Abstract
Background and objective
Topical aminophylline, caffeine, yohimbe, l‐carnitine, and gotu kola (Centella asiatica) may aid in reducing body fat. Lipoxyderm™ contains these ingredients and was used to test if fat loss of the thigh, in conjunction with a low intensity exercise program and restricted calorie intake, was enhanced via the topical application of this lotion.
Methods
This was a double‐blind, placebo‐controlled, within‐group study that investigated the effects of Lipoxyderm™ on thigh fat mass, circumference, and skinfold thickness. Seven participants underwent pre/post‐exercise testing for weight, bilateral thigh circumference/skinfold thickness, and body composition/thigh fat mass assessment via dual‐energy X‐ray absorptiometry. Participants followed a hypocaloric diet, walked 150 minutes/wk, and were randomly assigned to apply a placebo to one leg and Lipoxyderm™ to their other leg for 28 days. Separate two‐way mixed factorial repeated measures ANOVAs were used to compare the effects of Lipoxyderm™ to the placebo on thigh circumference, skinfold thickness, and fat mass.
Results
A significant time x group interaction was found for thigh circumference (F 1,6 = 18.2, P = 0.005), skinfold thickness (F 1,6 = 14.6, P = 0.009), and fat mass (F 1,6 = 37.1, P = 0.001).
Conclusions
A twice‐daily topical application of Lipoxyderm™ for 28 days compared to a placebo combined with a walking program and a restricted caloric intake is more effective at reducing thigh circumference (1.2 vs 0.8 cm), thigh skinfold thickness (3.7 vs 2.0 mm), and thigh fat mass (100.0 g vs 57.3 g).
Keywords: aminophylline, caffeine, fat reduction, gotu kola, l‐carnitine, yohimbe
1. INTRODUCTION
Obesity is a problem that has been on the rise throughout the world over the last several decades. High rates of obesity have naturally led to an increase in the development of weight loss products that are marketed to the public for weight loss. According to the National Institutes of Health, Americans spend approximately $2 billion dollars per year on weight loss products.
Topical fat loss and defining lotions have been developed and sold worldwide as one of many categories of products that are marketed to facilitate weight loss. One product in this category, Lipoxyderm™ (LD), is advertised as a topical lotion that helps to enhance fat loss. The product contains several active ingredients that have been shown to be effective in improving body composition including aminophylline, l‐carnitine, gotu kola (Centella asiatica), yohimbe, and caffeine.1, 2, 3, 4 The lotion also contains glycolic acid and vitamin E, which may potentially improve the absorption of the other active ingredients and act as a skin antioxidant, respectively.5, 6, 7 One application of the lotion (3.93 mL) contains 39 mg of aminophylline, 39 mg of gotu kola, 39 mg of l‐carnitine, 39 mg of vitamin E, 39 mg of glycolic acid, 75 mg of yohimbe, and 196 mg of caffeine.
Caffeine is the active ingredient found in the greatest quantity in LD. Acheson et al8 reported that caffeine supplementation alone enhances lipolysis as well as fat oxidation; however, researchers have suggested that its effectiveness appears to be magnified when combining caffeine with other thermogenic agents.9 Diepvens et al10 suggested that caffeine may affect the secretion of catecholamines, which activate β‐2 adrenergic receptors and in turn increase the concentration of cyclic adenosine monophosphate (cAMP) in cells that activates lipase in the lipolysis process. Researchers have also reported that caffeine can prevent an accumulation of fats and may speed up lipolysis by blocking α‐adrenergic receptors.11, 12 Furthermore, caffeine stimulates lipolysis via the inhibition of phosphodiesterase (PDE) and has been shown to be effective at penetrating through the skin.3
Like caffeine, aminophylline is a PDE inhibitor and lowers the lipolytic threshold by inhibiting the breakdown of cAMP in the fat cell.13 In a study investing the effects of aminophylline cream on waist circumference with 50 men and women between 21 and 65 years of age that were classified as overweight, investigators reported a significant reduction in waist circumference compared to the placebo group with no adverse events in either group.13 Although aminophylline cream application has occasionally shown side effects such as heart palpitations and anxiety, it has been demonstrated to offer a safe and effective method for cosmetic fat reduction from the waist and the thigh.1, 13
Gotu kola, also known as Centella asiatica, has active compounds such as pentacyclic triterpenes and has been used in treating small wounds, burns, psoriasis, and scleroderma.2 The mechanism of action involves increasing fibroblast production, the synthesis of collagen, and the fibronectin content.2 Furthermore, researchers reported that it can improve the tensile strength of newly formed skin and reduces the inflammatory phase of hypertrophic scars and keloids.2 In a study of 60 people with cellulite, Brinkhaus et al14 reported that application of gotu kola showed a beneficial effect on reducing the progression of cellulite as well as a significant improvement in 85% of the participants with no adverse reactions.
Yohimbine, the active ingredient in yohimbe, is an α‐2 adrenergic receptor antagonist that increases the activity of the sympathetic nervous system and can increase the amount of epinephrine and norepinephrine in the blood stream. Although the efficacy of oral administration of this product on improving body composition is controversial in the literature, Greenway et al1 reported that the combination of lipolytic compounds such as aminophylline and yohimbine together give greater stimulation of lipolysis than the individual components alone. Greenway et al1 reported that topical administration of yohimbine on the thigh demonstrated no rashes, changes in blood pressure, or changes in pulse.
l‐carnitine is an amino acid used by the body for β‐oxidation by transporting long‐chain fatty acids across the membrane of the mitochondria. It is used for energy production and fat metabolism in the heart and skeletal muscle. l‐carnitine has been used for various purposes inclusive of improving diabetes15 and obesity.16 Although some studies have reported that l‐carnitine supplementation led to increases in fat oxidation,16 the effectiveness of l‐carnitine at reducing body fat remains controversial.17 Despite these conflicting findings, l‐carnitine supplementation has been shown to be safe in humans.17
Vitamin E and Glycolic acid are the other active ingredients in LD. Vitamin E is widely used in many topical skin care products due to its antioxidant properties. Although there is a lack of strong evidence from controlled studies regarding Vitamin E's effectiveness in treating specific skin conditions, small trials and case reports have shown positive results with the application of Vitamin E for the treatment of yellow nail syndrome, collagen synthesis, and wound healing.18 Glycolic acid has been used for the treatment of various dermatological diseases such as acne, aging/photoaging, keratosis, age spots, plaque psoriasis, and warts.6 Glycolic acid has also been shown to have the capability of disrupting the skin barrier function and enhance percutaneous absorption.5
To the authors’ knowledge, no current research exists on the effects of combining caffeine, aminophylline, gotu kola, yohimbe, and l‐carnitine on enhancing fat loss. Furthermore, the effectiveness of topical lotions to enhance the reduction in body fat is not well accepted or thoroughly investigated. The purpose of this study was to investigate the effectiveness of a lotion that contains caffeine, aminophylline, gotu kola, yohimbe, and l‐carnitine to facilitate the reduction in thigh fat mass, thigh circumference, and thigh skinfold thickness. Although fat loss in the thigh and the abdominal region has been reported with the use of aminophylline alone,1, 13 research on the effectiveness of topical multi‐ingredient lotions is lacking. This study was a 28‐day double‐blind, placebo‐controlled, within‐group study designed to investigate the effects of topical LD, in conjunction with a moderate intensity exercise program and a hypocaloric diet, on thigh fat mass, thigh circumference, and subcutaneous thigh skinfolds in healthy and sedentary women between 18 and 54 years of age.
2. MATERIALS AND METHODS
2.1. Experimental design
In order to test the hypothesis of enhancing fat loss via the application of a topical lotion containing lipolytic active ingredients, a 28‐day double‐blind, placebo‐controlled, within‐group study was performed with women's thighs selected as the region of the body to test. Women's thighs were selected as the region of the body to test to enable each woman to serve as their own control because a placebo was applied to one thigh and the active ingredient was applied to the other thigh; moreover, the hypocaloric diet and moderate intensity exercise program was completed by the same person that was applying a different lotion to each thigh. Hence, the primary difference between one thigh and the other thigh was the type of lotion being applied to each leg. This region was also selected due to the presence of estrogen in females. Investigators previously connected estrogen with greater α‐2 receptors and fewer β‐2 receptors on fat cells of the hips/thighs of women, creating a higher lipolytic threshold that subsequently results in a higher concentration of fat in this region.19, 20, 21
2.2. Subjects
Ten healthy women signed an informed consent approved by the California State University Institutional Review Board and began the study. Fourteen to seven days prior to the study, participants met with the research team to fill out the Physical Activity Readiness Questionnaire, a medical screening questionnaire, and receive study instructions/procedures that were to be followed prior to arriving for testing and during the study. Participants were also asked to apply a small amount of the placebo (PLA) and LD lotion to their forearm to ensure there were no allergic or adverse reactions. In order to be included in the study, participants could not be pregnant or take medications or supplements (other than a multivitamin). Furthermore, the participants could not have any neurological or musculoskeletal injuries, no skin allergies/conditions/sensitivity that would prevent them from applying a lotion to their skin, and reported exercising less than 60 minutes a week at a moderate intensity for the last 6 months. Due to time constraints, seven of the 10 participants completed the study.
Subjects served as their own controls over the 28‐day protocol since the PLA was applied to one of their thighs, and the LD was applied to their other thigh as randomly assigned by a member of the research team not involved in the data collection or data analysis. A 28‐day intervention was selected in an effort to maximize adherence to a walking regimen and a restricted caloric intake; however, this came at the expense of examining the effects of the intervention over a longer period of time and may be considered a limitation. Participants were also required to abstain from consuming any fat loss supplements (eg, l‐carnitine) or pre/post‐workout supplements during the study. Measures of weight, height, lean body mass, fat mass, thigh fat mass, thigh circumference, and subcutaneous fat skinfolds were taken within five days prior to, and within five days following, the walking and lotion application protocol. A walking program of 30 minutes 5 days per week was prescribed to each participant and was monitored via a physical activity log that was turned in weekly for four weeks to a member of the research team. All seven participants completed the 20 days of walking over the four week period.
2.3. Lean body mass, fat mass, thigh fat mass, and thigh muscle mass assessment
A total body DXA (GE Lunar Prodigy, Chicago, IL) scan was performed to measure total body composition. Participants laid supine on the scanning bed and the GE Lunar Prodigy computer software (GE Healthcare Lunar, Madison, WI) provided estimations of total lean body mass and fat mass. A rectangle that measured 29.9 cm × 8 cm (height × width) that began superior to the superior patellar border of each thigh was identified as a region of interest; thigh fat mass was measured in this region. All DXA analyses were performed by the same investigator.
2.4. Thigh circumference and thigh subcutaneous fat skinfold
Participants wore loose fitting shorts that could be rolled up the thigh on both sides. Each thigh was measured 20 cm above the superior patellar border where it was marked with a marker. Participants were provided with a marker so that they could trace the same mark every day after they showered in order to keep the mark visible. The investigator then measured the thigh circumference with a tape measure and skinfolds with a Lange Skinfold Caliper (Beta Technology, Santa Cruz, CA) at the level of the mark on the initial testing day and final testing day upon completion of the program. Measurements of thigh circumference and skinfold were taken twice at each location to ensure intra‐rater reliability, and the average of the two measurements was used as the measurement. The same experienced investigator performed all thigh circumference and skinfold measurements. The test‐retest intra‐rater correlation coefficient for the investigator utilizing a 2‐way mixed‐effects model with absolute agreement was 0.984 (95% CI 0.992‐0.995, P < 0.001) and 0.998 (95% CI 0.993‐0.999, P < 0.001) for measurements of the thigh circumference and skinfold thickness, respectively.
2.5. Dietary/lotion supervision
Participants were asked to consume a 1200 Calorie diet throughout the 28‐day study. The diet was tracked via the free website/app MyFitnessPal® (UnderArmour, Baltimore, MD). Participants were given a tutorial of the tracking software during the orientation meeting 7‐14 days before the study began. After the initial meeting, a member of the research team oversaw the diet logs of the participants via weekly emails from the participants. All participants received two sets of lotions in small containers; one set was LD and the other set the PLA (Cetaphil®; Galderma, Fort Worth, TX). A research assistant not involved in the data collection, data entry, or data analysis tracked and distributed the lotions accordingly and randomized the thigh to which each participant applied the lotion. This information was not revealed to the research members collecting/analyzing data or to the participants until all data were collected. The lotions were similar in appearance, texture, and smell. Each small container of lotion had the equivalent of one application portion (3.93 mL). Participants were instructed to apply the lotion every morning and every night (after showering if they showered) with no application of any other lotion on either thigh; they were also instructed to wash their hands between applying the lotion to the appropriate thigh to avoid contamination of the area. Since a member of the research team had weekly interaction with the subjects via email, all subjects were monitored for compliance throughout the study for their diet and lotion application. The research assistant in charge of distributing the lotions had a master list of corresponding data that represented which subject received a PLA or LD on which thigh. Each participant was provided with their lotion containers (PLA and LD) on the first day of testing and had to return all 28 empty containers every week to the laboratory to ensure compliance; a new set of containers were provided to the participants every week. The participants were asked to restate the thigh in which the PLA and the LD were applied on a weekly basis as a way to ensure that they had applied the lotion as initially instructed; all participants complied with the correct application of each lotion on the assigned thigh.
2.6. Data analysis
Descriptive data were calculated for all variables, the data were analyzed for normality, and the data met the assumptions for being normally distributed. Differences among trials were analyzed with separate 2 (group) × 2 (time) repeated measures ANOVAs for thigh circumference (TC), thigh skinfold thickness (SKF), and thigh fat mass (TFM). Upon finding a significant F‐ratio, pairwise comparisons were examined with Bonferroni's correction. Statistical significance was set at P < 0.05. Data were presented as mean ± SD unless otherwise noted. All analysis was performed using IBM Statistics 24.0 (IBM Corp., Armonk, NY).
3. RESULTS
The seven participants that completed the study were all compliant with the walking and lotion application protocol, but the average caloric intake was 1520 ± 321 kcal/d instead of 1200 kcal/d. The participant's mean age, height, and mean pre‐intervention/post‐intervention body weight, fat mass, and fat free mass are presented in Table 1. Mean TC, SKF, and TFM for the LD thigh and the PLA thigh are presented in Table 2. A significant time × group interaction was found for TC (F 1,6 = 18.2, P = 0.005), SKF (F 1,6 = 14.6, P = 0.009), and TFM (F 1,6 = 37.1, P = 0.001). A significant main effect for time was found for TC (F 1,6 = 86.0, P < 0.001), SKF (F 1,6 = 14.7, P = 0.009), and TFM (F 1,6 = 28.0 P = 0.002). No significant main effect was found for the group for TC, SKF, or TFM. Figures 1, 2, 3 represent the comparison of the LD thigh to the PLA thigh for pre‐intervention vs post‐intervention measurements.
Table 1.
Participant age, height, weight, fat mass (FM), and fat free mass (FFM)
| Age (y) | Height (cm) | Weight pre‐int. (kg) | Weight post‐int. (kg) | FM pre‐int. (kg) | FM post‐ int. (kg) | FFM pre‐int. (kg) | FFM post‐int. (kg) | |
|---|---|---|---|---|---|---|---|---|
| Mean ±SD | 28.1 ± 14.3 | 162.9 ± 5.0 | 88.7 ± 20.0 | 84.8 ± 18.9* | 41.5 ± 13.8 | 39.8 ± 13.0* | 45.0 ± 6.9 | 44.9 ± 6.8 |
cm, centimeters; kg, kilograms; pre‐int., pre‐intervention; post‐int., post‐intervention.
Significant difference pre‐int. vs post‐int. (P < 0.05)
Table 2.
Thigh circumference, skinfold, and fat mass before vs after
| Thigh circumference (cm) | Thigh skinfold (mm) | Thigh fat mass (g) | ||||
|---|---|---|---|---|---|---|
| Pre‐int. | Post‐int. | Pre‐int. | Post‐int. | Pre‐int. | Post‐int. | |
| Lipoxyderm™ | 66.3 ± 8.5 | 65.1 ± 8.2* , † | 44.6 ± 10.6 | 40.9 ± 9.2* , † | 803.6 ± 233.3 | 703.6 ± 207.1* , † |
| Placebo | 65.7 ± 8.1 | 64.9 ± 8.0* | 44.4 ± 10.8 | 42.9 ± 9.9* | 788.6 ± 234.7 | 731.3 ± 220.1* |
cm, centimeters; mm, millimeters; g, grams; pre‐int., pre‐intervention; post‐int., post‐intervention.
Significant difference pre‐intervention vs post‐intervention (P < 0.05)
Significant difference LD vs PLA (P < 0.05)
Figure 1.
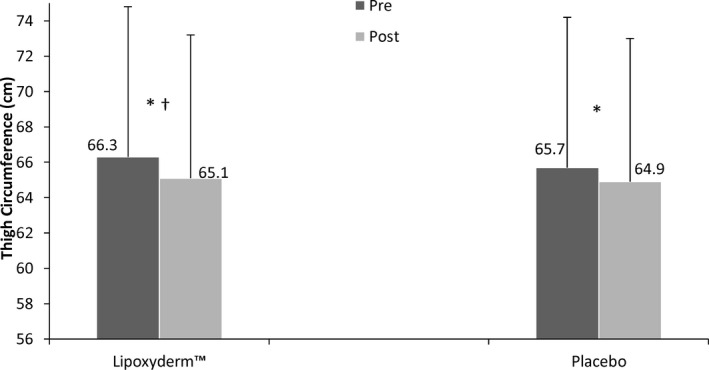
Thigh circumference pre and post comparison of LD and PLA. *Significant difference pre to post (P < 0.001); †Significant difference for Lipoxyderm™ vs placebo pre to post (P = 0.005)
Figure 2.
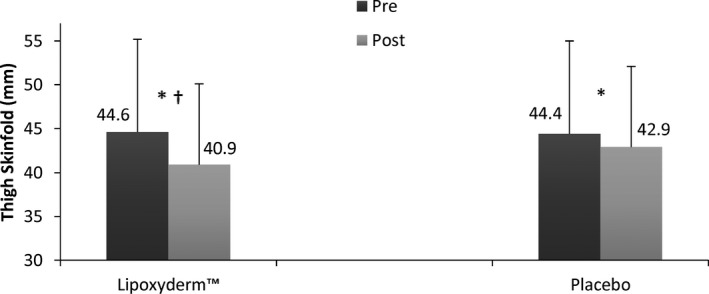
Thigh skinfold pre and post comparison of LD and PLA. *Significant difference pre to post (P < 0.009); †Significant difference for Lipoxyderm™ vs placebo pre to post (P = 0.009)
Figure 3.
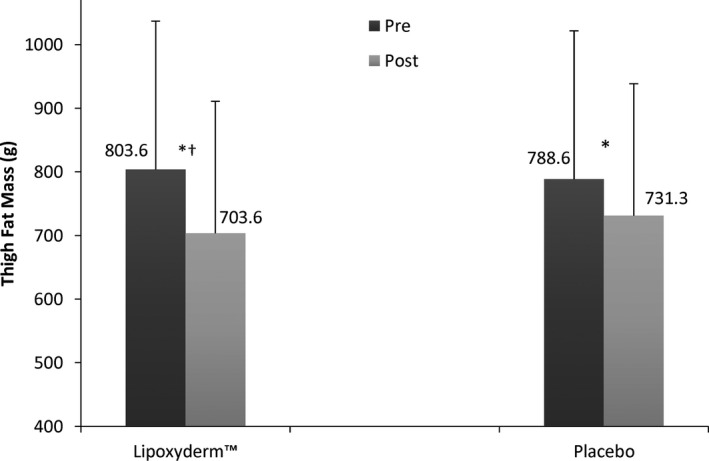
Thigh fat mass pre and post comparison of LD and PLA. *Significant difference pre to post (P < 0.002); †Significant difference for Lipoxyderm™ vs placebo pre to post (P = 0.001)
4. DISCUSSION
The results of this study show that a twice daily topical application of a lotion containing aminophylline, caffeine, yohimbe, l‐carnitine, and gotu kola for 28 days is more effective at reducing thigh circumference (1.2 cm vs 0.8 cm), thigh skinfold thickness (3.7 mm vs 2.0 mm), and thigh fat mass (100.0 g vs 57.3 g) compared to a placebo when both interventions are combined with a walking program and a restricted caloric intake. In comparing the percentage differences between the LD thigh and the PLA thigh, the LD thigh had an average loss of 33.3% more thigh circumference, 45.9% more skinfold thickness, and 42.7% more thigh fat mass.
Similar to other studies that investigated the effectiveness of aminophylline1, 13 at reducing thigh circumference and waist circumference, the current study demonstrated that a topical application of a lotion containing aminophylline as one of the active ingredients also helps to reduce the circumference of the treated area. Akin to the study performed by Greenwell et al,1 this study also used the thighs of females as the region of interest to compare to each other due to the reduced lipolytic receptor activity of this body region. Since each woman served as her own control because the PLA was applied to one thigh and LD was applied to the other thigh, the effects of the PLA compared to the LD could be compared more effectively and with better confidence.
In comparison with previous studies investigating the effects of aminophylline at enhancing fat loss, this study had more than one assessment tool to measure its potential effects. Greenway et al,1 for example, only measured the participant's thigh circumference in their investigation. Similarly, Caruso et al13 only measured the participant's waist circumference and body mass index in their 12‐week investigation utilizing aminophylline. Conversely, the current investigation reported changes in overall body composition and changes in thigh fat mass as measured with a DEXA. Additionally, changes in thigh circumference and skinfold thickness were measured in this investigation. Hence, this investigation provided various measurement tools to quantify the effectiveness of the multi‐ingredient lotion.
Another difference between this investigation and previous investigations performed to assess the effectiveness of topical lotions to enhance fat reduction is that LD has a combination of five active ingredients that may assist with lipolysis that have never been investigated together to the author's knowledge. There are potentially synergistic properties of the individual active ingredients in LD that may further enhance the effectiveness of the lotion. One example of this was reported by Greenway et al1 where the authors suggest that the combination of lipolytic compounds such as aminophylline and yohimbine together give greater stimulation of lipolysis than the individual components alone. Similarly, both caffeine and aminophylline are PDE inhibitors and lower the lipolytic threshold by inhibiting the breakdown of cAMP in the fat cell. Finally, researchers have reported that caffeine's effectiveness appears to be magnified when combining it with other thermogenic agents.9
The ability to reduce body fat from a specific region of the body has been investigated in the past with some ambiguous results. Ramirez‐Campillo et al22 reported that a localized muscle endurance unilateral lower body resistance training protocol in men and women decreased fat mass in the upper extremities and the trunk but did not decrease fat mass in the trained leg. In another study investigating regional body composition changes in women after 6 months of resistance and aerobic training, Nindl et al23 reported a 31% and 12% reduction in fat in the arms and trunk, respectively, but regional fat loss in the legs did not occur. Part of this phenomenon could be explained by the variations in local adipose tissue metabolism. Smith et al24 and Kral et al25 reported that fat was absorbed slower in the femoral region than the abdominal region in women losing weight after the jejuno‐ileal bypass operation for severe obesity. The presence of estrogen may partially explain the regional variation in adipose tissue metabolism in women. As previously stated, investigators have connected estrogen with greater α‐2 receptors and fewer β‐2 receptors on fat cells of the hips/thighs of women, creating a higher lipolytic threshold that subsequently results in a higher concentration of fat in this region.19, 20, 21 Lipase is a hormone‐sensitive enzyme that is responsible for lipolysis, which is the process of hydrolyzing triaglycerol into glycerol and fatty acids. When cyclic adenosine monophosphate (cAMP) activates the protein kinase‐A, the enzyme lipase is activated by becoming phosphorylated. In order to activate cAMP, the stimulatory guanine nucleotide‐binding proteins (GTP‐binding proteins) must be activated to stimulate the enzyme adenylate cyclase. If the inhibitory GTP‐binding proteins become activated, adenylate cyclase will also be inhibited and in turn inhibit the lipolytic process. Stimulation of the β‐2 adrenergic receptors stimulates the GTP stimulatory binding protein that activates adenylate cyclase which then activates cAMP; ultimately, this increases lipolysis. Conversely, the α‐2 adrenergic receptors and the adenosine receptors stimulate the GTP inhibitory binding proteins; in turn, these GTP inhibitory binding proteins inhibit adenylate cyclase and thus inhibit lipolysis. Hence, by controlling lipolysis, hormones such as estrogen can influence body fat distribution. Since LD contains a combination of lipolytic compounds such as caffeine, aminophylline, and yohimbine, they may improve lipolysis by stimulating the β‐2 adrenergic receptors and inhibiting the α‐2 adrenergic receptors, and PDE. These proposed mechanisms of action require further study.
Despite the strength of this study design as a double‐blind, placebo‐controlled, within‐group investigation and the additional variables that were measured to investigate the effectiveness of LD on enhancing fat loss, this study has several limitations. First, there was a small sample size of seven participants that completed the study. A larger follow‐up study with more participants with various activity levels, starting values of body composition, and investigating its effects on males is warranted. Furthermore, the multi‐ingredient nature of the lotion makes it difficult to decipher the individual contributions that each ingredient played in making the product effective. It is possible that only some of the ingredients found in LD are responsible for the changes seen while others played an insignificant role. Lastly, since participants participated in a low‐calorie diet and walking program for 28 days, it is not clear how much of the results seen were the result of LD or of the diet/walking intervention. While this might appear as a major limitation, it should be noted that both legs lost significantly more thigh circumference, skinfold thickness, and fat mass over the course of the 4 weeks; however, the LD thigh lost significantly more compared to the PLA thigh despite the same person undergoing the same walking program and dietary intake.
5. CONCLUSION
A 28‐day BID topical application of LD, combined with exercise and restricted calories, can help to reduce body mass and fat mass. Furthermore, fat loss of the thighs appears to be facilitated with the use of LD as demonstrated by the LD thigh decreasing more compared to the PLA thigh in TC, SKF, and TFM. Similar to previous studies,1, 13 topical application of aminophylline can help to facilitate fat loss. The combination of aminophylline, gotu kola, l‐carnitine, vitamin E, glycolic acid, yohimbe, and caffeine, as found in LD, may be more effective at reducing body fat than the topical application of aminophylline alone; further research is warranted to test this hypothesis. Future research should also individually test the active ingredients as a topical application for the reduction of body fat as well as utilize more subjects and include males in the study. In order for any fat loss ingredient to be effective, it is likely that a caloric deficit must be implemented. Future research of LD or other topical lipolytic ingredients without exercise and a hypocaloric diet is also warranted.
ACKNOWLEDGMENTS
The authors would like to acknowledge that Fitwell Health, LLC is the manufacturer of Lipoxyderm™. Fitwell Health donated all products and placebos for this investigation. The authors would like to acknowledge Dr. Jason Ng for his feedback in this manuscript.
Escalante G, Bryan P, Rodriguez J. Effects of a topical lotion containing aminophylline, caffeine, yohimbe, l‐carnitine, and gotu kola on thigh circumference, skinfold thickness, and fat mass in sedentary females. J Cosmet Dermatol. 2019;18:1037–1043. 10.1111/jocd.12801
REFERENCES
- 1. Greenway FL, Bray GA, Heber D. Topical fat reduction. Obes Res. 1995;3(4):561‐568. [DOI] [PubMed] [Google Scholar]
- 2. Bylka B, Znajdek‐Awiżeń P, Studzińska‐Sroka E, Brzezińska M. Centella asiatica in cosmetology. Postep Derm Alergol. 2013;30(1):46‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Herman A, Herman AP. Caffeine’s mechanisms of action and its cosmetic use. Skin Pharmacol Physiol. 2013;26(8):8‐14. [DOI] [PubMed] [Google Scholar]
- 4. Roure R, Oddos T, Rossi A, Vial F, Bertin C. Evaluation of the efficacy of a topical cosmetic slimming product combining tetrahydroxypropyl ethylenediamine, caffeine, carnitine, forskolin and retinol, In vitro, ex vivo and in vivo studies. Int J Cosmet Sci. 2011;33:519‐526. [DOI] [PubMed] [Google Scholar]
- 5. Suswardana H, Radiono S, Chusniyati N, et al. The effect of 50% glycolic acid on the percutaneous absorption of eutectic mixture of local anesthetics: a study of the electrofulguration‐induced pain. Int J Dermatol. 2008;47:280‐283. [DOI] [PubMed] [Google Scholar]
- 6. Cotellessa C, Peris K, Chimenti S. Glycolic acid and its use in dermatology. J Eur Acad Dermatol Venereol. 1995;5:215‐217. [Google Scholar]
- 7. Chiu A, Kimball AB. Topical vitamins, minerals, and botanical ingredients as modulators of environmental and chronological skin damage. Br J Dermatol. 2003;149:681‐691. [DOI] [PubMed] [Google Scholar]
- 8. Acheson KJ, Zahorska‐Markiewicz B, Pittet P, Anantharaman K, Jequier E. Caffeine and coffee: their influence on metabolic rate and substrate utilization in normal weight and obese individuals. Am J Clin Nutr. 1980;33(5):989‐997. [DOI] [PubMed] [Google Scholar]
- 9. Haller CA, Jacob P, Benowitz NL. Enhanced stimulant and metabolic effects of ephedrine and caffeine. Clin Pharmacol Ther. 2004;75:259‐273. [DOI] [PubMed] [Google Scholar]
- 10. Diepvens K, Westerterp KR, Westerterp‐Plantenga MS. Obesity and thermogenesis related to the consumption of caffeine, ephedrine, capsaicin, and green tea. Am J Physiol Regul Integr Comp Physiol. 2007;292:77‐85. [DOI] [PubMed] [Google Scholar]
- 11. Dodd SL, Herb RA, Powers SK. Caffeine and exercise performance. An update. Sports Med. 1993;15:14‐23. [DOI] [PubMed] [Google Scholar]
- 12. Panchal SK, Poudyal H, Waanders J, Brown L. Coffee extract attenuates changes in cardiovascular and hepatic structure and function without decreasing obesity in high‐carbohydrate, high‐fat diet‐fed male rats. J Nutr. 2012;142:690‐697. [DOI] [PubMed] [Google Scholar]
- 13. Caruso MK, Pekarovic S, Raum WJ, Greenway F. Topical fat reduction from the waist. Diabetes Obes Metab. 2007;9:300‐303. [DOI] [PubMed] [Google Scholar]
- 14. Brinkhaus B, Lindner M, Schuppan D, Hahn EG. Chemical, pharmacological and clinical profile of the East Asian medical plant Centella asiatica . Phytomedicine. 2000;75:427‐448. [DOI] [PubMed] [Google Scholar]
- 15. Mingrone G, Greco AV, Capristo E, et al. L‐carnitine improves glucose disposal in type 2 diabetic patients. J Am Coll Nutr. 1999;18:77‐82. [DOI] [PubMed] [Google Scholar]
- 16. Wutzke KD, Lorenz H. The effect of l‐carnitine on fat oxidation, protein turnover, and body composition in slightly overweight subjects. Metabolism. 2004;53:1002‐1006. [DOI] [PubMed] [Google Scholar]
- 17. Kraemer WJ, Volek JS, Dunn‐Lewis C. L‐carnitine supplementation: influence on physiological function. Curr Sports Med Rep. 2008;7(4):218‐223. [DOI] [PubMed] [Google Scholar]
- 18. Thiele JJ, Ekanayake‐Mudiyaneselage S. Vitamin E in human skin: organ‐specific physiology and considerations for its use in dermatology. Mol Aspects Med. 2007;28(5–6):646‐667. [DOI] [PubMed] [Google Scholar]
- 19. Arner P. Adrenergic receptor function in fat cells. Am J Clin Nutr. 1992;55:228S–236S. [DOI] [PubMed] [Google Scholar]
- 20. Arner P, Hellstrom L, Wahrenberg H, Bronnegard M. Beta‐adrenoceptor expression in human fat cells from different regions. J Clin Invest. 1990;86(5):1595‐1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lafontan M, Berlan M. Fat cell adrenergic receptors and the control of white and brown fat cell function. J Lipid Res. 1993;34:1057‐1091. [PubMed] [Google Scholar]
- 22. Ramirez‐Campillo R, Andrade DC, Campos‐Jara C, Henriquez‐Olguin C, Alvarez‐Lepin C, Izquierdo M. Regional fat changes induced by localized muscle endurance resistance training. Journal of Strength and Conditioning Research. 2013;27(8):2219‐2224. [DOI] [PubMed] [Google Scholar]
- 23. Nindl BC, Harman EA, Marx JO et al. Regional body composition changes in women after 6 months of periodized physical training. J Appl Physiol. 2000;88:2251‐2259. [DOI] [PubMed] [Google Scholar]
- 24. Smith J, Hammersten J, Bjorntorp P, Kral JG. Regional differences in the effect of weight reduction in human fat cell metabolism. Eur J Clin Invest. 1979;9:327‐332. [DOI] [PubMed] [Google Scholar]
- 25. Kral JG, Bjorntorp P, Schesten T, Sjostrom L. Body composition in adipose tissue cellularity before and after jejuno‐ileostomy in several obese subjects. Eur J Clin Invest. 1977;7:414‐419. [DOI] [PubMed] [Google Scholar]


