Summary
Essentials.
Performance of the one‐stage clotting (OSC) assay varies with the clotting activator used.
Recombinant FIX‐albumin fusion protein (rIX‐FP) was reliably monitored with most OSC reagents.
rIX‐FP shows comparable reagent‐dependent variability to other rFIX products in the OSC assay.
Actin® FS and kaolin‐based reagents underestimated rIX‐FP activity by around 50% in the OSC assay.
Summary
Background
Measuring factor IX activity (FIX:C) with one‐stage clotting (OSC) assays, based on the activated partial thromboplastin time (APTT), is the current mainstay of diagnostic techniques for hemophilia B. Assessing the performance of new recombinant FIX (rFIX) products in OSC assays is essential, as APTT reagents from different manufacturers yield different potency estimates for rFIX.
Objectives
To evaluate the extent to which choice of reagent composition influences rFIX potency measurements of recombinant FIX–albumin fusion protein (rIX‐FP, IDELVION) activity in OSC assays.
Methods
rIX‐FP was added to FIX‐deficient plasma, and FIX:C was assessed centrally and locally in a multicenter international field study with a variety of commercial OSC APTT reagents. Paired sample analysis of clinical samples was performed to compare values of FIX:C from local and central laboratories. In‐house bioanalytical investigations with spiked samples were conducted to compare the APTT‐reagent dependent variability of rIX‐FP with unmodified rFIX and rFIX Fc fusion protein (rFIXFc).
Results
Central and local assessments of FIX:C from 10 countries and 21 participating centers showed comparable results to those from the central laboratory across the majority of 18 different APTT reagents from both clinical and spiked samples. There was a consistent underestimation of rIX‐FP activity of ≈ 50% with OSC assays using Actin FS or kaolin‐based APTT reagents. In the bioanalytical study, rIX‐FP showed comparable variability in OSC assays to unmodified rFIX and rFIXFc.
Conclusions
rIX‐FP activity can be accurately measured by the use of OSC assays with the majority of commercial reagents. Actin FS or kaolin‐based reagents will probably lead to a 50% underestimation of activity.
Keywords: activated partial thromboplastin time, blood coagulation tests, factor IX, hemophilia B, multicenter study
Introduction
Hemophilia B, which is commonly referred to as factor IX deficiency, is a hereditary X‐linked bleeding disorder that leads to excessive internal bleeding into joints and muscles or as a result of minor wounds and trauma 1. Treatment of hemophilia B involves FIX replacement with plasma‐derived or recombinant FIX (rFIX) concentrates in either on‐demand or prophylactic regimens 1. Accurate measurement of FIX activity (FIX:C) levels in hemophilia B patients is needed for clinical monitoring, and is most commonly performed by the use of a one‐stage clotting (OSC) assay with commercially available pooled normal plasma standards calibrated against the World Health Organization (WHO) FIX plasma International Standard 2. The OSC assay is the established European Pharmacopeia method, and consequently has been used to assign potency labeling for new rFIX products 3.
Despite the widespread use of OSC assays in clinical settings, interlaboratory and intralaboratory variations in FIX:C have been reported with this method 4, 5, 6, 7. There are a variety of commercial reagent options available that contain different forms of clotting activators and sources of phospholipid that may contribute to the aforementioned assay variability. For unmodified rFIX products, the results of OSC assays are known to be influenced by the type of activated partial thromboplastin time (APTT) reagent used for potency assignment by the manufacturer 8. Substantial variability in the performance of OSC assays has also been reported with rFIX products modified with Fc fusion (rFIX Fc fusion protein [rFIXFc]) or glycoPEGylation to extend the half‐life 5, 6, 7. Consequently, the ISTH recommends that the potency of new rFIX products be assessed in OSC assays with different APTT reagents, as potency estimates may be highly dependent on the reagent 9. Therefore, for accurate postinfusion monitoring, it is preferable to use an assay that gives similar results to that used to assign potency of the FIX product 3, 10.
rIX‐FP (IDELVION) is a fusion protein linking human rFIX with recombinant human albumin designed with an improved pharmacokinetic profile as compared with conventional rFIX 11. In the PROLONG‐9FP clinical trial program, prophylaxis with rIX‐FP demonstrated hemostatic efficacy with dosing intervals of up to 14 days in adults and 7 days in pediatric patients, with a median annualized spontaneous bleeding rate of 0 with all regimens 12, 13. The potency assignment of rIX‐FP was based on an OSC assay.
During rIX‐FP clinical studies, a reagent‐dependent FIX:C determination in OSC assays was observed. Here, we report the findings of a multicenter international field study conducted to assess the variability between different OSC assay reagents for measuring FIX:C of rIX‐FP. In addition, parallel OSC assay FIX:C data from local and central laboratories collected in the rIX‐FP clinical studies are presented. Third, in‐house bioanalytical investigations with spiked samples were conducted to compare OSC assay variability with two other licensed rFIX concentrates.
Materials and methods
To understand the variation in FIX:C of rIX‐FP attributable to the different APTT reagents used in OSC assays, an investigation was initiated to evaluate the consistency in FIX:C measurements with a variety of commercial reagents.
Assessment of variability in the OSC assay
FIX:C of rIX‐FP concentrate was determined with the standard central laboratory assay reagent Pathromtin SL and eight additional APTT reagents, containing a variety of different surface activator compounds: HemosIL APTT‐SP, PTT reagent (kaolin), Actin, Actin FS, Actin FSL, PTT [a], STA‐aPTT and STA‐Cephascreen (as detailed in Table S1). The tests were performed on a Behring Coagulation System (BCS) analyzer (Siemens Healthcare, Erlangen, Germany). For all reagents, the same test parameters were used, which is comparable to the routine test setting for the determination of FIX on the BCS. Each assay sample contained 15 μL of buffer, 5 μL of sample (rIX‐FP concentrate), 50 μL of FIX‐deficient plasma (FIX‐DPL), and 75 μL of APTT reagent, and was incubated for 120 s before the addition of 75 μL of CaCl2 solution. No further modifications to the test settings (e.g. incubation time) were performed to optimize the behavior of the different APTT reagents in this preliminary investigation. FIX:C of rIX‐FP was estimated by comparison with the International Standard FIX Concentrate (WHO 07/182). This reference standard and the rIX‐FP concentrate sample were each diluted to three different concentrations within the working range of the assay (0.2–1 IU mL−1). Linear regression analysis was applied to the results, and FIX:C of rIX‐FP was calculated by line comparison against the reference standard (WHO 07/182).
Multicenter international field study (locally prepared and spiked samples)
Participating centers in a phase I/II study (NCT01233440) and a phase II/III study (NCT01496274) of previously treated hemophilia B patients (FIX:C of ≤ 2%) aged ≥ 12 years and additional external laboratories were provided with either lyophilized rIX‐FP reference plasma or spiked samples, both of which were spiked in a central laboratory. Lyophilized rIX‐FP reference plasma samples were reconstituted and diluted at the participating centers to yield rIX‐FP concentrations of 0.9, 0.5 and 0.25 IU mL−1 (locally prepared spiked samples). Centrally prepared samples were spiked at concentrations of 0.625, 0.25 and 0.1 IU mL−1 rIX‐FP, and provided to participating centers. This approach provided a broad range of concentrations for testing. Centers were blinded to the rIX‐FP concentration of samples. All centers were instructed to assay samples with the instrumentation, reagents and local OSC assay that had been validated in their clinical laboratory for clinical evaluation of patients. A validated OSC assay was performed with Pathromtin SL (Siemens Healthcare Diagnostics, Marburg, Germany) as an activator, and the BCS was used to determine rIX‐FP activity in the central laboratory. The measured values were interpreted by use of a reference curve prepared from standard human plasma (Siemens Healthineers) calibrated by the manufacturer against the WHO standard for FIX. FIX:C levels were considered to be comparable if they fell within ± 30% of the spiked concentration.
Analysis of paired clinical samples
In addition to the field study, centers participating in the phase II/III rIX‐FP clinical study (NCT01496274) and a phase III clinical study (NCT01662531) on previously treated pediatric hemophilia B patients (FIX ≤ 2%) aged < 12 years collected paired patient samples for analyses in both their local laboratory and the central laboratory. Sites that obtained a subject sample and measured FIX:C, by using an OSC assay with reagents and instruments validated for clinical monitoring and used routinely in their local laboratory, were instructed to send a paired sample to the central laboratory for analysis. Paired samples were considered to be comparable if the measured value in the local laboratory was within 30% of the measured value in the central laboratory.
In‐house bioanalytical investigation of spiked samples of rFIX‐FP in OSC assays by the use of a normal human plasma (NHP) standard diluted in FIX‐deficient plasma and comparison with other rFIX concentrates
rIX‐FP, rFIXFc (Alprolix) and rFIX (BeneFIX) were spiked into FIX‐DPL obtained by immunodepletion at nominal concentrations of 1.0, 0.6, 0.3 and 0.05 IU mL−1, based on the label potency for each product. Fourteen different APTT reagents that are commercially available and frequently used in clinical laboratories were investigated in this study (for details, see Table S1). Clotting time was determined on the BCS XP, with the generic test setting FIX.PSL.d (Pathromtin SL) and with reagent‐specific adjusted incubation times, or, if available, the test setting recommended by the reagent manufacturer. More specifically, the settings for each reagent were defined on the basis of the following strategy. The four settings explicitly recommended by Siemens Healthcare Diagnostics for their reagents, which represent a variety of APTT reagents, as they consist of different phospholipids and varying surface‐active components, display the identical pipetting scheme but different incubation times. Hence, this pipetting scheme was also applied to the other investigated APTT reagents with modified incubation times as recommended by each respective manufacturer. Moreover, the CaCl2 concentration was adapted as recommended by the respective manufacturer. Two exceptions from this general strategy with respect to the setting were the APTT reagents Cephen and CK Prest. For Cephen, the manufacturer provides an explicit setting for the BCS XP instrument, which was therefore applied. Measurements conducted with CK Prest must be performed with an instrument that operates on a mechanical rather than an optical measurement principle. Consequently, data for this reagent were collected on a Schnitger/Gross coagulometer according to the recommendation of the reagent manufacturer.
The clotting time was evaluated against an NHP standard (Siemens Healthcare Diagnostics; lot 503252), which consists of a pool of citrated platelet‐poor plasma samples collected from at least 20 healthy individuals, and diluted in FIX‐DPL. Six independent measurements were conducted for each spike concentration. As FIX activities of spiked samples were evaluated on the basis of a calibration with NHP, results are calculated as percentages of NHP. To facilitate comparisons, the data were divided by the nominal (label) value of the spike concentration to yield percentage recovery. Although there is no clear agreement in the literature as to what constitutes acceptable recovery in the clinical setting, a target range of ± 25% from the optimum has been proposed 10, 14, 15. However, differences of up to 40% are observed with licensed rFIX products 3. In the presented analyses, accuracy thresholds of ± 25% and ±35% are thus highlighted (Fig. 4).
Figure 4.
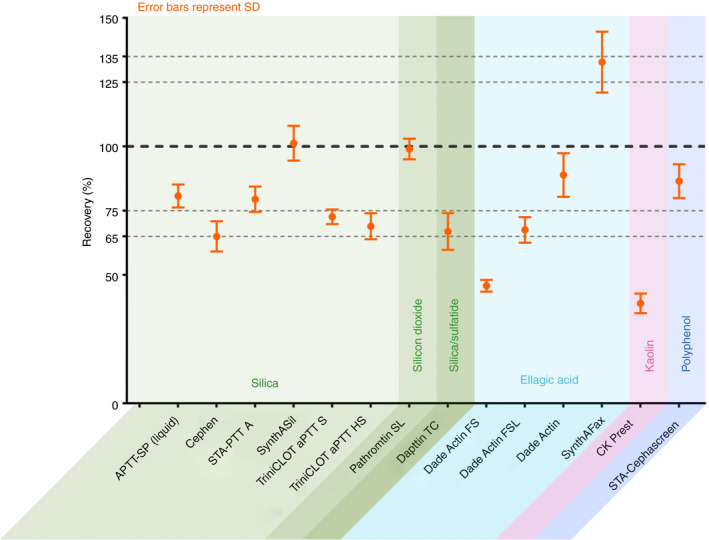
Recombinant FIX–albumin fusion protein (rIX‐FP) activity in the one‐stage clotting assay with 14 different activated partial thromboplastin time (APTT) reagents: mean ± standard deviation (SD) percentage recovery for rIX‐FP across the range of spiked concentrations (1.0, 0.6, 0.3 and 0.05 IU mL −1) tested. The SDs thus represent the dilution linearity obtained with each APTT reagent. The black dashed line indicates the expected value of 100% of the nominal, and the gray dashed lines indicate the ± 25% and ± 35% interval around this expectation.
Results
Variability in OSC assays by APTT reagent
When samples of rIX‐FP concentrate were analyzed with the WHO International Standard for FIX, with the standard central laboratory assay reagent Pathromtin SL and eight additional APTT reagents, APTT reagent‐dependent variability in rIX‐FP activity was identified. As compared with the standard central laboratory assay reagent Pathromtin SL, Actin FS and PTT reagent (kaolin) led to an underestimation of the mean rIX‐FP activity by up to 50% when a plasma‐derived standard was used for calibration (Fig. 1).
Figure 1.
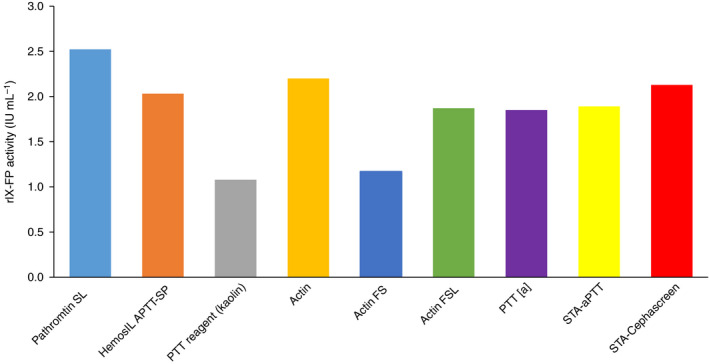
Mean FIX activity of recombinant FIX–albumin fusion protein (rIX‐FP) concentrate when measured against the World Health Organization (WHO) International Standard concentrate with different activated partial thromboplastin time (APTT) reagents. FIX activity of rIX‐FP concentrate was measured in the one‐stage clotting assay against the WHO International Standard FIX concentrate with eight different APTT reagents and the central laboratory assay reagent Pathromtin SL.
Multicenter field study
The rIX‐FP activity of locally prepared spiked samples was measured with the OSC assay in five centers in four countries, with four different APTT reagents, i.e. Actin FS, Platelin‐LS, aPTT‐SP, and Pathromtin SL, and in the central laboratory (Pathromtin SL), to support clinical study activities. FIX:C determination showed overall comparable (within ± 30% of target) results to those of the central laboratory across the range of concentrations measured in centers using aPTT‐SP and Pathromtin SL; however, rIX‐FP activity was underestimated by ≈ 50% across the range of concentrations measured in those laboratories with Actin FS as the activator as compared with the central laboratory (Fig. 2A). Platelin‐LS also showed an underestimation of > 30% at spiked concentrations of 0.5 IU mL−1 and 0.25 IU mL−1.
Figure 2.
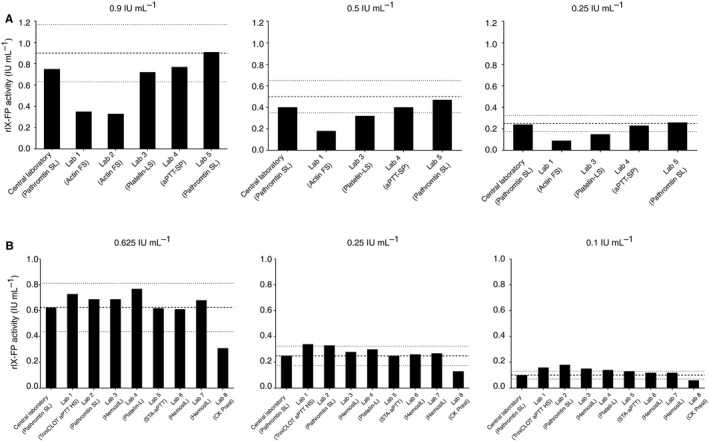
Individual laboratory one‐stage clotting assay results for recombinant FIX–albumin fusion protein (rIX‐FP). (A) Use of locally prepared spiked plasma samples from lyophilized rIX‐FP by further dilution at rIX‐FP‐spiked concentrations of 0.9, 0.5 and 0.25 IU mL −1. (B) Use of centrally prepared spiked plasma samples at rIX‐FP‐spiked concentrations of 0.625, 0.25 and 0.1 IU mL −1. Target spikes are shown by dashed lines, and ± 30% thresholds by dotted lines.
The rIX‐FP activity of centrally prepared spiked samples was measured in eight centers in five countries, with six different commercial APTT reagents, i.e. TriniCLOT aPTT HS, Platelin‐L, HemosIL, STA‐aPTT, CK Prest (kaolin), and Pathromtin SL, and in the central laboratory (Pathromtin SL). Results were comparable (within ± 30% of target) across laboratories at concentrations of 0.625 IU mL−1 and 0.25 IU mL−1, and with the central laboratory. The one exception was the laboratory using a kaolin‐based reagent (CK Prest) as the activator, which showed ≈ 50% underestimation of rIX‐FP activity as compared with the central laboratory. At the lowest concentration (0.1 IU mL−1), variability was greater, with a number of laboratories showing overestimation of ≥ 30%. However, the laboratory using the kaolin‐based reagent CK Prest was the only laboratory to show underestimation of ≥ 30% at a spike of 0.1 IU mL−1 (Fig. 2B).
Paired sample analysis
Twenty‐one study centers in 10 countries provided paired samples measured with seven different APTT reagents, including the central laboratory reagent Pathromtin SL. In line with the central laboratory assessment and field study data, the comparison of local and central laboratory results identified variability (more than ± 30% of the value measured in the central laboratory) in local laboratories using Actin FS as the APTT reagent (Fig. 3), with the majority of samples showing underestimation. The kaolin‐based reagent (NPF Renam) also showed underestimation of rIX‐FP in some samples. All other APTT reagents used generated comparable results between the central and local laboratories for the majority of the paired samples. In addition to the APTT reagents used, the different methods and instruments used locally would have contributed to the variability observed.
Figure 3.
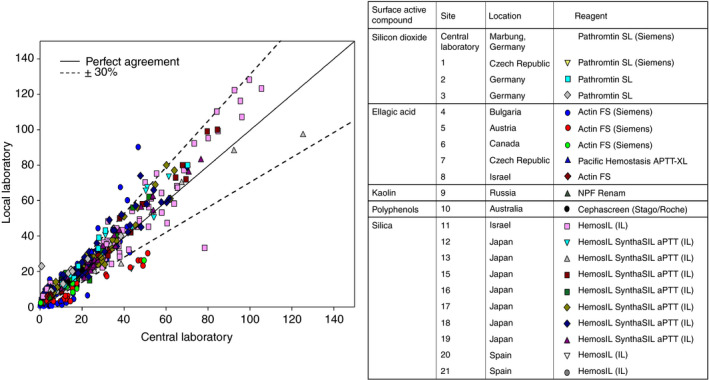
Analysis of adult and pediatric paired samples of recombinant FIX–albumin fusion protein (rIX‐FP) with the one‐stage clotting assay between a central laboratory and local laboratories. [Color figure can be viewed at wileyonlinelibrary.com]
In‐house bioanalytical investigation of spiked samples of rIX‐FP in OSC assays by the use of an NHP standard diluted in FIX‐deficient plasma and comparison with other rFIX concentrates
Fourteen different commercially available APTT reagents with six different reagent classes distinguished by the contained surface‐active compounds, i.e. ellagic acid (Dade Actin FS, Dade Actin FSL, Dade Actin, and SynthAFax), kaolin (CK Prest), silica (aPTT‐SP [liquid], Cephen, STA‐PTT A, SynthASil, TriniCLOT aPTT HS, and TriniCLOT aPTT S), silicon dioxide particles (Pathromtin SL), silica and sulfatide (Dapttin TC), and a polyphenolic activator (STA‐Cephascreen), were evaluated with rIX‐FP. rIX‐FP activity results are presented as percentage recovery (percentage of NHP divided by the nominal [label] value of the spike concentration). Across the range of spiked concentrations (1.0–0.05 IU mL−1) tested, differences were observed between the APTT reagents used (Fig. 4). In line with previous findings, Actin FS and CK Prest (kaolin) were confirmed to generate underestimated rIX‐FP activity estimates, in this case by > 50%. As stated on the product label, using Actin FS and kaolin‐based APTT reagents in an OSC assay will probably result in underestimation of rIX‐FP activity 16; therefore, these APTT reagents were excluded from all statistical evaluation. The mean percentage recovery was within ± 35% for the remaining 12 APTT reagents tested, and within ± 25% for six APTT reagents tested, with respect to the expected value (Fig. 4).
The spike recoveries ranged from 65.0% with Cephen to 132.8% with SynthAFax. The mean (percentage coefficient of variation [%CV]) percentage spike recovery with rIX‐FP for the 12 APTT reagents was 84.1 (23.4%), across the range of spiked concentrations (Fig. 5). When the same 12 APTT reagents were used in OSC assays with rFIXFc and rFIX, variability in FIX:C activity measurements was comparable with that with rIX‐FP, with mean (%CV) percentage spike recovery of 110.9 (27.0%) for rFIXFc and 117.5 (22.0%) for rFIX (Fig. 5). APTT reagents underestimating and overestimating FIX:C were identified for all three FIX concentrates (Fig. 5). Depending on the investigated FIX product, specific but different APTT reagents resulted in falsely high or falsely low measured activity. Particularly interesting are the overall results obtained with SynthAFax, which overestimated the activity of rIX‐FP (132.8%) while yielding a particularly low recovery of 58.1% for rFIX. This highlights the fact that the choice of a particular APTT reagent might have opposite effects on the accuracy of results obtained for different rFIX concentrates, and thus that a strong and specific reagent–product interaction exists. For rFIXFc, spike recovery ranged from 74.7% with TriniCLOT aPTT HS to 174.6% with Dapttin TC, and for rFIX it ranged from 58.1% with SynthAFax to 170.2% with STA PTT A. rFIXFc and rFIX tended to show overestimation, whereas rIX‐FP showed underestimation, relative to the nominal (label) value.
Figure 5.
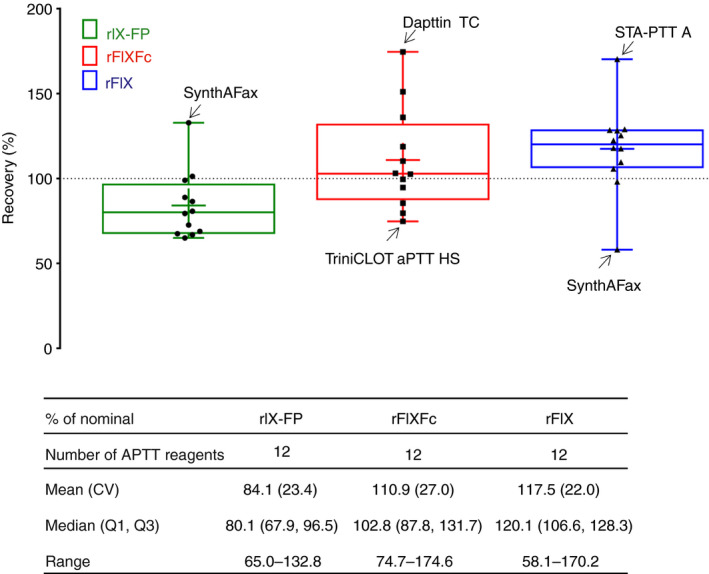
FIX activity in the one‐stage clotting assay by FIX concentrate: percentage of nominal (label) FIX activity across the range of spiked concentrations (1.0, 0.6, 0.3 and 0.05 IU mL −1) measured with 12 different activated partial thromboplastin time (APTT) reagents in the one‐stage clotting assay for recombinant FIX–albumin fusion protein (rIX‐FP), recombinant FIX Fc fusion protein (rFIXFc), and recombinant FIX (rFIX). APTT reagents showing the greatest variability are indicated. In each box, the middle horizontal line represents the median, and the lower and upper borders of the box represent the first and third quartiles. The whiskers indicate the minimum and maximum, and the ‘+’ sign indicates the arithmetic mean. CV, coefficient of variation.
Even at low plasma concentrations (0.05 IU mL−1), when the accuracy of FIX:C measurements is of particular importance, FIX:C was accurately measured for rIX‐FP in OSC assays with the majority of APTT reagents (Fig. 6). The mean FIX:C values were 4.4% NHP for rIX‐FP, 6.3% NHP for rFIXFc and 6.4% NHP for rFIX across the 12 suitable reagents. These data demonstrate accurate measurement of rIX‐FP at low concentrations, e.g. a recovery of 88% of the expected value. APTT reagents leading to underestimation and overestimation of FIX:C in the OSC assay were also identified for rFIXFc and rFIX, when spiked at a concentration of 0.05 IU mL−1 (Fig. 6). These findings were consistent with the findings across all spiked concentrations, with the same APTT reagents showing the greatest variability (Fig. 5). Maximal overestimations of FIX:C levels in percentage NHP (percentage recovery) determined were 7.0% NHP (140%) for rIX‐FP, 12.2% NHP (244%) for rFIXFc, and 9.8% NHP (196%) for rFIX. Maximal underestimations of FIX:C levels in percentage NHP (percentage recovery) determined were 3.2% NHP (64%) for rIX‐FP, 3.8% NHP (76%) for rFIXFc, and 2.3% NHP (46%) for rFIX.
Figure 6.
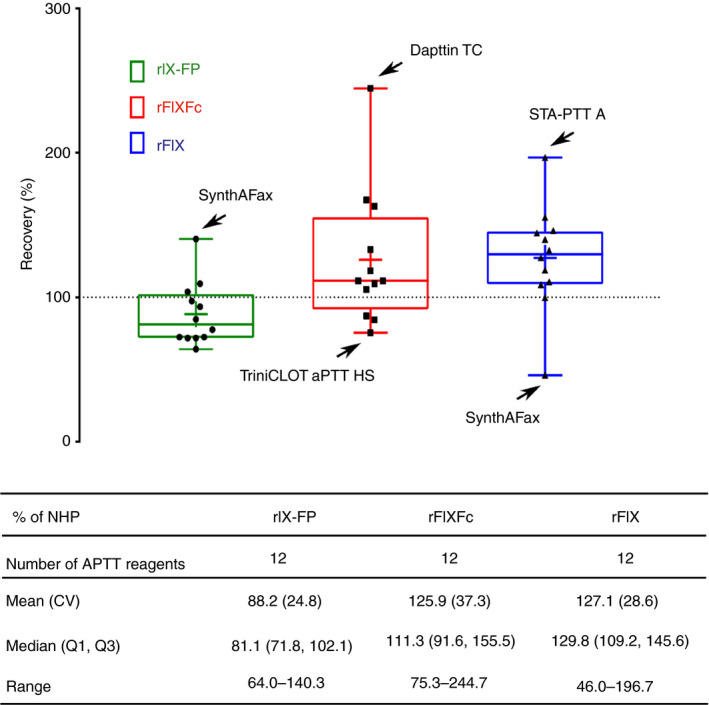
FIX activity in the one‐stage clotting assay by FIX concentrate at a FIX concentration of 0.05 IU mL −1: percentage of normal human plasma (NHP) FIX activity at a spiked concentration of 0.05 IU mL −1 measured with 12 different activated partial thromboplastin time (APTT) reagents in the one‐stage clotting assay for recombinant FIX–albumin fusion protein (rIX‐FP), recombinant FIX Fc fusion protein (rFIXFc), and recombinant FIX (rFIX). APTT reagents showing the greatest variability are indicated. Suboptimal accuracies were observed for TriniCLOT aPTT S, STA PTT and Dapttin TC for all products (data not shown). In each box, the middle horizontal line represents the median, and the lower and upper borders of the box represent the first and third quartiles. The whiskers indicate the minimum and maximum, and the ‘+’ sign indicates the arithmetic mean. CV, coefficient of variation.
Discussion
The objective of the studies presented here was to establish the extent to which different clinical hemostasis laboratories are able to accurately and reliably monitor FIX:C levels in hemophilia B patients treated with rIX‐FP by using OSC assays. During the clinical development of rIX‐FP, reagent‐dependent activity determination was observed in OSC assays when calibration against a plasma‐derived standard was performed (data not shown). An assessment of variability with eight different APTT reagents showed that Actin FS and a kaolin‐based APTT reagent both underestimated rIX‐FP activity by ≈ 50%. To further investigate these findings, a multicenter international field study was undertaken in 13 laboratories in seven countries. With the use of spiked samples at a variety of concentrations, the majority of the nine commercially available APTT reagents tested reliably measured rIX‐FP activity in the field study. This finding was further supported by an analysis of paired samples obtained from 21 centers participating in the PROLONG‐9FP phase III studies, for which OSC assay results were largely comparable between central and local laboratories.
The findings of an in‐house bioanalytical investigation into APTT reagent‐dependent variability in OSC assays using spiked samples with 14 different APTT reagents were consistent with the findings in clinical samples. The majority of APTT reagents resulted in a recovery that was within 35% of that expected for rIX‐FP, with Actin FS and CK Prest (kaolin) falling outside this range. There is some disagreement in the literature as to what constitutes acceptable recovery, with a target of ± 25% having been proposed 14; however, differences of up to 40% are observed with licensed rFIX products 3. Here, we observed a similar pattern of variability at low concentrations (0.05 IU mL−1) as across the entire range of concentrations tested (1.0–0.05 IU mL−1).
The measured activity of spiked samples of rIX‐FP in OSC assays using an NHP standard averaged 84.1% of the nominal activity (%CV of 23.4%) across the range of spiked concentrations and the APTT reagents assessed, not including Actin FS and CK Prest (see the section on in‐house bioanalytical investigation above). The underestimation observed when some APTT reagents were used may be attributable to their chemical nature in this artificial assay system. APTT reagents consist of a surface‐active compound and a mixture of lipids. The type of surface‐active compound probably impacts on the rate of FXIa formation in the OSC assay, which, in turn, determines the activation rate of FIX. In case of rIX‐FP, the activation requires cleavage of the FIX activation peptide within the FIX molecule, and cleavage of the linker connecting FIX with recombinant albumin 17. As FIX:C is measured over a short timeframe in OSC assays, small differences in activation may be sufficient to result in a relevant offset if measurement is performed against an unmodified FIX standard, and finally result in an apparently lower assigned potency of rIX‐FP. Also, the variability of lipids used in APTT reagents may potentially impact on the catalytic efficiency of the tenase complex. In this study, it has been also shown that the type of diluent used for dilution of the NHP standard, i.e. FIX‐DPL, was key to obtaining the most precise measurements over the range of spiked concentrations. It is of note that these effects appear to be purely laboratory phenomena, as clinical studies have demonstrated the efficacy of rIX‐FP 13. An alternative explanation is that the underestimation or overestimation may be attributable to variation in the filling of vials, as nominal values were based on reported IU within commercial vials. For rIX‐FP, the FIX:C value obtained with Pathromtin SL, the APTT reagent used in the OSC standard assay of the central laboratory and, most importantly, also used for potency assignment of rIX‐FP, was very close to the expected value (99% NHP), ruling out such an explanation.
The spectrum of APTT reagents assessed in this study contained a variety of activators, such as ellagic acid, silica, and kaolin, as well as variable phospholipid sources (synthetic, purified soy phosphatides, and rabbit brain cephalin extract). These constituents may also explain, to some extent, the discrepancies observed between assays. Overall, the findings presented in this study reinforce the suggestion that certain APTT reagents utilized in OSC assays lead to discrepancies in the assessments of FIX:C in samples containing FIX‐DPL and various rFIX products 5, 6, 7, 18, 19.
An assessment of OSC assay FIX:C activity in spiked samples with the same set of APTT reagents for rFIXFc and rFIX showed that the extent of APTT‐reagent dependent variability was comparable between FIX concentrates, with each product showing underestimation and overestimation with different APTT reagents. Given the known reagent‐dependent variability in OSC assays with rFIX 4, 6, 7, 8, the discrepancies observed in this study were not unexpected. A field study evaluating OSC assay variability for rFIXFc, with rFIX (BeneFIX) as a comparator, found that ellagic‐acid based APTT reagents tend to overestimate rFIXFc activity; however, variability with ellagic acid‐based and silica‐based reagents was considered to be within acceptable levels (± 30% of expected). Consistent with the findings reported here, kaolin‐based reagents underestimated rFIXFc activity by up to 50% 6. Therefore, the use of kaolin‐based reagents in OSC assays is not optimal for monitoring both rIX‐FP and rFIXFc. Sommer et al. also found that rFIX concentrations were consistently overestimated 6, similarly to the findings reported here.
GlycoPEGylated rFIX (N9‐GP) activity in OSC assays has been shown to be dramatically overestimated (five‐fold) with most silica‐containing APTT reagents, because of an assay artefact facilitated by a combination of silica and the conjugated PEG 5, 7, indicating that, to avoid underdosing, these reagents should not be used for monitoring N9‐GP activity. However, a small number of specific APTT reagents, e.g. SynthAFax, DG Synth, and STA‐Cephascreen, which show acceptable recovery, have been identified for monitoring N9‐GP 5, 7, as have suitable reagent instrument systems 20.
A recent assessment of assay variability with rFIX products reported that, for unmodified rFIX, interlaboratory variability was reduced from > 20% to < 2% when FIX:C was measured against a National Institute for Biological Standards and Control (NIBSC) recombinant reference standard as compared with the plasma‐derived International Standard, for common APTT reagents. However, for the extended half‐life rFIX products, there was variability between laboratories that was not improved by calibration against the NIBSC rFIX standard 21. This study highlights the importance of demonstrating the degree of assay variability for a given rFIX product, the complexities involved in laboratory assessment, and the need to provide guidance to clinical laboratories to ensure optimal monitoring of FIX:C activity in their patients.
Here, we have shown that rIX‐FP activity can be monitored with a wide variety of APTT reagents and with a plasma standard, indicating that there is no clinical need for the development of a product‐specific standard. However, it should be noted that not all APTT reagents that are available for use in OSC assays were tested in the studies reported here. Overestimation or underestimation of rIX‐FP activity with those APTT reagents not included in the study can therefore not be ruled out. Additionally, lot‐to‐lot variation in APTT reagents may account for additional variability in FIX:C activity in OSC assays, as such variations (e.g. in the phospholipid component of the APTT reagent) may alter the clotting time measured for a recombinant FIX sample differently from the clotting time measured for the FIX standard, which is derived from plasma and thus shows different biochemical properties. These potential effects have not been investigated in this study. Recent results have suggested that certain patient‐specific factors, such as genetic mutations of the FIX molecule, can also influence assay discrepancies 22. The current study did not account for these aspects, and therefore cannot rule out their contribution to the results reported. A further limitation of this field study is that it did not assess the chromogenic substrate assay for monitoring rIX‐FP 2; therefore, the findings of this study are restricted to those from OSC assays.
In conclusion, following infusion of rIX‐FP, plasma FIX:C can be accurately and reliably monitored by the use of OSC assays with the majority of commercially available APTT reagents, and with current plasma standards. However, the use of a kaolin‐based APTT reagent or Actin FS will probably result in an underestimation of the FIX:C levels.
Addendum
C. Horn, C. Négrier, U. Kalina, W. Seifert, and K. D. Friedman contributed to the design of the study, and analysis and interpretation of data, drafted the manuscript, and reviewed and approved the final version. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure of Conflict of Interests
C. Horn, U. Kalina and W. Seifert are employees of CSL Behring. C. Négrier has received personal fees from Alnylam, Bayer, and Roche, and grant/research support and personal fees from CSL Behring, LFB, Novo Nordisk, Octapharma, Pfizer, Shire, and Sobi. K. D. Friedman has served as a consultant to Shire, Novo Nordisk, Bioverativ, Bayer, and Genentech, and as a speaker and consultant for CSL Behring.
Supporting information
Table S1. APTT reagents used for determination of OS clotting FIX activities with rIX‐FP by study.
Acknowledgements
The authors would like to thank the PROLONG‐9FP study investigators for providing paired samples for analysis and contributing to the field study, and also the additional external laboratories who performed the OSC assay on spiked samples for the field study. The authors also thank K. Holliday of Meridian HealthComms, Plumley, UK for providing medical writing support, which was funded by CSL Behring GmbH, Marburg, Germany in accordance with Good Publication Practice (GPP3).
Horn C, Négrier C, Kalina U, Seifert W, Friedman KD. Performance of a recombinant fusion protein linking coagulation factor IX with recombinant albumin in one‐stage clotting assays. J Thromb Haemost 2019; 17: 138–48.
Manuscript handled by: P. Toulon
Final decision: F. R. Rosendaal, 15 October 2018
References
- 1. Peyvandi F, Garagiola I, Young G. The past and future of haemophilia: diagnosis, treatments, and its complications. Lancet 2016; 388: 187–97. [DOI] [PubMed] [Google Scholar]
- 2. Kitchen S, Signer‐Romero K, Key NS. Current laboratory practices in the diagnosis and management of haemophilia: a global assessment. Haemophilia 2015; 21: 550–7. [DOI] [PubMed] [Google Scholar]
- 3. Dodt J, Hubbard AR, Wicks SJ, Gray E, Neugebauer B, Charton E, Silvester G. Potency determination of factor VIII and factor IX for new product labelling and postinfusion testing: challenges for caregivers and regulators. Haemophilia 2015; 21: 543–9. [DOI] [PubMed] [Google Scholar]
- 4. Sørensen MH, Andersen S, Ezban M. Factor IX‐deficient plasma spiked with N9‐GP behaves similarly to N9‐GP post‐administration clinical samples in N9‐GP ELISA and FIX activity assays. Haemophilia 2015; 21: 832–6. [DOI] [PubMed] [Google Scholar]
- 5. Bowyer AE, Hillarp A, Ezban M, Persson P, Kitchen S. Measuring factor IX activity of nonacog beta pegol with commercially available one‐stage clotting and chromogenic assay kits: a two‐center study. J Thromb Haemost 2016; 14: 1428–35. [DOI] [PubMed] [Google Scholar]
- 6. Sommer JM, Buyue Y, Bardan S, Peters RT, Jiang H, Kamphaus GD, Gray E, Pierce GF. Comparative field study: impact of laboratory assay variability on the assessment of recombinant factor IX Fc fusion protein (rFIXFc) activity. Thromb Haemost 2014; 112: 932–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rosen P, Rosen S, Ezban M, Persson E. Overestimation of N‐glycoPEGylated factor IX activity in a one‐stage factor IX clotting assay owing to silica‐mediated premature conversion to activated factor IX. J Thromb Haemost 2016; 14: 1420–7. [DOI] [PubMed] [Google Scholar]
- 8. Kitchen S, Gray E, Mertens K. Monitoring of modified factor VIII and IX products. Haemophilia 2014; 20(Suppl. 4): 36–42. [DOI] [PubMed] [Google Scholar]
- 9. Hubbard AR, Dodt J, Lee T, Mertens K, Seitz R, Srivastava A, Weinstein M. Recommendations on the potency labelling of factor VIII and factor IX concentrates. J Thromb Haemost 2013; 11: 988–9. [DOI] [PubMed] [Google Scholar]
- 10. Kitchen S, Kershaw G, Tiefenbacher S. Recombinant to modified factor VIII and factor IX – chromogenic and one‐stage assays issues. Haemophilia 2016; 22(Suppl. 5): 72–7. [DOI] [PubMed] [Google Scholar]
- 11. Santagostino E, Negrier C, Klamroth R, Tiede A, Pabinger‐Fasching I, Voigt C, Jacobs I, Morfini M. Safety and pharmacokinetics of a novel recombinant fusion protein linking coagulation factor IX with albumin (rIX‐FP) in hemophilia B patients. Blood 2012; 120: 2405–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Santagostino E, Martinowitz U, Lissitchkov T, Pan‐Petesch B, Hanabusa H, Oldenburg J, Boggio L, Negrier C, Pabinger I, von Depka Prondzinski M, Altisent C, Castaman G, Yamamoto K, Alvarez‐Roman MT, Voigt C, Blackman N, Jacobs I. Long‐acting recombinant coagulation factor IX albumin fusion protein (rIX‐FP) in hemophilia B: results of a phase 3 trial. Blood 2016; 127: 1761–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kenet G, Chambost H, Male C, Lambert T, Halimeh S, Chernova T, Mancuso ME, Curtin J, Voigt C, Li Y, Jacobs I, Santagostino E. Long‐acting recombinant fusion protein linking coagulation factor IX with albumin (rIX‐FP) in children. Results of a phase 3 trial. Thromb Haemost 2016; 116: 659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kitchen S, Tiefenbacher S, Gosselin R. Factor activity assays for monitoring extended half‐life FVIII and factor IX replacement therapies. Semin Thromb Hemost 2017; 43: 331–7. [DOI] [PubMed] [Google Scholar]
- 15. Peyvandi F, Oldenburg J, Friedman KD. A critical appraisal of one‐stage and chromogenic assays of factor VIII activity. J Thromb Haemost 2016; 14: 248–61. [DOI] [PubMed] [Google Scholar]
- 16. CSL Behring . IDELVION Summary of Product Characteristics. 9 February 2018. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003955/WC500207380.pdf. Accessed 12 June 2018.
- 17. Schulte S. Half‐life extension through albumin fusion technologies. Thromb Res 2009; 124(Suppl. 2): S6–8. [DOI] [PubMed] [Google Scholar]
- 18. Toulon P, Eloit Y, Smahi M, Sigaud C, Jambou D, Fischer F, Appert‐Flory A. In vitro sensitivity of different activated partial thromboplastin time reagents to mild clotting factor deficiencies. Int J Lab Hematol 2016; 38: 389–96. [DOI] [PubMed] [Google Scholar]
- 19. Pouplard C, Trossaert M, Le Querrec A, Delahousse B, Giraudeau B, Gruel Y. Influence of source of phospholipids for APTT‐based factor IX assays and potential consequences for the diagnosis of mild haemophilia B. Haemophilia 2009; 15: 365–8. [DOI] [PubMed] [Google Scholar]
- 20. Tiefenbacher S, Bohra R, Amiral J, Bowyer A, Kitchen S, Lochu A, Rosen S, Ezban M. Qualification of a select one‐stage activated partial thromboplastin time‐based clotting assay and two chromogenic assays for the post‐administration monitoring of nonacog beta pegol. J Thromb Haemost 2017; 15: 1901–12. [DOI] [PubMed] [Google Scholar]
- 21. Gray E, Wilmot HV, Hogwood J, Dougall T, Rigsby P. Assay discrepancies for new generation FIX products. Haemophilia 2016; 22(Suppl. 4): 70. [Google Scholar]
- 22. Kihlberg K, Strandberg K, Rosen S, Ljung R, Astermark J. Discrepancies between the one‐stage clotting assay and the chromogenic assay in haemophilia B. Haemophilia 2017; 23: 620–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. APTT reagents used for determination of OS clotting FIX activities with rIX‐FP by study.


