Abstract
Aims
Endocrinological abnormalities, including low testosterone levels, are prevalent in cirrhosis. We assessed sexual hormone status in regard to hemodynamic abnormalities and its impact on hepatic decompensation and survival.
Methods
Males with cirrhosis were prospectively included in this study since 2010. Sexual hormones including bioavailable testosterone, total testosterone, luteinizing hormone, follicle‐stimulating hormone, prolactin, and sex hormone‐binding globulin as well as Child–Pugh score, Model for End‐stage Liver Disease (MELD) score, and hepatic venous pressure gradient were recorded. Sarcopenia was also assessed in patients with available computed tomography scans. Clinical follow‐up for hepatic decompensation, liver transplantation, and death was recorded until May 2017.
Results
One hundred fourteen male cirrhotic patients were included: age 55 ± 9.4 years, MELD 13.5 (range, 7–20.7). Etiologies were alcoholic liver disease in 61(53.5%) patients, viral in 30 (26.3%) patients, and other in 23 (20.2%). Child–Pugh scores were A in 32 (28.1%) patients, B in 48 (42.1%), and C in 34 (29.8%). Levels of bioavailable testosterone and total testosterone decreased with advanced Child–Pugh score (P < 0.001 and P < 0.001) whereas prolactin increased (P = 0.002). Median bioavailable testosterone (0.8 ng/mL [0.1–2] vs. 1.68 ng/mL [0.07–2.65]; P = 0.004) and total testosterone (2.7 ng/mL [0.23–12.34] vs. 7 ng/mL [0.25–10]; P = 0.041) levels were lower in patients with severe portal hypertension (hepatic venous pressure gradient >12 mmHg). Median bioavailable testosterone (0.25 ng/mL [0.07–1.7] vs. 0.97 ng/mL [0.15–2.74)]; P = 0.017) and total testosterone levels (1.28 ng/mL [0.25–7.32] vs. 4.32 ng/mL [0.43–13.47]; P = 0.031) were significantly lower in sarcopenic patients. Median follow‐up was 13 months (0.2–75 months) and liver‐related events were recorded in 46 patients (40.4%; death, 31 [27.2%]). Low total testosterone was associated with an increased risk for hepatic decompensation and/or death, even after adjusting for Child–Pugh score, MELD, and other relevant factors (Child–Pugh score model: hazard ratio 2.503, 95% confidence interval, 1.214–5.157, P = 0.013; MELD model: hazard ratio 3.065, 95% confidence interval, 1.523–6.169, P = 0.002).
Conclusion
In parallel to increasing severity of cirrhosis, levels of testosterone decline whereas prolactin levels increase. However, low testosterone levels are independently associated with a higher risk for hepatic decompensation and mortality.
Keywords: cirrhosis, mortality, sexual hormone, testosterone
Introduction
The hypothalamic–pituitary–gonadal (HPG) axis is pathologically profoundly altered in cirrhotic patients. This is attributed to both a gonadal and hypothalamus–pituitary dysfunction, although the process itself is not yet fully understood.1, 2, 3, 4, 5 Low serum testosterone (T) levels are frequently found in cirrhotic men and correlate well with severity of liver disease.1, 6, 7 Primary hypogonadism due to direct suppression of the testis, for example, through ethanol, as well as secondary/central hypogonadism due to inadequately normal luteinizing hormone (LH)/downregulated gonadotropin‐releasing hormone (GnRH) release have been described.1, 2, 3, 4, 8 Conflicting results have been published regarding LH levels in cirrhosis. Physiologically LH levels should be increased in the state of low T, however, in cirrhosis, reports vary between normal, increased, or even decreased LH.1, 2, 8, 9, 10 Along with Leydig cell dysfunction, hypothalamic dysfunction and direct inhibition of LH secretion through hyperestrogenism, which is known to occur due to a combination of increased peripheral aromatization of androgens and portosystemic shunting,11,12 are thought to contribute towards this abnormality.5
More recent studies have focused on the prognostic impact of T in the course of advanced liver disease. Low androgens and elevated serum estrone were associated with higher Model for End‐stage Liver Disease (MELD) score and individual adverse health outcomes.6 Association of low T with mortality13 within 12 months of follow‐up was also described, but no impact on hepatic decompensation.14 Furthermore, hepatic steatosis and presence of non‐alcoholic fatty liver disease is significantly associated with low serum T levels.15 However, data on the predictive value of sex hormones, and especially T, for liver‐related decompensation (ascites, hepatic encephalopathy [HE], spontaneous bacterial peritonitis [SBP], variceal hemorrhage, and hepatorenal syndrome) is scarce and data on the association of sex hormones with portal pressure are lacking.
The aim of our study was to prospectively evaluate the predictive potential of sex hormones regarding decompensation and mortality and its association with markers of portal hypertension in a thoroughly characterized cohort of cirrhotic patients.
Methods
Patients
One hundred fifty one male patients were prospectively screened between May 2010 and December 2012 in the outpatient clinic and ward of the Division of Gastroenterology and Hepatology at the Medical University of Vienna (Vienna, Austria) as part of a study evaluating prevalence of erectile dysfunction in cirrhosis. One hundred fourteen patients were finally included in statistical analysis (29 did not meet inclusion/exclusion criteria, eight excluded due to missing laboratory data on sexual hormones). Inclusion criteria were: cirrhosis irrespective of etiology (diagnosis of cirrhosis was based on either clinical or radiological parameters or on liver histology). Exclusion criteria were: overt HE, previous urologic surgery, active alcohol abuse, malignancy other than hepatocellular carcinoma, T supplementation, previous liver transplantation, and severe cardiac conditions. Child–Pugh score (CPS), MELD score, and routine laboratory markers along with clinical data (age, body mass index, comorbidities, and medication) were recorded at inclusion. In patients with available abdominal computed tomography (CT) scans, sarcopenia was assessed (see below). Additionally, the following sex hormones were analyzed: bioavailable testosterone (BT), total T (TT), LH, follicle‐stimulating hormone (FSH), prolactin (PRL), and sex hormone‐binding globulin (SHBG). Hepatic venous pressure gradient (HVPG) values were recorded if available.
Patients were routinely seen every 3–6 months in the outpatient clinic of our department and any liver‐related clinical events were recorded. Decompensation was defined as hospitalization for acute decompensation of cirrhosis such as: variceal bleeding, jaundice, occurrence or worsening of ascites, HE, spontaneous bacterial peritonitis, and hepatorenal syndrome. In those who were already diagnosed with ascites at baseline, significant worsening of ascites was also considered to denote acute decompensation of cirrhosis. Follow‐up was obtained until May 2017.
Treatment strategies
The treatment strategy of our outpatient clinic was based on the national consensus statements that were available during the study period.16, 17 The Billroth I and II consensuses were based on the international clinical practice recommendations at the time of publication. In brief, non‐selective β‐blockers (NSBB) were preferred over endoscopic variceal ligation (EVL) for primary prophylaxis of variceal bleeding. In case of contraindications for NSBB therapy, EVL was carried out. For secondary prophylaxis, NSBB in combination with EVL was the therapy of choice; treatment failures were evaluated for transjugular intrahepatic portosystemic shunt (TIPS). Patients with ascites were treated with paracentesis plus albumin and diuretics, as well as TIPS, if indicated.18 Patients with HE were treated with lactulose and/or rifaximin.17
Nutritional status, as part of diagnostic work‐up, was routinely assessed in inpatients and outpatients with cirrhosis. Patients were counseled to meet their daily energy intake of 35–40 kcal/kg and a protein intake of 1.2–1.5 g/kg body weight, according to the recommendations available during the study period.19, 20 High‐energy protein drinks were recommended to patients at risk of malnutrition.19 Furthermore, dietary counselling was available for all patients at their own request.
Measurement of sex hormones
Total testosterone, SHBG, LH, FSH, and PRL were analyzed using electrochemiluminescence immunoassay (ECLIA; Roche Diagnostics, Vienna, Austria). Bioavailable T (= free T plus albumin‐bound T) was then calculated automatically through the Department of Laboratory Medicine using the Vermeulen formula.21 To avoid laboratory deviation due to the circadian rhythm of sexual hormones and especially T, blood used for analyzing sex hormones was assessed during routine laboratory analysis in fasting conditions in the morning.
Hepatic venous pressure gradient measurement
The right internal jugular vein was accessed under ultrasound guidance and local anesthesia with the Seldinger technique using a catheter introducer set (8.5 Fr; Arrow International, Reading, PA, USA). Then a balloon catheter (7 Fr Ferlitsch HVPG catheter; Pejcl Medizintechnik, Vienna, Austria) was chosen to cannulate the liver vein through the transjugular access as described previously.22, 23 Clinically significant portal hypertension (CSPH) was defined as an HVPG ≥10 mmHg.17
Assessment of sarcopenia
Computed tomography scans within 1 year of study inclusion were taken into account. All measurements were obtained on axial contrast‐enhanced CT scans of the abdomen. The cross‐sectional muscle area was calculated in all patients at the level of the third lumbar vertebrae (L3) using OsiriX medical imaging software for iOS (version 7.5; Pixmeo, Bernex, Switzerland) and syngo.via software (version VB30; Siemens Healthcare, Erlangen, Germany). The skeletal muscle index was defined as the total cross‐sectional area of all abdominal muscles (rectus abdominis, oblique and transverse abdominal muscles, paraspinal muscles, and the psoas muscles) at the level of L3 on a single scan image normalized by height. Results are shown in cm2/m2. A senior radiology resident and an attending specialized in abdominal radiology analyzed all variables independently and the mean value of both measurements was taken into account for statistical analysis. Sarcopenia was defined according to a previously proposed cut‐off: <42 cm2/m2.24
Statistical analysis
Continuous variables were reported as mean ± standard deviation or median (95% confidence interval [CI]), and categorical variables were reported as number (n) of patients with the certain characteristic (proportion of patients with the certain characteristic [%]). Spearman rho‐coefficient was used to detect correlations between two variables where at least one was distributed non‐parametric, and/or categorized as an ordinal variable. Student's t‐test was used for group comparisons of normally distributed data, and the Mann–Whitney U‐test where data were not normally distributed. Kruskal–Wallis H‐test and post‐hoc comparisons were used to compare medians in groups of three or more. The significance level was adjusted using the Bonferroni method. Pearson's χ2‐test or Fisher's exact test were used for group comparisons. The Youden index was used to determine optimal cut‐offs regarding death and decompensation for BT, TT, and PRL. The impact of T on mortality, decompensation, and transplant‐free survival was analyzed using semiparametric proportional hazard Cox models. Univariate Cox regression was carried out to determine factors significantly associated with the composite end‐point (hepatic decompensation and/or death). Then all univariate significant factors were included in four separate multivariate models to avoid multicollinearity between CPS/MELD and BT/TT. Patients entered the model on the day that blood was drawn for analyzing sexual hormone levels and were followed until either death or liver transplantation. Hepatic decompensation during follow‐up was recorded as defined above. Patients who received liver transplantation were censored at the day of surgery. The log–rank test was used to find differences in survival times between the groups that were stratified according to the previously determined cut‐offs (Youden index). Two‐sided P‐values <0.05 were considered statistically significant. The IBM spss 24.0 statistics software (SPSS, Armonk, NY, USA) was used for all statistical analyses and Kaplan–Meier curves.
Ethics
This study was approved by the ethics committee of the Medical University of Vienna (study no. 450/2010) and undertaken in accordance to the current version of the Helsinki Declaration. All patients signed an informed consent form prior to study inclusion.
Results
A total of 114 patients were included. Main patient characteristics are listed in Table 1 and univariate correlation of sex hormones and parameters of liver function in Table 2. We determined optimal cut‐offs regarding hepatic decompensation and mortality using the Youden index: BT < 1.59 ng/mL, TT <6.25 ng/mL, and PRL >13.8 ng/mL.
Table 1.
Main patient characteristics
|
All n = 114 |
CPS A n = 32 |
CPS B n = 48 |
CPS C n = 34 |
P‐value overall | |
|---|---|---|---|---|---|
| Age, mean ± SD | 55 ± 9.2 | 51.7 ± 9.4 | 57.4 ± 9.1 | 56.2 ± 8.5 | A vs. B, 0.018 |
| A vs. C, 0.113 | |||||
| B vs. C, 0.816 | |||||
| Etiology, n (%) | |||||
| ALD | 61 (53.5) | 16 (26.2) | 25 (41.0) | 20 (32.8) | 0.634 |
| Viral | 30 (26.3) | 10 (33.3) | 15 (50.0) | 5 (16.7) | |
| COMB | 9 (7.9) | 2 (22.2) | 4 (44.4) | 3 (33.3) | |
| Other | 14 (12.3) | 4 (28.6) | 4 (28.6) | 6 (42.9) | |
| HCC, n (%) | 20 (17.5) | 3 (15.0) | 12 (60.0) | 5 (25.0) | 0.173 |
| Ascites, n (%) | 62 (54.0) | 2 (6.3) | 28 (58.0) | 32 (94.0) | <0.001 |
| Grade 0–1 | 52 (45.6) | 30 (57.7) | 20 (38.5) | 2 (3.8) | |
| Grade 2 | 35 (30.7) | 2 (5.7) | 19 (54.3) | 14 (40.0) | |
| Grade 3 | 27 (23.7) | 0 (0.0) | 9 (33.3) | 18 (66.7) | |
| Death, n (%) | 31 (27.0) | 5 (16.1) | 16 (51.6) | 10 (32.3) | 0.206 |
| Decompensation during follow‐up, n (%) | 35 (30.7) | 7 (20.0) | 16 (45.7) | 12 (34.3) | 0.435 |
| LTX during follow‐up, n (%) | 39 (34.2) | 7 (21.9) | 16 (33.3) | 16 (47.1) | 0.097 |
| Days of follow‐up, n (%) | 397 (5.75–2299.25) | 1421.5 (19.5–2423.4) | 449.5 (14.1–2274.2) | 192.5 (5–1060.5) | A vs. B, 0.019 |
| A vs. C, <0.001 | |||||
| B vs. C, 0.004 | |||||
| MELD, median (95% CI) | 13.56 (7–20.7) | 8.4 (6.43–15.3) | 13.1 (9.7–18.86) | 17.1 (13.5–23) | <0.001 |
| HVPG, mmHg, mean ± SD† | 16.8 ± 6 | 13 ± 5.5 | 19.3 ± 4.8 | 19.8 ± 5.4 | A vs. B, 0.001 |
| A vs. C, 0.004 | |||||
| B vs. C, 0.963 | |||||
| CSPH, n (%) | 45 (91.8) | 16 (35.6) | 19 (42.2) | 10 (22.2) | 0.043 |
| No CSPH, n (%) | 4 (8.2) | 4 (100.0) | 0 (0.0) | 0 (0.0) | |
| Bioavailable T, median (95% CI) | 1.12 (0.12–2.58) | 1.66 (0.68–3.41) | 1.07 (0.14–1.95) | 0.39 (0.1–1.5) | <0.001 |
| Total T, median (95% CI) | 4.38 (0.32–11.62) | 7.18 (2.97–16.33) | 4.39 (0.37–9.6) | 1.49 (0.25–8) | <0.001 |
| LH, median (95% CI) | 6.2 (1.72–15.18) | 6.9 (2.56–39.56) | 6.45 (1.57–15.26) | 5.1 (0.1–15.55) | A vs. B, 0.861 |
| A vs. C, 0.068 | |||||
| B vs. C, 0.072 | |||||
| SHBG, mean ± SD | 93.65 ± 43.5 | 109.1 ± 49.86 | 94.29 ± 38.9 | 78.2 ± 38.5 | |
| A vs. C, 0.011 | |||||
| A vs. B, 0.384 | |||||
| B vs. C, 0.279 | |||||
| FSH, median (95% CI) | 6.9 (1.63–23.62) | 7.5 (2.44–30) | 6.55 (2–30.86) | 5.6 (0.6–23) | A vs. B, 0.272 |
| A vs. C, 0.115 | |||||
| B vs. C, 0.351 | |||||
| PRL, median (95% CI)‡ | 13.45 (5.24–42.23) | 8.4 (3.4–38, n = 24) | 13.45 (5.85–34, n = 34) | 16 (7.65–73.61, n = 30) | A vs. B, 0.039 |
| A vs. C, 0.001 | |||||
| B vs. C, 0.040 | |||||
Available in 49 patients.
Available in 88 patients.
ALD, alcoholic liver disease; COMB, Viral + ALD; CI, confidence interval; CPS, Child–Pugh score; CSPH, clinically significant portal hypertension; FSH, follicle‐stimulating hormone; HCC, hepatocellular carcinoma; HVPG, hepatic venous pressure gradient; LH, luteinizing hormone; LTX, liver transplantation; MELD, Model for End‐stage Liver Disease; PRL, prolactin; SHBG, sex hormone‐ binding globulin; T, testosterone.
Table 2.
Univariate correlation of sex hormones and liver‐related parameters in patients with liver cirrhosis
| CPS | MELD | Ascites | Albumin | Prothrombin time | Bilirubin | vWF‐Ag | HVPG† | |
|---|---|---|---|---|---|---|---|---|
| Bioavailable T | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.003 |
| r −0.674 | r −0.536 | r −0.595 | r 0.568 | r 0.353 | r −0.450 | r‐0.480 | r −0.424 | |
| Total T | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.036 |
| r −0.645 | r −0.536 | r −0.564 | r 0.494 | r 0.347 | r −0.397 | r‐0.413 | r −0.303 | |
| SHBG | 0.003 | 0.001 | 0.014 | 0.348 | 0.027 | 0.252 | 0.453 | 0.992 |
| r −0.280 | r −0.309 | r −0.231 | r 0.089 | r 0.208 | r −0.109 | r −0.74 | r 0.001 | |
| LH | 0.059 | 0.511 | 0.240 | 0.350 | 0.006 | 0.157 | 0.427 | 0.824 |
| r −0.178 | r −0.063 | r −0.111 | r 0.089 | r 0.258 | r −0.134 | r 0.078 | r −0.033 | |
| FSH | 0.184 | 0.686 | 0.022 | 0.734 | 0.724 | 0.289 | 0.503 | 0.610 |
| r −0.126 | r −0.039 | r −0.216 | r −0.032 | r 0.034 | r −0.101 | r −0.066 | r −0.076 | |
| PRL‡ | <0.001 | <0.001 | 0.002 | <0.001 | 0.050 | 0.011 | 0.005 | 0.095 |
| r 0.390 | r 0.379 | r 0.321 | r −0.399 | −0.209 | r 0.269 | r 0.306 | r 0.283 |
Available in 49 patients.
Available in 88 patients.
CPS, Child–Pugh score; FSH, follicle‐stimulating hormone; HVPG, hepatic venous pressure gradient; LH, luteinizing hormone; MELD, Model for End‐stage Liver Disease; PRL, prolactin; SHBG, sex hormone‐binding globulin; T, testosterone; vWF‐Ag, von Willebrand‐Factor antigen.
Sex hormones and parameters of liver function
As shown in Table 1, BT and TT significantly decreased over CPS stages (P < 0.001). Prolactin significantly increased over CPS stages (P = 0.002). Sex hormone‐binding globulin was significantly lower between CPS A vs. C (P = 0.014), but no difference between A vs. B or B vs. C was found. For correlations of sex hormones with markers of liver disease see Table 2. Figure 1 shows median levels of sexual hormones over CPS. Furthermore, the distribution of patients found with BT, TT, and PRL levels below respectively above the threshold (determined via Youden index) showed significantly more patients with low BT (P < 0.001 and P < 0.001), low TT (P < 0.001 and P < 0.001), and elevated PRL levels (P < 0.001 and P = 0.025) with increasing CPS and ascites stages (Fig. 2).
Figure 1.
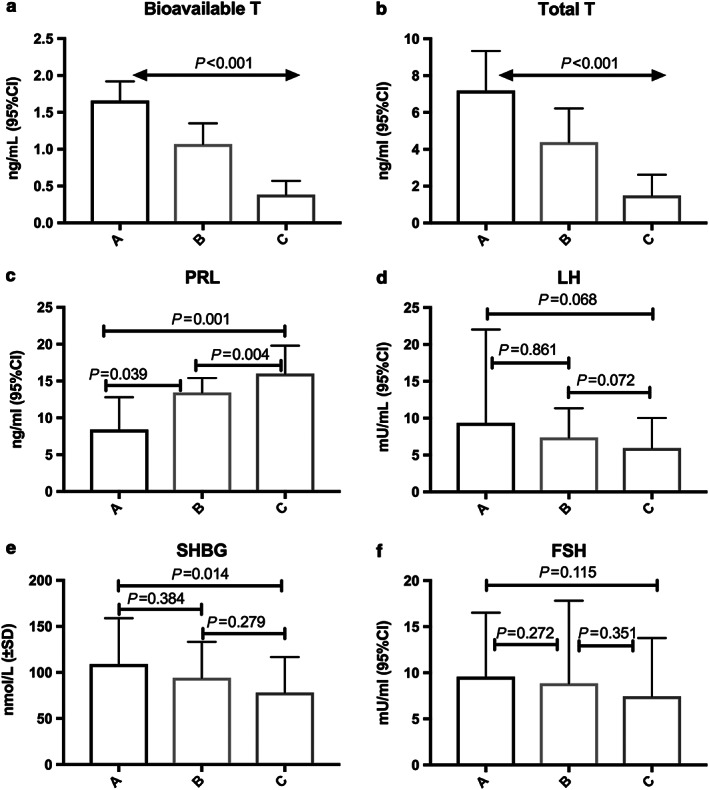
Median levels of sexual hormones over Child–Pugh score in male patients with cirrhosis. CI, confidence interval; FSH, follicle‐stimulating hormone; LH, luteinizing hormone; PRL, prolactin; SD, standard deviation; SHBG, sex hormone‐binding globulin; T, testosterone.
Figure 2.
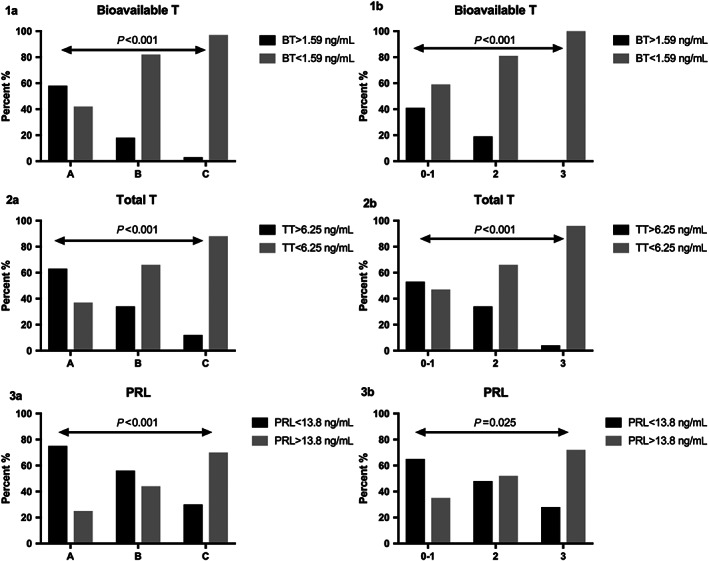
Distribution of bioavailable testosterone (BT) (1a), total testosterone (TT) (2a), and high prolactin (PRL) (3a) over Child–Pugh score stages and grades of ascites (1b, 2b, and 3b) (according to cut‐offs determined by the Youden index) in men with cirrhosis.
Sex hormones and ascites
Median levels of BT (ascites grade 0–1 vs. 2 P = 0.001; 0–1 vs. 3 P ≤ 0.001; 2 vs. 3 P = 0.001) and TT (ascites grade 0–1 vs. 2 P = 0.009; 0–1 vs. 3 P ≤ 0.001; 2 vs. 3 P ≤ 0.001) significantly decreased with increasing severity of ascites. Prolactin levels increased (ascites grade 0–1 vs. 2 P = 0.113; 0–1 vs. 3 P = 0.005; 2 vs. 3 P = 0.052). In addition, distribution of patients found with BT, TT, and PRL levels below respectively above the threshold (determined by the Youden index) showed significantly more patients with low BT (P < 0.001), low TT (P < 0.001), and elevated PRL (P = 0.025) with increasing ascites grades (Fig. 2).
Sex hormones and portal hypertension
Forty‐nine patients had available data on HVPG. Bioavailable T (r −0.424, P = 0.003) and TT (r −0.303, P = 0.036) correlated significantly with absolute HVPG values. Clinically significant portal hypertension was present in 45 (91.8%) patients. Thirty‐eight patients were found with HVPG ≥ 12 mmHg (77.6%). Median BT (0.8 ng/mL [0.1–2] vs. 1.68 ng/mL [0.07–2.65]; P = 0.004) and TT (2.7 ng/mL [0.23–12.34] vs. 7 ng/mL [0.25–10]; P = 0.041) levels were significantly lower in patients with HVPG ≥ 12 mmHg.
Sex hormones and sarcopenia
Abdominal CT scans were available in 66 (58%) patients. Sarcopenia was present in 14 patients (21%). The prevalence of sarcopenia was not different between CPS (A, 13.3%; B, 24.1%, C, 22.7%; P = 0.692), although there was a trend towards higher median CPS in sarcopenic patients (P = 0.075). Significantly more patients with MELD >15 points were found with sarcopenia (MELD >15, 38.9% vs. MELD <15, 14.6%; P = 0.031). Bioavailable T (0.25 ng/mL [0.07–1.7] vs. 0.97 ng/mL [0.15–2.74]; P = 0.017) and TT levels (1.28 ng/mL [0.25–7.32] vs. 4.32 ng/mL [0.43–13.47]; P = 0.031) were significantly lower in sarcopenic patients, whereas PRL (P = 0.460), FSH (P = 0.539), LH (P = 0.442), and SHBG (P = 0.900) levels were not different.
Sex hormones and hepatic decompensation and mortality
Forty‐six (40.4%) patients had a liver‐related event during a median follow‐up of 13 months (0.2–75 months). Among them, 31 (27.2%) patients died; cause of death was liver failure in 17 (55%), sepsis in 6 (19%), HCC in 6 (19%), and variceal bleeding in 2 (7%). Median months of follow‐up were not significantly different between the groups (decompensated/died 8.5 months [0.6–60 months] vs. others 9.1 months [0.1–77 months]; P = 0.344). During follow‐up, 5 patients developed variceal bleeding (14.3%), 3 SBP (8.6%), 11 refractory ascites (31.4%), 13 HE (37.1%), and 3 jaundice (8.6%). Significantly more patients in the group with low BT and TT met the composite end‐point during follow‐up (BT < 1.59 ng/mL 88.4% vs. BT > 1.59 ng/mL 11.6%, P = 0.009; TT < 6.25 ng/mL 76.1% vs. TT > 6.25 ng/mL 23.9%, P = 0.027). Kaplan–Meier survival estimation found significant difference in median time to hepatic decompensation/death between patients with low BT (35.6 months [27–44] vs. 62.5 months [50–75], P = 0.002) low TT (30.5 months [21–40] vs. 58 months [48–68], P < 0.001), and elevated PRL (32.3 months [21–44] vs. 51.2 months [40–63], P = 0.018) (Fig. 3). For univariate analysis regarding the composite endpoint see Table 3. Multivariate analysis was undertaken with four models including both CPS and MELD and/or BT and TT and found that a TT level <6.25 ng/mL was associated with an increased risk for the composite end‐point, even after adjusting for CPS and MELD and other relevant factors (model 1b: HR, 2.503; 95% CI, 1.214–5.157; P = 0.013; Model 2b: HR, 3.065; 95% CI, 1.523–6.169; P = 0.002). In contrast, BT below the threshold of <1.59 ng/mL did not attain statistical significance in any model (see Table 3).
Figure 3.
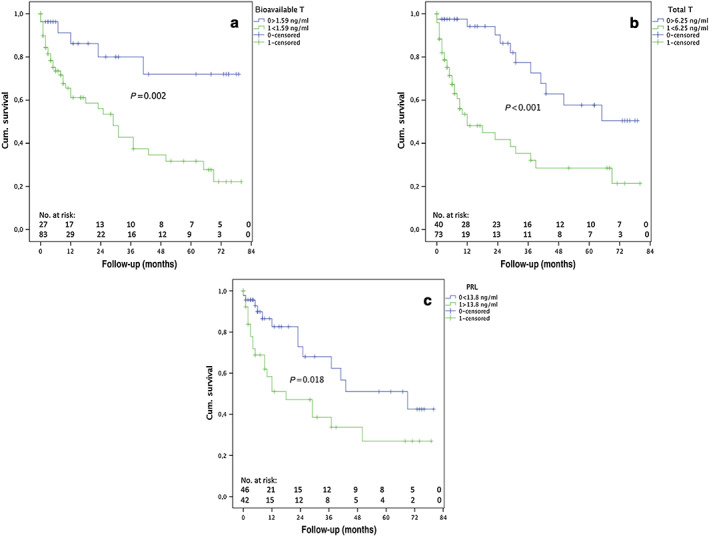
Kaplan–Meier curves for bioavailable testosterone (T) (a), total T (b), and prolactin (PRL) (c) in men with cirrhosis, stratified according to optimal Youden index‐determined cut‐offs. Cum., cumulative. [Color figure can be viewed at wileyonlinelibrary.com]
Table 3.
Univariate and multivariate Cox regression models to determine risk factors for mortality or decompensation during follow‐up
| HR (95% CI) | P‐value | MV model 1a | MV model 1b | MV model 2a | MV model 2b | |
|---|---|---|---|---|---|---|
| HR (95%CI); P‐value | HR (95% CI); P‐value | HR (95% CI); P‐value | HR (95% CI); P‐value | |||
| Age | 1.025 (0.992–1.058) | 0.139 | NS | NS | NS | NS |
| CPS | B: 2.349 (1.07–5.15) | 0.033 | B: 2.08 (0.89–4.89); P = 0.092 | B: 2.31 (1.01–5.3); P = 0.048 | NS | NS |
| C: 6.65 (2.577–17.19) | <0.001 | C: 5.38 (1.89–15.32); P = 0.002 | C: 5.28 (1.89–14.71); P = 0.001 | NS | NS | |
| HCC | 2.079 (0.944–4.579) | 0.069 | NS | NS | NS | NS |
| MELD | 1.124 (1.045–1.209) | 0.002 | NS | NS | NS | NS |
| Bioavailable T < 1.59 ng/mL | 3.933 (1.54–10) | 0.004 | 2.59 (0.97–6.93); P = 0.059 | NS | 2.47 (0.93–6.57); P = 0.069 | NS |
| Total T < 6.25 ng/mL | 3.493 (1.757–6.942) | <0.001 | NS | 2.50 (1.21–5.16); P = 0.013 | NS | 3.07(1.52–6.17); P = 0.002 |
| PRL >13.8 ng/mL | 2.282 (1.14–4.56) | 0.020 | NS | NS | NS | NS |
| Creatinine | 1.924 (1.068–3.466) | 0.029 | NS | NS | NS | NS |
| Albumin | 0.910 (0.863–0.961) | 0.001 | NS | NS | 0.93(0.88–0.99); P = 0.036 | NS |
| Presence of ascites | 3.634 (1.918–6.884) | <0.001 | NS | NS | 2.28 (1.12–4.64); P = 0.023 | 3.15 (1.52–6.17); P = 0.001 |
| Previous hepatic decompensation | 1.815 (0.996–3.307) | 0.052 | NS | NS | NS | NS |
MV model 1a: Significant variables in the multivariate Cox regression model (included variables: Child–Pugh score [CPS], hepatocellular carcinoma [HCC], bioavailable testosterone [T], creatinine, and previous hepatic decompensation).
MV model 1b: Significant variables in the multivariate Cox regression model (included variables: CPS, HCC, total T, creatinine, and previous hepatic decompensation).
MV model 2a: Significant variables in the multivariate COX regression model (included variables: Model for End‐stage Liver Disease [MELD], HCC, bioavailable T, albumin, presence of ascites [yes/no], and previous hepatic decompensation).
MV model 2b: Significant variables in the multivariate Cox regression model (included variables: MELD, HCC, total T, albumin, presence of ascites [yes/no], and previous hepatic decompensation).
CI, confidence interval; HCC, hepatocellular carcinoma; HR, hazard ratio; MV, multivariate; NS, non significant (P > 0.1); PRL, prolactin.
Discussion
In this study, we showed the significant potential of low total T levels as an independent marker for the prediction of hepatic decompensation and death in patients with cirrhosis. For the first time we described the association between portal hypertension and low T levels. We also found increasing PRL levels associated with liver dysfunction. The other sex hormones measured in our study (LH, FSH, and SHBG) showed only mild correlation with liver dysfunction (Tables 1, 2).
According to current concepts, the deficiency of T in cirrhosis is thought to be due to multiple factors including both primary and secondary hypogonadism.5, 7 The inappropriately low LH levels over CPS in our data confirm this hypothesis (see Table 1 and Fig. 1). However, the underlying cause for the alterations of the HPG axis in cirrhosis is not yet fully understood. Studies that evaluated sex hormone status pre‐ and post‐liver transplantation found that the HPG axis function improves after liver transplantation, suggesting that hepatic dysfunction plays an important role.5, 9, 10, 25 Limited data exists on the prognostic value of estrogen in cirrhosis; in an Australian study,6 high circulating serum estrone levels were associated with higher MELD and adverse outcomes. In another study evaluating estrogen levels pre‐ and post‐TIPS implantation, increased estrogen levels were found in patients after TIPS,11 which was explained by the impact of portosystemic shunting on hyperestrogenism in cirrhosis. Estrogens are generally elevated in cirrhosis3, 26, 27 due to portosystemic shunting,11, 12 decreased hepatic metabolism, and increased peripheral aromatization of androgens into estrogens.1, 5 Hyperestrogenism inhibits central gonadotropin (LH) production and stimulates SHBG production, which contributes to hypotestosteronism.2, 5 Accordingly, further studies about the prognostic impact of estrogen levels in cirrhosis would be of interest. Approximately 45% of T is bound to SHBG, another 50% to albumin, and approximately 2% circulates as free T.5, 7 Hyperestrogenism and low androgens contribute towards increased production of SHBG, which then leads to further reduction of biologically active free T due to increased binding.5, 7 Prolactin is elevated in chronic liver disease, mostly due to dopaminergic system alterations and hyperestrogenism, and correlates well with parameters of liver dysfunction (also see Tables 1, 2) as previously described.4, 8, 10 Central inhibition of the GnRH secretion through PRL is thought to further contribute towards hypogonadism.5
Low T has been previously described as an independent risk factor for mortality13, 14, 28 and major infection14 in cirrhosis. Low median T levels have been described in patients with severe HE, who were admitted to hospital, as well as patients with ascites.3, 6 We found significant difference in median time to hepatic event in groups with low TT, low BT, and high PRL and significantly more patients in the group with low BT and TT met the composite end‐point during follow‐up. Hence, we could for the first time show that low TT is an independent risk factor for hepatic decompensation and mortality during a long follow‐up period. Bioavailable T did not attain statistical significance and this could be explained by the fact that TT measures all components of serum T: free T, albumin‐bound T, and SHBG‐bound T. Hence, in states of altered albumin and SHBG production, total T levels are a more stable predictive marker. For the first time, an association between HVPG low BT and TT is described by our data. This can certainly be explained due to the reflection of cirrhosis severity through HVPG but also due to increased portosystemic shunting in patients with elevated HVPG, where ultimately estrogen increasingly bypasses the liver and centrally inhibits GnRH production, which then leads to secondary hypogonadism.12 Whether low T levels are a cause or just a significant biomarker for hepatic decompensation and mortality is not known yet. The most consequential hypothesis would be that T accurately reflects liver disease severity and therefore solely acts as a predictive biomarker. This hypothesis is partly accredited in a study29 in which intramuscular T injection vs. placebo in cirrhotics with low T showed no significant difference in mortality. Another hypothesis suggests a link between T and systemic inflammation. Testosterone has been shown to suppress the innate and adaptive immune system, such as reduction of macrophage activation, lymphocyte numbers, and cytokine levels.30, 31, 32 However, the downregulation of GnRH in the hypothalamus in severe chronic disease due to increased inflammatory markers (interleukin‐1 and ‐6 and tumor necrosis factor‐α)30 perpetually leads to secondary hypogonadism. Similarly, inflammatory markers are commonly elevated in patients with cirrhosis, which is a consequence of endotoxemia due to pathological bacterial translocation from the gut.33 Low T was found as an independent risk factor for major infection,14 therefore confirming the pathophysiologic rationale in a clinical study and suggesting low T as a marker not only of disease severity but also increased inflammation. Furthermore, an inverse relationship between C‐reactive protein and T has been described.34 Sarcopenia has been described as an independent risk factor for mortality28, 35, 36 in cirrhosis. Testosterone has an important anabolic function in muscle mass homeostasis, hence, low T directly impairs prognosis by contributing towards evolvement of sarcopenia. In our study, both BT and TT levels were significantly lower in sarcopenic patients. As sarcopenia is independently associated with adverse outcomes in patients with cirrhosis, the implementation of specific treatments for sarcopenia (e.g. T supplementation) might improve prognosis. In addition, it has been reported that low total T was an even better predictor for mortality than sarcopenia.28 Similar to our study, sarcopenia significantly correlated with MELD but not with CPS.28 In a recent randomized controlled trial, intramuscular T therapy increased bone and muscle mass, however, there was no difference in clinical events during follow‐up.29 Accordingly, future studies should aim to assess whether T supplementation‐induced amelioration of sarcopenia improves the outcome of patients with cirrhosis.
Several other treatment strategies for sarcopenia have been evaluated, and more recently, guidelines for diagnosis and nutritional support have been published.24, 37 It is recommended that, in all patients with cirrhosis and malnutrition, optimal daily protein intake should not be lower than 1.2–1.5 g/kg per day and overall energy intake should not fall below 35 kcal/kg.37 Late evening oral nutritional supplementation is key; moreover, supplementation with branched‐chain amino acids has shown positive results.37, 38, 39 Other treatment options include physical exercise or ammonia‐lowering strategies, although further studies in these fields are needed before they can be recommended for daily clinical practice.37 Interestingly, low T levels have been associated with increased risk for all‐cause mortality within several other chronic diseases.40, 41
Limitations of our study certainly are the relatively small number of patients included, although the prospective setting and tight follow‐up in the outpatient clinic as well as the long‐term follow‐up period puts this into perspective. Also excluding patients with HE comprises a possible selection bias. Nevertheless, previous studies have described significantly low T levels in patients with HE,3, 26 hence we would expect similar results if patients with HE had been included. Additionally, data on estrogen levels are missing, and association with HVPG in our study would have been interesting. Strengths of our study are the long‐term follow‐up and detailed record of hepatic decompensation, liver transplantation, and mortality. Furthermore, we could for the first time show the association between HVPG and low T levels, indicating a negative effect of increased portosystemic shunting on (secondary) hypogonadism.
In conclusion, sex hormone status in cirrhotic men is frequently deranged. Total T is an independent risk factor for hepatic decompensation and mortality. Patients below the threshold of 6.25 ng/mL for TT are at increased risk and should be monitored closely. Taking into account recent results that T therapy in cirrhotic men is safe, further studies are warranted to determine whether reversing hypotestosteronism and sarcopenia has a beneficial effect on long‐term clinical outcomes.
Paternostro, R. , Heinisch, B. B. , Reiberger, T. , Mandorfer, M. , Bardach, C. , Lampichler, K. , Seeland, B. , Schwarzer, R. , Trauner, M. , Peck‐Radosavljevic, M. , and Ferlitsch, A. (2019) Dysbalanced sex hormone status is an independent predictor of decompensation and mortality in patients with liver cirrhosis. Hepatol Res, 49: 201–211. 10.1111/hepr.13253.
Conflict of interest: The authors have no conflict of interest.
Financial support: None declared.
References
- 1. Zietz B, Lock G, Plach B et al Dysfunction of the hypothalamic–pituitary–glandular axes and relation to Child–Pugh classification in male patients with alcoholic and virus‐related cirrhosis. Eur J Gastroenterol Hepatol 2003; 15: 495–501. [DOI] [PubMed] [Google Scholar]
- 2. Mowat NA, Edwards CR, Fisher R, McNeilly AS, Green JR, Dawson AM. Hypothalamic–pituitary–gonadal function in men with cirrhosis of the liver. Gut 1976; 17: 345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. De Besi L, Zucchetta P, Zotti S, Mastrogiacomo I. Sex hormones and sex hormone binding globulin in males with compensated and decompensated cirrhosis of the liver. Acta Endocrinol 1989; 120: 271–276. [DOI] [PubMed] [Google Scholar]
- 4. Mooradian AD, Shamma'a M, Salti I, Cortas N. Hypophyseal–gonadal dysfunction in men with non‐alcoholic liver cirrhosis. Andrologia 1985; 17: 72–79. [DOI] [PubMed] [Google Scholar]
- 5. Foresta C, Schipilliti M, Ciarleglio FA, Lenzi A, D'Amico D. Male hypogonadism in cirrhosis and after liver transplantation. J Endocrinol Invest 2008; 31: 470–478. [DOI] [PubMed] [Google Scholar]
- 6. Sinclair M, Gow PJ, Angus PW et al High circulating oestrone and low testosterone correlate with adverse clinical outcomes in men with advanced liver disease. Liver Int 2016; 36: 1619–1627. [DOI] [PubMed] [Google Scholar]
- 7. Sinclair M, Grossmann M, Gow PJ, Angus PW. Testosterone in men with advanced liver disease: abnormalities and implications. J Gastroenterol Hepatol 2015; 30: 244–251. [DOI] [PubMed] [Google Scholar]
- 8. Wang YJ, Wu JC, Lee SD, Tsai YT, Lo KJ. Gonadal dysfunction and changes in sex hormones in postnecrotic cirrhotic men: a matched study with alcoholic cirrhotic men. Hepatogastroenterology 1991; 38: 531–534. [PubMed] [Google Scholar]
- 9. Handelsman DJ, Strasser S, McDonald JA, Conway AJ, McCaughan GW. Hypothalamic–pituitary–testicular function in end‐stage non‐alcoholic liver disease before and after liver transplantation. Clin Endocrinol (Oxf) 1995; 43: 331–337. [DOI] [PubMed] [Google Scholar]
- 10. Madersbacher S, Ludvik G, Stulnig T, Grunberger T, Maier U. The impact of liver transplantation on endocrine status in men. Clin Endocrinol (Oxf) 1996; 44: 461–466. [DOI] [PubMed] [Google Scholar]
- 11. Nolte W, Schindler CG, Figulla HR et al Increase of serum estradiol in cirrhotic men treated by transjugular intrahepatic portosystemic stent shunt. J Hepatol 2001; 34: 818–824. [DOI] [PubMed] [Google Scholar]
- 12. Van Thiel DH, Gavaler JS, Cobb CF, McClain CJ. An evaluation of the respective roles of portosystemic shunting and portal hypertension in rats upon the production of gonadal dysfunction in cirrhosis. Gastroenterology 1983; 85: 154–159. [PubMed] [Google Scholar]
- 13. Grossmann M, Hoermann R, Gani L et al Low testosterone levels as an independent predictor of mortality in men with chronic liver disease. Clin Endocrinol (Oxf) 2012; 77: 323–328. [DOI] [PubMed] [Google Scholar]
- 14. Sinclair M, Gow PJ, Grossmann M, Shannon A, Hoermann R, Angus PW. Low serum testosterone is associated with adverse outcome in men with cirrhosis independent of the Model for End‐stage Liver Disease score. Liver Transpl 2016; 22: 1482–1490. [DOI] [PubMed] [Google Scholar]
- 15. Kim S, Kwon H, Park JH et al A low level of serum total testosterone is independently associated with nonalcoholic fatty liver disease. BMC Gastroenterol 2012; 12: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peck‐Radosavljevic M, Trauner M, Schreiber F. Austrian consensus on the definition and treatment of portal hypertension and its complications. Endoscopy 2005; 37: 667–673. [DOI] [PubMed] [Google Scholar]
- 17. Peck‐Radosavljevic M, Angermayr B, Datz C et al Austrian consensus on the definition and treatment of portal hypertension and its complications (Billroth II). Wien Klin Wochenschr 2013; 125(7–8): 200–219. [DOI] [PubMed] [Google Scholar]
- 18. European Association for the Study of the Liver . EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol 2010; 53: 397–417. [DOI] [PubMed] [Google Scholar]
- 19. Plauth M, Cabre E, Riggio O et al ESPEN guidelines on enteral nutrition: liver disease. Clin Nutr 2006; 25: 285–294. [DOI] [PubMed] [Google Scholar]
- 20. Plauth M, Merli M, Kondrup J et al ESPEN guidelines for nutrition in liver disease and transplantation. Clin Nutr 1997; 16: 43–55. [DOI] [PubMed] [Google Scholar]
- 21. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999; 84: 3666–3672. [DOI] [PubMed] [Google Scholar]
- 22. Ferlitsch M, Reiberger T, Hoke M et al von Willebrand factor as new noninvasive predictor of portal hypertension, decompensation and mortality in patients with liver cirrhosis. Hepatology 2012; 56: 1439–1447. [DOI] [PubMed] [Google Scholar]
- 23. Ferlitsch A, Bota S, Paternostro R et al Evaluation of a new balloon occlusion catheter specifically designed for measurement of hepatic venous pressure gradient. Liver Int 2015; 35: 2115–2120. [DOI] [PubMed] [Google Scholar]
- 24. Nishikawa H, Shiraki M, Hiramatsu A et al Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol Res 2016; 46: 951–963. [DOI] [PubMed] [Google Scholar]
- 25. Seehofer D, Steinmueller T, Graef KJ et al Pituitary function test and endocrine status in patient with cirrhosis of the liver before and after hepatic transplantation. Ann Transplant 2002; 7: 32–37. [PubMed] [Google Scholar]
- 26. Sinclair M, Gow PJ, Angus PW et al High circulating estrone and low testosterone correlate with adverse clinical outcomes in men with advanced liver disease. Liver Int 2016; 36: 1619–1627. [DOI] [PubMed] [Google Scholar]
- 27. Pignata S, Daniele B, Galati MG et al Oestradiol and testosterone blood levels in patients with viral cirrhosis and hepatocellular carcinoma. Eur J Gastroenterol Hepatol 1997; 9: 283–286. [DOI] [PubMed] [Google Scholar]
- 28. Sinclair M, Grossmann M, Angus PW et al Low testosterone as a better predictor of mortality than sarcopenia in men with advanced liver disease. J Gastroenterol Hepatol 2016; 31: 661–667. [DOI] [PubMed] [Google Scholar]
- 29. Sinclair M, Grossmann M, Hoermann R, Angus PW, Gow PJ. Testosterone therapy increases muscle mass in men with cirrhosis and low testosterone: a randomised controlled trial. J Hepatol 2016; 65: 906–913. [DOI] [PubMed] [Google Scholar]
- 30. Maggio M, Basaria S, Ble A et al Correlation between testosterone and the inflammatory marker soluble interleukin‐6 receptor in older men. J Clin Endocrinol Metab 2006; 91: 345–347. [DOI] [PubMed] [Google Scholar]
- 31. Muehlenbein MP, Bribiescas RG. Testosterone‐mediated immune functions and male life histories. Am J Hum Biol 2005; 17: 527–558. [DOI] [PubMed] [Google Scholar]
- 32. Gold SM, Chalifoux S, Giesser BS, Voskuhl RR. Immune modulation and increased neurotrophic factor production in multiple sclerosis patients treated with testosterone. J Neuroinflammation 2008; 5: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reiberger T, Ferlitsch A, Payer BA et al Non‐selective betablocker therapy decreases intestinal permeability and serum levels of LBP and IL‐6 in patients with cirrhosis. J Hepatol 2013; 58(5): 911–921. [DOI] [PubMed] [Google Scholar]
- 34. Kupelian V, Chiu GR, Araujo AB, Williams RE, Clark RV, McKinlay JB. Association of sex hormones and C‐reactive protein levels in men. Clin Endocrinol (Oxf) 2010; 72(4): 527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Montano‐Loza AJ, Meza‐Junco J, Prado CM et al Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol 2012; 10: 166–173. [DOI] [PubMed] [Google Scholar]
- 36. Kim HY, Jang JW. Sarcopenia in the prognosis of cirrhosis: going beyond the MELD score. World J Gastroenterol 2015; 21: 7637–7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. European Association for the Study of the Liver . EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol 2018. 10.1016/j.jhep.2018.06.024. [DOI] [Google Scholar]
- 38. Nakaya Y, Harada N, Kakui S et al Severe catabolic state after prolonged fasting in cirrhotic patients: effect of oral branched‐chain amino‐acid‐enriched nutrient mixture. J Gastroenterol 2002; 37: 531–536. [DOI] [PubMed] [Google Scholar]
- 39. Yoshida T, Muto Y, Moriwaki H, Yamato M. Effect of long‐term oral supplementation with branched‐chain amino acid granules on the prognosis of liver cirrhosis. Gastroenterol Jpn 1989; 24: 692–698. [DOI] [PubMed] [Google Scholar]
- 40. Hyde Z, Norman PE, Flicker L et al Low free testosterone predicts mortality from cardiovascular disease but not other causes: the Health in Men Study. J Clin Endocrinol Metab 2012; 97: 179–189. [DOI] [PubMed] [Google Scholar]
- 41. Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: endogenous testosterone and mortality in men: a systematic review and meta‐analysis. J Clin Endocrinol Metab 2011; 96: 3007–3019. [DOI] [PMC free article] [PubMed] [Google Scholar]


