Abstract
BACKGROUND:
Although pharmacotherapy is not the cornerstone of obesity treatment, it is a valuable tool that could be considered for patients who have not had adequate benefit from lifestyle interventions or who have difficulty maintaining initial weight loss over longer periods.
CONTENT:
This review focuses on the role of antiobesity drugs, the mechanisms by which the drugs work, potential pharmacological targets in the neural control of food intake and regulation of body weight, the history of antiobesity drugs, a summary of efficacy and safety data from clinical trials, and the clinical application of pharmacotherapy. Currently, 5 approved drug therapies are available in the US for long-term weight management, with only 2 of these meeting the stronger Food and Drug Administration (FDA) criteria of 5% weight loss relative to a placebo after 1 year and others receiving approval based on the categorical criterion of the proportions of patients achieving 5% weight loss. Interpretation of the results of clinical trials conducted before regulatory agency approval is limited by high dropout rates; thus, the results might not be replicable in clinical practice settings. Many patients who are suitable candidates for pharmacotherapy are not using the new drugs due to lack of insurance coverage and high out-of-pocket costs.
SUMMARY:
With the availability of 4 new drugs since 2012, clinicians in the US now have more tools for long-term weight management. The quality of pharmacotherapy clinical investigations needs considerable improvement. Future research should focus on examining the mediators and moderators of response.
Contributors to the development and persistence of obesity in humans include genetic, epigenetic, biological, behavioral, psychosocial, environmental, cultural, and dietary factors, with the scenario being generally multifactorial (1). Therefore, although lifestyle modification, with an emphasis on caloric restriction and increased physical activity, is recommended as the first-line therapy for the prevention and treatment of obesity, most patients, especially those with more severe forms of obesity and medical comorbidities, need additional interventions. Furthermore, given that the most commonly encountered chronic medical problems in clinical practice—type 2 diabetes (T2D),2 hypertension, and dyslipidemia—tend to improve with weight reduction (2), increased emphasis on obesity management can reduce the burden of medical management of various comorbidities individually.
Bariatric surgery is a very effective intervention for achieving weight loss and ameliorating obesity-related comorbidities, but is associated with greater risks and higher costs relative to nonsurgical interventions, and thus it is not feasible or desirable for millions of individuals with obesity. Bariatric surgery is currently recommended for patients with a body mass index (BMI) of ≥40 kg/m2 or ≥35 mg/m2 in the presence of weight-related comorbidities (3). Pharmacotherapy, with an efficacy level that falls between that of lifestyle and surgical interventions, can thus bridge the gap that exists.
WHO ARE CANDIDATES FOR ANTIOBESITY DRUG THERAPY?
Drugs approved for weight management should be considered for patients with a BMI of ≥30 kg/m2 and those with a BMI of at least 27 kg/m2 in the presence of weight-related comorbidities when added to lifestyle counseling (3). Pharmacotherapy may be considered for patients with excess bodyweight who (i) achieve modest benefit with lifestyle intervention and need additional weight loss; (ii) lose some weight with lifestyle intervention but have difficulty maintaining weight loss; (iii) have made numerous unsuccessful attempts at losing weight with diet and exercise; and (iv) are unable to comply with recommended lifestyle changes due to chronic severe medical conditions.
WHO RECEIVES ANTIOBESITY DRUG THERAPY?
Although nearly half of obesity patients meet BMI eligibility criteria for antiobesity drugs, it is estimated that ≤3.5% receive prescriptions for these drugs in the US (4–6). The majority of users of antiobesity drugs are women (85%), white (86%), and aged <44 years (5, 7), with the possible explanation being a higher level of body image distress among these groups.
WHO PRESCRIBES ANTIOBESITY DRUGS AND WHICH ARE THE MOST PRESCRIBED?
Primary care physicians account for most prescriptions for antiobesity drugs, ranging from 70% to 84%, depending on the period of survey (6, 8). Despite the availability of 5 medications for chronic weight management since 1999, phentermine, approved in 1959 for short-term weight loss, remains the most commonly used antiobesity drug in the US, accounting for 74%–89% of all prescriptions (6, 7). High costs, lack of insurance coverage, and the negative attitudes of prescribers toward pharmacotherapy and the treatment of obesity in general might be factors contributing to underutilization of newer antiobesity drugs. Furthermore, removal of some approved antiobesity drugs (fenfluramine, dexfenfluramine, sibutramine, rimonabant) because of safety issues might be a possible explanation for the reluctance of some physicians to prescribe this class of drugs.
HOW DOES PHARMACOTHERAPY ASSIST IN WEIGHT LOSS AND WEIGHT MANAGEMENT?
Losing weight requires creation of a negative energy balance. With this in mind, patients with obesity are generally counseled to decrease their caloric intake and increase physical activity. Antiobesity drugs, via reduction of hunger and food cravings and enhancement of satiety, help patients to limit the amount of food they eat, thereby increasing their adherence to the prescribed diet plan (9). Although increased energy expenditure is suggested as a possible additional mechanism for weight loss associated with some antiobesity drugs, reduction in energy intake explains most of the weight loss achieved with all available antiobesity drugs. With the exception of orlistat, which reduces the absorption of fat in the gut, currently approved drugs for weight management primarily work via their effects on the central nervous system (CNS). Therefore, it is important to have a basic understanding of the CNS pathways involved in the regulation of energy homeostasis.
NEURAL CONTROL OF ENERGY HOMEOSTASIS
Neural control of energy balance is complex, redundant, and flexible.
It has become increasingly clear that the neural pathways controlling food intake and energy expenditure that lead to the regulation of energy balance, body weight, and fat distribution are complex, redundant, and flexible. The idea of a relatively simple hypothalamic adipostat that had emerged about 20 years ago following the discovery of leptin has been replaced with an extensive neural network that encompasses most areas of the brain, including the cortico-limbic systems that make up >80% of the human brain. This expanded system (Fig. 1) is thought to integrate both the classical homeostatic as well as the hedonic, emotional, and cognitive aspects of food intake and energy expenditure (10). Clearly, with a larger system comes increased heterogeneity of neurotransmitters and modulators as potential targets for pharmacotherapy. Furthermore, the redundancy and flexibility of the system require treatments that address >1 pathway. To avoid compensation, treatments ideally impinge on both the energy intake and the energy expenditure arms of the system. This can be achieved by combining drugs with different pharmacodynamic profiles, e.g., a phentermine/topiramate combination. A number of second-generation unimolecular polypharmacy agents are under development (11).
Fig. 1. Schematic diagram showing potential pharmacological targets in the neural controls of food intake and regulation of body weight.
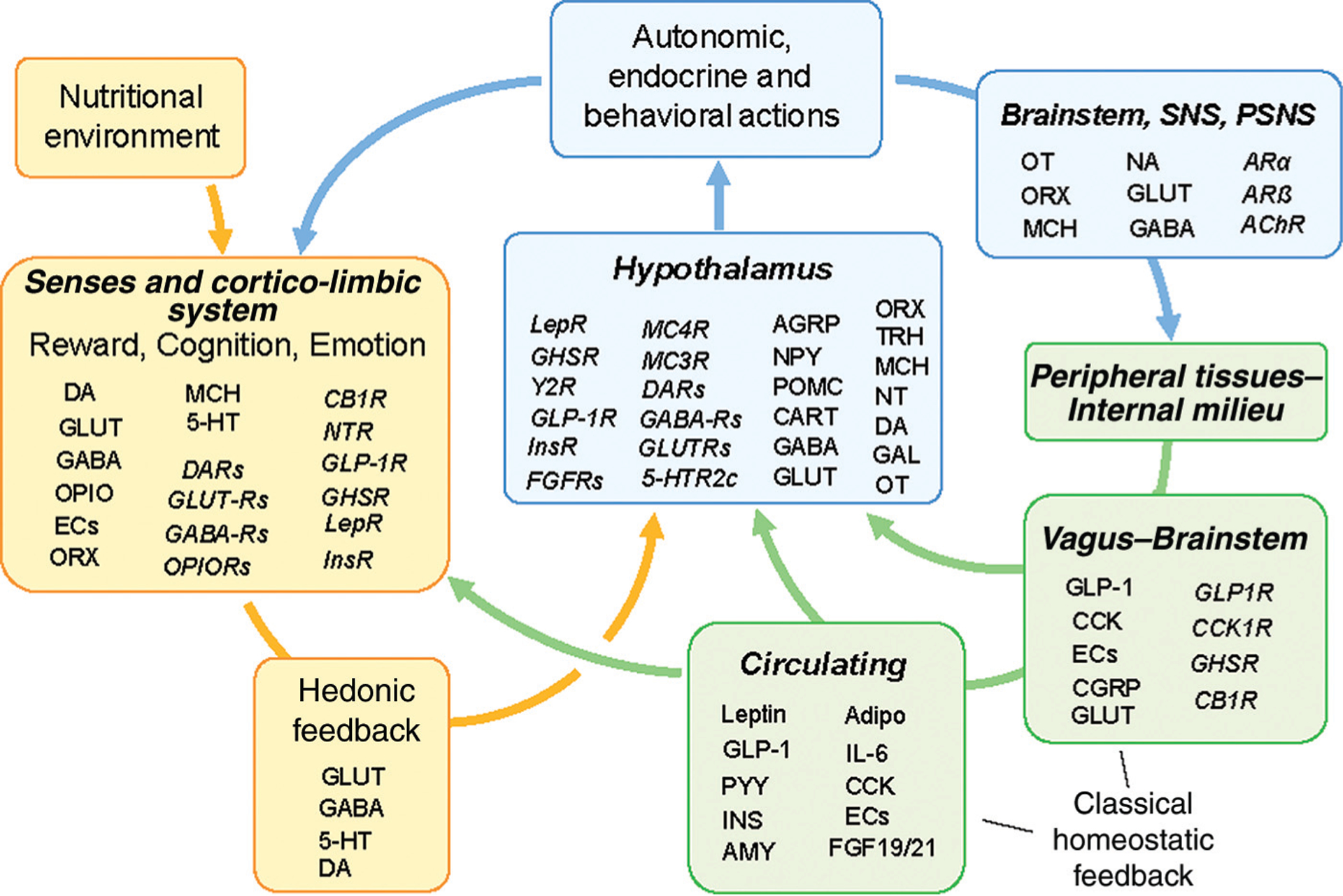
A selection of only key neurotransmitters, hormones, and other factors (normal font) and receptors (italic) are shown at their major sites of action. Abbreviations: AChR, cholinergic acetylcholine receptor; Adipo, adiponectin; AGRP, Agouti-related protein; AMY, amylin; ARα, α-adrenergic receptor; ARß, ß-adrenergic receptor; CART, cocaine- and amphetamine-related transcript; CB1R, cannabinoid receptor-1; CCK, cholecystokinin; CGRP, calcitonin gene-related peptide; DA, dopamine; DARs, dopamine receptors; ECs, endocannabinoids; FGF19/20, fibroblast growth factors 19 & 21; FGFRs, fibroblast growth factor receptors; GABA, gamma-aminobutyric acid; GABARs, GABA receptors; GLUT, glutamate; GUTRs, glutamate receptors; GLP-1, glucagon-like peptide 1; GLP-1R, glucagon-like peptide-1 receptor; IL-6, interleukin-6; INS, insulin; InsR, insulin receptor; Lep, leptin; LepR, leptin receptor; MCH, melanin-concentrating hormone; NT, neurotensin; NTR, neurotensin receptor; OPIO, opioids; OPIORs, opioid receptors; ORX, orexin/hypocretin; OT, oxytocin; POMC, proopiomelanocortin (α-MSH); PYY, polypeptide tyrosine-tyrosine; 5-HT, serotonin; 5-HTR2c, serotonin 2c receptor.
Biological defense mechanisms are a major obstacle to weight loss.
Another fundamental new insight is the recognition that it is the defense of a particular body weight that is at the core of the near-global obesity epidemic. Specifically, the powerful counterregulatory and biologically adaptive mechanisms of increased hunger and decreased metabolism that kick into action upon calorie restriction are likely the major obstacles to preventing or reversing obesity (12). For any obesity prevention or treatment strategy, including pharmacotherapy, to be successful, it will have to be able to suppress these adaptive responses and permanently change the defended level of body weight/adiposity. Although we can accurately measure these adaptive biological responses, we do not fully understand their mechanisms and, more importantly, we neither understand the molecular mechanisms that constitute defense of the set point nor how the set point can be changed. Bariatric surgeries in patients with obesity and in rodent models strongly suggest that this treatment leads to sustained weight loss because it changes the defended body weight/adiposity level (13). Therefore, understanding the mechanisms by which bariatric surgeries achieve resetting the body weight set point is a major research goal as it may lead to nonsurgical interventions with similar efficacy.
A powerful basomedial hypothalamus is key to body weight regulation.
A major candidate for body weight set point regulation is the basomedial hypothalamus, where nutrient availability is sensed and translated into an optimal behavioral and metabolic action pattern (14). The basomedial hypothalamus, more than other regions, is exquisitely sensitive to any kind of manipulation, leading to either extreme obesity or starvation. Agonists and antagonists to the melanocortin receptors MC4R and MC3R are among the most powerful drugs for changing body weight in rodents, but application to humans has not been successful because of strong undesirable side effects. However, melanocortin-signaling is just one aspect of basomedial hypothalamic functions, with a recent study identifying 50 transcriptionally distinct cell populations in the arcuate nucleus-median eminence alone (15). The effects of the 5-HT2c agonist, lorcaserin, on food intake appear to be primarily due to its activation of basomedial hypothalamic proopiomelanocortin neurons (16), although additional action on the mesolimbic reward circuit has been suggested (17). Thus, targeting basomedial hypothalamic signaling mechanisms remains a hot topic in obesity drug development.
Importance of manipulating the reward value of food.
The literature on nonhuman animals very clearly identifies palatable food as the major cause of obesity. More than 40 years ago it was demonstrated that rats become rapidly obese on a palatable cafeteria diet, an effect that was moderated by access to physical activity and completely reversed by changing the diet back to regular laboratory chow (18). How is diet-induced obesity possible in the presence of powerful hypothalamic body weight regulation? One possibility for the corruption of hypothalamic body weight regulatory mechanisms by palatable diets rich in saturated fats and sugar is their proinflammatory action mediated by increased numbers of microglia and astrocytes in critical hypothalamic areas. However, current evidence suggests that the rapid initial hypothalamic gliosis is rather a neuroprotective response to cope with neural stress induced by an increased load of saturated fats and that only long-term overnutrition eventually changes inflammatory signaling of hypothalamic microglia and astrocytes to a more neurotoxic phenotype (19). This clearly puts the blame for the initial cause of this vicious cycle to overeating of palatable diets high in saturated fats and sugar. There is considerable evidence for a role of the meso-corticolimbic reward pathways (20). Although dopamine is a crucial neurotransmitter in the reward system, it also depends on opioidergic, glutamatergic, γ-aminobutyric acid (GABA)-ergic, and nicotinic cholinergic signaling, as well as signaling via orexin and the melanin-concentrating hormone. Although currently marketed CNS-acting anorexigenic drugs affect the food reward system, there are further opportunities for developing drugs that could selectively manipulate food reward without having undesirable effects on mood, cognition, and executive functions.
Fighting obesity through improvement of cognitive functions.
Several lines of evidence in both rodents and humans have shown strong interactions between obesity and cognitive functions, particularly with learning and memory (21, 22). The rescue of impaired learning and memory functions may be an effective strategy to modulate food intake and reduce body weight in obese subjects (23). Because habitual and “mindless” behavior routines such as instinctively responding to environmental food cues with eating or automatically taking the elevator instead of the stairs are less accessible to cognitive modulation (24), a case can be made for shifting from subconscious procedural to more conscious, associative forms of memory and more mindful behavior. Because habit formation appears to depend on a shift of activity from progressively more ventral to more dorsal striatal loops (25), a potential strategy would be to prevent or attenuate this shift. It will thus be important to identify the molecular signatures of these different striatal domains to ultimately pharmacologically target specific subsets and thereby enhance associative memories and cognitive attention.
HISTORY OF ANTIOBESITY DRUGS
Drugs approved to treat obesity have been in existence since the 1950s (Table 1). Until the approval of dexfenfluramine in 1996 for chronic weight management, weight loss drugs were approved in the US for short-term use only, about 12 weeks. With the exception of fenfluramine, all drugs approved before 1996 were structurally related to amphetamine, although they differed in their effects on enhancing the turnover of norepinephrine and dopamine, thus differing in their abuse potential. Although randomized controlled trials (RCTs) of at least 1 year were lacking, these sympathomimetic drugs showed a fair amount of efficacy, averaging approximately 3.0–3.6 kg weight loss relative to a placebo (26); of these drugs, mazindol was discontinued by the manufacturer in 1999. Phentermine, diethylpropion, phendimetrazine, and benzphetamine have remained on the US market, with phentermine being the most prescribed weight loss drug in this class, as well as among all approved antiobesity drugs. Fenfluramine, first approved in 1973, was withdrawn worldwide 24 years later, along with its newly-approved isomer, dexfenfluramine, in 1997 following echocardiographic demonstration of increased risk of mitral and aortic regurgitation among patients treated with these drugs. It was also in 1997 that sibutramine, a serotonin and norepinephrine uptake inhibitor, was approved for long-term treatment of obesity; however, the manufacturer voluntarily withdrew the drug worldwide at the request of the US Food and Drug Administration (FDA) in 2010 when results of a long-term trial (27) in high-risk patients revealed a slightly increased risk of major adverse cardiovascular events (MACE) with sibutramine treatment relative to a placebo (hazard ratio, 1.16; 95% confidence interval 1.03–1.31; P = 0.02). There was considerable enthusiasm for cannabinoid receptor-1 antagonists when rimonabant, the first drug in that class, was approved in the European Union in 2006; however, due to increased frequency of mood disorders and suicidal behaviors (28), it received a negative recommendation from an FDA advisory committee, resulting in the manufacturer withdrawing the new drug application (NDA). In 2008, the European Medicines Agency ordered removal of rimonabant from European markets. Following withdrawal of rimonabant, further clinical development of at least 3 other cannabinoid receptor-1 antagonists—taranabant (Merck), CP-945 598 (Pfizer), and BMS-646256 (Bristol Myers Squibb)—was halted without NDA submission.
Table 1.
History of prescription antiobesity drugs.
| Drug | Year initially approved | Comments |
|---|---|---|
| Phentermine | 1959 | Short-term use; most prescribed drug in the US; withdrawn in Europe in 2000 for unfavorable benefit-to-risk |
| Diethylpropion | 1959 | Short-term use |
| Phendimetrazine | 1959 | Short-term use |
| Benzphetamine | 1960 | Short-term use |
| Mazindol | 1973 | Short-term use; discontinued in 1999 |
| Fenfluramine | 1973 | Short-term use; withdrawn in 1997 due to increased risk of valvular heart disease |
| Dexfenfluramine | 1996 | Long-term use; withdrawn in 1997 due to increased risk of valvular heart disease |
| Sibutramine | 1997 | Long-term use; withdrawn in 2010 due to increased risk of major adverse cardiovascular events |
| Orlistat | 1999 | Long-term use; Approved in 2003 for pediatric obesitya |
| Rimonabant | 2006 | Long-term use; approved in Europe only; withdrawn in 2008 due to serious psychiatric adverse events |
| Phentermine + Topiramate | 2012 | Long-term use; marketed under REMSb to reduce teratogenicity risk |
| Lorcaserin | 2012 | Long-term use; marketing delayed by a year due to DEA classification process |
| Naltrexone + Bupropion | 2014 | Long-term use |
| Liraglutide 3.0 mg | 2014 | Long-term use; also approved at a lower dose for type 2 diabetes in 2010 |
Alli is lower-dose (60 mg) orlistat approved in 2007 for use without prescription.
REMS, Risk Evaluation and Mitigation Strategy.
CURRENTLY MARKETED ANTIOBESITY DRUGS APPROVED FOR SHORT-TERM USE
Phentermine, approved in 1959 for short-term use, is a norepinephrine-releasing agent, with possible norepinephrine uptake inhibition with lower abuse potential than amphetamine due to lack of significant dopaminergic effects (29). It is the most prescribed weight loss drug in the US despite the availability of 5 approved drugs for long-term use in recent years. A 2002 metaanalysis estimated that treatment with phentermine 30 mg/day for 8–24 weeks achieved a mean weight loss of 3.6 kg relative to a placebo (26). Other drugs in this class have fewer published clinical trials. Although most clinical trials of phentermine were of short duration, there was a 1968 publication (30) that reported a 36-week RCT that compared phentermine 30 mg/day continuously, phentermine 30 mg/day intermittently (1 month on, 1 month off), and a placebo continuously. All subjects (n = 108, women) were instructed to follow an energy-restricted diet of 1000 kcal/day. Results were reported only for the 64 women who completed the study. Weight changes with continuous phentermine (n = 17), intermittent phentermine (n = 22), and a placebo (n = 25) were −12.2 kg, −13.0 kg, and −4.8 kg, respectively. A significant placebo-subtracted weight loss of 6.4–6.7 kg was reported for phentermine 30–37.5 mg/day in two 12-week RCTs among Korean obese patients (31, 32). In a recently reported 28-week RCT that compared various treatments, phentermine 15 mg/day led to weight loss of 6.1% vs 1.7% for a placebo (33). Common side effects of phentermine are dry mouth, constipation, and insomnia. There is no scientific evidence that phentermine used alone increases the risk of valvular heart disease (34).
CURRENTLY MARKETED DRUGS APPROVED FOR LONG-TERM WEIGHT MANAGEMENT
Orlistat.
Orlistat, a pancreatic lipase inhibitor that reduces intestinal absorption of fat by about one-third, has been available since 1999. Orlistat 120 mg three-times-daily achieves a placebo-subtracted weight loss of approximately 3% (Table 2) and somewhat less in RCTs of longer duration (35, 36). Low-dose orlistat (60 mg three-times-daily), approved for use without a prescription, achieved a placebo-subtracted weight loss of 1.2 kg (1.6%) after 4 months in the nonprescription NDA study and 2.3 kg (2.4%) at 6 months in 2 prescription NDA studies (37). In December 2003, orlistat was approved for weight management of obese adolescents age 12 years and above, and to date it remains the only antiobesity drug approved for pediatric patients. In a 1-year study of obese adolescents, BMI decreased by 0.55 kg/m2 with orlistat treatment compared with an increase of 0.31 kg/m2 with a placebo; weight increased only 0.5 kg with orlistat but 3.1 kg with a placebo (38).
Table 2.
1-Year weight loss and secondary efficacy of currently available antiobesity drugs.
| Drug | Weight loss relative to placebo | Glycemic measures | Blood pressure | Lipids |
|---|---|---|---|---|
| Orlistat | Approximately 3.0% | +++a | ++ | ++ |
| Lorcaserin | 3.0 to 3.6% | +++ | + | + |
| Liraglutide | 4.0 to 5.4% | ++++ | ++ | ++ |
| Phentermine/Topiramate | 8.6 to 9.3% | +++ | ++ | ++ |
| Naltrexone/Bupropion | 3.3 to 4.8% | ++ | Unfavorable | + |
+ least efficacy; +++++ most efficacy.
When several doses have been studied, data are shown for the most effective dose.
Lorcaserin.
Lorcaserin, a selective serotonin 5-HT2C receptor agonist, was approved in the US in 2012 and became available almost a year later due to a delay in receiving DEA classification for abuse potential. At the recommended dose in humans, lorcaserin has minimal affinity for 5-HT2B receptors, stimulation of which is suspected to increase the risk of valvular heart disease (39) that led to the withdrawal of fenfluramine and dexfenfluramine. On reviewing the lorcaserin NDA in 2010, the FDA noted (40) that the mean weight loss associated with lorcaserin was about 3% greater than the mean weight loss associated with a placebo and therefore did not satisfy the first (5% difference in weight between active drug and a placebo) of 2 efficacy criteria set forth in the FDA guidelines; however, lorcaserin 10 mg twice daily dose satisfied, by a slim margin (47% vs 23%), the second efficacy criterion (the proportion of patients losing ≥5% weight be at least 35% and at least double the proportion of the placebo-treated group). Concerns were also expressed about the increase in mammary tumors in preclinical studies in rats. Following rereview in 2012, the FDA approved lorcaserin with certain postmarketing requirements (41) that included the conduct of a long-term cardiovascular outcomes trial (CVOT), which began in 2014 and completed enrollment of approximately 12 000 patients in December 2015; the results are pending. In the largest of the three phase-3 trials of lorcaserin, patients on lorcaserin had a mean weight loss of 5.8 kg during the first year, but they gained 2.5 kg in the second year, whereas patients who were blindly switched from lorcaserin to a placebo gained a mean 4.8 kg (42). These observations suggest that patients continuing on lorcaserin beyond 1 year regain significant weight and that almost all of the lost weight is regained when the drug is discontinued. FDA recommends discontinuation of lorcaserin (Table 3) after 12 weeks if weight loss of at least 5% is not achieved (41). However, a recently published RCT showed that only 28% of obese patients treated with lorcaserin 10 mg twice daily achieved 5% weight loss after 12 weeks (43), thus raising concern about the utility of this drug in clinical practice because only 1 of 4 patients would be suitable for continuing the drug for longer than 3 months.
Table 3.
Dosing of currently available antiobesity drugs.
| Drug | Trade Name (s) | Recommended Dose | DEA schedule | Comments |
|---|---|---|---|---|
| Phentermine | Adipex-P | 18.75–37.5 mgQDa | IV | Recommended TID dosing is 30–60 min before meals. QD dosing is in the morning. Avoid dosing close to bedtime. |
| Fasti n | 30 mg QD | |||
| Suprenza | 15–30 mg QD | |||
| Lomaira | 8 mgTID | |||
| Diethylpropion | Tenuate | 25 mgTID | IV | As above |
| Tenuate Dospan | 75 mg QD | |||
| Phendimetrazine | Bontril | 35 mgTID | III | As above |
| Prelu-2 | 105 mg QD | |||
| Benzphetamine | Didrex | 25–50 mgTID | III | Recommended TID dosing is 30–60 min before meals. |
| Orlistat | Xenical | 120 mgTID | None | Recommended to take at mealtime. Dosing is same for adults and adolescents. |
| Alii | 60 mgTID | |||
| Phentermine/Topiramate | Qsymia | 7.5/46 mg QD 15/92 mg QD | IV | Start at 3.75/23 mg QD. Increase to 7.5/46 mg after 2 weeks. If weight loss is <3% after 3 months, discontinue or increase dose to 15/92 mg QD. Consider using a transition dosing of 11.25/69 mg while moving to 15/92 mg. Discontinue if weight loss is <5% after 3 months on the 15/92 mg dose. |
| Lorcaserin | Belviq | 10 mg BID | IV | Discontinue if weight loss is <5% after 3 months. |
| Belviq XR | 20 mg QD | |||
| Naltrexone/Bupropion | Contrave | 16/180 mg BID | None | Available as 8/90 mg tablets only. Start 1 tablet every morning. Increase to 1 tablet BID during second week. Increase to 2 in the morning and 1 in the evening during third week. From Week 4, dose 2 tablets BID. |
| Liraglutide 3.0 mg | Saxenda | 3 mg QD | None | Available as a pre-filled, multi-dose injector pen that delivers solution in selected doses. Start 0.6 mg QD. At weekly intervals, increase the dose by 0.6 mg until 3.0 mg QD dose is reached. Inject subcutaneously in the abdomen, thigh or upper arm. Discontinue if weight loss is <4% after 4 months. |
QD, once daily; BID, two times daily; TID, three times daily.
The most frequent adverse effects of lorcaserin (Table 4) are headache, dizziness, fatigue, nausea, dry mouth, and constipation. The dropout rate due to adverse events was low (8.4% vs 6.8% with a placebo) for patients assigned to lorcaserin in phase-3 trials (40). The FDA accepted a relative risk of 1.16 (95% confidence interval, 0.81–1.67) for valvular heart disease from exposure to lorcaserin over 2 years (44). Because obesity and depression are both common presentations in clinical practice, it would be ideal that a drug prescribed to induce weight loss is compatible with commonly used antidepressants. However, because the safety of lorcaserin coadministered with selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, and other drugs that affect serotonergic system has not been established or systematically evaluated, FDA recommends caution when using lorcaserin in patients taking these drugs (45). During review of lorcaserin, FDA advisory committee members expressed a concern that given the minimal efficacy of lorcaserin, physicians might combine it with phentermine (46). A 12-week study (43) that compared lorcaserin alone and in combination with phentermine 15 mg/day or 30 mg/day reported that twice as many patients dropped out due to adverse events in the group that received lorcaserin and phentermine 30 mg/day. Mean weight loss with lorcaserin was 3.3% with only 28% patients achieving a weight loss of ≥5%. The study did not have placebo- or phentermine-only arms.
Table 4.
Most frequent adverse events and limitations of use of currently available antiobesity drugs.
| Drug | Adverse events | Limitations of use and precautions |
|---|---|---|
| Phentermine | Dry mouth, constipation, insomnia | Contraindicated in patients with advanced cardiovascular disease, moderate to severe hypertension, hyperthyroidism, glaucoma, and agitate states. Small increases in heart rate and blood pressure may be observed. Mild to moderate abuse potential. |
| Diethyl prop ion | ||
| Phendimetrazine | ||
| Benzphetamine | ||
| Orlistat | Fecal urgency, fecal incontinence, flatus with discharge, oily spotting | Contraindicated in chronic malabsorption syndrome, and cholestasis. Must take a multivitamin supplement containing fat-soluble vitamins. Rare cases of liver injury. |
| Lorcaserin | Headache, dizziness, fatigue, nausea, dry mouth, constipation, cough, hypoglycemia in patients with diabetes | Safety of coadministration with antidepressants has not been established. Monitor for symptoms of toxicity related to serotonin excess. Potential for serotonin syndrome, a rare but serious condition. Monitor for signs and symptoms of valvular heart disease. |
| Liraglutide 3.0 mg | Nausea, vomiting, diarrhea, constipation, dyspepsia, abdominal pain, headache, fatigue, hypoglycemia, increased lipase | Contraindicated in patients with personal or family history of medullary thyroid carcinoma or Multiple Endocrine Neoplasia syndrome type 2. Should not be used with insulin. Do not use with other GLP-1 agonists. Causes thyroid C-cell tumors in rats and mice. Discontinue if pancreatitis is suspected. |
| Phentermine/Topiramate | Paresthesia, dizziness, insomnia, dysguesia, constipation, dry mouth | Contraindicated in patients with glaucoma, hyperthyroidism, and during and within 2 weeks of taking monoamine oxidase inhibitors. |
| Available under a Risk Evaluation and Mitigation Strategy (REMS) that requires negative pregnancy test before treatment and monthly thereafter to reduce the risk of teratogenicity. Small increase in heart rate. Monitor electrolytes to detect metabolic acidosis and elevated creatinine. Monitor closely for depression, anxiety, and memory problems. | ||
| Naltrexone/Bupropion | Nausea, vomiting, headache, dizziness, insomnia, dry mouth, diarrhea | Contraindicated in patients with uncontrolled hypertension, chronic opioid use, seizure disorders, anorexia nervosa or bulimia, during withdrawal from alcohol, barbiturates, benzodiazepines, and antiepileptic drugs. Should not use with other bupropion-containing products and during or within 2 weeks of taking monoamine oxidase inhibitors. Monitor for suicidal ideation and behavior. Monitor for increases in heart rate and blood pressure. Rare cases of hepatotoxicity. |
All antiobesity drugs are contraindicated in pregnancy.
Liraglutide 3.0 mg.
Liraglutide is an injectable glucagon-like peptide-1 agonist initially approved in 2010 for treatment of T2D at the dose of 1.8 mg daily. Data suggest that liraglutide decreases appetite and enhances satiety via its effects on the CNS (47). These findings led to the development of liraglutide for treatment of obesity. A 20-week phase-2B trial in patients with obesity demonstrated that liraglutide treatment led to a dose-dependent weight loss of −4.8 kg, −5.5 kg, −6.3 kg, and −7.2 kg with 1.2 mg, 1.8 mg, 2.4 mg, and 3.0 mg doses, respectively, compared with −2.8 kg with a placebo and −4.1 kg with orlistat (48). The liraglutide NDA for obesity included three phase-3 trials of 1 year or more that primarily examined the efficacy of liraglutide 3.0 mg/day. One of these trials randomized 3731 overweight/obese patients in a 2:1 ratio to receive daily liraglutide 3.0 mg or placebo for 1 year (49). The study excluded patients with diabetes but enrolled those with prediabetes who were followed for an additional 2 years for a total of 3 years to examine whether liraglutide treatment could reduce the risk of developing T2D. The mean weight change with liraglutide was −8.0% vs −2.6% with a placebo at 1 year. For patients with prediabetes at baseline, liraglutide treatment was associated with a mean weight change of −6.1% vs −1.9% with a placebo (50). The time to onset of T2D over 3 years was estimated to be 2.7 times longer with liraglutide than with a placebo (95% CI 1.9–3.9). In a separate 1-year RCT, weight changes of −6.0% with liraglutide 3.0 mg, −4.7% with liraglutide 1.8 mg, and −2.0% with a placebo were reported among overweight/obese patients with T2D, confirming that the higher dose is more effective for weight loss (51). In another RCT, overweight/obese patients without diabetes were randomized to liraglutide 3.0 mg or a placebo to examine weight maintenance after they had achieved an average 6.0% weight loss with a low-calorie diet (52). During the maintenance phase, mean weight changes were −6.2% for liraglutide vs −0.2% for a placebo. Although liraglutide is associated with an increase in resting heart rate of 2–3 bpm compared to a placebo, a long-term CVOT of 9340 patients with T2D revealed that those assigned to liraglutide 1.8 mg had a lower MACE incidence compared to a placebo group after a median follow-up of 3.8 years (53).
Nausea, vomiting, and diarrhea are common adverse effects of liraglutide, especially nausea, which has an incidence of almost 40%. Liraglutide caused thyroid C-cell tumors in rats and mice. It is contraindicated in patients with a personal or family history of medullary thyroid cancer or in patients with type 2 Multiple Endocrine Neoplasia Syndrome.
Phentermine/Topiramate.
As discussed above, phentermine has been in use for nearly 6 decades in the US. The combination of phentermine and topiramate has been well investigated for treatment of obesity, and it received FDA approval in 2012. Topiramate, an unusual drug with several pharmacological actions, including blockade of sodium and L-type calcium channels, antagonism of glutamate α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/kainate receptors, facilitation of GABA-mediated chloride fluxes, and inhibition of carbonic anhydrase activity, is approved for treatment of epilepsy and migraine prophylaxis. Weight loss has been a frequent finding among patients treated with topiramate, although it is not precisely known which mechanism of topiramate contributes to its effects on weight (54). A 2011 metaanalysis of 10 RCTs of 16–60 weeks duration revealed that topiramate treatment led to robust weight loss, relative to a placebo, of 5.3 kg in overweight/obese adults (55). In addition to weight loss, topiramate treatment led to a reduction in blood pressure and significant improvement of glycemia in studies of overweight/obese patients with T2D and hypertension (55). Although topiramate demonstrated consistent and clinically meaningful benefits for obese patients, the risk associated with it at the doses that resulted in weight loss, especially cognitive and psychiatric adverse effects, hindered further development of this drug for weight management. Therefore, efforts focused on combining low-dose topiramate with a low dose of another weight loss drug. In this context, phentermine appeared to be suitable candidate to combine with topiramate due to their varied pharmacological mechanisms and dissimilar adverse effect profiles.
Gadde et al. (56) examined the efficacy and safety of combining topiramate 100 mg/day with phentermine 15 mg/day in comparison with individual components in a 24-week RCT in 200 obese adults. Generic phentermine was administered in the morning and topiramate was given in the evening. Combination therapy led to robust weight loss (−10.7%) that was significantly superior to the weight loss achieved by each of the other 3 comparisons (placebo −2.1%, phentermine −4.6%, topiramate −6.3%). Subsequently, a once-daily formulation was tested in a 28-week RCT in which 2 doses of combination therapy (phentermine/topiramate 7.5/46 mg or 15/92 mg) were compared against individual components (topiramate 46 mg or 92 mg; or, phentermine 7.5 mg or 15 mg) and a placebo (33). Weight changes (intention-to-treat analysis) were −1.7% for a placebo, −5.5% for phentermine 7.5 mg, −6.1% for phentermine 15 mg, −5.1% for topiramate 46 mg, −6.4% for topiramate 92 mg, −8.5% for phentermine/topiramate 7.5/46 mg, and −9.2% for phentermine/topiramate 15/92 mg. Although both doses of combination therapy led to robust weight loss, the 7.5/46 mg dose had better tolerability than the 15/92 mg dose. Unfortunately, the addition of phentermine did not lower the incidence of neuropsychiatric adverse events as hoped.
The long-term safety and efficacy of phentermine/topiramate was examined in 2 large phase-3 trials, one that enrolled patients with moderate to severe obesity (57) and another that enrolled overweight/obese patients with weight-related comorbidities (58). Both RCTs demonstrated robust efficacy with phentermine/topiramate with placebo-subtracted weight losses of −6.6% for the 7.5/46 mg and −8.6% to −9.3% for the 15/92 mg dose. In addition to weight loss, there were clinically significant improvements in blood pressure, triglycerides, and glycemic measures with phentermine/topiramate treatment.
The most frequent adverse effects of phentermine/topiramate in phase-3 trials were paresthesia, dry mouth, constipation, insomnia, dizziness, and dysguesia (59). Cognitive and psychiatric adverse events occurred 2–3 times more frequently with phentermine/topiramate relative to a placebo, leading to twice (18% vs 9%) as many patients dropping out due to tolerability issues. Topiramate increases the risk of oral clefts among babies born to mothers who were exposed to topiramate during the first trimester of pregnancy (60); therefore, to minimize the risk, a negative pregnancy test is required before starting phentermine/topiramate and monthly thereafter (61). At the time of approval in 2012, based on the observation of a slight increase in heart rate, especially in the first two months of treatment with phentermine/topiramate, the FDA required (62) that a postmarketing CVOT be conducted and completed by 2017; a review of clinical trials registry in June 2017 revealed that such a study has not been conducted.
Naltrexone/Bupropion.
Bupropion, a marketed antidepressant that is also approved for smoking cessation, has been shown to promote clinically significant weight loss in 3 RCTs in obese patients (63). Naltrexone, an opioid-receptor antagonist, decreases food intake in animals and decreases food cravings in obese humans, with weight loss reported for female subjects (64, 65). A 16-week proof-of-concept trial (66) revealed no difference in weight loss achieved with bupropion alone (−3.6%) vs a naltrexone/bupropion combination (−4.0%). In a subsequent RCT of 24-weeks duration, in which study dropout rates were extremely high (63% withdrew early in one of the groups), additional weight loss was shown with bupropion combined with 2/3 doses of naltrexone (67).
In phase-3 RCTs, bupropion 360 mg/day combined with naltrexone 32 mg/day or 16 mg/day achieved placebo-subtracted weight losses of 4.2% and 3.7%, respectively (68). However, FDA reviewers expressed concern about heart rate and systolic and diastolic blood pressure increases relative to a placebo, which were 2.4 bpm, 2.4 mmHg, and 2.0 mmHg, respectively, in the first 8 weeks. Heart rate and blood pressure remained somewhat increased even at 1 year. Further concern was that naltrexone/bupropion treatment was associated with mean daytime systolic blood pressure and diastolic blood pressure increases of 3.3 mmHg and 3.1 mmHg, respectively, relative to a placebo (68). The FDA required a preapproval CVOT but granted approval for naltrexone/bupropion in 2014 following review of interim data from that trial. However, after the sponsor made an inappropriate public release of confidential interim data, the study’s steering committee recommended its termination (69). End-of-study data showed there was no difference in MACE incidence between patients assigned to naltrexone/bupropion or a placebo (2.8% and 2.7% events). Notably, the authors of the study expressed concern that only 17.3% of placebo patients and 27% of naltrexone/bupropion patients were still taking the study drug at 2 years. Study discontinuation rates attributed to adverse events were significantly higher among patients assigned to naltrexone/bupropion vs a placebo (28.1% vs 8.7%).
Naltrexone/bupropion is associated with a high incidence of gastrointestinal (nausea, vomiting, constipation, dry mouth) adverse effects in addition to headache, dizziness, insomnia, and anxiety. Naltrexone/bupropion is contraindicated in patients with uncontrolled hypertension, seizure disorders, and anorexia nervosa or bulimia, and it should be avoided in patients receiving chronic treatment with opioids and in those abruptly stopping alcohol, benzodiazepines, barbiturates, or antiepileptic drugs.
ISSUES OF INTEREST TO CLINICIANS WHEN USING ANTIOBESITY DRUGS
What factors predict weight loss with antiobesity drugs?
Weight loss in the first 3–6 months appears to be the best predictor of weight loss after 1 year (70–73). Although we do not have substantial information from research studies to guide us with regard to treatment selection, there are a few factors clinicians could consider based on the pharmacological actions of the various drugs. For example, for patients who report intense food cravings, phentermine/topiramate and naltrexone/bupropion may be good options based on published studies of topiramate and naltrexone (64, 74), lorcaserin for patients who struggle with impulse control (75), phentermine/topiramate for eating disorders (76), naltrexone/bupropion for patients who struggle with addiction (77) and/or depression, liraglutide or lorcaserin to induce weight loss in overweight/obese patients with prediabetes or T2D, and orlistat for obese individuals who need to reduce their fat intake.
How effective are antiobesity drugs beyond 1 year?
As is the case with all nonsurgical interventions for treating obesity, some weight is regained when antiobesity drugs are continued beyond 1 year (42, 50, 69, 70, 78), resulting in a net placebo-subtracted mean weight loss of 2%–4% after 2–4 years, with liraglutide demonstrating the most efficacy and lorcaserin and naltrexone/bupropion being less effective.
What happens when an antiobesity drug is stopped?
Similar to the observations made with lifestyle interventions, lost weight is regained when drug therapy for weight management is stopped, although there is some net weight loss when followed for few months (42, 79, 80). Thus, for patients who are tolerating the drug well and continuing to benefit, further continuation of the drug therapy should be considered.
Conclusions
Currently, marketed drug therapies for chronic weight management achieve 3% to 9% weight loss, relative to a placebo, after 1 year among patients with obesity when all participants are provided some level of lifestyle counseling. Weight loss achieved with lorcaserin, orlistat, and naltrexone/bupropion is at the lower end of the range and phentermine/topiramate at the upper end, with liraglutide falling in between. Despite much enthusiasm for the newer drugs at the time of approval, only a small proportion of eligible patients are using them due to their high cost. Thus, it is not surprising that phentermine, which has been available in the US for well over 5 decades, remains the most prescribed drug for weight management, although it has never been studied in a randomized controlled trial of at least 1 year. Weight loss achieved with drug therapy generally seems to improve glycemic control, but improvements of blood pressure and lipids are smaller and inconsistent.
Acknowledgment:
The authors thank Katelyn Daigle for editorial assistance in preparing and revising this manuscript.
Footnotes
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:
Employment or Leadership: None declared.
Consultant or Advisory Role: K.M. Gadde, AstraZeneca for a diabetes study with all payments made to his academic institution.
Stock Ownership: None declared.
Honoraria: None declared.
Research Funding: K.M. Gadde, NIH, AstraZeneca; J.W. Apolzan, NIH, USDA; H.-R. Berthoud, NIH.
Expert Testimony: None declared.
Patents: K.M. Gadde, awarded several patents related to obesity and body weight. The inventions disclosed in Gadde’s patents have not been discussed in this manuscript.
Nonstandard abbreviations: T2D, type 2 diabetes; CNS, central nervous system; RCT, randomized controlled trial; FDA, Food and Drug Administration; MACE, major adverse cardiovascular events; NDA, new drug application; CVOT, cardiovascular outcomes trial.
References
- 1.Bray GA, Frühbeck G, Ryan DH, Wilding JPH. Management of obesity. Lancet 2016;387:1947–56. [DOI] [PubMed] [Google Scholar]
- 2.Blackburn GL. Benefits of weight loss in the treatment of obesity. Am J Clin Nutr 1999;69:347–9. [DOI] [PubMed] [Google Scholar]
- 3.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol 2014;63:2895–3023. [DOI] [PubMed] [Google Scholar]
- 4.Nicklas JM, Huskey KW, Davis RB, Wee CC. Successful weight loss among obese U.S. adults. Am J Prev Med 2012;42:481–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xia Y, Kelton CML, Guo JJ, Bian B, Heaton PC. Treatment ofobesity: pharmacotherapy trends in the United States from 1999 to 2010. Obesity 2015;23:1721–8. [DOI] [PubMed] [Google Scholar]
- 6.Thomas CE, Mauer EA, Shukla AP, Rathi S, Aronne LJ. Low adoption of weight loss medications: a comparison of prescribing patterns of antiobesity pharmacotherapies and SGLT2s. Obesity 2016;24:1955–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hampp C, Kang EM, Borders-Hemphill V. Use of prescription antiobesity drugs in the United States. Pharmacotherapy 2013;33:1299–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stafford RS, Radley DC. National trends in antiobesity medication use. Arch Intern Med 2003;163:1046–50. [DOI] [PubMed] [Google Scholar]
- 9.Gadde KM. Current pharmacotherapy for obesity: extrapolation of clinical trials data to practice. Expert Opin Pharmacother 2014;15:809–22. [DOI] [PubMed] [Google Scholar]
- 10.Berthoud HR, Münzberg H, Morrison CD. Blaming the brain for obesity: integration of hedonic and homeostatic mechanisms. Gastroenterology 2017;15: 1728–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tschop MH, Finan B, Clemmensen C, Gelfanov V, Perez-Tilve D, Muller TD, DiMarchi RD. Unimolecular polypharmacy for treatment of diabetes and obesity. Cell Metab 2016;24:51–62. [DOI] [PubMed] [Google Scholar]
- 12.Fothergill E, Guo J, Howard L, Kerns JC, Knuth ND, Brychta R, et al. Persistent metabolic adaptation 6 years after “the biggest loser” competition. Obesity; 2016; 24:1612–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hao Z, Mumphrey MB, Townsend RL, Morrison CD, Münzberg H, Ye J, Berthoud HR. Reprogramming of defended body weight after roux-en-y gastric bypass surgery in diet-induced obese mice. Obesity 2016;24: 654–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sternson SM, Eiselt AK. Three pillars for the neural control of appetite. Annu Rev Physiol 2017;79:401–23. [DOI] [PubMed] [Google Scholar]
- 15.Campbell JN, Macosko EZ, Fenselau H, Pers TH, Lyu-betskaya A, Tenen D, et al. A molecular census of arcuate hypothalamus and median eminence cell types. Nat Neurosci 2017;20:484–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burke LK, Heisler LK. 5-hydroxytryptamine medications for the treatment of obesity. J Neuroendocrinol 2015; 27:389–98. [DOI] [PubMed] [Google Scholar]
- 17.Valencia-Torres L, Olarte-Sanchez CM, Lyons DJ, Georgescu T,Greenwald-Yarnell M,Myers MG Jr.,et al. Activation of ventral tegmental area 5-ht2c receptors reduces incentive motivation. Neuropsychopharmacology 2017;42:1511–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sclafani A, Springer D. Dietary obesity in adult rats: similarities to hypothalamic and human obesity syndromes. Physiol Behav 1976;17:461–71. [DOI] [PubMed] [Google Scholar]
- 19.Thaler JP, Guyenet SJ, Dorfman MD, Wisse BE, Schwartz MW. Hypothalamic inflammation: marker or mechanism of obesity pathogenesis? Diabetes 2013; 62:2629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berridge KC, Ho CY, Richard JM, Difeliceantonio AG. The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Res 2010;1350: 43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hargrave SL, Jones S, Davidson TL. The outward spiral:a vicious cycle model of obesity and cognitive dysfunction. Curr Opin Behav Sci 2016;9:40–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang J, Matheson BE, Kaye WH, Boutelle KN. Neurocognitive correlates of obesity and obesity-related behaviors in children and adolescents. Int J Obes 2014; 38:494–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson E, Kersbergen I, Higgs S. Eating ‘attentively’ reduces later energy consumption in overweight and obese females. Br J Nutr 2014;112:657–61. [DOI] [PubMed] [Google Scholar]
- 24.Horstmann A, Dietrich A, Mathar D, Possel M, Villringer A, Neumann J. Slave to habit? Obesity is associated with decreased behavioural sensitivity to reward devaluation. Appetite 2015;87:175–83. [DOI] [PubMed] [Google Scholar]
- 25.Knowlton BJ, Patterson TK. Habit formation and the striatum. [Epub ahead of print] Curr Top Behav Neurosci September 28, 2016. as doi: 10.1007/7854_2016_451. [DOI] [PubMed] [Google Scholar]
- 26.Haddock CK, Poston WSC, Dill PL, Foreyt JP, Ericsson M. Pharmacotherapy for obesity: a quantitative analysis of four decades of published randomized clinical trials. Int J Obes 2002;26:262–73. [DOI] [PubMed] [Google Scholar]
- 27.James WP, Caterson ID, Coutinho W, Finer N, Van Gaal LF, Maggioni AP, et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med 2010;363:905–15. [DOI] [PubMed] [Google Scholar]
- 28.Gadde KM, Allison DB. Cannabinoid-1 receptor antagonist, rimonabant, for management of obesity and related risks. Circulation 2006;114:974–84. [DOI] [PubMed] [Google Scholar]
- 29.Alexander M, Rothman RB, Baumann MH, Endres CJ, Brasić JR, Wong DF. Noradrenergic and dopaminergic effects of (+)-amphetamine-like stimulants in the baboon Papio Anubis. Synapse 2005;56:94–9. [DOI] [PubMed] [Google Scholar]
- 30.Munro JF, MacCuish AC, Wilson EM, Duncan LJ. Comparison of continuous and intermittent anorectic therapy in obesity. BMJ 1968;1:352–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim KK, Cho HJ, Kang HC, Youn BB, Lee KR. Effects on weight reduction and safety of short-term phentermine administration in Korean obese people. Yonsei Med J 2006;47:614–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang JG, Park CY, Kang JH, Park YW, Park SW. Randomized controlled trial to investigate the effects of a newly developed formulation of phentermine diffuse-controlled release for obesity. Diabetes Obes Metab 2010;12:876–82. [DOI] [PubMed] [Google Scholar]
- 33.Aronne LJ, Wadden TA, Peterson C, Winslow D, Odeh S, Gadde KM. Evaluation of phentermine and topiramate versus phentermine/topiramate extended-release in obese adults. Obesity 2013;21:2163–71. [DOI] [PubMed] [Google Scholar]
- 34.Jick H, Vasilakis C, Weinrauch LA, Meier CR, Jick SS, Derby LE. A population-based study of appetite-suppressant drugs and the risk of cardiac-valve regurgitation. N Engl J Med 1998;339:719–24. [DOI] [PubMed] [Google Scholar]
- 35.Rucker D, Padwal R, Li SK, Curioni C, Lau DCW. Long term pharmacotherapy for obesity and overweight: updated meta-analysis. BMJ 2007;335:1194–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leblanc ES, O’Connor E, Whitlock EP, Patnode CD, Kapka T. Effectiveness of primary care-relevant treatments for obesity in adults: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med 2011;155:434–47. [DOI] [PubMed] [Google Scholar]
- 37.Food and Drug Administration. Orlistat nonprescription briefing document: NDA 21–887. Endocrine and metabolic drugs advisory committee meeting, January 23, 2006. http://www.fda.gov/ohrms/dockets/ac/06/briefing/2006-4201B1_02_03-FDA-Clinical-Review.pdf (Accessed June 2017). [Google Scholar]
- 38.Chanoine JP, Hampl S, Jensen C, Boldrin MS, Hauptman J. Effect of orlistat on weight and body composition in obese adolescents. JAMA 2005;293:2873–83. [DOI] [PubMed] [Google Scholar]
- 39.Rothman RB, Baumann MH, Savage JE, Rauser L, McBride A, Hufeisen SJ, Roth BL. Evidence for possible involvement of 5-HT (2B) receptors in the cardiac valvulopathy associated with fenfluramine and other serotonertic medications. Circulation 2000;102:2836–41. [DOI] [PubMed] [Google Scholar]
- 40.Food and Drug Administration. FDA briefing document, NDA 22529. Advisory committee meeting for lorcaserin. Endocrinologic & Metabolic Drugs Advisory Committee, 16 September 2010. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM225631.pdf (Accessed June 2017). [Google Scholar]
- 41.Food and Drug Administration: FDA news release. FDA approves Belviq to treat some overweight or obese adults. June 27, 2012. https://wayback.archive-it.org/7993/20170112023940/, http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm309993.htm (Accessed June 2017). [PubMed]
- 42.Smith SR, Weissman NJ, Anderson CM, Sanchez M, Chuang E, Stubbe S, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med 2010;363:245–56. [DOI] [PubMed] [Google Scholar]
- 43.Smith SR, Garvey WT, Greenway FL, Zhou S, Fain R, Pilson R, et al. Coadministration of lorcaserin and phentermine for weight management: a 12-week, randomized, pilot safety study. Obesity 2017;25:857–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Colman E, Golden J, Roberts M, Egan A, Weaver J, Rose-braugh C. The FDA’s assessment of two drugs for chronic weight management. N Engl J Med 2012;367: 1577–8. [DOI] [PubMed] [Google Scholar]
- 45.Prescribing information for Belviq® (lorcaserin HCl) for oral use, CIV. Arena Pharmaceuticals, Zofingen, Switzerland, and Eisai, Inc., Woodcliff Lake, NJ, May 2017. [Google Scholar]
- 46.Food and Drug Administration. Endocrinologic and Metabolic Drugs Advisory Committee meeting, May 10, 2012, Transcript. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM309522.pdf (Accessed June 2017). [Google Scholar]
- 47.Sisley S,Gutierrez-Aguilar R,Scott M,D’Alessio DA,Sandoval DA, Seeley RJ Neuronal GLP1R mediates liraglutide’s anorectic but not glucose-lowering effect. J Clin Invest 2014;124:2456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Astrup A, Rössner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet 2009;374:1606–16. [DOI] [PubMed] [Google Scholar]
- 49.Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med 2015;373:11–22. [DOI] [PubMed] [Google Scholar]
- 50.leRoux CW, Astrup A, Fujioka K, Greenway FL, Lau DCW, Van Gaal L, et al. 3 years of liraglutide versus placebo for type2diabetesriskreductionandweightmanagement in individuals with prediabetes: a randomised, double-blind trial. Lancet 2017;389:1399–1409. [DOI] [PubMed] [Google Scholar]
- 51.Davies MJ, Bergenstal R, Bode B, Kushner RF, Lewin A, Skøth TV, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE Diabetes randomized clinical trial. JAMA 2015;314:687–99. [DOI] [PubMed] [Google Scholar]
- 52.Wadden TA, Hollander P, Klein S, Niswender K, Woo V, Hale PM, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet induced weight loss: The SCALE Maintenance randomized study. Int J Obes 2013;37:1443–51. [DOI] [PubMed] [Google Scholar]
- 53.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375:311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Verrotti A, Scaparrotta A, Agostinelli S, Di Pillo S, Chiarelli F, Grosso S. Topiramate-induced weight loss: a review. Epilepsy Res 2011;95:189–99. [DOI] [PubMed] [Google Scholar]
- 55.Kramer CK, Leitão CB, Pinto LC, Canani LH, Azevedo MJ, Gross JL. Efficacy and safety of topiramate on weight loss: a meta-analysis of randomized controlled trials. Obes Rev 2011;12:e338–47. [DOI] [PubMed] [Google Scholar]
- 56.Gadde KM, Yonish GM, Foust MS, Tam PY, Najarian T. A 24-week randomized controlled trial of VI-0521, a combination weight loss therapy, in obese adults. Obes Res 2006;14(9 Suppl):A17. [Google Scholar]
- 57.Allison DB, Gadde KM, Garvey WT, Peterson CA, Schwiers ML, Najarian T, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity 2012;20: 330–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gadde KM, Allison DB, Ryan DH, Peterson CA, Troupin B, Schwiers ML, Day WW. Effects of low-dose, controlled-release phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet 2011;377:1341–52. [DOI] [PubMed] [Google Scholar]
- 59.Food and Drug Administration. Advisory Committee Meeting for Phentermine/Topiramate (Qnexa),July15, 2010. Division of Metabolism and Endocrinology Products (DMEP), Office of Drug Evaluation II, Center for Drug Evaluation and Research, Silver Spring, MD: http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/ucm218819.htm (Accessed June 2017). [Google Scholar]
- 60.Alsaad AM, Chaudhry SA, Koren G. First trimester exposure to topiramate and the risk of oral clefts in the offspring: a systematic review and meta-analysis. Reprod Toxicol 2015;53:45–50. [DOI] [PubMed] [Google Scholar]
- 61.Vivus, Inc. NDA 22580. Qsymia (phentermine and topiramate extended-release) Capsules: risk evaluation and mitigation strategy (REMS). Initial REMS approval, 07/2012; most recent modification, 09/2014. http://www.fda.gov/downloads/Drugs/DrugSafety/postmarketdrugsafetyinformationforpatientsandproviders/UCM312598.pdf (Accessed June 2017).
- 62.Food and Drug Administration. Center for Drug Evaluation and Research. Application number 22580Orig1s000, approval letter, July 17, 2012. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/022580Orig1s000Approv.pdf (Accessed June 2017).
- 63.Gadde KM, Xiong GL. Bupropion for weight reduction. Expert Rev Neurother 2007;7:17–24. [DOI] [PubMed] [Google Scholar]
- 64.Sternbach HA, Annitto W, Pottash AL, Gold MS. Anorexic effects of naltrexone in man. Lancet 1982;1: 388–9. [DOI] [PubMed] [Google Scholar]
- 65.Atkinson RL, Berke LK, Drake CR, Bibbs ML, Williams FL, Kaiser DL. Effects of long-term therapy with naltrexone on body weight in obesity. Clin Pharmacol Ther 1985; 38:419–22. [DOI] [PubMed] [Google Scholar]
- 66.Greenway FL, Whitehouse MJ, Guttadauria M, Anderson JW, Atkinson RL, Fujioka K, et al. Rational design of a combination medication for the treatment of obesity. Obesity 2008;17:30–9. [DOI] [PubMed] [Google Scholar]
- 67.Greenway FL, Dunayevich E, Tollefson G, Erickson J, Guttadauria M, Fujioka K, et al. Comparison of combined bupropion and naltrexone therapy for obesity with monotherapy and placebo. J Endocrinol Metab 2009;94:4898–906. [DOI] [PubMed] [Google Scholar]
- 68.Food and Drug Administration. FDA briefing document, NDA 200063 Advisory committee meeting for naltrexone/bupropion (Contrave), Endocrinologic & Metabolic Drugs Advisory Committee, 7 December 2010. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM235671.pdf (Accessed June 2017). [Google Scholar]
- 69.Nissen SE, Wolski KE, Prcela L, Wadden T, Buse JB, Bakris G, et al. Effect of naltrexone-bupropion on major adverse cardiovascular events in overweight and obese patients with cardiovascular risk factors: a randomized clinical trial. JAMA 2016;315:990–1004. [DOI] [PubMed] [Google Scholar]
- 70.Toplak H, Ziegler O, Keller U, Hamann A, Godin C, Wittert G, et al. X-PERT: weight reduction with orlistat in obese subjects receiving a mildly or moderately reduced-energy diet. Early response to treatment predicts weight maintenance. Diabetes Obes Metab 2006; 7:699–708. [DOI] [PubMed] [Google Scholar]
- 71.Finer N, Ryan DH, Renz CL, Hewkin AC. Prediction of response to sibutramine therapy in obese non-diabetic and diabetic patients. Diabetes Obes Metab 2006;8: 206–13. [DOI] [PubMed] [Google Scholar]
- 72.Smith SR, O’Neil PM, Astrup A, Finer N, Sanchez-Kam M, Fraher K, et al. Early weight loss while on lorcaserin, diet and exercise as a predictor of week 52 weight-loss outcomes. Obesity 2014;22:2137–46. [DOI] [PubMed] [Google Scholar]
- 73.Fujioka K, O’Neil PM, Davies M, Greenway FL, Lau DCW, Claudius B, et al. Early weight loss with liraglutide 3.0 mg predicts 1-year weight loss and is associated with improvements in clinical markers. Obesity 2016;24: 2278–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Butsch WS. Obesity medications: what does the future look like? Curr Opin Endocrinol Diabetes Obes 2015; 22:360–6. [DOI] [PubMed] [Google Scholar]
- 75.Higgins GA, Silenieks LB, Rossmann A, Rizos Z, Noble K, Soko AD, Fletcher PJ. The 5-HT2C receptor agonist lorcaserin reduces nicotine self-administration, discrimination, and reinstatement: relationship to feeding behavior and impulse control. Neuropsychopharmacology 2012;37: 1177–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arbaizar B, Gomez-Acebo I, Llorca J. Efficacy of topiramate in bulimia nervosa and binge-eating disorder: a systematic review. Gen Hosp Psychiatry 2008;30: 471–5. [DOI] [PubMed] [Google Scholar]
- 77.Yip SW, Potenza MN. Treatment of gambling disorders. Curr Treat Options Psychiatry 2014;1:189–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Torgerson JS, Hauptman J, Boldrin MN, Sjöström L. XENical in the Prevention of Diabetes in Obese Subjects (XENDOS) Study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004;27: 155–61. [DOI] [PubMed] [Google Scholar]
- 79.Bray GA, Blackburn GL, Ferguson JM, Greenway FL, Jain AK, Mendel CM, et al. Sibutramine produces dose-related weight loss. Obes Res 1999;7:189–98. [DOI] [PubMed] [Google Scholar]
- 80.Shin JH, Gadde KM, Østbye T, Bray GA. Weight changes in obese adults 6-months after discontinuation of double-blind zonisamide or placebo treatment. Diabetes Obes Metab 2014;16:766–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


