Summary
Objective
The euglycemic‐hyperinsulinemic clamp is the gold standard to evaluate insulin resistance (IR), but there are only a few studies on the prevalence of IR in Chinese Han women with polycystic ovary syndrome (PCOS). This study investigated: (a) the prevalence of IR in Chinese Han women with PCOS by clamp, (b) the degree of reduction in insulin sensitivity (IS) and the contribution of body mass index (BMI).
Design
Retrospective cross‐sectional analysis.
Patients
Chinese Han women with PCOS (n = 448) visiting the Department of Endocrinology or the Department of Obstetrics and Gynaecology of the First Affiliated Hospital of Chongqing Medical University. Chinese Han women without PCOS (controls) from the same area (n = 40).
Measurements
Clamp‐measured IS, age, BMI, and total testosterone.
Results
The prevalence of IR and reduction in IS were 56.3% and 30.3%, respectively, in Chinese Han women with PCOS (both P < 0.001). The inherent reduction in IS was 18.8% in lean women with PCOS and BMI independently reduced IS by 37.9% in obese women with PCOS. The prevalence of IR estimated by homeostatic model assessment (HOMA) was lower than that determined by clamp. The multivariable analysis showed that IR by clamp (R 2 = 0.48, P < 0.001) was independently associated with BMI (β = −0.52, P < 0.001), waist‐hip ratio (β = −0.23, P < 0.001), total testosterone (β = −0.07, P = 0.045) and age (β = 0.17, P < 0.001), while IR by HOMA was only associated with BMI (R 2 = 0.25, β = 0.50, P < 0.001). There were no differences in BMI groups distribution, HOMA‐IR and M values among the four PCOS subtypes (all P > 0.05).
Conclusions
56.3% of Chinese Han women with PCOS had IR and their reduction in IS was 30.3%. Obesity exacerbated the reduction in IS. When being evaluated by HOMA, the prevalence and the risk factors of IR in Chinese women with PCOS were underestimated.
1. INTRODUCTION
Polycystic ovary syndrome (PCOS) is the most common endocrine and metabolic disturbance in women of reproductive age. PCOS is characterized by hyperandrogenism and anovulatory infertility, and has substantial short‐ and long‐term reproductive, psychological and metabolic implications.1, 2 The prevalence of PCOS varies between 5% and 15% with ethnicity.3 The mechanism is complex and remains unclear.3
Insulin resistance (IR) is defined as reduced insulin sensitivity (IS) and refers to an increased amount of insulin needed to perform its metabolic actions.4 IR occurs in 40%‐70% of women with PCOS.5, 6, 7 It is usually accepted that obese women with PCOS have IR. Obesity may increase PCOS prevalence and exacerbate IR in women with PCOS.1, 8 Consensus is yet to be achieved in lean women with PCOS since IR is not consistently demonstrated.9, 10
The euglycemic‐hyperinsulinemic clamp is considered the gold standard to directly measure IS in research settings, but the complexity of the clamp technique has directed clinical researchers towards the use of fasting parameters of glucose homeostasis as surrogate measures of IR.10 Usually, only small studies use the euglycemic‐hyperinsulinemic clamp,9, 11, 12, 13 while indirect methods such as the homeostatic model assessment (HOMA) are broadly used in large‐scale metabolic studies on PCOS.14, 15, 16
A systematic review and meta‐analysis of euglycemic‐hyperinsulinemic clamp studies showed that the inherent reduction in IS is 27% and body mass index (BMI) exacerbates the reduction in IS by 15% in women with PCOS.17 Only a few studies on the prevalence of IR are reported in Chinese Han women with PCOS. Li et al 11 showed that IR was a common finding in women with PCOS and normal glucose tolerance and that BMI >25.545 kg/m2 indicated impaired β‐cell function in these women. Chen et al 18 showed that 23.7% of the Chinese patients with PCOS had dysregulated glucose metabolism. It is unclear whether small BMI in Chinese Han women with PCOS is associated with IR. Therefore, the study aimed to address the following key questions about Chinese Han women with PCOS: what is the prevalence of IR and degree of reduction in IS measured by euglycemic‐hyperinsulinemic clamp? What is the relative contribution of BMI to the reduction in IS? As secondary objectives, the study also aimed to analyse the difference of IS evaluated by euglycemic‐hyperinsulinemic clamp and HOMA, as well as the differences in clamp and HOMA‐IR among the different subtypes of PCOS.
2. MATERIALS AND METHODS
2.1. Participants
In this cross‐sectional study, 448 consecutive Chinese Han women with PCOS were recruited when visiting the Department of Endocrinology or the Department of Obstetrics and Gynaecology of the First Affiliated Hospital of Chongqing Medical University from July 2003 to May 2014. The diagnosis of PCOS and its subtypes were based on the 2003 Rotterdam consensus.19 For women with no history of sexual life, abdominal B‐ultrasound (frequency of 2‐5 MHz) was performed to assess the ovarian morphology; for those with sexual life history, transvaginal ultrasound (frequency of 5‐9 MHz) was performed. PCOS was diagnosed if there were >12 follicles and if the diameter of each follicle were 2‐9 mm, and/or the ovarian volume was >10 cm3. According to previous studies,20, 21 hirsutism was defined as coarse hair growing on the upper lip, lower jaw, areola surrounding, and the midline of the lower abdomen, and in the presence of a FG score ≥2 points in Chinese women. If infertile women had hirsutism and/or acne on the forehead, cheek, nose, or jaw, they would be determined as having hyperandrogenic clinical manifestations. If infertile women of childbearing age had a level of total serum testosterone higher than the upper limit of the 95% confidence interval (CI) of the controls (>75.62 ng/dL), they were clinically diagnosed with hyperandrogenism. Anovulation or oligo‐ovulation was defined as: regular menstruation failed to be achieved within 2‐3 years after menarche; amenorrhoea (>3 months of menopause or previous menstrual cycle >6 months); or oligomenorrhoea (menstrual cycle ≥35 days and >3 months of anovulation each year). PCOS was confirmed by excluding other disorders with a similar clinical presentation such as congenital adrenal hyperplasia, Cushing's syndrome and androgen‐secreting tumours. Serum 17‐hydroxyprogesterone, thyroid‐stimulating hormone, FSH/LH, prolactin and cortisol were measured to exclude related disorders.
Forty healthy women were selected as controls. All control subjects had a normal menstrual cycle. None had clinical and/or biochemical hyperandrogenism.
According to the guidelines for the prevention and control of overweight and obesity in Chinese adults,22 BMI of 24‐28 kg/m2 indicated overweight and BMI ≥28 kg/m2 indicated obesity. All women with PCOS were divided into the overweight, obese and lean (<24 kg/m2) groups. All subjects were Han. All controls had no first‐degree familial history of diabetes, while a first‐degree familial history of diabetes was present in 53 PCOS patients (11.8%). The exclusion criteria for all subjects were: (a) use of hormone medication (including oral contraceptives) within the past month; or (b) use of medication affecting insulin sensitivity (eg, metformin or thiazolidinediones) within the past three months. Informed consent was obtained from all participants, and the study was approved by the First Affiliated Hospital of Chongqing Medical University Ethical Committee. The study met the requirements of the 1975 Helsinki guidelines.
2.2. Data collection
All patients underwent anthropometric measurements: height, weight, waist circumference (WC) and hip circumference (HC). BMI was determined as the ratio between weight in kilograms and the square of height in metres. Waist‐hip ratio (WHR) was determined as the ratio between WC and HC, in centimetres.
2.3. Biochemical measurements
Fasting blood was sampled for the measurement of serum total testosterone levels, anytime between days 8 and 10 of the menstrual cycle or during amenorrhoea after excluding pregnancy. All blood samples were kept at −80°C. Fasting plasma glucose was measured by the hexokinase‐UV/NAD method (Olympus, Tokyo, Japan). Fasting serum insulin and total testosterone were determined by electrochemiluminescence (Roche, Basel, Switzerland). HOMA‐IR was calculated using the equation HOMA‐IR = fasting serum insulin (mIU/L) × fasting plasma glucose (mmol/L)/22.5.HOMA‐IR ≥ 2.69 was the cut‐off for abnormal values.23
2.4. Euglycemic‐hyperinsulinemic clamp
Euglycemic‐hyperinsulinemic clamps were performed in all subjects, as in our previous reports.12 After fasting overnight, subjects were admitted to the hospital. Venous catheters were placed in both arms for insulin infusion, glucose infusion and blood sampling. The insulin infusion was performed with a 10‐minutes priming dose of short‐acting human insulin (Humulin, Lilly) and maintained at a rate of 120 mU/(m2 × min) for 170 min. A variable infusion of 20% glucose was started on the fourth minute to maintain the plasma glucose levels at 5.2 mmol/L (4.9‐5.5 mmol/L). Blood samples for the measurement of plasma glucose were obtained at 5‐min intervals throughout the clamp. Plasma glucose was measured by the glucose oxidase method using a Biosen 5030 Glucose Analyzer (EKF Industrie, Elektronik GmbH, Barleben, Germany). The M value (mg/min/kg) was calculated from the glucose infusion rates during the last 60 minutes of the clamp. The lower limit of the M value was defined as the mean of the values in control subjects minus 1.96 times the standard deviation. In this study, the lower and upper limits of the M value were 9.01 and 15.47 mg/min/kg.
2.5. Statistical analysis
All statistical analyses were performed using SPSS 13.0 (SPSS Inc, Chicago, IL, USA). Data were presented as means ± SD. Variables with more than two groups were analysed by Welsh's ANOVA [with the Games Howell test (in case of heterogeneity of variance) or the Student‐Newman‐Keuls test (in case of homogeneity of variance) for post hoc analysis]. The independent sample t test was used for comparisons between two groups. Total testosterone, HOMA‐IR and fasting serum insulin were SQRT‐transformed before analysis due to non‐normal distribution. Categorical variables were analysed using the Chi‐square test and logistic regression analysis. Correlations were tested using Pearson correlation coefficients. Analysis of covariance and multiple linear regression was performed to adjust for covariates and confounders. The agreement in the prevalence of IR according to the M value and to HOMA‐IR was evaluated using the Cohen's kappa statistic (<0: no agreement; 0‐0.20, slight agreement; 0.21‐0.40, fair agreement; 0.41‐0.60, moderate agreement; 0.61‐0.80, substantial agreement; and 0.81‐1.00, almost perfect agreement). P value < 0.05 was considered statistically significant.
3. RESULTS
3.1. General characteristics
Compared with control subjects, the women with PCOS were younger and had higher BMI, WC, WHR, total testosterone, fasting plasma glucose and fasting serum insulin (all P < 0.05). As expected, compared with controls, women with PCOS had a lower M value (P < 0.001) and higher HOMA‐IR (P = 0.001) (Table 1). The M value averaged 12.47 mg/min/kg in control subjects and was 30.3% lower in women with PCOS.
Table 1.
General characteristics of PCOS women and control subjects
| Variables | PCOS women (n = 448) | Controls (n = 40) | P value |
|---|---|---|---|
| Age (years) | 25.2 ± 4.5 | 26.5 ± 3.5 | 0.029 |
| BMI (kg/m2) | 23.2 ± 4.2 | 21.0 ± 2.2 | 0.001 |
| WC (cm) | 77.2 ± 10.8 | 67.3 ± 5.6 | <0.001 |
| WHR | 0.83 ± 0.07 | 0.76 ± 0.05 | <0.001 |
| Total testosterone (ng/dL) | 77.3 ± 64.8 | 37.7 ± 19.3 | <0.001 |
| Fasting plasma glucose (mmol/L) | 5.1 ± 0.7 | 4.9 ± 0.5 | <0.001 |
| Fasting serum insulin (mU/L) | 10.5 ± 8.8 | 6.2 ± 3.3 | <0.001 |
| M value (mg/min/kg) | 8.69 ± 3.01 | 12.47 ± 1.71 | <0.001 |
| HOMA‐IR | 2.45 ± 2.19 | 1.36 ± 0.84 | 0.001 |
3.2. Subgroup analysis
According to BMI, the women with PCOS were divided into the lean (BMI <24 kg/m2), overweight (BMI 24‐28 kg/m2), and obese (BMI ≥28 kg/m2) subgroups. After excluding one control with obesity and three with overweight, 36 lean controls were included in the analyses. As expected, compared with the lean controls, women with PCOS had lower M value (P < 0.001) and higher HOMA‐IR (P < 0.001; Figure 1). M value averaged 12.24 mg/min/kg in lean controls, lower by 18.8% in lean women with PCOS, by 42.5% in overweight women with PCOS, and by 56.7% in obese women with PCOS (P < 0.05, unmatched subjects) (Table 2). Similar results were observed in the matched analysis (Supporting Information Table S1).
Figure 1.
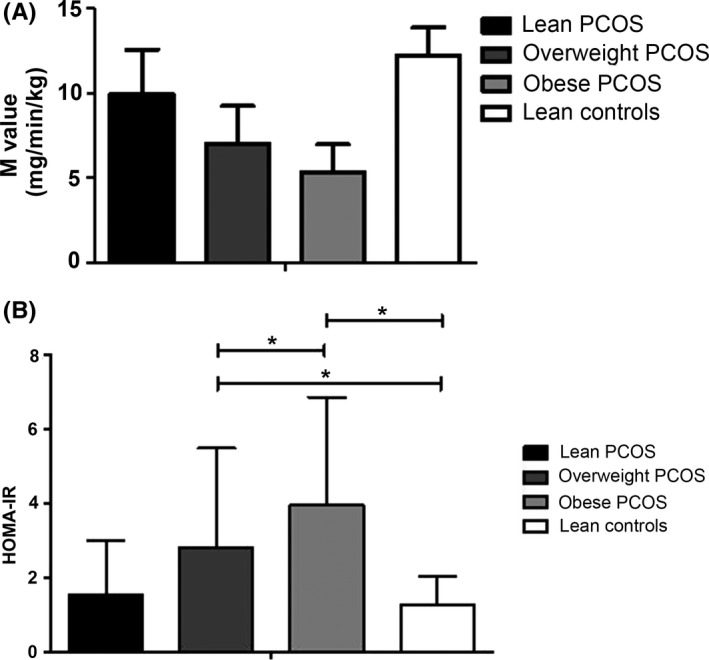
A, M value of the four groups of women. General P < 0.001; all P < 0.05 between each pair of groups. B, HOMA‐IR of the four groups of women. General P < 0.001; *P < 0.05
Table 2.
Parameters of insulin resistance of lean, overweight, and obese PCOS women
| Variables | PCOS women | Controls | P value | ||
|---|---|---|---|---|---|
| Lean group (n = 290) | Overweight group (n = 100) | Obese group (n = 58) | Lean group (n = 36) | ||
| Age (years) | 24.7 ± 4.3a | 25.7 ± 4.6a,b | 26.7 ± 4.5b | 26.2 ± 3.1a,b | 0.005 |
| Total testosterone (ng/dL) | 72.6 ± 48.8a | 75.9 ± 48.8a | 76.8 ± 62.1a | 39.6 ± 19.5b | 0.009 |
| Fasting plasma glucose (mmol/L) | 5.1 ± 0.6b | 5.2 ± 0.7b,c | 5.4 ± 0.9c | 5.1 ± 0.7a | <0.001 |
| Fasting serum insulin (mU/L) | 7.9 ± 5.9a | 13.7 ± 11.0b | 17.8 ± 10.4c | 5.9 ± 3.1a | <0.001 |
| M value (mg/min/kg) | 9.94 ± 2.64b | 7.04 ± 2.21c | 5.30 ± 1.68d | 12.24 ± 1.65a | <0.001 |
| HOMA‐IR | 1.53 ± 1.48a | 2.81 ± 2.68b | 3.97 ± 2.89c | 1.29 ± 0.76a | <0.001 |
| Prevalence of IR based on the M value | 114 (34.5%) | 81 (80.2%) | 57 (98.3%) | – | – |
| Prevalence of IR based on HOMA‐IR | 45 (15.6%) | 42 (41.6%) | 38 (58.6%) | – | – |
| Kappa value between IR by M value and HOMA‐IR | 0.069 | 0.139 | 0.390 | – | – |
| P value between IR by M value and HOMA‐IR | <0.001 | <0.001 | <0.001 | – | – |
Values denoted by different letters on the same row were significantly different.
Table 2 shows that the prevalence of IR was lower when using HOMA‐IR than when using the M value. The prevalence of IR estimated by the M value was 56.3% in women with PCOS, or 34.5%, 80.2% and 98.7% in lean, overweight, and obese women with PCOS, respectively (P < 0.05). The prevalence of IR estimated by HOMA‐IR was 27.9% in women with PCOS, or15.6%, 41.6% and 58.6% in lean, overweight and obese women with PCOS, respectively (P < 0.05) (Table 2).
A logistic regression analysis showed that when using the M value and compared with lean women with PCOS, overweight and obese women with PCOS had higher risk of IR (overweight: OR = 5.76, 95%CI: 3.38‐9.84, P < 0.001; obese: OR = 86.24, 95%CI: 11.78‐631.59, P < 0.001). In the same manner, using HOMA‐IR and compared with lean women with PCOS, overweight and obese women with PCOS had higher risk of IR (overweight: OR = 4.11, 95% CI: 2.38‐7.11, P < 0.001; obese: OR = 10.99, 95%CI: 5.82‐20.76, P < 0.001).
3.3. Bivariate analysis
The Pearson correlation analysis showed that in all subjects, the M value correlated with BMI, WC, WHR and total testosterone (r = −0.64, r = −0.64, r = −0.54 and r = −0.13, respectively, P < 0.001, P < 0.001, P < 0.001 and P = 0.004, respectively), but not with age (P = 0.069). HOMA‐IR correlated with BMI, WC and WHR (r = 0.50, r = 0.47 and r = 0.37, respectively, P < 0.001, P < 0.001 and P < 0.001), but not with total testosterone (P = 0.162) and age (P = 0.825).
3.4. Multivariate analysis
Two multiple linear regression analyses were performed with two models, respectively, including the M value and HOMA‐IR as the dependent variables. The M value (R 2 = 0.48, P < 0.001) was independently associated with BMI (β = −0.52, P < 0.001), WHR (β = −0.23, P < 0.001), total testosterone (β = −0.07, P = 0.045), and age (β = 0.17, P < 0.001). After adjustment for WHR, total testosterone and age, HOMA‐IR was only associated with BMI (R 2 = 0.25, β = 0.50, P < 0.001).
3.5. PCOS subtypes
A subgroup analysis was performed among 337 patients with available PCOS subtype data. Table 3 shows that there were no differences in BMI groups distribution (P = 0.56), HOMA‐IR (P = 0.95), and M value (P = 0.18) among the four PCOS subtypes.
Table 3.
Overweight/obesity according to the PCOS subtypes
| OH (n = 72) | HP (n = 15) | OHP (n = 187) | OP (n = 63) | P value | |
|---|---|---|---|---|---|
| BMI groupsa | |||||
| Lean | 48 (66.7%) | 8 (53.3%) | 117 (62.6%) | 44 (69.8%) | 0.56 |
| Overweight | 16 (22.2%) | 4 (26.7%) | 44 (23.5%) | 8 (12.7%) | |
| Obese | 8 (11.1%) | 3 (20.0%) | 26 (13.9%) | 11 (17.5%) | |
| HOMA‐IR (95%CI)b | 1.86 (1.50‐2.33) | 1.66 (1.05‐2.67) | 1.74 (1.54‐1.98) | 1.78 (1.42‐2.24) | 0.95 |
| M value (95%CI)b | 8.10 (7.40‐8.86) | 7.62 (6.24‐9.07) | 7.70 (7.28‐8.15) | 8.69 (7.89‐9.57) | 0.18 |
O, oligo/anovulation; H, hyperandrogenism; P, polycystic ovary morphology; BMI, body mass index; HOMA‐IR, homeostatic model assessment for insulin resistance.
Fisher's exact test.
Welsh's ANOVA.
4. DISCUSSION
In this cross‐sectional study of IR determined by euglycemic‐hyperinsulinemic clamp, the prevalence of IR was 56.3% in Chinese women with PCOS and their reduction in IS was 30.3%. The inherent reduction in IS was 18.8% in lean women with PCOS and BMI independently exacerbated the reduction in IS by 37.9% in obese women with PCOS. Compared with euglycemic‐hyperinsulinemic clamp, HOMA underestimated the prevalence of IR In addition, euglycemic‐hyperinsulinemic clamp was more useful than HOMA to identify IR‐associated risk factors other than obesity.
As in ours and others' previous reports,11, 12, 13, 24 the present study showed that women with PCOS have intrinsic reduction in IS on euglycemic‐hyperinsulinemic clamp and almost all obese women with PCOS have more serious IR than lean women with PCOS. This relationship between BMI and IR in PCOS was also observed in Caucasians.24 A systematic review and meta‐analysis of euglycemic‐hyperinsulinemic clamp studies by Cassar et al showed that the inherent reduction in IS is 27% and BMI exacerbates the reduction in IS by 15% in women with PCOS.17 Therefore, weight loss should be very important to improve IS and attenuate the complications of PCOS. It is interesting that WHR is independently associated with IR after adjusting for BMI in the present study. Therefore, the role of central obesity on PCOS‐related IR should also draw our attention.
Same as in previous studies,25, 26, 27 the present study showed that the average BMI in Chinese patients with PCOS was within the normal range (23.2 kg/m2) and that up to 64.5% were lean. On the contrary, most studies from Western countries showed that the average BMI of women with PCOS was usually >30 kg/m2.14, 24, 28, 29 Because the prevalence and degree of IR increase with BMI,24 it can be speculated that low BMI may be the main cause for the lower prevalence and degree of IR in Chinese women with PCOS compared with their Western counterparts. In addition, the multiple linear regression analysis showed that increasing age adversely impacted IR, while high levels of total testosterone positively impacted it, but the impact of testosterone was trivial after adjustment for BMI and WHR. The different subtypes of PCOS were not associated with differences in HOMA‐IR or M value. This is in contradiction to a previous study by Zhang et al 30 that showed that the patients with the OHP and OH subtypes had the highest IR, followed by the HP and then by the OP subtypes. Xu et al 31 and Sobti et al 32 showed that patients with and without hyperandrogenism had different glucose metabolism parameters. Fica et al 33 highlighted that patients with different PCOS subtypes respond differently to metformin. Further study is necessary to rule out these discrepancies.
The prevalence of IR in women with PCOS shows a marked variation among ethnic groups, being 83.6% in Arabs, 76% in Americans, 72% in Italians, 70% in Mexican‐Americans, 75% in British and 68% in Japanese.15, 24, 34 In the present study, the prevalence of IR in Chinese Han women with PCOS was lower, at 56.3%. In addition to differences in diet, lifestyle and genetic factors between Chinese and other ethnicities, different methods were used to determine IR in the different populations. HOMA was often used to investigate IR in clinical studies, but cut‐off values of HOMA to define IR vary with ethnicity. Using the intravenous glucose tolerance test, Ascaso et al selected the 75th percentile of HOMA‐IR value, which was 2.5 in Spaniards, with normal glucose regulation as the cutoff point to define IR35 In the same way, Xing et al showed that the 75th percentile of HOMA‐IR values was 2.69 in Chinese with normal glucose regulation.23 Thus, 14.2% of Chinese women with PCOS had IR according to HOMA‐IR > 2.69,27 while up to 86.3% of Arab women with PCOS had IR according to HOMA‐IR > 2.5.15 The present study in Chinese Han women with PCOS showed that the prevalence of IR by euglycemic‐hyperinsulinemic clamp was almost 3 folds higher compared with the prevalence of IR when determined by HOMA. A recent study in Caucasians also showed that the surrogate markers of IR resulted in a lower prevalence of IR compared with the M value.24 These results suggest that the underestimated prevalence of IR by HOMA should be corrected in women with PCOS. Nevertheless, it must also be stressed that the HOMA‐IR cut‐off points cannot be generalized since they depend upon the insulin assay being used. Additional studies are necessary to examine this issue.
The strengths of the present study were that the sample size was large and from a single geographic area, limiting the variability in genetics and lifestyle habits. Nevertheless, there are limitations. It was based on retrospective cross‐sectional data from Chinese Han women. These results may not apply to populations of other ethnic origin. Since the inclusion period of the study was very long, it was not possible to control potential confounders on IR such as lifestyle and diet. In addition, we had to use control subjects to determine the cut‐off for IS. We did so because the cut‐off for IR varies with ethnicity15, 24, 34 and because of a lack of data from Chinese patients. In addition, obesity, lifestyle habits, economic status and genetics vary widely across China and we desired to obtain a reference value that was more suitable to our specific study population. Finally, the study was conducted on a selected group of patients with PCOS who were admitted to an endocrinology clinic and obstetrics and gynecology clinic. Studying unselected patients with PCOS using a population‐based survey might be the optimal way to determine the real prevalence of IR in this population.
In conclusion, 56.3% of Chinese Han women with PCOS had IR and their reduction in IS was 30.3%. IR was an intrinsic characteristic of Chinese women with PCOS and obesity exacerbated reduction in IS. When being evaluated by HOMA, the prevalence and the risk factors of IR in Chinese women with PCOS were underestimated.
CONFLICT OF INTEREST
The authors have no conflict of interests to declare.
Supporting information
ACKNOWLEDGEMENTS
The authors thank all the research participants and their parents, without whom science would not advance.
Li W, Chen Q, Xie Y, Hu J, Yang S, Lin M. Prevalence and degree of insulin resistance in Chinese Han women with PCOS: Results from euglycemic‐hyperinsulinemic clamps. Clin Endocrinol. 2019;90:138–144. 10.1111/cen.13860
Weiping Li and Qingfeng Chen contributed equally to the manuscript.
YikaiXie, JinboHu, ShuminYang and Miaozhi Lin made equal senior contribution to the manuscript.
Funding information
This work was supported by grants from the National Natural Science Foundation of China (NSFC) (81300310) and the Basic and Frontier Research Project of Chongqing, China (cst2015jcyjA10092).
REFERENCES
- 1. Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010;8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anagnostis P, Tarlatzis BC, Kauffman RP. Polycystic ovarian syndrome (PCOS): Long‐term metabolic consequences. Metabolism. 2018;86:33‐43. [DOI] [PubMed] [Google Scholar]
- 3. Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta‐analysis. Hum Reprod. 2016;31:2841‐2855. [DOI] [PubMed] [Google Scholar]
- 4. Macut D, Bjekic‐Macut J, Rahelic D, Doknic M. Insulin and the polycystic ovary syndrome. Diabetes Res Clin Pract. 2017;130:163‐170. [DOI] [PubMed] [Google Scholar]
- 5. Nawrocka‐Rutkowska J, Ciecwiez S, Marciniak A, et al. Insulin resistance assessment in patients with polycystic ovary syndrome using different diagnostic criteria–impact of metformin treatment. Ann Agric Environ Med. 2013;20:528‐532. [PubMed] [Google Scholar]
- 6. Tabassum R, Imtiaz F, Sharafat S, Shukar‐Ud‐Din S, Nusrat U. Prevalence and clinical profile of insulin resistance in young women of poly cystic ovary syndrome: A study from Pakistan. Pak J Med Sci. 2013;29:593‐596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. DeUgarte CM, Bartolucci AA, Azziz R. Prevalence of insulin resistance in the polycystic ovary syndrome using the homeostasis model assessment. Fertil Steril. 2005;83:1454‐1460. [DOI] [PubMed] [Google Scholar]
- 8. Yang S, Li Q, Zhong L, et al. Serum pigment epithelium‐derived factor is elevated in women with polycystic ovary syndrome and correlates with insulin resistance. J Clin Endocrinol Metab. 2011;96:831‐836. [DOI] [PubMed] [Google Scholar]
- 9. Gennarelli G, Rovei V, Novi RF, et al. Preserved insulin sensitivity and {beta}‐cell activity, but decreased glucose effectiveness in normal‐weight women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:3381‐3386. [DOI] [PubMed] [Google Scholar]
- 10. Diamanti‐Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33:981‐1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li W, Li Q. Dysregulation of glucose metabolism even in Chinese PCOS women with normal glucose tolerance. Endocr J. 2012;59:765‐770. [DOI] [PubMed] [Google Scholar]
- 12. Li W, Ma L, Li Q. Insulin resistance but not impaired beta‐cell function: a key feature in Chinese normal‐weight PCOS women with normal glucose regulation. Gynecol Endocrinol. 2012;28:598‐601. [DOI] [PubMed] [Google Scholar]
- 13. Stepto NK, Cassar S, Joham AE, et al. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic‐hyperinsulaemic clamp. Hum Reprod. 2013;28:777‐784. [DOI] [PubMed] [Google Scholar]
- 14. van Hooff MH, Voorhorst FJ, Kaptein MB, Hirasing RA, Koppenaal C, Schoemaker J. Predictive value of menstrual cycle pattern, body mass index, hormone levels and polycystic ovaries at age 15 years for oligo‐amenorrhoea at age 18 years. Hum Reprod. 2004;19:383‐392. [DOI] [PubMed] [Google Scholar]
- 15. Al‐Jefout M, Alnawaiseh N, Al‐Qtaitat A. Insulin resistance and obesity among infertile women with different polycystic ovary syndrome phenotypes. Sci Rep. 2017;7:5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liang P, Xi L, Shi J, et al. Prevalence of polycystic ovary syndrome in Chinese obese women of reproductive age with or without metabolic syndrome. Fertil Steril. 2017;107:1048‐1054. [DOI] [PubMed] [Google Scholar]
- 17. Cassar S, Misso ML, Hopkins WG, Shaw CS, Teede HJ, Stepto NK. Insulin resistance in polycystic ovary syndrome: a systematic review and meta‐analysis of euglycaemic‐hyperinsulinaemic clamp studies. Hum Reprod. 2016;31:2619‐2631. [DOI] [PubMed] [Google Scholar]
- 18. Chen C, Jing G, Li Z, Juan S, Bin C, Jie H. Insulin resistance and polycystic ovary syndrome in a Chinese population. Endocr Pract. 2017. 10.4158/EP171849.OR. [DOI] [PubMed] [Google Scholar]
- 19. Rotterdam EA‐SP. Revised 2003 consensus on diagnostic criteria and long‐term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19:41‐47. [DOI] [PubMed] [Google Scholar]
- 20. Escobar‐Morreale HF, Carmina E, Dewailly D, et al. Epidemiology, diagnosis and management of hirsutism: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome Society. Hum Reprod Update. 2012;18:146‐170. [DOI] [PubMed] [Google Scholar]
- 21. Zhao JL, Chen ZJ, Shi YH, et al. Investigation of body hair assessment of Chinese women in Shandong region and its preliminary application in polycystic ovary syndrome patients. Zhonghua Fu Chan Ke Za Zhi. 2007;42:590‐594. [PubMed] [Google Scholar]
- 22. Chen C, Lu FC, Department of Disease Control Ministry of Health of the People's Republic of China . The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed Environ Sci. 2004;17(Suppl):1‐36. [PubMed] [Google Scholar]
- 23. Xing X, Yang ZJ. The diagnostic significance of homeostasis model assessment of insulin resistance in metabolic syndrome among subjects with different glucose tolerance. Chin J Diab. 2004;1:182‐186. [Google Scholar]
- 24. Tosi F, Bonora E, Moghetti P. Insulin resistance in a large cohort of women with polycystic ovary syndrome: a comparison between euglycaemic‐hyperinsulinaemic clamp and surrogate indexes. Hum Reprod. 2017;32:2515‐2521. [DOI] [PubMed] [Google Scholar]
- 25. Ni RM, Mo Y, Chen X, Zhong J, Liu W, Yang D. Low prevalence of the metabolic syndrome but high occurrence of various metabolic disorders in Chinese women with polycystic ovary syndrome. Eur J Endocrinol. 2009;161:411‐418. [DOI] [PubMed] [Google Scholar]
- 26. Wei HJ, Young R, Kuo IL, Liaw CM, Chiang HS, Yeh CY. Prevalence of insulin resistance and determination of risk factors for glucose intolerance in polycystic ovary syndrome: a cross‐sectional study of Chinese infertility patients. Fertil Steril. 2009;91:1864‐1868. [DOI] [PubMed] [Google Scholar]
- 27. Li R, Yu G, Yang D, et al. Prevalence and predictors of metabolic abnormalities in Chinese women with PCOS: a cross‐ sectional study. BMC Endocr Disord. 2014;14:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ehrmann DA, Kasza K, Azziz R, Legro RS, Ghazzi MN. Effects of race and family history of type 2 diabetes on metabolic status of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:66‐71. [DOI] [PubMed] [Google Scholar]
- 29. Kauffman RP, Baker VM, DiMarino P, Castracane VD. Hyperinsulinemia and circulating dehydroepiandrosterone sulfate in white and Mexican American women with polycystic ovary syndrome. Fertil Steril. 2006;85:1010‐1016. [DOI] [PubMed] [Google Scholar]
- 30. Zhang HY, Zhu FF, Xiong J, Shi XB, Fu SX. Characteristics of different phenotypes of polycystic ovary syndrome based on the Rotterdam criteria in a large‐scale Chinese population. BJOG. 2009;116:1633‐1639. [DOI] [PubMed] [Google Scholar]
- 31. Xu TT, Wang Y, Saphariny J, Hu ZP. Study on the differences of endocrine and metabolic characteristics in clinical subtypes of PCOS. J Reprod Contraception. 2012;23:81‐92. [Google Scholar]
- 32. Sobti S, Dewan R, Ranga S. Metabolic syndrome and insulin resistance in PCOS phenotypes. Int J Reprod Contraception Obst Gynecol. 2017;6(11):5067‐51 10. [Google Scholar]
- 33. Fica S, Albu A, Constantin M, Dobri GA. Insulin resistance and fertility in polycystic ovary syndrome. J Med Life. 2008;1:415‐422. [PMC free article] [PubMed] [Google Scholar]
- 34. Legro RS, Castracane VD, Kauffman RP. Detecting insulin resistance in polycystic ovary syndrome: purposes and pitfalls. Obstet Gynecol Surv. 2004;59:141‐154. [DOI] [PubMed] [Google Scholar]
- 35. Ascaso JF, Pardo S, Real JT, Lorente RI, Priego A, Carmena R. Diagnosing insulin resistance by simple quantitative methods in subjects with normal glucose metabolism. Diabetes Care. 2003;26:3320‐3325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


