Abstract
The aim of the present review was to assess the prognostic impact of lymphovascular invasion (LVI) in transurethral resection (TUR) of bladder cancer (BCa) specimens on clinical outcomes. A systematic review and meta‐analysis of the available literature from the past 10 years was performed using MEDLINE, EMBASE and Cochrane library in August 2017. The protocol for this systematic review was registered on PROSPERO (Central Registration Depository: CRD42018084876) and is available in full on the University of York website. Overall, 33 studies (including 6194 patients) evaluating the presence of LVI at TUR were retrieved. LVI was detected in 17.3% of TUR specimens. In 19 studies, including 2941 patients with ≤cT1 stage only, LVI was detected in 15% of specimens. In patients with ≤cT1 stage, LVI at TUR of the bladder tumour (TURBT) was a significant prognostic factor for disease recurrence (pooled hazard ratio [HR] 1.97, 95% CI: 1.47–2.62) and progression (pooled HR 2.95, 95% CI: 2.11–4.13), without heterogeneity (I 2 = 0.0%, P = 0.84 and I 2 = 0.0%, P = 0.93, respectively). For patients with cT1–2 disease, LVI was significantly associated with upstaging at time of radical cystectomy (pooled odds ratio 2.39, 95% CI: 1.45–3.96), with heterogeneity among studies (I 2 = 53.6%, P = 0.044). LVI at TURBT is a robust prognostic factor of disease recurrence and progression in non‐muscle invasive BCa. Furthermore, LVI has a strong impact on upstaging in patients with organ‐confined disease. The assessment of LVI should be standardized, reported, and considered for inclusion in the TNM classification system, helping clinicians in decision‐making and patient counselling.
Keywords: lymphovascular invasion, transurethral resection, recurrence, progression, upstaging, meta‐analysis, #blcsm, #BladderCancer
Introduction
Bladder cancer (BCa) is the fourth most commonly diagnosed malignancy in men, with significant morbidity and mortality worldwide 1. In western countries, ~70% of patients with BCa are diagnosed with non‐muscle‐invasive BCa (NMIBC) 2. Transurethral resection of the bladder tumour (TURBT) is necessary for the diagnosis, risk stratification and treatment of patients with NMIBC; however, up to 61% and 17% of patients who undergo TURBT and adjuvant treatment with BCG, respectively, for high‐risk cT1 BCa experience disease recurrence or progression to carcinoma invading the bladder muscle (MIBC) within the first year 2, 3. Identification of patients with NMIBC who are at high risk of experiencing progression to MIBC would allow selection for intensified therapy such as early radical cystectomy (RC) or inclusion in clinical trials of new therapies.
Lymphovascular invasion (LVI) is a crucial step in the initiation of tumour dissemination and metastasis 4. Although LVI has been established as a feature of biologically and clinically aggressive disease in patients treated with RC 5, 6, 7, 8, its role at TUR is not yet clear. The results from a previous meta‐analysis suggested an increased risk of pathological upstaging and poor clinical outcomes in patients with LVI at TURBT 9; however, the selection criteria included all studies regardless of the patients’ tumour stage. Moreover, new studies have added further data with which to analyse the prognostic impact of LVI at TUR in patients with organ‐confined BCa 10, 11, 12, 13, 14, 15, 16, 17, 18.
The aim of the present study was to perform a systematic review and meta‐analysis to assess the role of LVI in TUR specimens, regardless of tumour stage, to investigate its prognostic impact on disease recurrence and progression in patients with NMIBC, and to assess its predictive value for upstaging at RC in patients with clinically organ‐confined (cT1–2) disease.
Evidence Acquisition
Table S1 shows a completed Preferred Reporting Items for Systematic reviews and Meta‐Analyses (PRISMA) 2015 checklist, which clearly describes the methodology of the review 19. The protocol has also been registered in the International Prospective Register of Systematic Reviews database (PROSPERO: CRD42018084876).
Literature Search
A systematic review and meta‐analysis of the English‐language literature was performed according to the PRISMA statement and the Cochrane Handbook for Systematic Reviews of Interventions 20, 21 . The MEDLINE, EMBASE and Cochrane Library were systematically searched on 9 August 2017 to identify studies published between January 2007 and August 2017 that investigated the impact of LVI in TUR specimens on oncological outcomes in patients with urothelial BCa. After a first screening based on study title and abstract, all papers were assessed based on full text and excluded with reasons when inappropriate. A further check of the appropriateness of the papers based on full‐text revision was performed after data extraction. Two reviewers (A.M. and S.K.) carried out this process independently. Disagreements were solved by a third party (B.F.). The following string terms were used: (((‘bladder’) AND (‘cancer’ OR ‘urothelial carcinoma’ OR ‘urothelial neoplasm’ OR ‘carcinoma’ OR ‘transitional cell carcinoma’)) AND (‘TUR’ OR ‘TURB’ OR ‘TRANSURETHRAL RESECTION’)) AND (‘LVI’ OR ‘lymphovascular invasion’ OR ‘lymphatic invasion’ OR ‘vascular invasion’). The process used to identify articles is summarized in Fig. 1. Disease recurrence, disease progression and upstaging were the primary outcomes of interest.
Figure 1.
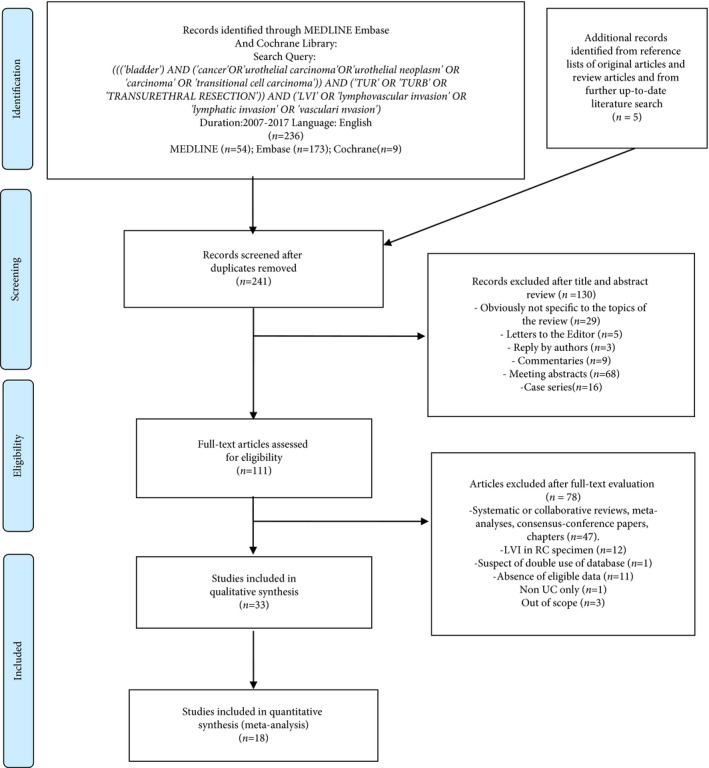
Flow chart for the article selection process. LVI, lymphovascular invasion; RC, radical cystectomy; UC, urothelial carcinoma.
Eligibility Criteria
As proposed by the PRISMA guidelines, we used the Population, Intervention, Comparator, Outcome and Study design approach to specify the eligibility criteria: studies were considered eligible if patients with LVI in BCa (population) treated with TUR with or without RC (intervention) were compared with patients without LVI (comparator) to investigate the prognostic value of LVI on disease recurrence, progression and upstaging (outcomes) in non‐randomized observation or cohort studies.
Inclusion and Exclusion Criteria for the Systematic Review
After article selection according to the eligibility criteria, the following types of study were excluded: review articles; editorials; commentaries; papers written in languages other than English; meeting abstracts; replies from authors; and case reports. If multiple articles published by the same author or group and based on similar patient cohorts were found, only the paper with the largest cohort was included. For the purposes of the present study, all eligible studies were included in the systematic review, regardless of the clinical stage involved.
Inclusion and Exclusion Criteria for the Meta‐Analysis
The studies selected for the systematic review were then screened for the quantitative analysis. Of these, studies analysing patients with ≤cT1 (cTa‐Tis‐T1) stage disease were included in the meta‐analysis of the prognostic impact of LVI at TUR on disease recurrence and progression. Studies analysing patients with organ‐confined (≤cT2) disease and subsequently treated with RC were included in a meta‐analysis of the prognostic impact of LVI at TUR on upstaging at RC.
Data Extraction
After full‐text evaluation, data were independently extracted by two authors (A.M. and S.K.) for further assessment of qualitative and quantitative evidence synthesis. All extracted variables were crosschecked to ensure their reliability. We recorded the baseline characteristics of the included participants, the use of peri‐operative chemotherapy and the median/mean follow‐up duration. Subsequently, the hazard ratios (HRs) and 95% confidence intervals (CIs) of LVI associated with each outcome were retrieved. Furthermore, we searched for methods and important confounders to establish comparability. All discrepancies regarding data extraction were resolved by consensus or finally decided on by the senior author (S.F.S.).
Statistical Analysis
Because of the observational nature of the included studies, we extracted the adjusted HR and odds ratio with 95% CI for cumulative effect size calculation from multivariable Cox regression analysis (for recurrence and progression outcomes) and logistic regression analysis (for upstaging outcome), respectively. Studies reporting only Kaplan–Meier log‐rank or univariable analyses were not considered for the meta‐analysis. Effect summary estimation methods were not used in these cases as a high level of additional selection bias would have been introduced. Statistical pooling of effect measures was based on the level of heterogeneity among studies, which was assessed with the Cochrane Q test and I 2 statistic. Significant heterogeneity was indicated by a P value <0.05 in Cochrane Q tests and a ratio >50% in I 2 statistics, which led to the use of random‐effect models according to the DerSimonian and Laird method 22, 23, 24. When these tests were negative for heterogeneity, fixed‐effect models were chosen for calculation of pooled HRs through the inverse‐variance method. Publication biases including small‐study effect were evaluated by visual inspection of funnel plots for all assessed comparisons. Statistical analyses were performed using stata/mp 14.2 (Stata Corp., College Station, TX, USA).
Risk of Bias
The risk‐of‐bias assessment is reported in Tables S2, S3, S4. The risk‐of‐bias evaluation of included studies was assessed according to the Cochrane Handbook for Systematic Reviews of Interventions for including non‐randomized studies 25, 26. Because of the inclusion of only non‐randomized comparative studies, risk of bias was determined by examining the risk of preassigned confounders. The main confounding factors were identified as the most important prognostic factors affecting disease recurrence, progression and upstaging. For this purpose, the Risk Of Bias In Non‐randomised Studies ‐ of Interventions (ROBINS‐I) was used for evaluating risk of bias in estimates of the comparative effectiveness of interventions from studies that did not use randomization to allocate units to comparison groups 27. The ROBINS‐I tool was used for each of the three outcomes analysed. The presence of confounders was determined by consensus between A.M. and B.F.
Evidence Synthesis
Study Selection
We identified a total of 236 articles from the search query and five from the reference lists. Overall 130 articles were excluded after title and abstract screening and 78 after full‐text evaluation. The remaining 33 articles were included in the systematic review. The flow chart of the study selection process is shown in Fig. 1.
Study Population
Study characteristics are reported in Table 1 10, 11, 12, 13, 14, 15, 16, 17, 18, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51. The 33 studies included 6194 patients treated with TUR with or without RC. The population examined was from the USA/Canada in 13 studies, Europe in 10 studies and Asia in 10 studies. All the studies had a retrospective design. LVI definition was provided in 16 studies (48.5%) and was found in 1 069 of 6194 patients (17.3%). Pathological characteristics are reported in Table 2 10, 11, 12, 13, 14, 15, 16, 17, 18, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51. Overall, 62% of patients had NMIBC, 32% had cT2 stage and 6% had cT3–4 stage. In 19 studies, only patients with NMIBC were analysed (n = 2 941) and LVI was detected in 15% of these patients. Extravesical disease and node involvement were reported in 633/1715 (36.9%) and 371/1537 patients (24.2%) subsequently treated after RC, respectively.
Table 1.
Characteristics of the eligible studies included in the systematic review
| First author of study and year | Country | Recruitment period | No. of patients | LVI, % | Definition of LVI | NOS | Main treatment |
|---|---|---|---|---|---|---|---|
| Andius P (2007) 28 | Sweden | 1987–1989 | 121 | 9.9 | Yes | 5 | TUR |
| Kassouf W (2007) 29 | USA | 1990–2005 | 120 | 31.7 | No | 6 | RC |
| Herr HW (2008) 30 | USA | 1995–2001 | 63 | 55.6 | No | 8 | TUR |
| Weizer AZ (2009) 31 | USA | 1995–2007 | 95 | 12.6 | No | 6 | RC |
| Cho KS (2009) 32 | Korea | 2001–2007 | 118 | 28.0 | Yes | 7 | TUR |
| Streeper N (2009) 33 | USA | 1995–2005 | 103 | 67.0 | Yes | 4 | RC |
| Seo HK (2010) 34 | Korea | 2001–2006 | 129 | 3.9 | No | 4 | TUR |
| Font A (2011) 35 | Spain | 1994–2007 | 98 | 14.3 | No | 6 | RC |
| Faba OR (2011) 36 | Spain | 1978–2002 | 141 | 19.9 | No | 5 | RC |
| Badalato G (2012) 37, * | USA | 1990–1999 | 90 | 24.4 | No | 6 | RC |
| Badalato G (2012) 37, * | USA | 2000–2010 | 259 | 10.0 | No | 6 | RC |
| Green DA (2012) 38 | USA | NR | 201 | 10.4 | Yes | 6 | RC |
| Xie HY (2012) 39 | China | 2003–2011 | 248 | 17.7 | Yes | 5 | RC |
| Kwon D (2012) 40 | Korea | 1999–2010 | 406 | 3.0 | No | 7 | TUR |
| Branchereau J (2013) 41 | France | 1994–2009 | 108 | 36.1 | Yes | 5 | TUR |
| Brimo F (2013) 42 | Canada/USA | 2004–2012 | 86 | 12.8 | Yes | 6 | TUR |
| Bolenz C (2013) 43 | EU | 2000–2006 | 111 | 18.0 | Yes | 6 | TUR |
| Levidou G (2013) 44 | Greece | 1985–1995 | 115 | 22.6 | No | 5 | NR |
| Olsson H (2013) 45 | Sweden | 1992–2001 | 211 | 7.6 | Yes | 6 | TUR |
| Kaimakliotis HZ (2014) 46 | USA | 2008–2013 | 308 | 9.1 | No | 5 | RC |
| Prelevic R (2014) 47 | Serbia | 2002–2012 | 233 | 69.5 | Yes | 4 | RC |
| Svatek RS (2014) 48 | USA | 2000–2008 | 545 | 9.0 | No | 4 | RC |
| Goldsmith B (2014) 49 | USA | 1987–2010 | 315 | 12.7 | No | 6 | RC |
| Go H (2015) 10 | Korea | 1996–2006 | 274 | 2.9 | Yes | 6 | TUR |
| Pietzak EJ (2015) 50 | USA | 1990–2009 | 275 | 12.0 | No | 5 | RC |
| Miyake M (2015) 11 | Japan | 1998–2013 | 106 | 38.7 | No | 6 | TUR |
| Patschan O (2015) 12 | Sweden | 1997–2003 | 156 | 24.2 | No | 7 | TUR |
| Weiss BE (2015) 13 | USA | 1977–2009 | 120 | 4.2 | No | 6 | TUR |
| Fukumoto K (2016) 14 | Japan | 1994–2013 | 116 | 25.9 | Yes | 6 | TUR |
| Haas CR (2016) 15 | USA | 1990–2012 | 117 | 8.5 | No | 7 | RC |
| Li G (2016) 16 | China | 2003–2014 | 206 | 27.7 | Yes | 7 | RC |
| Sha N (2016) 17 | China | 2006–2010 | 155 | 21.9 | Yes | 6 | TUR |
| Lucca I (2016) 51 | EU | 2001–2014 | 350 | 9.7 | Yes | 6 | RC |
| Ukai R (2017) 18 | Japan | 2000–2012 | 86 | 17.4 | Yes | 4 | TUR |
EU, European Union; LVI, lymphovascular invasion; NOS, Newcastle–Ottawa score; NR, not reported; RC, radical cystectomy; TUR, transurethral resection. *Two different cohorts of patients treated from 1990 to 1999 and from 2000 to 2010 analysed in a single study.
Table 2.
Pathological characteristics of the eligible studies included in the systematic review
| Study and year | cT stage (%) | High grade or G2–3 (%) | Muscle in TUR speci men (%) | Re‐staging TUR (%) | Intravesical adjuvant treatment | NAC | AC | Upstaging (pT3–4 at RC) (%) | pN stage at RC (%) | Median follow‐up (months) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cTa‐Tis‐T1 | cT2 | cT3–4 | BCG | Chemotherapy | Immediate | |||||||||
| Andius P (2007) 28 | 100 | 0 | 0 | 96.0 | 88 | 17 | 1 | 3 | NR | NR | NR | NR | NR | NR |
| Kassouf W (2007) 29 | 17.0 | 54.0 | 29 | 100 | NR | NR | NR | NR | NR | 64 | NR | NR | NR | 32 |
| Herr HW (2008) 30 | 0 | 57.0 | 43 | NR | 100 | 100 | NR | NR | NR | 100 | 0 | NR | NR | 86 |
| Weizer AZ (2009) 31 | 100 | 0 | 0 | NR | 100 | 24.2 | NR | NR | NR | excl | NR | 13.7 | 8.4 | 45.6 |
| Cho KS (2009) 32 | 100 | 0 | 0 | 97.4 | NR | 26.3 | 62 | 23 | NR | NR | NR | NR | NR | 35 |
| Streeper N (2009) 33 | 5.8 | 65.0 | 29 | NR | NR | NR | NR | NR | NR | excl | 11.3 | 37.9 | NR | NR |
| Seo HK (2010) 34 | 100 | 0 | 0 | 76.0 | 100 | NR | 100 | 0 | NR | NR | NR | NR | NR | 48.6 |
| Font A (2011) 35 | 0 | 3.0 | 97 | NR | 100 | NR | NR | NR | NR | 100 | NR | NR | NR | 45 |
| Faba OR (2011) 36 | 40.0 | 39.0 | 21 | 93.6 | NR | NR | NR | NR | NR | NR | 5.7 | NR | 13.4 | 42.5 |
| Badalato G (2012) 37 | 100 | 0 | 0 | NR | 31 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Badalato G (2012) 37 | 100 | 0 | 0 | NR | 47.5 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Green DA (2012) 38 | 50.2 | 49.8 | 0 | 98.5 | NR | NR | 35.3 | NR | excl | NR | 35.4 | 18.9 | NR | |
| Xie HY (2012) 39 | 47.0 | 53.0 | 0 | 87.0 | NR | NR | NR | NR | NR | excl | NR | NR | NR | NR |
| Kwon D (2012) 40 | 100 | 0 | 0 | 59.4 | NR | NR | NR | 100 | 0.2 | excl | NR | NR | NR | 76.9 |
| Branchereau J (2013) 41 | 100 | 0 | 0 | 100 | 100 | 72.2 | 0.1 | 100 | 0.1 | NR | NR | NR | NR | 47.8 |
| Brimo F (2013) 42 | 100 | 0 | 0 | NR | 74 | 0 | NR | NR | NR | NR | NR | NR | NR | 29 |
| Bolenz C (2013) 43 | 68.4 | 31.5 | 0 | 81.1 | NR | NR | 5.3 | 22.4 | 27.6 | NR | NR | NR | NR | 30 |
| Levidou G (2013) 44 | 63.5 | T2–3: 36.5 | 47.8 | NR | NR | 33.6 | NR | excl | NR | NR | NR | NR | ||
| Olsson H (2013) 45 | 100 | 0 | 0 | 100 | NR | 14.7 | NR | 18.5 | 0 | excl | NR | NR | NR | 60 |
| Kaimakliotis HZ (2014) 46 | 0 | 100 | 0 | NR | NR | NR | NR | NR | NR | 22.1 | 14.6 | 43.5 | 29.2 | 30 |
| Prelevic R (2014) 47 | 24.9 | 75.1 | 0 | 100 | NR | NR | NR | NR | NR | excl | NR | 56.6 | NR | NR |
| Svatek RS (2014) 48 | 40.9 | 41.7 | 17 | NR | NR | NR | NR | NR | NR | 36.9 | NR | NR | 15.8 | 49.3 |
| Goldsmith B (2014) 49 | 13.0 | 64.1 | 23 | NR | NR | NR | NR | NR | NR | excl | NR | 45.6 | 25.7 | NR |
| Go H (2015) 10 | 100 | 0 | 0 | 50.4 | NR | NR | 5.9 | 38.3 | NR | NR | NR | NR | NR | NR |
| Pietzak EJ (2015) 50 | 0 | 100 | 0 | NR | 100 | NR | NR | NR | NR | 8.7 | NR | 45.6 | 30.2 | 23.2 |
| Miyake M (2015) 11 | 100 | 0 | 0 | 100 | 100 | 12 | 13.0 | 54.0 | NR | NR | NR | NR | NR | 23 |
| Patschan O (2015) 12 | 100 | 0 | 0 | 100 | NR | 60.3 | 3.2 | 25.6 | NR | NR | NR | NR | NR | 78 |
| Weiss BE (2015) 13 | 100 | 0 | 0 | 100 | NR | NR | 19.2 | 80.8 | NR | NR | NR | NR | NR | 53 |
| Fukumoto, K (2016) 14 | 100 | 0 | 0 | 100 | NR | NR | 13.8 | 73.3 | 0 | NR | NR | NR | NR | 53 |
| Haas CR (2016) 15 | 100 | 0 | 0 | NR | NR | 42.7 | 0.0 | 100 | NR | NR | NR | 12.8 | 18.8 | 60.7 |
| Li G (2016) 16 | 100 | 0 | 0 | 41.7 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Sha N (2016) 17 | 100 | 0 | 0 | 39.4 | NR | NR | 97.4 | NR | NR | NR | NR | NR | 78.4 | |
| Lucca I (2016) 51 | 29.0 | 71.0 | 0 | 79.0 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Ukai R (2017) 18 | 100 | 0 | 0 | 100 | 91 | NR | 4.7 | 46.5 | NR | NR | NR | NR | NR | 49 |
AC, adjuvant chemotherapy; adj: adjuvant; CT: chemotherapy, excl: excluded, NAC: neoAC, NR: not reported, RC: radical cystectomy, TUR: transurethral resection.
Meta‐Analysis
Association of LVI in cT1 TUR Specimens with Disease Recurrence
The impact of LVI on disease recurrence was investigated in six studies including 1 412 patients treated with TURBT for cT1 BCa. The forest plot (Fig. 2) shows that LVI was significantly associated with disease recurrence (pooled HR 1.97, 95% CI: 1.47–2.62; z = 4.61). The Cochrane Q test (χ2 = 2.06; P = 0.841) and I 2 test (I 2 = 0.0%) did not show any significant heterogeneity. The funnel plot did not identify any study over the pseudo 95% CI (Fig. S1).
Figure 2.
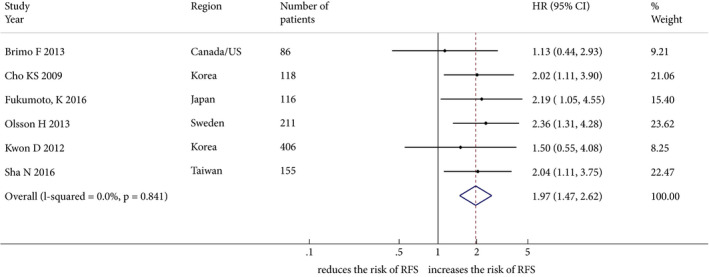
Forest plot showing the association between lymphovascular invasion and disease recurrence in studies including patients treated with transurethral resection for non‐muscle‐invasive bladder cancer. HR, hazard ratio; RFS, recurrence‐free survival.
Association of LVI in cT1 TUR Specimens with Disease Progression
The impact of LVI on disease progression was investigated in nine studies including 1334 patients treated with TURBT for cT1 BCa. The forest plot (Fig. 3) shows that LVI was significantly associated with disease progression (pooled HR 2.95, 95% CI: 2.11–4.13; z = 6.30). The Cochrane Q test (χ2 = 3.15; P = 0.925) and I 2 test (I 2 = 0.0%) did not show any significant heterogeneity. The funnel plot did not identify any study over the pseudo 95% CI (Fig. S2).
Figure 3.
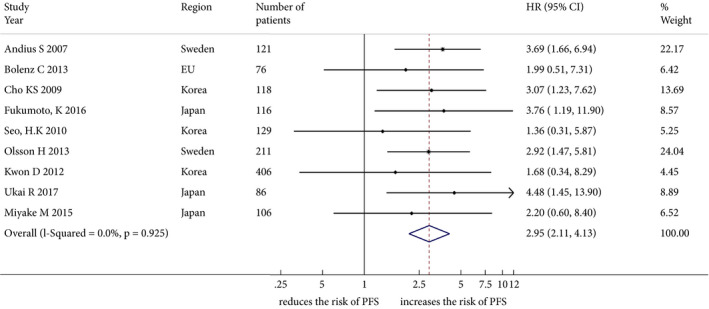
Forest plot showing the association between lymphovascular invasion and disease progression in studies including patients treated with transurethral resection for non‐muscle‐invasive bladder cancer. HR, hazard ratio; PFS, progression‐free survival.
Association of LVI in cT1–2 TUR Specimens with Upstaging at RC
The impact of LVI on upstaging after RC was investigated in seven studies including 1710 patients treated with TURBT for cT1–T2 BCa. The forest plot (Fig. 4) shows that LVI was significantly associated with upstaging after RC (pooled HR 2.39, 95% CI: 1.45–3.96; z = 3.40) in all of the seven studies. Subgroup analysis showed that LVI was not significantly associated with upstaging after RC in studies evaluating cT2 patients alone (pooled HR 1.25, 95% CI: 0.35–4.44; z = 0.35), but it was significantly associated in studies evaluating patients with cT1–2 disease together (pooled HR 3.08, 95% CI: 1.99–4.78; z = 3.67). The Cochrane Q test (χ2 = 12.93; P = 0.044) and I 2 test (I 2 = 53.6%) showed significant heterogeneity. The funnel plot identified one study over the pseudo 95% CI (Fig. S3).
Figure 4.
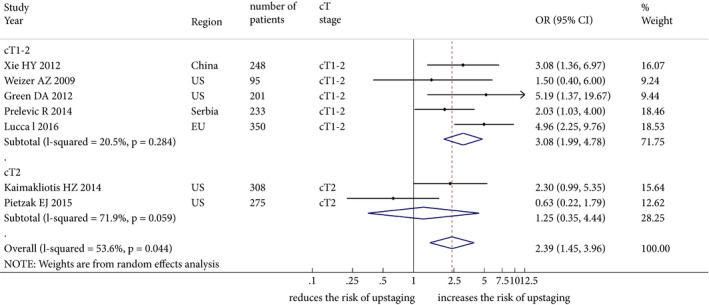
Forest plot showing the association between lymphovascular invasion and disease upstaging in studies including patients treated with transurethral resection and subsequently radical cystectomy for organ‐confined (≤cT2) bladder cancer. OR, odds ratio.
Discussion
Non‐muscle invasive BCa is a disease with highly variable behaviour and outcome. Patients with high‐risk T1 BCa have a greater risk of disease recurrence and progression compared with patients with T1 BCa without high‐risk features 3. The inherently heterogeneous course of T1 high grade/G3 disease makes this entity of NMIBC challenging to treat. An accurate risk stratification would allow the identification of those patients who should receive intensified therapies, such as early RC and preoperative systemic therapy, with the attempt to control micrometastatic disease. LVI is a histological feature of biologically and clinically aggressive BCa; it is associated with poor oncological outcomes if detected in RC specimens 5, 6, 7, 8 as well as in patients with upper tract urothelial carcinoma 52. In non‐seminomatous testicular germ cell cancer, LVI presence upstages the tumour according to the TNM classification 53.
In the present study, a systematic review and meta‐analysis was conducted to assess the role of LVI in TUR specimens, to investigate its prognostic impact on disease recurrence and progression in patients with NMIBC and to evaluate its predictive value for upstaging at RC in patients with organ‐confined disease. We identified 6194 patients treated with TUR, for whom LVI status was evaluated in 33 studies. Overall, LVI was reported in 17.3% of cases and in 15% of patients in studies analysing only patients with ≤cT1 stage. We found that, in patients with NMIBC, the presence of LVI at TUR doubled the risk of developing disease recurrence (pooled HR 1.97, 95% CI: 1.47–2.62) and increased the risk of disease progression to MIBC threefold (pooled HR 2.95, 95% CI: 2.11–4.13) compared with patients without LVI.
The currently recommended scoring models for individualized prediction of disease recurrence and progression are the European Organization for Research and Treatment of Cancer (EORTC) [3] and the Spanish Urological Club for Oncological Treatment (CUETO) 54 risk tables. The two scoring models are based on the most relevant clinical and pathological predictors, sex, age, tumour stage and grade, number of tumours, size, carcinoma in situ and prior recurrence rate. These tools aim to help in personalized risk assessment, thereby guiding clinical decision‐making regarding follow‐up and therapy; however, an external validation of these scores exhibited poor discrimination of both tools regarding disease recurrence and progression 55. Additional factors not included in the EORTC or the CUETO models might enhance their usefulness. Hydronephrosis as well as micropapillary or neuroendocrine variant histology could help identify patients who are likely to need multimodal intensified therapy 56.
An additional key feature with which to identify patients with aggressive biology and poor outcomes is LVI. The invasion of the numerous blood and lymphatic vessels in the lamina propria allow early haematogenous and lymphatic cancer cell dissemination 57. In this context, LVI might indicate a histological pattern associated with a higher propensity to muscle‐invasive, non‐organ‐confined disease and micrometastases. We found that LVI was associated with a more than double risk of upstaging after RC. Unfortunately, we could not identify any study analysing the impact of LVI on upstaging in NMIBC at TURBT; however, subgroup analysis showed that the association between LVI and upstaging was even higher in cT1–2 patients, while it was not significant in patients with cT2 disease. These results suggest that the presence of LVI is an independent prognostic factor of upstaging in cT1 disease, while it lost its significance in cT2 stage disease because of the greater aggressiveness of the cT2 disease itself. Thus, cT1 disease with LVI should be considered as an intermediate stage between cT1 and cT2 disease in terms of prognosis, thereby prognostically upstaging cT1. Whether this is because of the micrometastatic potential conferred by LVI remains to be determined. Further prospective studies could assess the indication to perform neoadjuvant systemic therapy prior to RC in patients with LVI in cT1 BCa. In patients with cT2 LVI, this has been already widely suggested 58, 59.
Critical for LVI assessment is the quality of the specimen. The rate of muscle presence in the specimen, which could be seen as a surrogate of quality of resection, ranged from 31% to 100% in the studies included in the review. Similarly, the rate of re‐staging TUR was often not reported and varied considerably among one‐third of studies included in the review. The depth of resection, the specimen size as well as the experience of the surgeon might influence the detection of LVI in the TUR specimen, the therapeutic decision‐making process and, therefore, the course of the disease.
The present meta‐analysis has some limitations. Only non‐randomized observational studies were included and all of them had a retrospective design. Furthermore, patients could not be controlled for the quality of the TUR, effect of repeat TUR and follow‐up schedules. In addition, a limitation was the different type of drug used for adjuvant therapy (BCG, mitomycin C, doxorubicin, epirubicin, etc.), the different treatment schedule and maintenance drug adopted. In addition, some studies did not provide a definition of LVI, usually described as the unequivocal presence of tumour cells within an endothelium‐lined space, with no underlying muscular walls 60. Studies separately analysing the combination of vascular and lymphatic invasion by tumour cells were excluded from the analysis as they may represent another disease entity, leading to heterogeneity. Pathology was performed by various pathologists and no study provided a centralized pathology assessment. Most of the studies did not report the long‐term oncological outcomes; this could have significantly influenced the results of the present meta‐analysis. Studies that did not identify independent predictors of outcomes are less likely to be published. All these limitations introduce a selection bias, as shown in the risk‐of‐bias assessments. Finally, in the analysis of the impact of LVI on upstaging (Figs 4 and S3) a significant heterogeneity was detected. This could be related to the different surgical indication to perform RC related to centres’ and surgeons’ preference according to the clinical features of each patient and to treatments other than RC not considered in this analysis, such as partial cystectomy and trimodality therapy.
In conclusion, despite the limitations inherent to the retrospective nature of studies included, the results of the present meta‐analysis suggest that LVI at TUR is a significant prognostic factor for disease recurrence and progression in patients with NMIBC. Furthermore, LVI seems to have a strong impact on upstaging. The assessment of LVI should be standardized and included in the TNM system. This readily accessible histological feature, together with other factors, may help clinicians to design more personalized management strategies helping in the decision‐making regarding intensified therapy, counselling and follow‐up for patients with NMIBC.
Conflict of Interest
Prof. Shahrokh F. Shariat owns or co‐owns the following patents: Methods to determine prognosis after therapy for prostate cancer, granted 2002‐09‐06; Methods to determine prognosis after therapy for bladder cancer, granted 2003‐06‐19; Prognostic methods for patients with prostatic disease, granted 2004‐08‐05; Soluble Fas: urinary marker for the detection of bladder transitional cell carcinoma, granted 2010‐07‐20. He is also an advisory board member of Astellas, Cepheid, Ipsen, Jansen, Lilly, Olympus, Pfizer, Pierre Fabre, Sanofi and Wolff and a speaker for Astellas, Ipsen, Jansen, Lilly, Olympus, Pfizer, Pierre Fabre, Sanochemia, Sanofi and Wolff. The remaining authors have no conflicts of interest to declare.
Abbreviations
- BCa
bladder cancer
- CUETO
Spanish Urological Club for Oncological Treatment
- EORTC
European Organization for Research and Treatment of Cancer () [3]
- HR
hazard ratio
- LVI
lymphovascular invasion
- MIBC
carcinoma invading the bladder muscle
- NMIBC
non‐muscle invasive bladder cancer
- PRISMA
Preferred Reporting Items for Systematic reviews and Meta‐Analyses
- RC
radical cystectomy
- ROBINS‐I
Risk Of Bias In Non‐randomised Studies ‐ of Interventions
- TURBT
transurethral resection of the bladder tumour
- TUR
transurethral resection
Supporting information
Fig. S1 Funnel plot showing the effect estimates of individual studies for the analysis of the impact of lymphovascular invasion on disease recurrence in patients treated with transurethral resection for non‐muscle‐invasive bladder cancer.
Fig. S2 Funnel plot showing the effect estimates of individual studies for the analysis of the impact of lymphovascular invasion on disease progression in patients treated with transurethral resection for non‐muscle‐invasive bladder cancer.
Fig. S3 Funnel plot showing the effect estimates of individual studies for the analysis of the impact of lymphovascular invasion on disease upstaging in patients treated with transurethral resection and consequently to radical cystectomy for organ‐confined (≤cT2) bladder cancer.
Table S1 PRISMA‐P (Preferred Reporting Items for Systematic review and Meta‐Analysis Protocols) 2015 checklist: recommended items to address in a systematic review protocol.
Table S2 The Risk of Bias In Non‐randomized Studies – of Interventions (ROBINS‐I) assessment of individual studies for the analysis of the impact of lymphovascular invasion on disease recurrence in patients treated with transurethral resection for non‐muscle‐invasive bladder cancer.
Table S3 The Risk of Bias In Non‐randomized Studies – of Interventions (ROBINS‐I) assessment of individual studies for the analysis of the impact of lymphovascular invasion on disease progression in patients treated with transurethral resection for non‐muscle‐invasive bladder cancer.
Table S4 The Risk of Bias In Non‐randomized Studies – of Interventions (ROBINS‐I) assessment of individual studies for the analysis of the impact of lymphovascular invasion on disease upstaging in patients treated with transurethral resection for non‐muscle‐invasive bladder cancer.
A.M. and S.K. contributed equally to this project and share first authorship.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68: 7–30 [DOI] [PubMed] [Google Scholar]
- 2. Babjuk M, Bohle A, Burger M et al. EAU guidelines on non‐muscle‐invasive urothelial carcinoma of the bladder: update 2016. Eur Urol 2017; 71: 447–61 [DOI] [PubMed] [Google Scholar]
- 3. Sylvester RJ, van der Meijden APM, Oosterlinck W et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 2006; 49: 466–7 [DOI] [PubMed] [Google Scholar]
- 4. Mathieu R, Lucca I, Roupret M et al. The prognostic role of lymphovascular invasion in urothelial carcinoma of the bladder. Nat Rev Urol 2016; 13: 471–9 [DOI] [PubMed] [Google Scholar]
- 5. Shariat SF, Karakiewicz PI, Palapattu GS et al. Nomograms provide improved accuracy for predicting survival after radical cystectomy. Clin Cancer Res 2006; 12: 6663–76 [DOI] [PubMed] [Google Scholar]
- 6. Kikuchi E, Margulis V, Karakiewicz PI et al. Lymphovascular invasion predicts clinical outcomes in patients with node‐negative upper tract urothelial carcinoma. J Clin Oncol 2009; 27: 612–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shariat SF, Svatek RS, Tilki D et al. International validation of the prognostic value of lymphovascular invasion in patients treated with radical cystectomy. BJU Int 2010; 105: 1402–12 [DOI] [PubMed] [Google Scholar]
- 8. Tilki D, Shariat SF, Lotan Y et al. Lymphovascular invasion is independently associated with bladder cancer recurrence and survival in patients with final stage T1 disease and negative lymph nodes after radical cystectomy. BJU Int 2013; 111: 1215–21 [DOI] [PubMed] [Google Scholar]
- 9. Kim HS, Kim M, Jeong CW et al. Presence of lymphovascular invasion in urothelial bladder cancer specimens after transurethral resections correlates with risk of upstaging and survival: a systematic review and meta‐analysis. Urol Oncol Orig Investig 2014; 32: 1191–9 [DOI] [PubMed] [Google Scholar]
- 10. Go H, Kim P‐J, Jeon YK et al. Sphingosine‐1‐phosphate receptor 1 (S1PR1) expression in non‐muscle invasive urothelial carcinoma: association with poor clinical outcome and potential therapeutic target. Eur J Cancer 2015; 51: 1937–45 [DOI] [PubMed] [Google Scholar]
- 11. Miyake M, Gotoh D, Shimada K et al. Exploration of risk factors predicting outcomes for primary T1 high‐grade bladder cancer and validation of the Spanish Urological Club for Oncological Treatment scoring model: long‐term follow‐up experience at a single institute. Int J Urol 2015; 22: 541–7 [DOI] [PubMed] [Google Scholar]
- 12. Patschan O, Holmang S, Hosseini A et al. Use of bacillus Calmette‐Guerin in stage T1 bladder cancer: long‐term observation of a population‐based cohort. Scand J Urol 2015; 49: 127–32 [DOI] [PubMed] [Google Scholar]
- 13. Weiss BE, Pietzak EJ, Wein AJ et al. Single instillation of mitomycin C plus bacillus Calmette‐Guerin (BCG) versus BCG alone in high grade non‐muscle invasive bladder cancer. Can J Urol 2015; 22: 7876–81 [PubMed] [Google Scholar]
- 14. Fukumoto K, Kikuchi E, Mikami S et al. Lymphovascular invasion status at transurethral resection of bladder tumors may predict subsequent poor response of T1 tumors to bacillus Calmette‐Guerin. BMC Urol 2016; 16: 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haas CR, Barlow LJ, Badalato GM et al. The timing of radical cystectomy for bacillus Calmette‐Guerin failure: comparison of outcomes and risk factors for prognosis. J Urol 2016; 195: 1704–9 [DOI] [PubMed] [Google Scholar]
- 16. Li G, Song H, Wang J et al. Poor prognostic value of lymphovascular invasion for pT1 urothelial carcinoma with squamous differentiation in bladder cancer. Sci Rep 2016; 6: 27586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sha N, Xie L, Chen T et al. Impact of lymphovascular invasion on recurrence and progression rates in patients with pT1 urothelial carcinoma of bladder after transurethral resection. Onco Targets Ther 2015; 8: 3401–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ukai R, Hashimoto K, Nakayama H, Iwamoto T. Lymphovascular invasion predicts poor prognosis in high‐grade pT1 bladder cancer patients who underwent transurethral resection in one piece. Jpn J Clin Oncol 2017; 47: 447–52 [DOI] [PubMed] [Google Scholar]
- 19. Moher D, Shamseer L, Clarke M et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev 2015; 4: 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liberati A, Altman DG, Tetzlaff J et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6: e1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Higgins JP. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). Cochrane Collab, 2011.
- 22. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986; 7: 177–88 [DOI] [PubMed] [Google Scholar]
- 23. DerSimonian R, Kacker R. Random‐effects model for meta‐analysis of clinical trials: an update. Contemp Clin Trials 2007; 28: 105–14 [DOI] [PubMed] [Google Scholar]
- 24. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003; 327: 557–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Deeks JJ, Dinnes J, D'Amico R et al. Evaluating non‐randomised intervention studies. 2003; Health Technol Assess 7:iii–x, 1–173. [DOI] [PubMed] [Google Scholar]
- 26. Reeves BC, Deeks JJ, Higgins JP, Wells GA. Chapter 13 Including non‐randomised studies, 2011.
- 27. Sterne JAC, Hernán MA, Reeves BC et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. BMJ 2016; 355: i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Andius P, Johansson SL, Holmang S. Prognostic factors in stage T1 bladder cancer: tumor pattern (solid or papillary) and vascular invasion more important than depth of invasion. Urology 2007; 70: 758–62 [DOI] [PubMed] [Google Scholar]
- 29. Kassouf W, Spiess PE, Brown GA et al. P0 Stage at radical cystectomy for bladder cancer is associated with improved outcome independent of traditional clinical risk factors. Eur Urol 2007; 52: 769–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Herr HW. Outcome of patients who refuse cystectomy after receiving neoadjuvant chemotherapy for muscle‐invasive bladder cancer. Eur Urol 2008; 54: 126–32 [DOI] [PubMed] [Google Scholar]
- 31. Weizer AZ, Wasco MJ, Wang R et al. Multiple adverse histological features increase the odds of under staging T1 bladder cancer. J Urol 2009; 182: 59–65 [DOI] [PubMed] [Google Scholar]
- 32. Cho KS, Seo HK, Joung JY et al. Lymphovascular invasion in transurethral resection specimens as predictor of progression and metastasis in patients with newly diagnosed T1 bladder urothelial cancer. J Urol 2009; 182: 2625–30 [DOI] [PubMed] [Google Scholar]
- 33. Streeper NM, Simons CM, Konety BR et al. The significance of lymphovascular invasion in transurethral resection of bladder tumour and cystectomy specimens on the survival of patients with urothelial bladder cancer. BJU Int 2009; 103: 475–9. [DOI] [PubMed] [Google Scholar]
- 34. Seo HK, Cho KS, Chung J et al. Prognostic value of p53 and Ki‐67 expression in intermediate‐risk patients with nonmuscle‐invasive bladder cancer receiving adjuvant intravesical mitomycin C therapy. Urology 2010; 76:512.e1‐7 [DOI] [PubMed] [Google Scholar]
- 35. Font A, Taron M, Gago JL et al. BRCA1 mRNA expression and outcome to neoadjuvant cisplatin‐based chemotherapy in bladder cancer. Ann Oncol 2011; 22: 139–44 [DOI] [PubMed] [Google Scholar]
- 36. Rodriguez Faba O, Palou J, Rosales A et al. Clinical predictive factors of poor outcome in patients with stage PT0 disease at radical cystectomy. J Urol 2011; 186: 442–7 [DOI] [PubMed] [Google Scholar]
- 37. Badalato GM, Gaya JM, Hruby G et al. Immediate radical cystectomy vs conservative management for high grade cT1 bladder cancer: is there a survival difference? BJU Int 2012; 110: 1471–7 [DOI] [PubMed] [Google Scholar]
- 38. Green DA, Rink M, Hansen J et al. Accurate preoperative prediction of non‐organ‐confined bladder urothelial carcinoma at cystectomy. BJU Int 2013; 111: 404–11 [DOI] [PubMed] [Google Scholar]
- 39. Xie HY, Zhu Y, Yao XD et al. Development of a nomogram to predict non‐organ‐confined bladder urothelial cancer before radical cystectomy. Int Urol Nephrol 2012; 44: 1711–9 [DOI] [PubMed] [Google Scholar]
- 40. Kwon DH, Song PH, Kim HT. Multivariate analysis of the prognostic significance of resection weight after transurethral resection of bladder tumor for non‐muscle‐invasive bladder cancer. Korean J Urol 2012; 53: 457–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Branchereau J, Larue S, Vayleux B et al. Prognostic value of the lymphovascular invasion in high‐grade stage pT1 bladder cancer. Clin Genitourin Cancer 2013; 11: 182–8 [DOI] [PubMed] [Google Scholar]
- 42. Brimo F, Wu C, Zeizafoun N et al. Prognostic factors in T1 bladder urothelial carcinoma: the value of recording millimetric depth of invasion, diameter of invasive carcinoma, and muscularis mucosa invasion. Hum Pathol 2013; 44: 95–102 [DOI] [PubMed] [Google Scholar]
- 43. Bolenz C, Auer M, Strobel P et al. The lymphatic system in clinically localized urothelial carcinoma of the bladder: morphologic characteristics and predictive value. Urol Oncol Semin Orig Investig 2013; 31: 1606–14 [DOI] [PubMed] [Google Scholar]
- 44. Levidou G, Thymara I, Saetta AA et al. TRAIL and osteoprotegerin (OPG) expression in bladder urothelial carcinoma: correlation with clinicopathological parameters and prognosis. Pathology 2013; 45: 138–44 [DOI] [PubMed] [Google Scholar]
- 45. Olsson H, Hultman P, Rosell J, Jahnson S. Population‐based study on prognostic factors for recurrence and progression in primary stage T1 bladder tumours. Scand J Urol 2013; 47: 188–95 [DOI] [PubMed] [Google Scholar]
- 46. Kaimakliotis HZ, Monn MF, Cary KC et al. Plasmacytoid variant urothelial bladder cancer: is it time to update the treatment paradigm? Urol Oncol 2014; 32: 833–8 [DOI] [PubMed] [Google Scholar]
- 47. Prelevic R, Stojadinovic M, Simic D et al. Scoring system development for prediction of extravesical bladder cancer. Vojnosanit Pregl 2014; 71: 851–7 [PubMed] [Google Scholar]
- 48. Svatek RS, Clinton TN, Wilson CA et al. Intravesical tumor involvement of the trigone is associated with nodal metastasis in patients undergoing radical cystectomy. Urology 2014; 84: 1147–51 [DOI] [PubMed] [Google Scholar]
- 49. Goldsmith B, Baumann BC, He J et al. Occult pelvic lymph node involvement in bladder cancer: implications for definitive radiation. Int J Radiat Oncol Biol Phys 2014; 88: 603–10 [DOI] [PubMed] [Google Scholar]
- 50. Pietzak EJ, Sterling ME, Smith ZL et al. Outcomes of radical cystectomy in potential candidates for bladder preservation therapy. Urology 2015; 85: 869–75 [DOI] [PubMed] [Google Scholar]
- 51. Lucca I, Hofbauer SL, Leitner CV et al. Development of a Preoperative Nomogram Incorporating Biomarkers of Systemic Inflammatory Response to Predict Nonorgan‐confined Urothelial Carcinoma of the Bladder at Radical Cystectomy. Urology 2016; 95: 132–8 [DOI] [PubMed] [Google Scholar]
- 52. Novara G, Matsumoto K, Kassouf W et al. Prognostic role of lymphovascular invasion in patients with urothelial carcinoma of the upper urinary tract: an international validation study. Eur Urol 2010; 57: 1064–71 [DOI] [PubMed] [Google Scholar]
- 53. Lago‐Hernandez CA, Feldman H, O'Donnell E et al. A refined risk stratification scheme for clinical stage 1 NSGCT based on evaluation of both embryonal predominance and lymphovascular invasion. Ann Oncol Off J Eur Soc Med Oncol 2015; 26: 1396–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fernandez‐Gomez J, Solsona E, Unda M et al. Prognostic factors in patients with non‐muscle‐invasive bladder cancer treated with bacillus Calmette‐Guerin: multivariate analysis of data from four randomized CUETO trials. Eur Urol 2008; 53: 992–1001 [DOI] [PubMed] [Google Scholar]
- 55. Xylinas E, Kent M, Kluth L et al. Accuracy of the EORTC risk tables and of the CUETO scoring model to predict outcomes in non‐muscle‐invasive urothelial carcinoma of the bladder. Br J Cancer 2013; 109: 1460–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Abufaraj M, Shariat SF, Foerster B et al. Accuracy and prognostic value of variant histology and lymphovascular invasion at transurethral resection of bladder. World J Urol 2018; 36: 231–40 [DOI] [PubMed] [Google Scholar]
- 57. Gakis G, Todenhofer T, Braun M et al. Immunohistochemical assessment of lymphatic and blood vessel invasion in T1 urothelial carcinoma of the bladder. Scand J Urol 2015; 49: 382–7. [DOI] [PubMed] [Google Scholar]
- 58. Millikan R, Dinney C, Swanson D et al. Integrated therapy for locally advanced bladder cancer: final report of a randomized trial of cystectomy plus adjuvant M‐VAC versus cystectomy with both preoperative and postoperative M‐VAC. J Clin Oncol 2001; 19: 4005–13. [DOI] [PubMed] [Google Scholar]
- 59. Moschini M, Soria F, Klatte T et al. Validation of preoperative risk grouping of the selection of patients most likely to benefit from neoadjuvant chemotherapy before radical cystectomy. Clin Genitourin Cancer 2017; 15: e267–73. [DOI] [PubMed] [Google Scholar]
- 60. Lapham RL, Grignon D, Ro JY. Pathologic prognostic parameters in bladder urothelial biopsy, transurethral resection, and cystectomy specimens. Semin Diagn Pathol 1997; 14: 109–22 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Funnel plot showing the effect estimates of individual studies for the analysis of the impact of lymphovascular invasion on disease recurrence in patients treated with transurethral resection for non‐muscle‐invasive bladder cancer.
Fig. S2 Funnel plot showing the effect estimates of individual studies for the analysis of the impact of lymphovascular invasion on disease progression in patients treated with transurethral resection for non‐muscle‐invasive bladder cancer.
Fig. S3 Funnel plot showing the effect estimates of individual studies for the analysis of the impact of lymphovascular invasion on disease upstaging in patients treated with transurethral resection and consequently to radical cystectomy for organ‐confined (≤cT2) bladder cancer.
Table S1 PRISMA‐P (Preferred Reporting Items for Systematic review and Meta‐Analysis Protocols) 2015 checklist: recommended items to address in a systematic review protocol.
Table S2 The Risk of Bias In Non‐randomized Studies – of Interventions (ROBINS‐I) assessment of individual studies for the analysis of the impact of lymphovascular invasion on disease recurrence in patients treated with transurethral resection for non‐muscle‐invasive bladder cancer.
Table S3 The Risk of Bias In Non‐randomized Studies – of Interventions (ROBINS‐I) assessment of individual studies for the analysis of the impact of lymphovascular invasion on disease progression in patients treated with transurethral resection for non‐muscle‐invasive bladder cancer.
Table S4 The Risk of Bias In Non‐randomized Studies – of Interventions (ROBINS‐I) assessment of individual studies for the analysis of the impact of lymphovascular invasion on disease upstaging in patients treated with transurethral resection for non‐muscle‐invasive bladder cancer.


