Abstract
The aim of this study was to determine the safety and effectiveness of dehydrated human umbilical cord allograft (EpiCord) compared with alginate wound dressings for the treatment of chronic, non‐healing diabetic foot ulcers (DFU). A multicentre, randomised, controlled, clinical trial was conducted at 11 centres in the United States. Individuals with a confirmed diagnosis of Type 1 or Type 2 diabetes presenting with a 1 to 15 cm2 ulcer located below the ankle that had been persisting for at least 30 days were eligible for the 14‐day study run‐in phase. After 14 days of weekly debridement, moist wound therapy, and off‐loading, those with ≤30% wound area reduction post‐debridement (n = 155) were randomised in a 2:1 ratio to receive a weekly application of EpiCord (n = 101) or standardised therapy with alginate wound dressing, non‐adherent silicone dressing, absorbent non‐adhesive hydropolymer secondary dressing, and gauze bandage roll (n = 54). All wounds continued to have appropriate off‐loading during the treatment phase of the study. Study visits were conducted for 12 weeks. At each weekly visit, the DFU was cleaned and debrided as necessary, with the wound photographed pre‐ and post‐debridement and measured before the application of treatment group‐specific dressings. A follow‐up visit was performed at week 16. The primary study end point was the percentage of complete closure of the study ulcer within 12 weeks, as assessed by Silhouette camera. Data for randomised subjects meeting study inclusion criteria were included in an intent‐to‐treat (ITT) analysis. Additional analysis was conducted on a group of subjects (n = 134) who completed the study per protocol (PP) (EpiCord, n = 86, alginate, n = 48) and for those subjects receiving adequate debridement (EpiCord, n = 67, alginate, n = 40). ITT analysis showed that DFUs treated with EpiCord were more likely to heal within 12 weeks than those receiving alginate dressings, 71 of 101 (70%) vs 26 of 54 (48%) for EpiCord and alginate dressings, respectively, P = 0.0089. Healing rates at 12 weeks for subjects treated PP were 70 of 86 (81%) for EpiCord‐treated and 26 of 48 (54%) for alginate‐treated DFUs, P = 0.0013. For those DFUs that received adequate debridement (n = 107, ITT population), 64 of 67 (96%) of the EpiCord‐treated ulcers healed completely within 12 weeks, compared with 26 of 40 (65%) of adequately debrided alginate‐treated ulcers, P < 0.0001. Seventy‐five subjects experienced at least one adverse event, with a total of 160 adverse events recorded. There were no adverse events related to either EpiCord or alginate dressings. These results demonstrate the safety and efficacy of EpiCord as a treatment for non‐healing DFUs.
Keywords: chronic wounds, dehydrated human umbilical cord allograft, diabetic foot ulcers, EpiCord
1. INTRODUCTION
Diabetes affects 9.4% of the population in the United States including approximately 23.1 million people with a known diagnosis and 7.2 million people who are undiagnosed.1 The estimated total cost of diagnosed diabetes in the United States is on the rise, as evidenced by a 41% increase in costs between 2007 and 2012, with 2012 costs estimated at $245 billion.2 The development of diabetic foot ulcers (DFUs) significantly influences costs related to managing a patient with diabetes. One‐quarter to one‐third of total annual expenses related to the care of people with diabetes is linked to peripheral vascular and neurological complications associated with DFUs.3 Up to 85% of lower‐extremity amputations are preceded by a chronic DFU, and it is estimated that 85% of these amputations may be preventable.4 Slow healing of lower‐extremity ulcers increases the risk of infection and potential for amputation. More than 80 000 amputations are performed each year on diabetic patients in the United States.5
Advanced wound therapies are perceived to be costly but have been shown to decrease long‐term costs through the reduction of foot complications and need for amputation.3, 4 It is recognised that, given the high frequency and high costs associated with treating DFUs, treatment strategies with the ability to promote more rapid and complete healing are warranted.6
The refractory nature of chronic DFUs is multifactorial. Factors contributing to delayed healing include disrupted signalling cascades that mediate cell recruitment and cross talk between cells, persistently elevated metalloproteinases (MMPs) levels, profound disturbances in bacteria‐host relationship, hyperglycaemia, neuropathy, peripheral arterial disease, and impaired immune function. Foot ulcers persisting for greater than 30 days are 4.7 times more likely to become infected compared with acute foot ulcers.7 What makes this increased risk of infection so impactful is that diabetics whose ulcers become infected are 56 times at greater risk of hospitalisation and 155 times more likely to undergo a lower‐extremity amputation than diabetics without an infected foot ulcer.7 Thus, expediting the closure of a chronic DFU becomes a high priority to avoid these costly complications.
The therapeutic and regenerative potential of allografts comprised of Wharton's jelly found in the umbilical cord (considered a foetal membrane) and amniotic fluid have recently been recognised in the context of treating diseases and injuries.8, 9, 10, 11 The umbilical cord connects the developing foetus and the placenta. In humans, the umbilical cord normally contains two arteries and one vein that carry essential nourishment and oxygenated blood to and from the mother and foetus. The outermost lining of the umbilical cord consists of amniotic membrane, while glycosaminoglycan‐rich Wharton's jelly within the umbilical cord protects the arteries and vein. Consisting of both amniotic epithelium and Wharton's jelly, human umbilical cord contains an extracellular matrix composed of collagen, proteoglycans, and hyaluronic acid, which have been shown to provide a protective environment for the healing process, and a connective tissue matrix to replace or supplement damaged or deficient integumental tissue.8 Immunogenicity of placental tissue12 lends credence to its use as an allograft material for difficult‐to‐heal wounds.
A dehydrated human umbilical cord allograft (EpiCord, MiMedx Group, Inc., Marietta, Georgia) (Figure 1) is processed through a patented PURION Plus process. The purpose of the present study is to investigate the efficacy and safety of using the EpiCord allograft to treat chronic DFUs.
Figure 1.
The EpiCord allograft comprised of dehydrated human umbilical cord. Available in multiple sizes. Five‐year shelf life in ambient conditions
2. MATERIALS AND METHODS
We conducted an Institutional Review Board (IRB)‐approved prospective, randomised controlled trial (RCT) at 11 clinical centres in the United States between August, 2016 and March, 2018. The study was conducted under the guidelines of Good Clinical Practice (GCP) and in accordance with the provisions of the Declaration of Helsinki. Tissue products used in the study were manufactured, handled, and stored in accordance with applicable Good Tissue Practices (GTPs). The study was reviewed and approved by the Chesapeake IRB or a site's local IRB and was pre‐registered in ClinicalTrials.gov (NCT02844660). The purpose of the study was to examine healing metrics of DFUs treated with EpiCord while maintaining a moist wound bed environment, compared with moist wound therapy with an alginate wound dressing. The study period was 18 weeks, consisting of a 2‐week run‐in phase, 12‐week treatment phase, and 4‐week follow‐up phase.
At each study site, potentially eligible subjects over the age of 18 were contacted and encouraged to participate, regardless of gender, race, or ethnicity. Eligibility was assessed via inclusion and exclusion criteria. (Table 1) Inclusion criteria included a documented history of Type 1 or Type 2 diabetes, presence of a DFU of 1 to 15 cm2 (post‐debridement) located below the ankle with a duration of at least 30 days, and adequate circulation to the affected extremity. Subjects with an ulcer penetrating down to the tendon, or bone, or the presence of another DFU within 3 cm of the study ulcer; active Charcot; wound infection; or DFU present for over 1 year without intermittent healing were ineligible for inclusion.
Table 1.
Major inclusion and exclusion criteria
| Inclusion criteria |
| 1. Known history of type 1 or type 2 diabetes |
| 2. Index ulcer characteristics: |
| a. Present for ≥30 d |
| b. Located below ankle |
| c. Area post‐debridement of 1 to 15 cm2 |
| 3. Subject has completed 14‐d run‐in period with ≤30% wound area reduction post‐debridement |
| 4. Adequate circulation to the affected extremity |
| 5. Age ≥ 18 |
| 6. Willing and able to provide informed consent and participate in all procedures and follow‐up evaluations necessary to complete the study |
| Exclusion criteria |
| 1. Index ulcer assessment |
| a. Penetrates down to tendon or bone |
| b. Another ulcer within 3 cm of index ulcer |
| c. Active Charcot deformity |
| d.. Major structural abnormalities of the foot |
| e. Clinical signs and symptoms of infection |
| f. Known or suspected ulcer malignancy |
| g. Wound duration > 1 y without intermittent closure |
| 2. Index ulcer treated with any of the following |
| a. in the last 7 d—negative pressure wound therapy, or hyperbaric oxygen (HBO) therapy |
| b. in the last 10 d—chemical debridement, Dakin's solution, medical honey therapy |
| c. in the last 30 d—cytotoxic chemotherapy, topical steroids, use of ≥14 d of immune suppressants, any biological skin substitutes, or subject has been on any investigational drug(s) or therapeutic device(s) |
| d. in the last 6 mo—amputation or revascularisation (surgical or stenting) to the affected leg or foot |
| 3. Known osteomyelitis or active cellulitis at wound site |
| 4. Haemoglobin A1C > 12 in the last 60 d prior to randomisation |
| 5. History of immune system disorders including systemic lupus erythematosus (SLE), fibromyalgia, acquired immunodeficiency syndrome (AIDS), or HIV |
| 6. Currently receiving radiation therapy or chemotherapy |
| 7. Currently on dialysis or planning to start dialysis |
Eligible subjects were enrolled in a 14‐day run‐in period, and the DFU was treated with moist dressings and offloaded using an appropriate sponsor‐approved device. Subjects whose DFU area did not reduce by at least 30% from baseline measurement during the run‐in period provided IRB‐approved signed consent prior to randomisation.
Subjects were randomised in a 2:1 ratio to receive a weekly application of EpiCord in addition to moist dressings and offloading or to receive standard care with alginate dressings and offloading. Randomisation to the study assignment was generated via sealed envelope group assignment. Envelopes containing the random group assignment were sequentially numbered, opaque, and sealed by the study sponsor prior to being delivered to the study site. At the point of entering a qualified subject into the study after signature was obtained acknowledging informed consent, site staff opened the next envelope in the sequential order indicating the study group assignment. Neither patient nor provider was blinded to group assignment.
All subjects were seen weekly at the study site. Dressings were changed at a minimum of once per week or as clinically indicated. Dressing changes were performed during scheduled visits by the investigational site staff. Per study protocol, sharp debridement of unhealed wounds was to be performed weekly or as deemed necessary by the site investigator. In subjects randomised to receive EpiCord, the EpiCord allograft was applied weekly, post‐debridement, on the wound bed and hydrated with sterile normal saline as needed, followed by a non‐adherent silicone dressing and a non‐adhesive absorbent hydropolymer secondary dressing, and wrapped with an outer layer of gauze. The standard care/alginate group had an alginate wound dressing applied to the debrided wound bed (silver or collagen alginates were prohibited); then, a non‐adherent silicone dressing (ADAPTIC TOUCH Acelity, San Antonio, Texas) was applied immediately above the alginate wound dressing, and an absorbent non‐adhesive hydropolymer secondary dressing (TIELLE Max, Acelity, San Antonio, Texas) was applied immediately above the ADAPTIC TOUCH layer. An outer gauze wrap was applied above the TIELLE Max layer.
All subjects were instructed on proper dressing care and the importance of keeping the secondary dressings dry at all times. Wounds were offloaded using a removable cast walker, and when a removable walker was unsuitable and/or not conducive to treatment, a standardised total contact cast kit was used. The Active Offloading Walker (boot and/or shoe) or a similar device was used by all subjects throughout the screening/run‐in and treatment phases of the study.
During the weekly visit, wounds were cleaned and debrided as necessary and then photographed (pre‐ and post‐debridement) and measured. The Silhouette camera (ARANZ Medical, Christchurch, New Zealand), an imaging device that precisely and consistently measures the area, depth, and volume of wounds and their healing progress, was used to obtain wound measurements at all study sites. In order to insure consistency across study sites, all images taken before and after debridement with the Silhouette camera were examined at study completion by a group of three wound care specialists who had not enrolled patients into the study. These specialists were blinded to group assignment, study sites, and the treating clinician. The image examination was performed as a group, and determination of timing of complete epithelialisation was reached by consensus. The group also made a judgement on whether the wound had been adequately debrided during the study visit.
2.1. Statistical methods
The PASS 2013 statistical software was used to determine the sample size needed to detect a difference of 30% between the two treatment groups in the percentage of healed subjects. Under the above assumption, 20 subjects for treatment group 1 (alginate controls) and 40 subjects for treatment group 2 (EpiCord) would be required to meet the Type I error rate (P‐value) of 0.05 with 80% power for a total of 60 subjects for the study, yet we chose to study over 100 subjects.
The intent‐to‐treat (ITT) population (all randomised subjects) was used as the basis for the primary efficacy analysis. All randomised subjects meeting study inclusion criteria who received at least one application of EpiCord were used for the analysis of safety data. Subjects who discontinued the trial before their wound healed were categorised as treatment failures for the primary efficacy analysis, and their last observation was carried forward.
Continuous variables were summarised as means and standard deviations (SDs); medians were also reported for data with non‐normal distribution. Categorical variables were reported as proportions/percentages. Parametric and non‐parametric tests were used as appropriate. Student's t‐test or the Kruskal‐Wallis test was used to test for differences in continuous variables. For categorical variables, Fisher's exact tests were performed to test for statistical differences. Kaplan‐Meier analysis was performed. Two‐sided P‐values < 0.05 were considered significant. SAS 9.4 (SAS institute, Inc., Cary, North Carolina) was used to perform statistical testing.
The primary efficacy end point examined was the percentage of subjects in the ITT population, with complete closure of the study ulcer within 12 weeks of treatment initiation. Twelve‐week healing rates were also examined in subjects completing the study per protocol and for only those wounds determined to have received consistent, adequate debridement.
Complete healing was defined as 100% epithelialisation of the wound. Pre‐debridement and post‐debridement Silhouette wound images were evaluated by a team of wound care specialists at study completion to determine if adequate debridement had occurred. Adequate debridement was defined as the exposure of healthy tissue in the ulcer bed, with no significant eschar, callous, necrotic tissue, or foreign material present in or around the wound.
3. RESULTS
Study subjects were enrolled at 11 study sites in the United States between August 2016 and March 2018. Study sites were located across the United States, and both hospital‐based and private clinic settings in urban and rural areas were represented. As indicated in the CONSORT flow diagram (Figure 2), a total of 202 subjects were screened and met the inclusion/exclusion criteria for entry into the study for the 2‐week run‐in period. At the conclusion of the run‐in period, there were 47 patients no longer eligible for study inclusion because of >30% reduction in wound size (n = 33), infection (n = 9), wound treated with other products not allowed per study protocol (n = 2), inaccurate baseline wound measurement (n = 2), or withdrawal of consent prior to randomization (n = 1). A total of 155 randomised subjects meeting study inclusion criteria were analysed per ITT analysis; 101 subjects received EpiCord, and 54 subjects received alginate dressings. Twenty‐one subjects failed to complete the study; 15 had been receiving EpiCord, and 6 had been receiving alginate dressings, yielding a per‐protocol EpiCord population of n = 86 and alginate population of n = 48.
Figure 2.
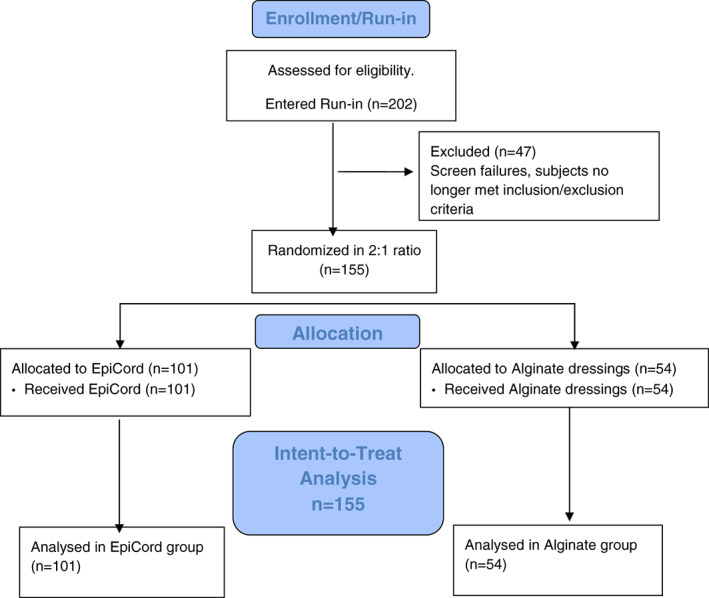
CONSORT flow diagram
In the overall study population (n = 155), 81.3% were male, 42.9% were smokers, 63.2% were obese, and 17.4% had a prior amputation. Demographics of the study groups are presented in Table 2. Characteristics of the study ulcers are presented for each study group in Table 3. The two study groups were well matched for demographic and clinical factors, as well as location, duration, and size of the study ulcer.
Table 2.
Clinical characteristics at study enrolment
| EpiCord (n = 101) | Alginate (n = 54) | P‐value | |
|---|---|---|---|
| Mean age, y (SD) | 58.3 (10.9) | 56.3 (10.2) | 0.2767 |
| Age ≥ 65 y (n, %) | 28 (27.7%) | 10 (18.5%) | 0.2427 |
| Male gender (n, %) | 82 (81.2%) | 44 (81.5%) | 1.000 |
| Race (n, %) | |||
| Caucasian | 81 (80.2%) | 44 (81.5%) | 0.6738 |
| African American | 12 (11.9%) | 8 (14.8%) | |
| Hispanic ethnicity (n, %) | 28 (27.7%) | 18 (33.3%) | 0.4675 |
| Mean BMI (SD) | 33.8 (7.3) | 32.9 (8.0) | 0.4627 |
| Obese BMI ≥ 30 (n, %) | 68 (67.3%) | 30 (55.6%) | 0.1646 |
| Mean A1C % (SD) | 8.0 (1.8) | 8.6 (2.0) | 0.0925 |
| Smoker (n, %) | 38 (37.6%) | 28 (52.8%) | 0.0869 |
| Alcohol use (n, %) | 50 (49.5%) | 25 (47.2%) | 0.8657 |
| Index ulcer is recurrent (n, %) | 26 (25.7%) | 15 (28.3%) | 0.8480 |
| History of cardiovascular abnormalities (n, %) | 39 (38.6%) | 20 (37.7%) | 1.0000 |
| Prior amputation (n, %) | 17 (16.8%) | 10 (18.5%) | 0.8260 |
BMI, body mass index.
Table 3.
Characteristics of study ulcer at baseline
| EpiCord (n = 101) | Alginate (n = 54) | P‐value | |
|---|---|---|---|
| Ulcer position (n, %) | |||
| Plantar | 77 (76.2%) | 45 (84.9%) | 0.3048 |
| Ulcer location (n, %) | |||
| Toe | 12 (11.9%) | 12 (22.6%) | 0.4112 |
| Forefoot | 58 (57.4%) | 27 (50.9%) | |
| Midfoot | 20 (19.8%) | 9 (17.0%) | |
| Hindfoot | 9 (8.9%) | 3 (5.7%) | |
| Mean ulcer size, cm2 (SD) | 2.6 (2.2) | 2.8 (2.6) | 0.6432 |
| Mean ulcer duration, weeks (SD) | 20.5 (13.7) | 20.3 (13.2) | 0.9265 |
3.1. Study outcomes (Figure 3)
Figure 3.
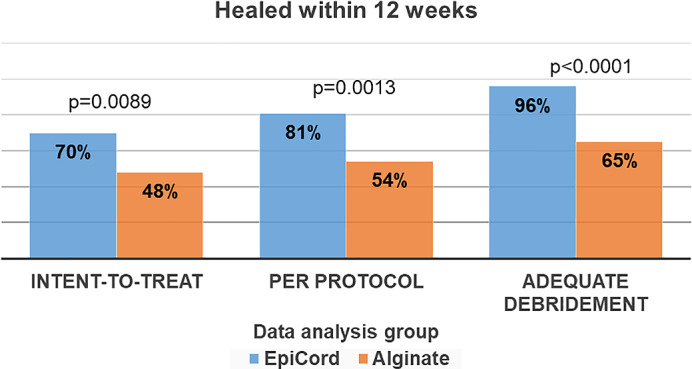
Primary study outcome. Complete healing within 12 weeks of treatment initiation
In the ITT population, at the end of the 12‐week treatment phase, 70% (71/101) of EpiCord‐treated ulcers had completely healed, a significantly greater number of healed ulcers compared with 48% (26/54) in the alginate group (P = 0.0089). For those subjects completing the study per protocol, healing rates at 12 weeks were 81% (70/86) for those in the EpiCord group and 54% (26/48) for those treated with alginates (P = 0.0013).
Overall, 69% (107/155) of study ulcers were determined to have received adequate debridement. Adequate debridement occurred in 67 of 101 (66.3%) and 40 of 54 (74.1%) of EpiCord and alginate‐treated ulcers, respectively, P = 0.3653. For those ulcers that received adequate debridement (n = 107), 64 of 67 (96%) of the adequately debrided and EpiCord‐treated ulcers healed completely within 12 weeks, compared with 26 of 40 (65%) of adequately debrided and alginate‐treated ulcers, P < 0.0001.
The median number of EpiCord allografts applied per healed wound was 7 (range 2‐12). Average cost per EpiCord‐healed ulcer was $3250.99 ± $2898.48.
3.2. Follow up at 16 weeks
At the 16‐week follow‐up visit, in the ITT population, 74 of 101 (73%) of EpiCord‐treated ulcers were healed, compared with 29 of 54 (54%) of alginate‐treated ulcers, P = 0.0199. For subjects completing the study per protocol, 73 of 86 (85%) of EpiCord‐treated ulcers were healed at 16‐week follow up, compared with 29 of 48 (60%) of ulcers treated with alginate dressings.
Of the 71 healed ulcers treated with EpiCord during the 12‐week treatment phase, 68 of 71 (96%) remained closed at week 16 follow up, while 22 of the 26 ulcers healed with alginate dressings (85%) had remained closed.
3.3. Kaplan‐Meier plot of time to heal (ITT population)
A Kaplan‐Meier plot of time‐to‐heal within 12 weeks by study group, demonstrated a superior wound‐healing trajectory for EpiCord‐treated ulcers compared with ulcers treated with alginate dressings. The log‐rank test of equality of the healing function over the two study groups produced a χ 2 test statistic of 5.89, with a P = 0.0152 (Figure 4).
Figure 4.
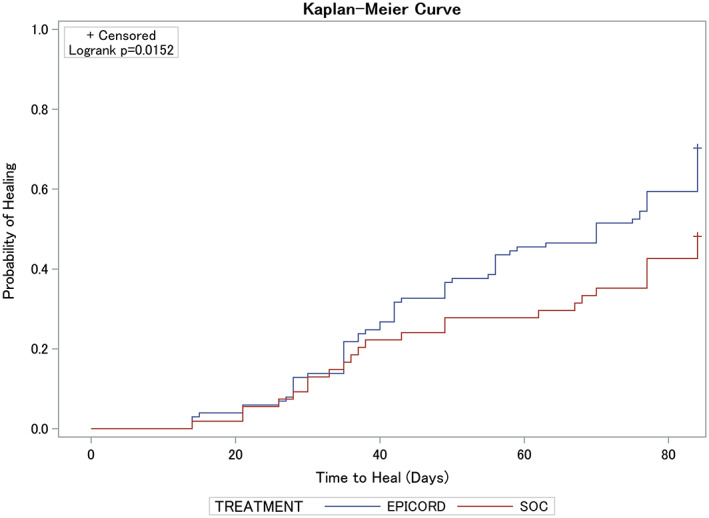
A Kaplan‐Meier plot of time to heal within 12 weeks by study group demonstrated a superior wound‐healing trajectory for EpiCord‐treated vs alginate‐treated (SOC) ulcers. The log‐rank test of equality of the healing function over the two study groups produced a χ 2 test statistic of 5.89, with a P = 0.0152
3.4. Adverse events/safety
All 155 subjects randomised were followed for evaluation of safety. Any unfavourable and unintended sign, symptom, or disease occurring during study enrolment, even those not necessarily having a causal relationship with study treatment, were documented as adverse events. An adverse event was classified as serious if it was determined to be life threatening or resulted in hospitalisation, prolonged disability, or death. An evaluation for adverse events was conducted and documented at each study visit. All adverse events were reviewed by site investigators and a Clinical Events Committee, including the study sponsor, to determine if the event was product or study related. Overall, 75 subjects experienced at least one adverse event, with a total of 160 adverse events recorded. (Table 4) There were no adverse events related to either EpiCord or alginate dressings.
Table 4.
Adverse events
| EpiCord (n = 101) | Alginate (n = 54) | |
|---|---|---|
| Subjects with at least 1 AE (n, %) | 42 (42%) | 33 (61%) |
| Adverse events (n) | 94 | 66 |
| Product‐related AEs (n) | 0 | 0 |
| Procedure‐related AEs (n) | 1 | 1 |
| Severe AEs (n) | 15 | 10 |
AE, adverse event.
4. DISCUSSION
EpiCord is a minimally manipulated, dehydrated, devitalised cellular umbilical cord allograft commercially available for homologous use, created through a proprietary PURION Plus process. The PURION Plus process results in an allograft material that can be stored in ambient conditions for 5 years. The results of the present study show that DFUs treated with the weekly application of EpiCord had significantly greater rates of complete healing within 12 weeks of treatment initiation than DFUs treated with alginate dressings, which by today's benchmarks are frequently considered part of the standard of care.
The umbilical cord consists of amniotic epithelium and Wharton's jelly that contains an extracellular matrix composed of collagen, fibroblasts, macrophages, proteoglycans, and hyaluronic acid, providing a protective milieu for healing, and a connective tissue matrix to replace or supplement damaged or deficient tissue. The ability to obtain mesenchymal stem cells (MSC) from the matrix of fresh umbilical cord tissue and use these cells in a variety of clinical applications has generated extensive interest in the development of regenerative therapies based on umbilical cord tissue.11, 13, 14 Contemporary advanced wound‐healing modalities are designed to stimulate angiogenesis and accelerate wound repair through regenerative mechanisms. A variety of stem cells may play a role in wound repair, including MSC, adipose stromal cells, and endothelial progenitor cells.15, 16 Soluble mediators generated by the wound‐healing cascade mobilise, recruit, and home these cells to sites of injury.
A recent study established that EpiCord possesses biological properties that stimulate cellular responses important for soft tissue healing.17 Tissue composition, evaluation of in vitro cellular response and in vivo bioresorption and tissue response was performed in a rat model. It was observed that EpiCord contains collagen I, hyaluronic acid, laminin, and fibronectin. In addition, 461 regulatory proteins that consist of growth factors, cytokines, inflammatory modulators, chemokines, proteases and inhibitors, adhesion molecules, signalling receptors, and other membrane‐bound and soluble proteins have been identified in PURION Plus‐processed EpiCord. Cell‐based assays demonstrated an increase in adipose‐derived stem cell and MSC proliferation, fibroblast migration, and endothelial progenitor cell vessel formation in a dose‐dependent manner after stimulation via EpiCord. In addition, rat subcutaneous implantation demonstrated biocompatibility as EpiCord allografts were resorbed without fibrous encapsulation.17
Although this is the first Level 1 RCT to examine the efficacy and safety of an allograft derived from umbilical cord as a treatment for chronic DFUs, our findings are not completely novel. In 2016, a single‐centre, retrospective case series on the use of a cryopreserved umbilical cord allograft as a treatment for chronic wounds of varying aetiology was published.18 The report was not an ITT analysis and excluded 23 patients, 29% of all patients treated, because of significant non‐compliance with instructions from the principal investigator, development of an infection resulting in amputation, and patients lost to follow up. Overall, 64 wounds were treated with the allograft, and 46 (71.9%) healed within 12 weeks. Of the 37 DFUs included in the retrospective case series, 29 (78.4%) healed after treatment with the umbilical cord allograft, although the 12‐week healing rate specifically for DFUs was not reported.18 In the present randomised controlled study, 86 DFUs were treated with EpiCord in patients completing the study per protocol, the group most comparable with those presented in the case series. Patients treated with EpiCord in the per‐protocol cohort achieved a healing rate of 81% within 12 weeks. Results of both studies are favourable for the application of umbilical cord allografts as a treatment for chronic wounds.
In the ITT population, the 12‐week healing rate of 70% reported with EpiCord is comparable with the 12‐week DFU healing rate of 70% achieved with another placental tissue product consisting of dehydrated human amnion/chorion membrane (EpiFix, MiMedx Group Inc., Marietta, Georgia).19 Although both products appear to produce similar healing results, the EpiCord allografts are thicker and more durable compared with the EpiFix allografts derived from amnion and chorion membranes, which when dehydrated are thinner and more brittle in texture until rehydrated into the wound bed. This variation in handling characteristics between the EpiCord and EpiFix products allows a clinician to determine which allograft is most appropriate to use based on wound type, depth, location, and presentation. A thicker and more durable allograft such as EpiCord may be a good choice for implantation into deeper wounds and in situations where suturing the allograft in place is desired.
Evaluating the benefits of available advanced wound care products compared with costs is a global challenge faced by both clinicians and health care policymakers. To justify the expense of advanced wound care products, they must be shown to be more effective and heal more wounds than less‐expensive alternatives. The results of this large, multicentre RCT provide additional Level 1 evidence regarding the efficacy of EpiCord overall but also illustrate the importance of wound preparation and debridement, which is in the hands of clinicians. The role of the clinician to select and execute the appropriate procedures to enhance rates of healing remains paramount to successful wound closure. Wound debridement has been reported to improve healing and is considered a vital adjunct in the treatment of DFUs.20, 21 To obtain the highest level of treatment success and ultimately reduce costs, advanced wound therapy must be used as an adjunct to, and not in lieu of, good wound care practices such as adequate debridement and off‐loading. Unfortunately, many wound care specialists have typically never received hands‐on standardised clinical training in surgical wound debridement, and there is often a wide variance in opinion among clinicians as to what constitutes adequate debridement and the amount of tissue removed during sharp debridement. In the current study, a blinded concurrent review was conducted to apply consistent standards and evaluate if wounds had been adequately debrided, with exposure of healthy tissue in the ulcer, with no significant eschar, callous, necrotic tissue, or foreign material present in or around the wound. Treatment with EpiCord in addition to adequate debridement resulted in a healing rate of 96% within 12 weeks. Adequately debrided wounds treated with alginate dressings had a healing rate of 65%, which, while impressive, still remained significantly lower than treatment with EpiCord, P < 0.0001. The appropriate use of strong evidence‐based products such as EpiCord, in the hands of a skilled clinician, is likely to provide the most benefits for patients and be among the most cost‐effective forms of health care expenditure.
The strength of the present study lies in its multicentre, RCT design, which is the gold standard from the clinical research paradigm. The RCT design, as well as the ITT analysis, allows for unbiased comparisons between study groups but may also reduce the actual treatment effect size through inclusion of drop‐outs and crossovers in their original study groups. The use of alginate dressings in the control group, instead of simple wet‐to‐dry gauze dressings, may have also reduced the treatment effect compared with EpiCord. A healing rate of 48% for wounds treated with alginate dressings in the ITT population compared with expected healing rates of approximately 24% reported with wet‐to‐dry dressings 20 years ago22 speaks to the overall advances being made in treating chronic wounds with contemporary management. Yet the results of the current study provide strong evidence that, while healing rates may be improved when more advanced dressings such as alginates are used, the clinicians role in adequate wound bed preparation continues to exist. Although the study groups were well matched for traditional factors influencing healing, other circumstances, typically problematic in the diabetic population, that we did not control for, such as nutrition, comorbidities, and polypharmacy, may have also influenced healing rates in the current study population.
DFUs are a major complication of patients with diabetes, and represent a growing problem in the health care system, imposing a substantial negative impact on individual's quality of life and increasing cost to the economy because of working days lost and upward trending financial burden on both public and private payers. The results of this first RCT on the use of EpiCord as a treatment for DFUs provide additional evidence of the safety and efficacy of dehydrated placental tissues.
ACKNOWLEDGEMENTS
The authors thank MiMedx Group Inc. for sponsoring the study and providing study oversight and data compilation and Niki Istwan, RN, for her administrative assistance with editing and formatting the manuscript.
Conflicts of interest
William Tettelbach, MD; Shawn Cazzell, DPM; Felix Sigal, DPM; Joseph M. Caporusso, DPM; Patrick S. Agnew, DPM; Jason Hanft, DPM; and Cyaandi Dove, DPM were among the clinical trial investigators and adjudicators for this study sponsored by MiMedx and received research funding. None of the authors report having a financial interest in any of the products mentioned in this manuscript during the course of the study. Although Dr.Tettelbach did not have any financial interest, or any other conflicts of interest, during the course of study, he is now an employee of MiMedx Group, Inc., the study sponsor.
Tettelbach W, Cazzell S, Sigal F, et al. A multicentre prospective randomised controlled comparative parallel study of dehydrated human umbilical cord (EpiCord) allograft for the treatment of diabetic foot ulcers. Int Wound J. 2019;16:122–130. 10.1111/iwj.13001
Funding information MiMedx Group Inc.
REFERENCES
- 1. Centers for Disease Control and Prevention National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2017. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed May 24, 2018.
- 2. American Diabetes Association . Economic costs of diabetes in the US in 2012. Diabetes Care. 2013;36(4):1033‐1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Driver VR, Fabbi M, Lavery LA, Gibbons G. The costs of diabetic foot: the economic case for the limb salvage team. J Vasc Surg. 2010;52(3):17S‐22S. [DOI] [PubMed] [Google Scholar]
- 4. Driver VR, de Leon JM. Health economic implications for wound care and limb preservation. J Manag Care Med. 2008;11(1):13‐19. [Google Scholar]
- 5. Kruse I, Edelman S. Evaluation and treatment of diabetic foot ulcers. Clin Diabetes. 2006. Apr;24(2):91‐93. 10.2337/diaclin.24.2.91. [DOI] [Google Scholar]
- 6. Holzer SE, Camerota A, Martens L, Cuerdon T, Crystal‐Peters J, Zagari M. Costs and duration of care for lower extremity ulcers in patients with diabetes. Clin Ther. 1998;20(1):169‐181. [DOI] [PubMed] [Google Scholar]
- 7. Lavery LA, Armstrong DG, Wunderlich RP, Mohler MJ, Wendel CS, Lipsky BA. Risk factors for foot infections in individuals with diabetes. Diabetes Care. 2006;29:1288‐1293. [DOI] [PubMed] [Google Scholar]
- 8. Joerger‐Messerli MS, Marx C, Oppliger B, Mueller M, Surbek DV, Schoeberlein A. Mesenchymal stem cells from wharton's jelly and amniotic fluid. Best Pract Res Clin Obstet Gynaecol. 2016. Feb;31:30‐44. 10.1016/j.bpobgyn.2015.07.006 Epub September 10, 2015. [DOI] [PubMed] [Google Scholar]
- 9. Nagamura‐Inoue T, He H. Umbilical cord‐derived mesenchymal stem cells: their advantages and potential clinical utility. World J Stem Cells. 2014;6(2):195‐202. http://www.wjgnet.com/1948-0210/full/v6/i2/195.htm, 10.4252/wjsc.v6.i2.195. Accessed September 17, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maslova O, Novak M, Kruzliak P. Umbilical cord tissue‐derived cells as therapeutic agents. Stem Cells Int. 2015;2015:150609. 10.1155/2015/150609 Epub July 12, 2015. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. El Omar R, Beroud J, Stoltz JF, Menu P, Velot E, Decot V. Umbilical cord mesenchymal stem cells: the new gold standard for mesenchymal stem cell‐based therapies? Tissue Eng Part B Rev. 2014. Oct;20(5):523‐544. 10.1089/ten.TEB.2013.0664 Epub April 22, 2014. [DOI] [PubMed] [Google Scholar]
- 12. Kubo M, Sonoda Y, Muramatsu R, Usui M. Immunogenicity of human amniotic membrane in experimental xenotransplantation. Invest Ophthalmol Vis Sci. 2001. Jun;42(7):1539‐1546. [PubMed] [Google Scholar]
- 13. Batsali AK, Kastrinaki MC, Papadaki HA, Pontikoglou C. Mesenchymal stem cells derived from Wharton's Jelly of the umbilical cord: biological properties and emerging clinical applications. Curr Stem Cell Res Ther. 2013. Mar;8(2):144‐155. Review. [DOI] [PubMed] [Google Scholar]
- 14. Zeddou M, Briquet A, Relic B, et al. The umbilical cord matrix is a better source of mesenchymal stem cells (MSC) than the umbilical cord blood. Cell Biol Int. 2010. Jul;34(7):693‐701. 10.1042/CBI20090414. [DOI] [PubMed] [Google Scholar]
- 15. Chen Z, Wang Y, Shi C. Therapeutic implications of newly identified stem cell populations from the skin dermis. Cell Transplant. 2015;24(8):1405‐1422. 10.3727/096368914X682431 Epub Jun 26, 2014. [DOI] [PubMed] [Google Scholar]
- 16. Kokai LE, Marra K, Rubin JP. Adipose stem cells: biology and clinical applications for tissue repair and regeneration. Transl Res. 2014. Apr;163(4):399‐408. [DOI] [PubMed] [Google Scholar]
- 17. Bullard JD, Lei J, Lim JJ, Massee M, Fallon AM, Koob TJ. Evaluation of dehydrated human umbilical cord biological properties for wound care and soft tissue healing. J Biomed Mater Res B Part B. 2018. Sep 10. 10.1002/jbm.b.34196. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Couture MA. Single‐center, retrospective study of cryopreserved umbilical cord for wound healing in patients suffering from chronic wounds of the foot and ankle. Wounds. 2016. Jul;28(7):217‐225. [PubMed] [Google Scholar]
- 19. Tettelbach W, Cazzell S, Reyzelman AM, et al. A confirmatory study on the efficacy of dehydrated human amnion/chorion membrane (EpiFix®) allograft in the management of diabetic foot ulcers: a prospective, multicenter, randomized, controlled study of 110 patients from 14 wound clinics. Int Wound J. 2018. Aug 22. 10.1111/iwj.12976. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wilcox JR, Carter MJ, Covington S. Frequency of debridements and time to heal: a retrospective cohort study of 312 744 wounds. JAMA Dermatol. 2013. Sep;149(9):1050‐1058. 10.1001/jamadermatol.2013.4960. [DOI] [PubMed] [Google Scholar]
- 21. Steed DL, Donohoe D, Webster MW, Lindsley L. Effect of extensive debridement and treatment on the healing of diabetic foot ulcers. Diabetic Ulcer Study Group. J Am Coll Surg. 1996. Jul;183(1):61‐64. [PubMed] [Google Scholar]
- 22. Margolis DJ, Kantor J, Berlin JA. Healing of diabetic neuropathic foot ulcers receiving standard treatment. A meta‐analysis. Diabetes Care. 1999. May;22(5):692‐695. [DOI] [PubMed] [Google Scholar]


