Abstract
Objective
To estimate the effect of locally tailored clinical guidelines on intrapartum care and perinatal outcomes among women with severe hypertensive disorders in pregnancy (sHDP).
Methods
A pre–post study at Zanzibar's low‐resource Mnazi Mmoja Hospital was conducted. All labouring women with sHDP were included at baseline (October 2014 to January 2015) and at 9–12 months after implementation of the ongoing intervention (October 2015 to January 2016). Background characteristics, clinical practice, and delivery outcomes were assessed by criterion‐based case file reviews.
Results
Overall, 188 of 2761 (6.8%) women had sHDP at baseline, and 196 of 2398 (8.2%) did so during the intervention months. The median time between last blood pressure recording and delivery decreased during the intervention compared with baseline (P=0.015). Among women with severe hypertension, antihypertensive treatment increased during the intervention compared with baseline (relative risk [RR] 1.37, 95% confidence interval [CI] 1.14–1.66). Among the neonates delivered (birthweight ≥1000 g), stillbirths decreased (RR 0.56, 95% CI 0.35–0.90) and Apgar scores of seven or more increased during the intervention compared with baseline (RR 1.17, 95% CI 1.03–1.33).
Conclusion
Although health system strengthening remains crucial, locally tailored clinical guidelines seemed to help work‐overloaded birth attendants at a low‐resource hospital to improve care for women with sHDP.
ClinicalTrials.org
Keywords: Birth asphyxia, Guidelines, Labor, PartoMa, Pre‐eclampsia, Severe hypertensive disorders, Stillbirth, Tanzania
Short abstract
Among women with severe hypertensive disorders at Zanzibar's referral hospital, locally tailored intrapartum guidelines were associated with care improvements and 44% risk reduction of stillbirth.
1. INTRODUCTION
Hypertensive disorders in pregnancy (HDP) cause one‐tenth of maternal deaths in Africa.1, 2 A previous facility‐based survey in low‐ and middle‐income countries found that the likelihood of maternal near‐miss was eightfold and perinatal death threefold higher in a group of women with pre‐eclampsia than in a reference group without HDP.2 Although most cases are preventable, undiagnosed or suboptimally treated HDP is repeatedly reported in sub‐Saharan Africa.3, 4, 5, 6, 7, 8, 9, 10 This violates human rights and is a key priority for safe motherhood.11, 12
Hypertensive disorders in pregnancy can rapidly progress in severity, with increased risk of organ failure such as cerebral hemorrhage, coagulopathy, eclampsia, and pulmonary edema. Lifesaving treatment includes induction and/or oxytocin augmentation of labor while stabilizing blood pressure (BP) and supporting vital organ functions.13, 14 In high‐volume low‐resource hospitals, urgent intrapartum diagnosis, surveillance, and management of such complex diseases are challenging and need realistic practical guidance.3, 5, 6, 7, 8, 9, 15, 16
In Tanzania, however, only one‐third of maternity units have emergency obstetric care guidelines available, and only one‐quarter have at least one birth attendant with adequate training in applying these.17 Even if available, guidelines are often not tailored to low‐resource settings.9, 15, 16, 18 Unachievable international guidelines limit implementation and may demoralize staff. Clinical guidelines tailored and disseminated to facilitate effective application in low‐resource settings are required.15, 17, 18
Zanzibar is a low‐income, semiautonomous archipelago of the United Republic of Tanzania, with a facility delivery rate of 55%. In its tertiary Mnazi Mmoja Hospital, approximately 12 000 births are attended to annually. There were 59 stillbirths per 1000 births in 2014–2015, and 52 neonates per 1000 live births had an Apgar score of 1–5. Approximately half of the stillborn fetuses were alive on admission, and the quality of intrapartum care was widely unacceptable.3 This prompted, in collaboration with doctors and nurse‐midwives, the development of locally achievable clinical guidelines for common intrapartum care. The resulting eight‐page PartoMa pocket booklet has subsequently been implemented among doctors and nurse‐midwives by quarterly seminars.18 In particular, doctors have reported finding the booklet's page on essential intrapartum management of HDP useful.18 The aim of the present study was therefore to estimate the effects of the PartoMa intervention of pocket guides and quarterly low‐dose training on care and delivery outcomes among women with severe HDP (sHDP).
2. MATERIALS AND METHODS
In a retrospective pre–post study, case files were reviewed for all women with sHDP (defined as systolic BP 160 mm Hg or higher and/or diastolic BP 110 mm Hg or higher and/or eclampsia pre‐delivery) who attended Mnazi Mmoja Hospital before the PartoMa intervention (October 1, 2014, to January 31, 2015) and 9–12 months after implementation (October 1, 2015, to January 31, 2016). The PartoMa study was approved by Mnazi Mmoja Hospital and Zanzibar Medical and Research Ethical Committee (ZAMREC/0001/JUNE/014), and registered with ClinicalTrials.org (NCT02318420). Patient identities were anonymized, and thus informed consent not required.
Throughout the study, each birth attendant (nurse‐midwife or doctor) simultaneously cared for an average of 3–6 laboring women, and 30% were intern doctors conducting their initial 6‐week rotation. Turnover was high among staff in permanent positions: 25% of nurse‐midwives and 40% of doctors during the initial 6 months of the intervention were no longer working there after 1 year.19 As a result, most staff had limited experience with childbirth care. Only two had a master in midwifery and there were only two obstetricians. At baseline, clinical intrapartum guidelines were not routinely used. The WHO composite partograph was recommended for all deliveries.20
The “PartoMa Pocket Guide” (File S1) was launched on January 27–28, 2015, at the first “PartoMa seminars”. These 3‐hour seminars were conducted quarterly in order to establish use of the guidelines via case‐based discussions. They were conducted outside working hours and without ‘per diems’ or other allowances. An average of 74% of doctors and 62% of nurse‐midwives from the obstetric division attended each of five seminar rounds during the first intervention year. One page of the pocket guide (Fig. 1) and one of five seminar cases concerned intrapartum care for women with sHDP. Detailed descriptions of guidelines development and the seminars are presented elsewhere.18, 19, 21
Figure 1.
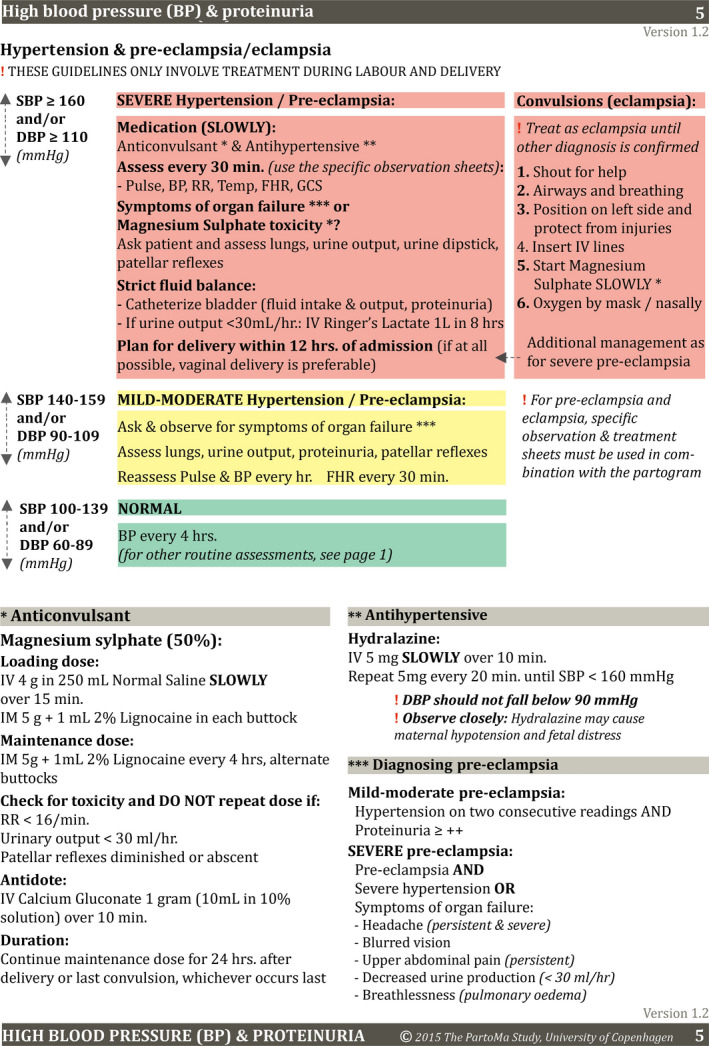
Intrapartum management of hypertensive disorders in pregnancy: page 7 of the PartoMa Pocket Guide version 1. A full overview of the PartoMa guidelines applied is available in File S1. Abbreviations: BP, blood pressure; CS, cesarean; FHR, fetal heart rate. Reproduced from [18].
Quality of intrapartum care for women with sHDP was delineated to diagnosis, surveillance, and treatment, and based on pre‐selected indicators (Fig. S1). In addition, maternal background characteristics, maternal mortality, neonatal Apgar score, and stillbirth were recorded, including the proportion of stillbirths with fetal heart rate (FHR) present on admission. The indicators were chosen to represent unambiguous audit criteria for low‐resource settings that were sensitive in monitoring progress in care and could be retrieved from case files.
Structural changes at the study site that might potentially explain outcome changes (or lack of changes) after implementation of the PartoMa intervention were assessed quarterly and have been previously described.19 In brief, staff numbers, supply shortages, and available emergency obstetric and neonatal care signal functions were similar throughout the baseline and first intervention year, but there were 16% fewer deliveries during the ninth to 12th intervention months compared with baseline. Daily clinical meetings were introduced 7 months before baseline and continued similarly throughout the study. No other formalized in‐house training or obstetric or early neonatal interventions were organized. Only one birth attendant received formalized training externally. The department started conducting maternal death audits in August 2015, but the audit findings were first presented for staff after the 12th intervention month.19
For the present study, the baseline period was limited to October 1, 2014, to January 31, 2015, because the hospital's case file storage system did not work properly before this period.19 The intervention period studied, October 1, 2015, to January 31, 2016 (the ninth to 12th intervention months), was previously used in the overall evaluation of PartoMa.19 For the assessment of sHDP, the calculated sample size for the intervention months was based on a two‐sided comparison of proportions. The proportion of women with sHDP treated with antihypertensives was used as a key indicator of care and was 86/184 (47%) at baseline. Post‐implementation, an improvement of at least 50% in the number of women treated was expected. Thus, the sample size of approximately 180 women in each group resulted in 99.9% power at a 5% significance level.
A structured form in Excel (Microsoft, Redmond, WA, USA) was used to collate data, with statistical analyses performed in RStudio version 1.1.383 (RStudio, Boston, MA, USA). Background characteristics were compared by using Fisher exact test. Care and delivery outcomes were compared by using relative risk (RR) and 95% confidence intervals (CIs) for dichotomous indicators, and Mann‐Whitney test for time intervals. A P value of less than 0.05 was considered statistically significant.
3. RESULTS
According to the hospital registers, 3599 women gave birth at baseline and 3002 did so during the four intervention months studied, but case files of 3609 and 3067 women were retrieved from the respective study periods. Of these, 2761 (76.5%) and 2398 (78.2%) women, respectively, had at least one BP measurement, or had eclampsia documented without a BP recording, of whom 188 (6.8%) and 196 (8.2%) fulfilled the criteria for sHDP and were included in the baseline and intervention groups. An additional 848 (23.5%) and 669 (21.8%) of the women retrieved from the study periods had no BP recorded during the whole admission period (RR 0.93, 95% CI 0.85–1.01), respectively, which could lead to an underestimation of sHDP (Fig. 2).
Figure 2.
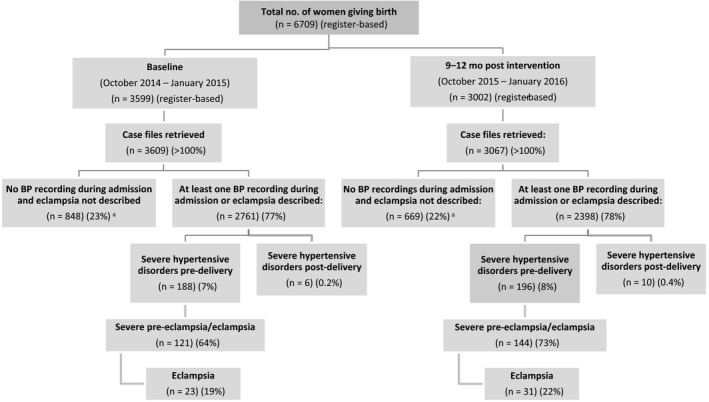
Flow chart showing women included in the study. The inclusion criteria were severe hypertension (systolic blood pressure [BP] ≥160 mm Hg and/or diastolic BP ≥110 mm Hg) and/or eclampsia. A subgroup of women with severe pre‐eclampsia/eclampsia were defined as having at least one recording of severe hypertension and a proteinuria measurement of at least 2+, and/or eclampsia recorded before or during admission. Abbreviations: BP, blood pressure; RR, relative risk; CI, confidence interval. aAmong all women in labor, routine BP assessments did not improve (RR 0.93, 95% CI 0.85–1.01).
The background characteristics of women with sHDP were comparable between the baseline and intervention groups: approximately 40% were nulliparous; 75% had completed 34 weeks (birthweight ≥2000 g) of pregnancy; more than 80% had attended prenatal care at least once and 40% had at least four prenatal visits. Among the multiparous women, 30% had previously lost one or more children (documentation was inadequate to distinguish perinatal deaths from deaths later in life). Labor progress on admission was similarly comparable between the two groups: 50% were admitted before active labor and 2% in the second stage. Fifteen percent were referred from lower level facilities. On admission, 9% were diagnosed with intrauterine fetal death (Table S1). Among the 188 women at baseline, 184 (97.9%) had severe hypertension (systolic BP ≥160 mm Hg and/or diastolic BP ≥110 mm Hg) and 70 (37.2%) had a systolic BP of 180 mm Hg or higher. These proportions were comparable with the intervention group: 193 (98.5%) and 85 (43.4%). The incidence of proteinuria (dipstick measurement ≥2+) was slightly higher during the intervention months, which might be due to fewer women in this group lacking a urine test (Table S1).
The routine BP assessments of all laboring women did not improve between the two periods (RR 0.93, 95% CI 0.85–1.01) (Fig. 2). Among women with sHDP, diagnosis was more frequent on admission in the intervention months (110 [56.1%]), compared with the baseline group (82 [43.6%]) (RR 1.29, 95% CI 1.05–1.58). Among the women not diagnosed on admission, only one had mild‐to‐moderate HDP diagnosed prior to severe disease. There was a tendency toward a decrease in women remaining undiagnosed despite recorded inclusion criteria (RR 0.72, 95% CI 0.51–1.00) (Fig. 3, Table 1). More women had proteinuria tested in the intervention group (177 [90.3%]) than in the baseline group (156 [83.0%]) (RR 1.09, 95% CI 1.01–1.18) (Table S1).
Figure 3.
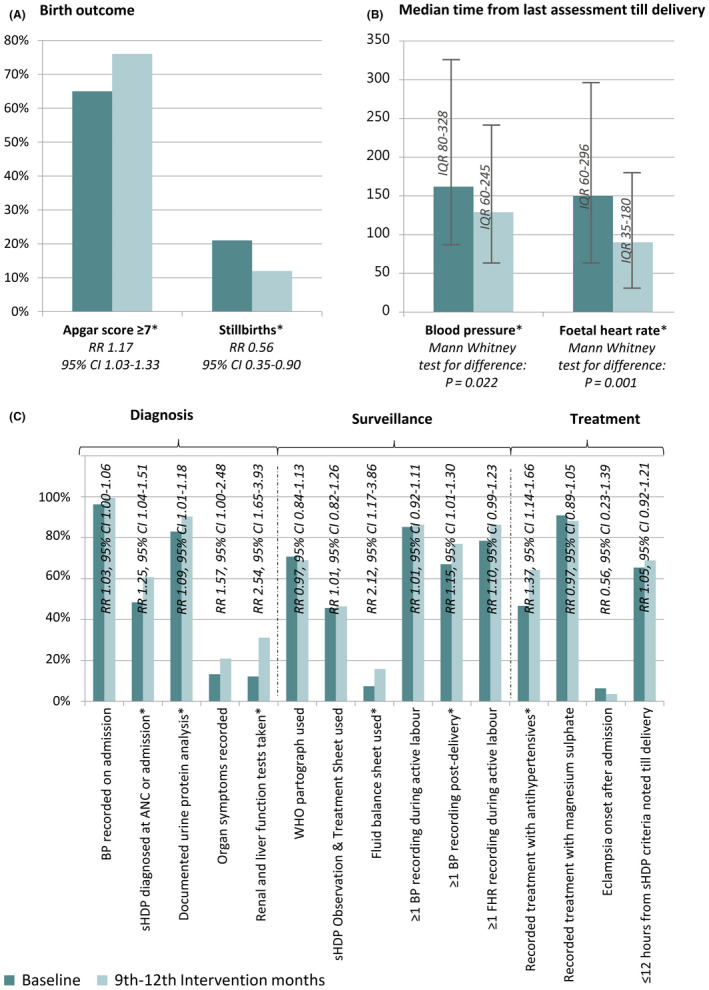
Comparison of key findings at baseline (October 2014–January 2015) and at 9–12 months of the PartoMa study (October 2015–January 2016). (A) Stillbirths (defined as fetal death with weight ≥1000 g) and newborns with an Apgar score of 7 or higher. (B) Median time between last recorded fetal heart rate (FHR) or blood pressure (BP) measurement and delivery. (C) Additional quality indicators of diagnosis, surveillance, and treatment of women with severe hypertensive disorders during pregnancy (sHDP). Abbreviations: CI, confidence interval; IQR, interquartile range; RR, relative risk. Asterisk indicates significant difference.
Table 1.
Univariable analysis of the diagnosis and surveillance of women with severe hypertensive disorders during childbirth, comparison of relative risk of outcomes during the ninth to 12th months of the intervention (October 1, 2015, to January 31, 2016) compared with during the baseline period (October 1, 2014, to January 31, 2015).a
| Characteristic | At baseline (n=188) | At 9–12 intervention months (n=196) | P value | RR (95% CI) |
|---|---|---|---|---|
| Time of diagnosis severe hypertensive disorder | ||||
| Prenatal care visit | 9 (4.8) | 9 (4.6) | 0.96 (0.39–2.36) | |
| During admission assessment | 82 (43.6) | 110 (56.1) | 1.29 (1.05–1.58) | |
| After admission assessment | 38 (20.2) | 33 (16.8) | 0.83 (0.55–1.27) | |
| Undiagnosed | 59 (31.4) | 44 (22.4) | 0.72 (0.51–1.00) | |
| Physical examination recorded on diagnosis | ||||
| Minimum: headache, epigastric pain, vision | 25 (13.3) | 41 (20.9) | 1.57 (1.00–2.48) | |
| Blood count: taken: renal and liver function tests as minimum | 23 (12.2) | 61 (31.1) | 2.54 (1.65–3.93) | |
| Documentation sheets at least partially appliedb | ||||
| Women in first stage of labor | 161 | 155 | ||
| Partograph | 114 (70.8) | 107 (69.0) | 0.97 (0.84–1.13) | |
| All study women | 188 | 196 | ||
| Specific observation and treatment sheet for HDP | 86 (45.7) | 91 (46.4) | 1.01 (0.82–1.26) | |
| Fluid balance sheet | 14 (7.4) | 31 (15.8) | 2.12 (1.17–3.86) | |
| Blood pressure | ||||
| BP recorded on admission | 181 (96.3) | 195 (99.5) | 1.03 (1.00–1.06) | |
| Women with known start of active phase of labor | 149 | 140 | ||
| BP recorded at least once during active phase of labor | 127 (85.2) | 121 (86.4) | 1.01 (0.92–1.11) | |
| All study women | 188 | 196 | ||
| BP at least once between delivery and dischargec | 126 (67.0) | 151 (77.0) | 1.15 (1.01–1.30) | |
| Women with vaginal delivery and ≥1 BP recording during admission | 126 | 130 | ||
| Time from last BP till delivery, min | 162 (80–328) | 129 (60–245) | 0.022d | |
| Fetal heart rate | ||||
| Women in active phase of labor, no IUFD | 144 | 131 | ||
| FHR at least once during active phase of labor | 113 (78.5) | 113 (86.3) | 1.10 (0.99–1.23) | |
| Women with recorded FHR | 140 | 164 | ||
| Time from last FHR till delivery, min | 150 (60–296) | 90 (35–180) | 0.001d | |
| Labor progression | ||||
| Women in active phase of labor | 165 | 156 | ||
| <5 h between any two cervical assessmentsd | 103 (62.4) | 112 (71.8) | 1.15 (0.99–1.34) | |
Abbreviations: BP, blood pressure; CI, confidence interval; FHR, fetal heart rate; HDP, hypertensive disorders in pregnancy; IUFD, intrauterine fetal death; relative risk.
Values are given as number (percentage), absolute number, or median (interquartile range), unless indicated otherwise.
The documentation sheets included a partograph sheet (at least the first cervical dilatation in active labor plotted correctly on the alert line), a specific sheet for HDP (at least one recorded observation and treatment, and fluid balance sheet (at least one recording of input and output).
Overall, 70/188 (37%) at baseline and 82/196 (42%) during the intervention had at least one BP recording every 24 h during the first 2 days postpartum (RR 1.12, 95% CI 0.88–1.44).
By Mann‐Whitney test for difference. This incude the interval between the last recording of cervical dilatation and delivery.
Among women with vaginal delivery and at least one recorded BP pre‐delivery, the median time between last BP and delivery was 162 minutes (interquartile range [IQR], 80–328 minutes; n=126) at baseline versus 129 minutes (IQR, 60–245 minutes; n=130) during the intervention months (Mann‐Whitney test, P=0.015). The proportion of women with at least one recorded BP post‐delivery improved from 126 (67.0%) at baseline to 151 (77.0%) during the intervention months (RR 1.15, 95% CI 1.01–1.30). Among 140 women at baseline and 164 during intervention months with at least one documented FHR, the median time between the last FHR and delivery (or identified intrauterine fetal death) decreased from 150 minutes (IQR, 60–296 minutes) to 90 minutes (IQR 35–180 minutes) (Mann‐Whitney test, P=0.001) (Fig. 3, Table 1).
Among the women with severe hypertension (184 at baseline and 193 during the intervention months), initiation of antihypertensive treatment increased, from 86 (46.7%) women at baseline to 124 (64.2%) during the intervention months (RR 1.37, 95% CI 1.14–1.66). In both periods, 90% of women with severe pre‐eclampsia/eclampsia had magnesium sulfate treatment initiated. Among the 110 women at baseline and 127 during the intervention months with magnesium sulfate initiated, 20 (18.2%) compared with 44 (34.6%), respectively, had patellar reflexes and respiratory rate recorded at least once for surveillance of toxicity (RR 1.91, 95% CI 1.20–3.03) (Fig. 3, Table 2). The median duration of labor from diagnosing sHDP to delivery was approximately 7 hours in both groups (IQR, 161–790 minutes and 225–745 minutes, respectively; Mann‐Whitney test, P=0.356) (Table 2).
Table 2.
Univariable analysis of the treatment of women with severe hypertensive disorders during childbirth, comparison of relative risk of outcomes during the ninth to 12th months of the intervention (October 1, 2015, to January 31, 2016) compared with during the baseline period (October 1, 2014, to January 31, 2015).a
| Characteristic | At baseline (n=188) | At 9–12 intervention months (n=196) | P value | RR (95% CI) |
|---|---|---|---|---|
| Antihypertensive treatment | ||||
| All women with severe hypertensionb | 184 | 193 | ||
| Hydralazine or nifedipine administered | 86 (46.7) | 124 (64.2) | 1.37 (1.14–1.66) | |
| Anticonvulsant treatment | ||||
| Women with urine protein ≥2 or eclampsiac | 121 | 144 | ||
| MgSO4 treatment initiated | 110 (90.9) | 127 (88.2) | 0.97 (0.89–1.05) | |
| Women receiving MgSO4 | 110 | 127 | ||
| Patellar reflexes and respiratory rate at least once | 20 (18.2) | 44 (34.6) | 1.91 (1.20–3.03) | |
| Labor progression | ||||
| All women with the required date and time recorded: | 173 | 189 | ||
| Time from inclusion criteria recorded till delivery, min | 390 (161–790) | 457 (225–745) | 0.356d | |
| All study women | 188 | 196 | ||
| Partograph action line crossed (prolonged first stage) | 22 (11.7) | 22 (11.2) | 0.96 (0.55–1.67) | |
| Induction of labore | 48 (25.5) | 75 (38.3) | 1.50 (1.11–2.03) | |
| Oxytocin for augmentation in active phase of laborf | 45 (23.9) | 36 (18.4) | 0.77 (0.52–1.13) | |
Abbreviations: CI, confidence interval; MgSO4, magnesium sulfate; RR, relative risk.
Values are given as absolute number, number (percentage), or median (interquartile range), unless indicated otherwise.
Diagnostic criteria for severe hypertension applied: systolic BP ≥160 mm Hg and/or diastolic BP ≥110 mm Hg.
Diagnostic criteria for severe pre‐eclampsia/eclampsia applied: severe hypertension on at least one reading and urine protein ≥2, or eclampsia/convulsions described (owing to poor data quality, women without severe hypertension or eclampsia, but fulfilling the WHO criteria of severe pre‐eclampsia due to symptoms of organ failure could not be identified). For an additional 28/188 (15%) women at baseline and 12/193 (6%) during the intervention, urine dipstick was not performed.
Mann‐Whitney test for difference.
One woman was induced by balloon catheter; all others were induced by misoprostol with or without subsequent oxytocin.
Women were excluded if induced. For 11/45 (24%) and 7/36 (19%), respectively, membranes were still intact when starting oxytocin augmentation (RR 0.80, 95% CI 0.34–1.84).
Among all deliveries (203 at baseline and 206 in the intervention months), the mode of delivery was comparable; 58 (28.6%) were delivered by cesarean at baseline compared with 63 (30.6%) during the intervention months. Among 65 (34.6%) women at baseline and 66 (33.7%) during the intervention months, there were no records in their medical file after the day of delivery; although it seems unlikely, it is unknown whether these women had been discharged.
Birthweight was 1000 g or more for 201 (99.0%) neonates at baseline and 196 (95.1%) during the intervention months. Of these, the incidence of stillbirth decreased from 42 (20.9%) at baseline to 23 (11.7%) during intervention months (RR 0.56, 95% CI 0.35–0.90), and the incidence of an Apgar score of seven or higher increased from 130 (64.7%) to 148 (75.5%), respectively (RR 1.17, 95% CI 1.03–1.33) (Table 3).
Table 3.
Birth outcomes among women with severe hypertensive disorders, comparison of relative risk of outcomes during the ninth to 12th months of the intervention (October 1, 2015, to January 31, 2016) compared with during the baseline period (October 1, 2014, to January 31, 2015).a
| Outcome | Baseline | At 9–12 intervention months | RR (95% CI) |
|---|---|---|---|
| Maternal outcomes | 188 | 196 | |
| Maternal death | 1 (0.5) | 5 (2.6) | 4.80 (0.57–40.67) |
| Day of discharge postpartumb | |||
| Delivery day | 65 (34.6) | 66 (33.7) | 0.97 (0.74–1.29) |
| 1–2 d postpartum | 35 (18.6) | 64 (32.7) | 1.75 (1.22–2.51) |
| ≥3 d postpartum | 88 (46.8) | 67 (34.2) | 0.73 (0.57–0.93) |
| Neonatal outcomes | 203 | 206 | |
| Mode of delivery | |||
| Spontaneous vaginal | 133 (65.5) | 130 (63.1) | 0.96 (0.83–1.11) |
| Vaginal breech | 10 (4.9) | 6 (2.9) | 0.59 (0.22–1.60) |
| Vacuum extraction | 0 | 4 (1.9) | |
| Cesarean | 58 (28.6) | 63 (30.6) | 1.07 (0.79–1.44) |
| Unknown | 2 (1.0) | 3 (1.5) | 1.48 (0.25–8.75) |
| Neonatal outcomes for those with birthweight ≥1000 gc | 201 | 196 | |
| Apgar score | |||
| ≥7 | 130 (64.7) | 148 (75.5) | 1.17 (1.03–1.33) |
| 6 | 5 (2.5) | 7 (3.6) | 1.44 (0.46–4.45) |
| 1–5 | 19 (9.5) | 12 (6.1) | 0.65 (0.32–1.30) |
| 0 (stillbirths) | 42 (20.9) | 23 (11.7) | 0.56 (0.35–0.90) |
| Information missing | 5 (3.0) | 6 (2.9) | 1.23 (0.38–3.97) |
| Stillbirths, time of death | |||
| Pre‐hospital (fetal death diagnosed on admission) | 18 (9.0) | 14 (7.1) | 0.80 (0.41–1.56) |
| Intra‐hospital (positive FHR on admission) | 18 (9.0) | 9 (4.6) | 0.51 (0.24–1.11) |
| Time unknown (no documented FHR during admission) | 6 (3.0) | 0 | |
Abbreviations: CI, confidence interval; FHR, fetal heart rate; RR, relative risk.
Values are given as absolute number or number (percentage), unless indicated otherwise.
The last recording in the file was treated as the last day of stay.
Of all neonates, 2/203 (1%) at baseline and 10/206 (4.9%) during the intervention had birthweight <1000 g approximate pregnancy duration <28 wk). At the study hospital, stillbirths at <28 weeks are classified as late spontaneous abortions. Although there might be a risk of mis‐categorizing stillbirths affected by severe intrauterine growth restriction, those stillbirths were excluded from the perinatal outcome data.
One woman at baseline and five during the intervention months died (RR 4.80, 95% CI 0.57–40.67). The woman at baseline died from obstetric hemorrhage. In the intervention group, one woman died from obstetric hemorrhage, and one had been referred from elsewhere and died on admission. The other three women experienced severe organ dysfunction postpartum in the study hospital; one patient had pulmonary edema and hypoxia, one had eclampsia and cerebral edema, and one had eclampsia and renal failure.
4. DISCUSSION
The present findings show that, although care remained substandard, several improvements had occurred during the ninth to 12th invention months, such as progress in surveillance, a 37% increase in antihypertensive treatment, and a 44% reduction in risk of stillbirth. Taken together with previous studies,3, 18, 19 the findings suggest that the PartoMa intervention was an important determinant of improvements. This indicates how effective locally tailored clinical guidelines and associated quarterly, low‐dose training can be in a weak healthcare system with critical shortages in human resources.
The study has both strengths and limitations. The low‐cost, pre–post approach had applicable process and outcome indicators. The comparable background characteristics of women between baseline and the intervention months strengthened its internal validity. Two baseline periods would have enabled potential time‐related changes to be examined, but the hospital's poor case file storage prevented this approach. There were few contextual confounders identified, but the 16% fewer deliveries in the intervention months might have allowed better care.19 Birth attendants were not paid to attend the seminars. Therefore, the high attendance after working hours appeared to indicate staff motivation to improve care, which was a mediator of improvements.19 Through retrieving all available case files from the months studied and reviewing them manually, the number of women in labor identified was 2% higher than if hospital registers alone had been used. This close approximation in a low‐resource hospital was a strength. The present findings are further strengthened by triangulation with similar results of the other PartoMa studies, emphasizing low inter‐observer bias and unambiguity of indicators.3, 18, 19 Although the effect of the PartoMa intervention was explored in terms of care and birth outcomes, the available case file data did not enable analyzing the frequency of organ failure and neonatal mortality, the in‐depth quality of monitoring and treatment, and whether the birth attendants’ interactions with the women were respectful. Furthermore, data from case file reviews might be biased owing to under‐ or over‐recording.3
The current study shows that the previously reported overall reduction in stillbirths from 59 to 39 per 1000 total births between baseline and the ninth to 12th intervention months19 was also true within the sHDP subgroup; stillbirths reduced from 20.9% to 11.7%. Although not reaching statistical significance, the results suggest that the reduction predominantly occurred among intra‐hospital stillbirths. Whereas a decrease in over‐use of oxytocin augmentation seemed to be a major contributor among non‐sHDP women,19 it did not explain the present reduction in stillbirths observed among sHDP women. Taken together with our previous evaluation,19 the improvements observed seem to relate to the three pages of the PartoMa pocket guide that were highest rated among birth attendants (FHR, labor progress, and intrapartum HDP).18 Furthermore, this is consistent with challenges identified in the baseline case–control study of stillbirth.3 It seems that the PartoMa intervention's integrated recommendations and training in routine and emergency intrapartum care have helped to trigger quality improvements. Qualitative implementation research is warranted to further identify organizational and individual aspects associated with the changes, and with lack of changes.22
Two other criterion‐based audits from East African referral hospitals also included locally tailored guidelines and similarly proved effective in strengthening care for women with HDP. However, their interventions were narrowly targeted at pre‐eclampsia/eclampsia and included additional structural changes.6, 8 To our knowledge, no other studies of broader guidelines have been shown to be effective in strengthening care and perinatal survival for women with HDP in low‐income countries.
Nevertheless, the five maternal deaths in the ninth to 12th intervention months reflect the unacceptable quality of care throughout the study. Moreover, the 7%–8% prevalence of severe hypertensive disorders, with 85% of affected women admitted directly from home and only one diagnosed with mild‐moderate disease prior to this, might in itself indicate missed opportunities for early detection and treatment, both prenatally and intrapartum. Data from another Tanzanian tertiary hospital reported an incidence of eclampsia of 6%,6 whereas a facility‐based survey of mild‐severe HDP in multiple low‐ and middle‐income countries found an overall prevalence of only 2.7%.2 The high prevalence of sHDP in the present study may also be associated with a high predisposition in the target population, which further emphasizes the need to strengthen early detection and management: 19% of Zanzibari women aged 25–44 years are affected by hypertension,23 with the risk of gestational aggravation and superimposed pre‐eclampsia.2, 24
In quality improvement processes, the potential effects, and adverse effects, of more or less broad focal points must be carefully considered. As part of the internationally formulated obstetric care signal functions, maximizing magnesium sulfate coverage has been repeatedly emphasized.25 At the study hospital, it was administered to 90% of women with severe pre‐eclampsia/eclampsia during both baseline and intervention months. Although evidence suggests that severe hypertension is a similarly important contributor to maternal mortality in sHDP,14 antihypertensives are not included in the signal functions, and this omission might have contributed to more than half of the women with sHDP at baseline not receiving antihypertensive treatment. A similar mismatch in pre‐eclampsia/eclampsia treatment was reported in Ghana, where health providers described how clinical guidelines were adhered to more closely if they were given more attention and if local adjustments were made.9 A second version of the brief and simple PartoMa pocket guide was launched in 2018, with the focal point broadened further by including additional essential intrapartum and postpartum care, and PartoMa seminars have now been conducted for nearly 4 years.21
It remains crucial to address the human resources crisis and substandard clinical competencies among staff, as well as unacceptable shortages in supplies and space. Nevertheless, in the meantime, locally tailored clinical guidelines and associated reoccurring, in‐house training, which takes account of the current resource constraints, are warranted in assisting work‐overloaded birth attendants in improving care. Achievable guidance is a basic right for birth attendants holding the lives of others in their hands.
AUTHOR CONTRIBUTIONS
NM contributed to the design of the study, data collection and analysis, data interpretation, and writing the manuscript. CBA contributed to the design of the study, data collection and analysis, and revising the manuscript. NH contributed to data collection and analysis, and revising the manuscript. TM contributed to data interpretation and revising the manuscript. ICB and JvR contributed to the design of the study and revising the manuscript. All authors approved the final manuscript and agreed to be accountable for all aspects of the work.
CONFLICTS OF INTEREST
The authors have no conflicts of interest.
Supporting information
File S1. PartoMa Pocket Guide version 1 applied in the present study.
Figure S1. Indicators analyzed to assess intrapartum quality of care and birth outcomes among women with severe hypertensive disorders of pregnancy (sHDP). The indicators are unambiguous research criteria that are sensitive in monitoring care progress at the low‐resource study site and can be retrieved from the case files. They do not represent acceptable quality of care. The following documentation sheets (asterisk) were used: a partograph sheet (at least the first cervical dilatation in active labor plotted correctly on the alert line), a specific sheet for HDP (at least one recorded observation), and a fluid balance sheet (minimum of one recording of input and output). Severe hypertension (double asterisk) was defined as systolic blood pressure (BP) 160 mm Hg or higher and/or diastolic BP 110 mm Hg or higher. Severe pre‐eclampsia (triple asterisk) was defined as severe hypertension on at least one reading and proteinuria of at least 2+, or recorded eclampsia/convulsions before or during admission. Abbreviations: FHR, fetal heart rate; IUFD, intrauterine fetal death.
Table S1. Background characteristics of women included at baseline and at 9–12 mo of the PartoMa intervention.
ACKNOWLEDGMENTS
The PartoMa study is financed by grants from the Lundbeck Foundation (R164‐2013‐16038), the Laerdal Foundation (40108), the Augustinus Foundation (14‐1059), and University of Copenhagen. The funding bodies have no role in the design, implementation, interpretation, or reporting of the study.
The copyright line for this article was changed on 6 September 2019 after original online publication.
REFERENCES
- 1. Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: A systematic review. Lancet. 2006;367:1066–1074. [DOI] [PubMed] [Google Scholar]
- 2. Abalos E, Cuesta C, Carroli G, et al. Pre‐eclampsia, eclampsia and adverse maternal and perinatal outcomes: A secondary analysis of the World Health Organization Multicountry Survey on Maternal and Newborn Health. BJOG. 2014;121:14–24. [DOI] [PubMed] [Google Scholar]
- 3. Maaløe N, Housseine N, Bygbjerg IC, et al. Stillbirths and quality of care during labour at the low resource referral hospital of Zanzibar: A case‐control study. BMC Pregnancy Childbirth. 2016;16:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nelissen EJT, Mduma E, Ersdal HL, Evjen‐Olsen B, van Roosmalen JJM, Stekelenburg J. Maternal near miss and mortality in a rural referral hospital in northern Tanzania: A cross‐sectional study. BMC Pregnancy Childbirth. 2013;13:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lumala A, Sekweyama P, Abaasa A, Lwanga H, Byaruhanga R. Assessment of quality of care among in‐patients with postpartum haemorrhage and severe pre‐eclampsia at st. Francis hospital nsambya: A criteria‐based audit. BMC Pregnancy Childbirth. 2017;17:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kidanto HL, Wangwe P, Kilewo CD, Nystrom L, Lindmark G. Improved quality of management of eclampsia patients through criteria based audit at Muhimbili National Hospital, Dar es Salaam, Tanzania. Bridging the quality gap. BMC Pregnancy Childbirth. 2012;12:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Herklots T, van Acht L, Meguid T, Franx A, Jacod B. Severe maternal morbidity in Zanzibar's referral hospital: Measuring the impact of in‐hospital care. PLoS ONE. 2017;12:e0181470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weeks AD, Alia G, Ononge S, Otolorin EO, Mirembe FM. A criteria‐based audit of the management of severe pre‐eclampsia in Kampala, Uganda. Int J Gynecol Obstet. 2005;91:292–297. [DOI] [PubMed] [Google Scholar]
- 9. Browne JL, van Nievelt SW, Srofenyoh EK, Grobbee DE, Klipstein‐Grobusch K. Criteria‐based audit of quality of care to women with severe pre‐eclampsia and eclampsia in a referral hospital in Accra, Ghana. PLoS ONE. 2015;10:e0125749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oladapo O, Adetoro O, Ekele B, Chama C, Etuk S, Aboyeji A, et al. When getting there is not enough: A nationwide cross‐sectional study of 998 maternal deaths and 1451 near‐misses in public tertiary hospitals in a low‐income country. BJOG. 2015;123:928–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meguid T. (Re)Humanising Health Care – placing dignity and agency of the patient at the centre. Nord J Hum Rights Routledge. 2016;34:60–64. [Google Scholar]
- 12. Chou D, Daelmans B, Jolivet RR, Kinney M, Say L; Every Newborn Action Plan (ENAP) and Ending Preventable Maternal Mortality (EPMM) working groups . Ending preventable maternal and newborn mortality and stillbirths. BMJ. 2015;351:h4255. [DOI] [PubMed] [Google Scholar]
- 13. World Health Organization . WHO recommendations for Prevention and treatment of pre‐eclampsia and eclampsia. Geneva, Switzerland: World Health Organization; 2011. [PubMed] [Google Scholar]
- 14. von Dadelszen P, Firoz T, Donnay F, et al. Preeclampsia in low and middle income countries‐health services lessons learned from the PRE‐EMPT (PRE‐eclampsia‐eclampsia monitoring, prevention and treatment) project. J Obstet Gynaecol Canada. 2012;34:917–926. [DOI] [PubMed] [Google Scholar]
- 15. Miller S, Abalos E, Chamillard M, et al. Beyond too little, too late and too much, too soon: A pathway towards evidence‐based, respectful maternity care worldwide. Lancet. 2016;388:2176–2192. [DOI] [PubMed] [Google Scholar]
- 16. Ameh CA, Ekechi CI, Tukur J. Monitoring severe pre‐eclampsia and eclampsia treatment in resource poor countries: Skilled birth attendant perception of a new treatment and monitoring chart (LIVKAN chart). Matern Child Health J. 2012;16:941–946. [DOI] [PubMed] [Google Scholar]
- 17. Ministry of Health and Social Welfare (Dar es Salaam) & Ministry of Health (Zanzibar) . Tanzania Service Provision Assessment Survey 2014–2015. Dar es Salaam; 2016. [Google Scholar]
- 18. Maaløe N, Housseine N, van Roosmalen J, et al. Labour management guidelines for a Tanzanian referral hospital: The participatory development process and birth attendants’ perceptions. BMC Pregnancy Childbirth. 2017;17:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maaløe N, Housseine N, Meguid T, et al. Effect of locally‐tailored labour management guidelines on intrahospital stillbirths and birth asphyxia at the referral hospital of Zanzibar: A quasi‐experimental pre‐post‐study (The PartoMa study). BJOG. 2017;125:235–245. [DOI] [PubMed] [Google Scholar]
- 20. Kwast BE, Lennox CE, Farley TMM; World Health Organization partograph in management of labour . World Health Organization Maternal Health and Safe Motherhood Programme. Lancet. 1994;343:1399–1404. [PubMed] [Google Scholar]
- 21. University of Copenhagen . The PartoMa Study – for saving lives at birth [Internet]. https://publichealth.ku.dk/partoma. Accessed June 1, 2018.
- 22. Bonell C, Fletcher A, Morton M, Lorenc T, Moore L. Realist randomised controlled trials: A new approach to evaluating complex public health interventions. Soc Sci Med. 2012;75:2299–2306. [DOI] [PubMed] [Google Scholar]
- 23. Jorgensen J. NCD Survey Report: Main findings from the National Non‐Communicable Disease Risk Factor Survey 2011. Zanzibar City; 2012. [Google Scholar]
- 24. Bramham K, Parnell B, Nelson‐Piercy C, Seed PT, Poston L, Chappell LC. Chronic hypertension and pregnancy outcomes: Systematic review and meta‐analysis. BMJ. 2014;348:g2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maine D. World Health Organization, Reproductive Health and Research . Monitoring emergency obstetric care : A handbook. Geneva, Switzerland: Dept. of Reproductive Health and Research, World Health Organization; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1. PartoMa Pocket Guide version 1 applied in the present study.
Figure S1. Indicators analyzed to assess intrapartum quality of care and birth outcomes among women with severe hypertensive disorders of pregnancy (sHDP). The indicators are unambiguous research criteria that are sensitive in monitoring care progress at the low‐resource study site and can be retrieved from the case files. They do not represent acceptable quality of care. The following documentation sheets (asterisk) were used: a partograph sheet (at least the first cervical dilatation in active labor plotted correctly on the alert line), a specific sheet for HDP (at least one recorded observation), and a fluid balance sheet (minimum of one recording of input and output). Severe hypertension (double asterisk) was defined as systolic blood pressure (BP) 160 mm Hg or higher and/or diastolic BP 110 mm Hg or higher. Severe pre‐eclampsia (triple asterisk) was defined as severe hypertension on at least one reading and proteinuria of at least 2+, or recorded eclampsia/convulsions before or during admission. Abbreviations: FHR, fetal heart rate; IUFD, intrauterine fetal death.
Table S1. Background characteristics of women included at baseline and at 9–12 mo of the PartoMa intervention.


