Abstract
Grading of prostate cancer has evolved substantially over time, not least because of major changes in diagnostic approach and concomitant shifts from late‐ to early‐stage detection since the adoption of PSA testing from the late 1980s. After the conception of the architecture‐based nine‐tier Gleason grading system more than 50 years ago, several changes were made in order to increase its prognostic impact, to reduce interobserver variation and to improve concordance between prostate needle biopsy and radical prostatectomy grading. This eventually resulted in the current five‐tier grading system, with a much more detailed description of the individual architectural patterns constituting the remaining three Gleason patterns (i.e. grades 3–5). Nevertheless, there is room for improvement. For instance, distinction of common grade 4 subpatterns such as ill‐formed and fused glands from the grade 3 pattern is challenging, blurring the division between low‐risk patients who could be eligible for deferred therapy and those who need curative therapy. The last few years have witnessed the publication of several studies on the prognostic impact of individual architectural subpatterns showing that, in particular, the cribriform pattern exceeded the prognostic impact of other grade 4 subpatterns. This review provides an overview of the changes in prostate cancer grading over time and provides a thorough description of the various Gleason subpatterns, the current evidence of their prognostic impact and areas of contention. Potential practical ways for improvements of the current grading system are also put forward.
Keywords: Gleason pattern, grading, prognostic biomarker, prostate cancer, tumour architecture
Introduction
In order for a grading system to be successful, three criteria must be met: (i) prognostic ability exceeding clinical parameters, (ii) reproducibility among pathologists and (iii) grading results on random biopsies sufficiently representative for the entire cancer.1 Despite the notable interobserver variation among pathologists, histopathological grading of prostate cancer remains the strongest prognosticator of disease recurrence and death, and the main stratification tool of patients for their various treatment options. Several grading systems were used over time, but since the late 1990s the Gleason grading system, conceived in 1966, was gradually adopted worldwide, replacing a multitude of competing grading systems.2 The capture of the complex architectural heterogeneity of prostate cancers in a single drawing by Gleason proved to be a very attractive concept for pathologists. Actually, to some extent the combining of the most dominant architectural patterns in one score seems to have overcome the heterogeneity issue which now may limit the utility of molecular–genetic biomarkers.3
Updates of Gleason grading were largely based on expert rather than evidence‐based opinion.4 However, in more recent years several studies have focused on the prognostic impact of individual prostate cancer growth patterns.5, 6, 7, 8 Further, interobserver variation for individual Gleason grade (sub)patterns was studied in greater detail.9, 10, 11 Employing molecular–genetic analysis of microdissected samples as a tool to gain insight into prostate cancer evolution shed some light on the clonal evolution of prostate cancer.12
After a description of historical aspects of prostate cancer grading, this review will provide an extensive overview of the various subpatterns constituting the individual Gleason grades. The main objectives are: (i) to summarise the current evidence for prognostic impact of each of these Gleason grade subpatterns, (ii) to point out areas where such data are lacking and (iii) discuss potential implications of recent insights in prostate cancer grading for future improvement.
History of Prostate Cancer Grading
Initially, prostate cancers were mainly graded using the universal four‐tiered Broders system published in 1926.13 This grading system was based on low microscopic power evaluation of the percentage of glandular differentiation. A detailed study published in the first decade of the 20th century had already mentioned the wide variability in appearance of prostate cancer, recognising several histological growth patterns, such as acinar, scirrhous and solid.14 This morphological heterogeneity challenges the development of a grading system in determining which histological features should be taken into account to assign a grade. Ahead of his time, in 1966 Donald Gleason developed a histological classification specifically for prostate cancer, which was entirely based on the various architectural patterns as depicted in his famous drawing.15 He distinguished five basic architectural patterns, numbered grades 1–5. Higher grades were considered to reflect more aggressive behaviour. Because the majority of prostate cancers showed more than one type of growth pattern, he assigned two patterns to each case in the order of predominance, adding the two most dominant patterns or grades to a single Gleason score or doubling the pattern number if only one pattern was present, resulting in a nine‐tier grading system. After validation of the prognostic relevance of the Gleason grading system in 197416 it slowly gained worldwide acceptance,17 and is now the recommended grading system for prostate cancer.
Next to grading systems requiring a low‐power evaluation of the glandular architecture the Mostofi grading system,18 adopted by the World Health Organisation (WHO) in 1980,19 also included the degree of cytonuclear atypia in the worst tumour area as a grading criterion in addition to the percentage glandular differentiation as originally defined by the Broders system. Others1, 20 refined this system by a more detailed description of the glandular patterns, including the distinctive cribriform pattern. The four‐tiered MD Anderson grading system, developed at around the same time, was entirely based on the percentage glandular differentiation, but the cribriform pattern was specifically assigned to the ‘grade 2’ category.21 Several grading systems were simultaneously in routine use until approximately 2000. This was an unsatisfactory situation, as it limited the comparison of different patient series and adoption of a standard risk classification.
Although several studies demonstrated the prognostic potential of cytonuclear grading,20, 22, 23 the lack of interobserver agreement and its sensitivity to variations in pathology processing reduced its routine applicability. Interestingly, a recent paper using an eye‐tracking device revealed that nuclear grading was subconsciously biased by the carcinoma architecture.24 This may explain why the cytonuclear atypia‐based now obsolete Mostofi (WHO) grading and the architecture‐based Gleason grading show a strong correlation,25 despite their fundamental conceptual differences.
Modifications were made to the Gleason grading system, a major one in 20054 and another in 201426 during two International Society of Urological Pathologists (ISUP) consensus conferences. Originally, Gleason did not describe and specifically grade ill‐formed glands. During the ISUP consensus conference in 2005, the ill‐formed pattern was added to Gleason grade 4. Consequently, from then on Gleason grade 3 only comprised well‐delineated malignant glands. This, and the recommendation for prostate biopsies to include any amount of grades 4 or 5 carcinoma in the Gleason score, even if less than 5% of the carcinoma extent, led to a substantial grade inflation.27 In 2005 it was also agreed that large cribriform glands should be diagnosed as a Gleason grade 4, while small cribriform glands could still be assigned a Gleason grade 3. Because of the poor interobserver reproducibility on diagnosing cribriform grade 3 glands,28 it was decided during the 2014 ISUP consensus conference to consider any cribriform pattern as a Gleason grade 4. For many years, grading glomeruloid architecture was also controversial. Its frequent association with other grade 4 patterns, including cribriform architecture,29 led in 2014 to its inclusion as a Gleason grade 4. Some studies reported an improvement in concordance between biopsy and prostatectomy Gleason score as well as observer reproducibility after the 2005 modification, while others failed to show such an improvement.9, 30 The latter could be attributed in part to the large number of possible Gleason scores.
A lowest Gleason score of 6 was perceived as an anomaly, increasing unnecessary anxiety of men diagnosed with this low‐risk prostate cancer and who were now offered deferred treatment. By 2000 Gleason patterns 1 and 2, typical of low‐grade transition zone carcinomas as encountered in transurethral resections, were hardly reported any more in biopsies31 and Berney suggested in 2007 that the lower scores should be removed from the Gleason system.32 Several groups noted that the proportion of high‐grade cancer might be more prognostic than the Gleason score per se.33, 34, 35 Subsequently, Stark et al. demonstrated convincingly that separation of Gleason score 7 into two subsets, that is, those with a predominant grade 3 component [i.e. 7 (3 + 4)] and those with a predominant grade 4 component [i.e. 7 (4 + 3)] strongly improved its prognostic impact.36 Over time, various combinations of Gleason score condensations were shown to be instrumental in the prediction of recurrent disease.37 In 2013 Epstein and Partin updated their pathological staging nomogram with tumours grouped as per Gleason scores 6, 7 (3 + 4), 7 (4 + 3), 8 and 9–10.38 This five‐tier ‘prognostic grade grouping’39 was subsequently endorsed by the ISUP in 2014 and recommended by the WHO and the American Joint Committee on Cancer (AJCC) tumour–node–metastasis (TNM) system:40, 41 Gleason scores ≤6 were compressed into ISUP grade (group) 1 and Gleason scores 9–10 into ISUP grade (group) 5, whereas Gleason score 7 was expanded to ISUP grade (group) 2, i.e. 7 (3 + 4) and ISUP grade (group) 3, i.e. 7 (4 + 3). Independent validation on historical data sets of this ‘renumbering’ of the Gleason score demonstrated its prognostic impact in biopsies and prostatectomies.42
ISUP 2014 Grading of Prostate Cancer
Henceforth, for simplicity we will refer to the individual grades of the grade group system as ISUP grades,43 a terminology now also adopted by the European Association of Urology (EAU) guideline committee of prostate cancer.44 An important advantage of this grading system is that it now ranges from 1 to 5. ISUP grade 1 carcinoma terminology may help acceptance of active surveillance, as it may be perceived as less ominous than Gleason score 6 (3 + 3) carcinoma. Another advantage is that the 2014 modification codifies the previously established substantial prognostic difference between Gleason scores 7 (3 + 4) and 7 (4 + 3). Unsurprisingly, comparison with a four‐tier Gleason score grouping (i.e. Gleason scores 6, 7, 8, 9–10) demonstrated the superior prognostic impact of the five‐tiered ISUP grading for both clinical and pathological outcome parameters after prostatectomy.45, 46 Commonly used clinical risk stratification systems such as D'Amico consider Gleason score 7 as a major criterion for intermediate‐risk prostate cancer. They may now need to adapt their risk stratification based on ISUP grades. Indeed, the current National Comprehensive Cancer Network (NCCN) guidelines on prostate cancer distinguishes between favourable and unfavourable intermediate risk based, among others, on the distinction between ISUP grades 2 and 3.47 Similarly, the Cancer of the Prostate Risk Assessment (CAPRA) score for biopsies and prostatectomy specimens distinguishes ISUP grades 2 and 3.48 A potential disadvantage of the ISUP 2014 grading system might be the loss of the linkage with the underlying heterogeneity in carcinoma architecture. For instance, differences between Gleason scores 3 + 5 and 5 + 3 versus 4 + 4 will no longer be apparent, as they will all be reported as ISUP grade 4.37
It is now recommended that the aggregated ISUP grade should be reported for each individually labelled biopsy site.49 If multiple cores of one biopsy site contain carcinoma, it is optional to report the grade for each core. Further, a global Gleason score is generally reported taking into account the grade patterns of the carcinoma foci encountered in all cores. Although it may seem counterintuitive, three recent studies showed that the global Gleason score outperformed the ‘worst’ Gleason score with regard to pathological features in corresponding prostatectomy specimens.50, 51, 52
Grading of prostatectomy specimens follows slightly different rules compared to biopsy grading: if no clearly spatially separate carcinoma foci with disparate grade are detected a ‘global’ ISUP grade is reported. If a predominant Gleason grade 3 carcinoma is identified with a minor (i.e. <5%) grade 4, the minor component is not accounted for in the ISUP grade, but recorded as a minor component. If Gleason grades 3 and 4 are the two dominant patterns and a grade 5 component comprises less than 5% of the carcinoma area, most would not include the grade 5 in the ISUP grade, but report it as minor grade 5 component. If, however, the grade 5 component represented the third most common pattern, while exceeding 5% of the carcinoma area, many pathologists would include the grade 5 component in the ISUP grade.
Description of ISUP 2014 Gleason grade 3 patterns
Gleason grade 3 pattern is defined by well‐differentiated glands, separated from each other by stroma. A few subpatterns can be distinguished, based on the density of neoplastic glands and on architectural variations (Table 1). The density of the neoplastic glands is defined by both the amount of intervening stroma and presence of intervening benign glands. A dense pattern is characterised by glands close to each other with a minimum amount of intervening stroma (Figure 1A). These grade 3 carcinomas can form prostate nodules, and if sufficiently large they are visible at imaging.53 A ‘sparse’ Gleason grade 3 pattern is characterised by carcinoma glands separated by benign prostatic glandular tissue, including stroma, and can be defined arbitrarily as comprising less than 50% of the tumour area (Figure 1B). As a consequence, sparse Gleason grade 3 pattern remains invisible in imaging.54 Rarely, a desmoplastic stroma may be present in a grade 3 carcinoma (Figure 1C), thought to portend an unfavourable prognosis.8 The usual architecture of grade 3 carcinoma glands is tubular of variable size, but sometimes an atrophic,55 branching,26 pseudohyperplastic,56, 57 PIN‐like,58 mucinous or collagenous micronodular (also termed mucinous fibroplasia)59 architecture may be seen (Table 1, Figure 1D–H). PIN‐like58 or PIN‐like ductal60 adenocarcinoma is characterised by several close by, often cystically dilated, glands architecturally resembling high‐grade prostatic intra‐epithelial neoplasia, lined by pseudostratified columnar epithelium with cytonuclear atypia, while entirely lacking a basal cell lining. Particularly when limited in extent, these lesions may be difficult to distinguish from high‐grade PIN. It was agreed at the ISUP 2005 consensus meeting4 that mucinous carcinomas (Figure 1F) would be graded on the basis of its glandular architecture. A recent paper on carcinomas with a mucinous component noted that they were often high‐grade, but confirmed that their prognosis depends on the underlying Gleason architecture.61 Similarly, it was concluded that the presence of large cytoplasmic vacuoles should not change the Gleason grade in an architecturally Gleason grade 3 carcinoma.
Table 1.
Summary of Gleason patterns and sub‐patterns*
| Grade 3 |
| Sub‐patterns based on density |
| Sparse |
| Intermediate |
| Dense |
| Sub‐patterns based on architecture |
| Atrophic |
| Branching |
| Mucinous fibroplasia (collagenous micronodular) |
| Paneth cell‐like |
| PIN‐like (ductal) |
| Pseudohyperplastic |
| Grade 4 |
| Sub‐patterns based on architecture |
| Ill‐formed |
| Abortive |
| Small‐ and large fused |
| Glomeruloid |
| Cribriform (small and large) |
| Papillary |
| Ductal adenocarcinoma |
| Non‐ductal (including PIN‐like cystic) |
| Grade 5 |
| Sub‐patterns based on architecture |
| Single cell |
| Single file |
| Cribriform with comedonecrosis |
| Pseudorosetting |
| Solid |
| Without comedonecrosis |
| With comedonecrosis |
Definitions provided in the text.
Figure 1.
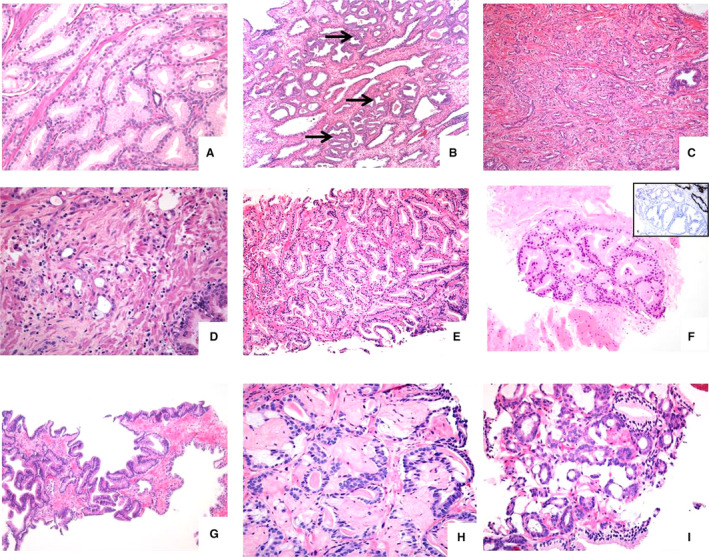
Gamut of Gleason grade 3 adenocarcinoma, including (A) densely packed medium‐sized well‐delineated tubules, typical of transition zone carcinoma, (B) ‘sparse’ carcinoma consisting of scattered acinar and tubular structures (arrows) widely separated from each other by intervening normal fibromuscular stroma and benign glands, (C) ‘stromogenic’ carcinoma showing well‐delineated small‐ to medium‐sized glands within a desmoplastic stroma, (D) atrophic variant carcinoma consisting of acini lined by flattened epithelial cells, (E) branching pattern carcinoma comprised of distinct medium‐sized glandular structures with branching outpouchings, not to be confused with fusing of glands, (F) pseudohyperplastic pattern adenocarcinoma with tumorous glands mirroring the architecture of normal benign glands, (G) PIN‐like adenocarcinoma, constituted of large‐sized glands lined by columnar neoplastic cells, lacking a layer of basal cells demonstrated by immunostaining, (H) miconodular collagenous nodule with entrapped acinar carcinoma cells with complex architecture and (I) mucinous carcinoma with distinct small‐sized neoplastic acini within extracellular mucin.
The biological behaviour of pure Gleason grade 3 pattern is well established: it may behave locally aggressively, but at the time of prostatectomy rarely, if ever, gives rise to metastatic disease.62, 63 Importantly, it is not known how frequently Gleason pattern 3 may transform into a more aggressive prostate cancer, although some have estimated that an approximately 20% grade transformation may occur over time.64, 65 A deep sequencing study revealed that a proportion of Gleason grade 3 pattern carcinomas are monoclonal, whereas a subset is composed of multiple subclones. This may imply that the latter are subject to genetic evolutionary changes.66
Description of ISUP 2014 Gleason grade 4 patterns
The 2014 ISUP consensus meeting established that Gleason pattern 4 comprises ill‐formed or poorly formed, (small and large) fused glands as well as glomeruloid and cribriform architecture (Table 1, Figure 2A–D). The overall prevalence of Gleason grade 4 subpatterns in biopsies of a prostate‐specific antigen (PSA)‐screened population was: fused 75%, ill‐defined 64%, cribriform 48% and glomeruloid 25%.6 In prostatectomy series, among the carcinomas with grade 4 component the frequency of fused glands was 38%, ill‐formed glands 40% and glomeruloid 21%,7 while in various publications the prevalence of cribriform architecture ranged in prostatectomy specimens between 30 and 55%.5, 7, 67 A few authors noted that particularly in Gleason grade 4 pattern the stroma component could vary strongly from normal to desmoplastic, carrying independent prognostic information.68, 69
Figure 2.
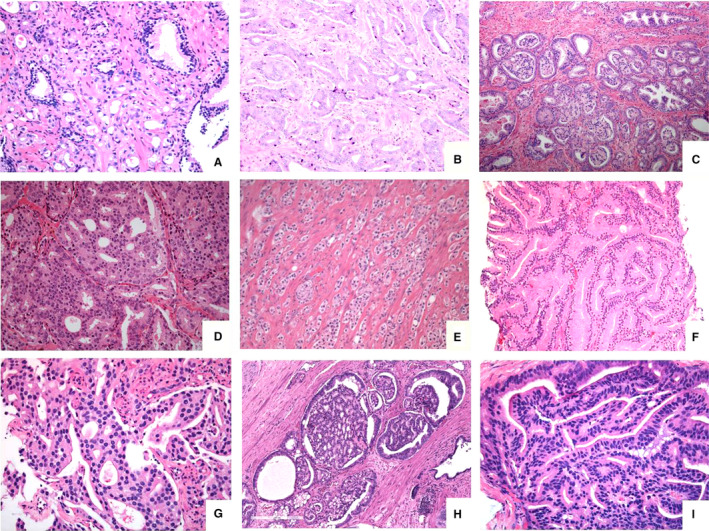
Depiction of grade 4 subpatterns: (A) haphazardly distributed poorly formed very small‐sized distinct glands showing some lumen‐formation, (B) fused small‐sized lumen‐containing glands in a retiform pattern, (C) focus of glomeruloid structures within small‐ to medium‐sized distinct glands, (D) expansile rounded tumour area with cribriform pattern lacking intervening stroma or capillaries, (E) abortive glands consisting of structures with glandular shape, but lacking a lumen, to be distinguished from solid pattern grade 5 carcinoma, (F) large‐sized glands which merge together, constituting the large‐fused pattern, (G) complex fused glands with irregular cribriform area, but with intervening stroma and capillaries, (H) large‐sized glands with glomeruloid features showing a cribriform pattern, adjacent to small‐sized glomeruloid glands and (I) papillary pattern lined by columnar tumour cells reminiscent of ductal adenocarcinoma.
Ill‐formed and fused glands
Ill‐ or poorly formed glands are defined as small‐sized discrete glands with no or rare lumens, elongated compressed or angulated glands and elongated nests visible at low power, while tangential sectioning of grade 3 glands cannot account for this histology.4, 11 Included in this definition of ill‐formed glands are the so‐called ‘abortive’ glands, larger discrete elongated nests or strands, lacking a lumen (Figure 2E). Fused glands can be small‐ or large‐sized (Figure 2F) and display multiple merged glandular strands and nests with lumen formation (Figure 2B). They are often combined with ill‐formed glands4 and may therefore be difficult to separate. Some fused glands with more complex architecture, labelled complex‐fused glands (Figure 2G), may have a pseudo‐cribriform appearance.10
At the time of their codification as a grade 4 pattern in 2005, no outcome data were available to confirm their unfavourable prognosis compared to Gleason grade 3 pattern. A prostatectomy study by Dong et al. demonstrated that patients upgraded due to the 2005 modification to Gleason score 7 (due mainly to presence of ill‐formed glands, but also due to cribriform pattern) were at intermediate risk for biochemical progression and metastasis compared to those with ISUP 2005 modified Gleason score 3 + 3 = 6 and patients with classical Gleason score 3 + 4 = 7.67 Conversely, Delahunt did not find a significant difference between pre‐ISUP 2005 and ISUP 2005 modified Gleason scores 6 and 7 carcinomas in biopsies.70 Some authors demonstrated in a large prostatectomy cohort that increased proportions of ill‐formed glands in a carcinoma would gradually increase the risk of carcinoma recurrence after prostatectomy.7, 8 Kweldam et al.63 revised the grading of a set of 796 Gleason score ≤7 (3 + 4) biopsies using the ISUP 2014 criteria and distinguished those with and without cribriform pattern. Patients who had biopsy ≤ISUP grade 2 without cribriform pattern (mainly ill‐formed or fused glands) had a similar biochemical recurrence rate after prostatectomy to men with ISUP grade 1. The above studies imply that the inclusion in the ISUP grade of any amount of grade 4, in particular of ill‐formed and fused glands as per the ISUP 2005 modification, needs further optimisation.
Glomeruloid architecture
The glomeruloid pattern is defined as dilated cancer glands of variable size, with cribriform cancer protruding into the lumen yet not attaching to the other side of the gland wall, superficially resembling a glomerulus.26 Because of its morphological resemblance to the cribriform pattern, particularly when larger‐sized (Figure 2H), some suggested that a glomeruloid pattern might represent the precursor for a cribriform pattern.29 A few subsequent studies showed that a glomeruloid pattern was actually associated with reduced risk of biochemical recurrence after radical prostatectomy,6, 7 but compared to a pure grade 3 glomeruloid pattern was more unfavourable, justifying its inclusion in the grade 4 category.7
Cribriform architecture
Cribriform carcinoma (Figure 2D) can be defined as an expansile area of carcinoma cells without intervening stroma or vasculature and with a diameter of at least an average‐sized (diameter approximately 200 μm) benign gland and with multiple punched‐out lumina,71 irrespective of its delineation. Earlier studies reported that proliferative activity was highest in this subpattern.72, 73 In 2011, Iczkowski et al. were the first to report that prostate cancer patients with cribriform growth (although also including the glomeruloid pattern) had a worse biochemical‐recurrence‐free survival than those with ‘poorly formed glands’.5 Subsequently, the adverse prognostic value of prostate cancers with a cribriform pattern was validated in several biopsy and prostatectomy studies, using different patient groups and outcome measures, including biochemical recurrence, metastasis and disease‐specific death.74 Notably, among Gleason score 8 cancers, the question of whether the highest Gleason grade was 3 + 5 versus 4 + 4 proved only mildly predictive of recurrence. However, the presence of cribriform cancer dichotomised cancer‐specific survival in Gleason 8 cancer,75 corroborating another large biopsy‐based study showing that across all Gleason score >6 carcinomas, the presence of a cribriform pattern predicted metastatic disease and death of disease.63 Together, these studies indicate that cribriform architecture in prostate cancer carries a much higher risk of disease progression compared to the other Gleason grade 4 patterns.76 Some papers also showed that the presence of cribriform architecture correlates strongly with the percentage of grade 4 carcinoma in biopsies6 and prostatectomy specimens.7
Papillary/ductal adenocarcinoma architecture
Papillary architecture (Figures 2I and 3) is not mentioned separately in the ISUP 2005/2014 grading system, but was included in the earlier descriptions of the Gleason grade 4 patterns. Papillary formations are characteristic for ductal adenocarcinoma when lined by tall columnar cells with elongated nuclei and showing pseudostratification (Figure 3A), but these structures can also be encountered in the setting of pseudohyperplastic variant carcinoma (Figure 3B), intraductal carcinoma, cystic PIN‐like ductal adenocarcinoma (Figure 3C)60 and in other Gleason grade 4 carcinomas lacking tall columnar cells typical of ductal adenocarcinoma (Figure 3D).77, 78
Figure 3.
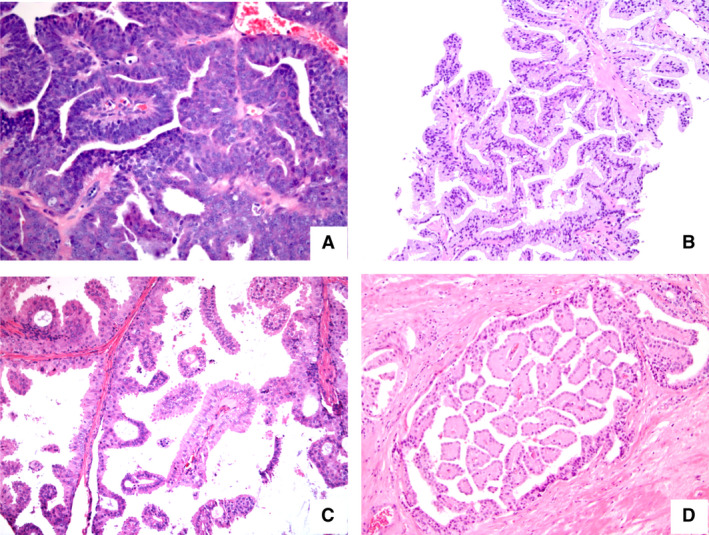
Composite of prostatic adenocarcinomas with papillary architecture: (A) classical ductal (‘endometrioid’) carcinoma, characterised by papillary formations, lined by tall columnar neoplastic cells, (B) papillary formations lined by tumour cells resembling benign prostate glandular luminal cells in a pseudohyperplastic variant carcinoma, (C,D) papillary formations protruding into larger cystic spaces and lined by columnar luminal cells with round (basal) nuclei (C) or with apical nuclei (D) distinct from the ductal adenocarcinoma as shown in (A).
Description of ISUP 2014 Gleason grade 5 patterns
Gleason grade 5 pattern represents carcinoma areas detectable at low power (at most, ×10 objective) which essentially lack glandular features such as lumen formation or glandular contours (Figure 4A–F). Its frequency in larger biopsy series is approximately 5%.79, 80 A few main patterns can be distinguished (Table 1), including comedonecrosis, single‐file/infiltrating cords and solid pattern.4, 81 The presence of comedonecrosis in a single carcinoma gland (focal comedonecrosis) is deemed sufficient for Gleason grade 5.4 Signet‐ring‐like cells, if arranged as single cells, solid larger nests (Figure 4F) or single files are also considered as Gleason 5 pattern. Single‐file/infiltrating cords without lumen formation and single cells are the most frequent and comedocarcinoma the least frequent grade 5 pattern in biopsies.82 Interobserver agreement is, however, lowest in biopsies for the patterns of infiltrating cords and single cells and highest for comedocarcinoma. The rare Paneth cell‐like differentiation in prostate cancer may show a nested or cord‐like architecture, but they seem to have a favourable prognosis and therefore should be graded as 3, instead of 4 or 5.83 Grade 5 pattern adenocarcinoma should be distinguished mainly on morphological grounds from poorly differentiated neuroendocrine carcinoma, as the latter show a much higher nucleus/cytoplasm ratio, with hyperchromasia of nuclei and nuclear moulding, while mitotic activity is very high. Immunostaining is an ancillary tool, as poorly differentiated neuroendocrine carcinomas often express one or more neuroendocrine markers, such as chromogranin A and synaptophysin, and have a MIB‐1 score of more than 50%, while mainly lacking expression of androgen receptor and PSA.
Figure 4.
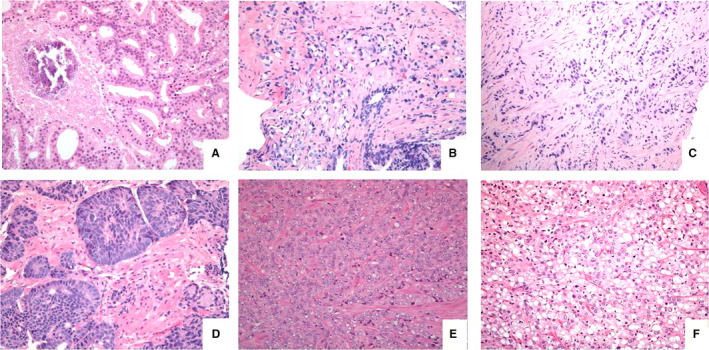
Most common grade 5 patterns, such as (A) comedocarcinoma, showing a necrotic central plug surrounded by fused glands, (B) single cells with vacuolisation with haphazard distribution between benign glands, (C) single file of tumour cells, lacking lumina, embedded in dense stroma, (D) smaller solid areas (upper right) with some acinar (pseudo‐rosetting) nuclear arrangement, but lacking lumen formation, (E) larger sheet of poorly differentiated tumour cells and (F) solid area of cells with signet ring‐like appearance.
Problematic areas
Distinction of grade 3 versus 4 patterns
Due to ‘grade inflation’ as a consequence of the decision at the 2005 modification of the Gleason grading to incorporate any amount of Gleason grade 4 pattern in the Gleason score of a biopsy,27 distinction of grades 3 and 4 has become even more pertinent. Discrepancies mainly arise from the distinction of grade 3 from ill‐formed (Figure 5A–C) and small‐fused glands (Figure 5D). Downgrading a biopsy Gleason score 7 to a Gleason score 6 at prostatectomy could be attributed in most cases to these two patterns in the biopsy.84 The sizes of well‐defined Gleason grade 3 glands range from large‐size, difficult to separate from benign glands, to ever smaller‐sized glands, and there is no abrupt distinction from ill‐formed glands (Figure 5A). Based on an interobserver variation study, Zhou et al. suggested that a minimum of 10 ‘ill‐formed’ glands constituting a separate tumour area in an otherwise Gleason grade 3 pattern tumour would be required to assign a grade 4 (Figure 5B,C). Ill‐formed glands intermingled with bona‐fide pattern 3 glands should not, in their view, lead to an upgrading of the biopsy.11 Further studies on the three‐dimensional architecture and outcome of non‐cribriform Gleason score 3 + 4 = 7 compared to Gleason score 3 + 3 = 6 prostate cancer might reveal whether distinction of ill‐formed Gleason pattern 4 from Gleason pattern 3 is objectively feasible based on specific differences85 and are of clinical relevance.86
Figure 5.
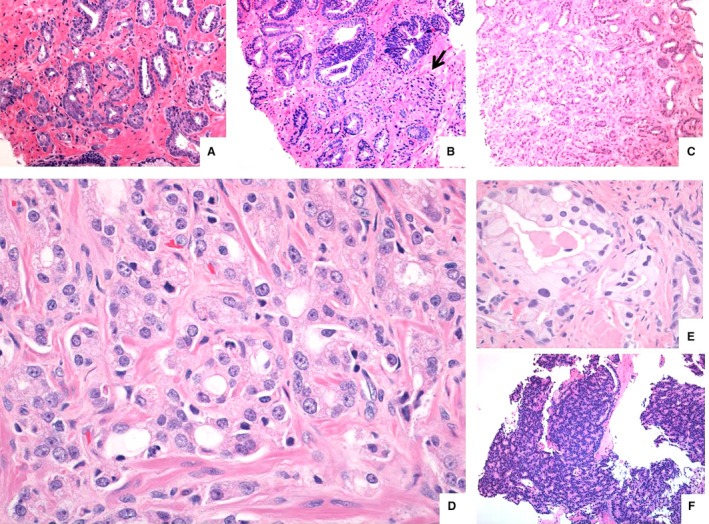
Architectural patterns which may be challenging for grading: (A) well‐defined grade 3 medium‐sized glands transitioning to and intermingling with increasingly smaller‐sized glands, but still considered as grade 3, contrasting with (B) medium‐sized grade 3 glands with an abrupt transition to a cluster of ill‐formed (grade 4) glands and (C) medium‐sized well‐described grade 3 glands slowly transitioning into a larger field (>10 structures) of ill‐formed (grade 4) glands. In (D) small‐sized closely packed circumscribed glands surrounded by thin wisps of stroma, most consistent with grade 3 glands, (E) well‐circumscribed pale glands with flocculated eosinophilic lumen content and considerable cytonuclear atypia (compared to gland on the right side) and (F) pseudo‐rosetting pattern in an otherwise solid carcinoma field in a biopsy considered as grade 5 pattern at the ISUP 2014 consensus meeting.
Small‐fused glands may not only be difficult to distinguish from ill‐formed glands, but also from closely packed Gleason grade 3 glands (Figure 5D). The presence of subtle intervening wisps of stroma separating each acinar structure is deemed sufficient to assign a Gleason grade 3. Further, the examination of multiple levels in a biopsy may help to distinguish tangentially cut pattern 3 and fused glands. We would suggest that, as a rule of thumb, manifestation as pattern 3 in one level is sufficient to abstain from grade 4 diagnosis at biopsy, even if in other levels a fused pattern seems to be present. Uncommonly, well‐defined carcinoma acinar structures may be embedded in a collageneous (desmoplastic) stroma (Figure 1C). McKenney et al.8 demonstrated its more aggressive behaviour, which would suggest that this variant may actually constitute a grade 4 pattern.
Pseudohyperplastic variant prostatic adenocarcinoma56, 57 is categorised as Gleason pattern 3, but due to its complex architecture it may mimic a fused gland or papillary pattern. Sometimes, immunostaining is required for distinction from high grade PIN (Figure 1F).
More rarely, well‐demarcated separate acini composed of tumour cells with severe cytonuclear atypia and a lumen filled with necrotic or eosinophilic material may occur.26 They would fulfil the criteria of a Gleason grade 3 pattern, but their cytonuclear atypia suggests otherwise (Figure 5E). ‘Abortive’ glands may also be confused with a grade 3 pattern, but can easily be distinguished by the absence of glandular lumina in multiple levels. Distinction of grade 3 and glomeruloid architecture should rarely cause confusion. Only distinction of telescoping in a grade 3 gland from a true glomeruloid pattern may occasionally cause uncertainty. Cribriform pattern and intraductal carcinoma are unlikely to be confused with a grade 3 pattern. Carcinomas with extracellular mucin and/or fibroplasia may manifest both as grade 3 or grade 4, dependent upon the complexity of the glandular structures. More recently, some authors suggested that mucinous carcinoma would behave in an indolent manner, just like grade 3, even if the glandular architecture would be more in keeping with grade 4 fused glands.8 Therefore, a conservative approach with respect to grading of mucinous variant carcinoma is recommended.26 The mucinous features often seem to merge with micronodular collagenous (mucinous fibroplasia) carcinoma areas, which may show a more complex glandular features (Figure 1H). The latter are not associated with aggressive carcinoma, and their behaviour was reported to be similar to grade 3 pattern carcinoma.59 A more recent paper observed, however, that this fibronodular pattern was frequently associated with cribriform pattern, but outcome data were not provided.87
Distinction within grade 4 architectural patterns
In a few interobserver studies, the distinction between the main Gleason grade 4 subpatterns was analysed.9, 10, 28 Ill‐formed and small‐fused glandular patterns are most difficult to separate, but they may be similar in clinical significance. To a lesser extent, glomeruloid and cribriform patterns were subject to interobserver disagreement. Glomeruloid architecture may vary in size, and in particular larger‐sized glomeruloid glands (Figure 2H) were considered by several pathologists as cribriform. Although at the 2005 Gleason grading modification small and large cribriforms were separated, based on the number of lumina (= <6 or >6, respectively), this distinction was abolished at the 2014 ISUP consensus meeting.
For the uncommon ‘complex‐fused’ glands pattern (Figure 2G), pathologists were often split between fused and cribriform,10 while its additional prognostic impact remains unclear.
Distinction of grades 4 and 5 architectural patterns
It has been common practice to be very conservative when assigning a Gleason grade 5. Most frequently, disagreement may arise when single cells or strands without apparent lumen formation are present, leading to a differential diagnosis of ill‐formed glands versus single‐file types of grade 5 carcinoma. An interobserver variation study noted that: ‘rare individual cells, strands, or nests identified only at less than ×40 lens magnification were considered sufficient to diagnose Gleason pattern 5 on needle biopsy by 17% of pathologists, whereas 83% required clusters of such structures seen at lower than ×40 magnification. The Gleason pattern 5 identified by occasional observers in some cases proved to be minute components rather than cohesive areas and should be overlooked according to the vast majority of observers’.9
A grade 5 area single‐file pattern should, in our opinion, be manifest as a morphologically distinct tumour area at low power. Intermingling of ill‐formed glands with minimal lumen formation, identified at any level of sectioning, should exclude a grade 5 diagnosis. A solid pattern carcinoma may sometimes merge with a cribriform pattern, giving rise to solid areas with so‐called rosette‐like spaces, defined as acinar structures, lacking a lumen (Figure 5F). At the 2014 ISUP consensus meeting this rosette‐like pattern, based on the nuclear arrangement, was included as a Gleason grade 5 pattern, although no specific outcome data were provided.26 Its assignment to grade 5 therefore remains somewhat debatable. It probably represents part of the gamut of solid well‐demarcated tumour foci ranging from cribriform–rosette‐like solid–comedocarcinoma. Necrosis may be seen in cribriform areas or in well‐described glands. Irregular (geographic) necrosis is not considered a feature of grade 5, in contrast to comedonecrosis (Figure 4A), defined as a central delineated plug of coagulative necrosis. Abortive gland pattern is uncommon, but should be considered grade 4 due to its ‘glandular contours’ (Figure 2E). Inclusion of quantitative criteria would probably improve the reproducibility among pathologists for some grade 5 patterns, as suggested by Shah et al.82 Thus, restricting a pattern 5 diagnosis to single cells/single cords if more than 10, concentrated in a cluster, would result in fair agreement among pathologists.
Quantitative grading
Prostatectomy findings of patients with a minimal amount of Gleason pattern 4 (<5%) in corresponding prostate biopsies may show a similar pathological stage and ISUP grade distribution to those with a biopsy ISUP grade 1.88 In this study, 63% of patients with biopsy ISUP grade 2 with less than a 5% grade 4 component were downgraded to ISUP grade 1 at prostatectomy. In order to mitigate the potential impact of the grade inflation due to the implementation of the 2005 ISUP modification on eligibility for active surveillance, the 2014 ISUP consensus/WHO classification of prostate cancer 201641 recommends that percentage Gleason grade 4 is routinely reported in biopsies with ISUP grades 2 and 3.26 Subsequent studies on quantitative grading showed the incremental, rather than acute, increase in risk of biochemical recurrence at higher proportions of the grade 4 component. It is thought that quantitative grading might limit the clinical impact of interobserver variation regarding borderline findings such as tumours with, for instance, very small Gleason grade 4 fractions impacting a man's eligibility for active surveillance.7, 51, 52 One study reported that interobserver reproducibility of percent Gleason grades 4 or 5 on prostate biopsies is at least as good as that of Gleason score.89 A subsequent reproducibility study on percentage Gleason grade 4 in prostate biopsies revealed that when reported in 10% increments, 32% had an exact match between fellow and expert urological pathologist and 75% were within a 10% range with a good (weighted kappa of 0.67) agreement.90 The agreement for percentage Gleason pattern 4 decreased significantly only when carcinoma comprised 10% or less of the core.
Intraductal carcinoma and grading
Recognition of intraductal carcinoma of the prostate (IDC‐P) as a strong independent prognosticator has taken several decades since its original discovery: interest in IDC‐P as a separate entity was raised in the 1970s and 1980s.91, 92, 93 In their landmark paper in 1986, McNeal and Kovi showed the unfavourable characteristics of associated prostate cancers in prostatectomy specimens harbouring IDC‐P. Gleason did not include IDC‐P as a separate pattern in his drawing and considered IDC‐P as cribriform pattern carcinoma or comedocarcinoma. At that time basal cell staining, a helpful adjunct to distinguish IDC‐P, was not available and IDC‐P was incorporated by default into the Gleason grading. Subsequently, IDC‐P was tacitly considered as one of the architectural patterns comprising the newly adopted (high‐grade) prostatic intra‐epithelial neoplasia (PIN) label. Because high‐grade PIN was lacking prognostic significance when found in association with prostate cancer, awareness of the prognostic impact of IDC‐P waned until O'Brien and Cohen subsequently noted its value as an independent unfavourable prognosticator, incorporating its presence in a nomogram used to predict biochemical recurrence after prostatectomy.94, 95 A few subsequent prostatectomy and prostate biopsy studies confirmed the independent prognostic value of IDC‐P.96, 97 The reporting of IDC‐P was discussed at the 2014 ISUP consensus meeting, and it was decided to report IDC‐P separately if present in isolation and not to include its presence in the Gleason grading.26 The arguments for this decision were the analogous situation in other malignancies and the rare finding of IDC‐P in the absence of adenocarcinoma (isolated IDC‐P) or IDC‐P in association with an ISUP grade 1 prostate cancer as highest grade.
Future implications
With the recognition of IDC‐P, invasive cribriform carcinoma and percentage Gleason patterns 4 and 5 as separate relevant prognostic parameters, the question arises as to how these should be incorporated into pathology reports and clinical decision‐making. Whereas the 2014 ISUP consensus only recommends reporting of IDC‐P if present in isolation, the 2018 EAU guideline on prostate cancer now recommends separate reporting of intraductal carcinoma when associated with carcinoma.44, 49 In one study it was shown that combining intraductal and invasive cribriform carcinoma on biopsy specimens predicted disease‐specific death more accurately than each separately.63 As invasive and intraductal carcinoma can be difficult to distinguish without basal cell immunohistochemistry, we and others have combined both entities into one group, labelled CR/IDC or cribriform architecture (CA).6, 63, 98 This would overcome the use of additional stainings for classification of both patterns.
Iczkowski et al. suggested adding ‘C’ to the ISUP grade, indicating the presence of invasive cribriform or intraductal carcinoma.74 For instance, ISUP grade 2 prostate cancer with cribriform growth would be classified as ISUP grade 2C. However, it is still unclear how the prognostic impact of the ISUP grades would be mutually related if CR/IDC is incorporated. In a biopsy cohort it was shown that men with ISUP grade 2 without CR/IDC had similar biochemical recurrence rates and disease‐specific survival to men with ISUP grade 1.6, 86 This might imply that one point could be subtracted if CR/IDC is absent in ISUP grades 2–5. Therefore, ISUP grade 2 without CR/IDC would then be labelled as modified ISUP grade 1. This would be an attractive and simple grading scheme, increasing the number of patients potentially eligible for active surveillance. Future studies should, however, demonstrate whether such a model is really clinically valid, in particular also for ISUP grades 3–5.
A few groups have advocated the implementation of desmoplastic stroma changes in routine reporting. As yet, lack of independent validation of stromal changes in prostate biopsies as an independent prognostic parameter and lack of information on its robustness precludes its routine implementation in prostate cancer grading.
In a detailed study of 13 261 radical prostatectomy specimens, Sauter and colleagues developed a ‘integrated quantitative’ Gleason score (‘IQ‐Gleason’) which was based entirely on percentages of Gleason patterns 4 and 5.99 In this model the absolute quantitative percentages of any Gleason patterns 4 and 5 are summed. If any pattern 5 is seen 10 points are added, and another 7.5 points if Gleason pattern 5 quantities are larger than 20%. The final score therefore ranges from 0 to 117.5. The advantages of this model are that identical grading rules are applied to biopsy and surgical specimens and that quantitation of Gleason patterns 4 and 5 are implemented, with less interobserver variability than in standard grading. Conversely, invasive cribriform and intraductal carcinoma are not accounted for in this model.
For the development of timely nomograms and decision models it is important to understand more clearly the mutual relation of the novel pathological parameters. Most studies only investigate one selected parameter. Thus, many studies do not elucidate if and how invasive cribriform and intraductal carcinoma are distinguished. One biopsy study demonstrated that the presence of invasive and/or intraductal carcinoma is strongly associated with percentage Gleason pattern 4. Among 370 ISUP grade 2 prostate cancers with <10% Gleason pattern 4, CR/IDC occurred in 6% of men, while this was 44% in patients with 25–50%.100 In multivariate analysis, biopsy CR/IDC was an independent parameter for postoperative biochemical recurrence in this study, while percentage grade 4 was not. Another subject for future study is the detailed analysis of individual Gleason 5 patterns, such as growth in cords, single cells, solid fields and the presence of comedonecrosis. We advocate that knowledge of the clinical impact of the distinct Gleason 5 patterns and elucidation of the mutual relations of these novel pathological parameters will finally result in more comprehensive and robust prostate cancer grading.
Kweldam C F, van Leenders G J & van der Kwast T (2019) Histopathology 74, 146–160. 10.1111/his.13767 Grading of prostate cancer: a work in progress
References
- 1. Bocking A, Kiehn J, Heinzel‐Wach M. Combined histologic grading of prostatic carcinoma. Cancer 1982; 50; 288–294. [DOI] [PubMed] [Google Scholar]
- 2. Epstein JI, Algaba F, Allsbrook WC Jr, Bastacky S, Boccon‐Gibod L, De Marzo AM. World Health Organization classification of tumours tumours of the urinary system and male genital organs 8. Lyon: IARC Press, 2004; 179–184. [Google Scholar]
- 3. Wei L, Wang J, Lampert E et al Intratumoral and intertumoral genomic heterogeneity of multifocal localized prostate cancer impacts molecular classifications and genomic prognosticators. Eur. Urol. 2017; 71; 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Epstein JI, Allsbrook WC Jr, Amin MB, Egevad LL; Committee IG . The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason grading of prostatic carcinoma. Am. J. Surg. Pathol. 2005; 29; 1228–1242. [DOI] [PubMed] [Google Scholar]
- 5. Iczkowski KA, Torkko KC, Kotnis GR et al Digital quantification of five high‐grade prostate cancer patterns, including the cribriform pattern, and their association with adverse outcome. Am. J. Clin. Pathol. 2011; 136; 98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kweldam CF, Wildhagen MF, Steyerberg EW, Bangma CH, van der Kwast TH, van Leenders GJ. Cribriform growth is highly predictive for postoperative metastasis and disease‐specific death in Gleason score 7 prostate cancer. Mod. Pathol. 2015; 28; 457–464. [DOI] [PubMed] [Google Scholar]
- 7. Choy B, Pearce SM, Anderson BB et al Prognostic significance of percentage and architectural types of contemporary Gleason pattern 4 prostate cancer in radical prostatectomy. Am. J. Surg. Pathol. 2016; 40; 1400–1406. [DOI] [PubMed] [Google Scholar]
- 8. McKenney JK, Wei W, Hawley S et al Histologic grading of prostatic adenocarcinoma can be further optimized: analysis of the relative prognostic strength of individual architectural patterns in 1275 patients from the canary retrospective cohort. Am. J. Surg. Pathol. 2016; 40; 1439–1456. [DOI] [PubMed] [Google Scholar]
- 9. Egevad L, Algaba F, Berney DM et al Interactive digital slides with heat maps: a novel method to improve the reproducibility of Gleason grading. Virchows Arch. 2011; 459; 175–182. [DOI] [PubMed] [Google Scholar]
- 10. Kweldam CF, Nieboer D, Algaba F et al Gleason grade 4 prostate adenocarcinoma patterns: an interobserver agreement study among genitourinary pathologists. Histopathology 2016; 69; 441–449. [DOI] [PubMed] [Google Scholar]
- 11. Zhou M, Li J, Cheng L et al Diagnosis of ‘poorly formed glands’ Gleason pattern 4 prostatic adenocarcinoma on needle biopsy: an interobserver reproducibility study among urologic pathologists with recommendations. Am. J. Surg. Pathol. 2015; 39; 1331–1339. [DOI] [PubMed] [Google Scholar]
- 12. Sowalsky AG, Kissick HT, Gerrin SJ et al Gleason score 7 Prostate cancers emerge through branched evolution of clonal Gleason pattern 3 and 4. Clin. Cancer Res. 2017; 23; 3823–3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Broders A. Carcinoma grading and practical application. Arch. Pathol. Lab. Med. 1926; 2; 376–381. [Google Scholar]
- 14. Young HHXV. Cancer of the prostate: a clinical, pathological and post‐operative analysis of 111 cases. Ann. Surg. 1909; 50; 1144–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gleason DF. Classification of prostatic carcinomas. Cancer Chemother. Rep. 1966; 50; 125–128. [PubMed] [Google Scholar]
- 16. Gleason DF, Mellinger GT; Veterans Administration Cooperative Urological Research Group . Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. 1974. J. Urol. 2002; 167; 953–958; discussion 9. [PubMed] [Google Scholar]
- 17. Eble JN, Sauter G, Epstein JI, Sesterhenn IA eds. World Health Organization classification of tumours. Tumours of the urinary system and male genital organs 6. Lyon: IARC, 2004; 179–184. [Google Scholar]
- 18. Mostofi FK. Grading of prostatic carcinoma. Cancer Chemother. Rep. 1975; 59; 111–117. [PubMed] [Google Scholar]
- 19. Mostofi FK, Sesterhenn IA, Sobin L, Organization WH. Histological typing of prostate tumours. Geneva: World Health Organization, 1980. [Google Scholar]
- 20. Schroeder FH, Blom JH, Hop WC, Mostofi FK. Grading of prostatic cancer (I): an analysis of the prognostic significance of single characteristics. Prostate 1985; 6; 81–100. [DOI] [PubMed] [Google Scholar]
- 21. Brawn PN, Ayala AG, Von Eschenbach AC, Hussey DH, Johnson DE. Histologic grading study of prostate adenocarcinoma: the development of a new system and comparison with other methods – a preliminary study. Cancer 1982; 49; 525–532. [DOI] [PubMed] [Google Scholar]
- 22. Veltri RW, Marlow C, Khan MA, Miller MC, Epstein JI, Partin AW. Significant variations in nuclear structure occur between and within Gleason grading patterns 3, 4, and 5 determined by digital image analysis. Prostate 2007; 67; 1202–1210. [DOI] [PubMed] [Google Scholar]
- 23. Helpap B, Otten J. Histologic–cytologic grading of uniform and pluriform prostate cancers. Pathologe 1982; 3; 216–222. [PubMed] [Google Scholar]
- 24. Bombari D, Mora B, Schaefer SC, Mast FW, Lehr HA. What was I thinking? Eye‐tracking experiments underscore the bias that architecture exerts on nuclear grading in prostate cancer. PLoS ONE 2012; 7; e38023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fandel TM, Pfnur M, Schafer SC et al Do we truly see what we think we see? The role of cognitive bias in pathological interpretation. J. Pathol. 2008; 216; 193–200. [DOI] [PubMed] [Google Scholar]
- 26. Epstein JI, Egevad L, Amin MB et al The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am. J. Surg. Pathol. 2016; 40; 244–252. [DOI] [PubMed] [Google Scholar]
- 27. Helpap B, Egevad L. Modified Gleason grading. An updated review. Histol. Histopathol. 2009; 24; 661–666. [DOI] [PubMed] [Google Scholar]
- 28. Latour M, Amin MB, Billis A et al Grading of invasive cribriform carcinoma on prostate needle biopsy: an interobserver study among experts in genitourinary pathology. Am. J. Surg. Pathol. 2008; 32; 1532–1539. [DOI] [PubMed] [Google Scholar]
- 29. Lotan TL, Epstein JI. Gleason grading of prostatic adenocarcinoma with glomeruloid features on needle biopsy. Hum. Pathol. 2009; 40; 471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Egevad L, Mazzucchelli R, Montironi R. Implications of the International Society of Urological Pathology modified Gleason grading system. Arch. Pathol. Lab. Med. 2012; 136; 426–434. [DOI] [PubMed] [Google Scholar]
- 31. Epstein JI. Gleason score 2–4 adenocarcinoma of the prostate on needle biopsy: a diagnosis that should not be made. Am. J. Surg. Pathol. 2000; 24; 477–478. [DOI] [PubMed] [Google Scholar]
- 32. Berney DM. The case for modifying the Gleason grading system. BJU Int. 2007; 100; 725–726. [DOI] [PubMed] [Google Scholar]
- 33. Cheng L, Davidson DD, Lin H, Koch MO. Percentage of Gleason pattern 4 and 5 predicts survival after radical prostatectomy. Cancer 2007; 110; 1967–1972. [DOI] [PubMed] [Google Scholar]
- 34. Stamey TA, McNeal JE, Yemoto CM, Sigal BM, Johnstone IM. Biological determinants of cancer progression in men with prostate cancer. JAMA 1999; 281; 1395–1400. [DOI] [PubMed] [Google Scholar]
- 35. Vis AN, Roemeling S, Kranse R, Schroder FH, van der Kwast TH. Should we replace the Gleason score with the amount of high‐grade prostate cancer? Eur. Urol. 2007; 51; 931–939. [DOI] [PubMed] [Google Scholar]
- 36. Stark JR, Perner S, Stampfer MJ et al Gleason score and lethal prostate cancer: does 3 + 4 = 4 + 3? J. Clin. Oncol. 2009; 27; 3459–3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Samaratunga H, Delahunt B, Yaxley J, Srigley JR, Egevad L. From Gleason to International Society of Urological Pathology (ISUP) grading of prostate cancer. Scand. J. Urol. 2016; 50; 325–329. [DOI] [PubMed] [Google Scholar]
- 38. Eifler JB, Feng Z, Lin BM et al An updated prostate cancer staging nomogram (Partin tables) based on cases from 2006 to 2011. BJU Int. 2013; 111; 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pierorazio PM, Walsh PC, Partin AW, Epstein JI. Prognostic Gleason grade grouping: data based on the modified Gleason scoring system. BJU Int. 2013; 111; 753–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Epstein JI. Prostate cancer grading: a decade after the 2005 modified system. Mod. Pathol. 2018; 31(Suppl. 1); S47–S63. [DOI] [PubMed] [Google Scholar]
- 41. Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO classification of tumours of the urinary system and male genital organs – part B. Prostate and bladder tumours. Eur. Urol. 2016; 70; 106–119. [DOI] [PubMed] [Google Scholar]
- 42. Epstein JI, Zelefsky MJ, Sjoberg DD et al A contemporary prostate cancer grading system: a validated alternative to the Gleason score. Eur. Urol. 2016; 69; 428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Egevad L, Delahunt B, Kristiansen G, Samaratunga H, Varma M. Contemporary prognostic indicators for prostate cancer incorporating International Society of Urological Pathology recommendations. Pathology 2018; 50; 60–73. [DOI] [PubMed] [Google Scholar]
- 44. Mottet N, Van d, Briers E et al EAU–ESTRO–ESUR–SIOG guidelines on prostate cancer. European Association of Urology guidelines. Arnhem, the Netherlands: European Association of Urology, 2018; 22–26. [Google Scholar]
- 45. Kirmiz S, Qi J, Babitz SK et al Grade groups provides improved predictions of pathologic and early oncologic outcomes compared with Gleason score risk groups. J. Urol. 2018. Epub ahead of print 10.1016/j.juro.2018.08.081. [DOI] [PubMed] [Google Scholar]
- 46. Wissing M, Brimo F, Chevalier S et al Optimization of the 2014 Gleason grade grouping in a Canadian cohort of patients with localized prostate cancer. BJU Int. 2018; 1–8. 10.1111/bju.14512. [DOI] [PubMed] [Google Scholar]
- 47. Zumsteg ZS, Zelefsky MJ, Woo KM et al Unification of favourable intermediate‐, unfavourable intermediate‐, and very high‐risk stratification criteria for prostate cancer. BJU Int. 2017; 120; E87–E95. [DOI] [PubMed] [Google Scholar]
- 48. Leapman MS, Ameli N, Shinohara K et al Validity of the Cancer of the Prostate Risk Assessment Score derived from targeted biopsy: modeling evidence from ultrasound lesion‐directed biopsy. Clin. Genitourin. Cancer 2017; 15; 93–99. [DOI] [PubMed] [Google Scholar]
- 49. Mottet N, Bellmunt J, Bolla M et al EAU–ESTRO–SIOG guidelines on prostate cancer. Part 1: Screening, diagnosis, and local treatment with curative intent. Eur. Urol. 2017; 71; 618–629. [DOI] [PubMed] [Google Scholar]
- 50. Trpkov K, Sangkhamanon S, Yilmaz A et al Concordance of ‘case level’ global, highest, and largest volume Cancer Grade Group on needle biopsy versus grade group on radical prostatectomy. Am. J. Surg. Pathol. 2018; 42; 1522–1529. 10.1097/pas.0000000000001137. [DOI] [PubMed] [Google Scholar]
- 51. Cole AI, Morgan TM, Spratt DE et al Prognostic value of percent Gleason grade 4 at prostate biopsy in predicting prostatectomy pathology and recurrence. J. Urol. 2016; 196; 405–411. [DOI] [PubMed] [Google Scholar]
- 52. Sauter G, Steurer S, Clauditz TS et al Clinical utility of quantitative Gleason grading in prostate biopsies and prostatectomy specimens. Eur. Urol. 2016; 69; 592–598. [DOI] [PubMed] [Google Scholar]
- 53. Downes MR, Gibson E, Sykes J, Haider M, van der Kwast TH, Ward A. Determination of the association between T2‐weighted MRI and Gleason sub‐pattern: a proof of principle study. Acad. Radiol. 2016; 23; 1412–1421. [DOI] [PubMed] [Google Scholar]
- 54. Langer DL, van der Kwast TH, Evans AJ et al Intermixed normal tissue within prostate cancer: effect on MR imaging measurements of apparent diffusion coefficient and T2 – sparse versus dense cancers. Radiology 2008; 249; 900–908. [DOI] [PubMed] [Google Scholar]
- 55. Egan AJ, Lopez‐Beltran A, Bostwick DG. Prostatic adenocarcinoma with atrophic features: malignancy mimicking a benign process. Am. J. Surg. Pathol. 1997; 21; 931–935. [DOI] [PubMed] [Google Scholar]
- 56. Humphrey PA. Variants of acinar adenocarcinoma of the prostate mimicking benign conditions. Mod. Pathol. 2018; 31(Suppl. 1); S64–S70. [DOI] [PubMed] [Google Scholar]
- 57. Arista‐Nasr J, Martinez‐Benitez B, Aguilar‐Ayala EL, Aleman‐Sanchez CN, Bornstein‐Quevedo L, Albores‐Saavedra J. Pseudohyperplastic prostate carcinoma: histologic patterns and differential diagnosis. Ann. Diagn. Pathol. 2015; 19; 253–260. [DOI] [PubMed] [Google Scholar]
- 58. Hameed O, Humphrey PA. Stratified epithelium in prostatic adenocarcinoma: a mimic of high‐grade prostatic intraepithelial neoplasia. Mod. Pathol. 2006; 19; 899–906. [DOI] [PubMed] [Google Scholar]
- 59. Bostwick DG, Wollan P, Adlakha K. Collagenous micronodules in prostate cancer. A specific but infrequent diagnostic finding. Arch. Pathol. Lab. Med. 1995; 119; 444–447. [PubMed] [Google Scholar]
- 60. Paulk A, Giannico G, Epstein JI. PIN‐like (ductal) adenocarcinoma of the prostate. Am. J. Surg. Pathol. 2018. 10.1097/pas.0000000000001139. [DOI] [PubMed] [Google Scholar]
- 61. Samaratunga H, Delahunt B, Srigley JR et al Mucinous adenocarcinoma of prostate and prostatic adenocarcinoma with mucinous components: a clinicopathological analysis of 143 cases. Histopathology 2017; 71; 641–647. [DOI] [PubMed] [Google Scholar]
- 62. Ross HM, Kryvenko ON, Cowan JE, Simko JP, Wheeler TM, Epstein JI. Do adenocarcinomas of the prostate with Gleason score (GS) </=6 have the potential to metastasize to lymph nodes? Am. J. Surg. Pathol. 2012; 36; 1346–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kweldam CF, Wildhagen MF, Bangma CH, van Leenders GJ. Disease‐specific death and metastasis do not occur in patients with Gleason score </=6 at radical prostatectomy. BJU Int. 2015; 116; 230–235. [DOI] [PubMed] [Google Scholar]
- 64. Draisma G, Postma R, Schroder FH, van der Kwast TH, de Koning HJ. Gleason score, age and screening: modeling dedifferentiation in prostate cancer. Int. J. Cancer 2006; 119; 2366–2371. [DOI] [PubMed] [Google Scholar]
- 65. Inoue LY, Trock BJ, Partin AW, Carter HB, Etzioni R. Modeling grade progression in an active surveillance study. Stat. Med. 2014; 33; 930–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Espiritu SMG, Liu LY, Rubanova Y et al The evolutionary landscape of localized prostate cancers drives clinical aggression. Cell 2018; 173(1003–1013); e15. [DOI] [PubMed] [Google Scholar]
- 67. Dong F, Wang C, Farris AB et al Impact on the clinical outcome of prostate cancer by the 2005 international society of urological pathology modified Gleason grading system. Am. J. Surg. Pathol. 2012; 36; 838–843. [DOI] [PubMed] [Google Scholar]
- 68. Ayala G, Tuxhorn JA, Wheeler TM et al Reactive stroma as a predictor of biochemical‐free recurrence in prostate cancer. Clin. Cancer Res. 2003; 9; 4792–4801. [PubMed] [Google Scholar]
- 69. Yanagisawa N, Li R, Rowley D et al Stromogenic prostatic carcinoma pattern (carcinomas with reactive stromal grade 3) in needle biopsies predicts biochemical recurrence‐free survival in patients after radical prostatectomy. Hum. Pathol. 2007; 38; 1611–1620. [DOI] [PubMed] [Google Scholar]
- 70. Delahunt B, Lamb DS, Srigley JR et al Gleason scoring: a comparison of classical and modified (International Society of Urological Pathology) criteria using nadir PSA as a clinical end point. Pathology 2010; 42; 339–343. [DOI] [PubMed] [Google Scholar]
- 71. Trudel D, Downes MR, Sykes J, Kron KJ, Trachtenberg J, van der Kwast TH. Prognostic impact of intraductal carcinoma and large cribriform carcinoma architecture after prostatectomy in a contemporary cohort. Eur. J. Cancer 2014; 50; 1610–1616. [DOI] [PubMed] [Google Scholar]
- 72. Gallee MP, Visser‐de Jong E, ten Kate FJ, Schroeder FH, Van der Kwast TH. Monoclonal antibody Ki‐67 defined growth fraction in benign prostatic hyperplasia and prostatic cancer. J. Urol. 1989; 142; 1342–1346. [DOI] [PubMed] [Google Scholar]
- 73. Helpap B. Cell kinetic and cytological grading of prostatic carcinoma. Virchows Arch. A Pathol. Anat. Histol. 1981; 393; 205–214. [DOI] [PubMed] [Google Scholar]
- 74. Iczkowski KA, Paner GP, Van der Kwast T. The new realization about cribriform prostate cancer. Adv. Anat. Pathol. 2018; 25; 31–37. [DOI] [PubMed] [Google Scholar]
- 75. Harding‐Jackson N, Kryvenko ON, Whittington EE et al Outcome of Gleason 3 + 5 = 8 prostate cancer diagnosed on needle biopsy: prognostic comparison with Gleason 4 + 4 = 8. J. Urol. 2016; 196; 1076–1081. [DOI] [PubMed] [Google Scholar]
- 76. Siadat F, Sykes J, Zlotta AR et al Not all gleason pattern 4 prostate cancers are created equal: a study of latent prostatic carcinomas in a cystoprostatectomy and autopsy series. Prostate 2015; 75; 1277–1284. [DOI] [PubMed] [Google Scholar]
- 77. Pickup M, Van der Kwast TH. My approach to intraductal lesions of the prostate gland. J. Clin. Pathol. 2007; 60; 856–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Varma M, Egevad L, Delahunt B, Kristiansen G. Reporting intraductal carcinoma of the prostate: a plea for greater standardization. Histopathology 2017; 70; 504–507. [DOI] [PubMed] [Google Scholar]
- 79. Shah RB, Tadros Y. Adenocarcinoma of the prostate with Gleason pattern 5 on core biopsy: frequency of diagnosis, morphologic subpatterns, and relation to pattern distribution based on the modified Gleason grading system. Hum. Pathol. 2014; 45; 2263–2269. [DOI] [PubMed] [Google Scholar]
- 80. Trpkov K, Zhang J, Chan M, Eigl BJ, Yilmaz A. Prostate cancer with tertiary Gleason pattern 5 in prostate needle biopsy: clinicopathologic findings and disease progression. Am. J. Surg. Pathol. 2009; 33; 233–240. [DOI] [PubMed] [Google Scholar]
- 81. Fajardo DA, Miyamoto H, Miller JS, Lee TK, Epstein JI. Identification of Gleason pattern 5 on prostatic needle core biopsy: frequency of underdiagnosis and relation to morphology. Am. J. Surg. Pathol. 2011; 35; 1706–1711. [DOI] [PubMed] [Google Scholar]
- 82. Shah RB, Li J, Cheng L et al Diagnosis of Gleason pattern 5 prostate adenocarcinoma on core needle biopsy: an interobserver reproducibility study among urologic pathologists. Am. J. Surg. Pathol. 2015; 39; 1242–1249. [DOI] [PubMed] [Google Scholar]
- 83. So JS, Gordetsky J, Epstein JI. Variant of prostatic adenocarcinoma with Paneth cell‐like neuroendocrine differentiation readily misdiagnosed as Gleason pattern 5. Hum. Pathol. 2014; 45; 2388–2393. [DOI] [PubMed] [Google Scholar]
- 84. Treurniet KM, Trudel D, Sykes J, Evans AJ, Finelli A, Van der Kwast TH. Downgrading of biopsy based Gleason score in prostatectomy specimens. J. Clin. Pathol. 2014; 67; 313–318. [DOI] [PubMed] [Google Scholar]
- 85. Tolkach Y, Thomann S, Kristiansen G. Three‐dimensional reconstruction of prostate cancer architecture with serial immunohistochemical sections: hallmarks of tumour growth, tumour compartmentalisation, and implications for grading and heterogeneity. Histopathology 2018; 72; 1051–1059. [DOI] [PubMed] [Google Scholar]
- 86. Kweldam CF, Kummerlin IP, Nieboer D et al Prostate cancer outcomes of men with biopsy Gleason score 6 and 7 without cribriform or intraductal carcinoma. Eur. J. Cancer 2016; 66; 26–33. [DOI] [PubMed] [Google Scholar]
- 87. Kim MJ, Divatia MK, Lee JH et al Collagenous micronodules in prostate cancer revisited: are they solely associated with Gleason pattern 3 adenocarcinomas? Int. J. Clin. Exp. Pathol. 2015; 8; 3469–3476. [PMC free article] [PubMed] [Google Scholar]
- 88. Huang CC, Kong MX, Zhou M et al Gleason score 3 + 4 = 7 prostate cancer with minimal quantity of gleason pattern 4 on needle biopsy is associated with low‐risk tumor in radical prostatectomy specimen. Am. J. Surg. Pathol. 2014; 38; 1096–1101. [DOI] [PubMed] [Google Scholar]
- 89. Glaessgen A, Hamberg H, Pihl CG, Sundelin B, Nilsson B, Egevad L. Interobserver reproducibility of percent Gleason grade 4/5 in prostate biopsies. J. Urol. 2004; 171; 664–667. [DOI] [PubMed] [Google Scholar]
- 90. Sadimin ET, Khani F, Diolombi M, Meliti A, Epstein JI. Interobserver reproducibility of percent Gleason pattern 4 in prostatic adenocarcinoma on prostate biopsies. Am. J. Surg. Pathol. 2016; 40; 1686–1692. [DOI] [PubMed] [Google Scholar]
- 91. Kovi J, Jackson MA, Heshmat MY. Ductal spread in prostatic carcinoma. Cancer 1985; 56(1566–157); 3. [DOI] [PubMed] [Google Scholar]
- 92. McNeal JE, Reese JH, Redwine EA, Freiha FS, Stamey TA. Cribriform adenocarcinoma of the prostate. Cancer 1986; 58(1714–171); 9. [DOI] [PubMed] [Google Scholar]
- 93. Rhamy RK, Buchanan RD, Spalding MJ. Intraductal carcinoma of the prostate gland. J. Urol. 1973; 109; 457–460. [DOI] [PubMed] [Google Scholar]
- 94. O'Brien BA, Cohen RJ, Wheeler TM, Moorin RE. A post‐radical‐prostatectomy nomogram incorporating new pathological variables and interaction terms for improved prognosis. BJU Int. 2011; 107; 389–395. [DOI] [PubMed] [Google Scholar]
- 95. O'Brien C, True LD, Higano CS, Rademacher BL, Garzotto M, Beer TM. Histologic changes associated with neoadjuvant chemotherapy are predictive of nodal metastases in patients with high‐risk prostate cancer. Am. J. Clin. Pathol. 2010; 133; 654–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Bonkhoff H, Wheeler TM, van der Kwast TH, Magi‐Galluzzi C, Montironi R, Cohen RJ. Intraductal carcinoma of the prostate: precursor or aggressive phenotype of prostate cancer? Prostate 2013; 73; 442–448. [DOI] [PubMed] [Google Scholar]
- 97. Van der Kwast T, Al Daoud N, Collette L et al Biopsy diagnosis of intraductal carcinoma is prognostic in intermediate and high risk prostate cancer patients treated by radiotherapy. Eur. J. Cancer 2012; 48; 1318–1325. [DOI] [PubMed] [Google Scholar]
- 98. Chua MLK, Lo W, Pintilie M et al A prostate cancer ‘nimbosus’: genomic instability and SChLAP1 dysregulation underpin aggression of intraductal and cribriform subpathologies. Eur. Urol. 2017; 72; 665–674. [DOI] [PubMed] [Google Scholar]
- 99. Sauter G, Clauditz T, Steurer S et al Integrating tertiary Gleason 5 patterns into quantitative Gleason grading in prostate biopsies and prostatectomy specimens. Eur. Urol. 2018; 73; 674–683. [DOI] [PubMed] [Google Scholar]
- 100. Kweldam CF, Kummerlin IP, Nieboer D et al Presence of invasive cribriform or intraductal growth at biopsy outperforms percentage grade 4 in predicting outcome of Gleason score 3 + 4 = 7 prostate cancer. Mod. Pathol. 2017; 30; 1126–1132. [DOI] [PubMed] [Google Scholar]


