Abstract
Background
Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR‐TKIs) are used to treat patients with non‐small cell lung cancer (NSCLC) and EGFR driver mutations. Although some patients discontinued these treatments because of adverse events, it is unclear whether switching EGFR‐TKI because of adverse events provides a benefit.
Methods
This retrospective study evaluated data from 22 patients with EGFR mutation‐positive NSCLC who received at least two EGFR‐TKIs that were switched because of adverse events (March 2011 to September 2017). Progression‐free survival 2 (PFS2) was defined as the time from starting of the first EGFR‐TKI treatment to disease progression during the second EGFR‐TKI treatment.
Results
Seventeen patients received gefitinib as the first EGFR‐TKI treatment, while four patients received afatinib and one patient received erlotinib. The median time to failure of the first EGFR‐TKI treatment was 1.6 months. The EGFR‐TKIs were switched because of hepatotoxicity (n = 16), interstitial lung disease (n = 3), and other reasons (n = 3). The median washout period was 1.1 months. Seventeen patients received erlotinib as the second EGFR‐TKI treatment, while three patients received gefitinib and two patients received afatinib. The median PFS for the second EGFR‐TKI treatment was 15.2 months. The median PFS2 was 17.7 months and the median overall survival was 32.8 months.
Conclusions
Switching EGFR‐TKIs because of adverse events provided a clinical benefit for patients with EGFR mutation‐positive NSCLC. Appropriate judgment regarding switching from one EGFR‐TKI to another may improve the performance status and prognosis of patients with EGFR mutation‐positive NSCLC.
Keywords: epidermal growth factor receptor, non‐small cell lung cancer, tyrosine kinase inhibitor
1. INTRODUCTION
Patients with advanced non‐small cell lung cancer (NSCLC) and epidermal growth factor receptor (EGFR) mutations can receive first‐line treatment using EGFR tyrosine kinase inhibitors (EGFR‐TKIs).1, 2 These drugs include gefitinib, erlotinib, and afatinib, which provide response rates of approximately 60‐70% and a median progression‐free survival (PFS) of approximately 12 months.3, 4, 5 However, a small proportion of patients with NSCLC and EGFR mutations discontinue EGFR‐TKI therapy because of adverse events (AEs).6, 7 In clinical practice, one approach to this development is switching from one EGFR‐TKI to another. However, to the best of our knowledge, only a few case series have evaluated treatment response after switching EGFR‐TKIs because of AEs, rather than disease progression (PD).8, 9 Therefore, this retrospective study evaluated the efficacy and outcomes of switching EGFR‐TKIs because of AEs.
2. PATIENTS AND METHODS
This retrospective study was approved by the institutional review board of Saiseikai Kumamoto Hospital (approved number: 597). We identified patients with advanced NSCLC who received at least two EGFR‐TKIs between March 2011 and March 2017 by searching our hospital's prescription drug database. All patients were followed‐up until September 2017. We identified 45 patients who underwent EGFR‐TKI switching, although 21 patients switched because of PD and were excluded. In addition, we excluded two patients who were lost to follow‐up. Thus, 22 patients who switched EGFR‐TKIs because of AEs were included. Treatment response was determined based on version 1.1 of the Response Evaluation Criteria in Solid Tumors. We defined patients without clear PD within 6 weeks as stable disease. Time to treatment failure (TTF) was assessed from the first EGFR‐TKI treatment start date to the date of the first instance of PD or treatment withdrawal because of AEs. PFS was calculated from the start date of EGFR‐TKI treatment to the date of the first instance of PD or death or lost to follow‐up. PFS2 was defined as the time from the start of the first EGFR‐TKI treatment to PD that was detected during the second EGFR‐TKI treatment. Overall survival (OS) was defined as the time from the start of the first EGFR‐TKI treatment to the date of death or data cut‐off and was estimated using the Kaplan‐Meier method. AEs were graded according to version 4.0 of the National Cancer Institute's Common Toxicity Criteria for Adverse Events. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R software (The R Foundation for Statistical Computing, Vienna, Austria).10
3. RESULTS
The characteristics and clinical outcomes of the 22 patients are summarized in Table 1. Two patients received platinum‐based chemotherapy as first‐line treatment. Seventeen patients received gefitinib as the first EGFR‐TKI treatment, while four patients received afatinib and one patient received erlotinib. The median TTF was 1.6 months (95% confidence interval [CI]: 1.1‐3.0 months). The reasons for switching EGFR‐TKIs were hepatotoxicity (n = 16), interstitial lung disease (ILD; n = 3), rash (n = 1), and combined factors (n = 2). Four out of 16 patients who had developed the first EGFR‐TKI (gefitinib)‐related hepatotoxicity had mild hepatotoxicity (less than Grade 2) while receiving the second EGFR‐TKI. Two patients who had stopped receiving gefitinib because of hepatotoxicity were continued on active surveillance for relatively long periods (11 and 7 months, respectively).
Table 1.
Characteristics of patients with EGFR mutation‐positive NSCLC who received both first‐ and second‐line EGFR‐TKI
| No. | Sex | Age | PS | EGFR mutation | TKI sequence | Reasons for discontinuation (according to CTCAE v4.0) | Response to first TKI | TTF | Interval between TKIs | Response to second TKI | PFS for second TKI | PFS2 | OS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 49 | 1 | 19del | G (1) → E (2) | Hepatotoxicity (Gr3) | PR | 5.8 | 1.2 | SD | 2.1 | 9.1 | 30.3 |
| 2 | M | 81 | 1 | 19del | G (1) → E (2) | ILD (Gr3) | PR | 3.6 | 3.0 | PR | 1.8 | 8.4 | 8.4 |
| 3 | M | 79 | 0 | 19del | G (2) → E (3) | Hepatotoxicity (Gr3) | SD | 1.7 | 3.7 | PR | 9.8 | 15.2 | 25.9 |
| 4 | F | 71 | 0 | 19del | G (1) → E (2) | Hepatotoxicity (Gr4) | PR | 1.1 | 7.7 | SD | 19.1 | 27.9 | 58.3a |
| 5 | F | 83 | 0 | L858R | G (1) → E (2) | Hepatotoxicity (Gr2) | CR | 3.0 | 1.7 | CR | 9.3 | 14.0 | 19.8 |
| 6 | F | 75 | 1 | L858R | G (1) → E (2) | Hepatotoxicity (Gr2) | PR | – | 0.6 | SD | 2.4 | 17.3 | 31.2 |
| 7 | M | 63 | 0 | L858R | G (1) → E (2) | Hepatotoxicity (Gr3) | PR | 2.4 | 0.8 | SD | 30.1 | 33.4 | 53.3a |
| 8 | F | 59 | 0 | L858R | G (1) → E (2) | Hepatotoxicity (Gr3) | PR | 1.1 | 1.1 | PR | 12.5 | 14.7 | 32.8 |
| 9 | M | 56 | 0 | 19del | G (1) → E (2) | Hepatotoxicity (Gr3) | PR | 1.6 | 0.5 | PR | 45.8a | 47.9a | 47.9a |
| 10 | F | 62 | 0 | L858R | G (1) → E (3) | Hepatotoxicity (Gr4) | PR | 0.9 | 11.2 | PR | 8.9 | 20.9 | 40.1 |
| 11 | F | 84 | 0 | 19del | G (1) → E (2) | Hepatotoxicity (Gr2) | PR | 1.4 | 0.9 | SD | 32.8 | 35.2 | 46.8a |
| 12 | F | 69 | 1 | L858R | G (1) → E (2) | Hepatotoxicity (Gr4) | PR | 4.6 | 0.9 | SD | 1.6 | 7.1 | 20.6 |
| 13 | F | 67 | 0 | 19del | A (2) → G (3) | Paronychia, anorexia, diarrhea (Gr2) | PR | 2.3 | 1.0 | SD | 30.5 | 33.8 | 36.8a |
| 14 | F | 58 | 0 | 19del | A (1) → E (2) | ILD (Gr2) | PR | 4.1 | 2.3 | PR | 30.3a | 36.7a | 36.7a |
| 15 | F | 61 | 1 | L858R | G (1) → E (2) | Hepatotoxicity (Gr4) | SD | 1.6 | 1.4 | SD | 17.3 | 20.3 | 22.4 |
| 16 | F | 57 | 0 | 19del | A (1) → E (2) | ILD (Gr2) | SD | 1.5 | 1.1 | SD | 1.7 | 4.4 | 7.4 |
| 17 | F | 70 | 0 | 19del | G (1) → E (2) | Hepatotoxicity (Gr3) | PR | 0.8 | 2.0 | SD | 3.7 | 6.5 | 27.3a |
| 18 | F | 67 | 0 | 19del | E (1) → G (2) | Rash (Gr3) | PR | 6.8 | 0.4 | SD | 18.4 | 25.6 | 27.1a |
| 19 | M | 69 | 0 | L858R | G (1) → E (2) | Hepatotoxicity (Gr4) | SD | 1.1 | 1.5 | PR | 15.2 | 17.7 | 24.2a |
| 20 | M | 74 | 0 | L858R | G (1) → A (2) | Hepatotoxicity (Gr3) | SD | 1.4 | 1.5 | SD | 10.3a | 13.2a | 13.2a |
| 21 | M | 74 | 0 | 19del | G (1) → A (2) | Hepatotoxicity (Gr2) | PR | 1.6 | 0.6 | SD | 11.0a | 13.2a | 13.2a |
| 22 | F | 50 | 0 | L858R | A (1) → G (2) | Rash, anorexia, diarrhea (Gr2) | SD | 0.3 | 0.5 | PR | 6.2a | 7.0a | 7.0a |
A, afatinib; CR, complete response; CTCAE, common terminology criteria for adverse events; E, erlotinib; EGFR, epidermal growth factor receptor; F, female; Gr, grade; G, gefitinib; ILD, interstitial lung disease; L858R, exon‐21 mutation L858R; M, male; OS, overall survival; PD, progressive disease; PFS, progression‐free survival; PR, partial response; PS, performance status; SD, stable disease; TK1, tyrosine kinase inhibitor; TTF, time to treatment failure; 19del, exon‐19 deletion.
Unit of time is months.
Patients have continued the second EGFR‐TKI treatment, or back‐line therapy, and the latest follow‐up data were collected on 30 September 2017.
Three patients developed the first EGFR‐TKI‐related ILD. One of the three patients relapsed due to ILD while receiving the second EGFR‐TKI, which resulted in the death of the patient.
Patient 2 was treated with gefitinib as first‐line chemotherapy. After 17 weeks of gefitinib treatment, the patient had a gefitinib‐induced Grade 3 ILD. The discontinuation of gefitinib and initiation of methylprednisolone (40 mg/day) treatment brought about improvement in the findings. Three months after the discontinuation of gefitinib and the tapering off of prednisolone (10 mg/day), the patient received and continued with erlotinib treatment for 8 weeks. However, the patient had recurrence and died of ILD.
Patient 14 was treated with afatinib as a first‐line chemotherapy. After 18 weeks of afatinib treatment, the patient had an afatinib‐induced asymptomatic ILD. The discontinuation of afatinib and initiation of prednisolone (20 mg/day) treatment also resulted in an improvement of the findings. Ten weeks after the discontinuation of afatinib and the tapering off of prednisolone (5 mg/day), the patient was commenced on erlotinib, which was continued while the steroid therapy was completed after 2 weeks. No recurrent ILD was observed during the observation period.
Patient 16 was treated with afatinib as first‐line chemotherapy. After 7 weeks of afatinib treatment, the patient developed difficulty in breathing, and chest computed tomography demonstrated localized ground‐glass opacity around the primary lung tumor. It was discovered that the patient had been treated in combination with hyperthermia treatment without the permission of the attending physician. Five weeks after the discontinuation of afatinib, the patient received erlotinib and continued on the treatment for 7 weeks. However, the patient hoped to receive alternative medicine and was lost to follow‐up.
The median interval between the first and second EGFR‐TKI treatments was 1.1 months (95% CI: 0.8‐1.7 months). Seventeen patients received erlotinib as the second EGFR‐TKI treatment, while three patients received gefitinib and two patients received afatinib. At the data cut‐off date (30 September 2017), five patients were continuing the second EGFR‐TKI treatment (Figure 1) and 12 patients had been treated using back‐line therapy. The median PFS for the second EGFR‐TKI treatment was 15.2 months (95% CI: 3.7‐30.1 months). The median PFS2 was 17.7 months (95% CI: 14.0‐27.9 months) (Figure 2), and the median OS was 32.8 months (95% CI: 22.4 months to not reached) (Figure 3).
Figure 1.
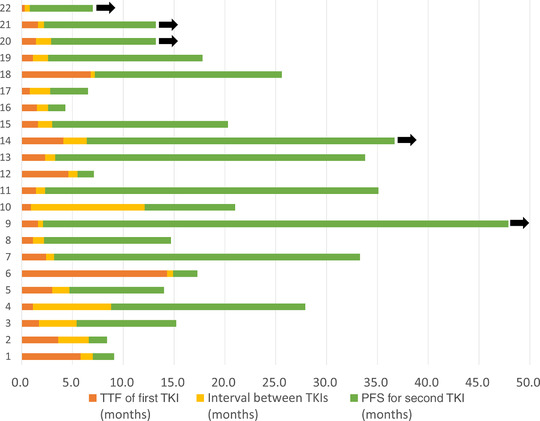
The swimmer plots for durations of the first and second EGFR‐TKI treatments. Black arrows indicate that the patient is still receiving treatment.
TTF, time to treatment failure; TKI, tyrosine kinase inhibitor; PFS, progression‐free survival [Color figure can be viewed at wileyonlinelibrary.com]
Figure 2.
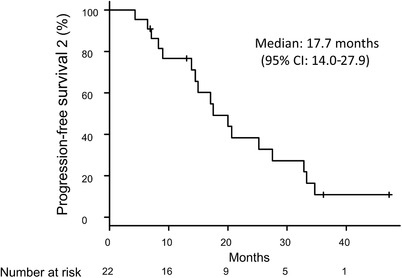
Progression‐free survival from the start of the first EGFR‐TKI treatment to progression during the second EGFR‐TKI treatment (PFS2).
CI, confidence interval
Figure 3.
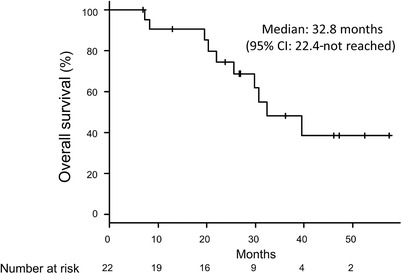
Overall survival among all patients.
CI, confidence interval
4. DISCUSSION
Patients with EGFR mutation‐positive NSCLC can receive EGFR‐TKIs as a standard treatment,1, 2 and most patients are treated with relatively controlled toxicity. However, some patients experience AEs and do not wish to continue treatment using the same drug. For example, two studies of patients with EGFR mutation‐positive NSCLC indicated that 6.1‐7.7% of patients refused to continue EGFR‐TKI treatment because of various AEs such as ILD and hepatitis.6, 7 Although there are some case reports describing successful EGFR‐TKI rechallenge after recovery from EGFR‐TKI‐induced AEs, few studies have examined switching EGFR‐TKIs because of AEs, rather than PD. For example, Takeda et al. reported five patients who discontinued treatment with first EGFR‐TKI, because of severe drug‐related toxicity (ILD in three cases, hepatotoxicity in one case, and rash in one case), and subsequently received a second EGFR‐TKI treatment.8 Kashiwabara et al. reported five patients who refused to continue treatment using first EGFR‐TKI, but were subsequently rechallenged using a second EGFR‐TKI.9 Neither report examined the safety of the second EGFR‐TKI treatment and whether it provided a beneficial effect or not. Thus, the present study builds on the experience of those researchers.
The present study evaluated PFS2, which is a surrogate endpoint for OS and is generally defined as the time from randomization to objective tumor progression or death during next‐line treatment.11 However, we defined PFS2 as the time from the start of the first EGFR‐TKI treatment to PD during the second EGFR‐TKI treatment. We believe that this approach provides a reasonable endpoint for evaluating the effect of switching EGFR‐TKI treatments, and we observed that the PFS and PFS2 outcomes were favorable, compared to the results from previous trials of EGFR‐TKI treatments. These results suggest that switching EGFR‐TKI treatments is a valid approach. In addition, hepatotoxicity was the main reason for switching EGFR‐TKIs in the present study, and the AEs were not always serious. Similarly, several case reports have suggested that switching EGFR‐TKIs may be possible in cases with severe hepatic dysfunction.12, 13, 14 Thus, our findings and those previous results indicate that EGFR‐TKI switching is likely effective for patients who develop hepatitis and have an EGFR driver mutation. Furthermore, switching EGFR‐TKIs before the patient develops severe hepatitis may protect their performance status for back‐line therapy.
Approximately 60% of patients with EGFR mutations have the T790M mutation when they experience PD during the first EGFR‐TKI treatment, and sequential treatment using a third‐generation EGFR‐TKI is critical for these patients.15 In addition, a previous study revealed that long periods of EGFR‐TKI treatment before rebiopsy may provide useful information regarding expression of the T790M mutation.16 Therefore, our results also provide useful data for treating patients who experience AEs during EGFR‐TKI treatment.
The present study has several limitations. First, we retrospectively searched for clinical records from a single institution, and the sample size was small. Furthermore, PFS may not be an adequate endpoint in retrospective study, because evaluation interval was not performed periodically. Second, there is a possibility of selection bias, as we only included patients who received a first and second EGFR‐TKI, although approximately 8% of patients do not respond to gefitinib.17 Thus, although these cases are rare, it is possible that we excluded patients who could not receive a second EGFR‐TKI treatment because of early PD or serious AEs. Third, the follow‐up period was short and 12 of the 22 patients were continuing treatment using a second EGFR‐TKI or back‐line chemotherapy at the data cut‐off point. In this context, retrospective analysis of real‐world Japanese clinical data revealed that the median OS after first‐line treatment was approximately 30.8 months in patients with an EGFR mutation.18 In contrast, the present study revealed a median OS of 32.8 months. Although it appears that our findings do not indicate inferior OS after EGFR‐TKI switching, further studies with longer follow‐ups are needed to evaluate this possibility.
In conclusion, switching EGFR‐TKI treatments because of AEs is an effective option for patients with EGFR mutation‐positive NSCLC.
ETHICAL CONSIDERATIONS
The study protocol was approved by the Research Ethics Committee of our institution, and the research complied with the 1964 Declaration of Helsinki and its later amendments.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
Supporting information
Appendix Table. Additional characteristics of patients with EGFR mutation‐positive NSCLC who received both first‐ and second‐line EGFR‐TKI
Sakata Y, Kawamura K, Shingu N, et al. The effects of switching EGFR‐TKI treatments for non‐small cell lung cancer because of adverse events. Asia‐Pac J Clin Oncol. 2020;16:e113–e117. 10.1111/ajco.13103
REFERENCES
- 1. Hanna N, Johnson D, Temin S, Jr , et al. Systemic therapy for stage IV Non–Small‐Cell lung cancer: American society of clinical oncology clinical practice guideline update. J Clin Oncol. 2017;35:3484–3515. [DOI] [PubMed] [Google Scholar]
- 2. Novello S, Barlesi F, Califano R, et al. Metastatic non‐small‐cell lung cancer: eSMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2016;27(Suppl 5):v1–v27. [DOI] [PubMed] [Google Scholar]
- 3. Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non‐small‐cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. [DOI] [PubMed] [Google Scholar]
- 4. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first‐line treatment for European patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (EURTAC): a multicentre, open‐label, randomized phase 3 trial. Lancet Oncol. 2012;13:239–246. [DOI] [PubMed] [Google Scholar]
- 5. Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–3334. [DOI] [PubMed] [Google Scholar]
- 6. Takeda M, Okamoto I, Nakagawa K. Pooled safety analysis of EGFR‐TKI treatment for EGFR mutation‐positive non‐small cell lung cancer. Lung Cancer. 2015;88:74–79. [DOI] [PubMed] [Google Scholar]
- 7. Ding PN, Lord SJ, Gebski V, et al. Risk of treatment‐related toxicities from EGFR tyrosine kinase inhibitors: a meta‐analysis of clinical trials of gefitinib, erlotinib, and afatinib in advanced EGFR‐mutated non‐small cell lung cancer. J Thorac Oncol. 2017;12:633–643. [DOI] [PubMed] [Google Scholar]
- 8. Takeda M, Okamoto I, Tsurutani J, Oiso N, Kawada A, Nakagawa K. Clinical impact of switching to a second EGFR‐TKI after a severe AE related to a first EGFR‐TKI in EGFR‐mutated NSCLC. Jpn J Clin Oncol. 2012;42:528–533. [DOI] [PubMed] [Google Scholar]
- 9. Kashiwabara K, Semba H, Fujii S, Tsumura S. Outcome in advanced non‐small cell lung cancer patients with successful rechallenge after recovery from epidermal growth factor receptor tyrosine kinase inhibitor‐induced interstitial lung disease. Cancer Chemother Pharmacol. 2017;79:705–710. [DOI] [PubMed] [Google Scholar]
- 10. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. EuropeanMedicine Agency . Guideline on the Evaluation of Anticancer Medicinal Products in Man. London, England: European Medicines Agency; 2012. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/01/WC500137128.pdf [Google Scholar]
- 12. Kijima T, Shimizu T, Nonen S, et al. Safe and successful treatment with erlotinib after gefitinib‐induced hepatotoxicity: difference in metabolism as a possible mechanism. J Clin Oncol. 2011;29:e588–590. [DOI] [PubMed] [Google Scholar]
- 13. Ku GY, Chopra A, Jr , LopesGde L. Successful treatment of two lung cancer patients with erlotinib following gefitinib‐induced hepatotoxicity. Lung Cancer. 2010;70:223–225. [DOI] [PubMed] [Google Scholar]
- 14. Kunimasa K, Yoshioka H, Iwasaku M, et al. Successful treatment of non‐small cell lung cancer with gefitinib after severe erlotinib‐related hepatotoxicity. Intern Med. 2012;51:431–434. [DOI] [PubMed] [Google Scholar]
- 15. Mok TS, Wu Y‐L, Ahn M‐J, et al. Osimertinib or Platinum‐Pemetrexed in EGFR T790M‐Positive lung cancer. N Engl J Med. 2017;376:629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kawamura T, Kenmotsu H, Omori S, et al. Clinical factors predicting detection of t790m mutation in rebiopsy for EGFR‐mutant non‐small‐cell lung cancer. Clin Lung Cancer. 2018;19:e247–252. [DOI] [PubMed] [Google Scholar]
- 17. Fukuhara T, Maemondo M, Inoue A, et al. Factors associated with a poor response to gefitinib in the NEJ002 study: smoking and the L858R mutation. Lung Cancer. 2015;88:181–186. [DOI] [PubMed] [Google Scholar]
- 18. Inoue A, Yoshida K, Morita S, et al. Characteristics and overall survival of EGFR mutation‐positive non‐small cell lung cancer treated with EGFR tyrosine kinase inhibitors: a retrospective analysis for 1660 Japanese patients. Jpn J Clin Oncol. 2016;465:462–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix Table. Additional characteristics of patients with EGFR mutation‐positive NSCLC who received both first‐ and second‐line EGFR‐TKI


