Abstract
Odorant‐binding proteins (OBPs) and chemosensory proteins (CSPs) of insects are thought to play roles in olfactory recognition affecting host choice, copulation, reproduction and other behaviors. Previous descriptions of OBPs and CSPs in the whitefly Bemisia tabaci often provided no or incomplete genetic information. In this study, we present a genome‐wide and transcriptome‐wide investigation of the OBPs and CSPs in B. tabaci MEAM1 (Middle East‐Asia Minor1 species). Eight OBP and 19 CSP genes were identified that covered all previous sequences. Phylogenetic analyses showed that the CSP genes had a lineage‐specific expansion (BtabBCSP1, BtabBCSP3, BtabBCSP13, BtabBCSP17, BtabBCSP18 and BtabBCSP19). Expression profiling of OBPs and CSPs by transcriptome sequencing and quantitative real‐time polymerase chain reaction (qPCR) revealed that expression patterns differed among developmental stages of B. tabaci MEAM1. Five OBP genes and 11 CSP genes significantly differed between males and females; four of the 19 CSP genes were highly expressed in adults, while two were highly expressed in nymphs. The expression profiles of the OBP and CSP genes in different tissues of B. tabaci MEAM1 adults were analyzed by qPCR. Four OBP genes found in B. tabaci MEAM1 were highly expressed in the head. Conversely, only two CSPs were enriched in the head, while the other six CSPs were specifically expressed in other tissues. Our results provide a foundation for future research on OBPs and CSPs in B. tabaci.
Keywords: Bemisia tabaci, CSPs, expression patterns, genome‐wide identification, OBPs, phylogenetic
Introduction
The olfactory recognition system plays a critical role in feeding, mating, oviposition, and other important behaviors of insects. The first step in olfactory recognition is the solubilization and transport of odor molecules from the external environment to the olfactory sensory neurons. In insects, this task is performed by two major families of small, soluble proteins: odorant‐binding proteins (OBPs) and chemosensory proteins (CSPs) (Vogt & Riddiford, 1981; Vogt et al., 1991; Angeli et al., 1999; Pelosi et al., 2006, 2014, 2018). OBPs provide the initial molecular interactions with chemical signals such as pheromones and host odors and are thought to ferry the semiochemical molecules across the antennal sensillum lymph to the olfactory receptors (Steinbrecht, 1998). OBPs are small (10–30 kDa), globular and abundant water‐soluble acidic proteins with a pattern of six conserved cysteine residues. These cysteine residues are paired into three interlocked disulfide bridges, which together with other amino acids form an odorant‐binding pocket that binds and protects small hydrophobic ligands (Leal et al., 1999; Scaloni et al., 1999; Laughlin et al., 2008). OBPs in insects have rich and expanded roles in pheromone signal transduction (Xu et al., 2005) and in the manipulation of host selection and mating behavior (Hooper et al., 2009; Pannure et al., 2012), and are also important for the identification of cryptic species (Lardeux et al., 2012; Gholizadeh et al., 2015). CSPs are small, soluble proteins that are abundant in the sensilla lymph and that have many functions (Wanner et al., 2004; Gong et al., 2007). Like OBPs, CSPs are odor‐binding proteins (Ban et al., 2003; Ozaki et al., 2005; Foret et al., 2007; Zhou et al., 2010; Li et al., 2013), but CSPs have fewer conserved cysteine residues and more conserved nucleotide sequences than OBPs across insect species (Pelosi et al., 2005). CSPs have more functions than OBPs in non‐sensory organs of insects, and these functions include pheromone delivery, solubilization of nutrients, and the development of insecticide resistance (Kamikouchi et al., 2004; Wanner et al., 2005; Maleszka et al., 2007; Xu et al., 2009; Liu et al., 2010; Guo et al., 2011, 2012; Zhou et al., 2013; Zhang et al., 2013; Jean‐François, 2014). CSPs can also act as effector proteins to trigger plant physiological defenses (Bos et al., 2010).
The sibling and cryptic species of the whitefly Bemisia tabaci include some of the world's most damaging agricultural pests and are considered among the World's Worst Invasive Species (Global Invasive Species Database: http://www.issg.org/database/welcome/). Among the B. tabaci sibling species, Bemisia Middle East‐Asia Minor 1 (MEAM1 or ‘B’) and Bemisia Mediterranean (MED or ‘Q’) are the most extensively studied. They are considered to be highly invasive and destructive pests in many parts of the world because of their broad host range and their ability to transmit viral pathogens of plants (Jones, 2003; De Barro et al., 2011; Gilbertson et al., 2015; Wan & Yang, 2016).
To date, OBPs and CSPs and the genes that encode them have been partly identified in B. tabaci based on expressed sequence tags and head transcriptome data (Li et al., 2012, 2014, 2016; Wang et al., 2016, 2017). However, the sequences obtained are incomplete. A comprehensive understanding of OBPs and CSPs in B. tabaci requires a more complete genome‐wide analysis. In this research, we used previously published data on B. tabaci genomes (Chen et al., 2016; Xie et al., 2017) and new antenna transcriptome data (obtained in the current study) to complete a genome‐wide analysis of OBP and CSP gene families in B. tabaci MEAM1. We systematically classified, characterized, and phylogenetically analyzed the OBP and CSP genes. By searching both genomic and transcriptomic data and experimentally validating the results, we identified eight candidate OBP genes and 19 candidate CSP genes in this species. We also used RPKM (reads per kilobase per million mapped reads) and quantitative real‐time polymerase chain reaction (qPCR) to determine the expression profiles of these genes in different developmental stages and different tissues. In addition to providing a framework for further research on B. tabaci OBPs and CSPs, the results will be useful for comparing OBP and CSP genes and proteins among different species.
Materials and methods
Insect rearing and sample preparation
A B. tabaci MEAM1 population was maintained on cotton plants at 27 ± 1 °C with an L : D 16 : 8 photoperiod and a relative humidity (RH) of 70% ± 10%. Every three to five generations, the purity of the strain was monitored using PCR and the sequence of mitochondrial cytochrome oxidase I (mtCO I) gene (Chu et al., 2010). Samples of stages (eggs, the four nymph stages, females, males) and tissues (head, abdomen and mixture of thorax, legs and wings) were separately collected from the B. tabaci MEAM1 population, rapidly frozen in liquid nitrogen, and stored at −80 °C.
RNA isolation, cDNA library construction, Illumina sequencing and antennae transcriptome assembly
RNA from the anatomical antennae tissues of thousands of adults of MEAM1 was extracted with Trizol reagent (Invitrogen, Carlsbad, CA, USA). according to the manufacturer's instructions, and RNA purity and degradation were checked on 1% agarose gels. RNA integrity was further confirmed using the 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) with a minimum RNA integrity number of 8. Poly (A)‐containing RNA was separated from the total RNA using the Dynabeads® mRNA purification kit (Invitrogen, Carlsbad, CA, USA), and the quality was verified on a denaturing gel. The messenger RNA (mRNA) was then used for SMARTer first‐strand complementary DNA (cDNA) synthesis using the SMARTer Ultra Low Input RNA for Illumina Sequencing Components – HV (cat. nos. 634822, 634825, 634827 and 634831). This was followed by full‐length double‐stranded cDNA (ds‐cDNA) amplification using limiting dilution PCR. PCR‐amplified cDNA was purified by using SPRI Ampure Beads, and purity was confirmed by using the Agilent 2100 BioAnalyzer. After covaris shearing of full‐length cDNA, the Low Input Library Prep Kit (cat. no. 634947) was used to create the final cDNA library. The paired‐end cDNA libraries (200 bp size) were prepared following the manufacturer's recommendations and sequenced on an Illumina GAII platform. The resulting high‐quality cleaned reads were assembled de novo into contigs using Trinity (trinityrnaseq_r20131110) with default parameters except that ‘min_kmer_cov’ was set to 2 (Friedman et al., 2011).
Identification of putative OBPs and CSPs in B. tabaci
The computational pipeline is detailed in Figure S3. The protein sequences of known OBPs and CSPs were used to search the B. tabaci (MEAM1) genome 1.0, the B. tabaci (MEAM1) genome (Chen et al., 2016), and the antenna transcriptome using the program TBLASTN with an e‐value threshold of 10−5. The sequences meeting the criteria were collected as candidate OBP/CSP sequences. After removal of the identical sequences, the remaining sequences were classified into two types (OBPs and CSPs). Putative OBP/CSP sequences were confirmed by subjecting them to BLASTX analysis with the non‐redundant protein sequence (NR) at GenBank (http://www.ncbi.nlm.nih.gov/). The conserved domains of these identified OBPs and CSPs were predicted using SMART (simple modular architecture research tool, http://smart.emblheidelberg.de/) (Letunic et al., 2015) and were confirmed using the National Center for Biotechnology Information conserved domain search service tool. All candidate OBP and CSP sequences were further validated by cloning and sequencing. Gene‐specific primers were designed and used to clone the open reading frame (ORF) or partial sequences of each OBP and CSP. The method of identification of putative OBPs and CSPs in B. tabaci MED is the same as above in B. tabaci MEAM1 except for MEAM1 antenna transcriptome application.
Sequence and phylogenetic analysis
The putative N‐terminal signal peptides and the most likely cleavage sites were predicted using the SignalP V4.1 program (http://www.cbs.dtu.dk/services/SignalP/). Sequences were aligned using the program ClustalW with default gap penalty parameters of gap opening 10 and extension 0.2. A neighbor‐joining tree was constructed using the program MEGA 6.0 with a p‐distance model and a pairwise deletion of gaps (Tamura et al., 2013). The bootstrap support of tree branches was assessed by re‐sampling amino acid positions 1000 times. Phylogenetic trees were then presented in circular shape and colored taxonomically using online tools provided by Evolview (He et al., 2016).
Motif analysis
A total of 120 of OBPs and 64 CSPs from B. tabaci MEAM1, B. tabaci MED and other insects (Supplementary file) were used for motif discovery and pattern analysis. The MEME (version 4.12.0) on the line server (http://meme-suite.org/index.html) was used to discover and analyze the motifs in this analysis. The parameters used were as follows: minimum width = 6, maximum width = 10, and the maximum number of motifs to find = 6.
Expression profiling of OBPs and CSPs
Expression profiles of OBPs and CSPs in different developmental stages of B. tabaci MEAM1 were obtained using transcriptome data. Samples were represented by three biological replicates that were independently processed. Total RNA was extracted using Trizol reagent according to the manufacturer's instructions (Invitrogen, Carlsbad, CA, USA). RNA was quantified using a Nanodrop 2000 (Thermo Scientific, Wilmington, DE, USA), and purity was checked on 1% agarose gels. RNA‐seq libraries were constructed as previously described and sequenced on a HiSeq 2500 system according to the manufacturer's instructions with sequencing at 125 bp (PE125, library size is 280–320 bp). The software Fastq_clean was used for RNA‐seq data cleaning and quality control (Zhang et al., 2014). The raw RNA‐seq reads were filtered with Fastq_clean software by trimming low‐quality (Q value < 20) nucleotides on both ends, clipping the adapter and barcode sequences from the 3′ end, and discarding the ribosomal RNA (rRNA) sequence. We then aligned the high‐quality cleaned RNA‐seq reads to the pre‐prepared RNA sequence data set with the Bowtie program allowing one mismatch. Following alignments, raw counts for each transcript and in each sample were derived and normalized to RPKM. Statistical analyses and plotting were conducted using the software R v2.15.3 with the Bioconductor packages (Gao et al., 2014). Differentially expressed genes (fold‐change > 2 and adjusted P‐value < 0.05) between two selected conditions were identified with the DESeq package. The transcript levels of B. tabaci CSPs and OBPs in different developmental stages were determined by calculating log2 (RPKM + 1) values.
Besides transcriptomic validation, qPCR analysis was used to confirm mRNA expression of CSPs in B. tabaci. Based on the RPKM value generated from the RNAseq data, we selected nine genes (differentially expressed genes between adult and egg, fold‐change > 2 and adjusted P‐value < 0.01) that are representative of all of the B. tabaci CSPs for the qPCR validation study. We also selected eight CSP genes and four OBP genes (differentially expressed genes between males and females, fold‐change > 2 and adjusted P‐value < 0.01) to confirm the transcript levels in different tissues. qPCR was conducted using an ABI PRISM 7500 Real‐time PCR System (Applied Biosystems, Foster City, CA, USA), and non‐treated B. tabaci adults were subjected to the analysis. All qPCR analyses included three technical replicates for each of three biological replicates. EF‐1α and SDHA were selected as the reference genes. The qPCR was carried out in a 20 mL reaction volume containing 10 μL of 2 × Super Real PreMix Plus, 0.4 μL of 50 × ROX Reference Dye, 0.5 μL of forward primer (10 μmol/L), 0.5 μL of reverse primer (10 μmol/L), 1.0 μL of cDNA (300 ng/μL) and 7.6 μL of ribonuclease‐free ddH2O. The instructions of the Super Real PreMix Plus (SYBR Green) kit (Tiangen, Beijing, China) were followed. The thermal cycling conditions were polymerase activation at 95°C for 15 min, followed by 40 cycles of denaturation at 95°C for 10 s, annealing at 60°C for 30 s and elongation at 72°C for 32 s. The amplification efficiency was estimated using the following equation: E = [10^ (−1/slope) −1] × 100%, in which the slope was derived by plotting the cycle threshold (Ct) value against six serially diluted template concentrations. The transcript levels of CSP and OBP genes were quantified according to the 2−ΔΔ method. SPSS 20.0 was used to analyze correlations between qPCR data and RNA‐seq data.
Results
Candidate odorant‐binding proteins and phylogenetic analyses in B. tabaci MEAM1
Among the eight candidate OBP genes that we identified in the B. tabaci genome, three (BtabBOBP1, BtabBOBP6 and BtabBOBP8) are located on the same scaffold and have the same orientation (Table 1; Fig. 1). Each of these three OBPs contains 6–7 exons within the 30 kb genomic region. By aligning the sequences and counting the cysteine motifs, we found that BtabBOBP6 lacks two cysteine residues (C2 and C5) and that BtabBOBP2 and BtabBOBP3 have two additional cysteine residues, that is, one after C4 and one after C6. These additional cysteine residues and a conserved proline residue are the key feature of Plus‐C (Fig. S1). We used all of the putative OBPs from B. tabaci representative homologous sequences from 20 hemipteran species to build a neighbor‐joining phylogenetic tree. The tree showed a clear cluster representing the Minus‐C OBP class, consisting of BtabBOBP6 and other similar genes, and a Plus‐C clade OBP class covering BtabBOBP2 and BtabBOBP3 (Fig. 2). Remaining OBPs (BtabBOBP1, BtabBOBP4, BtabBOBP5, BtabBOBP7 and BtabBOBP8) were grouped and belonged to the classic clade according to their percentage of similarity among hemipteran species (Fig. 2).
Table 1.
List of genes encoding odorant‐binding proteins (OBPs) in Bemisia tabaci genome
| Location | |||||
|---|---|---|---|---|---|
| Gene name | ORF (bp) | Signal peptide (aa) | Orientation | Start | End |
| BtabBOBP1 | 426 | 1‐24 | scaffold_135− | 465930 | 459692 |
| BtabBOBP2 | 741 | 1‐22 | Scaffold_24− | 1533820 | 1523195 |
| BtabBOBP3 | 747 | 1‐26 | Scaffold_7− | 1994480 | 1961838 |
| BtabBOBP4 | 426 | 1‐19 | Scaffold_267− | 547353 | 508414 |
| BtabBOBP5 | 633 | 1‐24 | Scaffold_277+ | 384969 | 402084 |
| BtabBOBP6 | 435 | 1‐25 | Scaffold_135− | 485754 | 472890 |
| BtabBOBP7† | 267 | ND | Scaffold_188− | 215970 | 204845 |
| BtabBOBP8 | 477 | 1‐21 | Scaffold_135− | 454393 | 438508 |
†Indicates that the gene is partial and lacks an intact open reading frame (ORF). ND indicates not detected.
Figure 1.
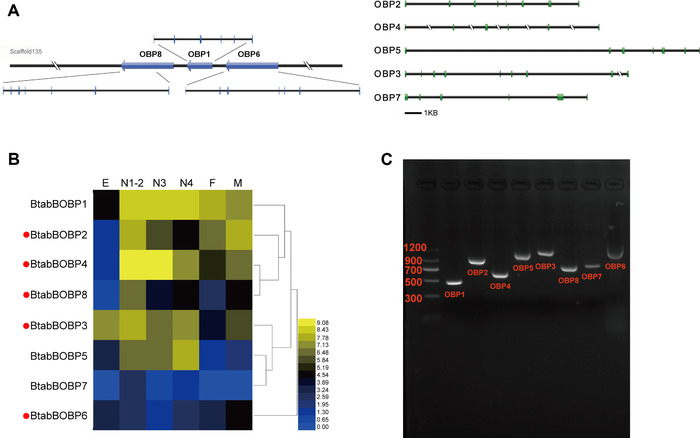
Structure and expression profiles of odorant‐binding protein (OBP) genes in Bemisia tabaci. (A) Structures and locations of OBP genes on scaffolds. The blue arrows indicate the transcription orientations of OBP genes on the scaffold. The transcript sequences of OBPs were matched to B. tabaci genomic sequences in order to identify the exons and introns. The exon regions are shown with blue boxes. (B) Expression profiles of OBPs in different developmental stages (E = egg, N = nymph stages 1 to 4 as indicated, F = adult female, and M = adult male). The transcript levels were determined by calculating log2 (reads per kilobase per million mapped reads + 1) values. Relative expression levels are indicated by a 15‐grade color scale. ● indicates differentially expressed genes (fold‐change > 2 and adjusted P‐value < 0.05) between males and females. (C) Electrophoretic separation of BtabOBPs on an agarose gel.
Figure 2.
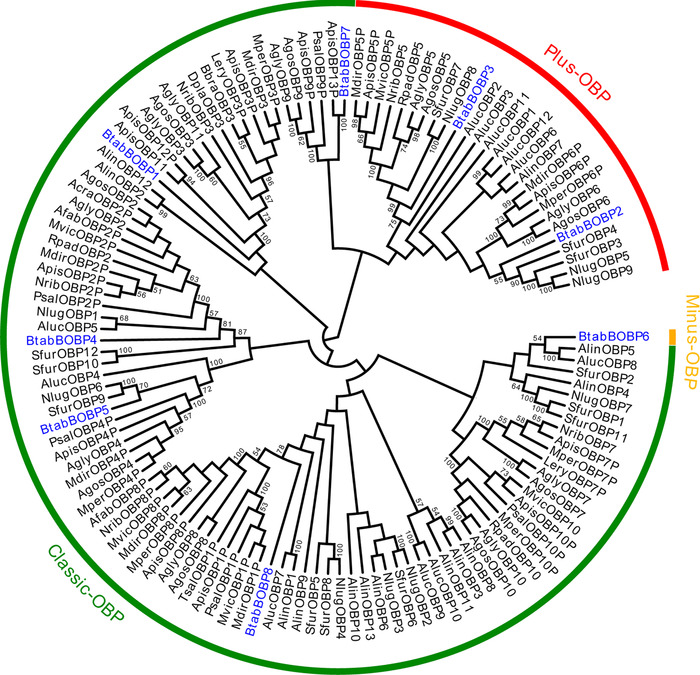
Phylogenetic analysis of the amino acid sequences of BtabBOBPs (indicated by blue) in the context of various hemipteran odorant‐binding proteins (OBPs). Neighbor‐joining tree of the odorant‐binding proteins (OBPs) based on amino acid sequences of Bemisia tabaci and other hemipterans. Bootstrap values were calculated with 1000 replications, and those larger than 50% are marked on the nodes. The protein names and sequences of the 112 OBPs used in this analysis are listed in a supplementary file. Btab = Bemisia tabaci, Apis = Acyrthosiphon pisum, Mper = Myzus persicae, Agos = Aphis gossypii, Sfur = Sogatella furcifera, Nlug = Nilaparvata lugens, Psal = Pterocomma salicis, Agly = Aphis glycines, Aluc = Apolygus lucorum, Alin = Adelphocoris lineolatus, Rpad = Rhopalosiphum padi, Mdir = Metopolophium dirhodum, Mvic = Megoura viciae, Bbra = Brevicoryne brassicae, Lery = Lipaphis erysimi, Afab = Aphis fabae, Acra = Aphis craccivora, Tsal = Tuberolachnus salignus, Dpla = Drepanosiphum platanoidis and Nrib = Nasonovia ribis‐nigri.
Expression profiles of the B. tabaci MEAM1 OBPs across developmental stages
Expression significantly differed between males and females for five OBP genes (BtabBOBP2, BtabBOBP3, BtabBOBP4, BtabBOBP6 and BtabBOBP8) (Fig. 1B). Among all developmental stages, expression was highest for BtabBOBP1 and BtabBOBP3 and lowest for BtabBOBP6 and BtabBOBP7. Expression of BtabBOBP2 and BtabBOBP4 was low in eggs but high in other stages. Expression of BtabBOBP5 was highest in nymphs followed by eggs.
mRNA expression of selected B. tabaci MEAM1 OBPs as determined by qPCR across different tissues
qPCR analyses were conducted to measure the expression levels of the four BtabBOBP genes in the head, thorax (mixture of thorax, legs and wings) and abdomen. The results indicated that four genes (BtabBOBP2, BtabBOBP3, BtabBOBP4 and BtabBOBP8) were approximately 2–100 times more expressed in head than in the other parts (Fig. 3). Furthermore, the two OBPs (BtabBOBP3 and BtabBOBP4) exhibited an expression level of 2.5 and 6.85 times difference between the thorax and abdomen, respectively (P < 0.05).
Figure 3.
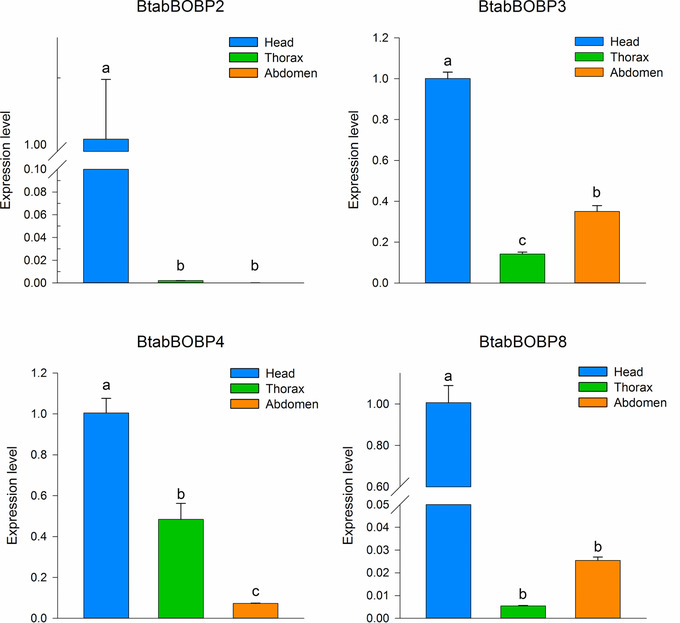
Bemisia tabaci odorant‐binding proteins (OBPs) transcript levels in different tissues as measured by quantitative real‐time polymerase chain reaction (qPCR). The expression levels were estimated using 2−Δ Δ method. Standard error for each sample is represented by error bar and the different letters (a, b, c) above each bar denote significant differences (P < 0.05).
Candidate chemosensory proteins and phylogenetic analyses in B. tabaci MEAM1
We identified 19 candidate CSP genes distributed across 11 scaffolds in the B. tabaci genome. More than half of them are located within clusters (Table 2). The largest cluster contains six CSP genes, which occur in both orientations on scaffold211 (Fig. 4). The alignment of the predicted B. tabaci CSP proteins showed high average pairwise sequence identity between CSP family members. All of the 19 candidate CSPs contain four intact, conserved cysteine residues (Fig. S2). The neighbor‐joining method was used to construct a phylogenetic tree for the CSPs of B. tabaci and of seven other hemipteran species (Fig. 5). CSPs in B. tabaci are represented in all major clades in phylogenetic trees constructed for these multi‐gene families in hemipterans. Phylogenetic analyses showed that the CSP genes had a lineage‐specific expansion (BtabBCSP1, BtabBCSP3, BtabBCSP13, BtabBCSP17, BtabBCSP18 and BtabBCSP19).
Table 2.
List of genes encoding chemosensory proteins (CSPs) in Bemisia tabaci genome
| Location | |||||
|---|---|---|---|---|---|
| Gene name | ORF (bp) | Signal peptide (AA) | Orientation | Start | End |
| BtabBCSP1 | 381 | 1‐19 | Scaffold_1728+ | 4851 | 6514 |
| BtabBCSP2 | 393 | 1‐18 | Scaffold_211+ | 417237 | 419266 |
| BtabBCSP4 | 321 | 1‐19 | Scaffold_1760− | 41222 | 37927 |
| BtabBCSP5 | 483 | 1‐16 | Scaffold_1760+ | 17122 | 27511 |
| BtabBCSP6 | 414 | 1‐20 | Scaffold_211+ | 319755 | 323826 |
| BtabBCSP7 | 384 | 1‐19 | Scaffold_4− | 522706 | 513251 |
| BtabBCSP8 | 327 | 1‐20 | Scaffold_893− | 64906 | 62662 |
| BtabBCSP9 | 372 | 1‐20 | Scaffold_14− | 2936398 | 2933040 |
| BtabBCSP10 | 408 | 1‐22 | Scaffold_211+ | 425352 | 428930 |
| BtabBCSP11 | 738 | ND | Scaffold_211+ | 368338 | 398455 |
| BtabBCSP12 | 408 | 1‐18 | Scaffold_211− | 296951 | 293093 |
| BtabBCSP13 | 381 | 1‐19 | Scaffold_1728− | 44016 | 41267 |
| BtabBCSP14 | 426 | 1‐22 | Scaffold_211+ | 340569 | 353913 |
| BtabBCSP15 | 336 | 1‐19 | Scaffold_95− | 900768 | 897021 |
| BtabBCSP16 | 453 | 1‐16 | scaffold74− | 114714 | 108425 |
| BtabBCSP17 | 381 | 1‐19 | Scaffold_135− | 89732 | 88317 |
| BtabBCSP18 | 381 | 1‐19 | Scaffold_708+ | 116559 | 117889 |
| BtabBCSP19 | 381 | ND | Scaffold_102+ | 531873 | 533667 |
ND indicates not detected. CSPs that could not be aligned well with the scaffold are not shown. ORF, open reading frame.
Figure 4.
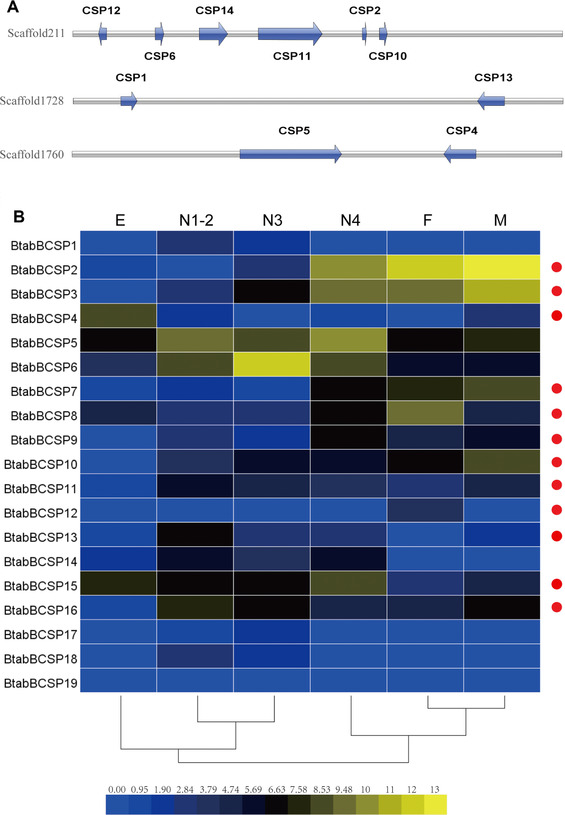
Locations and expression profiles of chemosensory protein (CSP) genes in Bemisia tabaci. (A) Locations of CSP genes on the scaffolds. Three gene clusters are located on scaffold211, scaffold1760 and scaffold1728, respectively. Each gene is depicted by arrowheads indicating the orientation of transcription in the scaffold. The exon regions are shown with green boxes. CSPs that could not be aligned well with the scaffold are not shown. (B) Expression profiles of CSPs in different developmental stages. The transcript levels were determined by calculating log2 (reads per kilobase per million mapped reads + 1) values. The levels of expression are indicated by a 15‐grade color scale. ● indicates differentially expressed genes (fold‐change > 2 and adjusted P‐value < 0.05) between males and females.
Figure 5.
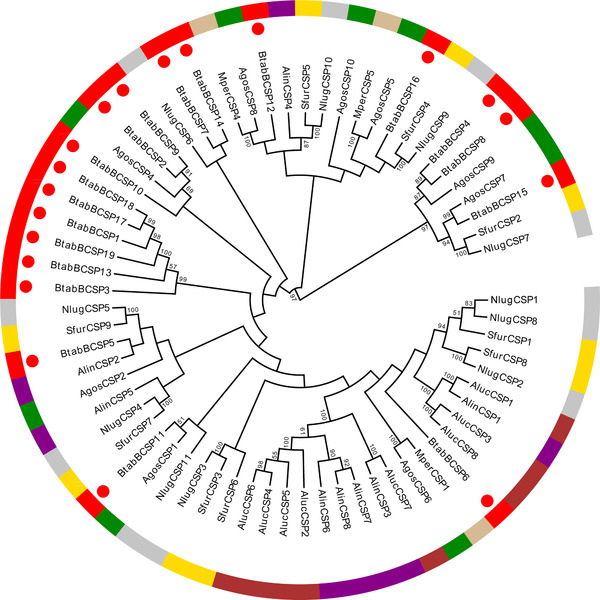
Phylogenetic analysis of the amino acid sequences of BtabBCSPs (indicated by ●) in the context of various hemipteran chemosensory proteins (CSPs). Neighbor‐joining tree of the CSP genes based on amino acid sequences of Bemisia tabaci and other insects. Bootstrap values were calculated with 1000 replications, and those larger than 50% are marked on the nodes. The protein names and sequences of the 48 CSPs used in this analysis are in a supplementary file. Alin = Adelphocoris lineolatus, Agos = Aphis gossypii, Aluc = Apolygus lucorum, Mper = Myzus persicae, Nlug = Nilaparvata lugens, Sfur = Sogatella furcifera and Btab = Bemsia tabaci.
Expression profiles of the B. tabaci MEAM1 CSPs across developmental stages
Of the 19 identified CSP genes, the expression of the following 11 significantly differed between females and males of B. tabaci: BtabBCSP2, BtabBCSP3, BtabBCSP4, BtabBCSP7, BtabBCSP8, BtabBCSP9, BtabBCSP10, BtabBCSP11, BtabBCSP13, BtabBCSP15 and BtabBCSP16) (Fig. 4B). Expression in adults was highest for BtabBCSP2, while expression in eggs was highest for BtabBCSP4. The latter gene had the lowest expression in females among the 19 genes. Expression of BtabBCSP11, BtabBCSP13 and BtabBCSP16 was highest in the 1st and 2nd instar nymphs.
mRNA expression of selected B. tabaci MEAM1 CSPs as determined by qPCR
The transcriptome expression profiles of nine selected CSP genes were confirmed by qPCR (Fig. 6). The transcriptome and qPCR expression profiles were highly consistent (P ≤ 0.05) for four of the nine CSPs (BtabBCSP2, BtabBCSP6, BtabBCSP7 and BtabBCSP12). The two kinds of expression profiles also tended to be similar for the other five CSP genes (Fig. 6).
Figure 6.
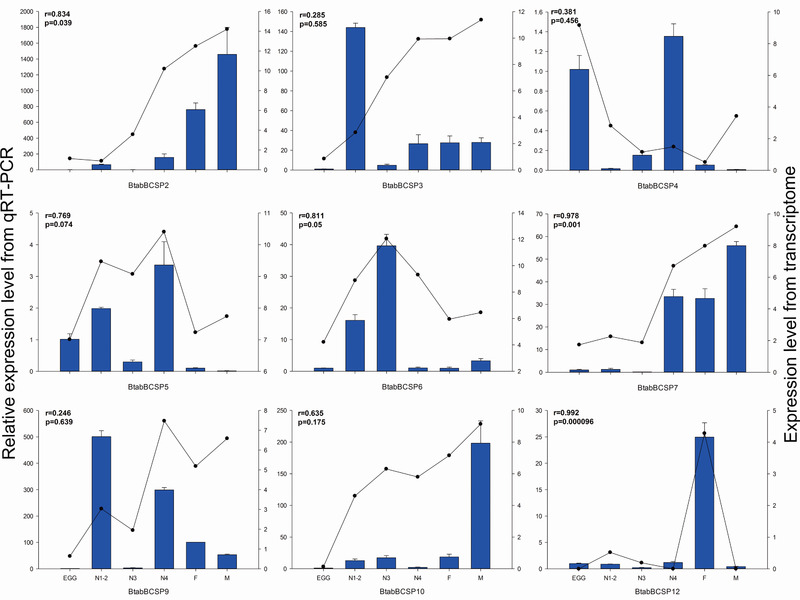
Quantitative real‐time polymerase chain reaction (qPCR)‐based expression profiling of nine selected chemosensory protein (CSP) genes in stages of Bemisia tabaci. Total RNA extracted from individual eggs, nymphs and adults of B. tabaci were used for the expression analysis of CSP genes by qPCR (dark blue bars) and RNA‐seq (black lines). The relative expression level of each CSP in each developmental stage was normalized to the reference gene SDHA. The transcript levels were determined by calculating log2 (reads per kilobase per million mapped reads + 1) values. E = egg, N = nymph stages 1 to 4 as indicated, F = adult female, and M = adult male.
qPCR analyses were conducted to measure the expression levels of the eight BtabBCSP genes in the head, thorax (mixture of thorax, legs and wings) and abdomen. The results showed that expression level of three genes (BtabBCSP2, BtabBCSP8 and BtabBCSP12) were significantly higher in abdomen than that in head and thorax (Fig. 7). Expression in thorax was highest for BtabBCSP3, BtabBCSP4, BtabBCSP9 and BtabBCSP10, while expression in head was highest for BtabBCSP7 (Fig. 7).
Figure 7.
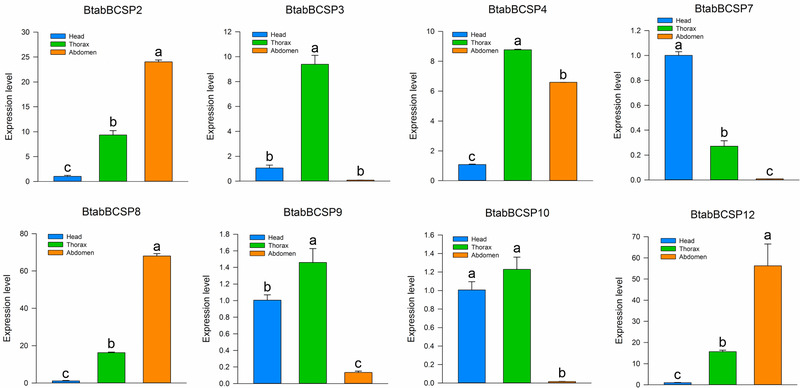
Bemisia tabaci chemosensory protein (CSP) transcript levels in different tissues as measured by quantitative real‐time polymerase chain reaction (qPCR). The expression levels were estimated using 2−Δ Δ method. Standard error for each sample is represented by error bar and the different letters (a, b, c) above each bar denote significant differences (P < 0.05).
Comparison of OBPs and CSPs between B. tabaci MEAM1 and MED
B. tabaci MEAM1 and MED both had eight OBP and 19 CSP genes, respectively. Full‐length amino acid sequences alignment showed the sequences of four OBPs and 11 CSPs of B. tabaci MEAM1 were completely the same as B. tabaci MED but different in OBP1, OBP4, OBP5, OBP8, CSP4, CSP5, CSP7, CSP10, CSP11, CSP13, CSP16 and CSP17 (Figs. S4 and S5).
Discussion
Before the current study, the cDNA sequences of OBPs and CSPs had been only partly identified in B. tabaci (only eight OBP and 13 CSP sequences had been identified in MED, and only three CSP sequences had been identified in MEAM1), and especially in many cases, the sequences were incomplete (Li et al., 2012; Liu et al., 2014, 2016; Wang et al., 2016a, 2016b, 2017). In the current study, we obtained complete sequences for the eight OBPs and 19 CSPs in MEAM1 (Table 1; Table 2; Table S3) based on genome and antennae transcriptome data sets; these covered (and in some cases completed) all previously published sequences, and added six CSP sequences. We also validated all of these OBP and CSP sequences by molecular cloning and sequencing. It follows that OBP and CSP sequences in this study are accurate and complete. In addition, genome‐wide (no antenna transcriptome) investigation of the OBPs and CSPs in another whitefly B. tabaci MED as the same method of MEAM1 was conducted. Sequences comparison between MEAM1 and MED indicated, as expected, that no evident difference were shown regardless of putative sequence number or sequences similarity (Fig. S4 and Fig. S5). This means the two invasive B. tabaci MEAM1 and MED may have similar evolution regarding olfactory recognition. These data substantially expand our knowledge of olfactory‐related genes in B. tabaci and will be useful for future research concerning the function of these genes and olfactory systems.
The number of OBPs detected in B. tabaci (eight) is substantially lower than that detected in Drosophila melanogaster (52), Anopheles gambiae (69), Bombyx mori (44), Tribolium castaneum (50) and Apis mellifera (21) (Table S4) (Lynch & Conery, 2000; Zhou, 2010; Vieira & Rozas, 2011; Xue et al., 2014; Benoit et al., 2015; Mesquita et al., 2015; Pelosi et al., 2018). The relatively low number of OBPs in B. tabaci has several possible explanations. First, species in the Hemiptera may in general have fewer OBPs than species in other orders (Table S4). However, Hemiptera species do not encounter a less diverse odorant complexity compared to other insect species. There's parallel relation between the number of OBPs and the degree of diversity of odor space. OBPs play important roles as carriers for odors through the sensillar lymph to transmembrane chemoreceptors. The odorant receptors (ORs) interact with odors, initiate downstream signaling, and ultimately lead to behavioral responses (Leal, 2013). Therefore, the number of ORs and OBPs is related to the diversity of odor. Second, the low number of OBPs may be compensated for the substantial number of CSPs in B. tabaci. Both OBPs and CSPs solubilize and transport odor molecules (Pelosi et al., 2006, 2014, 2018). Compared to Cimex lectularius, Nilaparvata lugens and Acyrthosiphon pisum, the number of OBPs in B. tabaci was lower while the number of CSPs was higher (Table S4). The combined number of OBPs and CSPs is similar in A. pisum, C. lectularius, N. lugens and B. tabaci.
We found that BtabBOBP1, BtabBOBP6 and BtabBOBP8 were arranged on scaffold 135 with the same transcription orientation. Our analysis also revealed that 10 CSPs were organized into three clusters on three scaffolds. This suggests that OBPs and CSPs may have undergone gene duplications in the genome. The duplication of individual genes has long been recognized as a major source of evolutionary novelties, including new genes and gene functions (Hanada et al., 2008; Kaessmann, 2010). Several OBPs and CSPs are organized in large clusters and are localized on the same scaffold in Rhodnius prolixus, C. lectularius and Papilio xuthus (Ozaki et al., 2008; Benoit et al., 2015; Mesquita et al., 2015). A previous study has shown that silkworm OBPs have undergone rapid evolution following a complex set of gene duplication events, which was hypothesized to have enhanced the ability to detect diverse sets of odorants (Gong et al., 2009). Therefore, we suspect that the OBPs and CSPs in B. tabaci evolved to detect specific odorants important to the species.
To explore OBP and CSP functions, we investigated expression patterns in different developmental stages, sexes and tissues. Some BtabBCSPs had unique expression patterns with respect to developmental stage, which suggests stage‐related functions. BtabBCSP2 and BtabBCSP3, for example, were mainly expressed in adults, while BtabBOBP4 and BtabBOBP5 were highly expressed in nymphs. B. tabaci nymphs normally feed on only one individual plant, while adults may disperse and feed on multiple plants. Therefore, BtabBCSP2 and BtabBCSP3 may be involved in the perception of plant volatiles. In N. lugens, an OBP (NlugOBP3) with a similar expression pattern as BtabBOBP4 and BtabBOBP5 is hypothesized to have non‐olfactory functions, such as the transporting of juvenile hormone (He et al., 2011). Perhaps BtabBOBP4 and BtabBOBP5 are involved in the metamorphosis of B. tabaci nymphs into adults. BtabBCSP4 is also highly expressed in eggs, which suggests that it is associated with B. tabaci development. In Locusta migratoria; 17 OBPs are abundantly expressed in female reproductive organs, and CSP91 was distinctly expressed in male organs (Jean‐François, 2014). In our study, the expression of BtabBOBP2, BtabBOBP3, BtabBOBP8, BtabBCSP8, BtabBCSP10 and BtabBCSP12 significantly differed between males and females, suggesting that these genes may be related to reproduction and mating. For BtabBCSP10 and BtabBCSP12, based on their high expression levels in different tissues, we can speculate that BtabBCSP12 has a potential function in recognition of semiochemicals and BtabBCSP10 has a potential function in reproduction. Moreover, our study showed that expression of BtabBOBP2, BtabBOBP3 and BtabBOBP8 was biased toward the head. In S. litura, female antennae‐biased expression of two OBP genes is consistent with their binding to the sex pheromones and plant volatiles with different binding affinities (Liu et al., 2015). Therefore, these three OBPs in B. tabaci are important to study further.
Finally, the OBP and CSP sequences and gene expression data presented in this report provide a foundation for the further study of olfactory functions in B. tabaci. In addition, the comparison of B. tabaci OBPs and CSPs with those of other insect species may provide insight into the evolution of insect chemosensory mechanisms and environmental adaptation.
Disclosure
The authors declare no conflict of interest.
Supporting information
Table S1 Primers used for polymerase chain reaction analysis of chemosensory proteins (CSPs).
Table S2 Primers used for polymerase chain reaction analysis of odorant‐binding proteins (OBPs).
Table S3 Currently available odorant‐binding proteins (OBPs) and chemosensory proteins (CSPs) of Bemisia tabaci.
Table S4 Numbers of validated peripheral chemoreception genes in insects.
Fig. S1 Alignment of B. tabaci odorant‐binding proteins (OBPs). Full‐length amino acid sequences of Bemisia tabaci OBPs were aligned by ClustalW and edited using BoxShade. Pink boxes show conserved cysteines, and blue boxes are features of Plus‐C. The conserved Cys residues are indicated. Shading indicates sequence identity >70%.
Fig. S2 Alignment of B. tabaci chemosensory proteins (CSPs). Full‐length amino acid sequences of Bemisia tabaci CSPs were aligned by ClustalW and edited using BoxShade. Pink boxes show conserved cysteines. The conserved Cys residues are indicated. Shading indicates sequence identity >70%.
Fig. S3 The computational pipeline used to identify the odorant‐binding proteins (OBPs) and chemosensory proteins (CSPs) in two MEAM1 Bemisia tabaci genomes (Chen et al., 2016, and another unpublished MEAM1 genome, FTP: http://111.203.21.119/download/B.gene.v3.cds.fa) and MEAM1 antenna transcriptome (FTP: http://111.203.21.119/download/B/antenna.fasta).
Fig. S4 Alignment and motif analysis of odorant‐binding proteins (OBPs) between Bemisia tabaci MEAM1 and MED. (A) Sequence alignment and motif information of four different full‐length OBPs (OBP1, OBP4, OBP5 and OBP8) between B. tabaci MEAM1 and MED. Pink boxes in alignment show different sites. (B) Summarized motifs conserved in insect OBPs but motif 5 missing in B. tabaci. The protein names and sequences of the 120 OBPs from different species were listed in a supplementary file.
Fig. S5 Alignment and motif analysis of chemosensory proteins (CSPs) between Bemisia tabaci MEAM1 and MED. (A) Sequence alignment and motif information of eight different full‐length CSPs (CSP4, CSP5, CSP7, CSP10, CSP11, CSP13, CSP16 and CSP17) between B. tabaci MEAM1 and MED. Pink boxes in alignment show different sites. (B) Motifs discovered in insect CSPs. The protein names and sequences of the 64 CSPs from different species were listed in a supplementary file.
Supplementary file Protein names and sequences of the odorant‐binding proteins (OBPs) and CSPs used in this analysis.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (31420103919, 31672032 and 31772172), Beijing Nova Program (Z171100001117039), Science and Technology Innovation Project from the Chinese Academy of Agricultural Sciences (CAAS‐XTCX2016017002) and the Beijing Key Laboratory for Pest Control and Sustainable Cultivation of Vegetables. Performed the analysis: YZ, WX, YJZ, YTY, SLW, QJW. Wrote the paper: YZ, WX.
The copyright line for this article was changed on 26 March 2019 after original online publication.
Contributor Information
Wen Xie, Email: xiewen@caas.cn.
You‐Jun Zhang, Email: zhangyoujun@caas.cn.
References
- Angeli, S. , Ceron, F. , Scaloni, A. , Monti, M. , Monteforti, G. , Minnocci, A. et al (1999) Purification, structural characterization, cloning and immunocytochemical localization of chemoreception proteins from Schistocerca gregaria . European Journal of Biochemistry, 262, 745–754. [DOI] [PubMed] [Google Scholar]
- Ban, L.P. , Scaloni, A. , Brandazza, A. , Angeli, S. , Zhang, L. , Yan, Y.H. et al (2003) Chemosensory proteins of Locusta migratoria . Insect Molecular Biology, 12, 125–134. [DOI] [PubMed] [Google Scholar]
- Benoit, J.B. , Adelman, Z.N. , Reinhardt, K. , Dolan, A. , Poelchau, M. , Jennings, E.C. et al (2015) Unique features of a global human ectoparasite identified through sequencing of the bed bug genome. Nature Communications, 7, 10165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos, J.I. , Prince, D. , Pitino, M. , Maffei, M.E. , Win, J. and Hogenhout, S.A. (2010) A functional genomics approach identifies candidate effectors from the aphid species Myzus persicae (green peach aphid). PLoS Genetics, 6, e1001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, W. , Hasegawa, D.K. , Kaur, N. , Kliot, A. , Pinheiro, P.V. , Luan, J. et al (2016) The draft genome of whitefly Bemisia tabaci MEAM1, a global crop pest, provides novel insights into virus transmission, host adaptation, and insecticide resistance. BMC Biology, 14, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, D. , Wan, F.H. , Zhang, Y.J. and Brown, J.K. (2010) Change in the biotype composition of Bemisia tabaci in Shandong Province of China from 2005 to 2008. Environmental Entomology, 39, 1028–1036. [DOI] [PubMed] [Google Scholar]
- De Barro, P.J. , Liu, S.S. , Boykin, L.M. and Dinsdale, A.B. (2011) Bemisia tabaci: astatement of species status. Annual Review of Entomology, 56, 1–19. [DOI] [PubMed] [Google Scholar]
- Foret, S. , Wanner, K.W. and Maleszka, R. (2007) Chemosensory proteins in the honey bee: insights from the annotated genome, comparative analyses and expressional profiling. Insect Biochemistry and Molecular Biology, 37, 19–28. [DOI] [PubMed] [Google Scholar]
- Friedman, N. , Haas, B.J. , Yassour, M. , Levin, J.Z. , Thompson, D.A. , Amit, I. et al (2011) Full‐length transcriptome assembly from RNA‐Seq data without a reference genome. Nature Biotechnology, 29, 644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, S. , Ou, J.H. and Xiao, K. (2014) R Language and Bioconductor in Bioinformatics Applications (Chinese Edition) Tianjin Science and Technology Translation and Publishing Co. [Google Scholar]
- Gholizadeh, S. , Firooziyan, S. , Ladonni, H. , Hajipirloo, H.M. , Djadid, N.D. , Hosseini, A. et al (2015) The Anopheles stephensi odorant binding protein 1 (AsteObp1) gene: a new molecular marker for biological forms diagnosis. Acta Tropica, 146, 101–113. [DOI] [PubMed] [Google Scholar]
- Gilbertson, R.L. , Batuman, O. , Webster, C.G. and Adkins, S. (2015) Role of the insect supervectors Bemisia tabaci and Frankliniella occidentalis in the emergence and global spread of plant viruses. Annual Review of Virology, 2, 67–93. [DOI] [PubMed] [Google Scholar]
- Gong, D.P. , Zhang, H.J. , Zhao, P. , Lin, Y. , Xia, Q.Y. and Xiang, Z.H. (2007) Identification and expression pattern of the chemosensory protein gene family in the silkworm. Bombyx mori. Insect Biochemistry and Molecular Biology, 37, 266–277. [DOI] [PubMed] [Google Scholar]
- Gong, D.P. , Zhang, H.J. , Zhao, P. , Xia, Q.Y. and Zhong, H.X. (2009) The odorant binding protein gene family from the genome of silkworm. Bombyx mori. BMC Genomics, 10, 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, W. , Wang, X.H. , Ma, Z.Y. , Xue, L.A. , Han, J.Y. , Yu, D. et al (2011) CSP and takeout genes modulate the switch between attraction and repulsion during behavioral phase change in the migratory locust. PLoS Genetics, 7, e1001291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, Z. , Cheng, Z.Y. , Huang, F. , Luttrell, R. and Leonard, R. (2012) Microarray analysis of global gene regulation in the cry1ab‐resistant and cry1ab‐susceptible strains of Diatraea saccharalis . Pest Management Science, 68, 718–730. [DOI] [PubMed] [Google Scholar]
- Hanada, K. , Zou, C. , Lehti‐Shiu, M.D. , Shinozak, K. and Shiu, S.H. (2008) Importance of lineage‐specific expansion of plant tandem duplicates in the adaptive response to environmental stimuli. Plant Physiology, 148, 993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, P. , Zhang, J. , Liu, N.Y. , Zhang, Y.N. , Yang, K. and Dong, S.L. (2011) Distinct expression profiles and different functions of odorant binding proteins in Nilaparvata lugens Stål. PLoS ONE, 6, e28921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Z. , Zhang, H.K. , Gao, S.H. , Lercher, M.J. , Chen, W.H. and Hu, S.N. (2016) Evolview v2: an online visualization and management tool for customized and annotated phylogenetic trees. Nucleic Acids Research, 44, W236–W241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper, A.M. , Dufour, S. , He, X.L. , Muck, A. , Zhou, J.J. , Almeida, R. et al (2009) High‐throughput ESI‐MS analysis of binding between the Bombyx mori pheromone‐binding protein BmorPBP1, its pheromone components and some analogues. Chemical Communication, 38, 5725–5727. [DOI] [PubMed] [Google Scholar]
- Jean‐François, P. (2014) Renaming Bombyx mori chemosensory proteins. International Journal of Bioorganic Chemistry & Molecular Biology, 2, 1–4. [Google Scholar]
- Jones, D.R. (2003) Plant viruses transmitted by whiteflies. European Journal of Plant Pathology, 109, 195–219. [Google Scholar]
- Kaessmann, H. (2010) Origins, evolution, and phenotypic impact of new genes. Genome Research, 20, 1313–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamikouchi, A. , Morioka, M. and Kubo, T. (2004) Identification of honeybee antennal proteins/genes expressed in a sex‐ and/or caste selective manner. Zoological Science, 21, 53–62. [DOI] [PubMed] [Google Scholar]
- Lardeux, F. , Aliaga, C. , Tejerina, R. and Ursic‐Bedoya, R. (2012) Development of exon‐primed intron‐crossing (epic) pcr primers for the malaria vector Anopheles pseudopunctipennis (Diptera: Culicidae). Comptes Rendus Biologies, 335, 398–405. [DOI] [PubMed] [Google Scholar]
- Laughlin, J.D. , Ha, T.S. , Jones, D.N.M. and Smith, D.P. (2008) Activation of pheromone‐sensitive neurons is mediated by conformational activation of pheromone‐binding protein. Cell, 133, 1255–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal, W.S. , Nikonova, L. and Peng, G.H. (1999) Disulfide structure of the pheromone binding protein from the silkworm moth. Bombyx mori. FEBS Letters, 464, 85–90. [DOI] [PubMed] [Google Scholar]
- Leal, W.S. (2013) Odorant reception in insects: roles of receptors, binding proteins, and degrading enzymes. Annual Review of Entomology, 58, 373–391. [DOI] [PubMed] [Google Scholar]
- Letunic, L. , Doerks, T. and Bork, P. (2015) SMART: recent updates, new developments and status in 2015. Nucleic Acids Research, 43, 257–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y.F. , Qin, Y.C. , Gao, Z.L. , Dang, Z.H. , Pan, W.L. and Xu, G.M. (2012) Cloning, expression and characterisation of a novel gene encoding a chemosensory protein from Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae). African Journal of Biotechnology, 11, 758–770. [Google Scholar]
- Li, Z.Q. , Zhang, S. , Ma, Y. , Luo, J.Y. , Wang, C.Y. , Lv, L.M. et al (2013) First transcriptome and digital gene expression analysis in neuroptera with an emphasis on chemoreception genes in Chrysopa pallens (Rambur). PLoS ONE, 8, e67151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, G.X. , Ma, H.M. , Xie, H.Y. , Xuan, N. , Guo, X. , Fan, Z.X. et al (2016) Biotype characterization, developmental profiling, insecticide response and binding property of Bemisia tabaci chemosensory proteins: role of csp in insect defense. PLoS ONE, 11, e0154706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, G.X. , Xuan, N. , Chu, D. , Xie, H.Y. , Fan, Z.X. , Bi, Y.P. et al (2014) Biotype expression and insecticide response of Bemisia tabaci chemosensory protein‐1. Archives of Insect Biochemistry and Physiology, 85, 137–151. [DOI] [PubMed] [Google Scholar]
- Liu, N.Y. , Yang, K. , Liu, Y. , Xu, W. , Anderson, A. , Dong, S.L. (2015) Two general‐odorant binding proteins in Spodoptera litura are differentially tuned to sex pheromones and plant odorants. Comparative Biochemistry and Physiology Part A, Molecular & Integrative Physiology, 180, 23–31. [DOI] [PubMed] [Google Scholar]
- Liu, X.L. , Luo, Q.A. , Zhong, G.H. , Rizwan‐Ul‐Haq, M. and Hu, M.Y. (2010) Molecular characterization and expression pattern of four chemosensory proteins from diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Journal of Biochemistry, 148, 189–200. [DOI] [PubMed] [Google Scholar]
- Lynch, M. and Conery, J.S. (2000) The evolutionary fate and consequences of duplicate genes. Science, 290, 1151–1159. [DOI] [PubMed] [Google Scholar]
- Maleszka, J. , Foret, S. , Saint, R. and Maleszka, R. (2007) RNAi‐induced phenotypes suggest a novel role for a chemosensory protein csp5 in the development of embryonic integument in the honeybee (Apis mellifera). Development Genes and Evolution, 217, 189–196. [DOI] [PubMed] [Google Scholar]
- Mesquita, R.D. , Vionette‐Amaral, R.J. , Lowenberger, C. , Rivera‐Pomar, R. , Monteiro, F.A. , Minx, P. et al (2015) Genome of Rhodnius prolixus, an insect vector of Chagas disease, reveals unique adaptations to hematophagy and parasite infection. Proceedings of the National Academy of Sciences USA, 112, 14936–14941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki, M. , Wada‐Katsumata, A. , Fujikawa, K. , Iwasaki, M. , Yokohari, F. , Satoji, Y. et al (2005) Ant nestmate and non‐nestmate discrimination by a chemosensory sensillum. Science, 309, 311–314. [DOI] [PubMed] [Google Scholar]
- Ozaki, K. , Utoguchi, A. , Yamada, A. and Yoshikawa, H. (2008) Identification and genomic structure of chemosensory proteins (CSP) and odorant binding proteins (OBP) genes expressed in foreleg tarsi of the swallowtail butterfly Papilio xuthus . Insect Biochemistry and Molecular Biology, 38, 969–976. [DOI] [PubMed] [Google Scholar]
- Pannure, A. , Mutthuraju, G.P. and Imran, S. (2012) The sense of smell in insects: a target for pest management? – A review. Current Biotica, 6, 399–419. [Google Scholar]
- Pelosi, P. , Calvello, M. and Ban, L.P. (2005) Diversity of odorant‐binding proteins and chemosensory proteins in insects. Chemical Senses, 30, i291–i292. [DOI] [PubMed] [Google Scholar]
- Pelosi, P. , Iovinella, I. , Felicioli, A. and Dani, F.R. (2014) Soluble proteins of chemical communication: an overview across arthropods. Frontiers in Physiology, 5, 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelosi, P. , Iovinella, I. , Zhu, J. , Wang, G.R. and Dani, F.R. (2018) Beyond chemoreception: diverse tasks of soluble olfactory proteins in insects. Biological Reviews, 93, 184–200. [DOI] [PubMed] [Google Scholar]
- Pelosi, P. , Zhou, J.J. , Ban, L.P. and Calvello, M. (2006) Soluble proteins in insect chemical communication. Cellular and Molecular Life Sciences, 63, 1658–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaloni, A. , Monti, M. , Angeli, S. and Pelosi, P. (1999) Structural analysis and disulfide‐bridge pairing of two odorant‐binding proteins from Bombyx Mori . Biochemical and Biophysical Research Communications, 266, 386–391. [DOI] [PubMed] [Google Scholar]
- Steinbrecht, R.A. (1998) Odorant‐binding proteins: expression and function. Annals New York Academy Sciences, 30, 323–332. [DOI] [PubMed] [Google Scholar]
- Tamura, K. , Stecher, G. , Peterson, D. , Filipski, A. and Kumar, S. (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30, 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira, F.G. and Rozas, J. (2011) Comparative genomics of the odorant‐binding and chemosensory protein gene families across the arthropoda: origin and evolutionary history of the chemosensory system. Genome Biology and Evolution, 3, 476–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt, R.G. , Prestwich, G.D. and Lerner, M. (1991) Odorant‐binding‐protein subfamilies associate with distinct classes of olfactory receptor neurons in insects. Journal of Neurobiology, 22, 74–84. [DOI] [PubMed] [Google Scholar]
- Vogt, R.G. and Riddiford, L.M. (1981) Pheromone binding and inactivation by moth antennae. Nature, 293, 161–163. [DOI] [PubMed] [Google Scholar]
- Wan, F.H. and Yang, N.W. (2016) Invasion and management of agricultural alien insects in China. Annual Review of Entomology, 61, 77–98. [DOI] [PubMed] [Google Scholar]
- Wang, R. , Li, F.Q. , Zhang, W. , Zhang, X.M. , Qu, C. , Tetreau, G. et al (2017) Identification and expression profile analysis of odorant binding protein and chemosensory protein genes in Bemisia tabaci MED by head transcriptome. PLoS ONE, 12, e0171739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, R. , Zhang, X.M. , Li, F.Q. , Wu, F. , Li, H.L. and Luo, C. (2016b) Cloning and prokaryotic expression of odorant binding protein OBP8 in MED cryptic species Bemisia tabaci and the binding characteristics with plant volatiles. Journal of Plant Protection, 43, 32–39. [Google Scholar]
- Wang, R. , Zhang, X.M. , Li, H.L. , Guo, X.J. and Luo, C. (2016a) Identification and expression profiling of five chemosensory protein genes in the whitefly MED. Bemisia tabaci. Journal of Asia‐Pacific Entomology, 19, 195–201. [Google Scholar]
- Wanner, K.W. , Isman, M.B. , Feng, Q. , Plettner, E. and Theilmann, D.A. (2005) Developmental expression patterns of four chemosensory protein genes from the eastern spruce budworm. Chroistoneura fumiferana. Insect Molecular Biology, 14, 289–300. [DOI] [PubMed] [Google Scholar]
- Wanner, K.W. , Willis, L.G. , Theilmann, D.A. , Isman, M.B. , Feng, Q. and Plettner, E. (2004) Analysis of the insect OS‐D‐like gene family. Journal of Chemical Ecology, 30, 889–911. [DOI] [PubMed] [Google Scholar]
- Xie, W. , Chen, C.H. , Yang, Z.Z. , Guo, L.T. , Yang, X. , Wang, D. et al (2017) Genome sequencing of the sweetpotato whitefly Bemisia tabaci MED/Q. GigaScience, 6, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, P.X. , Atkinson, R. , Jones, D.N. and Smith, D.P. (2005) Drosophila OBP lush is required for activity of pheromone‐sensitive neurons. Neuron, 45, 193–200. [DOI] [PubMed] [Google Scholar]
- Xu, Y.L. , He, P. , Zhang, L. , Fang, S.Q. , Dong, S.L. , Zhang, Y.J. et al (2009) Large‐scale identification of odorant‐binding proteins and chemosensory proteins from expressed sequence tags in insects. BMC Genomics, 10, 632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue, J. , Zhou, X. , Zhang, C.X. , Yu, L.L. , Fan, H.W. , Wang, Z. et al (2014) Genomes of the rice pest brown planthopper and its endosymbionts reveal complex complementary contributions for host adaptatio. Genome Biology, 15, 521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, M. , Sun, H. , Fei, Z. , Zhan, F. , Gong, X. and Gao, S. (2014) Fastq_clean: An optimized pipeline to clean the Illumina sequencing data with quality control. International Conference on Bioinformatics and Biomedicine (BIBM).
- Zhang, Y.N. , Jin, J.Y. , Jin, R. , Xia, Y.H. , Zhou, J.J. , Deng, J.Y. et al (2013) Differential expression patterns in chemosensory and non‐chemosensory tissues of putative chemosensory genes identified by transcriptome analysis of insect pest the purple stem borer Sesamia inferens (Walker). PLoS ONE, 8, e69715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, J.J. (2010) Odorant‐binding proteins in insects Vitamins and Hormones (ed. Litwack G.), pp. 241–272. Academic Press, Burlington, Massachusetts. [DOI] [PubMed] [Google Scholar]
- Zhou, J.J. , Vieira, F.G. , He, X.L. , Smadja, C. , Liu, R. , Rozas, J. et al (2010) Genome annotation and comparative analyses of the odorant‐binding proteins and chemosensory proteins in the pea aphid Acyrthosiphon pisum . Insect Molecular Biology, 19, 113–122. [DOI] [PubMed] [Google Scholar]
- Zhou, X.H. , Ban, L.P. , Iovinella, I. , Zhao, L.J. , Gao, Q. , Felicioli, A. et al (2013) Diversity, abundance and sex‐specific expression of chemosensory proteins in the reproductive organs of the locust Locusta migratoria manilensis . Biological Chemistry, 394, 43–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Primers used for polymerase chain reaction analysis of chemosensory proteins (CSPs).
Table S2 Primers used for polymerase chain reaction analysis of odorant‐binding proteins (OBPs).
Table S3 Currently available odorant‐binding proteins (OBPs) and chemosensory proteins (CSPs) of Bemisia tabaci.
Table S4 Numbers of validated peripheral chemoreception genes in insects.
Fig. S1 Alignment of B. tabaci odorant‐binding proteins (OBPs). Full‐length amino acid sequences of Bemisia tabaci OBPs were aligned by ClustalW and edited using BoxShade. Pink boxes show conserved cysteines, and blue boxes are features of Plus‐C. The conserved Cys residues are indicated. Shading indicates sequence identity >70%.
Fig. S2 Alignment of B. tabaci chemosensory proteins (CSPs). Full‐length amino acid sequences of Bemisia tabaci CSPs were aligned by ClustalW and edited using BoxShade. Pink boxes show conserved cysteines. The conserved Cys residues are indicated. Shading indicates sequence identity >70%.
Fig. S3 The computational pipeline used to identify the odorant‐binding proteins (OBPs) and chemosensory proteins (CSPs) in two MEAM1 Bemisia tabaci genomes (Chen et al., 2016, and another unpublished MEAM1 genome, FTP: http://111.203.21.119/download/B.gene.v3.cds.fa) and MEAM1 antenna transcriptome (FTP: http://111.203.21.119/download/B/antenna.fasta).
Fig. S4 Alignment and motif analysis of odorant‐binding proteins (OBPs) between Bemisia tabaci MEAM1 and MED. (A) Sequence alignment and motif information of four different full‐length OBPs (OBP1, OBP4, OBP5 and OBP8) between B. tabaci MEAM1 and MED. Pink boxes in alignment show different sites. (B) Summarized motifs conserved in insect OBPs but motif 5 missing in B. tabaci. The protein names and sequences of the 120 OBPs from different species were listed in a supplementary file.
Fig. S5 Alignment and motif analysis of chemosensory proteins (CSPs) between Bemisia tabaci MEAM1 and MED. (A) Sequence alignment and motif information of eight different full‐length CSPs (CSP4, CSP5, CSP7, CSP10, CSP11, CSP13, CSP16 and CSP17) between B. tabaci MEAM1 and MED. Pink boxes in alignment show different sites. (B) Motifs discovered in insect CSPs. The protein names and sequences of the 64 CSPs from different species were listed in a supplementary file.
Supplementary file Protein names and sequences of the odorant‐binding proteins (OBPs) and CSPs used in this analysis.


