Abstract
Mesenchymal stem cells (MSCs) are multipotent cells that can differentiate into mature cells of various cell types. Although the differentiation process of MSCs requires lineage‐specific transcription factors, the exact molecular mechanism that determines MSCs differentiation is not clearly addressed. Here, we demonstrate a Smad4‐Taz axis as a new intrinsic regulator for adipo‐osteogenic differentiation of MSCs and show that this function of Smad4 is independent of the transforming growth factor‐β signal. Smad4 directly bound to the Taz protein and facilitated nuclear localization of Taz through its nuclear localization signal. Nuclear retention of Taz by direct binding to Smad4 increased expression of osteogenic genes through enhancing Taz‐runt‐related transcription factor 2 (Runx2) interactions in the C3H10T1/2 MSC cell line and preosteoblastic MC3T3‐E1 cells, whereas it suppressed expression of adipogenic genes through promoting Taz‐peroxisome proliferator‐activated receptor‐γ (PPARγ) interaction in C3H10T1/2 and preadipogenic 3T3‐L1 cells. A reciprocal role of the Smad4 in osteogenic and adipogenic differentiation was also observed in human adipose tissue‐derived stem cells (hASCs). Consequently, Smad4 depletion in C3H10T1/2 and hASCs reduced nuclear retention of Taz and thus caused the decreased interaction with Runx2 or PPARγ, resulting in delayed osteogenesis or enhanced adipogenesis of the MSC. Therefore, these findings provide insight into a novel function of Smad4 to regulate the balance of MSC lineage commitment through reciprocal targeting of the Taz protein in osteogenic and adipogenic differentiation pathways. Stem Cells 2019;37:368–381
Keywords: Smad4, Taz, Mesenchymal stem cells, Adipocytes, Osteoblasts
Smad4 directly binds to Taz and induces the retention of Taz in the nucleus, resulting in enhancement of Taz interaction with runt‐related transcription factor 2 or peroxisome proliferator‐activated receptor‐γ. This increased interaction augments the expression of osteogenic genes or reduces the expression of adipogenic genes, respectively. Therefore, Smad4‐Taz axis reciprocally regulates osteogenic and adipogenic differentiation of mesenchymal stem cells.
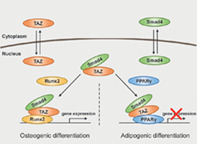
Significance Statement.
Although the Smad4 protein has been suggested to act as a common Smad in the transforming growth factor‐β (TGF‐ß) superfamily signaling pathway in human embryonic stem cells, it has been unclear whether Smad4 has a noncanonical role in adipo‐osteogenic differentiation of mesenchymal stem cells (MSCs), independent of the TGF‐ß and bone morphogenic protein pathways. The study demonstrated that Smad4 plays a crucial role in the regulation of lineage commitment of the MSCs, including human adipose tissue‐derived stem cells, into osteoblasts and adipocytes through modulating the retention of Taz in the nucleus during MSC differentiation. The Smad4 is specific to Taz but not YAP. Therefore, the findings provide new insight into a novel mechanism of the Smad4‐Taz axis in adipo‐osteogenic differentiation of MSCs and demonstrate a reciprocal role of Smad4 as a positive and negative factor in osteogenesis and adipogenesis of MSCs, respectively.
Introduction
Mesenchymal stem cells (MSCs) are adult stem cells that have self‐renewal activity and can differentiate into multiple cell lineages, including osteoblasts and adipocytes 1, 2. As the common progenitor cells of osteoblasts and adipocytes, MSC differentiation needs to be tightly regulated to maintain the homeotic balance of osteoblasts and adipocytes within organs such as the bones.
Accumulating evidence over the past decades demonstrates that differentiation of MSCs into osteoblasts and adipocytes requires important signaling pathways, including transforming growth factor‐β (TGF‐β)/bone morphogenic protein (BMP), Wnt, and Hedgehog signals, and specific transcription factors such as runt‐related transcription factor 2 (Runx2) and peroxisome proliferator‐activated receptor‐γ (PPARγ) 2. In addition, it has been reported that adipogenic induction factors inhibit osteogenesis, and conversely, osteogenic induction factors hinder adipogenesis, indicating that the decision process of MSCs into these cells is competing and reciprocal 2, 3. This reciprocal process is well demonstrated by PPARγ, which is known to induce the adipogenic differentiation of MSCs and suppress osteogenic differentiation 4, 5.
Among the major signaling pathways, the TGF‐β and BMP pathways have been studied by many groups regarding MSC differentiation as well as the pluripotency of stem cells 6. Although the TGF‐β and BMP signaling pathways have been particularly known to be involved in the osteogenic differentiation of MSCs 7, 8, 9, different outcomes occur depending on the subtypes, concentrations, and treatment time 10. For example, TGF‐β promotes the osteogenesis of MSCs at early time points, whereas it suppresses the process at later time points 6. In the C3H10T1/2 cell line, which is an MSC cell line, BMP2 accelerates osteogenesis at a high concentration and also promotes adipogenesis at a low concentration 10.
TGF‐β and BMP ligands transmit their signals through intracellular signal transducers called the Smad proteins 11. Among the Smad proteins, Smad4, acting as a common Smad, is essential for both signaling pathways that regulate various biological phenomena through complexing with receptor‐activated Smads, Smad2/3 or Smad1/5/8, depending on the ligands. Recently, several findings indicated the importance of Smad proteins in stem cell biology. Smad2/3 and Smad4 are required for the stabilized state of human embryonic stem cells (hESCs) through maintaining the balance of TGF‐β and BMP signals 12. Moreover, interaction between the Smad family and a transcriptional coactivator with a PDZ‐binding motif, called Taz, has been known to be required for regulating the cell fate 13 and maintaining the self‐renewal of hESCs 14. In addition, a certain report suggested that Taz forms a complex with Smad2/3 to bind to a TEA domain family member or Octamer‐binding transcription factor 4 required for hESC pluripotency 15.
Although these findings emphasize the importance of Smad proteins, including Smad4, in hESC biology, it has been unclear whether Smad4 has a noncanonical role in the differentiation of MSCs into osteoblasts and adipocytes, independent of the TGF‐β and BMP pathways. Here, we provide experimental evidence that Smad4 plays a crucial role in the regulation of lineage commitment of the MSCs into osteoblasts and adipocytes through modulating the retention of Taz in the nucleus during MSC differentiation, and show that this novel function is independent of the TGF‐β superfamily ligands.
Materials and Methods
Plasmids
Full‐length mouse Smad4, Taz, Runx2, and PPARγ complementary DNAs (cDNAs) were amplified by reverse transcription‐polymerase chain reaction (RT‐PCR) and subcloned into the pcDNA3‐Flag, pcDNA3‐HA, and pcDNA3.1 vectors (Invitrogen, Carlsbad, CA). Plasmids encoding different regions of the Smad4 protein (HA‐S4‐MH1, HA‐S4‐Linker, HA‐S4‐MH2, HA‐S4‐ΔMH1, HA‐S4‐ΔMH2, Flag‐S4‐MH1, and Flag‐S4‐MH2) were amplified from full‐length Flag‐Smad4 cDNA by PCR and subcloned into the EcoRI and XhoI sites of the pcDNA3‐Flag and pcDNA3‐HA vector. The mouse osteocalcin (OC) promoter region (−657/+13) was generated by PCR using C3H10T1/2 genomic DNA and subcloned into the pGL3 vector. A Smad4 mutant with nuclear localization signal (NLS) sequences substituted with alanine was generated using the Muta‐Direct Site‐Directed Mutagenesis Kit (iNtRON, Seoul, Korea). All constructs generated by PCR were verified by sequencing. Primer sequences for PCR and site‐directed mutagenesis are described in Supporting Information Table S1. pGL3‐adipocyte protein2 (aP2) promoter DNA was purchased from Addgene (Watertown, MA).
Cell Culture and Reagents
Murine mesenchymal C3H10T1/2 cells were cultured in Basal Media Eagle with 10% fetal bovine serum (FBS), 10% l‐glutamine, and 1% antibiotics (GIBCO‐BRL, Grand Island, NY). MC3T3‐E1 cells were maintained in Roswell Park Memorial Institute medium without ascorbic acid with 10% FBS and 1% penicillin/streptomycin (GIBCO‐BRL). 3T3‐L1 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) with 10% FCS and 1% penicillin/streptomycin (GIBCO‐BRL). Human adipose tissue‐derived stem cells (hASCs) were provided by Dr. Sun U. Song (Inha University College of Medicine, Korea) with material transfer agreement 16. The hASCs were maintained in low‐glucose DMEM with 10% FBS and 1% antibiotics (GIBCO‐BRL), and hASCs at passages 7–10 were used in this study. HEK293 cells and human colorectal cancer SW620 cells were maintained in DMEM with 10% FBS and 1% penicillin/streptomycin. The antibodies used in this study are described in Supporting Information Table S2. For MSC differentiation, dexamethasone (D2915), insulin (I9278), troglitazone (T2573), IBMX (I7018), β‐glycerolphosphate (G9422), l‐ascorbic acid (A4403), and proline (P5607) were obtained from Sigma (St. Louis, MO). Insulin‐transferrin‐selenium premix (354352) was purchased from BD Bioscience (San Jose, CA). TGF‐β1 (240‐B) and TGF‐β3 (243‐B) were obtained from R&D Systems (Minneapolis, MN).
Cell Differentiation and Staining
For osteogenic differentiation, hASCs and C3H10T1/2 and MC3T3‐E1 cells were cultured in the presence of β‐glycerophosphate (10 mM) and ascorbic acid (50 μg/ml) for 6 or 18 days. The alkaline phosphatase (ALP) staining to confirm osteogenic differentiation was performed as previously described 17. The colorimetric measurement of ALP‐specific activity of cells was performed according to the manufacturer's instruction (Anaspec, Fremont, CA). Alizarin red S (ARS) staining was performed on differentiated hASCs and MC3T3‐E1 cells on day 18 with 1% ARS solution for 20 minutes. To achieve adipocyte differentiation of hASCs and C3H10T1/2 cells, cells were plated and cultured until they were confluent, at which time the medium was replaced with differentiation medium containing insulin (10 μg/ml), dexamethasone (1 μM), troglitazone (1 μM), and IBMX (500 μM). For adipocyte differentiation of 3T3‐L1 cells, the same medium except for troglitazone was used. For adipogenic differentiation of C3H10T1/2 and 3T3‐L1 cells, the medium was replaced with differentiation medium every 2 days. For adipogenic differentiation of hASCs, the medium was changed every 3 days. Oil red O (ORO) staining and its quantitation were performed as previously described 17. Chondrogenic differentiation of hASCs was performed as previously described 18 and confirmed by safranin O staining.
Construction of Small Hairpin RNAs and Lentiviral and Retroviral Infections
The short hairpin RNA (shRNA) and small interfering RNA (siRNA) sequences specific for endogenous SMAD4 are described in Supporting Information Table S3. Specific shRNAs were purchased from Mission‐shRNA (Sigma). Recombinant lentiviruses expressing each shRNA or retroviruses respectively expressing Flag‐Smad4, Flag‐S4‐MH1, Flag‐S4‐MH2, Flag‐hSmad4, and Flag‐S4‐NLSm were generated, according to the protocols previously described 19.
Statistical Analyses
All experiments, including immunoblots, were performed in three independent biological replicates. Results are expressed as the mean ± SD. The public GSE12267 and GSE74209 data were downloaded from the National Center for Biotechnology Information Gene Expression Omnibus. Statistical analyses were made with one‐way analysis of variance for more than three groups using Prism software (GraphPad Inc., La Jolla, CA). A p value of <.05 was regarded as a statistically significant difference. Differences of p < .05 are annotated as *, p < .01 are annotated as **, and p < .001 as ***.
Details of the transfection, reporter assay, subcellular fractionation, immunoblot, immunoprecipitation (IP), RNA extraction, quantitative real‐time RT‐PCR, immunofluorescence (IF), fluorescence‐activated cell sorting, and chromatin IP (ChIP) assay are provided in the Supporting Information.
Results
Smad4 Expression Is Increased During Osteogenic Differentiation
To identify the physiological roles of Smad4 in the lineage commitment of MSCs, we initially examined the expression of Smad4 in osteogenic differentiation. Quantitative real‐time RT‐PCR analysis indicated that Smad4 mRNA is increased during osteogenic differentiation of C3H10T1/2 MSC cell line, together with ALP mRNA, a marker gene of osteogenesis (Fig. 1A). Immunoblot analysis also showed increased expression of the Smad4 and Runx2 proteins (Fig. 1A). Similar results were obtained for the osteogenic differentiation of preosteoblast MC3T3‐E1 cell line and primary hASCs (Fig. 1B, 1C). The capacity of hASCs used in this study to differentiate into adipocytes, osteoblasts, and chondrocytes and MSC‐specific cell surface markers of hASCs were confirmed by ORO, ARS, safrarin O staining methods, as well as flow cytometry (Supporting Information Fig. S1).
Figure 1.
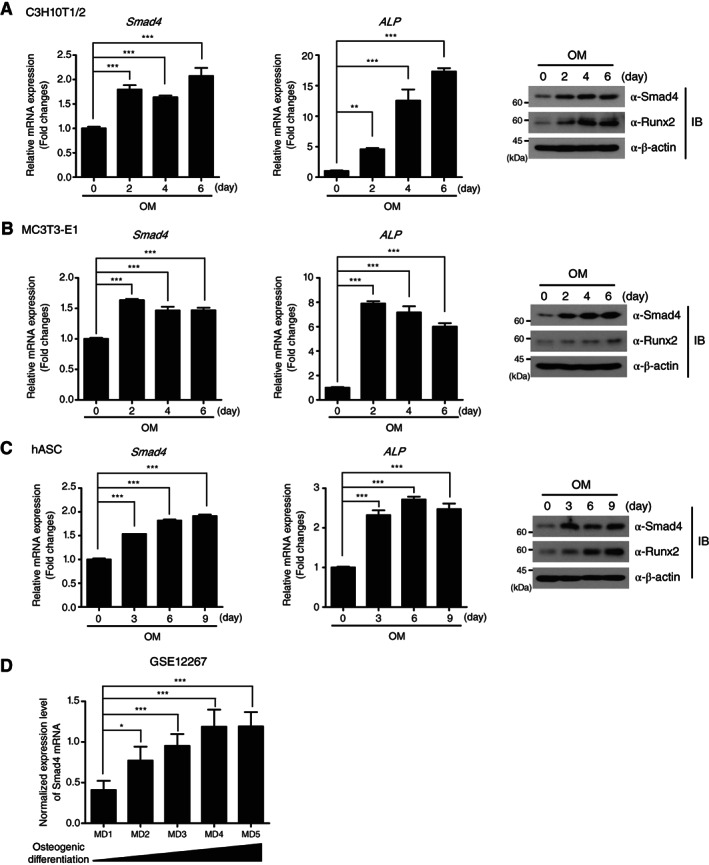
Transcriptional and translational expression of Smad4 is upregulated in osteogenesis. (A–C): Smad4 mRNA and protein expression during osteogenic differentiation of C3H10T1/2, MC3T3‐E1, and hASCs at the indicated time points were measured by quantitative reverse transcription‐polymerase chain reaction and IB analysis. The data were statistically analyzed by one‐way analysis of variance (ANOVA) followed by Tukey's multiple comparison test (**p < .01 and ***p < .001 compared to day 0, n = 3). Bars represent the mean ± SD. Expression of ALP mRNA and Runx2 protein were used as positive controls to prove successful osteogenesis. β‐actin expression was used as a loading control in IB analysis. The images in IB analysis are representative of three independent experiments. (D): Expression levels of Smad4 mRNA in osteogenic differentiation of human mesenchymal stem cells from a Gene Expression Omnibus data set (GSE12267, MD1 n = 5, MD2 n = 5, MD3 n = 5, MD4 n = 5, MD5 n = 5). The data were statistically analyzed by one‐way ANOVA followed by Tukey's multiple comparison test (*p < .05 and ***p < .001 compared to MD1). Bars represent the mean ± SD. Abbreviations: ALP, alkaline phosphatase; hASCs, human adipose tissue‐derived stem cells; IB, immunoblot; MD1; before MSC differentiation, MD2–5; culture endpoints of MSC differentiation into osteoblasts; OM, osteogenic differentiation medium; Runx2, runt‐related transcription factor 2.
To validate the increase of Smad4 expression in osteogenesis, we analyzed a public microarray data set (GSE12267) 20 displaying the gene expression profile in the osteogenic differentiation of human bone marrow‐derived MSCs, which showed a gradual augmentation of Smad4 expression during the progression of osteogenic differentiation (Fig. 1D). To support this statistical analysis, we analyzed another public microRNA array data set (GS74299) 21, investigating the inverse relationship of Smad4 to miR‐146a expression 22. miR‐146a was significantly increased in osteoporetic bones compared to healthy ones (Supporting Information Fig. S2), implying that Smad4, which is targeted by miR‐146a, may be reduced in osteoporosis. These results collectively suggest the possibility that Smad4 is involved in osteogenic differentiation of MSCs, independent of TGF‐β superfamily ligands.
Smad4 Depletion Inhibits Osteogenesis and Promotes Adipogenesis of C3H10T1/2 Cells
The capacity of MSCs to differentiate into several cell types makes it difficult to generate MSC‐specific knockout (KO) mice. Smad4 has the same hurdle in isolating Smad4‐KO MSCs, because Smad4‐KO mice fail to gastrulate and die at an early stage 23, 24. Therefore, to investigate the function of Smad4 in the osteogenic commitment of MSCs, we generated Smad4‐knockdown cell lines by infection of lentiviruses expressing two independent Smad4‐specific shRNAs in C3H10T1/2 cells. The efficiency of Smad4 depletion was confirmed by quantitative RT‐PCR and immunoblot analysis (Fig. 2A). C3H10T1/2 cells expressing green fluorescent protein (GFP)‐specific shRNA (shGFP) were used as a negative control. Before investigating the role of Smad4 in the osteogenic differentiation of MSCs, we examined expression of MSC‐specific cell surface markers in Smad4‐knockdown C3H10T1/2 cells to exclude the possibility that Smad4 depletion influences the characteristics of MSCs. MSCs have been reported to express positive and negative markers depending on species, isolation methods, and purified tissues 25, 26. Expressions of positive markers (CD44, CD105, and Sca‐1) and negative markers (CD11b, CD45, and MHC‐II) were not altered in Smad4‐knockdown C3H10T1/2 cells (shSmad4), compared to control cells (Fig. 2B).
Figure 2.
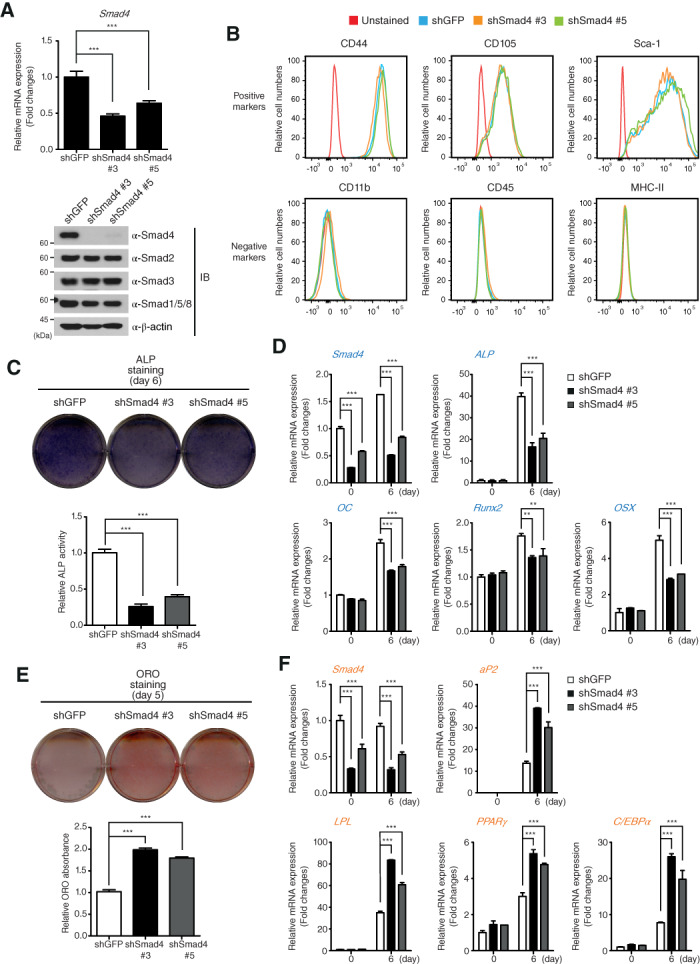
Smad4 reciprocally affects the osteogenic and adipogenic differentiation of C3H10T1/2 cells. (A): Expression of Smad4 mRNA and protein detected by quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR) and IB analysis in Smad4‐knockdown C3H10T1/2 cells depleted by different short hairpin RNAs (shRNAs) (shSmad4 #3 and shSmad4 #5). C3H10T1/2 cells expressing control shRNA against GFP mRNA (shGFP) were used as a negative control. β‐actin expression was used as a loading control in immunoblotting. (B): Expression of mesenchymal stem cell markers was analyzed by flow cytometry. (C): Smad4‐knockdown and shGFP‐expressing C3H10T1/2 cells were differentiated into osteoblasts under osteogenic differentiation conditions for 6 days. After cells were fixed, ALP was stained and ALP activity was measured. (D): Total RNAs of shSmad4‐ and shGFP‐expressing C3H10T1/2 cells were isolated at the indicated time points during osteogenic differentiation. Expression of Smad4, ALP, OC, Runx2, and OSX mRNAs were analyzed by qRT‐PCR. (E): Smad4‐knockdown and shGFP‐expressing C3H10T1/2 cells were differentiated into mature adipocytes for 5 days. Lipid droplet accumulation in the differentiated cells was identified by ORO staining and quantitated by measuring the ORO in the dissolved solution. (F): Total RNAs of shSmad4‐ and shGFP‐expressing C3H10T1/2 cells were isolated at the indicated time points during adipogenic differentiation. Expression of Smad4, aP2, LPL, PPARγ, and C/EBPα mRNAs were analyzed by qRT‐PCR. All data in this figure were statistically analyzed by one‐way analysis of variance followed by Tukey's multiple comparison test (***p < .001 compared to shGFP, n = 3). Bars represent the mean ± SD. The images shown in (A), (B), (C), and (E) are representative of three independent experiments. Abbreviations: ALP, alkaline phosphatase; C/EBPα, CCAAT/enhancer‐binding protein α; LPL, lipoprotein lipase; OC, osteocalcin; ORO, oil red O; OSX, osterix; PPARγ, peroxisome proliferator‐activated receptor‐γ; shGFP, green fluorescent protein‐specific short hairpin RNA.
We next investigated the differentiation of Smad4‐knockdown C3H10T1/2 cells into osteoblasts. ALP staining revealed that ALP activity is decreased in Smad4‐knockdown C3H10T1/2 cells, suggesting that osteogenesis is delayed by Smad4 depletion (Fig. 2C). Moreover, expression of target genes induced by osteogenic differentiation, including Smad4, ALP, OC, Runx2, and osterix (OSX), were significantly decreased at day 6 in Smad4‐knockdwon C3H10T1/2 cells (Fig. 2D).
Next, we confirmed the role of Smad4 in adipogenic differentiation of C3H10T1/2 MSC cells. Unlike osteogenic differentiation, expression of Smad4 mRNA and protein was unchanged during adipogenic differentiation of C3H10T1/2 cells (Supporting Information Fig. S3A). However, adipogenic differentiation was significantly promoted in Smad4‐knockdown C3H10T1/2 cells, which were identified by the detection of accumulating triglycerides in the cytosol by measurement of ORO staining (Fig. 2E). In addition, expression of adipogenic markers such as aP2, lipoprotein lipase, PPARγ, and CCAAT/enhancer‐binding protein α during adipogenic differentiation at day 6 was increased in Smad4‐knockdwon C3H10T1/2 cells (Fig. 2F). These results indicate that Smad4 not only acts as a positive factor in the osteogenic differentiation of MSCs but also as a negative factor in adipogenic differentiation.
Smad4 Regulates Adipo‐Osteogenic Differentiation of Human hASCs
To exclude the possibility that the effect of Smad4 in osteogenic differentiation is only specific to C3H10T1/2 MSC cell line, we generated the Smad4‐depleted hASCs by Smad4‐specific siRNAs (Fig. 3A) and subsequently investigated the differentiation of hASCs into osteoblasts under Smad4 depletion. Both ALP and ARS staining indicated that osteogenic differentiation of Smad4‐depleted hASCs is significantly delayed (Fig. 3B). In contrast to osteogenic differentiation of hASCs (Fig. 1C), expression of Smad4 mRNA and protein was unchanged during adipogenic differentiation of hASCs, similar to the results of C3H10T1/2 cells (Supporting Information Fig. S3). However, ORO staining indicated that adipogenic differentiation is promoted in Smad4‐knockdown hASCs (Fig. 3C). These results suggest that Smad4 acts as an important regulator in adipo‐osteogenic differentiation of MSCs, including C3H10T/12 cells and hASCs.
Figure 3.
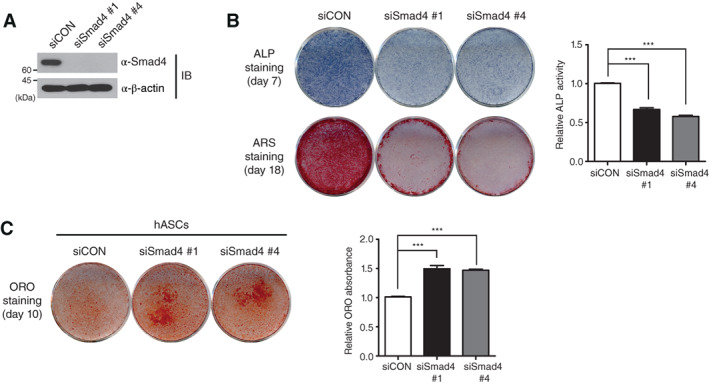
Smad4 reciprocally influences the osteogenic and adipogenic differentiation of hASCs. (A): Smad4 depletion by two independent small interfering RNA (siRNAs: siSmad4 #1 and shSmad4 #4) in hASCs was confirmed by IB analysis. (B): Smad4‐knockdown hASCs were differentiated into osteoblasts under osteogenic differentiation conditions. As a control, siCON‐expression hASCs were used. After 7 days, differentiation of hASCs into osteoblasts was verified by ALP staining together with measuring ALP activity. ARS staining was performed at day 18 after differentiation. The data regarding ALP activities were statistically analyzed by one‐way analysis of variance (ANOVA) followed by Tukey's multiple comparison test (***p < .001 compared to siCON, n = 3). Bars represent the mean ± SD. (C): siCON‐expressing and Smad4‐knockdown hASCs were differentiated into mature adipocytes for 10 days. Adipogenic differentiation was confirmed by ORO staining and quantitated by measuring the ORO in the dissolved solution. Data were statistically analyzed by one‐way ANOVA followed by Tukey's multiple comparison test (***p < .001 compared to siCON, n = 3). Bars represent the mean ± SD. The images shown in this figure are representative of three independent experiments. Abbreviations: ALP, alkaline phosphatase; ARS, alizarin red S; hASCs, human adipose tissue‐derived stem cells; IB, immunoblot; ORO, oil red O; siCON, control siRNA.
Smad4 Physically Interacts with Taz in Osteogenic and Adipogenic Differentiation
Our finding that Smad4 depletion in C3H10T1/2 cells shows opposite effects on osteogenesis and adipogenesis prompted us to focus on the Taz protein. The Taz protein acting as a transcriptional cofactor has been reported to promote osteogenesis and repress adipogenesis in MSC differentiation 27. We first examined whether Smad4 interacts with Taz in the differentiation of C3H10T1/2 cells. After plasmids encoding HA‐tagged Smad4 and Flag‐tagged Taz were transiently cotransfected into HEK293 cells, co‐IP assays indicated that Smad4 binds to Taz protein (Fig. 4A). IP assays revealed that endogenous Smad4 protein interacts with endogenous Taz under osteogenic differentiation conditions, with increased expressions of Runx2 and Taz proving successful progression of osteogenesis (Fig. 4B). Furthermore, endogenous interaction between Smad4 and Taz was also detected during adipogenic differentiation together with PPARγ expression (Fig. 4C). However, YAP protein, which is a paralog of Taz to be involved in MSC differentiation 28, did not interact with Smad4 in both osteogenic and adipogenic differentiation of MSCs (Supporting Information Fig. S4).
Figure 4.
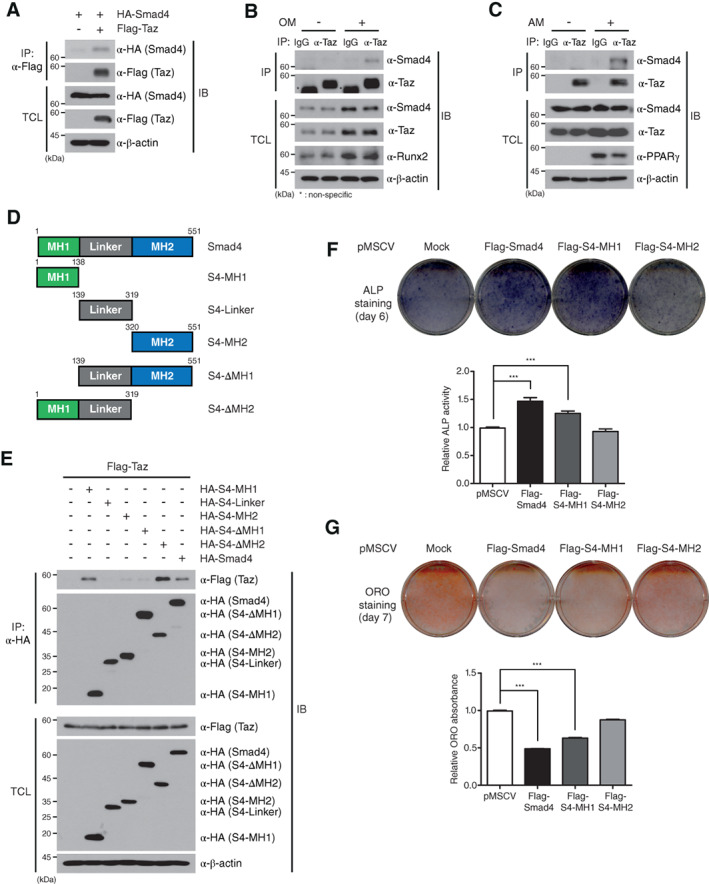
Smad4 interacts with Taz in osteogenic and adipogenic differentiation. (A): Plasmids encoding HA‐Smad4 was cotransfected with Flag‐Taz into HEK293 cells. Cell lysates were IP with the indicated antibodies and subsequently analyzed by IB. (B, C): C3H10T1/2 cells were differentiated into osteoblasts and adipocytes. To confirm the interaction of endogenous Smad4 and Taz proteins, IP and IB analysis were performed with the indicated antibodies against endogenous proteins. (D): Schematic representations of truncated Smad4 mutants encoding specific domains of Smad4 (MH1, Linker, MH2, ΔMH1, and ΔMH2). (E): Truncated Smad4 mutants were respectively cotransfected with the Flag‐Taz plasmid into HEK293 cells. IP and IB assays were performed with the indicated antibodies. (F, G): Full‐length Flag‐Smad4, Flag‐S4‐MH1, and Flag‐S4‐MH2 plasmids were, respectively, overexpressed in C3H10T1/2 cells by the retroviral system. After osteogenic differentiation, ALP activity was analyzed on day 6 to determine the osteogenic potential (F). A blue color indicates ALP activity. After adipogenic differentiation, cells were fixed on day 7 and stained with ORO (G). After the last washing, the stained plates were scanned and presented. The data in (F) and (G) were statistically analyzed by one‐way analysis of variance followed by Tukey's multiple comparison test (***p < .001 compared to shGFP, n = 3). Bars represent the mean ± SD. The data, except for (D), are representative of three independent experiments. Abbreviations: ALP, alkaline phosphatase; AM, adipogenic differentiation medium; IB, immunoblot; IgG, immunoglobulin G; IP, immunoprecipitated; OM, osteogenic differentiation medium; ORO, oil red O; PPARγ, peroxisome proliferator‐activated receptor‐γ; shGFP, green fluorescent protein‐specific short hairpin RNA; TCL, total cell lysates.
To gain further insight into the interaction between Smad4 and Taz, we generated five Smad4 deletion mutants: HA‐tagged N‐terminal MH1 domain (HA‐S4‐MH1), linker domain (HA‐S4‐Linker), C‐terminal MH2 domain (HA‐S4‐MH2), deletion of MH1 domain (HA‐S4ΔMH1), and deletion of MH2 domain (HA‐S4ΔMH2) (Fig. 4D). Co‐IP assays revealed that HA‐Smad4, HA‐S4‐MH1, and HA‐S4ΔMH2 bind to the Taz protein, whereas mutants without the MH1 domain (HA‐S4‐Linker, HA‐S4‐MH2, and HA‐S4ΔMH1) do not (Fig. 4E). Furthermore, we generated three truncated Taz mutants: HA‐tagged N‐terminal domain (HA‐ND), HA‐tagged middle domain, and HA‐tagged C‐terminal domain (Supporting Information Fig. S5A). These Taz deletion mutants were subsequently cotransfected with full‐length Flag‐tagged Smad4 (Flag‐Smad4) into HEK293 cells. Co‐IP assays showed that Flag‐Smad4 binds to full‐length HA‐tagged Taz (HA‐Taz) and HA‐ND (Supporting Information Fig. S5B).
Next, we examined whether interaction of the Smad4 MH1 domain with Taz protein is required for osteogenesis and adipogenesis. To this end, stable C3H10T1/2 cell lines ectopically expressing full‐length Flag‐Smad4, Flag‐tagged MH1 domain (Flag‐S4‐MH1), and Flag‐tagged MH2 domain (Flag‐S4‐MH2) were generated by infection of the respective recombinant retroviruses. The stable C3H10T1/2 cell lines expressing Flag‐Smad4 or Flag‐S4‐MH1 showed increased ALP activity and reduction of triglyceride accumulation in the cytoplasm during osteogenic and adipogenic differentiation, respectively, whereas the cells expressing Flag‐S4‐MH2 did not (Fig. 4F, 4G). Because these results were consistent with the osteogenic and adipogenic differentiation of Smad4‐knockdown C3H10T1/2 cells, our findings suggest that Smad4, as a binding partner of Taz, cooperates with the N‐terminal domain of Taz through its MH1 domain and contributes to osteogenic and adipogenic differentiation.
Smad4 Promotes Nuclear Localization of Taz
To understand the molecular mechanism of how Smad4 differentially regulates osteogenic and adipogenic differentiation through interacting with the Taz protein, we examined whether Smad4 regulates the expression of Taz mRNA or its protein in Smad4‐knockdown C3H10T1/2 cells. Quantitative RT‐PCR and immunoblot analysis indicated that Taz expression is unchanged by two independent Smad4 depletions (Supporting Information Fig. S6). These results promoted us to focus on the functional activity of Taz, because Taz has been known to be engaged in nuclear‐cytoplasmic shuttling to modulate the transcriptional activity of transcription factors 27, 29. To test the effect of Smad4 on Taz localization, an HA‐Taz plasmid was transiently transfected into HEK293 cells with a Flag‐Smad4 in a dose‐dependent manner and a GFP‐expressing plasmid was used as a negative control. After cytoplasmic and nuclear fractions were separated, immunoblot analysis indicated that Taz localization into the nucleus was significantly augmented by dose‐dependent increases of the Smad4 protein, but cytoplasmic localization of Taz was not affected (Fig. 5A).
Figure 5.
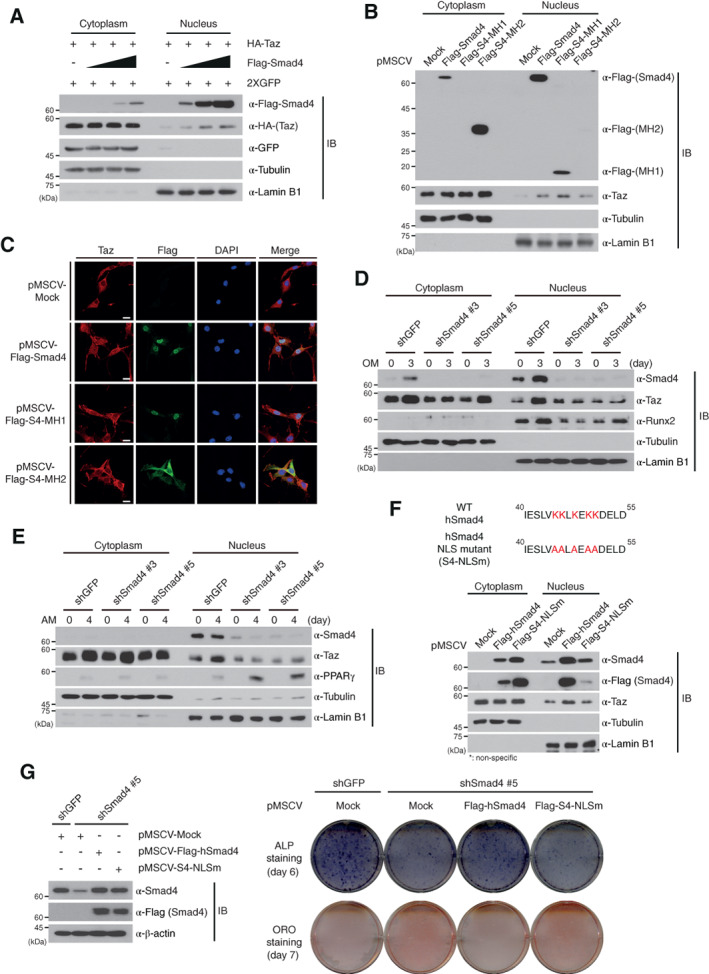
Smad4 induces nuclear localization of Taz. (A, B): A plasmid encoding HA‐Taz was cotransfected with dose‐dependent increases of the Flag‐Smad4 plasmid into HEK293 cells, which were subsequently fractionated (A). Stable C3H10T1/2 cells expressing the indicated plasmids were fractionated into cytoplasm and nuclei. The localization of Taz was analyzed by IBs with the indicated antibodies. The empty vector, pMSCV, was used as a negative control in (B) (Mock). (C): Localization of endogenous Taz in stable C3H10T1/2 cells expressing the indicated plasmids was detected by immunofluorescence analysis. The empty vector, pMSCV, was used as a negative control (Mock). Nuclei were stained with DAPI. Scale bars, 20 μm. (D, E): Smad4‐knockdown and shGFP‐expressing C3H10T1/2 cells in osteogenic (D) or adipogenic (E) differentiation medium were fractionated on day 3 or day 4, respectively, and the Taz localization was analyzed by immunoblotting. (F): Stable C3H10T1/2 cells expressing wild‐type human Smad4 (Flag‐hSmad4) and Smad4 NLS mutant (Flag‐S4‐NLSm) were fractionated and the localization of Taz was detected by immunoblotting. (G): Ectopic expression of Flag‐hSmad4 or Flag‐S4‐NLSm in Smad4‐knockdown C3H10T1/2 cells was detected by IB analysis (left). β‐actin expression was used as a loading control in IBs. Osteogenesis and adipogenesis of Smad4‐knockdown cells ectopically expressing Flag‐hSmad4 or Flag‐S4‐NLSm were observed at the indicated time points with ALP or ORO staining. The empty vector, pMSCV (Mock). The images in this figure are representative of three independent experiments. Expressions of α‐tubulin and α‐Lamin B1 in (A), (B), and (D)–(F) were used as cytoplasmic and nuclear markers and loading controls. Abbreviations: ALP, alkaline phosphatase; DAPI, 4′,6‐diamidino‐2‐phenylindole; GFP, green fluorescent protein; IB, immunoblot; NLS, nuclear localization signal; OM, osteogenic differentiation medium; ORO, oil red O; Runx2, runt‐related transcription factor 2; shGFP, green fluorescent protein‐specific short hairpin RNA.
To further validate the changes in Taz localization by Smad4 in C3H10T1/2 cells, subcellular fractionation and IF assays were performed in stable C3H10T1/2 cells ectopically expressing full‐length Flag‐Smad4, Flag‐S4‐MH1, or Flag‐S4‐MH2, respectively. C3H10T1/2 cells expressing the empty pMSCV vector were used as a negative control. Taz localization into the nucleus was increased in only C3H10T1/2 cells expressing full‐length Smad4 and its MH1 domain, compared to MH2 domain‐expressing and control C3H10T1/2 cells (Fig. 5B, 5C). However, these results do not exclude the possibility that Smad4, endogenously expressed in these overexpression cell lines, can influence the nuclear localization of Taz. To exclude this possibility, Taz localization into the nucleus was observed in Smad4‐null SW620 cells ectopically expressing Flag‐Smad4, Flag‐S4‐MH1, and Flag‐S4‐MH2, respectively (Supporting Information Fig. S7). Expressions of Smad4 and its MH1 domain significantly increased the retention of Taz in the nucleus of SW620 cells but the MH2 domain did not (Supporting Information Fig. S7). These data imply that Smad4 increases localization of the Taz protein into the nucleus through the interaction between the Smad4 MH1 domain and Taz.
We next examined localization of the Taz protein into the nucleus during osteogenesis and adipogenesis of C3H10T1/2 cells. When Smad4‐expressing control C3H10T1/2 cells were differentiated into osteoblasts, a time‐dependent increase of Taz protein was observed in both the cytoplasm and nucleus (Supporting Information Fig. S8A). To confirm whether Smad4 depletion affects Taz localization, we analyzed Taz expression in both cytosolic and nuclear fractions of shGFP‐ and shSmad4‐expressing C3H10T1/2 cells on day 0 and day 3 during osteogenic differentiation. When Smad4 was depleted, Taz localization in the nucleus was significantly decreased on day 3, compared to the increased Taz nuclear localization of control C3H10T1/2 cells (Fig. 4D). In addition, the increased Runx2 expression in the nucleus observed in osteogenic differentiation of shGFP‐expressing C3H10T1/2 cells was not detected in Smad4‐knockdown cells (Fig. 5D).
Like osteogenic differentiation, time‐dependent accumulation of Taz in the nucleus was identical in adipogenic differentiation of Smad4‐expressing C3H10T1/2 cells (Supporting Information Fig. S8B). However, the increased Taz localization in the nucleus of shGFP‐expressing C3H10T1/2 cells on day 4 of adipogenic differentiation was not observed in Smad4‐knockdown cells (Fig. 5E). In contrast, PPARγ expression in the nucleus was higher in Smad4‐knockdown C3H10T1/2 cells during adipogenesis compared to shGFP‐expressing C3H10T1/2 cells (Fig. 5E). The decreased localization of Taz into the nucleus of Smad4‐knockdown C3H10T1/2 cells was similarly observed during osteogenic and adipogenic differentiation of Smad4‐depleted hASCs (Supporting Information Fig. S8C, S8D), suggesting that Smad4 may bring Taz protein into the nucleus during osteogenic and adipogenic differentiation, because Smad4 shuttles between the cytoplasm and nucleus through its nuclear localization and nuclear export signals (NES) 30.
To confirm this possibility, we generated a Smad4 mutant with NLS sequences substituted with alanine (Fig. 5F). Stable C3H10T1/2 cells expressing Flag‐tagged human Smad4 (Flag‐hSmad4) or Flag‐tagged human Smad4 NLS mutant (Flag‐S4‐NLSm) were fractionated into cytoplasmic and nuclear extracts. Mutation of the NLS sequences within Smad4 clearly decreased Smad4 translocation into the nucleus, as expected (Fig. 5F). Interestingly, expression of wild‐type Flag‐hSmad4 increased the nuclear localization of Taz whereas the Flag‐S4‐NLSm mutant did not (Fig. 5F).
To test whether Smad4 NLS sequences are crucial for Taz nuclear localization during osteogenic and adipogenic differentiation, we generated stable C3H10T1/2 cells expressing wild‐type Flag‐hSmad4 or the Flag‐S4‐NLSm mutant in a Smad4‐knockdown background by infection of recombinant retroviruses. Ectopic expression of wild‐type Smad4 or the NLS mutant in Smad4‐knockdown C3H10T1/2 cells was confirmed by immunoblot analysis (Fig. 5G). ALP staining analysis showed that expression of wild‐type Flag‐hSmad4 restores osteogenic differentiation of C3H10T1/2 cells initially repressed by Smad4 depletion, whereas expression of the Smad4 NLS mutant (Flag‐S4‐NLSm) does not (Fig. 5G). In contrast, ORO staining showed that expression of wild‐type Smad4 represses adipogenic differentiation of C3H10T1/2 cells facilitated by Smad4‐depletion whereas the Smad4 NLS mutant does not (Fig. 5G). Therefore, these results indicate that Smad4 induces Taz retention in the nucleus through its NLS within MH1 domain and is thus a crucial factor regulating the reciprocal adipo‐osteogenic differentiation of C3H10T1/2 cells.
Smad4 Promotes Runx2‐Mediated Osteogenic Differentiation by Interacting with Taz
We next examined whether Smad4 affects the interaction between the Taz and Runx2 protein in osteogenic differentiation. Taz is known to interact with Runx2, a master transcription factor in osteogenic differentiation 27. Co‐IP assays indicated that interaction of Flag‐Taz with HA‐Runx2 was significantly increased in the presence of Flag‐Smad4 (Fig. 6A). To exclude the possibility that this result is because of direct interaction of Smad4 with Runx2, plasmids encoding Flag‐Smad4 and HA‐Runx2 were transfected into HEK293 cells in the absence or presence of Flag‐Taz. Co‐IP assays revealed that Flag‐Smad4 indirectly interacts with HA‐Runx2 in the presence of Flag‐Taz (Fig. 6B; lane 2, 3). Furthermore, IP assays showed that endogenous Taz protein interacts with endogenous Runx2 in the osteogenic differentiation of control C3H10T1/2 cells, but this interaction was decreased in Smad4‐knockdown C3H10T1/2 cells (Fig. 6C).
Figure 6.
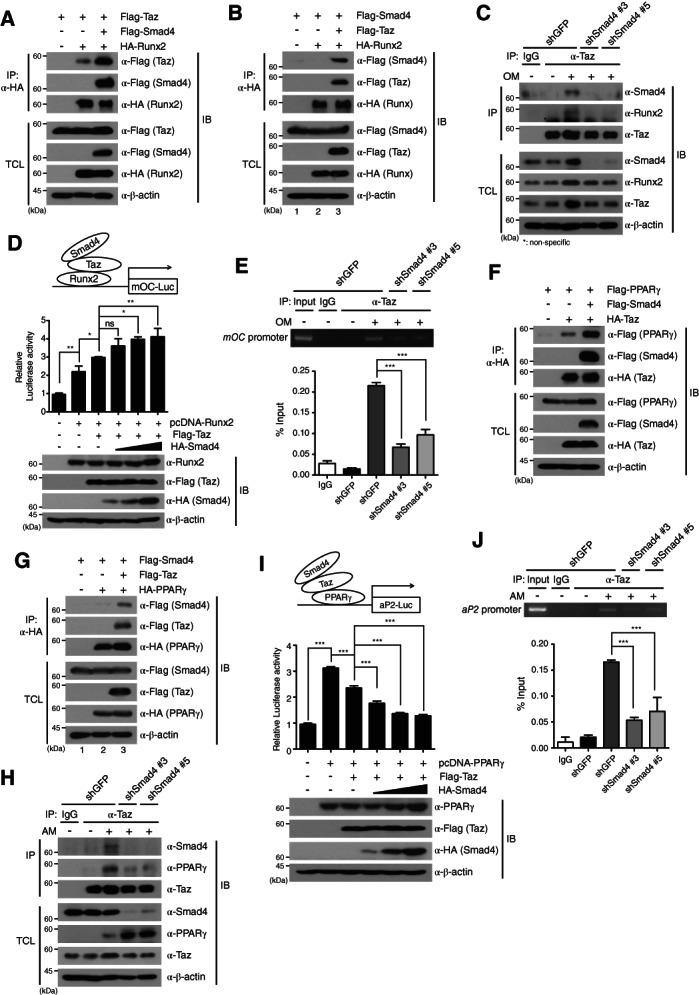
Smad4 enhances the Taz interaction with Runx2 or PPARγ. (A, F): HA‐Runx2 or Flag‐PPARγ plasmid was cotransfected with the Flag‐Taz or HA‐Taz plasmid into HEK293 cells in the absence or presence of Flag‐Smad4, according to the indicated combinations. (B, G): Flag‐Smad4 plasmid was cotransfected with the HA‐Runx2 (B) or HA‐PPARγ (G) into HEK293 cells in the absence or presence of Flag‐Taz. (C, H): After osteogenic differentiation for 3 days (C) or adipogenic differentiation for 4 days (H) of Smad4‐knockdown and shGFP‐expressing C3H10T1/2 cells, cell lysates were IP with anti‐Taz antibody and immunoblotted. (D, I): pcDNA‐Runx2 (D) or pcDNA‐PPARγ (I) plasmid was cotransfected with the mOC‐Luc or aP2‐Luc reporter, together with Flag‐Taz, into HEK293 cells under dose‐dependent expression of Flag‐Smad4. Expressions of the indicated proteins were confirmed by IB. Luciferase activity was normalized to the expression of Renilla luciferase. (E, J): After osteogenic (E) or adipogenic (J) differentiation of Smad4‐knockdown and control C3H10T1/2 cells, chromatin immunoprecipitation assays were performed on day 3 (E) or day 4 (J). Chromatin fragments were IP with anti‐Taz antibody. Polymerase chain reaction primers for the osteocalcin promoter region or aP2 promoter were used to amplify the DNAs. The amplified DNAs from input samples were used for normalizing the data. IgG: negative control. The data were analyzed by one‐way analysis of variance followed by Tukey's multiple comparison test (*p < .05, **p < .01 and ***p < .001 compared to the indicated controls, n = 3). Bars represent the mean ± SD. Images in this figure are representative of three independent experiments. Abbreviations: AM, adipogenic differentiation medium; IB, immunoblot; IgG, immunoglobulin G; IP, immunoprecipitated; OM, osteogenic differentiation medium; PPARγ, peroxisome proliferator‐activated receptor‐γ; Runx2, runt‐related transcription factor 2; shGFP, green fluorescent protein‐specific short hairpin RNA; TCL, total cell lysates.
We next investigated whether the Smad4 requirement in the direct interaction between Taz and Runx2 is involved in transcriptional regulation of osteogenic‐specific genes. A mouse OC‐luciferase (mOC‐Luc) reporter containing Runx2 binding sites, pcDNA‐Runx2, and Flag‐Taz plasmids were cotransfected into HEK293 cells, together with dose‐dependent expression of the HA‐Smad4 plasmid (Fig. 6D). Flag‐Taz enhanced activity of the mOC‐Luc reporter induced by Runx2 expression. Interestingly, dose‐dependent increases of Smad4 further increased the luciferase activity of mOC‐Luc, indicating that Smad4 increases Runx2‐mediated transcriptional activity through enhancing the interaction of the Taz‐Runx2 complex (Fig. 6D). This observation was proved by ChIP assays. ChIP assays with anti‐Taz antibody during osteogenic differentiation of shGFP‐expressing and shSmad4‐expressing C3H10T1/2 cells showed that Taz binds to the OC promoter during osteogenic differentiation of shGFP‐expressing control cells, but this binding was significantly decreased upon Smad4 depletion (Fig. 6E). These results suggest that Smad4 enhances transcriptional activity by the Taz‐Runx2 axis in osteogenic differentiation through promoting the interaction of Taz with Runx2.
Smad4 Suppresses PPARγ‐Mediated Adipogenic Differentiation by Interacting with Taz
Next, we investigated how the increase in Taz nuclear localization by Smad4 affects PPARγ‐mediated transcriptional activity required for adipogenic differentiation. Taz has been reported to be a transcriptional co‐repressor of PPARγ, a master transcription factor in adipogenic differentiation 27, and Smad4 was observed to interact with Taz in adipogenic differentiation (Fig. 4C). To address this question, we examined the interrelationship of the Smad4, Taz, and PPARγ proteins in adipogenesis. Plasmids encoding Flag‐tagged PPARγ (Flag‐PPARγ), Flag‐Smad4, and HA‐Taz were transiently transfected into HEK293 cells (Fig. 6F). HA‐Taz specifically bound to Flag‐PPARγ and this interaction was significantly augmented by expression of Flag‐Smad4 (Fig. 6F). Flag‐Smad4 did not directly bind to HA‐PPARγ2, but indirectly interacted with HA‐PPARγ through binding to Flag‐Taz (Fig. 6G; lane 2, 3). These results were supported by IP assays against endogenous Taz and PPARγ proteins. Although endogenous Taz protein interacted with endogenous Smad4 and PPARγ during adipogenic differentiation of control shGFP‐expressing C3H10T1/2 cells, the interaction between Taz and PPARγ was decreased in Smad4‐knockdown cells (Fig. 6H).
These results prompted us to hypothesize that Smad4 can modulate PPARγ‐mediated transcriptional downregulation of genes required for adipogenesis through interaction with Taz‐PPARγ, because the Taz protein is a known negative regulator of adipogenesis 27. A plasmid encoding an aP2‐luciferase (aP2‐Luc) reporter containing PPARγ binding sites, pcDNA‐PPARγ, and Flag‐Taz plasmids were cotransfected into HEK293 cells, together with dose‐dependent expression of the HA‐Smad4 plasmid. Taz expression suppressed activity of the aP2‐Luc reporter in the presence of PPARγ (Fig. 6I) Dose‐dependent increases of Smad4 expression further inhibited the luciferase activity of aP2‐Luc (Fig. 6I). ChIP assays with anti‐Taz antibody in the adipogenic differentiation of shGFP‐expressing and shSmad4‐expressing C3H10T1/2 cells indicated that Taz binds to the aP2 promoter during adipogenic differentiation of shGFP‐expressing control cells, but this binding was significantly reduced in Smad4‐depleted C3H10T1/2 cells (Fig. 6J). These results propose that the increased interaction of Taz with Smad4 in the nucleus enhances the interaction of Taz with PPARγ, contributing to the reduction of adipogenic differentiation through augmenting the inhibitory function of Taz.
Smad4 Regulates Differentiation of Preosteoblast and Preadipocyte Cells
Our present findings that Smad4 plays a crucial role in the reciprocal regulation of adipo‐osteogenic differentiation of the C3H10T1/2 MSC cell line led us to verify that this observation is not due to cell‐type specificity. Thus, we examined whether Smad4 shows the same effects in the osteogenic differentiation of MC3T3‐E1 cells and adipogenic differentiation of preadipocyte 3T3‐L1 cells. Subcellular fractionation assays of MC3T3‐E1 and 3T3‐L1 cells indicated that endogenous Smad4 is mainly localized in the nucleus, similar to C3H10T1/2 cells (Fig. 7A). We next induced the osteogenic differentiation of Smad4‐knockdown MC3T3‐E1 and adipogenic differentiation of Smad4‐knockdwon 3T3‐L1 cells, respectively. Smad4‐knockdown MC3T3‐E1 cells showed delayed osteogenic differentiation, measured by ALP staining and ARS staining (Fig. 7B). In contrast, ORO staining indicated that Smad4‐knockdown 3T3‐L1 cells promote more adipogenic differentiation than shGFP‐expressing 3T3‐L1 cells (Fig. 7C).
Figure 7.
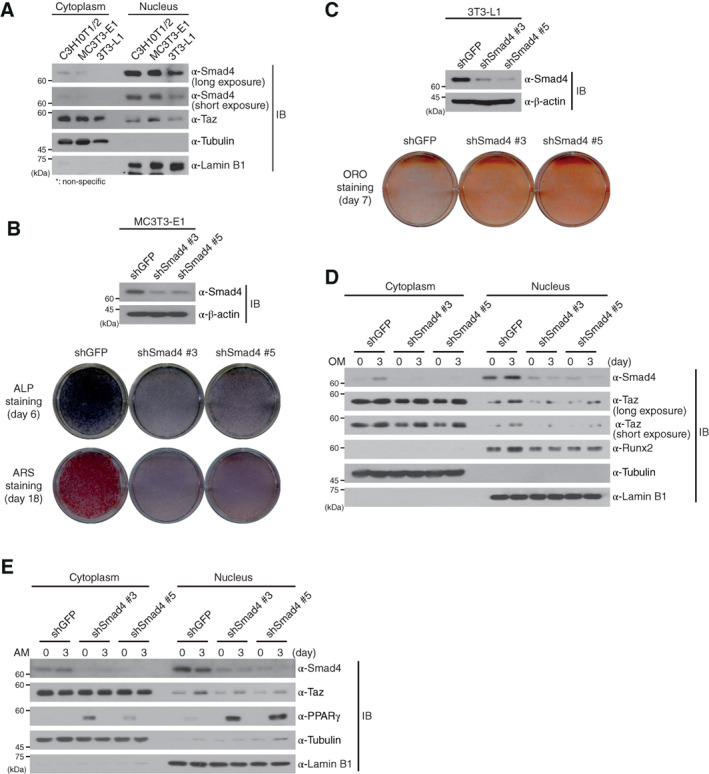
Smad4 is required for the differentiations of preosteoblasts and preadipocyes cells. (A): C3H10T1/2, preosteoblastic MC3T3‐E1 and preadipogenic 3T3‐L1 cells were fractionated into cytoplasm and nuclei. Expression of endogenous Smad4 and Taz were observed by immunoblotting with the indicated antibodies. (B): Smad4 depletion by lentiviruses expressing two independent short hairpin RNAs (shRNAs) specific to Smad4 was confirmed by immunoblotting in Smad4‐knockdown and shGFP‐expressing MC3T3‐E1 cells (upper panel). These cells were incubated with osteogenic medium, and osteogenic differentiation was confirmed by ALP and ARS staining. (C): Smad4 depletion by lentiviruses expressing two independent shRNAs specific to Smad4 was confirmed by immunoblotting in Smad4‐knockdown and shGFP‐expressing 3T3‐L1 cells (upper panel). Adipogenic differentiation of these cells was measured by ORO staining at day 7. (D): Smad4‐knockdown and shGFP‐expressing MC3T3‐E1 cells were incubated in OM for 3 days. Cells were fractionated into cytoplasm and nuclear extracts at the indicated times and both extracts were immunoblotted with the indicated antibodies. (E): Smad4‐knockdown and shGFP‐expressing 3T3‐L1 cells were differentiated into adipocytes for 3 days. Cells were fractionated into cytoplasm and nuclear extracts at the indicated times and both extracts were immunoblotted with the indicated antibodies. Expression of α‐tubulin and α‐Lamin B1 in (A), (D), and (E) was used as cytoplasmic and nuclear markers and loading controls. Images shown in this figure are representative of three independent experiments. Abbreviations: ALP, alkaline phosphatase; AM, adipogenic differentiation medium; ARS, alizarin red S; IB, immunoblot; OM, osteogenic differentiation medium; ORO, ORO, oil red O; Runx2, runt‐related transcription factor 2; shGFP, green fluorescent protein‐specific short hairpin RNA.
We also performed subcellular fractionation assays in shGFP‐expressing and shSmad4‐expressing MC3T3‐E1 and 3T3‐L1 cells under osteogenic or adipogenic differentiation conditions. Both Smad4‐knockdown MC3T3‐E1 and Smad4‐knockdown 3T3‐L1 cells showed a marked reduction of Taz retention in the nucleus 3 days after the start of osteogenic and adipogenic differentiation (Fig. 7D, 7E). Together with the results regarding osteogenic and adipogenic differentiation of hASCs (Fig. 3 and Supporting Information Fig. S8), our findings support that the opposing effects of Smad4 in osteogenic and adipogenic differentiation are not due to cell‐type specificity.
Discussion
MSCs can differentiate into osteoblasts and adipocytes, and the differentiation into either cell type is reversely exclusive 2. In this study, we demonstrate a noncanonical role of Smad4 independent of TGF‐β and BMP signals regarding MSC differentiation, like TGF‐β‐independent functions of Smad4 reported by several groups 31, 32, 33, 34. Our work revealed that Smad4 is reciprocally involved in osteogenic and adipogenic differentiation in C3H10T1/2 MSC cell line and primary hASCs and modulates the nuclear localization of Taz in all cells used in this study. Although the importance of Smad4 in osteogenic differentiation has been proposed in certain reports, including a study describing the reduction of bone mineral density, bone volume, and osteoblast numbers in osteoblast‐specific Smad4 conditional KO mice 35 and one on the role of miR‐144‐3p‐targeting Smad4 in osteogenic differentiation 36, our findings extend our knowledge about the molecular mechanism of Smad4 as a positive factor in the osteogenic differentiation of MSCs. In contrast, a possible role of Smad4 in adipogenic differentiation was predicted by a previous report that miR‐125b‐5p could potentially target Smad4 in preadipocyte 3T3‐L1 cells 37, but this report did not demonstrate the exact mechanism of Smad4 as a negative factor in adipogenic differentiation. Therefore, our results provide a clue about the inverse reciprocal regulation between the osteogenesis and adipogenesis of MSCs. That is, Smad4‐Taz availability to interact with Runx2 in the promotion of osteogenic differentiation simultaneously suppresses adipogenic differentiation through interacting with PPARγ.
One interesting finding in this study is the role of Smad4 regulating Taz localization into the nucleus. Smad4 is well known to be present mainly in the cytoplasm and to enter the nucleus when TGF‐β signaling occurs 11. However, endogenous and ectopically expressed Smad4 was mainly localized to the nucleus in all cell lines we tested. The predominant nuclear localization of Smad4 in C3H10T1/2, hASCs, MC3T3‐E1, and 3T3‐L1 cells can be speculated to be regulated by the relative strengths of NLS and NES within Smad4, because Smad4 is continuously shuttling between the cytoplasm and nucleus by its own NLS and NES without the TGF‐β signal 30 and its localization is also determined by the relative predominance between NLS and NES depending on cell type. What we note in this study is that Smad4 depletion decreases Taz retention in the nucleus and ectopic expression of Smad4 significantly increases Taz nuclear localization. This role of Smad4 in regulating the nuclear localization of Taz requires the NLS within Smad4. Therefore, decreased retention of Taz in the nucleus upon Smad4 depletion causes reduced interactions of Taz with Runx2 and PPARγ, required for osteogenic and adipogenic differentiation.
Taz is a well‐known protein regulating MSC differentiation. Several cytokines and natural products are involved in the differentiation of MSCs through regulating the activity of Taz. FGF2 signaling induces increased Taz expression and Wnt signaling leads to increased Taz stability 29, 38. Furthermore, kaempferol (KMP), a phytoestrogen abundant in tea, has been reported to facilitate the physical interaction between Taz and Runx2 and subsequently increase the transcriptional activities of Runx2 17. In addition, KMP also enhances the association of Taz with PPARγ, thereby resulting in diminished adipocytes through suppressing PPARγ transcriptional activity 17. Although these recent findings suggest that nuclear Taz activity is important in determining MSC lineage commitment, there have been no reports addressing the endogenous protein that induces the retention of Taz in the nucleus. Therefore, this is the first report about a protein, Smad4, which induces the retention of Taz in the nucleus during osteogenic and adipogenic differentiation.
Taz nuclear localization is also known to be regulated by phosphorylation. Taz phosphorylation at serine 89 by the large tumor suppressor kinase induces interaction with the 14‐3‐3 protein and subsequent cytosolic sequestration of Taz, and further phosphorylation by CK1ε causes Taz degradation by β‐TrCP 39, 40. Our results indicated that Smad4 does not affect the interaction of Taz with the 14‐3‐3 protein and the stability of Taz in osteogenic and adipogenic differentiation (Supporting Information Fig. S9), suggesting that nuclear localization of Taz by Smad4 is a unique mechanism independent of Taz phosphorylation.
Conclusion
We here demonstrate that nuclear retention of Taz by direct binding to Smad4 facilitates the osteogenic differentiation of the MSCs through enhancing Taz‐Runx2 interaction, whereas it suppresses the adipogenic differentiation of MSCs through promoting Taz‐PPARγ interaction. In addition, the nuclear localization of Taz requires the NLS of Smad4. Therefore, further understanding of the Smad4‐Taz axis in MSC differentiation may help in the artificial modulation of certain human diseases regarding osteogenic and adipogenic differentiation.
Author Contributions
J.S.P.: designed the research, experimental work, data analysis, manuscript writing; M.K., N.‐J.S., J.‐H.K., D.S., J.‐H.L., S.M.J., J.Y.L., and J.L.: experimental work, data analysis and interpretation; Y.S.L. and K.W.P.: conception/design, experimental work; S.H.P.: conception/design, experimental work, data analysis, manuscript writing, final approval of the manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Supporting information
Appendix S1: Supporting information
Acknowledgments
We thank Dr. Min Sung Choi for critical reading of the manuscript. This work was supported by the National Research Foundation grant of Korea (SRC 2017R1A5A1014560) funded by the Ministry of Science and ICT and in part by a grant from the National R&D Program for Cancer Control (1520120 to S.H.P) funded by Ministry for Health and Welfare, Republic of Korea.
References
- 1. Pittenger MF, Mackay AM, Beck SC et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143–147. [DOI] [PubMed] [Google Scholar]
- 2. Chen Q, Shou P, Zheng C et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ 2016;23:1128–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gimble JM, Zvonic S, Floyd ZE et al. Playing with bone and fat. J Cell Biochem 2006;98:251–266. [DOI] [PubMed] [Google Scholar]
- 4. Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol 2006;7:885–896. [DOI] [PubMed] [Google Scholar]
- 5. Moerman EJ, Teng K, Lipschitz DA et al. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: The role of PPAR‐gamma2 transcription factor and TGF‐beta/BMP signaling pathways. Aging Cell 2004;3:379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roelen BA, Dijke P. Controlling mesenchymal stem cell differentiation by TGFBeta family members. J Orthop Sci 2003;8:740–748. [DOI] [PubMed] [Google Scholar]
- 7. Lai CF, Cheng SL. Signal transductions induced by bone morphogenetic protein‐2 and transforming growth factor‐beta in normal human osteoblastic cells. J Biol Chem 2003;277:15514–15522. [DOI] [PubMed] [Google Scholar]
- 8. Lee MH, Kim YJ, Kim HJ et al. BMP‐2‐induced Runx2 expression is mediated by Dlx5, and TGF‐beta 1 opposes the BMP‐2‐induced osteoblast differentiation by suppression of Dlx5 expression. J Biol Chem 2003;278:34387–34394. [DOI] [PubMed] [Google Scholar]
- 9. Lee KS, Kim HJ, Li QL et al. Runx2 is a common target of transforming growth factor beta 1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast‐specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Mol Cell Biol 2000;20:8783–8792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. zur Nieden NI, Kempaja G, Rancourt DE et al. Induction of chondro‐, osteo‐, and adipogenesis in embryonic stem cells by bone morphogenic protein‐2: Effects of cofactors on differentiating lineages. BMC Dev Biol 2005;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Massague J. TGFbeta signalling in context. Nat Rev Mol Cell Biol 2012;13:616–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Avery S, Zafarana G, Gokhale PJ et al. The role of SMAD4 in human embryonic stem cell self‐renewal and stem cell fate. Stem Cells 2010;28:863–873. [DOI] [PubMed] [Google Scholar]
- 13. Mo JS, Park HW, Guan KL. The Hippo signaling pathway in stem cell biology and cancer. EMBO Rep 2014;15:642–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Varelas X, Sakuma R, Samavarchi‐Tehrani P et al. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem‐cell self‐renewal. Nat Cell Biol 2008;10:837–848. [DOI] [PubMed] [Google Scholar]
- 15. Beyer TA, Weiss A, Khomchuk Y et al. Switch enhancers interpret TGF‐beta and Hippo signaling to control cell fate in human embryonic stem cells. Cell Rep 2013;5:1611–1624. [DOI] [PubMed] [Google Scholar]
- 16. Shim G, Im S, Lee S et al. Enhanced survival of transplanted human adipose‐derived stem cells by co‐delivery with liposomal apoptosome inhibitor in fibrin gel matrix. Eur J Pharm Biopharm 2013;85:673–681. [DOI] [PubMed] [Google Scholar]
- 17. Byun MR, Jeong H, Bae SJ et al. TAZ is required for the osteogenic and anti‐adipogenic activities of kaempferol. Bone 2012;50:364–372. [DOI] [PubMed] [Google Scholar]
- 18. Jung KH, Song SU, Yi T et al. Human bone marrow‐derived clonal mesenchymal stem cells inhibits inflammation and reduce acute pancreatitis in rats. Gastroenterology 2011;3:998–1008. [DOI] [PubMed] [Google Scholar]
- 19. Lee JH, Jung SM, Yang KM et al. A20 promotes metastasis of aggressive basal‐like breast cancers through multi‐monoubiquitylation of Snail1. Nat Cell Biol 2017;19:1260–1273. [DOI] [PubMed] [Google Scholar]
- 20. Granchi D, Ochoa G, Leonardi E et al. Gene expression patterns related to osteogenic differentiation of bone marrow‐derived mesenchymal stem cells during ex vivo expansion. Tissue Eng Part C‐Methods 2010;16:511–524. [DOI] [PubMed] [Google Scholar]
- 21. De‐Ugarte L, Yoskovitz G, Balcells S et al. MiRNA profiling of whole trabecular bone: Identification of osteoporosis‐related changes in MiRNAs in human hip bones. BMC Med Genomics 2015;8:75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kuang W, Zheng L, Xu X et al. Dysregulation of the miR‐146a‐Smad4 axis impairs osteogenesis of bone mesenchymal stem cells under inflammation. Bone Res 2017;5:17037–17045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sirard C, de la Pompa JL, Elia A et al. The tumor suppressor gene Smad4/Dpc4 is required for gastrulation and later for anterior development of the mouse embryo. Genes Dev 1998;12:107–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang X, Li C, Xu X et al. The tumor suppressor SMAD4/DPC4 is essential for epiblast proliferation and mesoderm induction in mice. Proc Natl Acad Sci U S A 1998;95:3667–3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dominici M, Le Blanc K, Mueller I et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315–317. [DOI] [PubMed] [Google Scholar]
- 26. Peister A, Mellad JA, Larson BL et al. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood 2004;103:1662–1668. [DOI] [PubMed] [Google Scholar]
- 27. Hong JH, Hwang ES, McManus MT et al. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science 2005;309:1074–1078. [DOI] [PubMed] [Google Scholar]
- 28. Seo E, Basu‐Roy U, Gunaratne PH et al. SOX2 regulates YAP1 to maintain stemness and determine cell fate in the osteo‐adipo lineage. Cell Rep 2013;3:2075–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Byun MR, Hwang JH1, Kim AR et al. Canonical Wnt signalling activates TAZ through PP1A during osteogenic differentiation. Cell Death Differ 2014;21:854–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pierreux CE, Nicolas FJ, Hill CS. Transforming growth factor beta‐independent shuttling of Smad4 between the cytoplasm and nucleus. Mol Cell Biol 2000;20:9041–9054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moskowitz IP, Wang J, Peterson MA et al. Transcription factor genes Smad4 and Gata4 cooperatively regulate cardiac valve development. Proc Natl Acad Sci U S A 2011;108:4006–4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lim SK, Hoffmann FM. Smad4 cooperates with lymphoid enhancer‐binding factor 1/T cell‐specific factor to increase c‐myc expression in the absence of TGF‐beta signaling. Proc Natl Acad Sci U S A 2006;103:18580–18585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gu AD, S1 Z, Wang Y et al. A critical role for transcription factor Smad4 in T cell function that is independent of transforming growth factor beta receptor signaling. Immunity 2015;42:68–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang S, M3 T, Zou L et al. Reversing SKI‐SMAD4‐mediated suppression is essential for TH17 cell differentiation. Nature 2017;551:105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tan XH, Weng T, Zhang J et al. Smad4 is required for maintaining normal murine postnatal bone homeostasis. J Cell Sci 2007;120:2162–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang C, Geng J, Wei X et al. MiR‐144‐3p regulates osteogenic differentiation and proliferation of murine mesenchymal stem cells by specifically targeting Smad4. FEBS Lett 2016;590:795–807. [DOI] [PubMed] [Google Scholar]
- 37. Ouyang D, Y1 Y, Guo D et al. MicroRNA‐125b‐5p inhibits proliferation and promotes adipogenic differentiation in 3T3‐L1 preadipocytes. Acta Biochim Biophys Sin (Shanghai) 2015;47:355–361. [DOI] [PubMed] [Google Scholar]
- 38. Byun MR, Kim AR, Hwang JH et al. FGF2 stimulates osteogenic differentiation through ERK induced TAZ expression. Bone 2014;58:72–80. [DOI] [PubMed] [Google Scholar]
- 39. Liu CY, Zha ZY, Zhou X et al. The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCF{beta}‐TrCP E3 ligase. J Biol Chem 2010;285:37159–37169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yu FX, Zhao B, Guan KL. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell 2015;163:811–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information


