Summary
Ex vivo machine perfusion of the liver after cold storage has found to be most effective if combined with controlled oxygenated rewarming up to (sub)‐normothermia. On disconnection of the warm graft from the machine, most surgeons usually perform a cold flush of the organ as protection against the second warm ischemia incurred upon implantation. Experimental evidence, however, is lacking and protective effect of deep hypothermia has been challenged for limited periods of liver ischemia in other models. A first systematic test was carried out on porcine livers, excised 30 min after cardiac arrest, subjected to 18 h of cold storage in UW and then machine perfused for 90 min with Aqix‐RSI solution. During machine perfusion, livers were gradually rewarmed up to 20 °C. One group (n = 6) was then reflushed with 4 °C cold Belzer UW solution whereas the second group (n = 6) remained without cold flush. All livers were exposed to 45 min warm ischemia at room temperature to simulate the surgical implantation period. Organ function was evaluated in an established reperfusion model using diluted autologous blood. Cold reflush after disconnection from the machine resulted in a significant increase in bile production upon blood reperfusion, along with a significant reduction in transaminases release alanine aminotransferase and of the intramitochondrial enzyme glutamate dehydrogenase. Interestingly, free radical‐mediated lipid peroxidation was also found significantly lower after cold reflush. No differences between the groups could be evidenced concerning histological injury and recovery of hepatic energy metabolism (tissue content of adenosine triphosphate). Post‐machine preservation cold reflush seems to be beneficial in this particular setting, even if the organs are warmed up only to 20 °C, without notion of adverse effects, and should therefore be implemented in the protocol.
Keywords: cold flush, controlled oxygenated rewarming, liver preservation, machine perfusion, organ reconditioning
Introduction
Liver transplantation is often the last therapeutic option for end‐stage liver diseases, like cirrhosis or hepatic cancer. However, the steadily increasing demand on donor organ grafts is driving the growing use of marginal grafts originating from older donors or patients with disease condition 1.
In order to cope with the reduced resilience of such organs towards ischemic preservation, dynamic preservation and reconditioning methods have found their way into clinical practice of organ storage.
These mainly focus on ex vivo machine perfusion of the retrieved organ, most often performed in hypothermia using perfusion solutions adapted for organ perfusion at low temperatures 2, 3.
It was also found that main tissue damage does not occur during static storage, but rather evolves upon reestablishment of the blood circulation upon surgical revascularization fostering the postponed use of machine perfusion even after preceding cold storage and easy transportation of the graft conventionally stored on ice 4, 5, 6, 7.
Subsequently, ex vivo organ perfusion had been tried at higher temperatures, in order to enhance metabolic (re‐)equilibration of tissue homeostasis and to improve graft quality assessment prior to implantation. Thus, normothermic 8, 9, 10 or subnormothermic 11, 12, 13 perfusion of the liver has been proposed as a method of preservation or beneficial adjunct following usual cold storage in clinical liver transplantation.
Recently, it has been suggested that abrupt rise in temperature after hypothermic preservation may represent a genuine trigger of tissue injury, aggravating mitochondrial dysfunction and negatively affecting graft function upon reperfusion 14. As a consequence, slow warming up of the cold liver while being perfused on an ex vivo perfusion machine—the so called “controlled oxygenated rewarming” has been proposed to circumvent this “rewarming injury” 15.
Clinical transplantation procedures, however, comprise a mandatory phase of ischemia at ambient body temperature during surgical implantation, during which the graft is not actively oxygenated.
This second warm ischemia between machine perfusion and actual reperfusion in vivo upon reestablishment of blood circulation is usually dealt with by an additional cold organ flush at the end of machine perfusion with the aim to cool down the organ and thereby protect the graft from ischemic injury.
However, experimental evidence for this habit to be beneficial is lacking and it is still elusive if this cold flush contributes to tissue protection or if additional temperature variations could have negative impacts on tissue recovery. Earlier studies already analyzed the effect of intraischemic temperatures on later organ function in rat livers and found that mild hypothermia at 32–26 °C already diminishes the oxidative stress effectively, which the authors attribute to a reduced initial production of reactive oxygen species 16.
Moreover, static organ storage at elevated temperatures has already been proposed with Aqix‐RSI solution as useful medium and might thus render it reasonable to avoid a final cold flush of the machine perfused organ 17, 18.
Therefore, the present study was aimed to systematically investigate the usefulness of an initial cold flush prior to second warm ischemia during implantation as strategy to protect the liver graft from further injurious events.
Methods
All experiments were performed in accordance with the federal law regarding the protection of animals. The principles of laboratory animal care (NIH publication no. 85‐23, revised 1985) were followed.
Livers of female German Landrace pigs weighing between 25 and 30 kg were premedicated with intramuscular injection of ketamine (20 mg/kg), xylazine (2 mg/kg), and atropine (0.25 mg/kg). General anesthesia on the operating table was induced by intravenous bolus of midazolam (0.5 mg/kg) and fentanyl (10 μg/kg). On intubation, anesthesia was maintained by continuous mechanical ventilation with isoflurane. After liver dissection, cardiac standstill was induced by bleeding to death and injection of 20 ml potassium chloride (7.5%). Blood was collected in commercial bags (Composelect; Fresenius Kabi, Bad Homburg, Germany) for later experiments.
Thirty minutes after cardiocirculatory standstill, the liver was rinsed in situ by gravity with 2 l of 4 °C cold UW‐solution (Bridge to Life, London, UK). Afterward, livers were removed and hepatic artery and portal vein were cannulated on the back table and flushed with another liter UW‐solution at 4 °C. Livers were finally cold stored overnight in UW‐solution for 18 h.
After static cold storage (CS), livers were subjected to oxygenated machine perfusion in a liver assist system as described previously 19. Livers were perfused with 2 l Aqix‐RSI solution, oxygenated with 95% O2 and 5% CO2 for 90 min. Temperature was controlled and gradually increased from 8 °C up to 20 °C within the first 60 min and kept constant for the remaining 30 min. Portal vein and hepatic artery were connected to separate perfusion circuits with integrated hollow fiber oxygenators, perfusion pumps, and pressure sensors. The hepatic artery was perfused in a pulsatile mode at a pressure of 25 mmHg. Perfusion of the portal vein was applied continuously at 3 mmHg.
Prior to reperfusion, grafts were randomly assigned to one of the following groups (n = 6, resp.):
At the end of ex vivo MP, livers were rinsed with 1 l cold UW‐ solution; afterward, grafts were exposed to room temperature for 45 min to simulate the implantation period.
Livers were exposed to 45 min at room temperature without additional cold flush after MP.
For the flush group, liver temperature measurements were done after cold flush and at the end of the 45 min implantation interval. All livers were flushed with balanced salt solution at room temperature immediately prior to reperfusion. Afterward, functional recovery of the grafts was tested using an established in vitro reperfusion model as described previously 20, 21.
Isolated liver reperfusion model
In brief, livers were transferred to a moist chamber and perfused at 37 °C with autologous blood that was retrieved during surgical intervention and stored overnight in commercial blood bags. One liter of blood was diluted with 500 ml Gelafundin 5% and supplemented with Ca2Cl and sodium bicarbonate to adjust physiological calcium concentration and pH.
Temperature was controlled by a circulating thermostat and perfusate was oxygenated by a temperature controlled hollow fiber oxygenator (Hilite LT 1000; Medos, Stolberg, Germany). Gas flow was adapted to physiological blood gas values (pO2 150–200 mmHg, pCO2 40–60 mmHg). Hepatic artery was perfused at a physiological pressure of 80 mmHg, controlled by an integrated servo‐controlled roller pump and a pressure sensor connected to the inflow line immediately prior to the liver artery. Portal vein was perfused constantly at 0.9 ml/g/min driven by a centrifugal blood pump and portal perfusion pressure was controlled by a water column connected to the inflow tract.
During perfusion, livers were continuously supplied with a mixture of 80 ml glucose 5%, 20 ml amino acids (RPMI 50×), 10 IU insulin, and 1 mg taurocholic acid, infused to the perfusion circuit at 15 ml/min to provide nutrient support and substrate for bile production.
Analytical procedures
Perfusion resistance of hepatic artery and portal vein was calculated using independently measured flow and pressure values in relation to liver mass, expressed in mmHg * l * min−1 * 100 g−1.
Oxygen partial pressure, Hb, and perfusate concentrations of lactate and glucose were measured in a pH‐blood gas analyzer (ABL 815flex acid‐base laboratory; Radiometer, Copenhagen, Denmark).
Serum enzyme activities of alanine aminotransferase (ALT) and glutamate dehydrogenase (GLDH) were determined in a routine fashion at the laboratory center of the University Hospital.
The common bile duct was cannulated using polyethylene tubing and bile was collected throughout the reperfusion period. Hepatic bile production was calculated as ml/kg/h.
Oxygen‐free radical‐induced tissue injury was approximated by the amount of thiobarbituric acid‐reactive substances (TBARS), breakdown products of lipid peroxidation (LPO), released into the circulation upon reperfusion. TBARS were evaluated by fluorimetry from deproteinized serum samples using the adduct formation with thiobarbituric acid as detailed elsewhere 22.
Energetic status
Tissue specimens for assessment of high‐energy phosphates were taken with precooled steel tongs, immersed in liquid nitrogen, and stored at −80 °C for later analysis. Wet weight of the frozen tissue samples was measured before they were lyophilized in a vacuum freezer (−60 °C; <0.025 mbar) for at least 7 days to evaporate tissue water. Freeze‐dried specimens were weighed again and proteins were extracted with perchloric acid as described previously 7. Aliquots of the neutralized supernatant were used for the determination of adenosine triphosphate (ATP) by means of a commercial test kit (Abcam, Cambridge, UK) according to the manufacturer's instructions. The results were corrected for the respective dry weight to wet weight ratio of the tissue samples and expressed as μmol/g dry weight (dw).
Histology
Liver tissue samples were obtained at the end of experiments and were fixed in 4% buffered formalin. Paraffin embedding, tissue sections, and hematoxylin–eosin (HE) staining were performed in the institute of pathology of university hospital Essen using standard procedures. Morphological evaluation of parenchymal integrity was done at 200‐fold magnification. The extent of injury was semiquantitatively graded in a four‐stage system ranging from 0 to 3 in a blinded fashion by two independent investigators.
Statistics
All values are expressed as means ± SEM for n = 6 animals per group. Differences between groups were tested by unpaired, two‐sided t‐test, unless otherwise indicated. Statistical significance was set at P < 0.05.
Results
Influence of cold reflush on liver core temperature
Liver temperature after machine perfusion at 20 °C declined to 12.9 ± 0.3 °C after reflush with 1 l of cold UW‐solution. During the ensuing 45 min of simulated second warm ischemia at room temperature, liver core temperature rose again to attain a mean value of 16.6 ± 0.3 °C. Temperature in the untreated group during the second warm ischemia remained around room temperature.
Cold flush reduces liver enzyme release
During reperfusion, perfusate samples were collected every 30 min and enzyme releases of ALT and GLDH during reperfusion were taken as general parameters of hepatocellular injury of the liver.
The release of liver enzymes was observed to steadily increase during 3 h reperfusion in both groups. However, leakage of cytosolic ALT as well as of the intramitochondrial GLDH could be significantly reduced by initial cold flush prior to second warm ischemia, as illustrated in Fig. 1.
Figure 1.
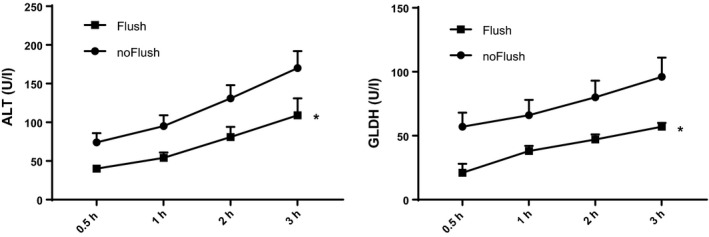
Release of liver enzymes during reperfusion after 18 h static cold storage and 90 min machine perfusion with controlled oxygenated rewarming up to 20 °C. Comparison of cold flush (Flush, n = 6) versus no‐flush (noFlush, n = 6) at the end of MP prior to 45 min implantation period. AST, aspartate‐aminotransferase; GLDH, glutamate dehydrogenase. Mean ± SEM, *P < 0.05.
Cold flush improves hepatic bile production
During experiments, hepatic bile production was observed as representative marker for hepatocyte functional integrity. Bile secretion was observed throughout 3 h reperfusion period (Fig. 2). The absence of cold flush after ex vivo MP diminished bile production of hepatocytes during reperfusion as presented in Fig. 2. A cold organ flush prior to second warm ischemia resulted in a significant increase for more than 30% of bile produced during the second half of perfusion time than observed for the no flush group.
Figure 2.
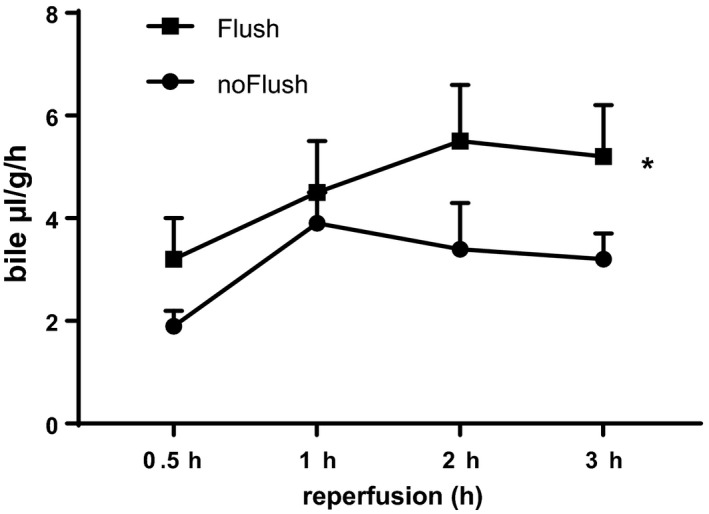
Hepatic bile production during 3 h reperfusion after 18 h static cold storage and 90 min machine perfusion with controlled oxygenated rewarming up to 20 °C. Comparison of cold flush (Flush, n = 6) versus no‐flush (noFlush, n = 6) at the end of controlled oxygenated rewarming prior to 45 min implantation period. Mean ± SEM, *P < 0.05.
Cold flush reduces oxidative stress
Oxidative stress incurred by the livers during the respective protocols was assessed by the measurement of free radical‐mediated LPO. LPO was analyzed in perfusate samples, collected at the end of 3 h reperfusion, and is shown in Fig. 3. Cold organ flush resulted in significantly lower LPO values than the control group without additional flush at the end of MP.
Figure 3.
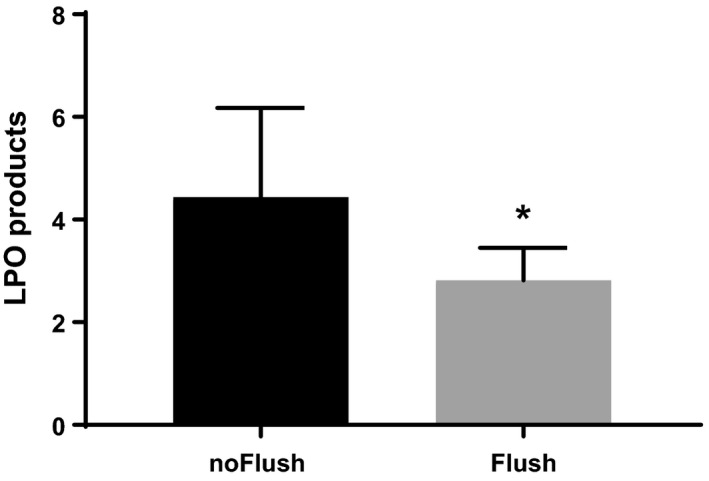
Lipid peroxidation products at the end of 3 h reperfusion. Cold stored livers were subjected to 90 min ex vivo machine perfusion with controlled oxygenated rewarming up to 20 °C. Comparison of cold flush (Flush, n = 6) versus no‐flush (noFlush, n = 6) at the end of controlled oxygenated rewarming prior to 45 min implantation period. Mean ± SEM, *P < 0.05.
Histology score
Structural alterations of the livers were analyzed using formalin fixed liver sections that were stained with hematoxylin–eosin (HE). Both treatment groups exhibited only moderate alterations of liver morphology and there were no obvious differences between the two groups (Fig. 4). Quantification of structural alterations yielded injury score values of 1.36 ± 0.24 for the cold flush group and 1.44 ± 0.15 for the control group without flush prior to the second warm ischemia (means ± SEM, differences being not significant).
Figure 4.
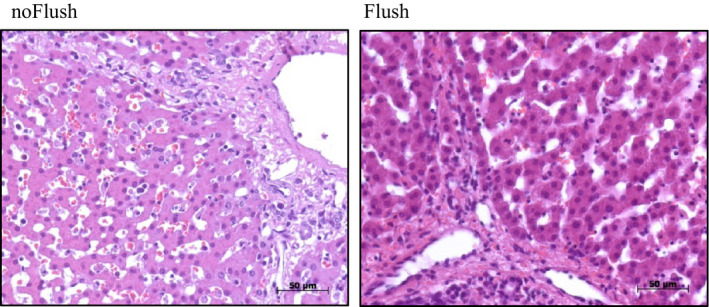
Liver tissue morphology at the end of 3 h reperfusion. Cold stored livers were subjected to 90 min ex vivo machine perfusion with controlled oxygenated rewarming up to 20 °C. Comparison of cold flush (Flush, n = 6, right) versus no‐flush (noFlush, n = 6, left) at the end of controlled oxygenated rewarming prior to 45 min implantation period.
Energetic recovery
Recovery of hepatic energy metabolism was assessed by tissue content of ATP at the end of the experiment as shown in Fig. 5. Flushed livers show by tendency more ATP production, but differences did not reach statistical significance.
Figure 5.
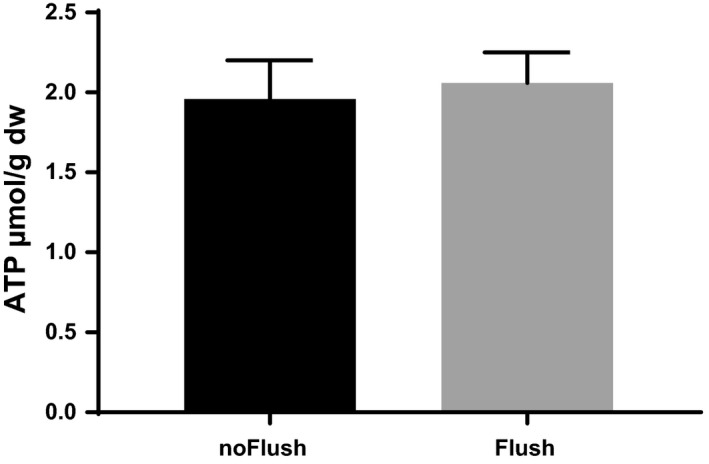
Tissue content of adenosine triphosphate at the end of 3 h reperfusion. Before reperfusion livers were subjected to 90 min controlled oxygenated rewarming to 20 °C with (n = 6) or without additional cold flush (n = 6) at the end of machine perfusion. Mean ± SEM.
Discussion
Recent upsurge of normothermic ex vivo graft perfusion prior to transplantation has brought up the question how to deal with the warm liver once disconnected from the perfusion machine.
In the case of subnormothermic machine perfusion, which has proven comparably effective by several groups 23, 24, 25, the need for secondary cooling of the previously rewarmed graft becomes even more controversial.
The perfusion solution used in this study—Aqix RSI—has already been proposed as useful medium for flush and static preservation of kidneys at 30 °C 17, 18. However, the results of the present study argue in favor of a cold flush to protect the organ during the second warm ischemia in the setting of engraftment.
Previous research has suggested that the thermal transit from cold to warm may, on its own, represent a potential inducer of cell injury 14, 26. This “rewarming injury” depends on cellular alterations occurring during hypothermia and seems to be mediated by iron‐dependent formation of reactive oxygen species 27. In this context, it might be of importance that the cold flush only lowers the core temperature of the livers but does not result in a severe cooling down to strictly hypothermic values of about 4 °C. It is known from systematic investigations in isolated cells that cellular release of LDH upon rewarming from the cold only takes place if the cells have previously been kept at temperatures below 13–16 °C 28 and if this exposure has lasted for longer time periods. Thus, the additional only mild thermal transition, encountered upon cold reflush of the organ, will conjecturally not be associated with notable side effects either. Moreover, observations of Khandoga et al. 16, using an in situ mouse model of partial liver ischemia, have shown, that the increase in tissue levels of reactive oxygen species mediated lipid peroxides was significantly reduced by lowering the intraischemic temperature to 26 °C, but completely abolished by intraischemic temperatures of 15 °C or less.
In line with these observations, we have found a significant reduction in lipid peroxide generation by implementing a cold flush of the organ prior to second warm ischemia and the small decrease in liver temperature from 20 to 13–16 °C might hence have been sufficient for a notable reduction in free radical generation. This is also translated into a significant reduction in consecutive liver injury, as evaluated by enzyme loss and improved bile production, but differences were not as prominent as to impact liver energetics or to induce morphological changes.
Interestingly, previous work of Biberthaler is in apparent contrast to our findings. The study showed an almost complete prevention of disintegration of hepatic parenchyma and release of transaminases after in situ warm ischemia of mouse livers for 90 min if parenchymal temperature was lowered to only 26 °C 29. No further protection was provided by more intense cooling of the organ. The different impact on transaminase release between our study and the results of Biberthaler et al. might be due to species differences or the divergences in the model as a healthy, blood perfused organ rendered ischemic by clamping the afferent vessels might react slightly different to a prealtered isolated perfused liver.
The model used in our study has been chosen as to resemble to the human situation as close as possible. Only large size organs will thermodynamically reflect a core temperature equilibration pattern similar to the clinical situation. Pilot investigations on rat livers showed an unrepresentatively swift passive rewarming after rinse with cold media (B. Lüer, personal communications) and were not adequate to delineate differences between reflushed and untreated livers.
The present experiments only addressed the subnormothermic situation, but clearly demonstrated an overall benefit obtained by reflushing the livers with cold media prior to second warm ischemia.
It can hence conjectured that the implementation of a secondary cold flush will also be useful after machine perfusion at normothermia in a comparable setting.
Authorship
CvH: wrote paper, performed research, and analyzed data. PH: analyzed data and wrote paper. TH: designed study and revised paper. HL: designed study and revised paper. AP: designed research and revised paper. TM: designed research, analyzed data, and wrote paper.
Funding
This research has received funding from the European Union's Seventh Framework Programme for Research, Technological Development and Demonstration under grant agreement no 305934.
Conflicts of interest
The authors have declared no conflicts of interest.
References
- 1. Gastaca M. Extended criteria donors in liver transplantation: adapting donor quality and recipient. Transplant Proc 2009; 41: 975. [DOI] [PubMed] [Google Scholar]
- 2. Yuan X, Theruvath AJ, Ge X, et al Machine perfusion or cold storage in organ transplantation: indication, mechanisms, and future perspectives. Transpl Int 2010; 23: 561. [DOI] [PubMed] [Google Scholar]
- 3. Guarrera JV, Henry SD, Samstein B, et al Hypothermic machine preservation in human liver transplantation: the first clinical series. Am J Transplant 2010; 10: 372. [DOI] [PubMed] [Google Scholar]
- 4. Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol 2012; 298: 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Minor T, Paul A. Hypothermic reconditioning in organ transplantation. Curr Opin Organ Transplant 2013; 18: 161. [DOI] [PubMed] [Google Scholar]
- 6. Dutkowski P, Graf R, Clavien PA. Rescue of the cold preserved rat liver by hypothermic oxygenated machine perfusion. Am J Transplant 2006; 6(5 Pt 1): 903. [DOI] [PubMed] [Google Scholar]
- 7. Stegemann J, Minor T. Energy charge restoration, mitochondrial protection and reversal of preservation induced liver injury by hypothermic oxygenation prior to reperfusion. Cryobiology 2009; 58: 331. [DOI] [PubMed] [Google Scholar]
- 8. Ravikumar R, Jassem W, Mergental H, et al Liver transplantation after ex vivo normothermic machine preservation: a phase 1 (first‐in‐man) clinical trial. Am J Transplant 2016; 16: 1779. [DOI] [PubMed] [Google Scholar]
- 9. Bral M, Gala‐Lopez B, Bigam D, et al Preliminary single centre Canadian experience of human normothermic ex vivo liver perfusion: results of a clinical trial. Am J Transplant 2017; 17: 1071. [DOI] [PubMed] [Google Scholar]
- 10. Fondevila C, Hessheimer AJ, Maathuis MH, et al Superior preservation of DCD livers with continuous normothermic perfusion. Ann Surg 2011; 254: 1000. [DOI] [PubMed] [Google Scholar]
- 11. Vairetti M, Ferrigno A, Carlucci F, et al Subnormothermic machine perfusion protects steatotic livers against preservation injury: a potential for donor pool increase? Liver Transpl 2009; 15: 20. [DOI] [PubMed] [Google Scholar]
- 12. Bruinsma BG, Yeh H, Ozer S, et al Subnormothermic machine perfusion for ex vivo preservation and recovery of the human liver for transplantation. Am J Transplant 2014; 14: 1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hoyer DP, Mathe Z, Gallinat A, et al Controlled oxygenated rewarming of cold stored livers prior to transplantation: first clinical application of a new concept. Transplantation 2016; 100: 147. [DOI] [PubMed] [Google Scholar]
- 14. Minor T, von Horn C, Paul A. Role of temperature in reconditioning and evaluation of cold preserved kidney and liver grafts. Curr Opin Organ Transplant 2017; 22: 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hoyer DP, Paul A, Luer S, Reis H, Efferz P, Minor T. End‐ischemic reconditioning of liver allografts: controlling the rewarming. Liver Transpl 2016; 22: 1223. [DOI] [PubMed] [Google Scholar]
- 16. Khandoga A, Enders G, Luchting B, et al Impact of intraischemic temperature on oxidative stress during hepatic reperfusion. Free Radic Biol Med 2003; 35: 901. [DOI] [PubMed] [Google Scholar]
- 17. Kay MD, Hosgood SA, Harper SJ, Bagul A, Waller HL, Nicholson ML. Normothermic versus hypothermic ex vivo flush using a novel phosphate‐free preservation solution (AQIX) in porcine kidneys. J Surg Res 2011; 171: 275. [DOI] [PubMed] [Google Scholar]
- 18. Kay MD, Hosgood SA, Harper SJ, et al Static normothermic preservation of renal allografts using a novel nonphosphate buffered preservation solution. Transpl Int 2007; 20: 88. [DOI] [PubMed] [Google Scholar]
- 19. Luer B, Fox M, Efferz P, Minor T. Adding pulsatile vascular stimulation to venous systemic oxygen persufflation of liver grafts. Artif Organs 2014; 38: 404. [DOI] [PubMed] [Google Scholar]
- 20. Koetting M, Lüer B, Efferz P, Paul A, Minor T. Optimal time for hypothermic reconditioning of liver grafts by venous systemic oxygen persufflation (VSOP) in a large animal model. Transplantation 2011; 91: 42. [DOI] [PubMed] [Google Scholar]
- 21. Minor T, Efferz P, Fox M, Wohlschlaeger J, Luer B. Controlled oxygenated rewarming of cold stored liver grafts by thermally graduated machine perfusion prior to reperfusion. Am J Transplant 2013; 13: 1450. [DOI] [PubMed] [Google Scholar]
- 22. Minor T, Koetting M. Gaseous oxygen for hypothermic preservation of predamaged liver grafts: fuel to cellular homeostasis or radical tissue alteration? Cryobiology 2000; 40: 182. [DOI] [PubMed] [Google Scholar]
- 23. Knaak JM, Spetzler VN, Goldaracena N, Louis KS, Selzner N, Selzner M. Technique of subnormothermic ex vivo liver perfusion for the storage, assessment, and repair of marginal liver grafts. J Vis Exp 2014; 90: e51419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tolboom H, Izamis ML, Sharma N, et al Subnormothermic machine perfusion at both 20 degrees C and 30 degrees C recovers ischemic rat livers for successful transplantation. J Surg Res 2012; 175: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. von Horn C, Baba HA, Hannaert P, et al Controlled oxygenated rewarming up to normothermia for pretransplant reconditioning of liver grafts. Clin Transplant 2017; 31(11). doi: 10.1111/ctr.13101. [Epub 2017 Sep 19]. [DOI] [PubMed] [Google Scholar]
- 26. Leducq N, Delmas‐Beauvieux MC, Bourdel‐Marchasson I, et al Mitochondrial permeability transition during hypothermic to normothermic reperfusion in rat liver demonstrated by the protective effect of cyclosporin A. Biochem J 1998; 336(Pt 2): 501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rauen U, de Groot H. New insights into the cellular and molecular mechanisms of cold storage injury. J Investig Med 2004; 52: 299. [DOI] [PubMed] [Google Scholar]
- 28. Rauen U, Kerkweg U, de Groot H. Iron‐dependent vs. iron‐independent cold‐induced injury to cultured rat hepatocytes: a comparative study in physiological media and organ preservation solutions. Cryobiology 2007; 54: 77. [DOI] [PubMed] [Google Scholar]
- 29. Biberthaler P, Luchting B, Massberg S, et al The influence of organ temperature on hepatic ischemia‐reperfusion injury: a systematic analysis. Transplantation 2001; 72: 1486. [DOI] [PubMed] [Google Scholar]


