Summary
Introduction
Mastication has been assessed in several ways in the past. Both patients reported and objective assessment methods have been developed. The University Medical Center (UMC) Utrecht has developed a mixing ability test (MAT) using a two‐coloured wax tablet. The present study investigates the association between the mixing ability test and a chewing related questionnaire in patients treated for oral malignancies.
Patients and methods
In a cohort study, patients treated for oral malignancies were assessed 4‐6 weeks before and 4‐6 weeks after treatment, as well as 6 months, 1 year and 5 years after treatment. The mixing ability test was assessed using 10 and 20 chewing strokes and was compared to seven questions about several aspects of mastication. Regression analysis was performed and density plots were drawn for statistical analysis.
Results
One hundred and twenty‐three patients were included in this study. The questionnaire was less predictive for the 10‐chewing stroke test and the test was less discriminatory for different food types than the 20‐chewing stroke mixing ability test. Three questions about the ability to chew solid, soft and thickened liquid food types were found to be significantly predictive for the 20‐chewing stroke test. Threshold values on the mixing ability index were around 20 for the ability to chew solid food types and 24 for soft food types.
Conclusion
The 10‐chewing stroke mixing ability test is less suitable than 20‐chewing strokes for patients with and treated for oral cancer. The 20‐chewing stroke mixing ability test has a fair association with self‐reported outcomes.
Keywords: head and neck cancer, masticatory performance, mixing ability, objective assessment, oral function, oral oncology, patient‐reported outcome
1. INTRODUCTION
Mastication, or chewing, comprises the process in which food is reduced in size and is formed into a bolus, ready for swallowing (deglutition). Chewing is a complex voluntary movement and it involves the infrahyoidal, suprahyoidal, facial, tongue, floor of the mouth, palatal and temporomandibular musculature. The anatomy, and thus the chewing efficacy deteriorates in patients treated for oral cancer.1 Masticatory performance has a profound impact on the Quality of Life (QoL) in patients treated for oral cancer.2, 3 The objective and subjective efficacy of the mastication process is referred to as masticatory performance and masticatory ability, respectively.4
Subjective testing of oral functioning has been performed with several questionnaires, eg the Oral Health Impact Profile‐14 (OHIP‐14),5 the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire 30 and Head & Neck Module 35 (EORTC‐QLQ30 and H&N35).6 Subjective testing also takes psychological and adaptational factors into account, which can be an advantage over objective outcomes, because it reflects a patients’ expectations and personal importance of masticatory performance for daily life satisfaction.7 Objective masticatory performance has been tested in several ways, and is able to detect smaller improvements, eg in evaluating the results of rehabilitation.8 For objective assessment, the degree of breakdown of chewed test foods, such as (pea)nuts,9, 10 corn chips,11 crackers12 and Optosil blocks 13, 14, 15 has been determined by sieving methods. Also, adenosine triphosphate (ATP)‐releasing gums have been used and tested in healthy subjects and oral cancer patients.16
Some of the patients after treatment for oral and oropharyngeal malignancies may not be able to complete some of the described masticatory performance tests, because the bite force needed to break down the test food particle is too high.13 Mixing ability tests (MATs) using paraffin wax tablets17 or colour changing chewing gum18, 19 are described and seem more appropriate for this patient group.20
Therefore, the University Medical Center in Utrecht a mixing ability test has been developed. It comprises a two‐coloured (red/blue) soft wax tablet which is analysed digitally after chewing.1 The outcome variable is called the “Mixing ability index”, or MAI. It ranges from 0 to 30, in which 30 is the worst possible outcome.
Since the development of the test, experience has been gained in its use.8, 21, 22, 23, 24, 25, 26 As it appears, the test is suitable for use in patients with compromised mastication, such as patients treated for oral malignancies.20 However, to date, it is unclear how patient‐reported chewing ability (ie to chew solid or soft food types) relates to MAI scores. This is of utmost importance for a better understanding of the dietary implications of the mixing ability test (MAT).
The longitudinal results of the MAI in this cohort have been previously studied.27 This study focuses only on the relationship between the MAI and self‐reported chewing ability. Thus, the primary aim of this study was to relate the index scores of the MAT to outcomes of chewing related items from a head and neck oncology questionnaire. This is done in order to search MAI cut‐off values for the ability to chew solid and soft food consistencies, and thus the dietary implications of the MAI scores. The secondary aim was to analyse the predictive value of a subset of questionnaire items on the outcomes of the mixing ability test in oral oncological patients. Finally, this study evaluates whether 10‐ and 20‐chewing strokes of mixing ability testing perform equally well for both the above described primary and secondary aim.
2. MATERIALS AND METHODS
2.1. Subjects
In this multicentre study, the patient population consists of patients with a primary malignant tumour involving the oral cavity referred to the University Medical Center Utrecht (UMCU) and Radboud University Medical Center (Radboudumc), from January 2007 through August 2009. Patients were included if they had a tumour involving the oral cavity, were treated with a curative intent, were able to understand Dutch, and were able to perform the mixing ability test.28 The experimental protocol was approved by the Ethics Committees of the UMCU and Radboudumc. All patients received written information and signed informed consent.
2.2. Assessments
Data was collected in a prospective cohort study,27 subjects were assessed prior to primary oral oncology treatment (t0), 4‐6 weeks after primary treatment and/or radiotherapy (respectively t1a and t1b), 6 months (t2), 12 months (t3) and 5 years (t5) after primary treatment.
2.3. Masticatory ability
For analysis of masticatory ability, patients completed seven chewing ability questions related to masticatory function. Respectively, patients were asked: (1). Have you had trouble eating solid food (eg carrots, peanuts or meat)?; (2) Have you had trouble eating soft food (eg cookies, bread or pasta)?; (3) Have you had trouble eating liquid food (eg custard or apple sauce)?; (4). Have you had trouble chewing?; (5) Have you had pain while chewing?; (6) Was your chewing ability an obstacle in your social life? and (7) Was your chewing ability an obstacle in your choice of food? For each question, there were four possible answers on an ordinal scale (respectively “Never,” “Sometimes,” “Often” and “Always”).
2.4. Masticatory performance
The mixing ability test (MAT) was used to measure masticatory performance.8 The test measures how well a subject mixes a two‐coloured wax tablet by chewing on it. The tablet has a diameter of 20 mm and consists of two 3 mm layers of red and blue wax. The wax is a soft material (Plasticine modelling wax, non‐toxic DIN EN‐71) that forms a compact bolus during chewing and was offered at room temperature (20°C). After being chewed, the wax is flattened between foil to a thickness of 2.0 mm to avoid shadows in the image by the oblique illumination of the scanner's lamp. The flattened wax is then photographed on both sides using a high‐quality scanner (Epson V750, Long Beach, California). The images of the wax are analysed and processed using a commercially available programme for image analysis (Adobe Photoshop CS3, San Jose, California). Chewing mixes the two colours and intermediate colour intensities appear. Thus, the spreads of the intensities for red and blue decrease. The outcome parameter is the Mixing Ability Index (MAI). The tablet is chewed for either 10‐ or 20‐chewing strokes (respectively MAI‐10 and MAI‐20). A lower score implies a better mixed tablet, hence better masticatory performance. The range of MAI is from 0 to 30. Naturally, it is expected that the MAI‐10 will have higher (thus worse) scores than the MAI‐20, which in groups with impaired chewing performance might lead to reduced sensitivity through a ceiling effect, or enhance the differences if the scores remain below the maximum value even after 20 strokes.25
2.5. Other functional assessments
The full description of the functional assessments has been published previously.27, 28 Briefly, the maximum bite force was measured using a strain gauge, mounted on a mouthpiece. The device was placed between the first molars and the subject was asked to bite as firmly as possible. This was repeated twice on each side and the mean of the highest value from the left and right side was presented as maximum bite force.
Maximum mouth opening was measured using an extra oral protocol. Placement of a sticker on the subjects’ nose and chin ensured a reference point. The subject was measured twice in a resting position, with closed lips and molars not in occlusion and twice when fully opened. The mean of the two resting positions was subtracted from the highest open value and presented as maximum mouth opening.
2.6. Statistics
Statistically, data were treated as being cross‐sectional. Differences in baseline demographics were analysed with a one‐way ANOVA for continuous variables and with a Chi‐square test for categorical variables. For the analysis of the reported ability to eat food consistencies, the outcomes of these three questions were dichotomized to be able to interpret the results in the following way: “Never” and “Sometimes” were converted into “Unlikely” and “Often” and “Always” were converted into “Likely” to report trouble with a certain food consistency. The dichotomization was done in order to be able to divide the participants according to their reported chewing ability in as few clinically meaningful groups as possible to reduce the amount of comparisons between the groups in chewing performance. The disadvantage of the dichotomization is that the grouping is based on the responses less directly than using each response category separately. For analysis of the ranges of MAI associated with food consistencies, three groups were formed based on the combination of answers on the question about the ability to eat solid and soft food. The group “solid and soft” (SaS) reported “unlikely” to have any problems with neither solid nor soft foods. The “only soft” (oS) group reported “likely” to have problems with solid food, but “unlikely” to have problems with other food consistencies. The “neither solid nor soft” (SnS) group reported “likely” to have problems with both solid and soft foods. The differences in mean MAI scores between these groups were analysed using an independent t test.
Histograms and density lines were created to visualise the distribution of MAI‐10 and MAI‐20 scores. Threshold values were derived from the density plots as intersection from the different lines. The ability of these threshold values of the MAI‐10 and MAI‐20 to classify the patients into different chewing ability groups was evaluated using a confusion matrix.
Two stepwise linear regression analyses with the MAI‐10 and MAI‐20 as dependent variables were performed. The original ordinal outcomes (ie “never,” “sometimes,” “often,” “always”) of the seven questions, as well as the variable “assessment moment” were added as independent factors to take the multiple assessments into account. These were removed in a backwards fashion if P > 0.05, until the model only contained factors with a significant effect on the model fit.
Tests with a P‐value less than 0.05 were considered to be statistically significant, but because of multiple comparisons (seven in total) the Bonferroni adjusted P‐value was set to 0.007. All tests were performed using SPSS 25 (IBM Corp. Armonk, NY, USA) and R statistics 3.4.2 (R Foundation for Statistical Computing, Vienna, Austria).
3. RESULTS
At baseline, 123 patients were included, of which 99 had completed the mixing ability tests before oral oncological intervention. Fifty‐three per cent of the included subjects were male. Demographic data of the included patients are presented in Table 1.
Table 1.
Demographic data of included patients
| Characteristic | Location of primary tumour | |||
|---|---|---|---|---|
| Categorical variables | Maxilla (n = 30) | Mandible (n = 48) | TFM (n = 45) | P‐value |
| Gender | ||||
| Male | 16 (53) | 23 (53) | 15 (33) | 0.179 |
| Female | 14 (47) | 25 (47) | 30 (67) | |
| Tumour size (pT/cT) | ||||
| T1 | 5 (17) | 14 (29) | 23 (51) | 0.006 |
| T2 | 11 (37) | 13 (27) | 14 (31) | |
| T3 | 1 (3) | 3 (6) | 4 (9) | |
| T4 | 13 (43) | 18 (38) | 4 (9) | |
| Dental status | ||||
| ED | 6 (21) | 16 (30) | 6 (11) | 0.212 |
| FD | 10 (29) | 9 (17) | 16 (29) | |
| FD+FDI | 0 (0) | 2 (4) | 5 (9) | |
| FD+D | 4 (12) | 8 (15) | 3 (5) | |
| FDI+FDI | 0 (0) | 0 (0) | 0 (0) | |
| FDI+D | 1 (3) | 0 (0) | 0 (0) | |
| D | 12 (35) | 19 (35) | 25 (45) | |
| Treatment | ||||
| Surgery | 12 (40) | 24 (50) | 23 (51) | 0.600 |
| Surgery & Radiotherapy | 18 (60) | 24 (50) | 22 (49) | |
| Obturator prosthesis | ||||
| Yes | 20 (59) | 0 (0) | 0 (0) | 0.003 |
| No | 14 (41) | 54 (100) | 55 (100) | |
| Surgical defect management | ||||
| Primary closure | 17 (57) | 16 (33) | 23 (51) | 0.000 |
| Local flap | 1 (3) | 2 (4) | 1 (2) | |
| Myocutaneous or free flap | 12 (40) | 12 (25) | 19 (42) | |
| Bone graft/flap | 0 (0) | 18 (38) | 2 (4) | |
| Continuous variables | ||||
| Mean age, years (SD) | 68.7 (12.3) | 66.7 (12.7) | 61.4 (13.1) | 0.033 |
| No. of occlusal units | 2.4 (4.1) | 2.3 (3.9) | 3.8 (5.1) | 0.230 |
| MMO [mm] (SD) | 52.9 (11.8) | 46.6 (11.4) | 56.0 (8.9) | 0.000 |
| MBF [N] (SD) | 223.8 (232.5) | 256.5 (329.8) | 376.7 (343.7) | 0.075 |
D, dentate; ED, edentulous; FD, full dentures; FDI, implant supported full denture; MBF, maximum bite force; MMO, maximum mouth opening; SD, standard deviation; TFM, tongue and/or floor of the mouth.
The number of patients available per assessment moment varied per question and assessment moment. When adding up all assessment moments, between 320 and 327 samples were available for analysis for each question (Table 2).
Table 2.
Details on number of samples per question and assessment moment
| t0 | t1a | t1b | t2 | t3 | t5 | Total | |
|---|---|---|---|---|---|---|---|
| Missing samples | |||||||
| Unable to perform test | 24 | 59 | 37 | 53 | 42 | 6 | 221 |
| Cumulative number of patients stopped participating | 0 | 7 | 5 | 10 | 19 | 24 | 24 |
| Cumulative number of patients deceased | 0 | 2 | 4 | 8 | 13 | 29 | 29 |
| Questions | |||||||
| 1. Have you had trouble eating solid food (eg carrots, peanuts or meat)? | 98 (1) | 49 (2) | 13 (4) | 49 (2) | 48 (1) | 63 (1) | 320 |
| 2. Have you had trouble eating soft food (eg cookies, bread or pasta)? | 98 (1) | 51 | 14 (3) | 49 (2) | 48 (1) | 63 (1) | 323 |
| 3. Have you had trouble eating liquid food (eg custard or apple sauce)? | 99 | 51 | 14 (3) | 51 | 49 | 63 (1) | 327 |
| 4. Have you had trouble chewing? | 99 | 51 | 14 (3) | 49 (2) | 47 (2) | 62 (2) | 322 |
| 5. Have you had pain while chewing? | 99 | 50 (1) | 14 (3) | 48 (3) | 47 (2) | 63 (1) | 321 |
| 6. Was your chewing ability an obstacle in your social life? | 99 | 51 | 13 (4) | 50 (1) | 48 (2) | 61 (3) | 322 |
| 7. Was your chewing ability an obstacle in your choice of food? | 99 | 51 | 14 (3) | 49 (2) | 48 (1) | 60 (4) | 321 |
t0: 4‐6 wk before treatment; t1a: 4‐6 wk after surgery; t1b: 4‐6 wk after radiotherapy; t2: 6 mo after treatment; t3: 1 y after treatment; t5: 5 y after treatment. The number between parentheses represents the number of patients who completed the mixing ability test but did not answer that question.
3.1. Masticatory performance vs food consistencies
The boxplots, and comparisons between the different food consistency groups of MAI‐10 and MAI‐20 are presented respectively in Figures 1 and 2. For the MAI‐10, SaS and oS are significantly different from each other (SaS‐oS: P = 0.001; oS‐SnS: P = 0.113) and for MAI‐20, all groups are significantly different from each other (SaS‐oS: P = 0.020; oS‐SnS: P = 0.006). In Figures 3A‐D and 4A‐D, the histograms and density plot of respectively MAI‐10 and MAI‐20 are displayed, showing only one intersection at 25.5 for MAI‐10 and intersections on 19.8 and 23.9 for MAI‐20.
Figure 1.
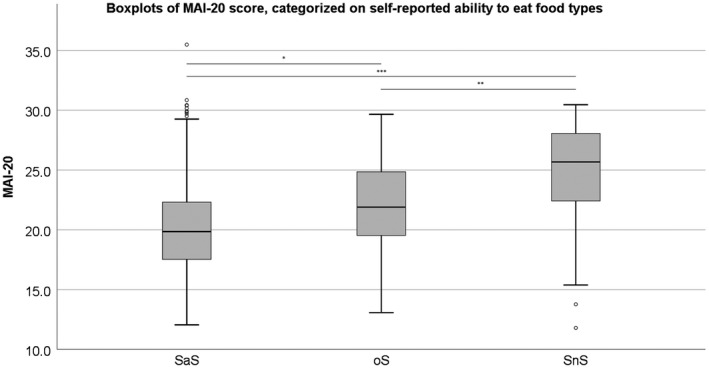
The outcome of the three food consistency groups when performing the MAI‐10. *P < 0.05; **P < 0.01; ***P < 0.001. °: Outlier. SaS: reported ‘Unlikely’ to have problems with solid or soft food types; oS: only ‘Likely’ to have problems with solid food types; Neither SnS: reported ‘Likely’ to have problems with both solid and soft food types
Figure 2.
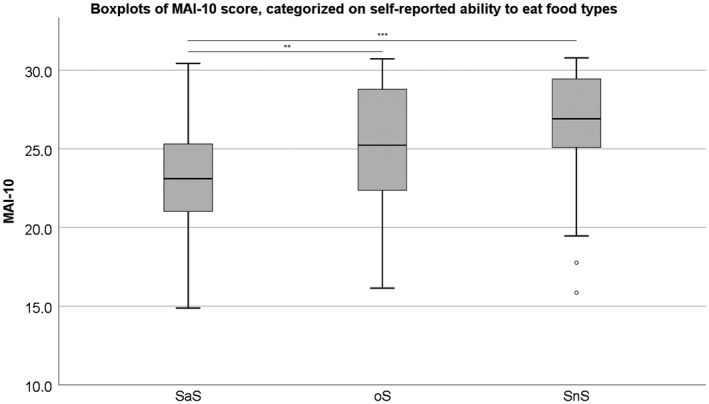
The outcome of the three food consistency groups when performing the MAI‐20. *P < 0.05; **P < 0.01; ***P < 0.001. °: Outlier. SaS: reported ‘Unlikely’ to have problems with solid or soft food types; oS: only ‘Likely’ to have problems with solid food types; Neither SnS: reported ‘Likely’ to have problems with both solid and soft food types
Figure 3.
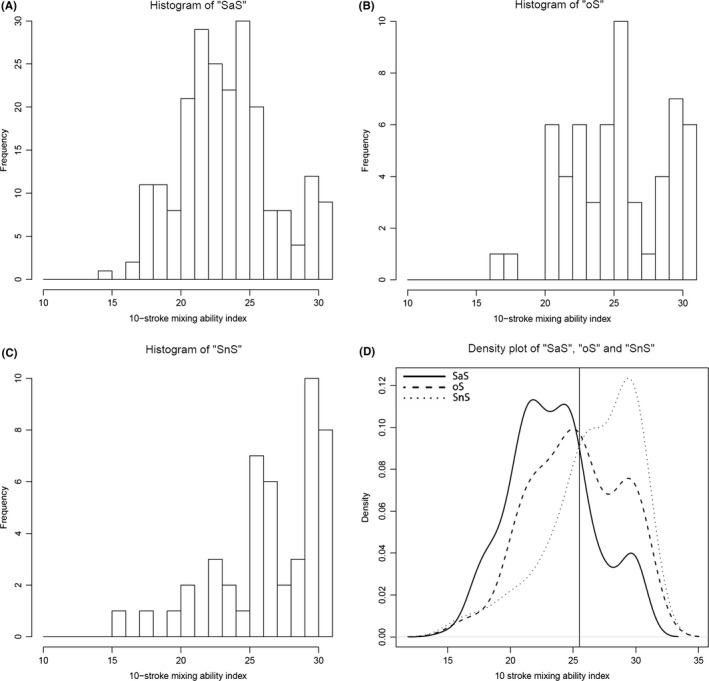
Histograms of the ‘SaS’ (A), ‘oS’ (B) and ‘SnS’ (C) groups and combined density lines (D) for the 10‐chewing stroke Mixing Ability Test. The ‘SaS’ group was unlikely to report any problems with chewing any food consistency. The ‘oS’ group was likely to report problems chewing solid foods and the ‘SnS’ group was likely to report problems with both solid and soft food consistencies. The solid line represents the ‘SaS’ group, the dashed line represents the ‘oS’ group and the dotted line represents the ‘SnS’ group. The vertical line through the intersection of the lines is at MAI = 25.5
Figure 4.
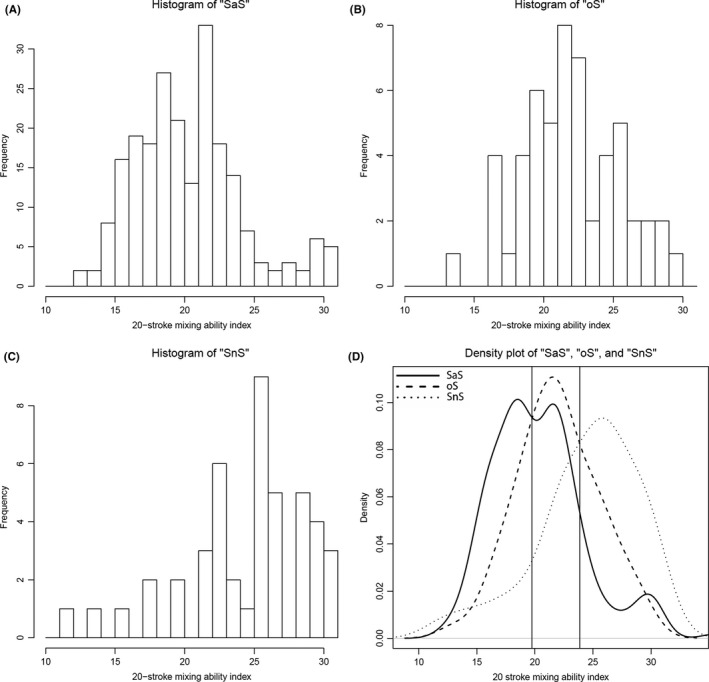
Histograms of the ‘SaS’ (A), ‘oS’ (B) and ‘SnS’ (C) groups and combined density lines (D) for the 20‐chewing stroke Mixing Ability Test. The ‘SaS’ group was unlikely to report any problems with chewing any food consistency. The ‘oS’ group was likely to report problems chewing solid foods and the ‘SnS’ group was likely to report problems with both solid and soft food consistencies. The solid line represents the ‘SaS’ group, the dashed line represents the ‘oS’ group and the dotted line represents the ‘SnS’ group. The vertical lines through the intersections are at MAI = 19.8 and MAI = 23.9
On the MAI‐10, 72% of the subjects scoring 25 or less reported to be in the SaS‐group. Of the MAI‐20, 51% of the subjects scoring below 20 reported to be in the SaS‐group. Most subjects (41%) who scored between 20 and below 24 on the MAI‐20 reported to be in the oS‐group, while the MAI‐10 was not able to determine a difference for this group. On the MAI‐10, 76% of the subjects who scored above 25 and on the MAI‐20, 60% of the subjects who scored above 24 reported to be in the SnS‐group (Tables 3 and 4).
Table 3.
Confusion matrix of the 10‐chewing stroke Mixing Ability Test and found food consistency thresholds
| Expected/answered MAI | Solid and soft N (%) | Only soft N (%) | Neither solid nor soft N (%) |
|---|---|---|---|
| ≤25 | 160 (72.4%) | 27 (46.6%) | 11 (23.4%) |
| >25 | 61 (27.6%) | 31 (53.4%) | 36 (76.6%) |
| Total | 100% | 100% | 100% |
MAI, Mixing Ability Index; Soft and solid: reported “Unlikely” to have problems with solid or soft food types; Only soft: only “Likely” to have problems with solid food types; Neither solid nor soft: reported “Likely” to have problems with both solid and soft food types.
The italic values represent the proportion of subjects of whom the reported chewing ability and chewing performance corresponded with the found thresholds for the MAI (true positives).
Table 4.
Confusion matrix of the 20‐chewing stroke MAT and found food consistency thresholds
| Expected/answered MAI range | Solid and soft N (%) | Only soft N (%) | Neither solid nor soft N (%) |
|---|---|---|---|
| <20 | 113 (51.5%) | 16 (29.6%) | 7 (15.6%) |
| 20 ≤ MAI <24 | 78 (35.5%) | 22 (40.8%) | 11 (24.4%) |
| 24≤ | 29 (13.1%) | 16 (29.6%) | 27 (60.0%) |
| Total | 220 | 54 | 45 |
MAI, Mixing Ability Index; Soft and solid: reported “Unlikely” to have problems with solid or soft food types; Only soft: only “Likely” to have problems with solid food types; Neither solid nor soft: reported “Likely” to have problems with both solid and soft food types.
The italic values represent the proportion of subjects of whom the reported chewing ability and chewing performance corresponded with the found thresholds for the MAI (true positives).
3.2. Regression analysis
In the regression analysis on the predictive value of the seven questions and factor time for the MAI‐10, only one question out of seven questions, namely “Have you had trouble eating solid food?” and the assessment moment were found to be significant factors in the variance of the MAI and these explain 46.2% of the total variance.
For the MAI‐20, two questions, namely “Have you had trouble eating solid food?” and “Have you had trouble eating liquid food?” were significant factors for the MAI‐20 and account for 39.6% of the total variance (Table 5).
Table 5.
Results of the linear regression analysis with the Mixing Ability Index with 10‐ and 20‐chewing strokes as dependent variable
| Linear regression | Significant factors | Β | SE | sig. | R | R 2 | SE Est | sig. |
|---|---|---|---|---|---|---|---|---|
| MAI‐10 | Constant | 24.883 | 0.386 | 0.000 | 0.462 | 0.213 | 3.220 | 0.000 |
| Assessment moment | −0.467 | 0.094 | 0.000 | |||||
| Trouble solid food | 1.068 | 0.170 | 0.000 | |||||
| MAI‐20 | Constant | 20.339 | 0.267 | 0.000 | 0.396 | 0.157 | 3.838 | 0.000 |
| Assessment moment | −0.259 | 0.112 | 0.022 | |||||
| Trouble solid food | 1.969 | 0.544 | 0.000 | |||||
| Trouble liquid food | 2.724 | 0.932 | 0.008 |
The dichotomized questions as well as the variable “assessment moment” were added as independent variables and non‐significant variables were removed in a backwards fashion if P > 0.05. MAI, Mixing ability Index; SE, Standard Error. In the MAI‐10 regression, removed factors were: trouble eating soft food; trouble eating liquid food; trouble chewing; pain chewing, impact on social life and impact on food choice.
In the MAI‐20 regression, removed factors were: trouble eating soft food, trouble chewing; pain chewing, impact on social life and impact on food choice.
4. DISCUSSION
In this cross‐sectional study, an effort has been made to relate the outcomes of the MAI‐10 and MAI‐20 to patient‐reported (chewing ability) data. According to present study, most patients with a MAI below 20 are unlikely to experience any problems with their diet. Between 20 and 24, most patients are likely to experience difficulties chewing solid foods. Most patients scoring 24 and above are likely to experience difficulties chewing both solid and soft food consistencies.
The questions about the ability to eat solid and liquid food consistency and the assessment moment significantly affect the outcome of the MAI obtained after 20‐chewing strokes.
For both the association with food consistency questions as well as the predictability by questionnaires, the MAI‐20 was more useful. The MAI‐10 only seems able to determine a difference in being able to chew all food types and having trouble with both solid and soft food types. The reasons might be that current study population of patients treated for oral cancer has such poor oral functioning, that they need more chewing strokes to achieve a certain degree of mixing. Even in healthy subjects the 20‐chewing stroke test is more capable of determining differences of masticatory performance.8
4.1. Comparison to existing literature
Efforts have been made to find an association between questionnaire outcome measures and objective masticatory performance. The association between the ability to masticate test foods until fit for swallowing and the outcome of a chewing gum mixing ability test has been studied before and cut‐off values with sensitivity/specificity values were described for different food consistencies.29 In another study, a correlation with ageing is seen for both masticatory performance using a sieving method and masticatory ability using a questionnaire mastication in full denture wearers.30 One study found a correlation between a chewing gum type mixing ability test and Japanese 35‐item questionnaire regarding food types in patients with glossectomy and marginal mandibulectomy.31 Another Japanese study on complete denture wearers showed a correlation between the chewing gum test and food questionnaire.32 A previous study on the association between food type related questionnaires and objective masticatory performance could not be found.
4.2. Strengths and limitations
The strength of present study is the number of analysed samples in such a specific patient group. However, this heterogenic patient population has its limitations regarding generalisation of the results. Results can therefore only be interpreted in oral cancer patients. In fact, especially the 20‐chewing stroke MAT is less suitable to use in younger, healthy subjects.20 Meticulous statistical analysis included usage of the Bonferroni correction due to multiple comparisons.33 However, this correction is often considered to be conservative, because the adjusted P‐value of 0.007 renders the probability of a significant finding just by chance to 1‐(1‐0.007)7 = 4.8%. Usually, an alpha of 5% is accepted in scientific literature. Without this correction, the results of the regression analysis would have been more favourable towards the 20‐chewing stroke MAT. Current data shows that there is a relationship between objective (mechanical) chewing performance and patient‐reported chewing ability. Consequently, the chewing performance test is a meaningful measure to describe chewing ability. Conversely, patient‐reported outcomes relate to mechanical chewing measures highlighting the importance of optimising oral function in oral rehabilitation. Hence, the results can be seen as mutually validating the two approaches. While questionnaires can be affected by multiple different subjective factors, eg psychological state,7 objective testing offers a complementary measure that has the potential of accurately detecting small improvements.
The mixing ability test is faster to administer than other objective chewing tests commonly used. Optosil blocks are often chewed 60 times34) Less human error can be expected in analyzing the wax tablets, as this is done digitally by specialised software. Other authors are developing digital methods for analyzing sieving methods as well.35 A disadvantage of this mixing ability test is that there is a “floor‐effect” when patients are not able to chew the wax tablet at all. They were assigned the worst possible outcome (MAI = 30) and therefore excluded from this study. This reflects a patients’ inability to chew in general, however, experience shows that these patients can develop skills to consume softer foods anyway. The presence of a significant predictive value of the ability to consume liquids makes less sense as this probably relates more to the tongue mobility and swallowing ability than to masticatory performance.36 Finally, the masticatory performance is also influenced by taste, as a study reported a decrease of chewing efficiency when the concentration of bitter tasting quinine was increased.37 The currently used questions did not explore the taste sensitivity, a possible confounding variable in the experiment. In addition, the participants were not asked about the taste of the wax tablet.
4.3. Future research
The outcomes of this study give insight in the abilities of patients with a certain MAI, but this should be interpreted with caution and more research is necessary to underline the association of the reported ability to consume food consistencies and the range of MAI. However, it could be useful to compare the outcome of the MAI to more extensive and validated food consistency questionnaires to further elaborate these findings. Eventually, this test has the potential to be used clinically for dietary advices to patients after oral oncological treatment. It could also act as an instrument to assess the need for improvement of masticatory performance, known to be an important aspect in health‐related quality of life.38 Performing the same study in different study populations is necessary to give insight in potential generalisability of, or differences in the cut‐off values. Finally, the reliability of the test needs to be evaluated by test‐retesting.
5. CONCLUSION
Enquiring a patients’ ability to chew solid foods has the most predictive value on the mixing ability index, and is easily done in a clinical setting. Ten chewing strokes tests in this patient group are less discriminatory and less predictive than 20‐chewing stroke tests. Therefore, in future studies, 20‐chewing strokes should be used in this patient group. Threshold values of MAI above which a difficulty to chew solid food types is to be expected in an oral cancer patient group is 20, and for soft food types an MAI of 24. However, the interpretation of these results must be done carefully as the evaluation has been done in this patient group only.
CONFLICT OF INTEREST
None of the authors report any conflict of interest.
de Groot RJ, Rosenberg AJWP, van der Bilt A, Aalto D, Merkx MAW, Speksnijder CM. The association between a mixing ability test and patient reported chewing ability in patients treated for oral malignancies. J Oral Rehabil. 2019;46:140–150. 10.1111/joor.12734
REFERENCES
- 1. Speksnijder CM, van der Glas HW, van der Bilt A, et al. Oral function after oncological intervention in the oral cavity: a retrospective study. J Oral Maxillofac Surg. 2010;68:1231‐1237. [DOI] [PubMed] [Google Scholar]
- 2. Dzioba A, Aalto D, Papadopoulos‐Nydam G, et al. Functional and quality of life outcomes after partial glossectomy: a multi‐institutional longitudinal study of the head and neck research network. J Otolaryngol Head Neck Surg. 2017;46:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Korfage A, Schoen PJ, Raghoebar GM, et al. Five‐year follow up of oral functioning and quality of life in patients with oral cancer with implant‐retained mandibular overdentures. Head Neck. 2011;33:831‐839. [DOI] [PubMed] [Google Scholar]
- 4. van der Bilt A. Assessment of mastication with implications for oral rehabilitation: a review. J Oral Rehabil. 2011;38:754‐780. [DOI] [PubMed] [Google Scholar]
- 5. Slade GD, Spencer AJ. Development and evaluation of the Oral Health Impact Profile. Community Dent Health. 1994;11:3‐11. [PubMed] [Google Scholar]
- 6. Bjordal K, Ahlner‐Elmqvist M, Tollesson E, et al. Development of a European organization for research and treatment of cancer (Eortc) questionnaire module to be used in quality of life assessments in head and neck cancer patients. Acta Oncol (Madr). 1994;33:879‐885. [DOI] [PubMed] [Google Scholar]
- 7. Ring L, Höfer S, Heuston F, Harris D, O'Boyle CA. Response shift masks the treatment impact on patient reported outcomes (PROs): the example of individual quality of life in edentulous patients. Health Qual Life Outcomes. 2005;3:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Speksnijder CM, Abbink JH, van der Glas HW, Janssen NG, Van Der Bilt A. Mixing ability test compared with a comminution test in persons with normal and compromised masticatory performance. Eur J Oral Sci. 2009;117:580‐586. [DOI] [PubMed] [Google Scholar]
- 9. Genden EM, Okay D, Stepp MT, et al. Comparison of functional and quality‐of‐life outcomes in patients with and without palatomaxillary reconstruction. Arch Otolaryngol Neck Surg. 2003;129:775. [DOI] [PubMed] [Google Scholar]
- 10. Roumanas ED, Garrett N, Blackwell KE, et al. Masticatory and swallowing threshold performances with conventional and implant‐supported prostheses after mandibular fibula free‐flap reconstruction. J Prosthet Dent. 2006;96:289‐297. [DOI] [PubMed] [Google Scholar]
- 11. Marunick MT, Mathog RH. Mastication in patients treated for head and neck cancer: a pilot study. J Prosthet Dent. 1990;63:566‐573. [DOI] [PubMed] [Google Scholar]
- 12. Huckabee M‐L, McIntosh T, Fuller L, et al. The Test of Masticating and Swallowing Solids (TOMASS): reliability, validity and international normative data. Int J Lang Commun Disord. 2017;53:144‐156. [DOI] [PubMed] [Google Scholar]
- 13. van Kampen FMCMC, van der Bilt A, Cune MSS, Fontijn‐Tekamp FAA, Bosman F. Masticatory function with implant‐supported overdentures. J Dent Res 2004;83:708‐711. [DOI] [PubMed] [Google Scholar]
- 14. Pereira LJ, Braga Caputo J, Midori Castelo P, et al. Oral physiology and quality of life in cancer patients. Nutr Hosp 2015;31:2161‐2166. [DOI] [PubMed] [Google Scholar]
- 15. Slagter AP, Bosman F, van der Glas HW, van der Bilt A. Human jaw‐elevator muscle activity and food comminution in the dentate and edentulous state. Arch Oral Biol. 1993;38:195‐205. [DOI] [PubMed] [Google Scholar]
- 16. Namaki S, Matsumoto M, Ohba H, Tanaka H, Koshikawa N, Shinohara M. Masticatory efficiency before and after surgery in oral cancer patients: comparative study of glossectomy, marginal mandibulectomy and segmental mandibulectomy. J Oral Sci. 2004;46:113‐117. [DOI] [PubMed] [Google Scholar]
- 17. Sato H, Fueki K, Sueda S, et al. A new and simple method for evaluating masticatory function using newly developed artificial test food. J Oral Rehabil. 2003;30:68‐73. [DOI] [PubMed] [Google Scholar]
- 18. Matsuyama M, Tsukiyama Y, Tomioka M, Koyano K. Clinical assessment of chewing function of obturator prosthesis wearers by objective measurement of masticatory performance and maximum occlusal force. Int J Prosthodont. 2006;19:253‐257. [PubMed] [Google Scholar]
- 19. Schimmel M, Christou P, Herrmann F, Müller F. A two‐colour chewing gum test for masticatory efficiency: development of different assessment methods. J Oral Rehabil. 2007;34:671‐678. [DOI] [PubMed] [Google Scholar]
- 20. van der Bilt A, Mojet J, Tekamp FA, Abbink JH. Comparing masticatory performance and mixing ability. J Oral Rehabil. 2010;37:79‐84. [DOI] [PubMed] [Google Scholar]
- 21. Jensen C, Speksnijder CM, Raghoebar GM, Kerdijk W, Meijer HJA, Cune MS. Implant‐supported mandibular removable partial dentures: functional, clinical and radiographical parameters in relation to implant position. Clin Implant Dent Relat Res. 2017;19:432‐439. [DOI] [PubMed] [Google Scholar]
- 22. Wetzels J‐WGH, Meijer GJ, Koole R, Adang EM, Merkx MAW, Speksnijder CM. Costs and clinical outcomes of implant placement during ablative surgery and postponed implant placement in curative oral oncology: a five‐year retrospective cohort study. Clin Oral Implants Res 2017;28:1433‐1442. [DOI] [PubMed] [Google Scholar]
- 23. Wetzels JW, Koole R, Meijer GJ, de Haan AFJ, Merkx MAW, Speksnijder CM. Functional benefits of implants placed during ablative surgery: a 5‐year prospective study on the prosthodontic rehabilitation of 56 edentulous oral cancer patients. Head Neck. 2016;38:E2103‐E2111. [DOI] [PubMed] [Google Scholar]
- 24. Kreeft AM, Krap M, Wismeijer D, et al. Oral function after maxillectomy and reconstruction with an obturator. Int J Oral Maxillofac Surg. 2012;41:1387‐1392. [DOI] [PubMed] [Google Scholar]
- 25. van der Bilt A, Speksnijder CM, de Liz Pocztaruk R, Abbink JH. Digital image processing versus visual assessment of chewed two‐colour wax in mixing ability tests. J Oral Rehabil. 2012;39:11‐17. [DOI] [PubMed] [Google Scholar]
- 26. Speksnijder CM, van der Bilt A, Abbink JH, Merkx MAW, Koole R. Mastication in patients treated for malignancies in tongue and/or floor of mouth: a 1‐year prospective study. Head Neck. 2011;33:1013‐1020. [DOI] [PubMed] [Google Scholar]
- 27. de Groot RJ, Wetzels JW, Merkx MAW, et al. Masticatory function and related factors after oral oncological treatment, a five‐year prospective study. Head Neck. 2018; (Accepted). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wetzels JGH, Merkx MAW, de Haan AFJ, Koole R, Speksnijder CM. Maximum mouth opening and trismus in 143 patients treated for oral cancer: a 1‐year prospective study. Head Neck. 2014;36:1754‐1762. [DOI] [PubMed] [Google Scholar]
- 29. Wada S, Kawate N, Mizuma M. Erratum to: what type of food can older adults masticate?: evaluation of mastication performance using color‐changeable chewing gum. Dysphagia. 2017;32:644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hirai T, Ishijima T, Koshino H, Anzai T. Age‐related change of masticatory function in complete denture wearers: evaluation by a sieving method with peanuts and a food intake questionnaire method. Int J Prosthodont. 1994;7:454‐460. [PubMed] [Google Scholar]
- 31. Aimaijiang Y, Otomaru T, Taniguchi H. Relationships between perceived chewing ability, objective masticatory function and oral health‐related quality of life in mandibulectomy or glossectomy patients with a dento‐maxillary prosthesis. J Prosthodont Res. 2016;60:92‐97. [DOI] [PubMed] [Google Scholar]
- 32. Ishikawa Y, Watanabe I, Hayakawa I, Minakuchi S, Uchida T. Evaluations of masticatory performance of complete denture wearers using color‐changeable chewing gum and other evaluating methods. J Med Dent Sci. 2007;54:65‐70. [PubMed] [Google Scholar]
- 33. Bonferroni CE. Teoria statistica delle classi e calcolo delle probabilità. Ist Super di Sci Econ e Commer di Firenze. 1936;8:3‐62. [Google Scholar]
- 34. van der Bilt A, Olthoff LW, Bosman F, Oosterhaven SP. Chewing performance before and after rehabilitation of post‐canine teeth in man. J Dent Res. 1994;73:1677‐1683. [DOI] [PubMed] [Google Scholar]
- 35. Eberhard L, Schindler HJ, Hellmann D, Schmitter M. Oral rehabilitation comparison of particle‐size distributions determined by optical scanning and by sieving in the assessment of masticatory performance. J Oral Rehabil. 2012;39:338‐348. [DOI] [PubMed] [Google Scholar]
- 36. Brown L, Rieger JM, Harris J, Seikaly H. A longitudinal study of functional outcomes after surgical resection and microvascular reconstruction for oral cancer: tongue mobility and swallowing function. J Oral Maxillofac Surg. 2010;68:2690‐2700. [DOI] [PubMed] [Google Scholar]
- 37. Neyraud E, Peyron MA, Vieira C, Dransfield E. Influence of bitter taste on mastication pattern. J Dent Res. 2005;84:250‐254. [DOI] [PubMed] [Google Scholar]
- 38. Said MM, Otomaru T, Aimaijiang Y, Li N, Taniguchi H. Association between masticatory function and oral health‐related quality of life in partial maxillectomy patients. Int J Prosthodont. 2016;29:561‐564. [DOI] [PubMed] [Google Scholar]


