Summary
Objective
In 2015, the International League Against Epilepsy (ILAE) proposed a new definition of status epilepticus (SE): 5 minutes of ongoing seizure activity to diagnose convulsive SE (CSE, ie, bilateral tonic–clonic SE) and 10 minutes for focal SE and absence SE, rather than the earlier criterion of 30 minutes. Based on semiology, several types of SE with prominent motor phenomena at any time (including CSE) were distinguished from those without (ie, nonconvulsive SE, NCSE). We present the first population‐based incidence study applying the new 2015 ILAE definition and classification of SE and report the impact of the evolution of semiology and level of consciousness (LOC) on outcome.
Methods
We conducted a retrospective population‐based incidence study of all adult patients with SE residing in the city of Salzburg between January 2011 and December 2015. Patients with hypoxic encephalopathy were excluded. SE was defined and classified according to the ILAE 2015.
Results
We identified 221 patients with a median age of 69 years (range 20‐99 years). The age‐ and sex‐adjusted incidence of a first episode of SE, NCSE, and SE with prominent motor phenomena (including CSE) was 36.1 (95% confidence interval [CI] 26.2‐48.5), 12.1 (95% CI 6.8‐20.0), and 24.0 (95% CI 16.0‐34.5; including CSE 15.8 [95% CI 9.4‐24.8]) per 100 000 adults per year, respectively. None of the patients whose SE ended with or consisted of only bilateral tonic–clonic activity died. In all other clinical presentations, case fatality was lower in awake patients (8.2%) compared with patients with impaired consciousness (33%).
Significance
This first population‐based study using the ILAE 2015 definition and classification of SE found an increase of incidence of 10% compared to previous definitions. We also provide epidemiologic evidence that different patterns of status evolution and LOCs have strong prognostic implications.
Keywords: classification, epidemiology, evolution, incidence, semiology, status epilepticus
Key Points.
Nonconvulsive status epilepticus (SE) had an annual incidence of 12.1 per 100 000 adults, SE with prominent motor phenomena 24.0 (including 15.8 CSE)
The detected incidence of SE increased due to the availability of emergency electroencephalography (EEG), clear diagnostic criteria, and an increasing elderly population
Evolution of semiology in status epilepticus affects outcome
Prominent motor phenomena predispose to good outcome and nonconvulsive parts in an SE episode to bad outcome; the sequence matters
Concerning outcome, distinguish SE patients fully awake or awake with reduced cognition from SE patients in somnolence, stupor, or coma
1. INTRODUCTION
Status epilepticus (SE) is a life‐threatening condition, with substantial mortality and morbidity in survivors.1 SE was defined traditionally as 30 minutes of ongoing epileptic activity or seizures without recovery in‐between. In 2015, a Task Force of the International League Against Epilepsy (ILAE) proposed to define SE as bilateral tonic–clonic activity lasting longer than 5 minutes, and absence SE and focal SE as exceeding 10 minutes.2 The new ILAE classification of SE 2015 distinguishes nonconvulsive SE (NCSE) from SE with prominent motor phenomena. This allows epidemiologic investigation of NCSE and its different subtypes in population‐based studies. The changes of semiology during one episode of SE, the level of consciousness (LOC), and their impact on outcome have not yet been investigated in a population‐based setting.
Previous studies on the epidemiology of SE found incidence rates ranging from 3.5 to 41 per 100 000 per year in North America,3, 4, 5, 6, 7, 8 9.9 to 27.2 in Europe,9, 10, 11, 12, 13, 14 1.3 to 5.2 in Asia,15, 16, 17 and 10.8 in Africa.18 As a consequence of the shorter diagnostic time with the ILAE 2015 definition, we expected to identify more patients with SE. The ILAE proposal also endorsed changes in categorization of etiology. Therefore, the aim of this retrospective epidemiologic study was to provide population‐based data on incidence, types, and causes of SE according to the 2015 ILAE definition and classification. In addition, we aimed to assess the impact on outcome of the evolution of the clinical presentation of SE and LOCs. This study was conducted according to the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement.19
The significance of this research is to obtain real‐world population‐based data on the incidence of SE and its subforms, in particular of NCSE, using the ILAE 2015 definition and classification. We also investigated the evolution of semiology and the LOC and their impact on outcome without any a priori assumptions or boundaries due to classification systems.
2. METHODS
2.1. Study design
We performed a retrospective population‐based incidence study of all adult patients (age 18 years or older) with a new diagnosis of a first episode of SE within the political borders of the city of Salzburg, Austria, from January 1, 2011, to December 31, 2015. Patients were identified retrospectively by searching the hospital patient management system for the terms “status epilepticus,” “convulsive status,” “non‐convulsive status,” “focal status,” or “aphasic status” in electroencephalography (EEG) reports or medical reports for inpatients and outpatients. Cases were identified at the Department of Neurology of Paracelsus Medical University, Christian Doppler Medical Center, which provided the only 24/7 EEG and neurologic emergency service in the city. All patients who presented at other hospitals in the city of Salzburg with acute neurologic symptoms or signs potentially qualifying for acute seizures and SE were admitted to our neuroemergency unit by ambulance or were seen acutely by our consultant neurologists in the respective hospitals. Medical and EEG reports of these consultations were included in this study. Patients residing in nursing homes with seizures or status were also referred to our department for treatment. Nurses were allowed to give antiseizure medication only after prescription by the treating physician. This practice is limited to the younger patient group (up to 18 years), which was not included in our study. Therefore, we expected to have complete coverage of all patients with SE in that area. At least 2 neurologists were on duty, and both they and the EEG service were available 24/7 (24/7 EEG service was established in our institution in 1995). Patients with SE in the epilepsy monitoring unit and those with hypoxic encephalopathy and status‐like EEG patterns after cardiac arrest were excluded. Pairs of 2 neurologists (ML and GG) who were experienced in epileptology and board‐certified neurophysiologists reviewed the patient charts independently and extracted the data. Consensus was reached by discussion or by third opinion (ET) in case of disagreement.
2.2. Diagnostic criteria
SE was diagnosed if diagnostic time exceeded 5 minutes of ongoing seizure activity for convulsive SE, or 10 minutes for absence status or focal status with or without impaired consciousness.2 For comparison, we also calculated the proportion of patients who met the traditional diagnostic time criterion of 30 minutes.
If one SE episode included bilateral tonic–clonic parts at any time, the event was classified as “convulsive” SE according to ILAE 2015 classification, which takes into consideration the most overt semiology.2 SE with prominent focal motor phenomena, tonic SE, hyperkinetic SE, and myoclonic SE, which had been classified traditionally in the groups of complex or simple partial SE, depending on the integrity of consciousness, were grouped together with CSE as “SE with prominent motor phenomena” (ILAE 2015).2
Nonconvulsive SE (or NCSE) was defined clinically as an enduring epileptic condition with reduced or altered consciousness, behavioral or vegetative abnormalities, or merely subjective symptoms like auras, but (by definition) without prominent focal or generalized convulsive movements at any time.20 We also investigated the evolution of semiology within one episode of SE and its impact on case fatality. Therefore, one SE episode may include more than one type of semiology, for instance nonconvulsive, focal motor, tonic, myoclonic, and convulsive parts in a semiologic sequence.
2.3. Clinical context
We applied the Salzburg consensus EEG criteria for confirmation of NCSE,21 but also included patients with subtle clinical phenomena such as minor jerks or conjugate gaze deviation and a very high clinical and paraclinical suspicion of NCSE based on history, clinical presentation, acute magnetic resonance imaging (MRI), or ictal hexamethylpropyleneamine oxime–single‐photon emission computed tomography (HMPAO‐SPECT) to prevent underascertainment. Salzburg criteria for NCSE are not 100% sensitive.21 Furthermore, if a patient presented with subtle motor phenomena (eg, periorally) after sustained bilateral tonic–clonic activity, then NCSE after CSE was diagnosed on clinical grounds and treatment was initiated immediately. All other patients with NCSE on presentation had to have EEG abnormalities, even if they did not strictly fulfill the Salzburg criteria. However, patients were excluded if NCSE could not be distinguished from a seizure with Todd’s phenomena. EEG studies were performed only in case of clinical suspicion, as continuous EEG had not yet been established during the study period. Full access to health care is free in Austria, due to mandatory government health care insurance. Thus we do not expect any referral bias in our series.
We defined etiology as either symptomatic (acute, remote, progressive, and SE defined in electroclinical syndromes) or unknown (ie, cryptogenic).2 Acute etiology referred to the first week after onset of the brain insult. Refractory SE (RSE) was defined when first‐line therapy with benzodiazepines and one second‐line treatment with antiepileptic drugs (AEDs) failed.22 In superrefractory SE (SRSE), status continued or recurred despite the use of anaesthetics for longer than 24 hours.22 Nonsurvivors were patients who died in the hospital or who were transferred to a hospice at discharge.
2.4. Statistical analysis
We included only patients residing in the census area “City of Salzburg” with the census code (“Zählsprengel”) 50101. We used standardization to adjust for age and sex, based on the 2016 population of Austria as reference population. The Austrian government statistical bureau, “Statistik Austria,” provided demographic data, with information stratified for sex and age groups of the populations of Salzburg and Austria during the period from January 1, 2011, to January 1, 2016.23
First, the cumulative incidence was calculated for the entire observation period of 5 years. Subsequently, that value was divided by 5 to obtain the average annual incidence expressed as incident cases per 100 000 adults per year. Case fatalities were calculated as percentages of all nonsurvivors in the group of patients with first SE in the 5‐year study period. For calculating age‐ and sex‐specific SE incidence and case fatality rates, we considered the population of Salzburg in the year 2011 as the population at risk. To investigate the impact of changes in the population over time, we also estimated SE incidence and case fatality rates separately for each year from 2011 to 2015 using the demographic information provided by “Statistik Austria” for the respective year. Subsequently, the age‐ and sex‐specific estimates for incidence rates were multiplied with the corresponding reference population weights and finally summed up to obtain age‐ and sex‐adjusted rates for the reference population of Austria in 2016. Details of statistical calculations are presented in Data S1, and a template for adjustment to populations other than Austria can be found in Table S1.
In addition, we calculated 95% confidence intervals (95% CIs) for both the crude (ie, unadjusted) and the age‐ and sex‐adjusted incidence rates, using the Agresti‐Coull and Dobson methods, respectively. Both methods are superior to classical approaches (eg, asymptotic normal confidence intervals) in the case of small numbers of events.24, 25 All statistical analyses were carried out using R version 3.4.1 (R Core Team 2017, https://www.R-project.org/).
For comparison of our results, we performed age and sex adjustment of data from previous studies to the reference population of Austria in 2016. We adjusted the data from the study by Hesdorffer and colleagues in the United States4 to the Austria population 2016.
The local ethics committee approved this retrospective noninterventional epidemiologic study. According to Austrian regulations, this study did not need written informed consent from the patients, since it involved a retrospective analysis of anonymized data, and was noninterventional.
3. RESULTS
In the year 2011, the census area 50 101 “City of Salzburg” comprised 121 727 adults. Based on our search algorithm, we identified 297 patients with SE, 238 of whom were diagnosed as a first episode; 59 episodes were recurrent status. Seventeen patients (7.1% of 238) were excluded because clinical or paraclinical information was insufficient to distinguish SE from a seizure with postictal Todd’s phenomenon; all of them were survivors. Thus, we included 221 patients with a median age of 69 years (range 20‐99) who had first episodes of SE. Demographic data and etiology of our patient population are shown in Table 1. We excluded 16 patients with NCSE related to anoxia during the study period. The EEG confirmation referring to Salzburg criteria for NCSE was available in 88.7% (102/115) of nonconvulsive semiology as the only or last semiology (Table 2). The crude, unadjusted, incidence of first episode of SE was 36.3 per 100 000 adults per year (95% CI 27.0‐48.8): 37.9 (95% CI 25.4‐56.3) in women and 34.5 (95% CI 21.8‐53.9) in men. Age and sex adjustment to the reference population, Austria 2016, revealed an incidence of 36.1 per 100 000 adults per year (95% CI 26.2‐48.5): 37.0 (95% CI 23.8‐54.9) in women and 35.1 (95% CI 21.2‐54.6) in men.
Table 1.
Demographic data of all first nonhypoxic status epilepticus episodes in adults
| All episodes of first SE (women N, %) | 221 (124, 56.0) |
| Age (y), median (IQR) | 69 (28) |
| Onset in hospital, N (%) | 58 (26.2) |
| History of epilepsy, N (%) | 90 (40.7) |
| Witnessed onset, N (%) | 101 (45.7) |
| Types of etiology, N (%) | |
| Symptomatic | 217 (98.2) |
| Acute | 80 (36.2) |
| Remote | 103 (46.6) |
| Progressive | 31 (14.0) |
| SE defined in electroclinical syndromes | 3 (1.4) |
| Cryptogenic (unknown) | 4 (1.8) |
| Etiology, N (%) | |
| Cerebrovascular | 100 (45.2) |
| Trauma | 37 (16.7) |
| Metabolic | 25 (11.3) |
| Tumors | 23 (10.4) |
| Toxic | 8 (3.6) |
| Degenerative disorders | 7 (3.2) |
| Infectious | 4 (1.8) |
| Immune‐mediated | 4 (1.8) |
| Others | 15 (6.8) |
| RSE episodes, N (%)/case fatality, % | 45 (20.4)/39.5 |
| RSE treated with a second or more AED, but no anesthetics | 41 (18.6)/41.5 |
| RSE treated with anesthetics | 4 (1.8)/25.0 |
| SRSE episodes, N (%)/case fatality, % | 8 (3.6)/37.5 |
Types of etiology are classified according to ILAE 2015. IQR, interquartile range; RSE, refractory status epilepticus; SE, status epilepticus; SRSE, super‐refractory status epilepticus.
Table 2.
Diagnostic EEG criteria for nonconvulsive status epilepticus,21 and nonconvulsive phase at the end in a semiologic sequence of one SE episode
| Diagnostic investigation | N |
|---|---|
| Diagnostic criteria for nonconvulsive SE in EEG:21 | 102 |
| Epileptiform discharges >25 per 10 s epoch | 10 |
| Spatiotemporal evolution | 34 |
| Epileptic discharges <25 per 10 s | 17 |
| Rhythmic activity | 13 |
| Epileptic discharges <25 per 10 s AND rhythmic activity | 4 |
| Subtle clinical phenomena | 26 |
| Epileptic discharges <25 per 10 s | 13 |
| Rhythmic activity | 3 |
| Epileptic discharges <25 per 10 s AND rhythmic activity | 1 |
| No EEG performed, observation in clinical context | 9 |
| Fluctuation | 55 |
| Epileptic discharges <25 per 10 s | 47 |
| Rhythmic activity | 5 |
| Epileptic discharges <25 per 10 s AND rhythmic activity | 3 |
| Rhythmic activity without fluctuation | 5 |
| Clinical improvement | 12 |
| Positive ictal HMPAO‐SPECT | 1 |
EEG, electroencephalography; HMPAO‐SPECT, hexamethylpropyleneamine oxime–single‐photon emission computed tomography.
Age‐ and sex‐adjusted incidence for CSE was 15.8 (95% CI 9.4‐24.8), which was included in 24.0 (95% CI 16.0‐34.5) “with prominent motor phenomena” (together with focal motor, tonic, clonic, and myoclonic SE); the incidence was 12.1 (95% CI 6.8‐20.0) for NCSE (ie, “without prominent motor phenomena”). Women represented 77.6% of NCSE and 44.8% of SE with prominent motor symptoms. Results for each of the 5 study years are shown in Figure 1. The total number of NCSE cases increased from 20 cases in the years 2011 and 2012, to 48 cases in the years 2014 and 2015; the number of cases of status with prominent motor symptoms increased by 13.0% over the same period.
Figure 1.
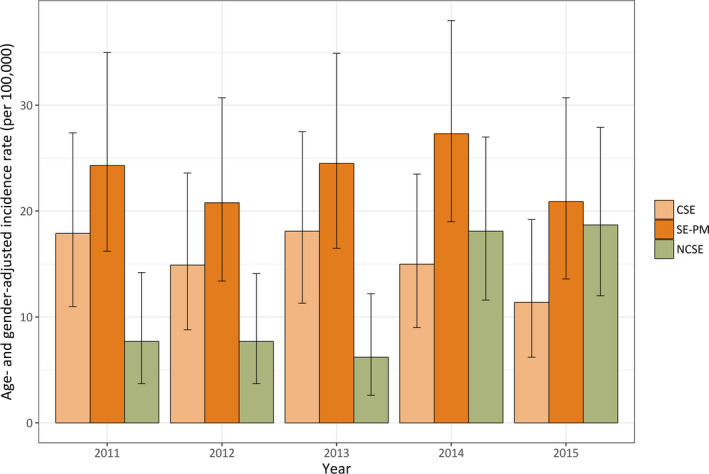
Incidence of first status epilepticus (SE) episode per 100 000 adults per year with relative contribution of nonconvulsive SE (NCSE), and “SE with prominent motor phenomena” (SE‐PM), which included convulsive SE (CSE). Bars indicate 95% confidence intervals
Patients age 60 years or older had a substantially increased incidence compared with adult patients younger than 60 years (Figure S1). We found an age‐ and sex‐adjusted incidence rate in the elderly of 79.9 (95% CI 53.4‐114.8): 89.6 (95% CI 54.0‐139.7) in elderly women and 67.6 (95% CI 32.3‐124.7) in elderly men. Patients younger than 60 years of age had an incidence of 18.1 (95% CI 10.1‐30.1): 12.8 (95% CI: 4.4‐29.0) in women, and 23.4 (95% CI 10.9‐44.0) in men.
The overall case fatality was 16.3% (95% CI 12‐21.8): 21.8% (95% CI 15.4‐29.9) in women and 9.3% (95% CI 4.8‐16.9) in men. In the elderly, case fatality was 22.5% (95% CI 16.4‐29.9): women 27.8% (95% CI 19.9‐37.5) and men 12.0% (95% CI 5.3‐24.2). In patients younger than 60 years, case fatality was 4.1% (95% CI: 0.92‐11.7): 0.0% (95% CI 0.0‐14.8) in women and 6.4% (95% CI 1.6‐17.8) in men. The relative distribution and case fatalities of subgroups of SE according to ILAE 2015 are presented in Table 3. Evolution of semiology during one episode of SE was found in 68 patients (30.8%). The impact of the evolution of semiology and LOC on outcome is presented in Table 4 and Figure 2. Case fatality rates for NCSE, nonconvulsive semiology at the end of the semiologic sequence, nonconvulsive semiology at the beginning of the sequence, and no nonconvulsive semiology at all (ie, only prominent motor phenomena) were 27.6%, 25.6%, 10.0%, and 3.5%, respectively.
Table 3.
Classification of status epilepticus according to ILAE 2015
| Classification of status epilepticus | First SE N = 221 (100%) | Case fatality N = 36 (16.3%) |
|---|---|---|
| (A) With prominent motor symptoms | 145 (65.6) | 15 (10.3) |
| A.1 CSE (synonym: bilateral tonic–clonic SE) | 94 (42.5) | 8 (8.5) |
| A.1.a. Generalized convulsive | 3 (1.4) | 0 |
| A.1.b. Focal onset evolving into bilateral convulsive SE | 88 (39.8) | 8 (9.1) |
| A.1.c. Unknown whether focal or generalized | 3 (1.4) | 0 |
| A.2 Myoclonic SE (prominent epileptic myoclonic jerks) | 0 | 0 |
| A.2.a. With coma | 0 | 0 |
| A.2.b. Without coma | 0 | 0 |
| A.3 Focal motor | 51 (23.1) | 7 (13.7) |
| A.3.a. Repeated focal motor seizures (including Jacksonian) | 38 (17.2) | 7 (18.4) |
| A.3.b. Epilepsia partialis continua | 6 (2.7) | 0 |
| A.3.c. Adversive status | 0 | 0 |
| A.3.d. Oculoclonic status | 0 | 0 |
| A.3.e. Ictal paresis (ie, focal inhibitory SE) | 1 (0.45) | 0 |
| A.4 Tonic status | 6 (2.7) | 0 |
| A.5 Hyperkinetic SE | 0 | 0 |
| (B) Without prominent motor symptoms (ie, NCSE) | 76 (34.4) | 21 (27.6) |
| B.1 NCSE with coma (including so‐called subtle SE) | 7 (3.2) | 3 (42.9) |
| B.2 NCSE without coma | 69 (31.2) | 18 (26.1) |
| B.2.a. Generalized | 0 | 0 |
| B.2.a.a. Typical absence status | 0 | 0 |
| B.2.a.b. Atypical absence status | 0 | 0 |
| B.2.a.c. Myoclonic absence status | 0 | 0 |
| B.2.b. Focal | 69 (31.2) | 18 (26.1) |
| B.2.b.a. Without impairment of consciousness | 1 (0.45) | 0 |
| B.2.b.b. Aphasic status | 10 (4.5) | 1 (10.0) |
| B.2.b.c. With impaired consciousness | 58 (26.2) | 17 (29.3) |
| B.2.c. Unknown whether focal or generalized | 0 | 0 |
| B.2.c.a. Autonomic SE | 0 | 0 |
CSE, convulsive status epilepticus; NCSE, nonconvulsive status epilepticus; SE, status epilepticus.
Table 4.
Outcome of patients with respect to the evolution of semiology and level of consciousness
| Level of consciousness | N | Case fatality, % (95% CI) | Age, median (range) | Etiology, acute, N (%) | Etiology, progressive, N (%) | |
|---|---|---|---|---|---|---|
| (A) Convulsive semiology at the end of SE, or as the only semiology | NA | 57 | 0.0 (0.0‐7.6) | 63 (20‐91) | 14 (24.5) | 6 (10.5) |
| (B) Convulsive semiology at the beginning of SE; focal motor‐, tonic‐, myoclonic‐, and nonconvulsive semiology in a semiologic sequence; focal motor SE, tonic SE, myoclonic SE | Fully awake and awake with reduced cognition | 36 | 2.8 (0.0‐15.4)a | 62 (25‐87) | 6 (16.7) | 5 (13.9) |
| Somnolence, stupor, coma | 52 | 26.9 (16.7‐40.4)a | 69 (23‐97) | 23 (44.2) | 6 (11.5) | |
| (C) Nonconvulsive SE | Fully awake, and awake with reduced cognition | 37 | 13.5 (5.4‐28.5) | 71 (20‐94) | 11 (29.7) | 10 (27.0) |
| Somnolence, stupor, coma | 39 | 41.0 (27.1‐56.6) | 78 (25‐99) | 25 (64.1) | 5 (12.8) | |
| (B and C) | Fully awake, and awake with reduced cognition | 73 | 8.2 (3.5‐17.1)a | 67 (20‐94) | 17 (23.3) | 15 (20.6) |
| Somnolence, stupor, coma | 91 | 33 (24.1‐43.2)a | 72 (23‐99) | 48 (52.8) | 11 (12.1) |
NA, not applicable; SE, status epilepticus.
Confidence intervals do not overlap.
Figure 2.
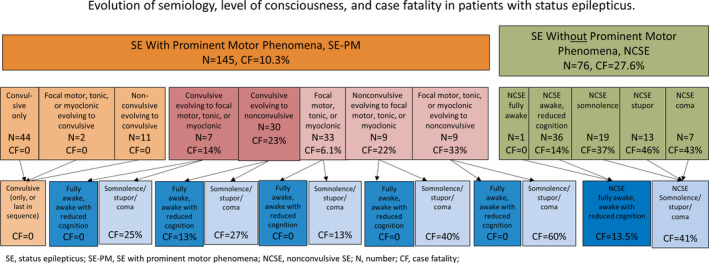
Systematic investigation of outcome in relation to semiologic sequence and level of consciousness. Arrows allow reader to follow particular semiologies with different levels of consciousness. Green shades denote nonconvulsive forms, orange and red shades denote prominent motor forms, and blue shades denote various levels of consciousness
The diagnostic time criterion of 5 minutes for convulsive SE and 10 minutes for focal SE and absences, compared with a 30‐minute diagnostic time, led to a 10% increase in the detection of first SE (20/201). Eighteen patients of these had convulsive SE, and the other 2 patients had NCSE. Twelve patients had remote etiology: six had acute symptomatic and 2 had cryptogenic etiology. Fourteen patients had preexisting epilepsy. The beginning of SE was observed in 14 patients. All 20 patients survived. Following 10‐300 minutes (median 180 minutes) of SE, cessation of SE without treatment occurred in 2.3% (5/221) of cases; all of those patients survived. During the 5‐year study period, there were 59 episodes of recurrent SE, that is, 21.1% of all SE (59/280), with a case fatality of 10.2%.
The population of Salzburg consisted of >95% Caucasian individuals. Age and sex adjustment of the data from the Rochester study4 to the current reference population, Austria 2016 (Table S1), revealed an incidence of 23.7 per 100 000 adults per year.
The incidence of RSE was 7.2 per 100 000 adults per year (95% CI 3.3‐13.8), which included all SE episodes refractory to one benzodiazepine and one AED, irrespective of further treatment with other AEDs or anaesthetics (Table 1). SRSE occurred with an incidence of 1.2 (95% CI 0.1‐5.1). RSE treated only with AEDs had 44% acute etiology, 25% remote, and 31% progressive disease, whereas proportions in RSE with anesthetics were 35%, 61%, and 4%, respectively.
Stays in the neurologic intensive care unit (NICU) lasted a median of 1 day (range 0‐91; mean 3.7). Comorbidities are presented in Table S2. Table S3 provides an overview of the various treatment forms. Table S4 presents the data concordant to the STROBE statement.
4. DISCUSSION
This study showed an average cumulative incidence of first nonhypoxic SE of 36.1 per 100 000 adults per year with the new ILAE 2015 definition and classification of SE. NCSE had an annual incidence of 12.1, whereas this rate was 24.0 for SE with prominent motor phenomena (including 15.8 for CSE).
The incidence of SE is considerably higher than previously reported in most epidemiologic studies (range 1.3 to 27.2/100 000 per year), with the exception of 41 in Richmond (USA), which included a high proportion of African Americans.3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 Reducing the diagnostic time from 30 to 5 minutes in CSE, or to 10 minutes in focal SE or absence SE, increased the resulting incidence only moderately (by 10%). Initially, we attributed the high incidence of SE to the substantial proportion (29.4%) of elderly, as the elderly showed a substantial increase in incidence after the age of 50 years in this, and several other studies (Figure S1).3, 4, 6, 7, 10, 12, 13, 14, 17, 18 We adjusted the incidence of 18.3 per 100 000 total population per year found in Rochester by Hesdorffer et al4 between 1965 and 1984, which included 23.2% elderly, to our reference population, Austria 2016, and revealed an age‐ and sex‐adjusted incidence of 23.7 per 100 000 adults per year. However, our results are still around 1.5 times higher than the adjusted data from Rochester, which were obtained by a records‐linkage system of the Rochester Epidemiology Project with subsequent maximal ascertainment.4 Our study also assumes an almost complete ascertainment, as the study area was a small area within a much larger catchment area, in which our clinic served all hospitals by liaison, with 24/7 availability of neurologists and EEG. We assume that the high incidence is due to a high proportion (34.4%) of NCSE in our study. This is more than 2.5 times higher than the 13% of “non‐motor” SE in the last of the 4 decades in the Rochester study.4 Similar to our investigation, a study in Ferrara, Italy, included 24/7 EEG service, and therefore presumably had the same chance of diagnosing NCSE, but the incidence of NCSE was not calculated separately because the ILAE 2015 classification was not available at the time.14 In our study, the incidence of NCSE more than doubled from 2011/2012 to 2014/2015, which coincided with the Salzburg consensus EEG criteria for NCSE becoming available in 2013. Increasing awareness and a learning curve might also be responsible for the increase in incidence over time. The current study, and the studies performed in Bologna and Lugo di Romagna (both Italy), are the only ones that reported a female preponderance of SE.12, 13 In Salzburg, we found an especially high proportion of women (73%) in our patients with NCSE. We have no clear explanation for this, but speculate that an urban setting, as opposed to rural services, facilitates women’s access to the health care system, which may influence case ascertainment.
The age‐ and sex‐adjusted incidences of RSE of 7.2 per 100 000 adults per year (19.5% of all first SE episodes) and of SRSE of 1.2 (3.6%) were moderately higher than in a recently published Finnish population (RSE 2.7 and SRSE 0.75, combined 3.4/100 000).26 Our case fatalities of RSE (39.5%, Table 1) and SRSE (37.5%) were influenced by the small number of events, but were substantially higher than in the Finnish study (7.4% in hospital),26 and in the global audit for RSE (22.0%),27 but of the same order of magnitude as in a large US study (31.8%).28 Overall case fatality was 16.3% in this study, whereas in other studies it ranged from 5% in Ferrara to 39% (30‐day) in Bologna, both in Northern Italy.12, 14
Our investigations revealed that poor outcome is not correlated with prominent motor phenomena, but with the occurrence of nonconvulsive phases in the semiologic sequence. NCSE had a case fatality of 27.6%; this rate was 25.6% for SE with nonconvulsive semiology at the end of the semiologic sequence, 10% for SE with nonconvulsive semiology at the beginning of the sequence, and 3.5% for only prominent motor phenomena. Therefore, it seems crucial to search for nonconvulsive phases in the semiologic sequence to estimate the risk for bad outcome, or, alternatively, to search for prominent motor phenomena for good outcome. The semiology that comes later determines the outcome. Case fatality was zero when semiologic sequence ended with or consisted of only bilateral tonic–clonic activity. Bilateral tonic–clonic activity might get noticed quickly by bystanders and might reflect the early full cerebral capacity of seizing without metabolic or electric exhaustion.5, 29 Indeed, our results challenge the historical view that bilateral tonic–clonic activity at any time during a SE episode should be classified as “convulsive” SE, irrespective of the position in the semiologic chain, the length of time, and the other semiologies during SE. Other large studies did not address this issue and failed to identify an influence of semiology on outcome.30 The semiology hierarchy should be reassessed and analyzed in future large studies, to provide precise semiology and associated outcome data.
With regard to LOCs, case fatality was 2.8% in patients fully awake or awake with reduced cognition in the case of SE with prominent motor phenomena, except CSE (LOC is not meaningful in CSE). However, case fatality was 26.9% if patients were somnolent, stuporous, or comatose (Table 4). Accordingly, in the NCSE group, we found similar case fatalities for fully awake (0.0%) and awake with reduced cognition (13.9%), in contrast with somnolence (36.8%), stupor (46.2%), and coma (42.9%) (Figure 2). We suggest that future studies should investigate the semiologic sequence with special emphasis on the identification of nonconvulsive phases, and determine the levels of consciousness (fully awake, awake with reduced cognition, somnolent, stuporous, or comatose).
This study has several limitations. First, the retrospective study design predisposed to underascertainment of spontaneously stopping episodes, and of those NCSE episodes that were treated successfully before EEG service arrived. However, only EEG could differentiate between SE and a seizure with Todd’s phenomenon in cases of otherwise missing clinical features, such as minor jerking or forced contralateral gaze deviation. Spontaneous cessation occurred in around 20% of cases in a placebo‐controlled trial,31 but only in 2.3% of cases in our study. We observed spontaneous cessation of SE up to 300 minutes after focal motor SE without LOC. Therefore, prospective studies should include spontaneously stopping SE at any time. We did not review patients with epilepsy without a diagnosis of SE for events meeting SE criteria. Such events may have been coded as epilepsy as opposed to SE, in particular with absence status. Second, one might argue that the retrospective design may result in inappropriate data acquisition relying on medical records. However, we have developed a data acquisition sheet32 which is standard operating procedure in our department. This SE documentation sheet accompanies the patient from our neuroemergency unit to the EEG lab, normal ward, and neurologic intensive care unit, ensuring the highest level of data reliability.32
Third, clinicians were free to decide in cases of SE refractory to one benzodiazepine and one AED whether to proceed with anesthetics or other AEDs. Case fatality was 41.0% in the group of RSE with further AED use, and 25.0% in RSE with anesthetics. We found no differences in the pattern of comorbidities between those groups; however, there was a tendency of a higher proportion of acute or progressive disease with nonsurvivors compared with a higher rate of remote etiologies with survivors. Physicians might have been reluctant to use anesthetics in patients with progressive disease (eg, tumor), or acute diseases (eg, severe stroke), who are already compromised and have an increased chance of death. This may have confounded the outcome data in our study. The use of intravenously applied anesthetics was identified as an independent risk factor in a recent 2‐center study.33 However, numbers in our study were too small to draw firm conclusions.
Fourth, patients with other major medical problems who were too ill to be transferred to their hospital could have been missed. Therefore we assume a slight underestimation of SE. However, we believe to have achieved the maximally possible coverage of SE.
Age strata of 5 years for study population and population of Salzburg are provided to perform age and sex adjustment of our data to any population (Table S1). This allows calculation of health care impact in populations with different population pyramids or in future populations estimated by census bureaus.
In summary, this first population‐based study applying the new ILAE (2015) diagnostic and classification criteria for SE in a small urban area over 5 years, with a very low probability of underascertainment, yielded an incidence of SE almost 1.5 times higher than previously reported. This finding is likely attributable to a high proportion of elderly and a high rate of NCSE in this study. The present study provides the first systematic epidemiologic evidence that the evolution of semiology has an impact on clinical outcome. Larger prospective multicenter studies are needed to allow an even deeper understanding of outcome‐relevant parameters. The current ILAE classification provides a good starting point to address this issue.
We highlight the practical advantage of the ILAE 2015 definition and classification, which improves analysis of NCSE, and the need for meticulous assessment of the evolution of semiology and LOCs to learn more about clinicopathologic correlations, which will improve outcome prediction.
DISCLOSURE OF CONFLICTS OF INTEREST
Markus Leitinger reports grants from Medtronic and UCB Pharma and personal fees from Everpharma and Eisai. Eugen Trinka reports personal fees from Eisai, personal fees from Everpharma, grants and personal fees from Biogen Idec, personal fees from Medtronics, personal fees from Bial, personal fees from Newbridge, grants and personal fees from UCB Pharma and Eisai, personal fees from GL Pharma, personal fees from GlaxoSmithKline, personal fees from Boehringer, personal fees from Viropharma and Actavis, grants from Red Bull, grants from Merck, grants from European Union, grants from FWF Österreichischer Fond zur Wissenschaftsförderung, grants from Bundesministerium für Wissenschaft und Forschung, and grants from the Jubiläumsfond der Österreichischen Nationalbank, outside the submitted work. Georg Zimmermann reports financial support from an Investigator‐Initiated Study grant from Eisai Ltd. (FYC‐IIS‐0M044‐1023) to Paracelsus Medical University, not related to the present work. Alexandra Rohracher reports travel support and speaker’s honoraria from Eisai. Gudrun Kalss reports travel support by UCB, Eisai, OEGN (Austrian Society of Neurology), Cyberonics, the Asian Oceanian Association of Neurology, CEA, and ILAE. Caroline Neuray reports speaker′s honoraria and travel expenses from Eisai. Julia Höfler reports speaker’s honoraria from UCB and travel grants from UCB, Eisai, and Gerot‐Lannach. Giorgi Kuchukhidze has received travel support from Eisai and UCB Pharma. Judith Dobesberger has received honoraria and travel support from UCB Pharma, Gerot‐Lannach, Eisai, GlaxoSmithKline, and Neurodata GmbH/Micromed Austria. Helmut F. Novak has received speaker’s honoraria from Baxter Austria, Astellas Pharma, SCS‐Angelini Pharmaceuticals, Fresenius Medical Care Austria and Orion Pharma, from UCB Pharma for clinical medication monitoring, travel grants from Fresenius Kabi Austria and as consultant to Hayward Medical Communications. Stefano Meletti reports personal fees as a scientific advisory board member for UCB and EISAI. Giada Giovannini, Christina Florea, Rudolf Kreidenhuber, Claudia Granbichler, Georg Pilz and Uwe Siebert report no disclosure. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
ACKNOWLEDGMENTS
We wish to thank Statistik Austria for their support, and all the teams of emergency ward and intensive care units for taking best care of their patients. We thank Dr Delia Randall for editing the English language, checking consistency, and formatting the manuscript. Dr Randall was paid by financial resources of the Department of Neurology Salzburg, which are dedicated for support of scientific work and publication and are free of commercial interests.
Leitinger M, Trinka E, Giovannini G, et al. Epidemiology of status epilepticus in adults: A population‐based study on incidence, causes, and outcomes. Epilepsia. 2019;60:53–62. 10.1111/epi.14607
Leitinger and Trinka joint first authors
REFERENCES
- 1. Neligan A, Shorvon SD. Frequency and prognosis of convulsive status epilepticus of different causes: a systematic review. Arch Neurol. 2010;67:931–40. [DOI] [PubMed] [Google Scholar]
- 2. Trinka E, Cock H, Hesdorffer D, et al. A definition and classification of status epilepticus—Report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia. 2015;56:1515–23. [DOI] [PubMed] [Google Scholar]
- 3. DeLorenzo RJ, Hauser WA, Towne AR, et al. A prospective, population‐based epidemiologic study of status epilepticus in Richmond, Virginia. Neurology. 1996;46:1029–35. [DOI] [PubMed] [Google Scholar]
- 4. Hesdorffer DC, Logroscino G, Cascino G, Annegers JF, Hauser WA. Incidence of status epilepticus in Rochester, Minnesota, 1965‐1984. Neurology. 1998;50:735–41. [DOI] [PubMed] [Google Scholar]
- 5. Logroscino G, Hesdorffer DC, Cascino G, Annegers JF, Hauser WA. Time trends in incidence, mortality, and case‐fatality after first episode of status epilepticus. Epilepsia. 2001;42:1031–5. [DOI] [PubMed] [Google Scholar]
- 6. Wu YW, Shek DW, Garcia PA, Zhao S, Johnston SC. Incidence and mortality of generalized convulsive status epilepticus in California. Neurology. 2002;58:1070–6. [DOI] [PubMed] [Google Scholar]
- 7. Dham BS, Hunter K, Rincon F. The epidemiology of status epilepticus in the United States. Neurocrit Care. 2014;20:476–83. [DOI] [PubMed] [Google Scholar]
- 8. Betjemann JP, Josephson SA, Lowenstein DH, Burke JF. Trends in status epilepticus‐related hospitalizations and mortality: redefined in US practice over time. JAMA Neurol. 2015;72:650–5. [DOI] [PubMed] [Google Scholar]
- 9. Jallon P, Coeytaux A, Galobardes B, Morabia A. Incidence and case‐fatality rate of status epilepticus in the Canton of Geneva. Lancet. 1999;353:1496. [DOI] [PubMed] [Google Scholar]
- 10. Coeytaux A, Jallon P, Galobardes B, Morabia A. Incidence of status epilepticus in French‐speaking Switzerland: (EPISTAR). Neurology. 2000;55:693–7. [DOI] [PubMed] [Google Scholar]
- 11. Knake S, Rosenow F, Vescovi M, et al. Incidence of status epilepticus in adults in Germany: a prospective, population‐based study. Epilepsia. 2001;42:714–8. [DOI] [PubMed] [Google Scholar]
- 12. Vignatelli L, Tonon C, D’Alessandro R, Bologna Group for the Study of Status Epilepticus . Incidence and short‐term prognosis of status epilepticus in adults in Bologna, Italy. Epilepsia. 2003;44:964–8. [DOI] [PubMed] [Google Scholar]
- 13. Vignatelli L, Rinaldi R, Galeotti M, de Carolis P, D’Alessandro R. Epidemiology of status epilepticus in a rural area of northern Italy: a 2‐year population‐based study. Eur J Neurol. 2005;12:897–902. [DOI] [PubMed] [Google Scholar]
- 14. Govoni V, Fallica E, Monetti VC, et al. Incidence of status epilepticus in southern Europe: a population study in the health district of Ferrara, Italy. Eur Neurol. 2008;59:120–6. [DOI] [PubMed] [Google Scholar]
- 15. Tiamkao S, Pranboon S, Thepsuthammarat K, Sawanyawisuth K. Incidences and outcomes of status epilepticus: a 9‐year longitudinal national study. Epilepsy Behav. 2015;49:135–7. [DOI] [PubMed] [Google Scholar]
- 16. Tiamkao S, Pranbul S, Sawanyawisuth K, Thepsuthammarat K; Integrated Epilepsy Research Group . A national database of incidence and treatment outcomes of status epilepticus in Thailand. Int J Neurosci. 2014;124:416–20. [DOI] [PubMed] [Google Scholar]
- 17. Ong CT, Sheu SM, Tsai CF, Wong YS, Chen SC. Age‐dependent sex difference of the incidence and mortality of status epilepticus: a twelve year nationwide population‐based cohort study in Taiwan. PLoS ONE. 2015;10:e0122350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bhalla D, Tchalla AE, Mignard C, et al. First‐ever population‐based study on status epilepticus in French Island of La Reunion (France) ‐ incidence and fatality. Seizure. 2014;23:769–73. [DOI] [PubMed] [Google Scholar]
- 19. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495–9. [DOI] [PubMed] [Google Scholar]
- 20. Drislane FW. Presentation, evaluation, and treatment of nonconvulsive status epilepticus. Epilepsy Behav. 2000;1:301–14. [DOI] [PubMed] [Google Scholar]
- 21. Leitinger M, Trinka E, Gardella E, et al. Diagnostic accuracy of the Salzburg EEG criteria for non‐convulsive status epilepticus: a retrospective study. Lancet Neurol. 2016;15:1054–62. [DOI] [PubMed] [Google Scholar]
- 22. Shorvon S, Ferlisi M. The outcome of therapies in refractory and super‐refractory convulsive status epilepticus and recommendations for therapy. Brain. 2012;135:2314–28. [DOI] [PubMed] [Google Scholar]
- 23. Statistik Austria . Statistik des Bevölkerungsstandes (census bureau, population statistics), Available from (https://www.statistik.at/web_de/klassifikationen/regionale_gliederungen/statistische_zaehlsprengel/index.html). Accessed August 1st 2017.
- 24. Agresti A, Coull BA. Approximate is better than ‘exact’ for interval estimation of binomial proportions. Am Stat. 1998;52:119–26. [Google Scholar]
- 25. Dobson AJ, Kuulasma K, Eberle E, Scherer J. Confidence intervals for weighted sums of Poisson parameters. Stat Med. 1991;10:457–62. [DOI] [PubMed] [Google Scholar]
- 26. Kantanen AM, Reinikainen M, Parviainen I, Kälviäinen R. Long‐term outcome of refractory status epilepticus in adults: a retrospective population‐based study. Epilepsy Res. 2017;133:13–21. [DOI] [PubMed] [Google Scholar]
- 27. Ferlisi M, Hocker S, Grade M, Trinka E, Shorvon S; International Steering Committee of the StEp Audit . Preliminary results of the global audit of treatment of refractory status epilepticus. Epilepsy Behav. 2015;49:318–24. [DOI] [PubMed] [Google Scholar]
- 28. Hocker SE, Britton JW, Mandrekar JN, Wijdicks EF, Rabinstein AA. Predictors of outcome in refractory status epilepticus. JAMA Neurol. 2013;70:72–7. [DOI] [PubMed] [Google Scholar]
- 29. Clark LP, Prout TP. Status epilepticus: a clinical and pathological study in epilepsy. [An article in 3 parts]. Am J Insanity. 1903;60:291–306. 60:645–75,61:81–108. [Google Scholar]
- 30. Baysal‐Kirac L, Feddersen B, Einhellig M, Rémi J, Noachtar S. Does semiology of status epilepticus have an impact on treatment response and outcome? Epilepsy Behav. 2018;83:81–6. [DOI] [PubMed] [Google Scholar]
- 31. Alldredge BK, Gelb AM, Isaacs SM, et al. A comparison of lorazepam, diazepam, and placebo for the treatment of out‐of‐hospital status epilepticus. N Engl J Med. 2001;345:631–7. [DOI] [PubMed] [Google Scholar]
- 32. Leitinger M, Kalss G, Rohracher A, et al. Predicting outcome of status epilepticus. Epilepsy Behav. 2015;49:126–30. [DOI] [PubMed] [Google Scholar]
- 33. Sutter R, De Marchis GM, Semmlack S, et al. Anesthetics and outcome in status epilepticus: a matched two‐center cohort study. CNS Drugs. 2017;31:65–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


