Abstract
Objective
18F FDG‐PET is superior to other imaging techniques in revealing residual laryngeal cancer after radiotherapy. Unfortunately, its specificity is low, due to FDG uptake in inflammation and in anaerobic conditions. PET imaging with the amino acid‐based radiopharmaceutical C11‐methionine (MET) should be less influenced by post‐radiation conditions. The aim of this study was to investigate the potential of MET in diagnosing recurrent laryngeal cancer after radiotherapy as compared to 18F‐FDG.
Methods
Forty‐eight patients with a clinical suspicion of local residual disease at least 3 months after completion of radiotherapy or chemoradiotherapy for a T2‐4 laryngeal carcinoma, along with an indication for direct laryngoscopy, were included. They received MET‐PET and FDG‐PET prior to the direct laryngoscopy. One senior nuclear medicine physician assessed both the FDG‐PET and MET‐PET images visually for the degree of abnormal uptake. The gold standard was a biopsy‐proven recurrence 12 months after PET. The nuclear physician had no access to the medical charts and was blinded to the results of the other PET. Sensitivity, specificity and positive and negative predictive value were calculated.
Results
The sensitivity of FDG was 77.3% and the specificity 56.0% after the conservative reading, with these values equalling 54.5% and 76.0% for MET. The positive predictive value of FDG was 60.7% and the negative predictive value 73.7%. The PPV of MET was 66.7%, and the NPV was 65.5%. The McNemar test within diseased (sensitivity comparison) shows a p‐value of 0.125, and the McNemar test within non‐diseased (specificity comparison) shows a P‐value of 0.180.
Conclusion
MET‐PET is not superior to FDG‐PET in terms of identifying recurrent laryngeal cancer.
Keywords: Methionine, FDG, PET, Post radiation, Recurrence, Laryngeal cancer
1. INTRODUCTION
In the Netherlands, more than 80% of laryngeal cancers are primarily irradiated. Salvage surgery is performed in case of residual disease, but detection of residual or recurrent disease can be difficult after radiotherapy. In the first half year after radiotherapy, residual or recurrent disease especially can have a scattered and sub‐mucosal growth pattern, embedded in oedema and inflammatory tissue (Figure 1).1 In some patients, these features will persist over the ensuing years.
Figure 1.
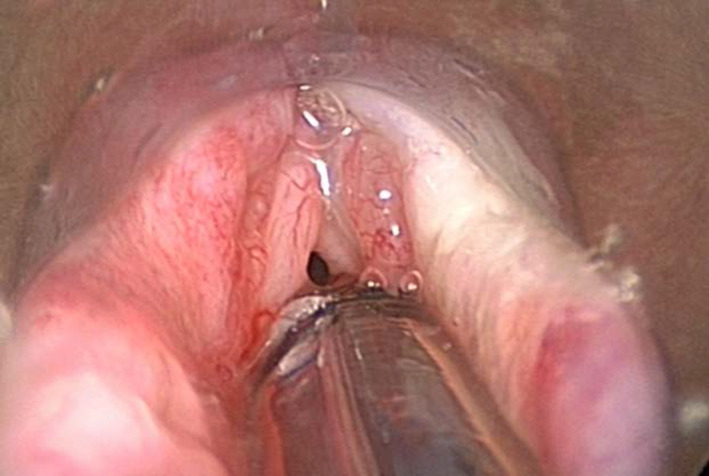
Direct laryngoscopy patient 26. Originally T1N2aM0 supraglottic laryngeal cancer treatment 70 Gy radiotherapy. Clinical suspicion recurrent disease due to deterioration quality of voice 2 y after finishing radiotherapy. The second biopsy showed squamous cell carcinoma
A positive biopsy, by means of endoscopy, is the gold standard for confirming residual or recurrent disease, but a negative biopsy does not necessarily exclude residual or recurrent disease. Several direct laryngoscopies may be necessary to prove the presence of residual or recurrent disease.2, 3, 4 In addition, the tissue damage caused by biopsies may exacerbate the already existing inflammation, oedema and fibrosis.5 Conventional imaging techniques to detect recurrent laryngeal carcinoma after radiotherapy include CT and MRI. The sensitivity of both imaging techniques ranges from 58% to 72%.6, 7 In clinical practice, these figures may indicate that one needs to perform a direct laryngoscopy, despite negative CT or MRI findings. FDG‐PET appeared to be helpful for select patients with clinical suspicion of recurrent laryngeal carcinoma after radiotherapy, when direct laryngoscopies under general anaesthesia with biopsies were indicated.8 A systematic review by Brouwer and colleagues shows that FDG‐PET can help to reveal residual disease after radiotherapy, with a sensitivity of 89% and a specificity of 74%.3 An explanation for the relatively low specificity could be the uptake of FDG in activated macrophages. As in tumour cells, activated macrophages have an abundance of GLUT‐1 receptors and will therefore have a high uptake of FDG.9, 10 The conditions after radiotherapy are characterised by non‐vital tumour cells and macrophages dominating the former tumour site, regardless of the presence of residual disease or not.11
The uptake of amino acids—methionine, for example—is high in tumour cells but low in inflammatory tissues and could therefore be a good alternative to FDG.12 C‐11 MET is an established radiopharmaceutical and has been widely used to visualise intracranial lesions.13 Methionine (MET) has also been successfully used in visualising primary head and neck cancer.14, 15, 16, 17, 18 In addition to preclinical studies validating MET in the evaluation of radiotherapy/chemoradiotherapy, preclinical studies also showed a fast decline for MET in the post‐radiation phase.19, 20 Autoradiography shows that MET uptake is predominantly located in viable tumour cells, with low uptake in macrophages and nonviable tumour cells.21
In this study, we hypothesise that MET‐PET is better than FDG‐PET in detecting recurrent disease in patients with clinical suspicion of recurrent laryngeal carcinoma after radiotherapy.
2. METHODS
2.1. Patients
The protocol was approved by the ethics committee as required in the Netherlands under the Medical Research Involving Human Subjects Act. All patients provided written informed consent. Two university hospitals recruited patients for the study.
Forty‐eight patients with a clinical suspicion—although no obvious local residual, recurrent disease or second primary at least 3 months after completing radiotherapy/chemoradiotherapy with curative intent for a resectable T2‐4 laryngeal squamous cell carcinoma—who had a clinical indication for direct laryngoscopy and biopsy under general anaesthesia were included. Suspicion of recurrent disease was raised by the patient's complaints and changes on physical examination that included fibre‐optic laryngoscopy. Exclusion criteria were no younger than 18 years, a clinically evident recurrence, and pregnancy. One patient had to be excluded because parts of the PET scan registration were lost. The patient received MET‐PET and FDG‐PET prior to direct laryngoscopy. The maximum allowed timeframe between scans and laryngoscopy was 1 month.
2.2. Ethical considerations
The protocol was approved by the ethics committee as required in the Netherlands under the Medical Research Involving Human Subjects Act. All patients provided written informed consent. Two university hospitals recruited patients for the study.
2.2.1. Procedures
C11‐methionine was prepared in our laboratory by 11C ‐methylation of L‐homocysteine thiolactone using a Zymark robotic system. To this end, a solution of L‐homocysteine thiolactone in a NaOH/ethanol mixture was put into a C18 cartridge followed by the passage of C11‐methyl iodide. When the radioactivity on the cartridge was maximal, C11‐methionine was eluted with a phosphate buffer through a second C18 cartridge and a sterile filter to a sterile vial containing saline. This end product was ready for injection and met the following radiopharmaceutical criteria—radiochemical purity > 95%, specific activity > 10 000 GBq/mmol, sterile and pyrogen free, pH 4.5‐8.5, ethanol <3.5%, D/L ratio >90%, osmolarity 200‐800 mosmol/kg. We achieved successful C11‐methionine production in>95% of syntheses.
F18‐FDG was produced according to the method of Hamacher and colleagues, using an automated synthesis module.14 The radiochemical yield was 65.9% ± 7.1% (decay corrected).
The patients were scheduled for separate C11‐MET‐PET only and F18‐FDGPET only scans, shortly before the direct laryngoscopy. For both scans, patients were instructed to fast for at least 6 hours. A5 MBq/kg C11‐MET or 5 MBq/kg F18‐FDG was injected intravenously and again after 20 or 60 minutes (for C11‐MET or F18‐FDG, respectively). The scanning was performed on an ECAT EXACT HR +PET camera (Siemens/CTI Inc) at both institutions, according to the Netherlands protocol for standardisation and quantification of FDG whole body PET studies in multi‐centre trials.22 The scanned trajectory included skull base to the pelvis (Figures 1 and 2). PET images were iteratively reconstructed (ordered subset expectation maximisation). Both MET‐PET and FDG‐PET images were analysed visually on a Leonardo workstation (Syngo Leonardo, Siemens AG, Berlin).
Figure 2.
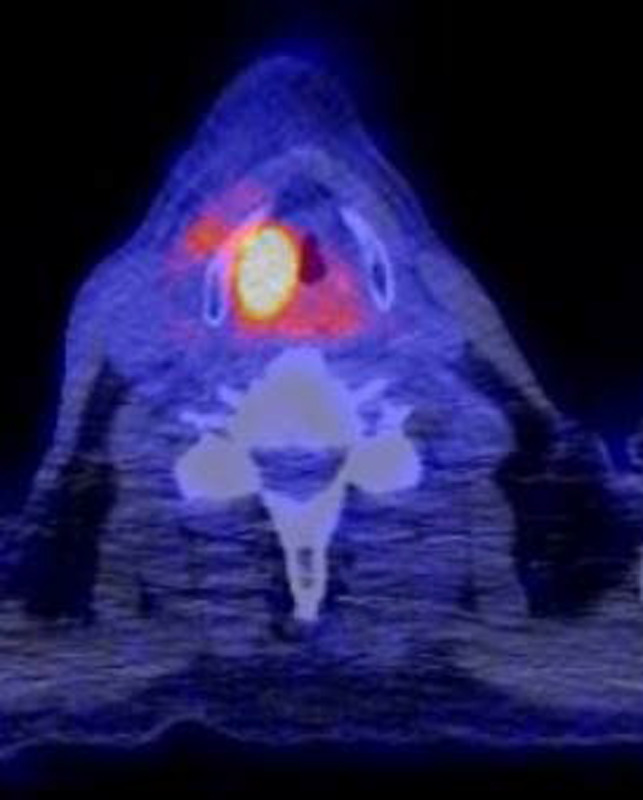
Positive FDG‐PET patient 26
Assessment of the PET images was performed visually for both FDG‐PET and MET‐PET by one senior nuclear medicine physician two years after inclusion was finished. He had no access to the medical charts and was blinded to the result of the paired scan. First all methionine PET images were assessed and in other session the FDG images. The larynx was assessed by the degree of abnormal uptake and side, and summarised in a three‐point scale: negative, equivocal or positive for local recurrent disease. The PET report also included information on lymph node involvement and distant metastases in the field of view.
All but two patients underwent direct laryngoscopy under general anaesthesia, combined with biopsies when indicated during laryngoscopy at the discretion of the attending head and neck surgeon (Figure 3). After a negative direct laryngoscopy, the procedure was repeated within two weeks if there was still a suspicion of residual disease. If not, the head and neck surgeon evaluated the patient every eight weeks, for a minimum period of 24 months. Two patients did not undergo a direct laryngoscopy because of the combination of poor general condition and a low level of suspicion. These two patients did not develop residual disease within 24 months after PET scanning.
Figure 3.
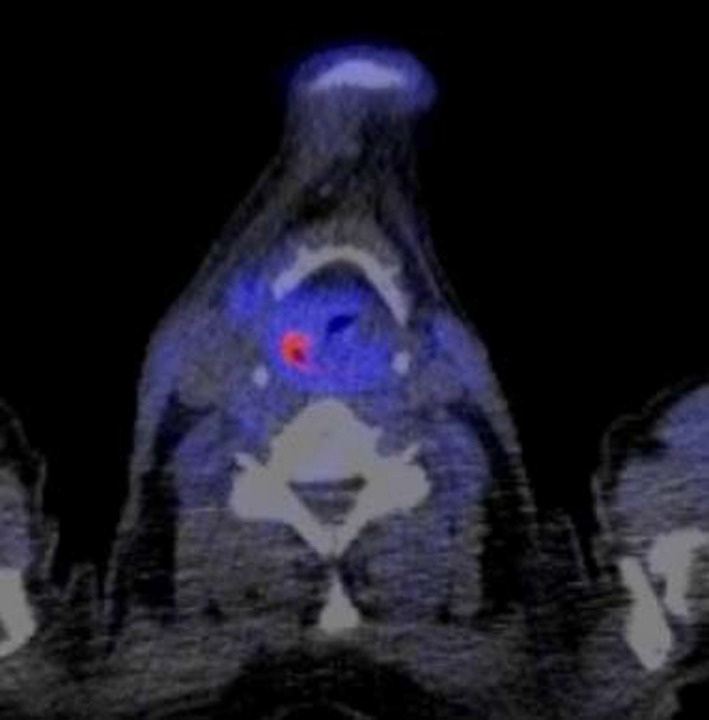
Equivocal MET PET patient 26
Outpatient clinic visits, hospital admission, operative procedures, additional imaging and histological recurrence of tumour, the results of any surgical procedure, and death were documented during the follow‐up period.
Data were collected by the first author, and he was responsible for the completeness and accuracy of the reported data and analyses.
PET scans were evaluated both conservatively and sensitively. Equivocal or positive was considered positive, and negative as negative, when evaluating the test characteristics sensitively. Positive was considered positive, and negative and equivocal as negative, when evaluating the test characteristics conservatively. In an attempt to reduce false‐positive findings, MET‐PET scans were also sensitively evaluated after excluding negative sensitive evaluated FDG‐PET registrations. Histologically proven squamous cell carcinoma of the larynx within 12 months after the MET‐PET registration was considered the positive gold standard.
Sensitivity, specificity, positive and negative predictive value and corresponding exact binomial 95% CIs were calculated using STATA (StataCorp. 2015. Stata Statistical Software: Release 14.0, College Station, TX: StataCorp LP.).
This study was powered to detect a difference of 10% in the paired proportion of the sensitivity/specificity of FDG‐PET and MET‐PET using the McNemar chi‐square test (power 80%; significance level <0.05). For this purpose, 48 patients had to be included.
3. RESULTS
Between November 1, 2008, and November 1, 2012, 48 patients were included, one of whom had to be excluded due to loss of PET data, which left 47 patients for analysis. Thirty‐eight of the patients were male and nine female. The average age was 61 years with a standard deviation of 6.7 years. Twenty patients had glottic, 24 supraglottic and 3 transglottic carcinoma. Twenty‐seven patients had T2, 17 patients T3 and 3 patients T4 primary tumours before primary radiotherapy. Initially, ten patients had positive lymph nodes, while none of the patients had distant metastases. Three patients received chemoradiation and the other 44 radiation therapy only. Details are listed in Table 1.
Table 1.
Baseline characteristics of the patients
| Variable | 47 |
|---|---|
| Male | 39 (82%) |
| Female | 8 (16%) |
| Age | |
| Mean (SD)—y | 62 (9) |
| <65 y—no. (%) | 29 (62%) |
| ≥65 y—no. (%) | 18 (38%) |
| Primary tumour site—% | |
| Supraglottic | 24 (51%) |
| Glottic | 20 (43%) |
| Transglottic | 3 (6%) |
| Primary tumour stage—% | |
| T2 | 26 (55%) |
| T3 | 17 (36%) |
| T4 | 4 (9%) |
| Primary node stage—% | |
| N0 | 37 (81%) |
| N1 | 4 (9%) |
| N2a | 1(2%) |
| N2b | 2 (4%) |
| N2c | 3(5%) |
| N3 | |
| Previous treatment—% | |
| Radiotherapy | 44 (94%) |
| Chemoradiotherapy | 3 (6%) |
Thirty patients experienced a deterioration of their voice, 16 in their swallowing function, 12 had a loss of weight and 24 experienced an increase in pain. In 22 patients, the suspicion of a recurrence was moderate and in 25 patients severe, although not evident, as assessed by their treating physician. The median time from completion of radiotherapy to the MET‐PET was 14 months, with a minimum of 3 months, and a maximum of 13 years and 3 months. In 29 direct laryngoscopies, a biopsy was taken. Four patients underwent more than one direct laryngoscopy; only in one patient were all (repeated) biopsies negative. Two residual cancers were not revealed by either FDG‐PET or MET‐PET, and one was revealed by FDG‐PET and not by MET‐PET. One residual disease was discovered after three consecutive direct laryngoscopies with biopsy taken, and the other two after two consecutive direct laryngoscopies. After 12 months, a total of 25 (53%) patients had not developed recurrent disease, and after 24 months 20 patients. Ten patients died of the disease, while six patients died of other causes. However, of the patients who died of other causes, two patients were already known to have incurable residual disease. Fourteen patients received a laryngectomy. Six of the patients died of the disease after laryngectomy. The remaining eight patients who received a laryngectomy were still alive without evidence of disease 24 months after inclusion.
The ten patients with initially positive lymph nodes did worse, only three of them are still alive without disease. In four patients, the FDG‐PET and MET‐PET showed distant metastases. In three patients, the metastases were located in the lung, and in one patient, bone metastases were visible. Although visible on both PET scans, the FDG‐PET signal was clearer.
Using a conservative reading, the sensitivity of FDG‐PET was 77.3% (95% CI = 54.6‐92.2) and the specificity 56.0% (95% CI = 34.9‐75.6). The sensitivity of MET‐PET was 54.5 (95% CI = 32.2‐75.6) and the specificity 76.0% (95% CI = 54.9‐90.6). FDG showed a positive predictive value of 60.7% and a negative predictive value of 73.7%. The positive predictive value of MET was 66.7% and the negative predictive value 65.5%. The McNemar test within diseased (sensitivity comparison) shows a P‐value of 0.125, and the McNemar test within non‐diseased (specificity comparison) shows a P‐value of 0.180.
Using the sensitive approach, the sensitivity of FDG‐PET was 95.5% (95% CI = 77.2‐99.9) and the specificity 24.0% (95% CI = 9.4‐45.1). The sensitivity of MET‐PET was 86.4% (95% CI = 65.1‐97.1) and the specificity 32.0% (95% CI = 14.9‐53.5). FDG showed a positive predictive value of 52.5% and a negative predictive value of 85.7%. The positive predictive value of MET was 52.8%, and the negative predictive value was 72.7%. The McNemar test within diseased (sensitivity comparison) shows a P‐value of 0.500, and the McNemar test within non‐diseased (specificity comparison) shows a P‐value of 0.625 (Table 2) .
Table 2.
The number positive, equivocal and negative PET registrations after one year
| Conservative approach | Sensitive approach | |||
|---|---|---|---|---|
|
FDGPET Negative and equivocal combined |
METPET Negative and equivocal combined |
FDGPET Positive and equivocal combined |
METPET Positive and equivocal combined |
|
| Sensitivity |
77.3% 54.6‐92.2 |
54.5% 32.2‐75.6 |
95.5% 77.2‐99.9 |
86.4% 65.1‐97.1 |
| Specificity |
56% 34.9‐75.6 |
76.0% 54.9‐90.6 |
24.0% 9.4‐45.1 |
32.0% 14.9‐53.5 |
| PPV |
60.7% 40.6‐78.5 |
66.7% 41.0‐86.7 |
52.5.% 36.1‐68.5 |
52.8% 35.5‐69.6 |
| NPV |
73.7% 48.8‐90.9 |
65.5% 45.7‐82.1 |
85.7% 42.1‐99.6 |
72.7% 39.0‐94.0 |
The combined approach using the FDG‐PET followed by the sensitive MET‐PET, only if the FDG‐PET was positive or equivocal (N = 40), led to a conversion of positive/equivocal evaluation to a negative evaluation five times. Three of these five were negative upon biopsy and therefore correctly classified as negative, while two were positive upon direct biopsy, so these would have been missed using this approach as compared to using FDG‐PET alone. The addition of MET‐PET therefore reduces the sensitivity, while only slightly reducing the number of unnecessary biopsies. Values for the test characteristics of this combined approach equalled: 86.4% sensitivity (95% CI: 65.1‐97.1), 36% specificity (95% CI: 18.0‐57.5), 54.3% PPV (95% CI: 36.8‐71.2) and 75% NPV (95%CI: 42.8‐94.5).
The patient who was included more than five years after finishing radiotherapy had a false‐positive FDG and MET‐PET.
Seventeen patients were included between 1 and 5 years after finishing radiotherapy. Using the conservative reading, the sensitivity of FDG‐PET was 80.0% (95% CI = 0.6‐1.0.), the specificity 60.1% (95% CI = 0.09‐1.00), the PPV 65.6% and the NPV 78.1%. Twenty‐six patients were included between three and twelve months after finishing radiotherapy. Using the conservative reading, the sensitivity of FDG‐PET was 75.3% (95% CI = 0.61‐0.89), the specificity 53.3% (95% CI = 0.05‐1.00), the PPV 61.5% and the NPV 73.2%.
Using the conservative reading, the sensitivity of MET‐PET in the group of patients who were included between 1 and 5 years was 57.4% (95% CI = 0.37‐0.77), the specificity 80.4% (95% CI = 0.32‐1.00), the PPV 70.80% and the NPV 69.30%.
The sensitivity in the group of patients who were included between three and twelve months after finishing radiotherapy was 52.8% (95% CI = 0.21‐0.83), the specificity 76.0% (95% CI = 0.36‐1.00), the PPV 65.4% and the NPV 63.3%.
4. DISCUSSION
Because the majority of recurrent laryngeal cancers after radiotherapy can be salvaged if detected in a timely fashion, early detection of recurrent disease is of importance.23 However, it may be difficult to differentiate between recurrence and post‐radiation changes.5 In the present study, FDG‐PET was able to detect recurrent laryngeal cancer after radiotherapy, with results worse than those results obtained in other studies.6, 8, 24, 25 This can be explained by the selection of our population. In most of the studies, patients with obvious residual/recurrent disease were included, while we excluded these patients.
Three recurrent diseases were not demonstrated by PET after the conservative reading: two were not demonstrated by MET‐PET, and one not by either FDG‐PET or MET‐PET. These recurrent diseases were also not diagnosed by a direct laryngoscopy with taking of biopsies. Two or more direct laryngoscopies were necessary to diagnose the residual cancer. This shows that a false‐negative PET had no more influence on the time of laryngectomy than a traditional workup. These findings are in agreement with the literature.5, 26
In addition to reliable detection of residual or recurrent laryngeal carcinoma after radiotherapy, PET is able to detect distant metastases.27, 28, 29 Although the metastases were revealed both by FDG and MET‐PET, they were more clearly visualised by FDG‐PET. FDG‐PET is our preference for detecting distant metastases.
To make a reliable selection for direct laryngoscopy, a combination of high sensitivity and high negative predictive value is mandatory. The sensitive reading of the FDG results meets these demands. Unfortunately, the positive predictive value is much lower. This could result in a considerable number of unnecessary direct laryngoscopies, if PET were used to select patients for this procedure.
To avoid unnecessary direct laryngoscopies under general anaesthesia, a higher positive predictive value is needed. The search for an alternative to FDG was mainly driven by a desire to improve the positive predictive value. The main goal of this study—an improvement in the positive predictive value without reducing the negative predictive value—was not achieved. The positive predictive value of MET for the conservative reading was slightly higher, although not significant, than the positive predictive value obtained with FDG. The negative predictive of 65.5% was too low, which implies that MET‐PET cannot be used to select patients for a direct laryngoscopy.
It would be interesting to know whether MET‐PET is able to distinguish false‐positive FDG‐PET scans from true‐positive FDG‐PET scans. The combined approach using sensitive MET‐PET only for FDG‐PET positive/equivocal scans shows that the number of unnecessary biopsies is slightly reduced at the cost of missing positive cases. These findings show that MET selects better, although not well enough to reduce the number of unnecessary direct laryngoscopies significantly and safely.
We have no explanation for these disappointing results. It is known that recurrent disease after radiotherapy is usually scattered and embedded in inflammatory tissue. This is why we conducted this pilot study. This small study did show that even early laryngeal cancers (T1‐2 glottic) were excellently visualised with MET.14 Since the major part of this study was carried out using an older generation PET camera, one would expect that the results in this study might have been better due to the improved sensitivity of the new generation of PET cameras. The limited size of recurrent disease found should therefore not be an important reason for the low sensitivity observed.
In contrast to FDG, MET visualises more than a single pathway. MET has a considerable non‐protein synthesis part, which makes MET unsuitable for quantitative analyses.12 However, the non‐protein synthesis pathways are more strongly activated by malignancies than inflammation. The negative effects of non‐protein pathways on the visualisation of recurrent disease should therefore be limited. Although high salivary gland activity is demonstrated by MET‐PET, it is unlikely that this hampered interpretation of PET, because the larynx is out of the field of the submandibular and parotid glands.
A more likely explanation could be the tumour‐to‐background ratio. The tumour‐to‐background ratio of FDG is higher than the ratio obtained with MET.12 Although the uptake of FDG in inflammatory tissues is thought to be higher than the uptake of MET, recurrent disease is probably better detected due to the absolutely stronger uptake of FDG in a malignancy (Figures 2 and 3.).
Most malignancies show, in addition to an increased glucose metabolism, an increased metabolism of amino acids, nucleosides and phospholipids. Parts of malignancies may be hypoxic and may have molecular targets on their cells.
Several amino acids, the nucleoside thymidine, the precursor for the biosynthesis of phospholipids choline, hypoxia tracers and labelled monoclonal antibodies are incorporated in radiopharmaceuticals, which could be alternatives to FDG. The literature shows only a few studies in which an alternative to FDG is used to visualise recurrent head and neck carcinoma after radiotherapy. These studies have their limitations because none of the studies include more than 20 patients, and they frequently deal with more than one sub‐site. These studies show that 11C‐choline and FLT visualise primary and recurrent head and neck cancer slightly worse than FDG does.28, 29, 30, 31 The amino acid tyrosine (TYR) and O‐(2‐[18F] fluor ethyl)‐L‐tyrosine (FET) are the only radiopharmaceuticals that show results that are equal or better than those obtained with FDG for visualising recurrent or residual head and neck carcinomas. Unfortunately the laborious production processes are a serious limitation to using TYR and FET on a larger scale.32, 33, 34
To answer the question of whether other radiopharmaceuticals are more suitable for revealing recurrent laryngeal disease after radiotherapy, studies designed like ours need to be conducted.
5. CONCLUSION
MET‐PET is not superior to FDG‐PET for identifying recurrent laryngeal cancer after radiotherapy.
Wedman J, Pruim J, van der Putten L, et al. Is C‐11 Methionine PET an alternative to 18‐F FDG PET for identifying recurrent laryngeal cancer after radiotherapy? Clin Otolaryngol. 2019;44:124–130. 10.1111/coa.13242
REFERENCES
- 1. Holm LE. Cancer occurring after radiotherapy and chemotherapy. Int J Radiat Oncol Biol Phys. 1990;19(5):1303‐1308. [DOI] [PubMed] [Google Scholar]
- 2. Brouwer J, deBree R, Comans EF, et al. Improved detection of recurrent laryngeal tumor after radiotherapy using (18)FDG‐PET as initial method. Radiother Oncol. 2008; 87: 217‐220. [DOI] [PubMed] [Google Scholar]
- 3. Brouwer J, Hooft L, Hoekstra OS, et al. Systematic review: Accuracy of imaging tests in the diagnosis of recurrent laryngeal carcinoma after radiotherapy. Head Neck. 2008;30(7):889‐897. [DOI] [PubMed] [Google Scholar]
- 4. Brouwer J, Bodar EJ, de Bree R, et al. Detecting recurrent laryngeal carcinoma after radiotherapy: Room for improvement. Eur Arch Otorhinolaryngol. 2003; 261(8):417‐422. [DOI] [PubMed] [Google Scholar]
- 5. Zbaren P, de Bree R, Takes RP, Rinaldo A, Ferlito A. Which is the most reliable diagnostic modality for detecting locally residual or recurrent laryngeal squamous cell carcinoma after (chemo)radiotherapy? Eur Arch Otorhinolaryngol. 2013;270(11):2787‐2791. [DOI] [PubMed] [Google Scholar]
- 6. Greven KM, Williams DW, Keyes JW Jr, McGuirt WF, Watson NE Jr, Case LD. Can positron emission tomography distinguish tumor recurrence from irradiation sequelae in patients treated for larynx cancer? Cancer J Sci Am. 1997;3(6):353‐357. [PubMed] [Google Scholar]
- 7. Tshering Vogel DW, Zbaeren P, Geretschlaeger A, Vermathen P, De Keyzer F, Thoeny HC. Diffusion‐weighted MR imaging including bi‐exponential fitting for the detection of recurrent or residual tumour after (chemo)radiotherapy for laryngeal and hypopharyngeal cancers. Eur Radiol. 2013;23(2):562‐569. [DOI] [PubMed] [Google Scholar]
- 8. de Bree R, van der Putten L, van Tinteren H, et al. Effectiveness of an (18)F‐FDG‐PET based strategy to optimize the diagnostic trajectory of suspected recurrent laryngeal carcinoma after radiotherapy: The RELAPS multicenter randomized trial. Radiother Oncol. 2016;118(2):251‐256. [DOI] [PubMed] [Google Scholar]
- 9. Kubota K, Furumoto S, Iwata R, Fukuda H, Kawamura K, Ishiwata K. Comparison of 18F‐fluoromethylcholine and 2‐deoxy‐D‐glucose in the distribution of tumor and inflammation. Ann Nucl Med. 2006;20(8):527‐533. [DOI] [PubMed] [Google Scholar]
- 10. Kubota R, Yamada S, Kubota K, Ishiwata K, Tamahashi N, Ido T. Intratumoral distribution of fluorine‐18‐fluorodeoxyglucose in vivo: High accumulation in macrophages and granulation tissues studied by microautoradiography. J Nucl Med. 1992;33(11):1972‐1980. [PubMed] [Google Scholar]
- 11. Borgmann K, Roper B, El Awady R, et al. Indicators of late normal tissue response after radiotherapy for head and neck cancer: Fibroblasts, lymphocytes, genetics, DNA repair, and chromosome aberrations. Radiother Oncol. 2002;64(2):141‐152. [DOI] [PubMed] [Google Scholar]
- 12. Cook GJ, Maisey MN, Fogelman I. Normal variants, artefacts and interpretative pitfalls in PET imaging with 18‐fluoro‐2‐deoxyglucose and carbon‐11 methionine. Eur J Nucl Med. 1999;26(10):1363‐1378. [DOI] [PubMed] [Google Scholar]
- 13. Zhao C, Zhang Y, Wang J. A meta‐analysis on the diagnostic performance of (18)F‐FDG and (11)C‐methionine PET for differentiating brain tumors. AJNR Am J Neuroradiol. 2014;35(6):1058‐1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wedman J, Pruim J, Langendijk JA, Van Der Laan BF. Visualization of small glottic laryngeal cancer using methyl‐labeled (11)C‐methionine positron emission tomography. Oral Oncol. 2009;45(8):703‐705. [DOI] [PubMed] [Google Scholar]
- 15. Lindholm P, Leskinen‐Kallio S, Minn H, et al. Comparison of fluorine‐18‐fluorodeoxyglucose and carbon‐11‐ methionine in head and neck cancer. J Nucl Med. 1993;34(10):1711‐1716. [PubMed] [Google Scholar]
- 16. Chesnay E, Babin E, Constans JM, et al. Early response to chemotherapy in hypopharyngeal cancer: Assessment with (11)C‐methionine PET, correlation with morphologic response, and clinical outcome. J Nucl Med. 2003;44(4):526‐532. [PubMed] [Google Scholar]
- 17. Lindholm P, Leskinen S, Lapela M. Carbon‐11‐methionine uptake in squamous cell head and neck cancer. J Nucl Med. 1998;39(8):1393‐1397. [PubMed] [Google Scholar]
- 18. Leskinen‐Kallio S, Nagren K, Lehikoinen P, Ruotsalainen U, Teras M, Joensuu H. Carbon‐11‐methionine and PET is an effective method to image head and neck cancer. J Nucl Med. 1992;33(5):691‐695. [PubMed] [Google Scholar]
- 19. Murayama C, Harada N, Kakiuchi T. Evaluation of D‐18F‐FMT, 18F‐FDG, L‐11C‐MET, and 18F‐FLT for monitoring the response of tumors to radiotherapy in mice. J Nucl Med. 2009;50(2):290‐295. [DOI] [PubMed] [Google Scholar]
- 20. Kubota R, Kubota K, Yamada S, et al. Methionine uptake by tumor tissue: A microautoradiographic comparison with FDG. J Nucl Med. 1995;36(3):484‐492. [PubMed] [Google Scholar]
- 21. Stöber B, Tanase U, Herz M, Seidl C, Schwaiger M, Senekowitsch‐Schmidtke R. Differentiation of tumour and inflammation: Characterisation of [methyl‐3H]methionine (MET) and O‐(2‐[18F]fluoroethyl)‐L‐tyrosine (FET) uptake in human tumour and inflammatory cells. Eur J Nucl Med Mol Imaging. 33, 932‐939. [DOI] [PubMed] [Google Scholar]
- 22. Boellaard R, Oyen WJ, Hoekstra CJ, et al. The netherlands protocol for standardisation and quantification of FDG whole body PET studies in multi‐centre trials. Eur J Nucl Med Mol Imaging. 2008;35(12):2320‐2333. [DOI] [PubMed] [Google Scholar]
- 23. Stoeckli SJ, Pawlik AB, Lipp M, Huber A, Schmid S. Salvage surgery after failure of nonsurgical therapy for carcinoma of the larynx and hypopharynx. Arch Otolaryngol Head Neck Surg. 2000;126(12):1473‐1477. [DOI] [PubMed] [Google Scholar]
- 24. Bongers V, Hobbelink MG, van Rijk PP, Hordijk GJ. Cost‐effectiveness of dual‐head 18F‐fluorodeoxyglucose PET for the detection of recurrent laryngeal cancer. Cancer Biother Radiopharm. 2002;17(3):303‐306. [DOI] [PubMed] [Google Scholar]
- 25. de Bree R, van der Putten L, Brouwer J, Castelijns JA, Hoekstra OS, Leemans CR. Detection of locoregional recurrent head and neck cancer after (chemo)radiotherapy using modern imaging. Oral Oncol. 2009;45(4‐5):386‐393. [DOI] [PubMed] [Google Scholar]
- 26. de Bree R, van der Putten L, Hoekstra OS, et al. A randomized trial of PET scanning to improve diagnostic yield of direct laryngoscopy in patients with suspicion of recurrent laryngeal carcinoma after radiotherapy. Contemp Clin Trials. 2007;28(6):705‐712. [DOI] [PubMed] [Google Scholar]
- 27. Senft A, deBree R, Hoekstra OS, et al. Screening for distant metastases in head and neck cancer patients by chest CT or whole body FDG‐PET: A prospective multicenter trial. Radiother Oncol. 2008;87(2):221‐229. [DOI] [PubMed] [Google Scholar]
- 28. Krabbe CA, Pruim J, Van Der Laan BF, Rodiger LA, Roodenburg JL. FDG‐PET and detection of distant metastases and simultaneous tumors in head and neck squamous cell carcinoma: A comparison with chest radiography and chest CT. Oral Oncol. 2008;45(3):234‐240. [DOI] [PubMed] [Google Scholar]
- 29. Ito K, Yokoyama J, Kubota K, Morooka M. Comparison of 18F‐FDG and 11C‐choline PET/CT for detecting recurrences in patients with nonsquamous cell head and neck malignancies. Nucl Med Commun. 2010;31(11):931‐937. [DOI] [PubMed] [Google Scholar]
- 30. Cobben DC, Van DerLaan BF, Maas B. 18F‐FLT PET for visualization of laryngeal cancer: Comparison with 18F‐FDG PET. J Nucl Med. 2004;45(2):226‐231. [PubMed] [Google Scholar]
- 31. Been LB, Hoekstra HJ, Suurmeijer AJ, Jager PL, Van Der Laan BF, Elsinga PH. [(18)F]FLT‐PET and [(18)F]FDG‐PET in the evaluation of radiotherapy for laryngeal cancer. Oral Oncol. 2009;45(12):e211‐e215. [DOI] [PubMed] [Google Scholar]
- 32. Miyakubo M, Oriuchi N, Tsushima Y, et al. Diagnosis of maxillofacial tumor with L‐3‐[18f]‐fluoro‐alpha‐methyltyrosine (FMT) PET: A comparative study with FDG‐PET. Ann Nucl Med. 2007;21(2):129‐135. [DOI] [PubMed] [Google Scholar]
- 33. de Boer J, Pruim J, Albers FW, Burlage F, Vaalburg W, Van Der Laan BF. Prediction of survival and therapy outcome with 11C‐tyrosine PET in patients with laryngeal carcinoma. J Nucl Med. 2004;45(12):2052‐2057. [PubMed] [Google Scholar]
- 34. de Boer J, Van Der Laan BF, Pruim J, et al. Carbon‐11 tyrosine PET for visualization and protein synthesis rate assessment of laryngeal and hypopharyngeal carcinomas. Eur J Nucl Med Mol Imaging. 2002;29(9):1182‐1187. [DOI] [PubMed] [Google Scholar]


