Abstract
Objectives
To investigate and further validate if two novel cancer‐related glycoproteins, discovered by a genetic‐guided proteomics approach, can distinguish benign disease from prostate cancer (PCa) in men with enlarged prostates.
Patients and Methods
A retrospective study was performed that included men with a total prostate‐specific antigen (PSA) concentration of 2.0–10 ng/mL, negative digital rectal examination and enlarged prostate (volume ≥35 mL). Serum samples were collected between 2011 and 2016 at a single centre from 474 men before they underwent prostate biopsy. Serum concentrations of thrombospondin 1 (THBS1) and cathepsin D (CTSD) glycoproteins were combined with the percentage of free PSA to total PSA ratio (%fPSA) to predict any or significant cancer at biopsy.
Results
The multivariable logistic regression model including THBS1, CTSD and %fPSA discriminated among biopsy‐positive and biopsy‐negative patients in the validation set with an area under the curve (AUC) of 0.86 (P < 0.001, 95% confidence interval (CI) 0.82–0.91), while %fPSA alone showed an AUC of 0.64 (P < 0.001, 95% CI 0.57–0.71). At 90% sensitivity for PCa, the specificity of the model was 62%, while %fPSA had a specificity of 23%. For high grade (Gleason score ≥ 7 in prostatectomy specimen) PCa, the specificity was 48% at 90% sensitivity, with an AUC of 0.83, (P < 0.001, 95% CI 0.77 to 0.88). Limitations of the study include the retrospective set‐up and single‐centre cohort.
Conclusions
A model combining two cancer‐related glycoproteins (THBS1 and CTSD) and %fPSA can improve PCa diagnosis and may reduce the number of unnecessary prostate biopsies because of its improved specificity for PCa when compared to %fPSA alone.
Keywords: biomarkers, thrombospondin 1, cathepsin D, prostate‐specific antigen, #ProstateCancer
Abbreviations
- PCa
prostate cancer
- THBS1
thrombospondin 1
- CTSD
cathepsin D
- %fPSA
percentage of free PSA to total PSA ratio
- AUC
area under the curve
- GS
Gleason score
- tPSA
total PSA
- fPSA
free PSA
Introduction
Benign prostatic hyperplasia is the most common disease of the prostate besides prostate cancer (PCa) 1 and is a major reason for the poor specificity of PSA‐based testing for PCa 2. This often leads to the decision to perform a prostate biopsy despite the underlying benign cause of PSA elevation. In support of this, it has been shown that the use of PSA in men with smaller prostates is associated with lower false‐positive rates than in men with larger prostate volumes 3. In addition to PSA, the percentage of free PSA (fPSA) to total PSA (tPSA) ratio (%fPSA) remains an important and widely accepted test for men with an elevated PSA concentration in the range of 2–10 ng/mL, often referred to as the ‘diagnostic grey zone’, and is used to improve specificity and thus, reduce the number of unnecessary biopsies 4.
We have identified men with elevated PSA, enlarged prostate volume and negative DRE as the largest and the most challenging patient population, in which a decision to conduct a biopsy is associated with the most uncertainty. This patient population mainly comprises elderly patients 1 and continues to grow as the population ages.
We originally used a conditional Pten knockout mouse model to discover new biomarkers for improved PCa diagnosis. Pten is in fact known to be involved in both BPH and PCa development 5. The mass‐spectrometry‐based biomarker discovery 6 was focused exclusively on glycoproteins because glycosylated proteins, such as those secreted or shed from the cell surface, are likely to reside and persist in blood 6. This proteomics approach led to the identification of new glycoprotein biomarkers differentiating men with BPH from those with PCa 7. Additional studies also highlighted the prognostic potential of these biomarkers 8 as well as their use in predicting response to therapy 9, 10. More recently, we showed that a refined selection of two glycoprotein biomarkers, thrombospondin 1 (THBS1) and cathepsin D (CTSD) aids in the discrimination between PCa‐positive and PCa‐negative men 11.
The aim of the present study was to test the combination of THBS1 and CTSD together with %fPSA to provide a more accurate method to identify patients with an elevated PSA but a low risk profile of harbouring PCa; therefore, we selected available serum samples from men with an elevated PSA who were referred to the Martini‐Klinik, Hamburg between 2011 and 2016 for prostate biopsy.
Patients and Methods
Study Design
In this retrospective study, pre‐biopsy serum samples were obtained from the biobank of the Martini‐Klinik, Hamburg‐Eppendorf, Germany. Patients underwent TRUS‐guided 10‐core (range 3–32 cores) prostate biopsy to investigate an elevated PSA. Serum samples were collected between April 2011 and January 2016 and were taken prior to any prostate manipulation, stored at room temperature for 30 min, and centrifuged at 2 800 g for 10 min in a serum separator tube. Samples were kept at −80°C for long‐term storage. To develop the best model and create training and validation datasets for testing, the cohort was split in half according to the collection date of the sample. The samples collected before mid March 2013 were assigned to the training set, while samples collected afterwards were used for validation.
Study Population
To be included in the study, patients needed to meet the following inclusion criteria: initial biopsy; an elevated total PSA (tPSA) concentration between 2 and 10 ng/mL; a negative DRE; an enlarged prostate, with a volume (determined by TRUS) ≥35 mL; an available serum sample; and provision of informed consent to using their sample for research purposes. In addition, all men with a positive biopsy outcome needed to have undergone subsequent in‐house radical prostatectomy, so that the Gleason score (GS) from both the biopsy and the prostatectomy specimen were available. Out of the 474 serum samples used in the present study, 67 samples were used in a previous study 11. The study was approved by the local ethics commission Medical Chamber Hamburg under the reference PV3652.
Assay Methods
The biomarkers THBS1 and CTSD were measured using a sandwich ELISA format. In brief, human THBS1 purified from platelets 12 and recombinant human CTSD were used as calibrators; both capture and detection antibodies were mouse monoclonals generated against the human mature protein of THBS1 and the full‐length recombinant protein of CTSD. For verification purposes, THBS1 and CTSD assays were re‐measured using a Luminex MAGPIX system (Luminex Corp., Austin, TX, USA), as described by Endt et al. 11. Serum tPSA and fPSA concentrations were re‐analysed for all samples using the ADVIA Centaur immunoassay system (Siemens Healthcare, Erlangen, Germany) to calculate the percentage of fPSA to tPSA ratio (%fPSA). The tPSA values reported in the present paper refer to the original values measured at the University Hospital Hamburg‐Eppendorf using the Dimension Vista system (Siemens Healthcare).
Statistical Methods
Statistical analysis was performed with GraphPad PRISM version 6.0, (GraphPad Software, La Jolla, CA, USA). We performed comparisons using the Mann–Whitney U‐test, assuming data were not normally distributed. P values <0.05 were taken to indicate statistical significance. Logistic regression analysis was performed using SPSS Statistics software (version 23). Comparisons of AUCs were determined using DeLong's method 13. Decision‐curve analysis was performed as described by Vickers and Elkin 14 using RStudio® (version 0.99.46), an integrated development environment for R (version 3.2.2) 15.
Results
Biopsy Outcome
The study population consisted of 474 men who underwent a prostate biopsy. In the case of a positive biopsy, these patients underwent prostatectomy. This resulted in 236 men with a negative biopsy result and 238 who were diagnosed with PCa. A median of 10 TRUS‐guided biopsies (range 3–32) were obtained and a median (range) of 2 (1–10) cores were positive in those men diagnosed with PCa. Eighty‐five men (64%) with a GS of 6 at biopsy were upgraded at prostatectomy to a GS of 3 + 4 (92%) or GS ≥ 4 + 3 (8%). No multiparametric MRI data were available.
The cohort was evenly divided into a training and a validation set according to a cut‐off date. In the training set, which consisted of samples collected from men undergoing prostate biopsy before mid‐March 2013, 130 of the 237 men (54.9%) had a positive biopsy for cancer compared with 108 of 237 men (45.6%) in the validation set (Table 1). More men had significant (GS ≥ 7) PCa diagnosed at biopsy in the validation set, but no statistically significant difference in grade was detected at prostatectomy. Neither age, prostate volume or tPSA was significantly different among the two sets.
Table 1.
Characteristics of the training and validation sets of men undergoing prostate biopsy
| Training set | Validation set | P | |
|---|---|---|---|
| Patients, n | 237 | 237 | − |
| Age range, years (median) | 41.8–84.5 (64.5) | 46.9–83.1 (63.6) | 0.599 |
| Prostate volume range, mL (median) | 35–220 (50) | 35–250 (50) | 0.946 |
| Total PSA range, ng/mL (median) | 2.08–10.00 (5.8) | 2.24–10.0 (6.14) | 0.536 |
| PCa diagnosis, n (%) | 130 (54.9) | 108 (45.6) | 0.044 |
| Biopsy GS | |||
| 3 + 3, n (%) | 78 (60) | 54 (50) | 0.004 |
| 3 + 4, n (%) | 32 (24.6) | 34 (31.5) | |
| ≥4 + 3, n (%) | 20 (15.4) | 20 (18.5) | |
| GS in prostatectomy specimen | |||
| 3 + 3, n (%) | 31 (23.8) | 22 (20.4) | 0.130 |
| 3 + 4, n (%) | 78 (60) | 74 (68.5) | |
| ≥4 + 3, n (%) | 21 (16.2) | 12 (11.1) | |
GS, Gleason score; PCa, prostate cancer.
All serum samples were measured using the original Luminex‐based assays 11 as well as the newly developed ELISAs. There was good correlation for both assays between Luminex and ELISA measurements. A Spearman correlation coefficient of 0.87 (P < 0.001) was observed for THBS1 and 0.73 (P < 0.001) for CTSD. The ELISA test showed improved reproducibility, with coefficients of variation below 5% compared with 8% for Luminex.
Predicting Biopsy Outcome
Univariate analysis of tPSA, fPSA, %fPSA, as well as the two glycoproteins THBS1 and CTSD, was performed independently on both the training and validation sets. Multivariable logistic regression models were developed using the training cohort and tested in the validation cohort. Table 2 shows the AUCs and specificities at sensitivities of 95% and 90% for the various variables in both sets and the two multivariable logistic regression models, including the combination of THBS1 and CTSD with and without %fPSA.
Table 2.
Univariate and multivariable analyses to assess the predictive value in prostate cancer detection and specificity at 95% and 90% sensitivity
| Specificity (%) | AUC | P | ||
|---|---|---|---|---|
| Sensitivity | 95%(CI) | 90%(CI) | ||
| Training set | ||||
| Univariate | ||||
| tPSA | 9 (5–17) | 18 (11–26) | 0.56 (0.49–0.63) | 0.115 |
| fPSA | 8 (3–14) | 12 (7–20) | 0.58 (0.51–0.65) | 0.036 |
| %fPSA | 1 (0–5) | 20 (13–28) | 0.66 (0.59–0.73) | <0.001 |
| CTSD | 4 (1–9) | 11 (6–19) | 0.57 (0.49–0.64) | 0.073 |
| THBS1 | 31 (22–41) | 53 (43–63) | 0.81 (0.75–0.87) | <0.001 |
| Multivariable | ||||
| THBS1 + CTSD | 33 (24–43) | 55 (45–65) | 0.82 (0.76–0.87) | <0.001 |
| %fPSA + THBS1 + CTSD | 32 (23–42) | 53 (43–63) | 0.83 (0.78–0.88) | <0.001 |
| Validation set | ||||
| Univariate | ||||
| tPSA | 12 (7–19) | 22 (14.9–29.8) | 0.52 (0.45–0.6) | 0.514 |
| fPSA | 11 (6–18) | 34 (26–43) | 0.59 (0.52–0.66) | 0.019 |
| %fPSA | 11 (6–18) | 23 (16–31) | 0.64 (0.57–0.71) | <0.001 |
| CTSD | 7 (3–13) | 12 (7–19) | 0.58 (0.5–0.65) | 0.044 |
| THBS1 | 35 (27–44) | 50 (42–60) | 0.84 (0.79–0.89) | <0.001 |
| Multivariable | ||||
| THBS1 + CTSD | 35 (27–44) | 57 (48–65) | 0.85 (0.8–0.9) | <0.001 |
| %fPSA + THBS1 + CTSD | 34 (26–43) | 62 (53–70) | 0.86 (0.82–0.91) | <0.001 |
%fPSA, percentage of free PSA to total PSA ratio; AUC, area under the curve; CTSD, cathepsin D; fPSA, free PSA; THBS1, thrombospondin 1; tPSA, total PSA.
Performing the analysis on both training and validation sets highlighted that the univariate variables fPSA, %fPSA and THBS1 were significantly different between biopsy‐negative and biopsy‐positive patients, while tPSA was not. The serum concentration of CTSD was only significantly different in the validation set (P = 0.04) but not in the training set (P = 0.07), probably as a result of subtle differences in the patient composition of the training and validation sets. In addition, both THBS1 and CTSD serum concentrations were lower in men with negative biopsies. As a reference, %fPSA differentiated men with a negative and positive biopsy outcome in the training set, with an AUC of 0.66 (95% CI, 0.59–0.73) and in the validation set with an AUC of 0.64 (95% CI, 0.57–0.71), respectively. The AUC for the multivariable logistic regression model for the combination of THBS1 and CTSD was 0.82 (95% CI 0.76–0.87) in the training set and 0.85 (95% CI, 0.80–0.90) in the validation set. The combination of %fPSA, THBS1 and CTSD was successfully validated, with an AUC of 0.86 (95% CI 0.82–0.91; Fig. 1); the AUC was not significantly different from that of the training set (P = 0.3). The specificity for the combination of %fPSA, THBS1 and CTSD at 95% sensitivity was 34.1% and 62.0% at a 90% sensitivity. In comparison, %fPSA showed specificities of only 10.9% and 22.5% at 95% and 90% sensitivity, respectively.
Figure 1.
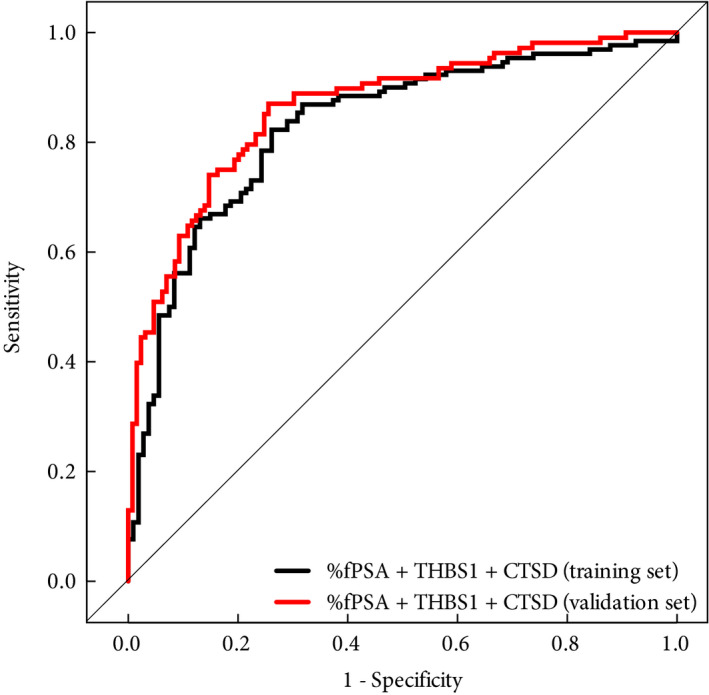
Receiver‐operating characteristic curves of the multivariate logistic regression model including percentage of free PSA to total PSA ratio ( %fPSA), thrombospondin 1 (THBS1 ) and cathepsin D (CTSD) in the training (black line) and validation (red line) sets for predicting biopsy outcome.
Table 3 shows the number of negative biopsies performed and the number of biopsies that could be avoided for %fPSA and the combination of THBS1 and CTSD with and without %fPSA in the training and validation sets. The cut‐offs chosen in both the training set and the validation set resulted in a 10% false‐negative rate (90% sensitivity) for any grade of cancer detected. While %fPSA alone avoided 31 (24%) out of 129 negative biopsies in the validation set, THBS1 and CTSD together avoided 73 (56%) negative biopsies. The addition of %fPSA to THBS1 and CTSD further increased the number of avoidable negative biopsies to 80 (62%). When looking at missed significant cancers in the validation set, seven (13%) out of 54 cancers with a GS ≥ 7 based on the biopsy grading were missed by the combination of all three variables and a single case with GS ≥ 4 + 3 (prostatectomy specimen grading), respectively. The results for the training set were very similar to those of the validation set, as shown in Table 3.
Table 3.
Impact of using percentage of free PSA to total PSA ratio (%fPSA) and the multivariate models of thrombospondin 1 + cathepsin D with and without %fPSA on negative biopsies avoided and any as well as significant cancers detected or missed
| Negative biopsies | Any cancer | Biopsy GS ≥7 | Prostatectomy GS ≥4 + 3 | |||||
|---|---|---|---|---|---|---|---|---|
| Performed, n | Avoided, n (%) | Detected, n (%) | Missed, n (%) | Detected, n (%) | Missed, n (%) | Detected, n (%) | Missed, n (%) | |
| Training | ||||||||
| Univariate | ||||||||
| %fPSA | 86 | 21 (20) | 117 (90) | 13 (10) | 48 (92) | 4 (8) | 20 (95) | 1 (5) |
| Multivariable | ||||||||
| THBS1 + CTSD | 48 | 59 (55) | 117 (90) | 13 (10) | 46 (88) | 6 (12) | 19 (90) | 2 (10) |
| %fPSA + THBS1 + CTSD | 50 | 57 (53) | 117 (90) | 13 (10) | 47 (90) | 5 (10) | 19 (90) | 2 (10) |
| Validation | ||||||||
| Univariate | ||||||||
| %fPSA | 98 | 31 (24) | 97 (90) | 11 (10) | 49 (91) | 5 (9) | 12 (100) | 0 (0) |
| Multivariable | ||||||||
| THBS1 + CTSD | 56 | 73 (57) | 97 (90) | 11 (10) | 48 (89) | 6 (11) | 12 (100) | 0 (0) |
| %fPSA + THBS1 + CTSD | 49 | 80 (62) | 97 (90) | 11 (10) | 47 (87) | 7 (13) | 11 (99) | 1 (8) |
%fPSA, percentage of PSA to total PSA ratio; CTSD, cathepsin D; GS, Gleason score; THBS1, thrombospondin 1.
In addition, instead of assigning patients by temporal order, we repeated the analysis by randomly assigning the samples to training (n = 237) and validation (n = 237) sets and obtained similar results. Using random assignment, the training set had an AUC of 0.86 (95% CI 0.81–0.91), which was again validated with an AUC of 0.85 (95% CI 0.80–0.90) as shown in Fig. S1.
Predicting Significant Cancer
The combination of %fPSA, THBS1 and CTSD was also tested on the ability to distinguish negative and insignificant (GS < 7) vs significant (GS ≥ 7) cancers. The multivariable logistic regression model showed an AUC of 0.77 (95% CI 0.69 to 0.84) for the training set and an AUC of 0.73 (95% CI 0.65 to 0.81) for the validation set based on biopsy grading (Fig. 2A); however, when based on grading of the prostatectomy specimen, a slightly higher accuracy was obtained for both the training set (AUC 0.78, 95% CI 0.72 to 0.84) and the validation set (AUC 0.83, 95% CI 0.77 to 0.88; Fig. 2B). The difference in the AUCs obtained in the training and validation sets were neither statistically significant for the biopsy‐based grading (P = 0.5) nor for the grading based on the prostatectomy specimen (P = 0.3). At 90% sensitivity, the specificity was 32% for biopsy‐based grading and 48% for grading based on the prostatectomy specimen.
Figure 2.
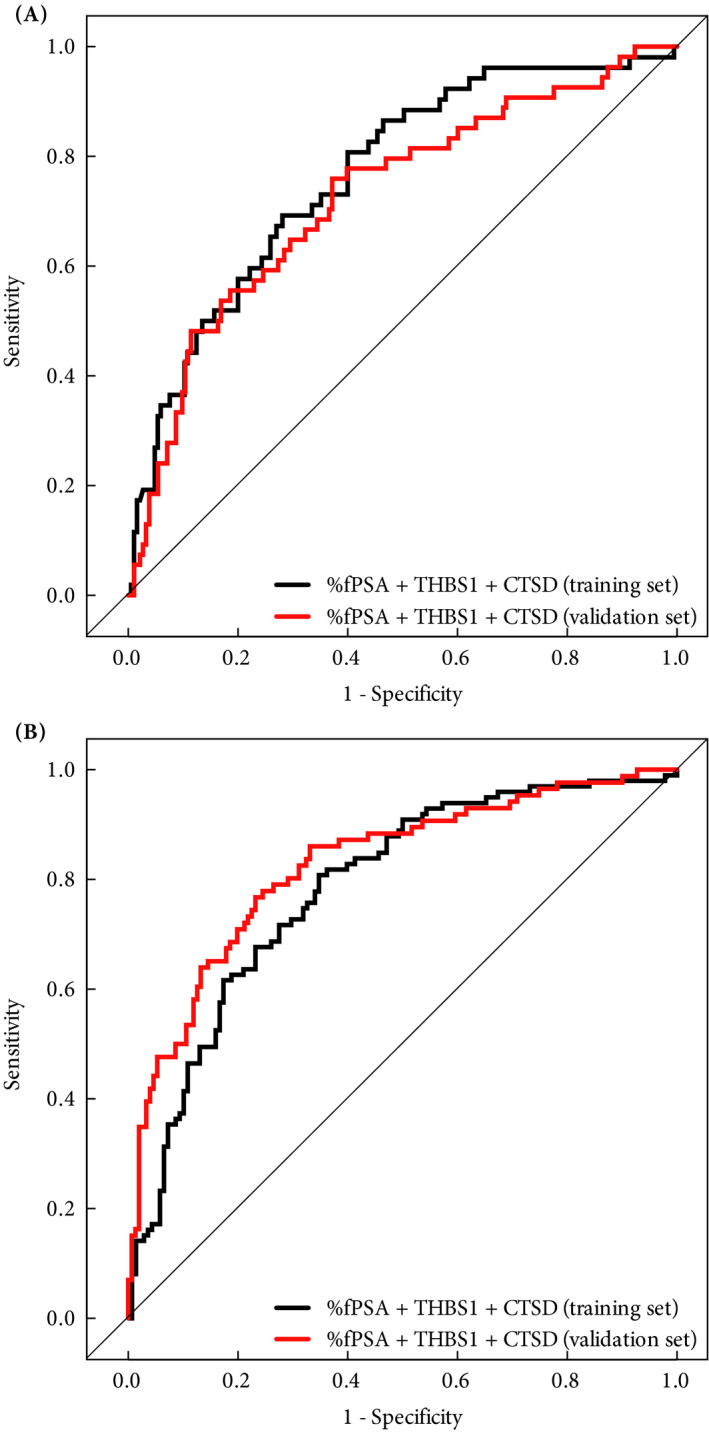
Receiver operating characteristic curves of the multivariate logistic regression model including percentage of free PSA to total PSA ratio (%fPSA), thrombospondin 1 (THBS1) and cathepsin D (CTSD) in the training (black line) and validation (red line) sets for predicting significant cancer (Gleason score ≥7) according to (A) biopsy Gleason score and (B) pathological Gleason score of the prostatectomy specimen.
In addition, we tested if patients with PCa with GS ≥ 4 + 3 based on prostatectomy specimen grading could be differentiated from the rest. The multivariable logistic regression model of %fPSA, THBS1 and CTSD showed an AUC of 0.79 (95% CI 0.69 to 0.90) for the training set and an AUC of 0.73 (95% CI 0.58 to 0.87) for the validation set. At 92% sensitivity, the specificity in the validation set was 40%.
Clinical Utility
To evaluate the clinical utility of the model comprising %fPSA, THBS1 and CTSD, decision‐curve analyses were performed on both the training and validation sets (Fig. 3). Determining which men should be biopsied using the proposed model was superior to biopsying all men or using %fPSA once the threshold probability reached ~30%, and was superior to the strategy of not biopsying anyone up to a threshold probability of ~80%.
Figure 3.
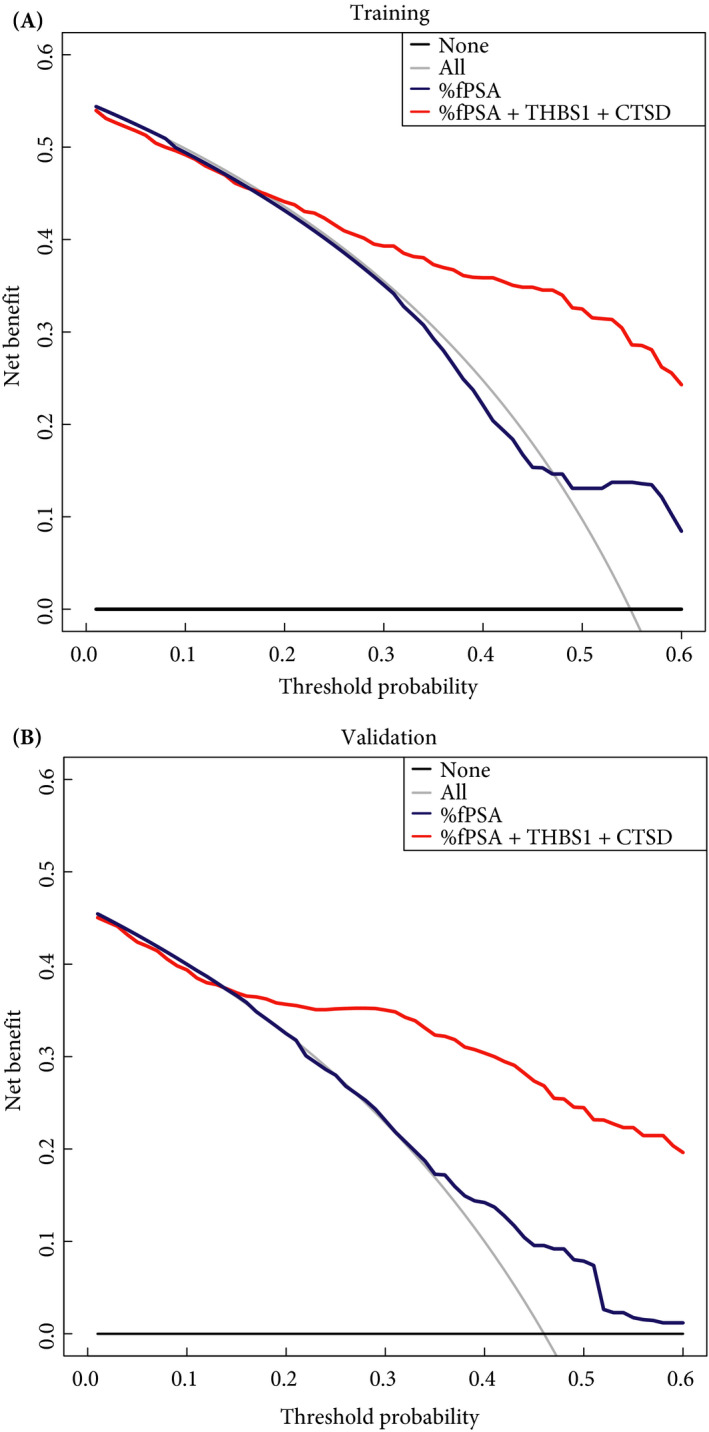
Decision‐curve analysis showing the effect of percentage of free PSA to total PSA ratio (%fPSA) and the model including %fPSA, thrombospondin 1 (THBS1) and cathepsin D (CTSD) in (A) the training and (B) the validation set for the detection of prostate cancer. Clinical net benefit for the models is plotted against the risk threshold at which a patient or clinician would opt for biopsy. As a comparison, the grey line represents the strategy of performing a biopsy in all men, and the black horizontal line represents the strategy of no men undergoing biopsy.
Discussion
The ability to distinguish benign disease, such as BPH, from PCa should improve early cancer detection as the elevated PSA level in benign disease often hampers PSA‐based screening. It has already been shown that fewer early‐stage cancers and, in particular, fewer high‐grade cancers are being detected because of reduced PSA‐based screening 16, 17. In the present paper, we describe a method that may improve PSA‐based screening as it offers the possibility to assess the likelihood of a positive prostate biopsy in a challenging and continually increasing subset of patients consisting of men with elevated PSA, enlarged prostate volume and negative DRE.
The proposed method is intended to reduce overdiagnosis and complements the current %fPSA measurement with the addition of two serum‐based glycoproteins, THBS1 and CTSD, that could form part of the current diagnostic workflow. THBS1 is an anti‐angiogenic factor that suppresses the neovascularization from the existing vascular system and inhibits tumour growth, and has been linked to PCa 18, 19. It is well known that pathological angiogenesis represents a crucial step in most forms of cancer and more specifically in PCa development where angiogenesis has been shown to play a fundamental role in PCa growth 20. The role of THBS1 in urological cancers and in particular with PCa has been reviewed in detail by Miyata and Sakai 21. Lower levels of THBS1 in biopsy‐negative men compared with men with PCa are in line with its reported negative correlation with PCa development 22. CTSD, an aspartic endoprotease, is involved in tumour metastasis and is commonly known to be overexpressed and secreted by cells of several tumour types including PCa 23. This is in contrast to the lower abundance of CTSD in biopsy‐positive serum samples measured in the present study.
The two proteins were previously tested in serum samples from 359 men from a combined cohort of two clinical centres: Martini‐Klinik, Hamburg‐Eppendorf, Germany and the Cantonal Hospital St. Gallen, Switzerland 11. In the present study, we further validated those findings in a larger cohort collected at the Martini‐Klinik, University Hospital Hamburg‐Eppendorf, Germany. The 474 serum samples collected from men before undergoing prostate biopsy were assigned to the training and validation sets according to the sample collection date to best represent the natural distribution of men having a positive or negative biopsy outcome 24. In addition, this approach provides proper cohort composition according to the longitudinal sample collection. The 67 samples used in the previous study and fitting the current inclusion criteria were re‐measured and used exclusively in the training set of this study. In addition, samples were randomly divided (50:50) for training and validation, and similar results were obtained when compared with temporal ordering. The immunoassays used for measuring THBS1 and CTSD were recently transferred from a Luminex to an ELISA platform, and all results were verified with the formerly developed Luminex assays and yielded similar results. The new ELISA‐based tests are highly reproducible and more amenable to routine clinical testing.
In the present study, we show that the multivariable model combining THBS1 and CTSD together with %fPSA improved the detection of PCa in a very challenging patient population: men with PSA in the grey zone (2–10 ng/mL), with enlarged prostate volume (≥35 mL) and negative DRE. In contrast to our previous study, age did not add any diagnostic value, perhaps because only patients with enlarged prostate glands were included. In the validation set, it was shown that the model could have avoided 62% of negative biopsies at a 10% false‐negative rate for any‐grade PCa. This result is supported by the data from decision‐curve analysis that suggested a clinical benefit. When training the model to predict high grade cancer, the model was better at predicting the GS found in the prostatectomy specimen than the one obtained from biopsy. This is an important finding as it is known that upgrading of GS from biopsy to prostatectomy occurs frequently 25 and is currently a challenge in managing localized PCa.
Similar multivariable approaches include the use of PSA isoforms such as the Prostate Health Index test which measures [−2]proPSA in addition to tPSA and fPSA 26 or the four‐kallikrein score test that includes the concentration of intact PSA and human kallikrein 2 besides tPSA and fPSA as well as patient age, DRE (nodules, no nodules), and prior negative biopsy (yes, no) 27. In addition, gene‐based methods were developed to improve PCa diagnosis. Most of these tests measure either PCA3 alone 28, in combination with TMPRSS2:ERG 29, 30, or gene expression measured directly from exosomes 30. More recently, a model was developed combining HOXC6 and DLX1 expression levels with known risk factors such as age, PSA, PSA density, family history of PCa and functions with or without DRE information 31; however, it is unknown how the new glycoprotein biomarkers presented in that study compare with other commercially available markers.
The main limitation of the present study is that all samples used in both the training set and the validation set were collected at a single clinical centre. Although the serum samples were collected prospectively, we retrospectively selected the samples from the biobank according to our specific inclusion criteria, which resulted in a highly selected patient group. Because of the inclusion of only biopsy‐positive patients who also underwent radical prostatectomy, there might be a bias towards patients harbouring PCa with a higher grade . In addition, the inclusion of patients with enlarged prostate volume was based on the volume obtained by TRUS and is not the current standard of care performed before the decision to biopsy is made; however, a prostate volume assessment based on DRE as discussed by Roobol et al. 32 would be sufficiently accurate as volume is not used in the mathematical model itself. The cut‐off of 35 mL for prostate volume was chosen because it has previously been shown that above a prostate volume of 38 mL, the proportion of cancers detected in men with a tPSA between 2 and 9 ng/mL was < 50% 3. In addition, a second study suggests that PSA density is a useful tool in the evaluation of patients with prostate volumes ≤35 mL 33 and both studies indicated the need for improved diagnosis above the chosen cut‐off.
In conclusion, the present study provides evidence that measurement of the two serum glycoproteins THBS1 and CTSD used in a multivariable model together with %fPSA is superior to %fPSA alone in predicting prostate biopsy outcome. While additional prospective multicentre studies are needed to support our findings, more than half of the negative prostate biopsies could have been avoided while delaying the diagnosis of only a few high grade cancers. Further prospective clinical validation is currently ongoing to confirm these results.
Acknowledgements
Funding for this work was provided by ProteoMediX.
Conflict of Interest
Some of the authors have received/held stock options (Silke Gillessen) and salaries (Annalisa Macagno, Alcibiade Athanasiou, Anja Wittig, Ramy Huber, Bruno Golding) and founder shares (Ralph Schiess) from ProteoMediX. Silke Gillessen and Thomas Steuber are advisors to ProteoMediX. Thomas Steuber, Annalisa Macagno, Alcibiade Athanasiou and Ralph Schiess are inventors of the patent application WO2018011212, and Silke Gillessen and Ralph Schiess have the patent application WO2009138392.
Supporting information
Fig. S1. Receiver‐operating characteristic curves of the multivariate logistic regression model including %fPSA, THBS1 and CTSD in randomly assigned training (black line) and validation (red line) sets for predicting biopsy outcome.
References
- 1. Lepor H. Pathophysiology, epidemiology, and natural history of benign prostatic hyperplasia. Rev Urol 2004; 6 (Suppl 9): S3–10 [PMC free article] [PubMed] [Google Scholar]
- 2. Hoffman RM. Clinical practice. Screening for prostate cancer. N Engl J Med 2011; 365: 2013–9 [DOI] [PubMed] [Google Scholar]
- 3. Al‐Azab R, Toi A, Lockwood G et al. Prostate volume is strongest predictor of cancer diagnosis at transrectal ultrasound‐guided prostate biopsy with prostate‐specific antigen values between 2.0 and 9.0 ng/mL. Urology 2007; 69: 103–7 [DOI] [PubMed] [Google Scholar]
- 4. Bangma CH, Rietbergen JB, Kranse R et al. The free‐to‐total prostate specific antigen ratio improves the specificity of prostate specific antigen in screening for prostate cancer in the general population. J Urol 1997; 157: 2191–6 [PubMed] [Google Scholar]
- 5. Trotman LC, Niki M, Dotan ZA et al. Pten dose dictates cancer progression in the prostate. PLoS Biol 2003; 1: E59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schiess R, Wollscheid B, Aebersold R. Targeted proteomic strategy for clinical biomarker discovery. Mol Oncol 2009; 3: 33–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cima I, Schiess R, Wild P et al. Cancer genetics‐guided discovery of serum biomarker signatures for diagnosis and prognosis of prostate cancer. Proc Natl Acad Sci USA 2011; 108: 3342–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kälin M, Cima I, Schiess R et al. Novel prognostic markers in the serum of patients with castration‐resistant prostate cancer derived from quantitative analysis of the pten conditional knockout mouse proteome. Eur Urol 2011; 60: 1235–43 [DOI] [PubMed] [Google Scholar]
- 9. Templeton A, Rothermundt C, Cathomas R et al. Everolimus as first‐line therapy in nonrapidly progressive metastatic castration‐resistant prostate cancer (mCRPC): a multicenter phase II trial (SAKK 08/08). J Clin Oncol 2011; 29: 1–3 21115866 [Google Scholar]
- 10. Rothermundt C, Hayoz S, Templeton AJ et al. Metformin in chemotherapy‐naive castration‐resistant prostate cancer: a multicenter phase 2 trial (SAKK 08/09). Eur Urol 2014; 66: 468–74 [DOI] [PubMed] [Google Scholar]
- 11. Endt K, Goepfert J, Omlin A et al. Development and clinical testing of individual immunoassays for the quantification of serum glycoproteins to diagnose prostate cancer. PLoS One 2017; 12: e0181557–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roberts DD, Cashel J, Guo N‐H. Purification of thrombospondin from human platelets. J Tissue Culture Method 1994; 16: 217–22 [Google Scholar]
- 13. DeLong ERE, DeLong DMD, Clarke‐Pearson DLD. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988; 44: 837–45 [PubMed] [Google Scholar]
- 14. Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making 2006; 26: 565–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. R Core Team . R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing, 2015. [Google Scholar]
- 16. Jemal A, Fedewa SA, Ma J et al. Prostate cancer incidence and PSA testing patterns in relation to USPSTF screening recommendations. JAMA 2015; 314: 2054–61 [DOI] [PubMed] [Google Scholar]
- 17. Sammon JD, Abdollah F, Choueiri TK et al. Prostate‐specific antigen screening after 2012 US preventive services task force recommendations. JAMA 2015; 314: 2077–9 [DOI] [PubMed] [Google Scholar]
- 18. Doll JA, Reiher FK, Crawford SE et al. Thrombospondin‐1, vascular endothelial growth factor and fibroblast growth factor‐2 are key functional regulators of angiogenesis in the prostate. Prostate 2001; 49: 293–305 [DOI] [PubMed] [Google Scholar]
- 19. Kwak C, Jin RJ, Lee C et al. Thrombospondin‐1, vascular endothelial growth factor expression and their relationship with p53 status in prostate cancer and benign prostatic hyperplasia. BJU Int 2002; 89: 303–9 [DOI] [PubMed] [Google Scholar]
- 20. Russo G, Mischi M, Scheepens W et al. Angiogenesis in prostate cancer: onset, progression and imaging. BJU Int 2012; 110: E794–808 [DOI] [PubMed] [Google Scholar]
- 21. Miyata Y, Sakai H. Thrombospondin‐1 in urological cancer: pathological role, clinical significance, and therapeutic prospects. IJMS 2013; 14: 12249–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vallbo C, Wang W, Damber J‐E. The expression of thrombospondin‐1 in benign prostatic hyperplasia and prostatic intraepithelial neoplasia is decreased in prostate cancer. BJU Int 2004; 93: 1339–43 [DOI] [PubMed] [Google Scholar]
- 23. Vetvicka V. Procathepsin D in cancer development. J Cancer Ther Res 2012; 1: 22 [Google Scholar]
- 24. Pepe M, Etzioni R, Feng Z et al. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst 2001; 93: 1054–61 [DOI] [PubMed] [Google Scholar]
- 25. Epstein JI, Feng Z, Trock BJ et al. Upgrading and downgrading of prostate cancer from biopsy to radical prostatectomy: incidence and predictive factors using the modified Gleason grading system and factoring in tertiary grades. Eur Urol 2012; 61: 1019–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lazzeri M, Haese A, Taille A de la et al. Serum Isoform [−2]proPSA Derivatives Significantly Improve Prediction of Prostate Cancer at Initial Biopsy in a Total PSA Range of 2–10 ng/ml: a Multicentric European Study. Eur Urol 2013; 63: 986–94 [DOI] [PubMed] [Google Scholar]
- 27. Parekh DJ, Punnen S, Sjoberg DD et al. A multi‐institutional prospective trial in the USA confirms that the 4Kscore accurately identifies men with high‐grade prostate cancer. Eur Urol 2014; 68: 464–70 [DOI] [PubMed] [Google Scholar]
- 28. Roobol MJ, Schröder FH, van Leeuwen P et al. Performance of the Prostate Cancer Antigen 3 (PCA3) Gene and Prostate‐Specific Antigen in Prescreened Men: exploring the Value of PCA3 for a First‐line Diagnostic Test. Eur Urol 2010; 58: 475–81 [DOI] [PubMed] [Google Scholar]
- 29. Tomlins SA, Aubin SMJ, Siddiqui J et al. Urine TMPRSS2:ERG fusion transcript stratifies prostate cancer risk in men with elevated serum PSA. Sci Transl Med 2011; 3: 94ra72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McKiernan J, Donovan MJ, O'Neill V et al. A novel urine exosome gene expression assay to predict high‐grade prostate cancer at initial biopsy. JAMA Oncol 2016; 2: 882 [DOI] [PubMed] [Google Scholar]
- 31. Van Neste L, Hendriks RJ, Dijkstra S et al. Detection of high‐grade prostate cancer using a urinary molecular biomarker‐based risk score. Eur Urol 2016; 70: 740–8 [DOI] [PubMed] [Google Scholar]
- 32. Roobol MJ, van Vugt HA, Loeb S et al. Prediction of prostate cancer risk: the role of prostate volume and digital rectal examination in the ERSPC risk calculators. Eur Urol 2012; 61: 577–83 [DOI] [PubMed] [Google Scholar]
- 33. Al‐Khalil S, Boothe D, Durdin T et al. Interactions between benign prostatic hyperplasia (BPH) and prostate cancer in large prostates: a retrospective data review. Int Urol Nephrol 2016; 48: 91–7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Receiver‐operating characteristic curves of the multivariate logistic regression model including %fPSA, THBS1 and CTSD in randomly assigned training (black line) and validation (red line) sets for predicting biopsy outcome.


