rodriguez‐Barbosa JI. HVEM/LIGHT/BTLA/CD160 cosignaling pathways as targets for immune regulation. J Leukoc Biol. 2010;87:223–235. [DOI] [PubMed] [Google Scholar]
- 52. Chen C, Gault A, Shen L, Nabavi N. Molecular cloning and expression of early T cell costimulatory molecule‐1 and its characterization as B7‐2 molecule. J Immunol. 1994;152:4929–4936. [PubMed] [Google Scholar]
- 53. Chen L, Flies DB. Molecular mechanisms of T cell co‐stimulation and co‐inhibition. Nat Rev Immunol. 2013;13:227–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pereira C, Tavares B, Loureiro G, et al. Dynamics of CD86 expression on allergic inflammation–new insights. Recent Pat Inflamm Allergy Drug Discov. 2009;3:128–131. [DOI] [PubMed] [Google Scholar]
- 55. Matsui H, Sopko NA, Hannan JL, et al. M1 macrophages are predominantly recruited to the major pelvic ganglion of the rat following cavernous nerve injury. J Sex Med. 2017;14:187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liu Y, Stewart KN, Bishop E, et al. Unique expression of suppressor of cytokine signaling 3 is essential for classical macrophage activation in rodents in vitro and in vivo. J Immunol. 2008;180:6270–6278. [DOI] [PubMed] [Google Scholar]
- 57. Ishizuka EK, Ferreira MJ, Grund LZ, et al. Role of interplay between IL‐4 and IFN‐gamma in the in regulating M1 macrophage polarization induced by Nattectin. Int Immunopharmacol. 2012;14:513–522. [DOI] [PubMed] [Google Scholar]
- 58. Li D, Duan M, Feng Y, et al. MiR‐146a modulates macrophage polarization in systemic juvenile idiopathic arthritis by targeting INHBA. Mol Immunol. 2016;77:205–212. [DOI] [PubMed] [Google Scholar]
- 59. Li J, Liu Y, Xu H, Fu Q. Nanoparticle‐delivered IRF5 siRNA facilitates M1 to M2 transition, reduces demyelination and neurofilament loss, and promotes functional recovery after spinal cord injury in mice. Inflammation. 2016;39:1704–1717. [DOI] [PubMed] [Google Scholar]
- 60. Tian L, Li W, Yang L, et al. Cannabinoid receptor 1 participates in liver inflammation by promoting M1 macrophage polarization via RhoA/NF‐kappaB p65 and ERK1/2 pathways, respectively, in mouse liver fibrogenesis. Front Immunol. 2017;8:1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mulder R, Banete A, Basta S. Spleen‐derived macrophages are readily polarized into classically activated (M1) or alternatively activated (M2) states. Immunobiology. 2014;219:737–745. [DOI] [PubMed] [Google Scholar]
- 62. Rojas J, Salazar J, Martinez MS, et al. Macrophage heterogeneity and plasticity: impact of macrophage biomarkers on atherosclerosis. Scientifica (Cairo). 2015;2015:851252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Liberale L, Dallegri F, Montecucco F, Carbone F. Pathophysiological relevance of macrophage subsets in atherogenesis. Thromb Haemost. 2017;117:7–18. [DOI] [PubMed] [Google Scholar]
- 64. Pyne S, Adams DR, Pyne NJ. Sphingosine 1‐phosphate and sphingosine kinases in health and disease: recent advances. Prog Lipid Res. 2016;62:93–106. [DOI] [PubMed] [Google Scholar]
- 65. Kasper B, Winoto‐Morbach S, Mittelstadt J, et al. CXCL4‐induced monocyte survival, cytokine expression, and oxygen radical formation is regulated by sphingosine kinase 1. Eur J Immunol. 2010;40:1162–1173. [DOI] [PubMed] [Google Scholar]
- 66. Lan T, Li C, Yang G, et al. Sphingosine kinase 1 promotes liver fibrosis by preventing miR‐19b‐3p‐mediated inhibition of CCR2. Hepatology. 2018;68:1070–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gabriel TL, Mirzaian M, Hooibrink B, et al. Induction of Sphk1 activity in obese adipose tissue macrophages promotes survival. Plos One. 2017;12:e0182075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bohm A, Flosser A, Ermler S, et al. Factor‐Xa‐induced mitogenesis and migration require sphingosine kinase activity and S1P formation in human vascular smooth muscle cells. Cardiovasc Res. 2013;99:505–513. [DOI] [PubMed] [Google Scholar]
- 69. Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte‐to‐macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–7311. [DOI] [PubMed] [Google Scholar]
- 70. Sudduth TL, Greenstein A, Wilcock DM. Intracranial injection of Gammagard, a human IVIg, modulates the inflammatory response of the brain and lowers Abeta in APP/PS1 mice along a different time course than anti‐Abeta antibodies. J Neurosci. 2013;33:9684–9692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wilcock DM. A changing perspective on the role of neuroinflammation in Alzheimer's disease. Int J Alzheimers Dis. 2012;2012:495243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Salomon BL, Leclerc M, Tosello J, et al. Tumor necrosis factor alpha and regulatory T cells in oncoimmunology. Front Immunol. 2018;9:444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Aggarwal BB. Signalling pathways of the TNF superfamily: a double‐edged sword. Nat Rev Immunol. 2003;3:745–756. [DOI] [PubMed] [Google Scholar]
- 74. Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. [DOI] [PubMed] [Google Scholar]
- 75. Ohlsson SM, Linge CP, Gullstrand B, et al. Serum from patients with systemic vasculitis induces alternatively activated macrophage M2c polarization. Clin Immunol. 2014;152:10–19. [DOI] [PubMed] [Google Scholar]
- 76. Frodermann V, van Duijn J, van Puijvelde GH, et al. Heat‐killed Staphylococcus aureus reduces atherosclerosis by inducing anti‐inflammatory macrophages. J Intern Med. 2016;279:592–605. [DOI] [PubMed] [Google Scholar]
- 77. Ortiz MC, Lefimil C, Rodas PI, et al. Neisseria gonorrhoeae modulates immunity by polarizing human macrophages to a M2 profile. Plos One. 2015;10:e0130713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Xiao P, Dong C, Yue Y, Xiong S. Dynamic expression of microRNAs in M2b polarized macrophages associated with systemic lupus erythematosus. Gene. 2014;547:300–309. [DOI] [PubMed] [Google Scholar]
- 79. Chen X, Wen Z, Xu W, Xiong S. Granulin exacerbates lupus nephritis via enhancing macrophage M2b polarization. Plos One. 2013;8:e65542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zhang W, Zhou Q, Xu W, et al. DNA‐dependent activator of interferon‐regulatory factors (DAI) promotes lupus nephritis by activating the calcium pathway. J Biol Chem. 2013;288:13534–13550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hunter CA, Jones SA. IL‐6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16:448–457. [DOI] [PubMed] [Google Scholar]
- 82. Schaper F, Rose‐John S. Interleukin‐6: biology, signaling and strategies of blockade. Cytokine Growth Factor Rev. 2015;26:475–487. [DOI] [PubMed] [Google Scholar]
- 83. Garbers C, Heink S, Korn T, Rose‐John S. Interleukin‐6: designing specific therapeutics for a complex cytokine. Nat Rev Drug Discov. 2018;17:395–412. [DOI] [PubMed] [Google Scholar]
- 84. Tanaka T, Narazaki M, Kishimoto T. IL‐6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6:a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kishimoto T, Kang S, Tanaka T. IL‐6: a new era for the treatment of autoimmune inflammatory diseases In: Nakao K, Minato N, Uemoto S, eds. Innovative Medicine: Basic Research and Development. Tokyo: Springer; 2015:131–147. [PubMed] [Google Scholar]
- 86. Heinrich PC, Castell JV, Andus T. Interleukin‐6 and the acute phase response. Biochem J. 1990;265:621–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Akira S, Taga T, Kishimoto T. Interleukin‐6 in biology and medicine. Adv Immunol. 1993;54:1–78. [DOI] [PubMed] [Google Scholar]
- 88. Cai Y, Zhang W, Xiong S. Mannose‐binding lectin blunts macrophage polarization and ameliorates lupus nephritis. Plos One. 2013;8:e62465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhang W, Xu W, Xiong S. Macrophage differentiation and polarization via phosphatidylinositol 3‐kinase/Akt‐ERK signaling pathway conferred by serum amyloid P component. J Immunol. 2011;187:1764–1777. [DOI] [PubMed] [Google Scholar]
- 90. Schulert GS, Fall N, Harley JB, et al. Monocyte microRNA expression in active systemic juvenile idiopathic arthritis implicates microRNA‐125a‐5p in polarized monocyte phenotypes. Arthritis Rheumatol. 2016;68:2300–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bumgardner SA, Zhang L, LaVoy AS, et al. Nod2 is required for antigen‐specific humoral responses against antigens orally delivered using a recombinant Lactobacillus vaccine platform. Plos One. 2018;13:e0196950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Walker DG, Lue LF. Immune phenotypes of microglia in human neurodegenerative disease: challenges to detecting microglial polarization in human brains. Alzheimers Res Ther. 2015;7:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Sung SJ, Ge Y, Dai C, et al. Dependence of glomerulonephritis induction on novel intraglomerular alternatively activated bone marrow‐derived macrophages and Mac‐1 and PD‐L1 in lupus‐prone NZM2328 mice. J Immunol. 2017;198:2589–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Xiao C, Rajewsky K. MicroRNA control in the immune system: basic principles. Cell. 2009;136:26–36. [DOI] [PubMed] [Google Scholar]
- 95. O'Connell RM, Rao DS, Baltimore D. microRNA regulation of inflammatory responses. Annu Rev Immunol. 2012;30:295–312. [DOI] [PubMed] [Google Scholar]
- 96. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zhang Y, Zhang M, Zhong M, Suo Q, Lv K. Expression profiles of miRNAs in polarized macrophages. Int J Mol Med. 2013;31:797–802. [DOI] [PubMed] [Google Scholar]
- 98. Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF‐kappaB‐dependent induction of microRNA miR‐146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103:12481–12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Cai X, Yin Y, Li N, et al. Re‐polarization of tumor‐associated macrophages to pro‐inflammatory M1 macrophages by microRNA‐155. J Mol Cell Biol. 2012;4:341–343. [DOI] [PubMed] [Google Scholar]
- 100. Nazari‐Jahantigh M, Wei Y, Noels H, et al. MicroRNA‐155 promotes atherosclerosis by repressing Bcl6 in macrophages. J Clin Invest. 2012;122:4190–4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Nie M, Liu J, Yang Q, et al. MicroRNA‐155 facilitates skeletal muscle regeneration by balancing pro‐ and anti‐inflammatory macrophages. Cell Death Dis. 2016;7:e2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zhuang G, Meng C, Guo X, et al. A novel regulator of macrophage activation: miR‐223 in obesity‐associated adipose tissue inflammation. Circulation. 2012;125:2892–2903. [DOI] [PubMed] [Google Scholar]
- 103. Yu F, Zheng J, Mao Y, et al. Long non‐coding RNA growth arrest‐specific transcript 5 (GAS5) inhibits liver fibrogenesis through a mechanism of competing endogenous RNA. J Biol Chem. 2015;290:28286–28298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Banerjee S, Cui H, Xie N, et al. miR‐125a‐5p regulates differential activation of macrophages and inflammation. J Biol Chem. 2013;288:35428–35436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Eigsti RL, Sudan B, Wilson ME, Graff JW. Regulation of activation‐associated microRNA accumulation rates during monocyte‐to‐macrophage differentiation. J Biol Chem. 2014;289:28433–28447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43:904–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Mattick JS, Rinn JL. Discovery and annotation of long noncoding RNAs. Nat Struct Mol Biol. 2015;22:5–7. [DOI] [PubMed] [Google Scholar]
- 108. Chen MT, Lin HS, Shen C, et al. PU.1‐regulated long noncoding RNA lnc‐MC controls human monocyte/macrophage differentiation through interaction with microRNA 199a‐5p. Mol Cell Biol. 2015;35:3212–3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Reddy MA, Chen Z, Park JT, et al. Regulation of inflammatory phenotype in macrophages by a diabetes‐induced long noncoding RNA. Diabetes. 2014;63:4249–4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Huang Z, Luo Q, Yao F, et al. Identification of differentially expressed long non‐coding RNAs in polarized macrophages. Sci Rep. 2016;6:19705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ye Y, Xu Y, Lai Y, et al. Long non‐coding RNA cox‐2 prevents immune evasion and metastasis of hepatocellular carcinoma by altering M1/M2 macrophage polarization. J Cell Biochem. 2018;119:2951–2963. [DOI] [PubMed] [Google Scholar]
- 112. Sun D, Yu Z, Fang X, et al. LncRNA GAS5 inhibits microglial M2 polarization and exacerbates demyelination. EMBO Rep. 2017;18:1801–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Cao Q, Wang N, Qi J, Gu Z, Shen H. Long non‐coding RNA‐GAS5 acts as a tumor suppressor in bladder transitional cell carcinoma via regulation of chemokine (C‐C motif) ligand 1 expression. Mol Med Rep. 2016;13:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Lawrence T, Gilroy DW, Colville‐Nash PR, Willoughby DA. Possible new role for NF‐kappaB in the resolution of inflammation. Nat Med. 2001;7:1291–1297. [DOI] [PubMed] [Google Scholar]
- 115. Brand K, Page S, Rogler G, et al. Activated transcription factor nuclear factor‐kappa B is present in the atherosclerotic lesion. J Clin Invest. 1996;97:1715–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Bonizzi G, Karin M. The two NF‐kappa B activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. [DOI] [PubMed] [Google Scholar]
- 117. Porta C, Rimoldi M, Raes G, et al. Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor kappaB. Proc Natl Acad Sci U S A. 2009;106:14978–14983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Zhang W, Xu W, Xiong S. Blockade of Notch1 signaling alleviates murine lupus via blunting macrophage activation and M2b polarization. J Immunol. 2010;184:6465–6478. [DOI] [PubMed] [Google Scholar]
- 119. Pearson G, Robinson F, Beers Gibson T, et al. Mitogen‐activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. [DOI] [PubMed] [Google Scholar]
- 120. Vergadi E, Ieronymaki E, Lyroni K, Vaporidi K, Tsatsanis C. Akt signaling pathway in macrophage activation and M1/M2 polarization. J Immunol. 2017;198:1006–1014. [DOI] [PubMed] [Google Scholar]
- 121. Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9:59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Fukao T, Koyasu S. PI3K and negative regulation of TLR signaling. Trends Immunol. 2003;24:358–363. [DOI] [PubMed] [Google Scholar]
- 123. Troutman TD, Bazan JF, Pasare C. Toll‐like receptors, signaling adapters and regulation of the pro‐inflammatory response by PI3K. Cell Cycle. 2012;11:3559–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Lopez‐Pelaez M, Soria‐Castro I, Bosca L, Fernandez M, Alemany S. Cot/tpl2 activity is required for TLR‐induced activation of the Akt p70 S6k pathway in macrophages: implications for NO synthase 2 expression. Eur J Immunol. 2011;41:1733–1741. [DOI] [PubMed] [Google Scholar]
- 125. Zhao X, Dai J, Xiao X, et al. PI3K/Akt signaling pathway modulates influenza virus induced mouse alveolar macrophage polarization to M1/M2b. Plos One. 2014;9:e104506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Krausgruber T, Blazek K, Smallie T, et al. IRF5 promotes inflammatory macrophage polarization and TH1‐TH17 responses. Nat Immunol. 2011;12:231–238. [DOI] [PubMed] [Google Scholar]
- 127. Liu YC, Zou XB, Chai YF, Yao YM. Macrophage polarization in inflammatory diseases. Int J Biol Sci. 2014;10:520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Satoh T, Takeuchi O, Vandenbon A, et al. The Jmjd3‐Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat Immunol. 2010;11:936–944. [DOI] [PubMed] [Google Scholar]
- 129. Leibowitz BJ, Wei L, Zhang L, et al. Ionizing irradiation induces acute haematopoietic syndrome and gastrointestinal syndrome independently in mice. Nat Commun. 2014;5:3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Dorresteijn LD, Kappelle AC, Boogerd W, et al. Increased risk of ischemic stroke after radiotherapy on the neck in patients younger than 60 years. J Clin Oncol. 2002;20:282–288. [DOI] [PubMed] [Google Scholar]
- 131. Russell NS, Hoving S, Heeneman S, et al. Novel insights into pathological changes in muscular arteries of radiotherapy patients. Radiother Oncol. 2009;92:477–483. [DOI] [PubMed] [Google Scholar]
- 132. Gabriels K, Hoving S, Gijbels MJ, et al. Irradiation of existing atherosclerotic lesions increased inflammation by favoring pro‐inflammatory macrophages. Radiother Oncol. 2014;110:455–460. [DOI] [PubMed] [Google Scholar]
- 133. Rothfuchs AG, Gigliotti D, Palmblad K, et al. IFN‐alpha beta‐dependent, IFN‐gamma secretion by bone marrow‐derived macrophages controls an intracellular bacterial infection. J Immunol. 2001;167:6453–6461. [DOI] [PubMed] [Google Scholar]
- 134. Rosenberger CM, Finlay BB. Phagocyte sabotage: disruption of macrophage signalling by bacterial pathogens. Nat Rev Mol Cell Biol. 2003;4:385–396. [DOI] [PubMed] [Google Scholar]
- 135. Ohama H, Asai A, Ito I, et al. M2b macrophage elimination and improved resistance of mice with chronic alcohol consumption to opportunistic infections. Am J Pathol. 2015;185:420–431. [DOI] [PubMed] [Google Scholar]
- 136. Kobayashi M, Jeschke MG, Asai A, et al. Propranolol as a modulator of M2b monocytes in severely burned patients. J Leukoc Biol. 2011;89:797–803. [DOI] [PubMed] [Google Scholar]
- 137. Kobayashi M, Jeschke MG, Shigematsu K, et al. M2b monocytes predominated in peripheral blood of severely burned patients. J Immunol. 2010;185:7174–7179. [DOI] [PubMed] [Google Scholar]
- 138. Kobayashi M, Asai A, Ito I, et al. Short‐term alcohol abstinence improves antibacterial defenses of chronic alcohol‐consuming mice against gut bacteria‐associated sepsis caused by enterococcus faecalis oral infection. Am J Pathol. 2017;187:1998–2007. [DOI] [PubMed] [Google Scholar]
- 139. Filardy AA, Pires DR, Nunes MP, et al. Proinflammatory clearance of apoptotic neutrophils induces an IL‐12(low)IL‐10(high) regulatory phenotype in macrophages. J Immunol. 2010;185:2044–2050. [DOI] [PubMed] [Google Scholar]
- 140. MacParland SA, Tsoi KM, Ouyang B, et al. Phenotype determines nanoparticle uptake by human macrophages from liver and blood. ACS Nano. 2017;11:2428–2443. [DOI] [PubMed] [Google Scholar]
- 141. Nakamura K, Ito I, Kobayashi M, Herndon DN, Suzuki F. Orosomucoid 1 drives opportunistic infections through the polarization of monocytes to the M2b phenotype. Cytokine. 2015;73:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Schiffer L, Bethunaickan R, Ramanujam M, et al. Activated renal macrophages are markers of disease onset and disease remission in lupus nephritis. J Immunol. 2008;180:1938–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Liu YH, Ding Y, Gao CC, et al. Functional macrophages and gastrointestinal disorders. World J Gastroenterol. 2018;24:1181–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol. 2008;209:378–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Zhou Z, Peng X, Insolera R, Fink DJ, Mata M. Interleukin‐10 provides direct trophic support to neurons. J Neurochem. 2009;110:1617–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Thompson CD, Zurko JC, Hanna BF, Hellenbrand DJ, Hanna A. The therapeutic role of interleukin‐10 after spinal cord injury. J Neurotrauma. 2013;30:1311–1324. [DOI] [PubMed] [Google Scholar]
- 147. Wilcock DM. Neuroinflammatory phenotypes and their roles in Alzheimer's disease. Neurodegener Dis. 2014;13:183–185. [DOI] [PubMed] [Google Scholar]
- 148. Wilcock DM, Hurban J, Helman AM, et al. Down syndrome individuals with Alzheimer's disease have a distinct neuroinflammatory phenotype compared to sporadic Alzheimer's disease. Neurobiol Aging. 2015;36:2468–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Lee BC, Lee J. Cellular and molecular players in adipose tissue inflammation in the development of obesity‐induced insulin resistance. Biochim Biophys Acta. 2014;1842:446–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Chylikova J, Dvorackova J, Tauber Z, Kamarad V. M1/M2 macrophage polarization in human obese adipose tissue. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2018;162:79–82. [DOI] [PubMed] [Google Scholar]
- 151. Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Ramkhelawon B, Hennessy EJ, Menager M, et al. Netrin‐1 promotes adipose tissue macrophage retention and insulin resistance in obesity. Nat Med. 2014;20:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Basu Mallik S, Jayashree BS, Shenoy RR. Epigenetic modulation of macrophage polarization‐ perspectives in diabetic wounds. J Diabetes Complications. 2018;32:524–530. [DOI] [PubMed] [Google Scholar]
- 155. Yeligar SM, Mehta AJ, Harris FL, Brown LA, Hart CM. Peroxisome proliferator‐activated receptor gamma regulates chronic alcohol‐induced alveolar macrophage dysfunction. Am J Respir Cell Mol Biol. 2016;55:35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Szanto A, Balint BL, Nagy ZS, et al. STAT6 transcription factor is a facilitator of the nuclear receptor PPAR gamma‐regulated gene expression in macrophages and dendritic cells. Immunity. 2010;33:699–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Tamura R, Tanaka T, Yamamoto Y, Akasaki Y, Sasaki H. Dual role of macrophage in tumor immunity. Immunotherapy. 2018;10:899–909. [DOI] [PubMed] [Google Scholar]
- 158. Lisi L, Stigliano E, Lauriola L, Navarra P, Dello Russo C. Proinflammatory‐activated glioma cells induce a switch in microglial polarization and activation status, from a predominant M2b phenotype to a mixture of M1 and M2a/B polarized cells. ASN Neuro. 2014;6:171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. da Fonseca AC, Badie B. Microglia and macrophages in malignant gliomas: recent discoveries and implications for promising therapies. Clin Dev Immunol. 2013;2013:264124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Bai J, Adriani G, Dang TM, et al. Contact‐dependent carcinoma aggregate dispersion by M2a macrophages via ICAM‐1 and beta2 integrin interactions. Oncotarget. 2015;6:25295–25307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Li H, Jiang T, Li MQ, Zheng XL, Zhao GJ. Transcriptional regulation of macrophages polarization by microRNAs. Front Immunol. 2018;9:1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Li Y, Cai L, Wang H, et al. Pleiotropic regulation of macrophage polarization and tumorigenesis by formyl peptide receptor‐2. Oncogene. 2011;30:3887–3899. [DOI] [PubMed] [Google Scholar]
- 163. Chen MM, Xiao X, Lao XM, et al. Polarization of tissue‐resident TFH‐like cells in human hepatoma bridges innate monocyte inflammation and M2b macrophage polarization. Cancer Discov. 2016;6:1182–1195. [DOI] [PubMed] [Google Scholar]
- 164. Fujiwara Y, Hizukuri Y, Yamashiro K, et al. Guanylate‐binding protein 5 is a marker of interferon‐gamma‐induced classically activated macrophages. Clin Transl Immunology. 2016;5:e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Li XY, Yue Y, Zhu YY, Xiong SD. Extracellular, but not intracellular HMGB1, facilitates self‐DNA induced macrophage activation via promoting DNA accumulation in endosomes and contributes to the pathogenesis of lupus nephritis. Mol Immunol. 2015;65:177–188. [DOI] [PubMed] [Google Scholar]
- 166. Chen M, Zhang WJ, Xu W, Zhang F, Xiong SD. Blockade of TLR9 signaling in B cells impaired anti‐dsDNA antibody production in mice induced by activated syngenic lymphocyte‐derived DNA immunization. Mol Immunol. 2011;48:1532–1539. [DOI] [PubMed] [Google Scholar]
- 167. Qiao B, Wu J, Chu YW, et al. Induction of systemic lupus erythematosus‐like syndrome in syngeneic mice by immunization with activated lymphocyte‐derived DNA. Rheumatology (Oxford). 2005;44:1108–1114. [DOI] [PubMed] [Google Scholar]
- 168. Wen Z, Xu L, Xu W, Xiong S. Production of anti‐double‐stranded DNA antibodies in activated lymphocyte derived DNA induced lupus model was dependent on CD4(+) T cells. Lupus. 2012;21:508–516. [DOI] [PubMed] [Google Scholar]
- 169. Herndon DN, Barrow RE, Rutan TC, et al. Effect of propranolol administration on hemodynamic and metabolic responses of burned pediatric patients. Ann Surg. 1988;208:484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Pedrazzi M, Patrone M, Passalacqua M, et al. Selective proinflammatory activation of astrocytes by high‐mobility group box 1 protein signaling. J Immunol. 2007;179:8525–8532. [DOI] [PubMed] [Google Scholar]


