Summary
Background
Evidence of immunomodulatory therapies to guide clinical management of atopic eczema (AE) is scarce, despite frequent and often off‐label use. Patient registries provide valuable evidence for the effects of treatments under real‐world conditions that can inform treatment guidelines, give the opportunity for health economic evaluation and the evaluation of quality of care, as well as pharmacogenetic and dynamic research, which cannot be adequately addressed in clinical trials.
Objectives
The TREatment of ATopic eczema (TREAT) Registry Taskforce aims to seek international consensus on a core set of domains and items (‘what to measure’) for AE research registries, using a Delphi approach.
Methods
Participants from six stakeholder groups were included: doctors, nurses, nonclinical researchers, patients, industry and regulatory body representatives. The eDelphi comprised three sequential online rounds, requesting participants to rate the importance of each proposed domain item. Participants could add domain items to the proposed list in round 1. A final consensus meeting was held to ratify the core set.
Results
Participants (n = 479) from 36 countries accessed the eDelphi platform, of whom 86%, 79% and 74% completed rounds 1, 2 and 3, respectively. At the face‐to‐face consensus meeting attended by 42 participants the final core set was established containing 19 domains with 69 domain items (49 baseline and 20 follow‐up items).
Conclusions
This core set of domains and items to be captured by national AE systemic therapy registries will standardize data collection and thereby allow direct comparability across registries and facilitate data pooling between countries. Ultimately, it will provide greater insight into the effectiveness, safety and cost‐effectiveness of photo‐ and systemic immunomodulatory therapies.
Short abstract
What's already known about this topic?
Evidence of photo‐ and systemic immunomodulatory therapies to guide clinical management for atopic eczema (AE) is scarce, despite frequent and often off‐label use.
There is a need to gather long‐term, comparative and real‐life data on the effectiveness, safety and cost‐effectiveness of these therapies beyond the confines of short‐term randomized controlled trials, especially when new biological and small‐molecule therapies are entering clinical practice.
Patient registries can provide valuable data to address these issues.
What does this study add?
By performing an international Delphi exercise, consensus was reached on a core set of domains and items to be captured by national AE patient registries.
This core set will standardize data collection and thereby allow direct comparability across registries and facilitate data pooling between countries.
What are the clinical implications of this work?
Ultimately, this core set will provide greater insight into the effectiveness, safety and cost‐effectiveness of photo‐ and systemic immunomodulatory therapies.
This may fill the current gaps of evidence and lead to new guidelines for daily clinical practice, and thereby may contribute to the improvement of the care of children and adults with AE.
Plain language summary available online
Systemic immunomodulatory therapies form a key part of the treatment of moderate‐to‐severe atopic eczema (AE, syn. ‘atopic dermatitis’) where topical or phototherapy alone is not sufficient to induce disease remission and maintain long‐term symptom control.1
With the exception of ciclosporin and dupilumab these therapies are prescribed off‐label;2, 3, 4 nevertheless, immunomodulatory therapies are frequently prescribed in both children and adults.5, 6, 7 However, long‐term use may be limited due to adverse effects. There is also only a small body of randomized controlled trials (RCTs) to guide clinical management.8, 9, 10, 11
There is a need to gather long‐term, comparative and real life data on effectiveness, safety and cost‐effectiveness of these therapies in large‐scale multicentre registries beyond the confines of short‐term RCTs12 at the time when novel biological and small‐molecule therapies are entering clinical practice.
The TREatment of ATopic eczema (TREAT) Registry Taskforce aims to find consensus on core domains and domain items for AE research registries and to harmonize data collection on patients receiving photo‐ and systemic immunomodulatory therapies. The ultimate goal is to reduce heterogeneity between national AE patient registries, allow direct comparability of individual country data and facilitate potential data pooling between countries.
Heterogeneity of outcomes (i.e. domains) used in disease (e.g. psoriasis) registries has been demonstrated to hinder comparing results and pooling of data between centres and countries.13 Therefore the development of an a priori internationally agreed core set of variables14 (i.e. an agreed‐on minimum set of variables that should be measured and reported by every registry) is essential, as indicated by the guideline of the European Commission‐funded PAtient REgistries iNiTiative joint action (PARENT JA).15
The objective of this Delphi study was to reach international consensus between different stakeholders on a core set of domains and items (‘what to measure’) for existing and future AE patient registries with a research focus, that collect data of children and adults on photo‐ and systemic immunomodulatory therapies.
Methods
This study was registered in the Core Outcome Measures for Effectiveness Trials (COMET) database (www.comet-initiative.org/studies/details/825?result=true) and reported following the recommended checklist of Sinha et al.16 and Core Outcome Set‐STAndards for Reporting (COS‐STAR) statement.17 The protocol has been published previously.18
In brief, an online Delphi exercise (i.e. ‘eDelphi’) and a subsequent consensus meeting were conducted to investigate domains and items of importance. The eDelphi included three sequential rounds answered anonymously by key stakeholder groups to avoid participants being influenced by opinions of other group members and to minimize response bias. After each round, a summary of the responses was fed back to the group. Individual participants could then decide to keep their original answers or to change their opinion in the next round, considering responses from the other participants. The total number of registered participants for each round was recorded as the number of participants who had completed the rating. Reasons for changes to scores were documented by asking participants at the end of rounds 2 and 3 to give a general view of why they changed their scores.
Participants and recruitment
Representatives from six key stakeholder groups19 were invited:
Healthcare professionals: doctors who care for patients with moderate‐to‐severe AE.
Healthcare professionals: nurses who care for patients with moderate‐to‐severe AE.
Nonclinical researchers with active research interest in AE, e.g. methodologists, epidemiologists, health economists.
Patients: adults and carers of children or adults with AE.
Industry representatives involved in the development of systemic immunomodulatory drugs for AE.
Regulatory body representatives from the European Medicines Agency (EMA), U.S. Food and Drug Administration (FDA) and national regulatory bodies, such as the U.K. National Institute for Health and Care Excellence (NICE).
Groups 1–3 had representation from members of relevant international societies registered with the International League of Dermatological Societies (ILDS) and other relevant special interest groups [e.g. International Eczema Council, European Taskforce for Atopic Dermatitis, European network of psoriasis patients registries (PSONET) as well as the German AE systemic therapy and hand dermatitis registries] from different parts of the world. These societies were asked to send out an e‐mail with the link to our eDelphi. Patient representatives were recruited from national eczema support groups (see Table 2 of the protocol published by Gerbens et al.18), again by e‐mail invitation. Groups 5 and 6 were identified through personal contacts from Europe and the U.S.A.
Table 2.
Dropout rates of the eDelphi
| Initial participation | Dropouts (%); R1 and R2 | Dropouts (%); R1–R3 |
|---|---|---|
| Doctors (n = 302) | 55 (18) | 69 (23) |
| Nurses (n = 21) | 6 (29) | 7 (33) |
| Patients (n = 52) | 12 (23) | 18 (35) |
| Researchers (n = 16) | 2 (13) | 2 (13) |
| Industry (n = 14) | 7 (50) | 8 (57) |
| Regulatory bodies (n = 5) | 3 (60) | 3 (60) |
| Overall (n = 410) | 85 (21) | 107 (26) |
R, round
Members of the TREAT research group (C.A., S.B., R.B., A.B., M.D., C.F., L.G., A.I., P.M‐H., A.R., J.S., P.S., C.V., D.W. and S.W.; see treat‐registry‐taskforce.org/centres) participated in the eDelphi as well as in the consensus meeting.
eDelphi questionnaire
Based on a systematic search of the literature, decisions already made on core outcomes by the Harmonising Outcome Measures for Eczema (HOME) initiative and round table discussion within the TREAT research group, 119 domains (i.e. high‐level data, e.g. physical examination) and items (i.e. more granular data, e.g. blood pressure) were initially identified; a proposed list of 89 domains items mapped to domains (Table S1; see Supporting Information) together with an obvious list of 30 domains and items (Table S2; see Supporting Information). The ‘obvious’ list included items that were considered obvious, e.g. age, and were therefore not included in the eDelphi but listed separately.
Patients additionally reviewed the questionnaire concerning content and language.
eDelphi survey
A pilot‐tested online e‐management survey system, DelphiManager, maintained by the COMET Initiative, was used.20 At the beginning of each round, the details of the study with key objectives were presented. Subsequently, participants were asked to rate each of the domain items using the Grading of Recommendations Assessment, Development and Evaluations (GRADE) scale, a 9‐point scale with 1 to 3 labelled ‘not important’; 4 to 6 ‘important but not critical’; and 7 to 9 ‘critical’ (see Fig. S1 for examples of how the results were presented to participants; Supporting Information).21 Participants had the option of selecting ‘unable to score’ if they felt unable to rate, and of providing feedback on a specific item or in general at the end of the survey. Hovering over the text provided an explanation for key terms.
The system asked participants to complete each round and reminder e‐mails were sent to increase the response rate. To reduce the risk of attrition bias, the importance of completing all rounds was highlighted at the outset of each round.
Definition of consensus and core set
The definition of consensus for the eDelphi was based on that proposed by Harman et al.,22 but amended to take into consideration the multiple stakeholder groups. This definition ensures that the vast majority considers an item critically important in the absence of a sizeable minority thinking the opposite. Consensus that a domain item should be included in the core set (‘consensus in’) was defined as 70% or more of participants in each stakeholder group scoring its importance as 7 to 9 and less than 15% scoring it as 1 to 3. For ‘consensus out’ it was the other way around. If there was no consensus ‘in’ or ‘out’, it was referred to as ‘no consensus’. Any item where all groups confirmed ‘consensus in’ was taken to be in the core set.
After round 3 the following definition of ‘consensus out’ was used for feasibility reasons (deviation from the protocol): domain items that had not met the threshold for ‘consensus in’ and were rated ‘critical’ by less than 50% in all groups (excluding regulatory bodies, as there were only two of them in round 3 and none at the meeting) were categorized as ‘no consensus, no voting required’ and assumed to be excluded from the core set. Further, those that had not met the threshold for ‘consensus in’ but were rated ‘critical’ by at least 50% in at least one group were categorized as ‘no consensus, voting required’. Overall this led to the classification of each domain item as ‘consensus in’, ‘no consensus, no voting required’ or ‘no consensus, voting required’ after round 3.
The definition of consensus for the consensus meeting was that used by the Outcome Measures in Rheumatology collaboration (OMERACT) group and HOME initiative.23, 24, 25 ‘Consensus in’ was defined as: less than 30% of the whole group of participants disagree.
eDelphi round 1
Information collected in round 1 included: (i) participants’ characteristics (Table 1); (ii) the obvious list to be reviewed and potentially commented on; (iii) the proposed list to be scored; and (iv) an option to add additional domains or items.
Table 1.
Characteristics of participants in the eDelphi and consensus meeting
| Participants registered (n = 479) (%) | Participants completing R1 and included in next rounds (n = 410) (%) | Participants consensus meeting (n = 42) (%) | |
|---|---|---|---|
| Stakeholder groupa | |||
| Doctors | 335 (69·9) | 302 (73·7) | 19 (45·2) |
| Nurses | 30 (6·3) | 21 (5·1) | 6 (14·3) |
| Patients | 76 (15·9) | 52 (12·7) | 6 (14·3) |
| Researchers | 16 (3·3) | 16 (3·9) | 3 (7·1) |
| Industry | 16 (3·3) | 14 (3·4) | 8 (19·1) |
| Regulatory bodies | 6 (1·3) | 5 (1·2) | – |
| Age (years) | |||
| 18–30 | 38 (7·8) | 24 (5·9) | – |
| 31–40 | 124 (25·9) | 102 (24·9) | – |
| 41–50 | 145 (30·3) | 125 (30·5) | – |
| 51–60 | 125 (26·1) | 114 (27·8) | – |
| > 60 | 47 (9·8) | 45 (11) | – |
| Sex | |||
| Female | 300 (62·6) | 249 (60·7) | 25 (60) |
| Male | 177 (37) | 159 (38·8) | 17 (40) |
| Other | 2 (0·4) | 2 (0·5) | – |
| Countries (according to continent) | |||
| Africa | 3 (0·6) | 1 (0·2) | – |
| Asia | 54 (11·3) | 49 (12) | – |
| Europe | 348 (72·7) | 292 (71·2) | 39 (92·8) |
| North America | 35 (7·3) | 33 (8) | 1 (2·4) |
| Oceania | 34 (7·1) | 30 (7·3) | – |
| South America | 5 (1) | 5 (1·2) | 2 (4·8) |
| Total countries participating | 39 | 36 | 13 |
| Characteristics of healthcare professionals (i.e. doctors and nurses) | |||
| Current position | |||
| University teaching hospital | 288 (78·9) | 260 (80·4) | – |
| Other hospital | 48 (13·2) | 38 (11·8) | – |
| Community setting | 19 (5·2) | 18 (5·6) | – |
| Other, e.g. private practice | 10 (2·7) | 7 (2·2) | – |
| Age group of patients with AEs that participants predominantly care for | |||
| Paediatric | 208 (57) | 183 (56·7) | – |
| Adult | 151 (41·4) | 137 (42·4) | – |
| N/A (retired, university setting without patients) | 6 (1·6) | 3 (0·9) | – |
| Experience in care of patients with AEs | |||
| < 10 years | 113 (31) | 98 (30·3) | – |
| > 10 years | 248 (67·9) | 224 (69·3) | – |
| Did not answer | 4 (1·1) | 1 (0·3) | – |
| Membership of international dermatology society or AE interest group | |||
| Yes | 246 (67·4) | 224 (69·3) | – |
| No | 116 (31·8) | 98 (30·3) | – |
| Did not answer | 3 (0·8) | 1 (0·3) | – |
AE, atopic eczema; N/A, not applicable; R, round
aParticipants themselves decided their stakeholder group from a drop‐down menu.
eDelphi round 2
All domain items were carried through to the second round, together with additional domain items listed by participants in round 1 after deduplication by the TREAT group. Participants were asked to rate them. They were presented with the number of participants who scored each category and the distribution of scores (%) for each domain item for their particular stakeholder group with a reminder of their own round 1 score.
Those who had not participated in or completed the first round were not invited to round 2.
eDelphi round 3
All domain items were carried forward into round 3. All participants received identical feedback, containing the distribution of scores (%) for each domain item for all stakeholder groups, along with a reminder of their own round 2 score.
All participants included in round 2 were invited for round 3.
Consensus meeting
A face‐to‐face meeting was held in Amsterdam on 25 October 2016. Representation of all eDelphi stakeholder groups was attempted, but healthcare professionals and patients were of particular importance. The travel expenses of participating patients was covered by the funds held by C.F. and P.S. A patient pre‐meeting, attended by six patients, was held to empower patients and to encourage them to express their views during the main consensus meeting. The main meeting was moderated by one nonvoting independent participant (P.W.), with extensive experience in the conduct of international Delphi exercises, who ensured that the voices of all representative groups were heard, and the discussions and decision‐making process were not dominated by individual participants. The nominal group technique was used.26, 27
The facilitator presented the response rates of each eDelphi round and the results of round 3 according to the three above‐mentioned categories of classification. The domain items in the categories ‘consensus in’ and ‘no consensus, no voting’ were not voted on, unless reasons were very strong and transparent. The same applied for opening discussion around new domains or items. For the last category ‘no consensus, voting required’, discussion and voting took place, anonymized by using TurningPoint© electronic handsets and software version 5·0 for Windows (Turning Technologies, Youngstown, OH, U.S.A.) to analyse results in real time.
Participants completed a conflict of interest form.
Data management
Confidentiality of survey data was ensured using unique numerical identifiers. Data were password protected and accessible only to the TREAT research group, who under no circumstances breached confidentiality. SPSS version 22·0 for Windows (SPSS, Inc., Chicago, IL, U.S.A.) and R version 3·3·3 (The R Foundation) were used to perform statistical analyses.
Ethical requirements
Consent to participate was assumed if individuals registered and completed rounds. Consent to list participants in the acknowledgments was assumed if participants completed all Delphi rounds as this was stated beforehand in the invitation letter for the consensus exercise.
The Medical Ethics Review Committee of the Academic Medical Centre in Amsterdam (reference number W15_249 # 15·0294) confirmed that the Medical Research Involving Human Subjects Act (WMO) did not apply to this study.
Results
Participants’ characteristics
Of the 479 registered participants, 410 completed round 1 and were included in the next round. Six stakeholder groups were involved; 302 (74%) doctors, 21 (5%) nurses, 52 (13%) patients, 16 (4%) researchers, 14 (3%) industry representatives and five (1%) regulatory body representatives. Participants originated from 36 countries, mainly from Europe. (See Table 1).
Response rates and change in participant score
Rounds 1–3 reached a response rate of 86% (410/479), 79% (325/410) and 74% (303/410), respectively. Dropout rates per stakeholder group are presented in Table 2.
Participants changed scores between the different subsequent rounds. Common reasons mentioned were: that responses of others convinced them; they'd had second thoughts; items were not important enough for the core set; or they misread the question the first time.
eDelphi rounds 1–3
A flowchart of identification and selection of core domain items is shown in Figure 1.
Figure 1.
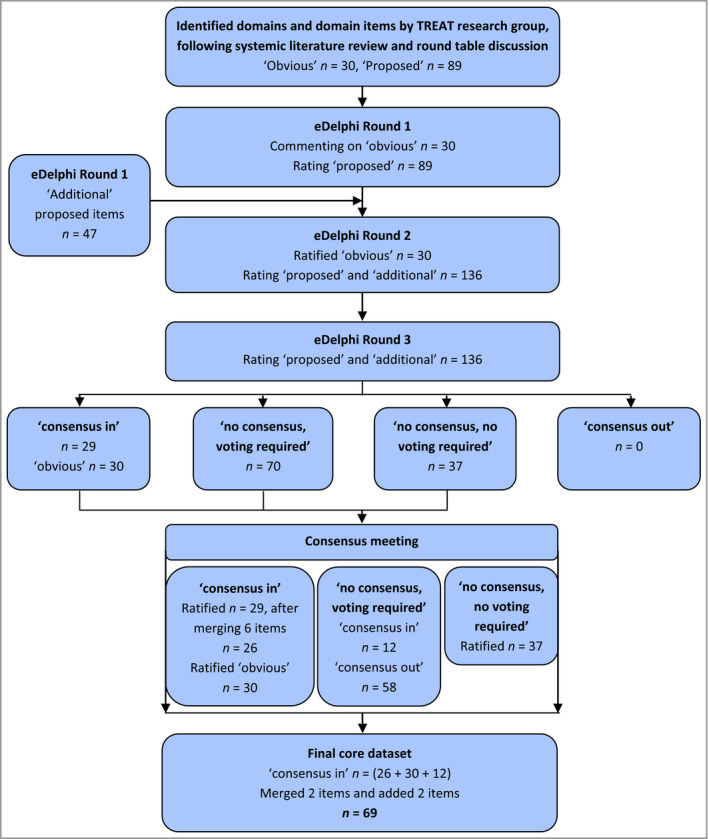
Flowchart of identification and selection of core domains and domain items. ‘Obvious’: domains and items that were considered obvious to be included in AE registries by the TREAT research group, e.g. age and sex, and were therefore not included in the eDelphi. ‘Proposed’: domain items proposed to register by the TREAT research group and included in the eDelphi. ‘Additional’: domain items added by participants in round 1 of the eDelphi. ‘Consensus in’: defined as 70% or more of participants in each stakeholder group scoring the importance of a domain item as 7–9 and less than 15% scoring it as 1–3; included in the core set. ‘Consensus out’: the other way around. ‘No consensus, voting required’: domain items that did not meet the threshold for ‘consensus in’ but were rated ‘critical’ by at least 50% in at least one group. ‘No consensus, no voting required’: domain items that did not meet the threshold for ‘consensus in’ and were rated critical by less than 50% in all groups; excluded from the core set. AE, atopic eczema; TREAT, TREatment of ATopic eczema.
A total of 119 domain items were included in round 1: the proposed list (n = 89) (Table S1; see Supporting Information) and obvious list (n = 30) (Table S2; see Supporting Information). The obvious list was not included for voting, but participants could comment on domains and items to be changed or excluded from the list and included for voting in round 2. However, no changes were made to the list. Participants suggested 105 additional domain items in round 1. After deleting duplicates, 47 were added to round 2 (Table S3; see Supporting Information).
In round 2 participants voted on 136 domain items (proposed and additional list). After voting, 19 domain items reached consensus to be included in the core set, in addition to the a priori defined obvious list (Table S3).
All items were then taken into round 3, including those that reached ‘consensus in’. There were 29 domain items that reached ‘consensus in’; 0 ‘consensus out’; and 37 ‘no consensus, no voting required’, leaving 70 domain items without consensus and required voting (Table S3). These were subsequently considered in the consensus meeting.
Consensus meeting
Minutes of the meeting are available online at treat‐registry‐taskforce.org. Forty‐two participants (Table 1), representing all stakeholder groups except regulatory bodies, ratified the 30 obvious and 29 domain items for which ‘consensus in’ was reached. Six of the 29 items were merged into three by discussion and voting, i.e. ‘follow‐up reporting of disease control by physician’ with ‘follow‐up reporting of disease control by patient’ into ‘follow‐up reporting of disease control’ (93% consensus), ‘dermatology‐specific quality of life (QoL) score’ with ‘AE‐specific QoL score’ into ‘skin‐specific QoL score’ for both baseline and follow‐up (77% consensus). The two ‘disease control’ items were combined as this core domain is still under research by the HOME initiative.28 Merging of ‘QoL scores’ was done as the opinion was that too many items on QoL in the core set would overburden patients.
Discussion and voting was performed for the remaining 70 domain items where ‘no consensus, voting required’ had been reached. Twelve (5 from the proposed and 7 from the additional list) were added, making a total of 38 domain items that reached consensus to be included in the core set together with the 30 obvious items (Table 3; Tables S3 and S4; see Supporting Information). The items concerning frequency of safety investigations and follow‐up frequency for registry data entry were not voted on, as they were considered to be part of the ‘when to measure’ instead of the ‘what to measure’.
Table 3.
Core set of domains and domain items
| Domains | Obvious/proposed/additional[Link] | Domain items |
|---|---|---|
| Demographics | Obvious | Date of birth and date of enrolment into registry |
| Sex | ||
| Ethnicity | ||
| Educational status | ||
| Current occupation or education | ||
| AE diagnosis | Obvious | How diagnosis AE is established |
| Use of validated diagnostic criteria | ||
| Date of onset AE | ||
| Past AE treatments | Obvious | Phototherapy |
| Systemic therapy | ||
| Proposed | Topical treatments for AE | |
| Day hospital care treatments for AE (outpatient) | ||
| Hospitalization for AE | ||
| Current AE treatments | Obvious | Phototherapy |
| Systemic therapy | ||
| Proposed | Topical treatments | |
| Additional | Amount of topical creams/ointments used per week | |
| Family history of AE or allergic diseases | Obvious | Family history of AE or allergic diseases |
| Allergic comorbidities | Obvious | Asthma |
| Allergic rhinoconjunctivitis | ||
| Atopic eye disease | ||
| Eosinophilic oesophagitis | ||
| Food allergies | ||
| Contact allergies | ||
| Other comorbidities | Obvious | Malignancies |
| Serious infections | ||
| Current concomitant medication | Proposed | Antihistamines, oral or topical |
| Antibiotics, oral or topical[Link] | ||
| Additional | Immunosuppressives for other inflammatory diseases | |
| Baseline general AE questions | Additional | Exposures that trigger disease flares |
| Episodes of skin infection (i.e. folliculitis, HSV, molluscum contagiosum) | ||
| Days lost from usual activities | ||
| Baseline physical examination | Proposed | Fitzpatrick skin type |
| Skin examination | ||
| Baseline physician‐ and patient‐reported domains | Proposed | Physician‐assessed clinical signs |
| Investigator/physician global assessment | ||
| Patient‐reported symptoms | ||
| Patient global assessment | ||
| Generic quality of life score | ||
| Skin‐specific quality of life score | ||
| Patient‐reported satisfaction with AE care received | ||
| Additional | Impact of AE on the family | |
| Baseline investigations | Obvious | Medical history (tuberculosis, HIV, hepatitis B or C) |
| Full blood count | ||
| Liver function | ||
| Kidney profile | ||
| Proposed | Evaluating TPMT level prior to azathioprine use | |
| Baseline management | Proposed | Main reasons for choosing specific treatment (systemic or phototherapy) |
| Relative contraindication(s) for selected treatment | ||
| Follow‐up general AE questions | Additional | Days lost from usual activities[Link] |
| Change in diagnosis after enrolment (e.g. from AE to CTCL) | ||
| Date of death and relation to AE | ||
| Follow‐up physical examination | Proposed | Skin examination |
| Follow‐up physician‐ and patient‐reported domains | Proposed | Physician‐assessed clinical signs |
| Investigator/physician global assessment | ||
| Patient‐reported symptoms | ||
| Patient global assessment | ||
| Generic quality of life score | ||
| Skin‐specific quality of life score | ||
| Reporting of disease control | ||
| Adherence to treatment between appointments | ||
| Patient‐reported satisfaction with AE care received | ||
| Additional | Impact of AE on the family | |
| Follow‐up investigations | Obvious | Safety bloods[Link] |
| Follow‐up adverse events | Obvious | Serious adverse events |
| Adverse events that cause stop or switch of therapy or change in dosage | ||
| For (serious) adverse events: probability of relationship with treatment | ||
| Follow‐up management | Obvious | Reason for switching therapy |
| Reason for discontinuation of therapy |
AE, atopic eczema; CTCL, cutaneous T‐cell lymphoma; HIV, human immunodeficiency virus; HSV, herpes simplex virus; TPMT, thiopurine methyltransferase.
Obvious: domains and items that were considered obvious to be included in AE registries by the TREAT research group, e.g. age and sex, and were therefore not included in the eDelphi. Proposed: domains and items proposed to register by the TREAT research group and included in the eDelphi. Additional: domains and items added by participants in round 1 of the eDelphi.
These items were merged (i.e. ‘antibiotics oral’ and ‘antibiotics topical’ to ‘antibiotics, oral or topical’) or added [i.e. ‘days lost from usual activities’ (follow‐up) and ‘safety bloods’ (follow‐up)] after the eDelphi and consensus meeting.
After a final discussion within the TREAT research group the items ‘antibiotics, oral’ and ‘antibiotics, topical’ were merged into one item (‘antibiotics, oral or topical’), and ‘days lost from usual activities’ and ‘safety bloods’ (as already included at baseline) were added as obvious follow‐up items making a total of 69 items mapped to 19 domains.
Discussion
This Delphi exercise identified a core set of 19 domains containing 69 items recommended to be captured in existing and future AE patient registries with a research focus, which collect data from children and adults on photo‐ and systemic immunomodulatory therapies. Domains and items are related to demographics, AE diagnosis and treatment (past and current), family history, (allergic) comorbidities, concomitant medication, physical examination, physician‐ and patient‐reported outcomes, investigations, management and adverse events.
Strengths and limitations
Firstly, we registered and published our protocol and followed the COMET and COS‐STAR guidelines.17 Secondly, we successfully involved a large number of participants from different stakeholder groups and countries, which strengthens the recommendations. Despite the strenuous challenge of keeping all participants on board, we maintained a good response rate. Further, by employing both an eDelphi exercise and a face‐to‐face consensus meeting, positive effects of anonymity (avoiding dominance of certain participants) and interaction were combined. Finally, patients were actively involved throughout the planning and conduct of the project.
Nevertheless, some limitations remain. Although we tried to avoid the risk of disproportionate representation among stakeholder groups, a higher number of clinicians participated compared with other groups. However, this overrepresentation was not a problem in the eDelphi because results were presented by stakeholder group. At the consensus meeting this point was consciously addressed by our facilitator who ensured all voices, especially of patients, were heard. Further, the number of researchers and regulatory body representatives was low. The dropout rates of both regulatory body and industry representatives were high; a possible explanation may be lower relevance of the eDelphi to these groups and lack of familiarity with the clinical area. Another potential limitation is that although dermatology societies from across the world were invited to participate, there was an overrepresentation of Western nationalities. This may limit the generalizability of the consensus and we cannot exclude the possibility that cross‐cultural differences were missed. Further, the survey was conducted only in English. To address this limitation, we involved patients and patient organizations in the development and pilot phase, used plain English where possible, included help texts in the survey, and provided a list with explanations of domains and items. Another consideration is the feasibility of this core set, given the number of domains and items included. In this context, it is important to highlight that this core set is not for clinical practice but for registries focused on research. Further, 49 domain items are measured only at baseline, leaving only 20 for follow‐up visits. Also, most of the domains and items are required by guidelines and many are already included as part of clinical visits. Above all, there is experience with the TREATgermany registry, which has been ongoing for a few years and has a bigger dataset than our core domains and items.
This internationally agreed core set has the potential to unify data collection across AE patient registries to allow for direct comparisons between countries as well as data sharing and pooling. Healthcare professionals and patients will benefit especially by the data generated on comparative effectiveness, safety and cost‐effectiveness of photo‐ and systemic immunomodulatory therapies across dermatology centres and country boundaries.
This core set is recommended to be used in all existing (currently only one, TREATgermany) and future AE patient registries with a research focus, and we encourage all stakeholders to endorse this recommendation as it will have impact only if it is consistently implemented.
Our research group is in the process of determining how the identified domains and items should be measured, defined and categorized. These results will be published separately. The final core set will be pilot tested for feasibility in 2018 to ensure we do not overburden colleagues. This may result in a more limited version of the current core set downstream. For updates of our work and for anyone who is interested in collaborating with us, please contact the TREAT Registry Taskforce via our website, treat‐registry‐taskforce.org.
Supporting information
Table S1. Proposed domains and domain items for round 1 of the eDelphi questionnaire.
Table S2. Obvious domains and domain items.Table S3. Domain items after eDelphi rounds 2 and 3 as well as the consensus meeting.Table S4. Domain items that have reached consensus (n = 38, excluding n = 30 from obvious list) to be included in the core dataset with percentage scoring 7–9 and 1–3 at consensus meeting.Fig S1. (a) Example of how each item was presented in round 1 of the eDelphi. (b) Example of how results were fed back to participants in round 3 of the eDelphi.
Acknowledgments
We would like to acknowledge the Dutch Atopic Eczema Patient Society (VMCE, Vereniging voor Mensen met Constitutioneel Eczeem), the U.K. National Eczema Society and the Irish Skin Foundation for their support. We would like to thank Paula Williamson for her excellent work as facilitator, Mariëlle Vermeulen for taking minutes of the consensus meeting, Mariska Schaap for the logistics of the consensus meeting and Gabriëlle Appel and Marleen van der Stok for assisting the patients during the consensus meeting. Above all, we would like to acknowledge the participants.
The following took part in both the eDelphi and the face‐to‐face meeting: Valeria Aoki, Christian Apfelbacher, Gabrielle Appel, Paula Beattie, Anna Belloni Fortina, Richard de Booij, Sara Brown, Caoimhe Fahy, Louise Gerbens, Gitta Giskes, Marijke van Hilten‐Cornelisse, Sanja Kezic, Willem Kouwenhoven, Sandra Lawton, Pina Middelkamp‐Hup, Marie‐Anne Morren, Pieter van Nederkassel, Raquel Orfali, Carle Paul, Cecilia A.C. Prinsen, Luis Puig, Jane Ravenscroft, Amanda Roberts, Michael Rudenko, Marit Saunes, Hilary Selles‐Basford, Anne Speirs, Phyllis Spuls, Marleen van der Stok, Efstratios Vakirlis, Christian Vestergaard, Elke Weisshaar and Anthea Wilson.
The following took part in the face‐to‐face meeting only: Fanneke Alkemade, Amit Bodhani, Remco Böing, Erik‐Jan Dammers, Laurent Eckert, Carsten Flohr, Richard Hudson, Simon van Leeuwen and Jan Slachmuylders.
The following completed the eDelphi: Susanne Abraham, Elaine Agius, Tove Agner, Karen Agnew, Alex Anstey, Michael Ardern‐Jones, Bernd Arents, Helene Aubert, Esther Serra Baldrich, Sebastien Barbarot, Susannah Baron, Eulalia Baselga, Jonathan Batchelor, Andrea Bauer, Lisa Beck, Diane van Beek, Teresa L. Berents, Isabel Betlloch, Anthony Bewley, Thomas Bieber, Robert Bissonnette, Julie Block, F.G. Bosma, Ann Boyapati, Aaron Boyce, Marijke Brouwer, Fiona Browne, Dusan Buchvald, Esther Burden‐The, Tim Burton, Celine Busard, Aideen Byrne, Yolanda Gilaberte Calzada, Baraka M. Chaula, Diana Chen, Chia‐Yu Chu, Tomoko Chubachi, Shen Chunping, Sheila Clark, Tim Clayton, Kim Clemmensen, Stuart Cohen, Sinead Collins, Harry Comber, Michael J. Cork, Pippa Cousen, Fiona Cowdell, Mary‐Margaret Chren, Paraic Curran, Wim de Cuyper, Robert Dawe, Mette Deleuran, A. Deniz Akkay, Miep van Dijk, Maj Dinesen, Hannah Drewel, Aaron Drucker, Veronika Dvorakova, Lawrence F. Eichenfield, Gan Yiping Emily, Ton Emmerik, Nienke Evers, Steven Feldman, Carlos Ferrandiz, Gayle Fischer, Wilma Fischer‐Barth, M. Fluggen, Catherine Foley, Peter Foley, Henry Foong Boon Bee, Lucretia Adina Frasin, Lars Frencg, Masaki Futamura, Joanna Gach, Floor Garritsen, Faith Kishi Generao, Jon Genuneit, Susannah George, Karen Gibbon, Elizabeth Gilmour, Luc van Gils, Douglas Gin, Giampiero Girolomoni, Mary Glover, Michelle Goh, Rebeca Goiriz, Richard Goodwin, Helen Goodyear, Carolina Gouveia, Diana de Graaf, Jill Green, Michelle Green, Danielle Greenblatt, Monisha Gupta, Suzanne Hadley, Anne Halbert, Maija Hansen, Kara Heelan, Almudena Hernandez, Peter H. Hoeger, Susannah Hoey, Colin Holden, L.M. Hollestein, Jonathan Hourihane, Kathryn Humphreys, Sally Ibbotson, John Ingram, Alan Irvine, Henrique Akira Ishii, Nathan Jetter, Emma Johansson, Catherine Jury, Kenji Kabashima, Ranbir Kaulsay, Jana Kazandjieva, Kyu Han Kim, Laura von Kobyletzki, Karen Koh, Tineke Komen, Sjors Koppes, Malcolm Lane‐Brown, Maria Angela M. Lavadia, Philip Laws, John Lear, Adriene Lee, Chih‐Hung Lee, Dong Hun Lee, Haur Yueh Lee, Kwang Hoon Lee, Irene Leigh, Yael Anne Leshem, Nick Levell, M. Lezaire, Yunzhu Li, M.A. Lin, Peter Lio, Junfeng Liu, Ying Liu, Antonia Lloyd‐Lavery, Yuan‐Hsin Lo, Yufang Lu, Maeve Lynch, Andrea Manca, Yasaman Mansouri, David Margolis, Lara Marin, Ute Mark, Pauline Marren, Gillian Marshman, Ana Martãn, Ludovic Martin, Anna Martinez, Maeve McAleer, Collette McCourt, Kathy McElhone, David McMahon, Ian McNicoll, Tess McPherson, Simon Meggitt, Alan Menter, Jasmina Mikeljevic, Blaithin Moriarty, Rachael Morris‐Jones, Celia Moss, Ruth Murphy, Makiko Nakahara, Takeshi Nakahara, Ibrahim Nasr, Iria Neri, A.M. Nooy, Audrey Nosbaum, Uffe Nygaard, Amanda Oakley, Graham Ogg, Yukihiro Ohya, Simone Oliwiecki, Arnold Oranje, David Orchard, Ott, David Paige, Amy Paller, Suzanne Pasmans, Annalisa Patrizi, Aikaterini Patsatsi, Bibiana Pérez, Li Ping, Maeve Mario Cezar Pires, Diana Purvis, Asha Rajeev, Anjna Rani, Gitte Susanne Rasmussen, Catherine Reid, Arthur Rhodes, Matthew Ridd, Menno de Rie, Jean Robinson, Alfonso Rodriguez‐Herrera, Caroline Roduit, R.M. Ross Hearn, Giorgio Rovatti, Diana Rubel, T. Rustemeyer, Malcolm Rustin, Tracey Sach, M. Santer, Laura Scardamaglia, Joost Schalkwijk, Jochen Smitt, Lynda Schneider, Mandy Schram, M.L.A. Schuttelaar, N. Seeder, Julien Seneschal, Fiona Shackley, Wang Shan, Heather Sharp, Mary Sharp, Yi‐Hsien Shih, Alexa Shipman, S. Sikking, J.H. Sillevis Smitt, Bernadette De Silva, Jonathan Silverberg, Dagmar Simon, Eric Simpson, Lone Skov, Lea Solman Kosutic, Julia Stainforth, J.F. Stalder, Mieke Stinkens, John Su, Desiree Sutman, Åke Svensson, Katherine Sweeney, Bob Swerlick, Saleem Taibjee, Alain Taieb, Eugene Tan, W.D. Taylor, Anne Thaysen, Jacob Thyssen, Jing Tian, Stephen Till, David Todd, Miriam Megha Tollefson, Magdalena Trzeciak, Ron Tupker, George Varigos, Nick Vlahakis, Annika Volke, Yin Vun, Carl‐Fredrik Wahlgren, Gorav Wali, Charlotte Walker, Dmitri Wall, Daniel Wallach, Shernaz Walton, Mandy Wan, Hua Wang, Tobias Weberschock, Aw Chen Wee, Stephan Weidinger, Richard Weller, Linzi Wildgust, Soren Wille, Hywel Williams, Miriam Wittmann, Andreas Wollenberg, Chu Yan, Ying Ye, Zenas Yiu, Xiao Yuanyuan, Lieke van der Zwan.
Conflicts of interest
A.D.I. has served as a consultant to AbbVie, Anacor, Chugai Pharma, Pfizer, Regeneron, Roche/Genentech, Sanofi‐Genzyme and UCB Pharma. S.B. has received research grants from La Fondation pour la Dermatite Atopique and Pierre Fabre Laboratories, has received personal fees from Bioderma, Ferring, La Roche Posay Laboratoire Dermatologique, Novalac and Sanofi‐Genzyme, and has received nonfinancial support from AbbVie, Janssen and Novartis. M.D. has been a speaker, advisory board member and/or investigator for AbbVie, CK‐Care Foundation, La Roche Posay Foundation, Leo Pharma, Meda Pharma, Pierre Fabre Laboratories, Regeneron and Sanofi‐Genzyme. L.F.E. has served as a consultant to Anacor/Pfizer, Leo Pharma, Lilly, Roche/Genentech and Sanofi/Regeneron. J.S. has received institutional funding for investigator‐initiated research from ALK, Novartis, Pfizer and Sanofi, and is Chief Investigator of the German AE registry – TREATgermany. C.V. has advised and given lectures for AbbVie, Leo Pharma, Novartis and Sanofi‐Genzyme, and has been involved in the development of PO‐SCORAD. S.W. has served as a consultant and/or lecturer to Novartis, Pfizer and Sanofi‐Genzyme, has received independent research grants from Biogen, Novartis, Pfizer, La Roche Posay Foundation and Sanofi‐Genzyme, and has been involved in performing clinical trials with many pharmaceutical companies that manufacture drugs used for the treatment of AE. C.F. has advised Roche/Genentech and Sanofi/Regeneron and is Chief Investigator of the UK–Irish Atopic eczema Systemic Therapy Registry – A*STAR. P.I.S. has served as a consultant to AbbVie, Anacor, Leo Pharma and Novartis, has received independent research grants from Leo Pharma and Schering‐Plough, has been involved in performing clinical trials with many pharmaceutical companies that manufacture drugs used for the treatment of AE, and is Chief Investigator of the Dutch AE registry – TREAT NL.
Participants of the consensus meeting declared the following conflicts of interest: Fanneke Alkemade and Remco Böing are employees of Roche. Paula Beattie has been involved in the validation of the CLQI/CDLQI. Amit Bodhani and Simon van Leeuwen are employees of AbbVie. Sara Brown has been involved in setting up a registry in the U.K. and in developing the EASI calculator application. Erik‐Jan Dammers is an employee of Sanofi‐Genzyme and has been involved in their EUROSTAD study. Richard Hudson and Laurent Eckert are employees of Sanofi‐Genzyme. Carle Paul has been involved in the development of the EASI and has served as a consultant to Astellas and Sanofi. Jan Slachmuylders is an employee of Novartis.
Funding sources The TREAT eDelphi is supported through an unconditional grant held by C.F. and through a research fund held by P.I.S.
Conflicts of interest C.F. and P.I. Spuls contributed equally and share last authorship.
Plain language summary available online
References
- 1. Weidinger S, Novak N. Atopic dermatitis. Lancet 2016; 387:1109–22. [DOI] [PubMed] [Google Scholar]
- 2. European Medicines Agency . Questions and answers on Sandimmun, Sandimmun Neoral and associated names (ciclosporin, 10, 25, 50 and 100 mg capsules, 100 mg/ml oral solution and 50 mg/ml concentrate for solution for infusion). 2013. Available at: www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Sandimmun_30/WC500144897.pdf (last accessed 9 May 2018).
- 3. U.S. Food and Drug Administration (FDA) . FDA approves new eczema drug Dupixent. 28 March 2017. Available at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm549078.htm (last accessed 9 May 2018).
- 4. European Medicines Agency . Dupixent. 2017. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/004390/human_med_002158.jsp&mid=WC0b01ac058001d124 (last accessed 9 May 2018).
- 5. Proudfoot LE, Powell AM, Ayis S et al The European TREatment of severe Atopic eczema in children Taskforce (TREAT) survey. Br J Dermatol 2013; 169:901–9. [DOI] [PubMed] [Google Scholar]
- 6. Taylor K, Swan DJ, Affleck A et al U.K. Translational Research Network in Dermatology and the U.K. Dermatology Clinical Trials Network. Treatment of moderate‐to‐severe atopic eczema in adults within the U.K.: results of a national survey of dermatologists. Br J Dermatol 2017; 176:1617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Totri CR, Eichenfield LF, Logan K et al Prescribing practices for systemic agents in the treatment of severe pediatric atopic dermatitis in the US and Canada: the PeDRA TREAT survey. J Am Acad Dermatol 2017; 76:281–5. [DOI] [PubMed] [Google Scholar]
- 8. Garritsen FM, Brouwer MW, Limpens J et al Photo(chemo)therapy in the management of atopic dermatitis: an updated systematic review with implications for practice and research. Br J Dermatol 2014; 170:501–13. [DOI] [PubMed] [Google Scholar]
- 9. Roekevisch E, Spuls PI, Kuester D et al Efficacy and safety of systemic treatments for moderate‐to‐severe atopic dermatitis: a systematic review. J Allergy Clin Immunol 2014; 133:429–38. [DOI] [PubMed] [Google Scholar]
- 10. Ring J, Alomar A, Bieber T et al Guidelines for treatment of atopic eczema (atopic dermatitis) Part II. J Eur Acad Dermatol Venereol 2012; 26:1176–93. [DOI] [PubMed] [Google Scholar]
- 11. Sidbury R, Davis DM, Cohen DE et al Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol 2014; 71:327–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spuls PI, Gerbens LAA, Apfelbacher CJ et al The international TREatment of ATopic Eczema (TREAT) registry taskforce: an initiative to harmonize data collection across national atopic eczema photo‐ and systemic therapy registries. J Invest Dermatol 2017; 137:2014–16. [DOI] [PubMed] [Google Scholar]
- 13. Ormerod AD, Augustin M, Baker C et al Challenges for synthesising data in a network of registries for systemic psoriasis therapies. Dermatology 2012; 224:236–43. [DOI] [PubMed] [Google Scholar]
- 14. Williamson PR, Altman DG, Bagley H et al The COMET handbook. Trials 2017; 18(Suppl. 3):280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zaletel M, Kralj M, eds. Methodological Guidelines and Recommendations for Efficient and Rational Governance of Patient Registries. Ljubljana: National Institute of Public Health, 2015. Available at: https://ec.europa.eu/health/ (last accessed 8 May 2018). [Google Scholar]
- 16. Sinha IP, Smyth RL, Williamson PR. Using the Delphi technique to determine which outcomes to measure in clinical trials: recommendations for the future based on a systematic review of existing studies. PLoS Med 2011; 8:e1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kirkham JJ, Gorst S, Altman DG et al Core Outcome Set‐STAndards for Reporting: the COS‐STAR statement. PLoS Med 2016; 13:e1002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gerbens LA, Boyce AE, Wall D et al TREatment of ATopic eczema (TREAT) registry taskforce: protocol for an international Delphi exercise to identify a core set of domains and domain items for national atopic eczema registries. Trials 2017; 18:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Williamson PR, Altman DG, Blazeby JM et al Developing core outcome sets for clinical trials: issues to consider. Trials 2012; 13:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harman NL, Bruce IA, Kirkham JJ et al The importance of integration of stakeholder views in core outcome set development: otitis media with effusion in children with cleft palate. PLoS One 2015; 10:e0129514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guyatt GH, Oxman AD, Kunz R et al GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol 2011; 64:395–400. [DOI] [PubMed] [Google Scholar]
- 22. Harman NL, Bruce IA, Callery P et al MOMENT – Management of Otitis Media with Effusion in Cleft Palate: protocol for a systematic review of the literature and identification of a core outcome set using a Delphi survey. Trials 2013; 14:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schmitt J, Spuls P, Boers M et al Towards global consensus on outcome measures for atopic eczema research: results of the HOME II meeting. Allergy 2012; 67:1111–17. [DOI] [PubMed] [Google Scholar]
- 24. Chalmers JR, Schmitt J, Apfelbacher C et al Report from the third international consensus meeting to harmonise core outcome measures for atopic eczema/dermatitis clinical trials (HOME). Br J Dermatol 2014; 171:1318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boers M, Kirwan JR, Tugwell P et al The OMERACT handbook. Outcome Measures in Rheumatology, 2017. Available from: https://omeract.org/resources (last accessed 9 May 2018).
- 26. Cantrill JA, Sibbald B, Buetow S. The Delphi and nominal group techniques in health services research. Int J Pharm Pract 1996; 4:67–74. [Google Scholar]
- 27. Jones J, Hunter D. Consensus methods for medical and health services research. BMJ 1995; 311:376–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chalmers JR, Simpson E, Apfelbacher CJ et al Report from the fourth international consensus meeting to harmonize core outcome measures for atopic eczema/dermatitis clinical trials (HOME initiative). Br J Dermatol 2016; 175:69–79. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Proposed domains and domain items for round 1 of the eDelphi questionnaire.
Table S2. Obvious domains and domain items.Table S3. Domain items after eDelphi rounds 2 and 3 as well as the consensus meeting.Table S4. Domain items that have reached consensus (n = 38, excluding n = 30 from obvious list) to be included in the core dataset with percentage scoring 7–9 and 1–3 at consensus meeting.Fig S1. (a) Example of how each item was presented in round 1 of the eDelphi. (b) Example of how results were fed back to participants in round 3 of the eDelphi.


