Summary
Complete graft thrombosis is the leading cause of early graft loss following pancreas transplantation. Partial thrombosis is usually subclinical and discovered on routine imaging. Treatment options may vary in such cases. We describe the incidence and relevance of partial graft thrombosis in a large transplant center. All consecutive pancreas transplantation at our center (2004–2015) were included in this study. Radiological follow‐up, type and quantity of thrombosis prophylaxis, complications and, graft and patient survival were collected. Partial thrombosis and follow‐up were also studied. All 230 pancreas transplantations were included in the analysis. Computed tomography was performed in most cases (89.1%). Early graft failure occurred in 23 patients (13/23 due to graft thrombosis, 3/23 bleeding, 1/23 anastomotic leakage, 6/23 secondary to antibody mediated rejection). There was evidence of partial thrombosis in 59 cases (26%), of which the majority was treated with heparin and a vitamin K antagonist with graft preservation in 57/59 patients (97%). Thrombosis is the leading cause of early graft loss following pancreas transplantation. Computed tomography allows for early detection of partial thrombosis, which is usually subclinical. Partial graft thrombosis occurs in about 25% of all cases. In this series, treatment with anticoagulant therapy (heparin and vitamin K antagonist) resulted in graft preservation in almost all cases.
Keywords: complications, pancreas transplantation, thrombosis
Introduction
Graft thrombosis is still considered the Achilles' heel of pancreas transplantation. Great successes have been achieved with this procedure in terms of curing patients from type 1 diabetes mellitus over the last 40 years, but thrombosis remains a challenging problem with a reported incidence of 3–10% 1, 2 Several risk factors are associated with complete graft thrombosis which usually leads to graft loss. A review on risk factors showed that donor age, cerebrovascular death, procurement related problems, type of preservation solution, and graft pancreatitis are risk factors 1. The Pancreas Donor Risk Index (PDRI), which was developed using data on 1 year graft survival, clearly shows that a higher donor risk leads to a higher risk of graft failure 3. Complete graft thrombosis, in most cases accompanied by marked hyperglycemia and/or graft tenderness, is the most common cause of early pancreas graft loss 2, 4
Little is known about the clinical significance of partial graft thrombosis. By some, this is believed to be a ‘physiological' phenomenon caused by ligation of the mesenteric and splenic veins and their side branches 5. Especially in pancreas transplantation, this ligating of smaller vessels contributes to Virchow's triad (hypercoagulable state, venous stasis, and endothelial injury), which may be one of the contributors to the relatively high incidence of thrombosis in pancreas transplantation, as compared to other organs 6. However, sometimes partial thrombosis extends from the ligated venous ends to larger and more centrally located veins. Partial graft thrombosis is usually subclinical (i.e. without hyperglycaemia) and discovered on routine ultrasound or computer tomography (CT) imaging in the early postoperative phase 7. It is unclear whether this form of thrombosis should be considered a precursor for complete thrombosis. If this were so, it would be necessary to detect its presence as early as possible, so antithrombotic treatment may salvage the graft. One recent study, where only donors younger than 40 years of age without other risk factors for graft thrombosis, showed a partial thrombosis incidence of 27%. All of these partial thrombosis were safely managed with unfractionated intravenous heparin, without any negative consequences 8. Another recent study proposed a CT‐based grading scheme for graft thrombosis, stating that not all graft thrombosis requires treatment 9. It is our aim to evaluate these findings by describing our experience regarding partial thrombosis. We evaluated the clinical relevance of this partial thrombosis, the incidence, clinical outcome, and treatment.
Study population and design
A retrospective analysis in which all consecutive pancreas transplantations [simultaneous pancreas kidney (SPK), pancreas after kidney (PAK), pancreas transplant alone (PTA)] from January 1st 2004 until December 31st 2015 performed at the Leiden University Medical Center were included. A minimum of 90 days follow‐up was registered.
Recipient surgical technique
Standard SPK transplantations were performed using a midline incision, where the kidney was first transplanted in the left iliac fossa without direct ureteric anastomosis, allowing for hemodynamic stability and reduction of edema, followed by the pancreas on the right, anastomosed on the common iliac artery and caval vein. Only then is the ureteric anastomosis completed. Since 2011, exocrine drainage is usually performed by duodeno‐enterostomy. Prior to 2011, duodeno‐cystostomy with secondary enteric conversion to duodeno‐enterostomy after 12 months was performed in most cases. For recipients with PRA≤6%, the transplantation commenced directly after blood type confirmation and crossmatch was performed retrospectively as soon as possible 10. Recipients received routine postoperative intravenous contrast enhanced CT imaging within the first week after transplantation to rule out any postoperative complications. This was performed sooner when indicated (e.g. two consecutive blood glucose levels above 10 mm) or later when impaired kidney function hindered early CT imaging. Indications for imaging (including per protocol imaging) and their respective outcome (whether thrombosis was diagnosed or not) are shown in Table 2. In most cases of complete thrombosis, our intention is to surgically salvage or remove the graft. In case of partial or peripheral thrombosis, patients are initially treated with therapeutic intravenous heparin, followed by conversion to vitamin K antagonists (VKA) for at least 3 months. At that moment follow‐up CT imaging was performed. In our center, no routine screening for thrombophilia is performed.
Table 2.
Indications for postoperative imaging associated with diagnosis of thrombosis
| Imaging reason | n | Thrombosis | |||||
|---|---|---|---|---|---|---|---|
| Yes | No | Uncertain | |||||
| n | % | n | % | n | % | ||
| Protocol | 122 | 30 | 25 | 80 | 66 | 12 | 10 |
| Hyperglycemia | 52 | 20 | 39 | 25 | 48 | 7 | 14 |
| Fever | 19 | 3 | 16 | 13 | 68 | 3 | 16 |
| Abdominal tenderness | 12 | 2 | 17 | 8 | 67 | 2 | 17 |
| Other | 20 | 6 | 30 | 11 | 55 | 3 | 15 |
Pearson Chi‐Square P = 0.48.
Post‐transplant medical therapy
Since 2008, recipient immunosuppressive therapy consists of alemtuzumab induction (15 mg subcutaneous, 1st dose preoperative, 2nd dose postoperative day 1), rapidly tapered steroids (3 days, 500–250–125 mg intravenous), followed by tacrolimus (trough levels 8–12 μg/l) and mycophenolate mofetil maintenance immunosuppressive therapy. Previous protocols (regarding induction and maintenance) were described elsewhere 4. Standard anticoagulation therapy consisted of a twice daily, low dose low molecular weight heparin (LMWH), based on the recipients weight: nadroparin 2 dd 5700 IE for patients weighing over 100 kg and nadroparin 2dd 2850 IE for patients below 100 kg. This was a once daily regime prior to 2007, as is our standard protocol to prevent deep venous thrombosis and pulmonary embolism in all surgical patients. The first dose is administered at the recovery room and no other anticoagulants, especially platelet inhibitors, are prescribed. The clinical protocol was changed after the data collection and currently states that patients are prescribed once daily 5700 IE LWMH, and adjusted in case of impaired kidney function. In all cases, LMWH was prescribed for duration of the hospital admission. No new anti‐platelet therapy was prescribed in the postoperative period.
Data collection
Donor, recipient, and transplant related risk factors are shown in Table 1. Follow‐up data include HbA1c levels, surgical interventions, imaging studies including the reason for imaging, as well as anticoagulation therapy during the first postoperative admission, date last seen, date of restart of exogenous insulin therapy. When thrombosis, either partial or complete occurred, clinical outcomes were registered. Only graft thrombosis within the first 90 days (early graft loss) was analyzed. Very peripheral thrombosis in ligated ends of veins, was not considered graft thrombosis, this is considered grade 1 pancreas graft thrombosis according to the recent study from Cambridge 9. When thrombus was found in the parenchymal part of either superior mesenteric or splenic vein but there was still passage of contrast and perfusion of the graft, this was considered partial thrombosis. Absence of contrast due to thrombus was considered complete thrombosis. The actual involved vessel was not recorded in the database. Antibody mediated rejection (AMR) was defined as positive C4d staining and signs of rejection on histological examination of the graft following explantation and the presence of donor specific antibodies (DSA). Suspected AMR was defined as the presence of either positive C4d or the presence of DSA 11. Graft thrombosis was considered to be secondary to AMR when AMR was suspected. Consequently, graft thrombosis was only considered primarily when rejection was not suspected and data were reported separately.
Table 1.
Demographics of (a) donors, (b) recipients and (c) transplantations
| n | % | No thrombosis | Partial thrombosis | Complete thrombosis | P value* | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||||
| (a) | |||||||||
| Gender | |||||||||
| Male | 100 | 44 | 69 | 45 | 26 | 44 | 5 | 26 | 0.29 |
| Female | 130 | 56 | 83 | 55 | 33 | 56 | 14 | 74 | |
| Cause of death | |||||||||
| Stroke | 131 | 57 | 84 | 55 | 32 | 54 | 15 | 79 | 0.49 |
| Trauma | 76 | 33 | 53 | 35 | 19 | 32 | 4 | 21 | |
| Anoxia | 15 | 6.5 | 10 | 7 | 5 | 9 | 0 | 0 | |
| Other | 8 | 3.5 | 5 | 3 | 3 | 5 | 0 | 0 | |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Age | 35 | 13 | 34 | 13 | 36 | 13 | 40 | 11 | 0.07 |
| BMI | 23 | 3 | 23 | 3 | 23 | 2 | 25 | 3 | 0.02 |
| PDRI | 1.36 | 0.44 | 1.34 | 0.43 | 1.4 | 0.47 | 1.48 | 0.40 | 0.32 |
| (b) | |||||||||
| Gender | |||||||||
| Male | 133 | 58 | 92 | 61 | 35 | 59 | 6 | 32 | 0.05 |
| Female | 97 | 42 | 60 | 39 | 24 | 41 | 13 | 68 | |
| Previous graft thrombosis | 13 | 6 | 8 | 5 | 4 | 7 | 1 | 5 | 0.91 |
| Sensitized (PRA>5%)† | 19 | 12 | 14 | 14 | 3 | 8 | 2 | 13 | 0.66 |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Age | 43 | 8 | 43 | 7 | 43 | 9 | 43 | 5 | 0.95 |
| BMI | 25 | 4 | 25 | 4 | 25 | 3 | 25 | 3 | 0.84 |
| (c) | |||||||||
| Transplant type | |||||||||
| SPK | 203 | 88 | 137 | 90 | 51 | 86 | 15 | 79 | 0.18 |
| PAK | 25 | 11 | 14 | 9 | 8 | 14 | 3 | 16 | |
| PTA | 2 | 1 | 1 | 1 | 0 | 0 | 1 | 5 | |
| Donation after circulatory death | 21 | 9 | 14 | 9 | 7 | 12 | 0 | 0 | 0.30 |
| Retransplant | 15 | 6.5 | 9 | 6 | 4 | 7 | 2 | 11 | 0.74 |
| Perfusion solution | |||||||||
| UW | 208 | 90 | 139 | 91 | 51 | 86 | 18 | 95 | 0.43 |
| HTK/Other | 22 | 10 | 13 | 9 | 8 | 14 | 1 | 5 | |
| Exocrine drainage | |||||||||
| Duodenocystostomy | 86 | 37 | 56 | 37 | 22 | 37 | 8 | 42 | 0.91 |
| Duodeno‐enterostomy | 144 | 63 | 96 | 63 | 37 | 63 | 11 | 58 | |
| Anticoagulant therapy | |||||||||
| Nadroparin 2850 IE | 71 | 31 | 43 | 30 | 21 | 36 | 7 | 37 | 0.87 |
| Nadroparin 5700 IE (2dd2850 IE) | 143 | 62 | 97 | 66 | 35 | 60 | 11 | 58 | |
| Nadroparin 11400 IE (2dd5700 IE)‡ | 9 | 4 | 6 | 4 | 2 | 2 | 1 | 5 | |
Chi‐square for categorical variables, anova for continuous variables.
PRA known 160/230.
Therapeutic dosage LMWH or iv heparin.
Statistical analysis
Risk factors associated with thrombosis were analyzed using Chi‐square analyses for categorical variables and Analysis of Variance (anova) tests for continuous variables. Whether partial thrombosis was associated with graft survival was analyzed using Cox‐regression analysis.
Results
Overall results
In the study period a total of 230 consecutive pancreas transplantations were performed, of which 203 (88%) were SPK, 25 (11%) were PAK, and two (0.9%) were PTA. Fifteen of 230 (6.5%) were retransplantations. Donation after circulatory death (DCD) pancreata were used in 21 (9.1%) transplantations. Median cold ischemia time for pancreata was 10.7 h, for kidneys 10 h. Donor and recipient characteristics are shown in Table 1. Mean hospital stay after transplantation was 26 days (SD 16 days). Median follow‐up was 4.5 years (0–12 years). Mean PDRI was 1.36 (SD 0.44). Early graft failure occurred in 23 (10%) cases (90 days graft survival 90.0%). Eighteen of these grafts were lost due to thrombosis (7.8%), three due to bleeding, one due to anastomotic leakage, and one due to T‐cell mediated rejection 11. One year graft survival was 87%, longer term results of our series have been published elsewhere recently 12. Follow‐up was completed in June 2016.
Postoperative imaging
In 205 (89%) patients, computed tomography was the first postoperative radiological study. In 21 cases (9.1%) this was ultrasound. In one case MRI was used and in three cases no imaging was performed. Median interval from transplantation until the first (sequential CT imaging was performed during follow‐up, but is not reported in this study) radiological investigation was 6 days (IQR 3–9 days). The reasons for imaging were as follows: majority per protocol (without (acute) clinical indication), 122/227 (54%), because of sudden progressive hyperglycemia in 52 cases (23%), because of persistent fever in 19 cases (8.4%) and because of abdominal tenderness in 12 cases (5.3%). Other indications included increase in serum amylase, hematuria in a bladder drained patient, or decreased hemoglobin levels.
There was no statistical significant association between reason for imaging and whether thrombosis was diagnosed (P = 0.48) (Table 2). In 25% of the per protocol scans (in the absence of clinical symptoms), thrombosis was diagnosed. In 10–17% of the performed CT scans the radiologist did or could not diagnose or exclude thrombosis.
Postoperative thrombosis
In 78/230 cases (34%) CT imaging showed signs of graft thrombosis (either complete occlusive graft thrombosis or non‐occlusive peripheral thrombosis requiring treatment) within 90 days (Fig. 1). Higher recipient BMI was associated with a higher risk of complete thrombosis (P = 0.019). Although our center does not routinely screen for hypercoagulable states (e.g. protein S or C deficiency) there were two recipients (one protein C deficiency and protein S deficiency) with hypercoagulable syndromes, both did not develop thrombosis. Also, previous graft thrombosis was not associated with renewed graft thrombosis in this series.
Figure 1.
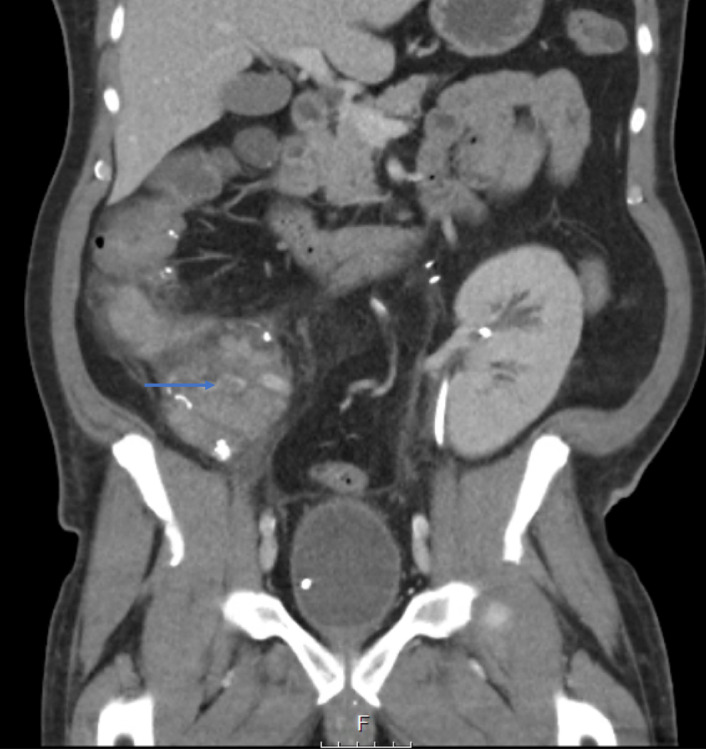
Computed tomography image of partial thrombosis in head of the pancreas (arrow).
In 19/230 cases (8.2%) complete venous thrombosis was found. In 2/19 there also was arterial thrombosis. This arterial thrombosis was considered to be secondary to venous thrombosis, since, during transplantectomy of the pancreas, arterial anastomoses were patent. Thrombosis was secondary to confirmed AMR in 2/19 cases and to suspected AMR in 4/19 cases 11. In 17/19 cases the graft had to be removed. In one case with both splenic and superior mesenteric venous occlusion, the patient was put on therapeutic anticoagulation therapy with intravenous heparin and later switched to VKA resulting in preserved graft function. This strategy was chosen because blood glucose levels remained normal and contrast CT showed normal parenchymal perfusion of the graft. In one case partial thrombosis had progressed to complete venous thrombosis at the 3‐month follow‐up CT scan. This patient was insulin independent and kept on anticoagulation. In 11/17 after transplantectomy, patients were relisted on the waiting list: two for islet transplantation and nine for PAK transplantation.
In 59/230 (25.6%) there was evidence of partial thrombosis on CT imaging (Fig. 1). Follow‐up data were available in 47 of 59 patients. All 59 patients were treated with intravenous heparin, followed by VKA (one patient received acetylsalicylic acid (ASA) instead of VKA, the reason was unknown). In 36/47, there was no evidence of remaining thrombus on follow‐up CT scan after a median of 94 days (4–284 days), VKA were ceased and patients were switched to ASA. Median duration of oral anticoagulant use was 122 days (6–1902 days). In seven patients, thrombus was still present at the end of follow‐up and patients were kept on OAC. In four cases, thrombus had progressed, with persistent functioning in two cases and graft failure in the other two. Figure S1 represents and an overview of patients and different forms/stages of thrombosis. Median duration of follow‐up after discovery of partial thrombosis was 125 days (range 4–804 days). When complete graft thrombosis was not the cause of graft failure, early graft failure occurred in 3/59 (5%) following partial thrombosis versus 3/149 (2%) when there was no evidence of thrombosis at all (P = 0.35). Adjusting for PDRI, using Cox‐regression analysis, partial thrombosis was not associated with pancreas graft survival (HR 0.89, 95% CI 0.36–2.24, P = 0.81), compared to no thrombosis.
Median interval between transplantation and diagnosis of complete graft thrombosis was 3 days, 84% occurred within the first week. Complete thrombosis that was believed to have occurred secondary to AMR was diagnosed after a median of 2 days. All transplantations were performed with negative retrospective crossmatch and only 1/6 patients had PRA>6% (in this case 12% at time of transplantation, 55% highest). Donor specific antibodies were positive in 2/6. Median interval between transplantation and diagnosis of partial thrombosis was 6 days. The rate of thrombosis did not increase over the years (P = 0.77). Total reoperation rate was 26% (59/230). In 22/230 cases (9.6%), surgical intervention was required for a bleeding complication (Table 3).
Table 3.
Indications for relaparotomy following transplantation
| n | % | |
|---|---|---|
| Thrombosis | 19 | 8.3 |
| Bleeding | 22 | 9.6 |
| Infection | 13 | 5.7 |
| Bowel anastomosis leakage | 3 | 1.3 |
| Other | 3 | 1.3 |
For seven recipients, the postoperative anticoagulation regime could not be identified from the patient records. Standard postoperative anticoagulation with LMWH in single dose (which was per protocol prior to 2007) was administered to 71 patients (31%) and 143 patients (62%) received double dose from the 1st postoperative day until discharge. Nine patients (3.9%) were on therapeutic anticoagulation (intravenous heparin or high dose LMWH), since they required anticoagulation prior to the transplantation due to cardiac arrhythmias or peripheral vascular disease. Seventeen patients received platelet aggregation inhibition after transplantation, all because this was prescribed to them prior to transplantation. Different anticoagulation is prescribed throughout the field (Table 4). Standard anticoagulation protocol with single or double dose LMWH was not significantly associated with complete thrombosis risk, 7/71 (9.9%) vs. 11/143 (7.7%) (P = 0.59) or partial thrombosis risk, 21/71 (30%) vs. 35/143 (25%) (P = 0.42).
Table 4.
Overview of reported anticoagulation (<1 week postoperative)
| Leiden University Medical Center | LMWH (nadroparin) 2850 IE, twice daily |
| Madison, Wisconsin | ASA |
| Oxford | ASA, subcutaneous heparin. Tailor‐made based on TEG |
| Bochum | Unfractionated heparin iv |
| Pisa | LMWH (nadoparin) 5700 IE, once daily for SPK; unfractionated heparin iv for PTA/PAK |
| Minnesota | Unfractionated heparin iv |
| Oslo, Norway | LMWH (dalteparin) 5000 IE, once daily. PO day 0 + 1, Dextran 500 ml + ASA |
| San Fransisco | Aspirin, dipyridamole and unfractionated heparin iv in non‐uremic |
| Cambridge | Epoprostenol, ASA |
Discussion
This study is an overview of diagnosis and treatment of thrombosis following pancreas transplantation. As shown in previous literature, graft thrombosis is the leading cause of early graft failure 1, 2. Our findings corroborate with those results. We also evaluated partial venous thrombosis, a complication following pancreas transplantation of which little is known 5, 8.
Standard radiological follow‐up in our center consists of contrast enhanced CT. This could be considered quite aggressive, especially since kidney function may still be impaired in the early postoperative phase. In our series, data on kidney DGF (hemodialysis within the first week) have been published elsewhere, and DGF is mostly related to DCD pancreas transplantation 12. In the case of DGF, CT imaging was usually postponed until kidney function was restored. Unfortunately, no data on acute kidney injury (25% increase in eGFR or 44 μm increase in serum creatinine) were available in our database. However, CT imaging allows for early detection of sub‐clinical partial thrombosis, which may be amenable for treatment 7, 9. This is supported by the finding that in 25% of the CT scans that were performed per protocol, some form of thrombosis was discovered. Furthermore, especially fever and abdominal tenderness appear to be aspecific clinical features accompanying thrombosis. Obviously, these may indicate other complications, which may be the indication for imaging. Some centers may prefer the use of ultrasound 13, 14. A disadvantage of ultrasound may be that not all vessels are visualized properly by overlying bowel gas and that an experienced radiologist has to be available, making results observer dependent. The proposed grading system of thrombosis by the Cambridge group is supported. Unfortunately, due to the retrospective nature of our study, the grading system was not incorporated in our database 9. Even though CT imaging in this study was inconclusive in 10–17% with regard to graft thrombosis, we do, however, believe that CT imaging should be part of routine follow‐up, following pancreas transplantation. It has to be noted however, that in our study, we did not consider very peripheral thrombosis (grade 1) amongst the cases of thrombosis. These forms of thrombosis were considered not to be clinically relevant. Further studies will focus on quantifying the grade of thrombosis in our center and which forms are clinically relevant and require treatment.
Complete thrombosis leading to graft loss occurred in 17 patients. In all cases, this was with venous thrombosis. The two cases of arterial thrombosis are believed to be secondary to the venous thrombosis. The percentage of graft thrombosis in our series is similar to that reported in literature although some centers report even lower thrombosis rates 15. The thrombosis rate, however, is likely related to intrinsic risks of the pancreatic graft, reflecting in for example the PDRI. As published before, due to scarcity of donors, the pancreata reported and accepted in our country have a relatively higher PDRI as compared to other countries 16. Also, as is shown in this study, thrombosis may be secondary to (antibody mediated) rejection, and thus, the incidence of ‘true' thrombosis was lower (in fact 13/230, 5.7%). It is not always clear from previously published reports whether thrombosis was secondary to rejection. In this study, the relationship of peripancreatic infection or pancreatitis was not studied, however, one of our previous reports did not show an association between pancreatitis and thrombosis (2/30) 12.
In 59 patients (26%), there was evidence of partial thrombosis. This is in line with recent results published by Harbell 8. Most patients were treated with heparin and VKA. During follow‐up, the majority of thrombus resolved with this treatment and most recipients remained insulin independent. In fact, only four progressed to complete thrombosis, of which only two required exogenous insulin. This data show that our current treatment of this partial thrombosis is effective and sufficient in preventing graft loss. However, we cannot predict outcome if no anticoagulants would have been given. Patients with partial thrombosis were treated with VKA after intravenous heparinization. Novel oral anticoagulants or directly acting oral anticoagulants (NOAC/DOAC) may also be used, however the experience with graft thrombosis is limited to our knowledge. Because of the risk of partial thrombosis, we suggest to include CT imaging in routine follow‐up, to evaluate the presence (or absence) of thrombus. In our series, VKA were ceased only after CT imaging had confirmed resolution of thrombus, which was substantially longer than 3 months in some cases.
We currently prescribe once daily LWMH (5700 IE) to most of our patients as thrombosis prophylaxis. Whether this is the optimal treatment remains up for debate. Clearly, there are as many possibilities as there are pancreas transplant centers: intravenous heparin, LWMH, acetylsalicylic acid, and a combination of either of them 2, 8, 15, 17, 18, 19, 20. We did not find an association between single or double dose LMWH prescription and thrombosis. It could however be that changes over time, especially in donor quality, may have masked such an association. It may be that the double dose LWMH masked an increased thrombosis risk with the increased willingness to accept higher risk donor grafts in more recent years. As was shown in this study, the change in protocol to a double dose of LMWH did come at the cost of a slightly higher bleeding risk, which on the other hand, may also have been caused by higher donor risk. Being even more aggressive in terms of anticoagulation, either by prescribing higher dosage of LMWH or prescribing intravenous heparin to each patient, does not seem justified in our series and may only be necessary in case of certain risk factors in a setting of tailor‐made anticoagulation, for example when using intra‐operative thromboelastograms (TEG) 6, 17. Since adequate modification into Virchow's triad is difficult in the setting of pancreas transplantation, optimal monitoring of the cascade of coagulation is paramount. A combination of intra‐operative TEG and postoperative CT imaging, may lead to the most optimal protocol in preventing both complete, as well as partial thrombosis. Furthermore, almost 75% of the patients in our current series (those that did not develop any form of thrombosis) would be ‘over‐treated' and thus be exposed to a potential higher bleeding risk.
Several limitations apply to our study. Due to the retrospective design, it was not possible to retrieve all the data. Also, protocol adjustments, in particular from once to twice daily LMWH as thrombosis prophylaxis, may have obscured results. As was stated prior in the discussion, it remains unclear which form of partial thrombosis is clinically significant. Whether these patients require anticoagulation, possibly associated with higher bleeding risk, would optimally be investigated in a randomized trial, where patients with grade 2 would be randomized to receive a particular dose of anticoagulation, or even none. The incorporation of CT imaging into clinical practice can't be supported by data from this study, but its usefulness has been studied and published in this journal 25 years ago, and has been part of our clinical protocol since then 21.
Conclusion
This study summarizes the single center outcome with regard to graft thrombosis following pancreas transplantation. We have shown that our current protocol to prevent graft thrombosis with once or twice daily low dose LMWH results in a low thrombosis incidence of 5.7%, similar to that reported in literature. Partial thrombosis is frequently discovered on routine CT imaging following transplantation. It is usually without clinical symptoms and may be adequately treated with heparin and VKA, with preservation of adequate graft function. Both postoperative CT imaging, as well as treatment with VKA for partial thrombosis, remain standard treatment at our transplant center.
Authorship
WK: designed the study. WK, CL, DL, VH, SS, and AB: collected the data. WK and AEB: analyzed the data. WK and CL: wrote the manuscript. All authors contributed significantly in revisions of the manuscript.
Funding
The authors have declared no funding.
Conflicts of interest
The authors have declared no conflicts of interest.
Supporting information
Figure S1. Flowchart of patients and stages of thrombosis through follow‐up.
References
- 1. Troppmann C. Complications after pancreas transplantation. Curr Opin Organ Transplant 2010; 15: 112. [DOI] [PubMed] [Google Scholar]
- 2. Finger EB, Radosevich DM, Dunn TB, et al A composite risk model for predicting technical failure in pancreas transplantation. Am J Transplant 2013; 13: 1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Axelrod DA, Sung RS, Meyer KH, Wolfe RA, Kaufman DB. Systematic evaluation of pancreas allograft quality, outcomes and geographic variation in utilization. Am J Transplant 2010; 10: 837. [DOI] [PubMed] [Google Scholar]
- 4. Kopp WH, Verhagen MJ, Blok JJ, et al Thirty years of pancreas transplantation at Leiden University Medical Center: long‐term follow‐up in a large eurotransplant center. Transplantation 2015; 99: e145. [DOI] [PubMed] [Google Scholar]
- 5. Ciancio G, Cespedes M, Olson L, Miller J, Burke G. Partial venous thrombosis of the pancreatic allografts after simultaneous pancreas–kidney transplantation. Clin Transplant 2000; 14: 464. [DOI] [PubMed] [Google Scholar]
- 6. Burke GW 3rd, Ciancio G, Figueiro J, et al Hypercoagulable state associated with kidney‐pancreas transplantation. Thromboelastogram‐directed anti‐coagulation and implications for future therapy. Clin Transplant 2004; 18: 423. [DOI] [PubMed] [Google Scholar]
- 7. Byrne M. The value of early protocol computer tomography and endovascular interventions in the pancreas transplant. 6th EPITA Winter Symposium; Igls, Austria: Transplant International; 2016.
- 8. Harbell JW, Morgan T, Feldstein VA, et al Splenic vein thrombosis following pancreas transplantation: identification of factors that support conservative management. Am J Transplant 2017; 17: 2955. [DOI] [PubMed] [Google Scholar]
- 9. Hakeem A, Chen J, Iype S, et al Pancreatic allograft thrombosis: suggestion for a CT grading system and management algorithm. Am J Transplant 2018; 18 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van de Linde P, van der Boog PJ, Baranski AG, de Fijter JW, Ringers J, Schaapherder AF. Pancreas transplantation: advantages of both enteric and bladder drainage combined in a two‐step approach. Clin Transplant 2006; 20: 253. [DOI] [PubMed] [Google Scholar]
- 11. de Kort H, Mallat MJ, van Kooten C, et al Diagnosis of early pancreas graft failure via antibody‐mediated rejection: single‐center experience with 256 pancreas transplantations. Am J Transplant 2014; 14: 936. [DOI] [PubMed] [Google Scholar]
- 12. Kopp WH, Lam HD, Schaapherder AF, et al Pancreas transplantation with grafts from donors deceased after circulatory death (DCD): 5 years single center experience. Transplantation 2018; 102: 333. [DOI] [PubMed] [Google Scholar]
- 13. Tolat PP, Foley WD, Johnson C, Hohenwalter MD, Quiroz FA. Pancreas transplant imaging: how I do it. Radiology 2015; 275: 14. [DOI] [PubMed] [Google Scholar]
- 14. Yates A, Parry C, Stephens M, Eynon A. Imaging pancreas transplants. Br J Radiol 2013; 86: 20130428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lindahl JP, Horneland R, Nordheim E, et al Outcomes in pancreas transplantation with exocrine drainage through a duodenoduodenostomy versus duodenojejunostomy. Am J Transplant 2018; 18: 154. [DOI] [PubMed] [Google Scholar]
- 16. Kopp WH, de Vries E, de Boer J, et al Donor risk indices in pancreas allocation in the Eurotransplant region. Transpl Int 2016; 29: 921. [DOI] [PubMed] [Google Scholar]
- 17. Vaidya A, Muthusamy AS, Hadjianastassiou VG, et al Simultaneous pancreas–kidney transplantation: to anticoagulate or not? Is that a question? Clin Transplant 2007; 21: 554. [DOI] [PubMed] [Google Scholar]
- 18. Sollinger HW, Odorico JS, Becker YT, D'Alessandro AM, Pirsch JD. One thousand simultaneous pancreas‐kidney transplants at a single center with 22‐year follow‐up. Ann Surg 2009; 250: 618. [DOI] [PubMed] [Google Scholar]
- 19. Walter M, Jazra M, Kykalos S, et al 125 cases of duodenoduodenostomy in pancreas transplantation: a single‐centre experience of an alternative enteric drainage. Transpl Int 2014; 27: 805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boggi U, Vistoli F, Signori S, et al Surveillance and rescue of pancreas grafts. Transpl Proc 2005; 37: 2644. [DOI] [PubMed] [Google Scholar]
- 21. Schaapherder AF, de Roos A, Chandie Shaw P, van der Woude FJ, Lemkes HH, Gooszen HG. The role of early baseline computed tomography in the interpretation of morphological changes after kidney‐pancreas transplantation. Transpl Int 1993; 6: 270. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flowchart of patients and stages of thrombosis through follow‐up.


