Abstract
In the early Pleistocene, global temperature cycles predominantly varied with ~41-kyr (obliquity-scale) periodicity. Atmospheric greenhouse gas concentrations likely played a role in these climate cycles; marine sediments provide an indirect geochemical means to estimate early Pleistocene CO2. Here we present a boron isotope-based record of continuous high-resolution surface ocean pH and inferred atmospheric CO2 changes. Our results show that, within a window of time in the early Pleistocene (1.38–1.54 Ma), pCO2 varied with obliquity, confirming that, analogous to late Pleistocene conditions, the carbon cycle and climate covaried at ~1.5 Ma. Pairing the reconstructed early Pleistocene pCO2 amplitude (92 ±13 μatm) with a comparably smaller global surface temperature glacial/interglacial amplitude (3.0 ±0.5 K), yields a surface temperature change to CO2 radiative forcing ratio of S[CO2]~0.75 (± 0.5) °C/Wm−2, as compared to the late Pleistocene S[CO2] value of ~1.75 (± 0.6) °C/Wm−2. This direct comparison of pCO2 and temperature implicitly incorporates the large ice sheet forcing as an internal feedback and is not directly applicable to future warming. We evaluate this result with a simple climate model, and show that the presumably thinner, though extensive, northern hemisphere ice sheets would increase surface temperature sensitivity to radiative forcing. Thus, the mechanism to dampen actual temperature variability in the early Pleistocene more likely lies with Southern Ocean circulation dynamics or antiphase hemispheric forcing. We also compile this new carbon dioxide record with published Plio-Pleistocene δ11B records using consistent boundary conditions and explore potential reasons for the discrepancy between Pliocene pCO2 based on different planktic foraminifera.
1. Introduction
The Pleistocene Epoch was characterized by a sequence of glacial/interglacial climate oscillations that are recorded in geochemical records from deep-sea sediments, continental deposits, and ice cores [e.g., Imbrie et al., 1984; Lüthi et al., 2008; Joannin et al., 2010]. These records show that Earth’s surface temperature covaried with the dominant periodicities of Earth’s orbit around the Sun: orbital precession, obliquity and/or eccentricity. Nevertheless, understanding how the climate system responds to and amplifies the initial orbital insolation forcing is a long-standing problem in the field of paleoclimatology [Hays et al., 1976; Hansen et al., 1984; Imbrie et al., 1992; Hansen et al., 2005]. High-resolution records of temperature and greenhouse gas concentrations are of paramount value to elucidate the pattern and drivers of global climate change, yet continuous high-resolution ice core records of trapped ancient air are thus far restricted to the late Pleistocene (0.8 Ma to present) [e.g., Lüthi et al., 2008]. Ancient air inclusions in late Pleistocene ice-cores suggest that the partial pressure of atmospheric CO2 (pCO2) lagged an initial temperature perturbation [Fischer et al., 2010] such that radiative greenhouse gas forcing at most amplified the orbital pacing of Pleistocene climate, via carbon cycle feedbacks, deep-sea carbon storage and release, and radiative forcing [Anderson et al., 2009; Shakun et al., 2012]. During the early Pleistocene (2.6–1.0 Ma), obliquity set the dominant tempo of climate, and cold glacial intervals were shorter and less extreme [e.g., Lisiecki and Raymo, 2005; Huybers, 2007]. The presence of North American glacial tills indicates that early Pleistocene ice sheets at glacial maxima extended as far south as 39°N [Roy et al., 2004; Balco and Rovey, 2010].
In the early Pleistocene, both tropical [e.g., Herbert et al., 2010] and extratropical [e.g., Lawrence et al., 2009; Martínez-Garcia et al., 2010] sea-surface temperature (SST) records reveal long-term cooling in both glacial and interglacial intervals (i.e., 2 K cooler in tropics and 3–4 K cooler in high latitudes). At the same time, the glacial-interglacial amplitude of surface temperature increased from ~3 K to ~5 K from the early to late Pleistocene, as glacial temperatures cooled [Lawrence et al., 2009; Herbert et al., 2010; Martínez-Garcia et al., 2010]. The gradual cooling of the early Pleistocene glacials culminated in the mid-Pleistocene transition (MPT) when surface temperature and benthic δ18O records shifted from a dominant 41-kyr periodicity to ~100-kyr pacing, albeit without an obvious corresponding change in the periodicity of incoming solar radiation [Ruddiman et al., 1989; Clark et al., 2006]. Mechanisms put forward to explain this shift in climate response at the MPT include the exposure of crystalline bedrock and thus a more solid foundation for the Laurentide ice sheet [e.g. Clark and Pollard, 1998], changing ocean dynamics that provide increased precipitation available to build larger northern hemisphere ice sheets [Tziperman and Gildor, 2003; McClymont et al., 2008], or the consequence of long-term cooling and glacial CO2 drawdown as a result of iron fertilization and/or reduced ocean ventilation which sequestered more carbon in the deep ocean [Köhler and Bintanja, 2008; Hönisch et al., 2009; Chalk et al., 2017]. Filling in the early Pleistocene pCO2 data gaps with orbitally-resolved records will help to evaluate these hypotheses.
While efforts to extend the continuous ice-core pCO2 record to the early Pleistocene are being actively pursued [Fischer et al., 2013; Higgins et al., 2015; Witze, 2015; Bibby et al., 2016], such records are not yet available. However, marine sediments provide an opportunity to estimate pCO2 changes from earlier and warmer periods of Earth history. One leading method for estimating atmospheric pCO2 from marine sediments is via the boron isotope ratio (δ11B) recorded in the shells of planktic foraminifera. Shell δ11B is primarily controlled by seawater acidity (i.e., pH). CO2 exchanges between the atmosphere and the surface ocean depend on the partial pressure in each medium after Henry’s law. Higher atmospheric pCO2 results in more CO2 dissolved in seawater and thereby lowers surface ocean pH. The pH dependency of the relative abundance and isotopic signature of the two main species of boron in seawater (borate ion and boric acid) permits the δ11B value of marine carbonates to reflect such changes in ocean pH (see Methods). Several studies have validated this proxy relative to ice core pCO2 in the late Pleistocene [Hönisch and Hemming, 2005; Henehan et al., 2013; Chalk et al., 2017] and presented estimates of pCO2 throughout portions of the Pliocene and Pleistocene [Hönisch et al., 2009; Bartoli et al., 2011; Henehan et al., 2013; Martínez-Botí et al., 2015a; Chalk et al., 2017]. Here we present estimates of pCO2 based on new δ11B measurements in planktic foraminifera shells within a time window spanning Marine Isotope Stages (MIS) 44–52 (1.38–1.54 Ma).
Published estimates of early Pleistocene pCO2 are as high as 410 (±50) μatm as recorded at ODP Site 999 at 2.37 Ma [Martínez-Botí et al., 2015a] and as low as 167 (±19) μatm at 0.65 Ma [Hönisch et al., 2009]. Bartoli et al. [2011] observed that atmospheric pCO2 decreased in a step-wise fashion at ~ 2.7 Ma, although that particular study did not measure a temperature proxy alongside each of the boron isotope analyses, and used modeled estimates of the carbonate ion concentration, [CO32-], as the second parameter of the carbonate system to translate pH into reconstructed pCO2. Here we present new high-resolution data from ODP 668B in the eastern tropical Atlantic, revise the dataset of Bartoli et al. [2011] with new temperature and geochemical constraints, and compile these records in a consistent manner with other published datasets of early Pleistocene pCO2. By minimizing the differences in the way boundary conditions are treated among these records, we are able to compare the combined δ11B and pCO2 records and to describe coherent features of the pCO2 decline from the late Pliocene through the Pleistocene.
2. Materials and Methods
The primary materials for this study come from ODP Site 668B in the eastern tropical Atlantic (4.77°N, 20.93°W, water depth 2693 m, average sedimentation rate 1.5 cm/kyr, Figure 1). The modern surface ocean at this location is near equilibrium with the atmosphere with respect to CO2 [Takahashi et al., 2009], and previously published late Pleistocene boron isotope data from this site show good agreement with ice core pCO2 over the past 0.8 Ma [Hönisch and Hemming, 2005; Hönisch et al., 2009], making this a promising location for extending the reconstruction of paleo-pCO2. Sediment samples were taken from core 668B every 5 cm (3.3 kyr) and were selected to include glacial and interglacial extrema in planktic δ18O [Hönisch et al., 2009]. Samples were washed and picked for the planktic foraminiferal species Globigerinoides ruber (300–355 μm size fraction) and Trilobatus (formerly Globigerinoides) sacculifer (>500 μm size fraction), which both live in the surface mixed layer [Spero et al., 2003].
Figure 1.
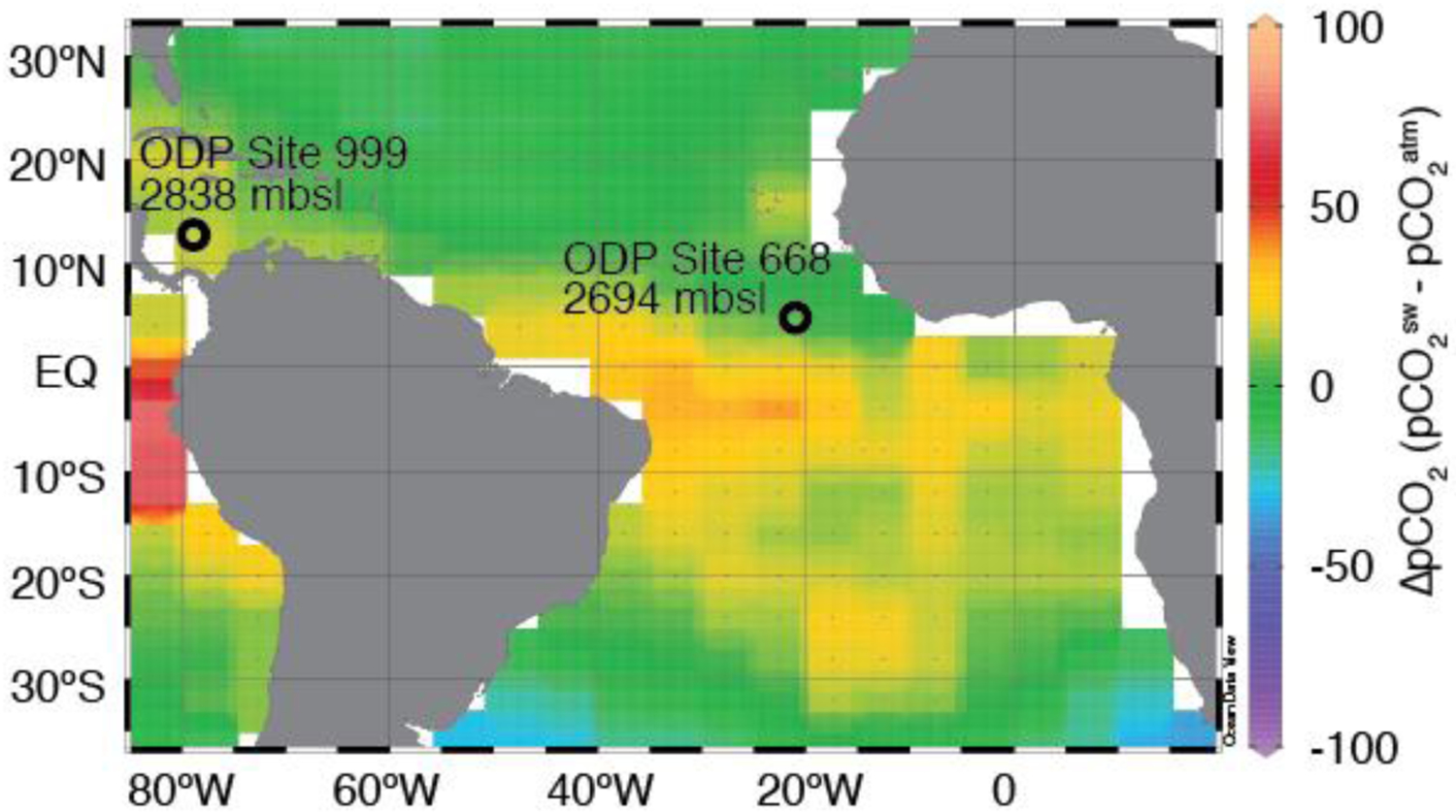
New geochemical data presented are from eastern equatorial Atlantic site ODP 668B and from Caribbean site ODP 999A. Background colors are modern mean annual ΔpCO2 (seawater pCO2 – atmosphere pCO2) [Takahashi et al., 2009] and are plotted using Ocean Data View 4 [Schlitzer, 2017]. Today surface water pCO2 in these locations is near equilibrium with the atmosphere.
2.1. δ11B-based pCO2 estimates
Tests of T. sacculifer from the >500 μm size fraction have been validated to reflect surface ocean conditions for pH and pCO2 estimates [Hönisch and Hemming, 2004; 2005; Hönisch et al., 2009]. Although partial dissolution has the potential to lower foraminiferal δ11B in corrosive bottom water, this effect is more pronounced in smaller foraminiferal test size classes [Hönisch and Hemming, 2004; Edgar et al., 2015]. The samples in this study come from the largest size fraction and from relatively shallow (2693 modern m depth) waters that are oversaturated with respect to calcite (Ώcalcite = 1.5, Δ[CO32-] ~35 μmol/kg). This depth is well above the regional lysocline and partial dissolution is unlikely [Regenberg et al., 2006]. For each δ11B sample, ~30–50 T. sacculifer shells were picked, gently crushed and cleaned by repeated sonication in MilliQ water and methanol, oxidation in a buffered H2O2 solution to remove organic material, and a final weak acid leach [Barker et al., 2003]. Cleaned material was rinsed in MilliQ water, dried to determine the calcite mass after cleaning, and finally dissolved in 2N hydrochloric acid just before analysis. All δ11B samples were measured at the Lamont-Doherty Earth Observatory using a TRITON thermal ionization mass spectrometer in negative mode (N-TIMS). Aliquots of the sample solution containing ≥1 ng boron were loaded onto outgassed zone-refined rhenium filaments, along with 1 μl of boron-free seawater to enhance ionization (see Hönisch et al. [2009] for details). Although analysis of boron isotopes in marine carbonates by the N-TIMS method yields ~1‰ higher δ11B values compared to the alternative MC-ICP-MS technique, the relative variability between different samples is similar between the two methods and yield equivalent pH and pCO2 results when technique-specific δ11Bforaminifera vs. δ11Bborate calibrations are applied [Foster et al., 2013; Farmer et al., 2016]. To minimize analytical uncertainty, each sample analysis was replicated 3–10 (average of 7) times. Of those replicates, individual analyses were rejected if excessive fractionation (δ11B >1‰) occurred over the 40 minutes of data acquisition; 14% of analyses were rejected on this basis. The majority of samples yielded 5 or more replicates that met the acceptance criteria. Only one sample, at 1.436 Ma, could not be replicated due to low abundance of T. sacculifer shells. Uncertainty in the δ11B measurement was determined as the larger of either: (1) the standard error in valid replicate analyses (i.e., 2σ = 2*standard deviation / √N, where N is the number of accepted replicates) or (2) the 2σ uncertainty of an equal number of repeat measurements of an in-house standard of NIST 951 precipitated in CaCO3 matrix (vaterite). Average 2σ uncertainty of all δ11B measurements is ±0.26‰.
The relative abundance of the two aqueous species of boron in seawater, borate ion (B(OH)4−) and boric acid (B(OH)3), is pH dependent [Dickson, 1990; Hemming and Hanson, 1992]. The isotopic fractionation between 11B (natural abundance ~80%) and 10B (~20%) generates a constant δ11B offset between these two aqueous boron species, such that δ11B of borate increases with the relative abundance of borate at higher pH. Since the borate ion is the primary species incorporated into foraminiferal calcite [e.g., Hemming and Hanson, 1992; Branson et al., 2015], in-situ seawater pH can be estimated from the δ11B of foraminiferal calcite. The boron isotopic composition of seawater (δ11Bsw) must also be known; in the modern ocean δ11Bsw is 39.61 (±0.04)‰ [Foster et al., 2010]. While this value likely increased over the Cenozoic through shifts in weathering and/or volcanic emissions [Lemarchand et al., 2000], attempts to reconstruct δ11Bsw over the past 5 million years have yielded disparate results. The combined evidence, whether from benthic foraminiferal δ11B alongside modeled ocean pH [Raitzsch and Hönisch, 2013] or paired δ11B measurements in the water column coupled with assumptions about water column pH gradient [Pearson and Palmer, 2000; Greenop et al., 2017], shows no clear indication of a trend in δ11Bsw over the past 5 Ma (Figure S1). As the residence time of boron in seawater is long (11–17 My), the maximum rate of change over this time period is likely <0.1‰ per million years [Lemarchand et al., 2000]; hence, we apply the modern δ11Bsw value (39.61‰) to all Plio-Pleistocene samples and increase the uncertainty of this assumed value at a constant rate of 0.1‰/Myr (Figure S1). Ocean pH is then determined via:
| (Eq. 1) |
where pK*B is the dissociation constant for boric acid at in situ temperature, salinity and pressure [Dickson, 1990; Millero, 1995], and the aqueous boron isotope fractionation factor is 11–10KB= 1.0272 (±0.0006) [Klochko et al., 2006]. Application of the boron isotope proxy also requires an understanding of any physiological or vital effects on the pH of the foraminiferal microenvironment, which have been documented for a variety of species. Here we use a calibration specifically established for T. sacculifer, which includes culture and core top sediment samples of T. sacculifer measured via N-TIMS at LDEO and Stony Brook [Sanyal et al., 2001; Hönisch and Hemming, 2004; 2005; Hönisch et al., 2009]. Values measured at Stony Brook have been cross-calibrated with N-TIMS [Hönisch et al., 2009] and a constant offset of −1.1‰ has been applied to all data measured in Stony Brook to shift them onto data measured on the N-TIMS at LDEO. A linear York fit [York et al., 2004] calculated for laboratory cultured specimens grown across a range of pH values includes uncertainties in the δ11Bcalcite measurement and the δ11 Bborate calculation for experimental seawater. This regression is then adjusted by a constant intercept offset of −0.84‰ so as to pass through the average of core top T. sacculifer δ11Bcalcite data and the corresponding preindustrial δ11Bborate (Figure 2 and Table S1). The offset accounts for the difference between cultured and sedimentary specimens, which is likely due to the gametogenic calcite crust that T. sacculifer secretes at greater water depths in the ocean [Bé, 1980]. The resulting calibration for T. sacculifer in the >500 μm size fraction (measured on N-TIMS), including 2σ uncertainty, is:
| (Eq. 2) |
Figure 2.

Calibration of δ11B of T. sacculifer as measured by N-TIMS versus δ11B of borate in seawater (data in Table S1). Culture data [Sanyal et al., 2001 and this study] define the slope of the relationship. Core top δ11B data are offset from the culture relationship, where the offset can be explained by secretion of gametogenic calcite at greater water depth. This offset (0.84‰) is subtracted from the intercept of the culture regression to define the sedimentary calibration of δ11BT. sacculifer calcite to δ11B borate. Linear regression is a York fit [York et al., 2004, Matlab script York_fit.m] which incorporates x and y uncertainty for each datum; slope and intercept uncertainties are 2σ.
In comparison, the published calibration for T. sacculifer using the MC-ICP-MS technique [Martínez-Botí et al., 2015b] is:
| (Eq. 3) |
While the slope of the MC-ICP-MS δ11Bborate calibration is steeper (0.83 vs. 0.73), the additional culture data points in our N-TIMS calibration (5 instead of 3) allow for a more precise calibration. The primary difference between these technique-specific calibrations is the intercept value, which is the most important factor in aligning pH estimates from N-TIMS and MC-ICP-MS δ11B analyses [Foster et al., 2013].
2.2. Temperature, salinity, and pressure estimates
Here we present new Mg/Ca-based estimates of SST using G. ruber from Site 668B and T. sacculifer from Site 999A. Due to the limited availability of large T. sacculifer tests from Site 668B, tests of G. ruber (sensu stricto, white) were used to estimate surface ocean temperature for this site. Globigerinoides ruber shares the near-surface ocean habitat of large T. sacculifer specimens [Ravelo and Fairbanks, 1992; Spero et al., 2003; Farmer et al., 2007]. For Site 668B, 40–60 G. ruber tests were picked from the 300–355 μm size fraction. For Site 999A, 40 shells of T. sacculifer were picked from the 425–500 μm size fraction, the same species and size fraction of planktic foraminifera as the original δ11B data [Bartoli et al., 2011]. Shells were then gently broken and cleaned following established protocol including both the oxidative and reductive steps [Boyle and Keigwin, 1985; Mashiotta et al., 1999]. Inclusion of the reductive step is generally preferred for Mg/Ca analysis as it is more effective at removing high-Mg contaminants such as authigenic metals precipitated after deposition in the sediment, even though it has been shown to lower Mg/Ca values beyond their original value [Martin and Lea, 2002; Weldeab et al., 2006]. Because the Mg/Ca-temperature calibration we use [Anand et al., 2003] is based on sediment trap samples and therefore did not apply reductive cleaning, we account for reductive cleaning bias by adding 10% to the measured Mg/Ca ratio of our samples [Martin and Lea, 2002]. Mg/Ca ratios were measured via ICPMS (iCAP-Q) at the Lamont-Doherty Earth Observatory. The 2σ standard error for repeated measurements of internal foraminifer reference standards is ±2.2%, or ~±0.08 mmol/mol. This SST calibration carries a larger uncertainty (±2.4 K, 2σ) than that of the Mg/Ca measurement uncertainty (~±0.24 K) and we adopt the larger calibration uncertainty for seawater temperature estimates. As our samples are derived from a time period when the Mg/Ca value of seawater (Mg/Casw) was likely lower than the modern value of 5.2 mmol/mol, a correction is needed for this change in seawater constituents [Delaney et al., 1985; Fantle and DePaolo, 2005; 2006; Medina-Elizalde et al., 2008; O’Brien et al., 2014; Evans et al., 2016]. To facilitate this correction, Mg/Casw values are taken from reported pore fluid chemistry and numerical modeling [Figure S7, Fantle and DePaolo, 2006]. This Mg/Casw dataset is within the uncertainty of Mg/Casw values estimated from fluid inclusions in marine evaporites, the most recent of which is Messinian in age (5–6 Ma, Mg/Casw ~ 3.6 mmol/mol) [Horita et al., 2002; Brennan et al., 2013]. If data from marine evaporite fluid inclusions were considered alone, and Mg/Casw values are linearly extrapolated from 5.5 Ma to present, the resulting SST would be ~0.7 K lower and calculated pCO2 would be ~5 μatm higher. The SST calibration we use includes the power-law modification suggested by Evans and Müller [2012] and the T. sacculifer exponential H-value of 0.41 [Delaney et al., 1985; Evans and Müller, 2012]:
| (Eq. 4) |
where (Mg/Ca)tsw is the Mg/Ca ratio of seawater at time t. This approach is commonly used to reconstruct temperature in this time period [e.g., Martínez-Botí et al., 2015a; Chalk et al., 2017]. The correction for dissolution with depth [Dekens et al., 2002] is omitted for all records in this study, as all study sites are well above the regional tropical Atlantic lysocline (4200 m water depth), and bottom water is saturated with respect to calcium carbonate [Key et al., 2004; Regenberg et al., 2006]. Hönisch et al. [2013] have demonstrated that adjusting Mg/Ca data from sediments above the lysocline effectively overestimates actual calcification temperatures.
Pleistocene salinity was calculated as a function of relative mean sea level (RMSL) as determined via a numerical ice-ocean model [Bintanja and van de Wal, 2008]. The change in local salinity is approximately proportional to the change in global average salinity due to glacial-interglacial sea level change. The equation used is
| (Eq. 5) |
where 35.8‰ is the modern salinity of the mixed layer (50 m water depth) at Site 668B [Zweng et al., 2013], 3800 m is the average depth of the ocean and 34.8‰ is the average salinity of the modern ocean. These estimates agree (within uncertainty) with local salinity estimates derived from using Mg/Ca-based SST and calcite δ18O to estimate the δ18O of seawater (δ18Osw, Figure 3) [Bemis et al., 1998; Legrande and Schmidt, 2006].
Figure 3.
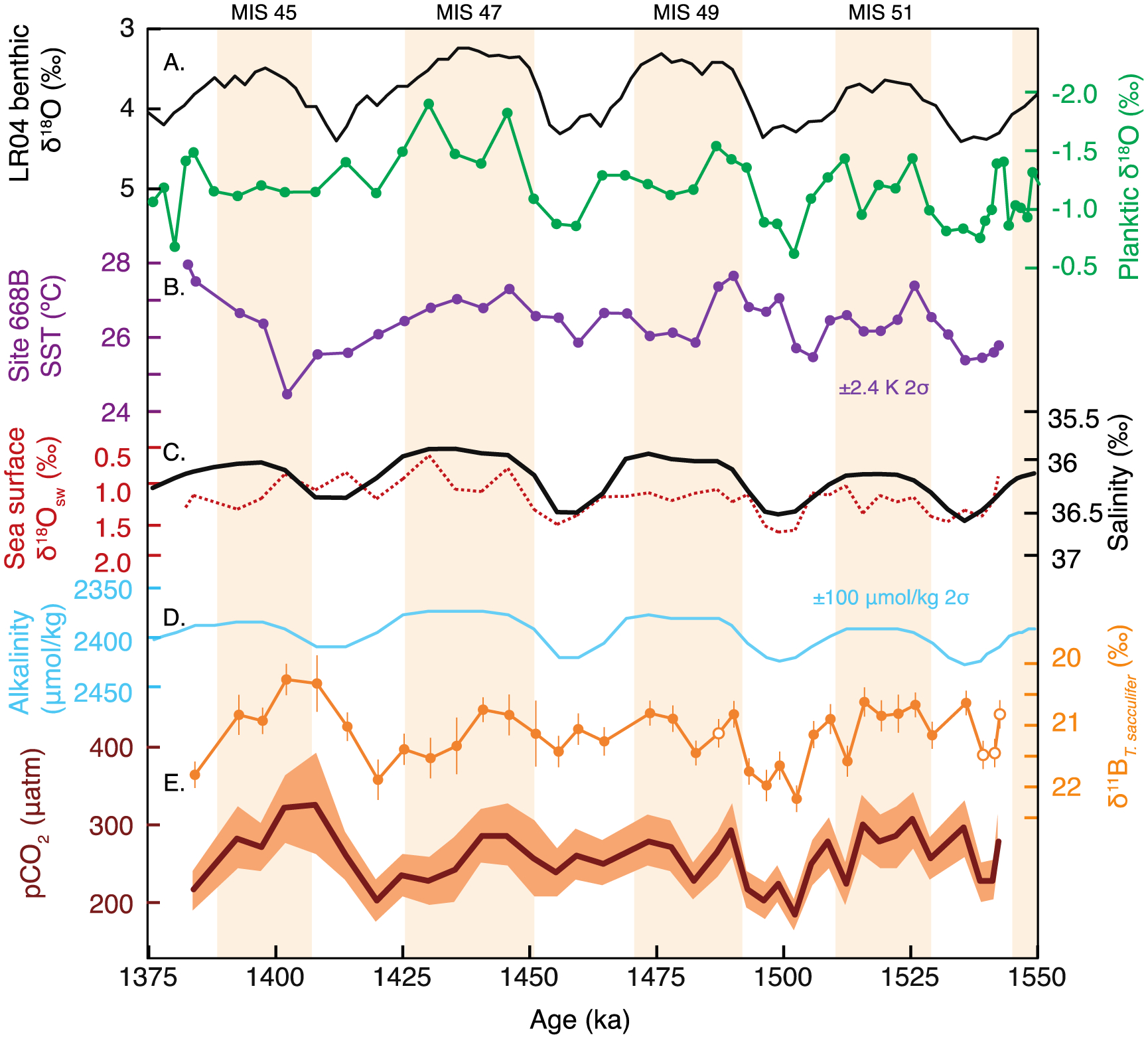
Site 668B orbital-scale records of the early Pleistocene from planktic foraminifera. (A) Site 668B planktic δ18O data [green, Hönisch et al., 2009] reflect local temperature and δ18Osw. (B) SST based on planktic Mg/Ca (purple, uncertainty is ±2.4 K (2σ)). (C) Calculated δ18Osw from planktic δ18O and Mg/Ca-based SST (red) and compared with salinity modeled from global sea level [black, Bintanja and van de Wal, 2008]. (D) Alkalinity based on local modern relationship with salinity. (E) Planktic δ11B data contain minima and maxima in line with glacial and interglacial periods; uncertainty is 2σ and open circles are from Hönisch et al. [2009]. Surface ocean pCO2 is calculated using pH (as estimated from δ11B, SST, and salinity) and estimated alkalinity; the uncertainty band reflects the propagated 2σ uncertainty, the largest source of which is contributed by the analytical uncertainty of δ11Bcalcite.
In situ seawater pressure is also required and is a function of the foraminiferal calcification depth. Here we assume a calcification depth for both G. ruber and T. sacculifer of 50 m [Schiebel and Hemleben, 2005; Farmer et al., 2007] and a corresponding seawater pressure of 50 decibars. The pressure effect on equilibrium constants is small; an uncertainty of ±50 m water depth results in an uncertainty in calculated pH of ±0.002 and pCO2 of ±1 μatm.
2.3. Alkalinity estimates
In order to calculate the partial pressure of CO2 in seawater, a second variable of the ocean carbonate system is required [e.g., Zeebe and Wolf-Gladrow, 2001]. Here, alkalinity is estimated from the modern local alkalinity-to-salinity relationship following the ‘constant alkalinity’ scenario of previous studies, which assumes that total alkalinity in the ocean remained constant in the past [Hönisch and Hemming, 2005; Hönisch et al., 2009]. The local alkalinity-to-salinity relationship was developed using WOCE and GLODAP databases [Schlitzer, 2000; Key et al., 2004] covering the area 0–10°N and 10–20°W [Hönisch and Hemming, 2005; Hönisch et al., 2009].
| (Eq. 6) |
Over long timescales (tens to hundreds of thousands of years) the local alkalinity-to-salinity relationship could deviate from the modern relationship as a function of the Canadian Shield silicate weathering contribution to global weathering rates, where increased weathering would have added alkalinity to the ocean [Clark et al., 2006]. Even though the relative contribution of such differential weathering is weakly constrained, the absolute impact on paleo-alkalinity is relatively small. Models show that varying the contribution of Canadian Shield weathering to the total ocean alkalinity budget of between 0 and 8% (Figure S2) only change the total ocean alkalinity in the Pleistocene by ~50 μmol/kg [Clark et al., 2006]. To account for the potential effect of differential weathering on the local alkalinity-to-salinity ratio for past time periods, we include an alkalinity uncertainty of ±100 μmol/kg for the past 1.8 Ma.
2.4. Calculation of pCO2
Aqueous CO2 (differentiated as PCO2) values are calculated from estimates of pH (total scale), alkalinity, salinity, temperature, and pressure using CO2SYS [version 2.3, Pierrot et al., 2006]. Within this program, we selected the recommended default values of pK1 and pK2 from Lueker et al. [2000], KHSO4 from Dickson [1990], KHF from Perez and Fraga [1987], and [B]T from Lee et al. [2010]. As described above, aqueous PCO2 at site 668B is in equilibrium with the atmosphere in the modern ocean [Takahashi et al., 2009] and comparison of boron isotope estimates with ice core records suggests it was also in equilibrium over the past 800 kyr [Hönisch and Hemming, 2005; Hönisch et al., 2009]. We therefore interpret our aqueous paleo-PCO2 estimates from Site 668B as equivalent to atmospheric paleo-pCO2. In order to directly compare with δ11B records from Site 999A, we translate δ11B-based aqueous PCO2 records from Site 999 to atmospheric pCO2 by subtracting the annual average pre-industrial disequilibrium value (21 μatm) as justified by Henehan et al. [2013] who accounted for the modern seasonal ΔpCO2 difference and a small correction for pre-industrial PCO2 [Gloor et al., 2003; Takahashi et al., 2009].
2.5. Uncertainty
Uncertainty in the pH estimate is a function of the propagated 2σ uncertainties in the δ11B measurement on δ11Bborate, the uncertainty in δ11 Bsw, and the effect of temperature and salinity uncertainties on the dissociation constants of boric and carbonic acid in seawater. We report uncertainty in pH as the root-mean-square error of the effect each parameter uncertainty has on pH: the δ11B measurement and δ11Bborate calibration (±0.03 pH units, ~80% coming from analytical uncertainty), the estimate of δ11Bsw (±0.01 pH units at 1.5 Ma), the temperature constraints (calibration uncertainty equates to ±0.02 pH units) and salinity (±0.01 pH units). Uncertainty is calculated on a sample-by-sample basis; average total pH uncertainty of the Site 668B data is ±0.043 (2σ). Uncertainty in the pCO2 calculation is likewise a function of uncertainty in the overall input parameters: the δ11B measurement and calibration (±23 μatm), alkalinity (±12 μatm), and the effects of temperature (±19 μatm) and salinity (±3 μatm). Uncertainty in PCO2 is similarly calculated on a sample-by-sample basis; average total PCO2 uncertainty is ±33 μatm (2σ) for the newly estimated PCO2 values at ~1.5 Ma.
2.6. Chronology
The age model for ODP Site 668B was initially constructed from microfossil occurrence data and estimates of the depths of geomagnetic reversals [Shipboard Scientific Party, 1988]. This initial chronology was later tuned through the alignment of glacial-interglacial cycles in the high-resolution planktic (G. ruber) δ18O values of Site 668B [Bird and Cali, 1998; 2002; Hönisch et al., 2009] with the well-dated planktic δ18O values of ODP Site 677 [Shackleton et al., 1990] and the LR04 benthic δ18O stack [Lisiecki and Raymo, 2005]. In order to resolve planktic δ18O mismatches between Sites 668B and 677 during MIS 48–52 (1.45–1.53 ka), we used AnalySeries software [Paillard et al., 1996] to add tie points which refine the planktic δ18O alignment. All ages younger than 1.3 Ma (19 m core depth) are unchanged from the previously published age model [Hönisch et al., 2009]. The revised age model for the period prior to 1.3 Ma (core depths 19–26 m) improves the planktic δ18O correlation coefficient (Pearson R2) between Sites 677 and 668B from ~0.31 to ~0.40 and reconstructed ages are up to 15% younger than previously estimated (Figure S3). The updated age model also conforms to the original ODP geomagnetic constraints, which placed the top of the Olduvai reversal (1.78 Ma) at 2.725 m core depth [Shipboard Scientific Party, 1988], just beyond the oldest available δ18O values from Site 668B (Figure S3). Unfortunately, the age uncertainty of the underlying benthic δ18O stack is ±6 kyr in the early Pleistocene [Lisiecki and Raymo, 2005]; this age uncertainty prevents the determination of leads or lags that are shorter than this confidence interval.
2.7. Comparison with other δ11B-based PCO2 records
For comparison between this new record and other previously published Pleistocene δ11B-based PCO2 records, we compile PCO2 estimates from δ11B data from Site 668B and Site 999A in the tropical Atlantic. These two sites present a good comparison as ocean-atmosphere disequilibrium is small at both locations. In order to rule out methodological differences, we calculated PCO2 from each published record using equivalent input parameters and technique-specific calibrations. The original Site 668B δ11BT. sacculifer data [Hönisch et al., 2009] are used to recalculate pH and PCO2 using the above methods (Section 2.1–2.6). The δ11B calibration for this dataset is updated (Eq. 2) and δ11Bsw is assumed to be 39.61‰ throughout. Temperature is calculated via the same Anand et al. [2003] calibration, after correcting for changes in seawater Mg/Ca (Eq. 4).
The δ11BT. sacculifer data of ODP 999A [Bartoli et al., 2011] were also revised using similar methods described above. To do so, we first generated new Mg/Ca data from the same or adjacent samples used for δ11B analysis (see section 2.2). In the original study, SST was interpolated from T. sacculifer Mg/Ca values [Groeneveld, 2005] with a different temporal resolution than the δ11B samples; our new temperature data reduce the interpolation of temperature estimates. Furthermore, the original SST record used the depth-based dissolution correction of Dekens et al. [2002], along with an outdated modern Mg/Casw value of 4.96 mmol/mol. Due to omission of the depth correction, the SST estimated for this record is on average 1.8 K cooler than SST in the original publication (Figure S4) which places interglacial SST in agreement with modern SST at this location, whether taken from an annual average of modern direct measurements (27.7 °C) [Locarnini et al., 2013] or estimated from core top Mg/Ca values (28.2 °C) [Henehan et al., 2013]. Site 999A (water depth 2839 m) is situated well above the regional lysocline (4200 m) and T. sacculifer shells are not subject to preferential high-Mg calcite dissolution at this water depth [Regenberg et al., 2006]. Taken together, the omission of the depth correction thus appears well justified.
Additionally, Bartoli et al. [2011] used modeled surface ocean [CO32-] estimates as the second parameter of the carbonate system and paired them with their δ11B-pH estimates and to calculate PCO2. Because pH and [CO32−] are closely related in carbonate equilibria, pairing them in carbon system calculations produces large variations in the calculated parameters. For instance, the pH and [CO32-] estimates of Bartoli et al. [2011] combine to yield glacial-interglacial total alkalinity variations of up ~800 μmol/kg (Figure S5). Such a large change in ocean alkalinity is unlikely over these timescales as alkalinity is stabilized by the distribution of calcium carbonate accumulation and dissolution (carbonate compensation) in the deep sea on scales of thousands of years [Broecker, 1971; Broecker and Peng, 1987; Boyle, 1988]. Extrapolating from LGM estimates of alkalinity and lysocline depth, an 800 μmol/kg decrease in alkalinity would correspond to a >2 km shallower lysocline, which is difficult to reconcile with observations of relatively small changes (<0.5 km) in lysocline depth over the Pleistocene [Farrell and Prell, 1991]. Geochemical models also suggest smaller alkalinity adjustments, e.g. <400 μmol/kg over the past 10 million years [Figures 3d and 4d of Tyrrell and Zeebe, 2004] and <250 μmol/kg over the past 2.6 Ma [Clark et al., 2006]. We therefore revise the PCO2 record of Bartoli et al. [2011] by using estimates of total alkalinity as the second carbonate system parameter; pH, salinity, and temperature are calculated in the same manner as for Site 668B (Sections 2.2, 2.4). Here alkalinity is calculated via the same regional relationship with salinity that was previously established for Site 999A [Foster, 2008].
| (Eq. 7) |
Figure 4.

Boron-based pCO2 record compared with orbital-scale records of the early Pleistocene climate. (A) LR04 benthic oxygen isotope stack [black, Lisiecki and Raymo, 2005] reflects high-latitude temperature and ice volume changes on the dominant ~41-kyr ice age cycle during this interval. (B) Compilations of sea surface temperature records used to estimate change in global average surface temperature (dashed line from Martínez-Botí et al. [2015a] and solid line from Snyder [Snyder, 2016]). (C) Benthic carbon isotope gradients between Pacific and North Atlantic basins (solid light green line, Δδ13CPacific-(North Atlantic)/2) [Lisiecki, 2010] and between intermediate and deep water in the south Atlantic (dashed line) [Hodell and Venz-Curtis, 2006]. (D) Marine accumulation rate of Fe from ODP Site 1090 (solid line) [Martínez-Garcia et al., 2011]. (E) Calculated δ11B-based surface ocean pCO2 from Figure 3. (F, G) Summer insolation at 65°N (blue) and 65°S (red) and orbital parameters [Laskar et al., 2004].
To account for the potential impact of differential weathering on the local alkalinity-tosalinity ratio for these older samples, we increase the alkalinity uncertainty to ±175 μmol/kg for all samples older than 1.8 Ma. Older time periods (> ~2 Ma are less well constrained and so larger uncertainty estimates are used. In contrast to Chalk et al. [2017] who used constant alkalinity and therefore assumed the larger 175 μmol/kg uncertainty throughout, our uncertainty estimates are superimposed on the alkalinity changes based on sea level, and are therefore even more conservative than those use by Chalk et al. [2017].
Published boron isotope data are also available from G. ruber from Site 999A [Foster, 2008; Seki et al., 2010; Henehan et al., 2013; Martínez-Botí et al., 2015a; Chalk et al., 2017]. Boron-based PCO2 reconstructions from both T. sacculifer and G. ruber have been extensively calibrated and both species provide convincing PCO2 estimates compared to ice core records. Here we apply minor revisions to the published δ11BG. ruber-based PCO2 calculations (namely, using a common SST calibration and assuming a constant δ11Bsw over the past 5 Ma) with the goal of ensuring compatible comparisons among the results. All the published δ11BG. ruber values are translated to δ11Bborate using the established species- and instrument-specific calibration of Henehan et al. [2013]:
| (Eq. 8) |
In each case G. ruber specimens are from the 300–355 μm size fraction so any additional offset for size fraction is not required [Henehan et al., 2013].
The other parameters needed (temperature, salinity, δ11Bsw, and alkalinity) are estimated consistently with Sections 2.1–2.6. In situ temperature is determined from Mg/Ca values as described by equation 4, except for the record of Seki et al. [2010] in which the alkenone unsaturation index is used for temperature reconstruction as in the original publication; alkenone-based temperatures are indistinguishable from Mg/Ca-based SST at this location. In all cases salinity is assessed from the modeled sea level estimates of Bintanja and van de Wal [2008], similar to equation 5, with modern salinity of the mixed layer (50 m water depth) at Site 999A of 36.2‰.
| (Eq. 9) |
For samples older than 3 Ma, the limit of the RMSL data set [Bintanja and van de Wal, 2008], the oldest value (St=3.0 Ma = 36.2‰) is used. An alternate method for calculating salinity used by previous publications is via the δ18O and Mg/Ca values of planktic calcite to find local δ18Osw and then translate δ18Osw to a salinity estimate. In practice, this method would result in average salinity ~0.4 lower than estimated above (Eq. 9), pH ~0.0002 lower, and PCO2 ~1 μatm higher. Equations for this alternative salinity estimate can be found in the supplemental material.
3. Results
The ODP Site 668B boron-based PCO2 record for the interval 1.38–1.54 Ma is presented in Figure 3. Within this time period δ11BT. sacculifer values range between a maximum of 22.19 (±0.21)‰ (1.502 Ma) and a minimum of 20.26 (±0.25)‰ (1.402 Ma) with an average δ11B value of 21.14 (±0.16)‰. Calculated PCO2 ranges between 183 (±21) μatm (at 1.502 Ma) and 327 (±61) μatm (at 1.408 Ma) with an average PCO2 value of 258 (±29) μatm. The average amplitude of minimum glacial to maximum interglacial PCO2 is 92 (±34) μatm (average of 4 interglacial maxima (304 (±46) μatm) minus average of 5 glacial minima (212 (±23) μatm); these uncertainties are based on the average uncertainty of the data points in question)).
The measured δ11BT. sacculifer value is the dominating parameter for the calculated PCO2 record (Figure S6). To evaluate the respective effects of temperature, alkalinity, and salinity on the calculated PCO2, we performed a simple sensitivity study in which δ11B was held constant at the average value (21.15‰) while all other factors (temperature, alkalinity, salinity) varied as evaluated previously. When δ11BT. sacculifer is held constant, the secondary driver of the PCO2 calculation is local SST, although even the full range of local SST (3.1 K) can only account for a maximum glacial-interglacial PCO2 range of 24 μatm (Figure S6). Thus, the calculation of paleo-PCO2 is driven primarily by changes in surface ocean pH as reflected in the raw δ11B data; temperature, salinity, and alkalinity exert only a minor additional influence on the pH and PCO2 estimates. This is in contrast to the potentially dominant effects other environmental parameters (i.e. [B(OH)4−], [HCO3−]) can have on B/Ca-based estimates of carbonate parameters [Tripati et al., 2011; Allen et al., 2012].
Published records of δ11B-based PCO2 were revised to be consistent with the given methodology above. Each parameter revision was tested individually to determine which parameter caused the greatest resulting PCO2 shift from the original publication. In each instance, T. sacculifer δ11B has been measured via N-TIMS and G. ruber δ11B via MC-ICP MS. For the longer (0–1.8 Ma) Site 668B T. sacculifer dataset, average PCO2 is +2 μatm higher than the published record [Hönisch et al., 2009]. This difference is composed of the assumption that δ11Bsw was 39.61‰ rather than 39.5‰ (+6 μatm), the updated δ11Bborate calibration (Eq. 2, −16 μatm), updated SST calibration (Eq. 4, +3 μatm), and alkalinity calculated via the ‘constant alkalinity scenario’ (Eq. 6, +14 μatm). For Site 999A (0–26 ka), average PCO2 is also +12 μatm higher than the published record [Henehan et al., 2013], primarily due to the revised salinity and alkalinity estimations (Eq. 7 and 9). For Site 999A (0–1.24 Ma), average PCO2 is +12 μatm higher than the published record [Chalk et al., 2017] also due to the revised salinity and alkalinity estimates. For the Site 999A study of Seki et al. [2010] (0–2.6 Ma), the revised average PCO2 is +26 μatm higher than the published record [Seki et al., 2010], due to the updated calibration for the boron isotope proxy in G. ruber [Henehan et al., 2013]. For the Plio-Pleistocene (2.3–3.3 Ma) Site 999A G. ruber dataset, average PCO2 is +12 μatm higher than the published record [Martínez-Botí et al., 2015b], primarily due to the revised assumption that seawater δ11Bsw was constant at 39.61‰ over the past 5 Ma (Figure S1), whereas the original publication assumed a lower δ11Bsw (~39.2‰) at the Plio-Pleistocene transition.
For the Plio-Pleistocene (1.97–4.6 Ma) Site 999A T. sacculifer dataset, average PCO2 is 41 μatm lower than the published record [Bartoli et al., 2011], primarily due to the revised SST record which omits the depth-based dissolution correction (Eq. 4, SST 1.8 K cooler, −15 μatm) and the application of the pre-industrial disequilibrium value at this site (−21 μatm). The choice of alkalinity, rather than [CO32-] as the second parameter of the carbonate system, reduces the glacial-interglacial PCO2 amplitude of this record by 25% compared to the original publication (Figure S5). This finding highlights the volatile nature of the pH and [CO32-] pair in constraining the carbonate system, i.e., a small uncertainty in [CO32-] translates into a large shift in PCO2 when paired with pH. The amplitude reduction is a result of both reducing the interglacial PCO2 estimates and increasing the glacial PCO2 estimates compared to the original record. All revisions were applied for consistency among calculations; the average PCO2 change in all cases is smaller than the average 2σ uncertainty reported in the original studies.
4. Discussion
Given that late Pleistocene pCO2 levels were closely correlated with both tropical SST [e.g., Lea, 2004; Herbert et al., 2010; Dyez and Ravelo, 2013] and high latitude surface temperatures [Petit et al., 1999; Jouzel et al., 2007], an equivalent relationship could be expected for early Pleistocene glacial-interglacial cycles. Here we add to the growing body of data evidence that a similar first-order relationship between global average temperature and pCO2 also existed in the early Pleistocene, specifically in the interval 1.38–1.54 Ma (Figure 4). During interglacial periods δ11B was lower (i.e., lower surface ocean pH and higher atmospheric pCO2) and during glacial periods δ11 B was higher (i.e., higher surface ocean pH and lower atmospheric pCO2), in line with similar findings for the late Pleistocene period [Hönisch and Hemming, 2005; Henehan et al., 2013; Chalk et al., 2017]. To verify that our data are directly related to variation in atmospheric pCO2, we need to evaluate a range of processes that could have biased our geochemical signals and interpretations.
First of all, ocean upwelling brings cooler and more CO2-rich water to the surface; if upwelling had increased during glacial intervals at this location, we would expect to observe a larger glacial-interglacial SST amplitude than other open-ocean sites; if upwelling had increased during interglacial intervals, we would expect a smaller SST amplitude. The local temperature amplitude we measure from Mg/Ca values (i.e., average of 4 interglacial SST maxima minus average of 5 glacial SST minima) is 1.4 (±0.3) K, equivalent to other early Pleistocene tropical SST records, whether derived from the south China Sea [Site 1146; 1.4 K, Herbert et al., 2010], the western Pacific [Site 806; 1.2 K, Medina-Elizalde and Lea, 2005; Site 871; 1.2 K, Dyez and Ravelo, 2014], the south Pacific [MD06–3018; 1.3K, Russon et al., 2010], the tropical Atlantic [Site 662; 1.6 K, Herbert et al., 2010], or the Arabian Sea [Site 722; 1.1 K, Herbert et al., 2010]. The similarity of the SST amplitude at Site 668B to other tropical records implies that glacial-interglacial upwelling changes at this site were not substantial and did not preferentially influence either glacial or interglacial δ11B values.
Secondly, shell diagenesis can occur after shells have been deposited on the ocean floor, potentially biasing our paleoenvironmental interpretations. However, in section 2 we already reasoned against potential dissolution at the sediment surface based on the shallow depth of the core tops and the much deeper Atlantic lysocline. Furthermore, the calcite saturation state of bottom water at this site is ΏCaCO3= 1.5 and bottom water Δ[CO32-] = 34.5, both of which are higher than any site previously used for δ11B-based pCO2 estimates and above the range suggested for the onset of partial dissolution [de Villiers 2005; Dai et al., 2016]. If diagenesis lowered δ11B values within the buried sediment, we would expect to also observe lower shell weights and higher planktic δ18O and δ13C values [e.g., Edgar et al., 2015]. We examined the correlations among these values and do not find any significant relationships to support the notion that diagenesis has occurred within the sediment column. Taken together with the expected range of glacial-interglacial SST presented above, we can reasonably infer that the δ11B-based PCO2 signal we measure is primary and has not been biased by incomplete preservation.
4.1. Early Pleistocene pCO2 varied with obliquity-related climate cycles
To examine the glacial-interglacial linkages between pCO2, temperature, and orbital climate parameters from the period 1.38–1.54 Ma, we created cross-plots of reconstructed pCO2 with physiochemical measurements, orbital parameters, and global stacks of temperature and benthic δ18O (Figure 5). In this analysis, only data that are clearly associated with the broad maxima or minima in temperature and marine isotope stage are plotted, as determined by visual comparison with both a benthic δ18O values [Lisiecki and Raymo, 2005] and a global stack of surface temperatures [Snyder, 2016]. Omitted are intermediate data that fall between these climate extrema and, due to age model uncertainty, cannot be easily associated with a particular stage; the MIS intervals and maximum/minimum pCO2 values are listed in Table S2.
Figure 5.
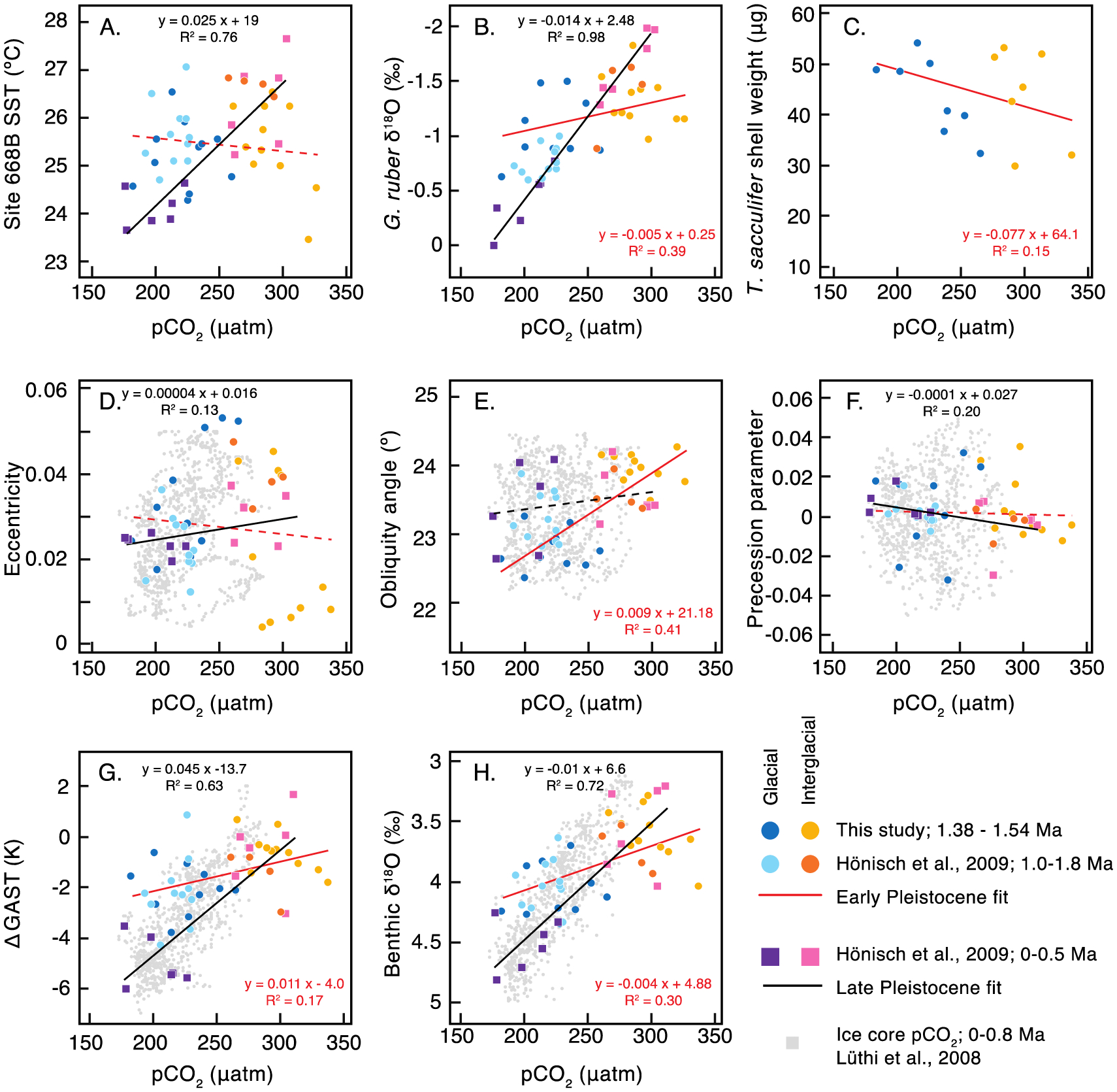
Cross plots of Site 668B peak MIS pCO2 with Mg/Ca-based SST (A), G. ruber δ18O (B), T. sacculifer shell weight (C), coeval orbital parameters (D-F) [Laskar et al., 2004], surface temperature (G) and benthic δ18O (H). Late Pleistocene ice core pCO2 is plotted for reference in gray [Bereiter et al., 2015], but not used for linear regressions. The linear fit of Early Pleistocene values (circles) is in red while the linear fit of late Pleistocene values (squares) is in black. Where R2 > 0.1, regression equations are plotted and the linear fit is a solid line. Late Pleistocene pCO2 is correlated with surface temperature and benthic δ18O, while early Pleistocene pCO2 is associated with obliquity forcing. Precession parameter is calculated as the standard definition of precession (i.e., where ϖ is the angle between perihelion and vernal equinox on the orbital plane, precession is e*sin(ϖ)).
With which orbital cycle is pCO2 most closely related in the early Pleistocene? Cross-plots show that in the late Pleistocene, periods of peak pCO2 are associated with orbital eccentricity, but in the early Pleistocene peak interglacial pCO2 is correlated with increased tilt (obliquity) in Earth’s rotation axis (Figure 5). The association of pCO2 and obliquity supports the notion that pCO2 was linked with the orbital insolation cycle and surface temperatures, even in the 41-kyr world before the mid-Pleistocene transition, but that the linkages took a different form, which resulted in climate that was in phase with obliquity. This finding is consistent with previously proposed hypotheses for maintaining ~41-kyr periodicity of glacial-interglacial change in the early Pleistocene, either through meridional temperature gradients or ice dynamics. In the early Pleistocene, the 41-kyr cycle could have been promoted by amplified equator-to-pole temperature gradients that carried additional moisture poleward to build ice sheets [Raymo and Nisancioglu, 2003] and/or by thinner, fast-spreading Laurentide ice sheets [Clark et al., 2006]. The glacial-interglacial temperature perturbations that drive glacial-interglacial climate would likely have been further amplified by obliquity-scale radiative pCO2 feedbacks.
4.2. Glacial pCO2 decreased through the Pleistocene
Site 668B δ11BT. sacculifer values indicate that early and late Pleistocene maximum interglacial pCO2 did not change across the MPT (average interglacial peak pCO2 at 1.2–1.8 Ma: 303 (±12) μatm, average interglacial peak pCO2 at 0–0.5 Ma: 299 (±16) μatm (two-tailed t-test of all peak interglacial pCO2 values, P>0.7). However, minimum glacial pCO2 values declined by ~25 μatm between the early and late Pleistocene (average minimum pCO2 at 1.2–1.8 Ma: 213 (±10) μatm, average minimum pCO2 at 0–0.5 Ma: 187 (±10) μatm (two-tailed t-test of glacial minimum values, P<0.05). These pCO2 estimates are congruent with proxy records of warmer glacial temperatures in the early Pleistocene than the late Pleistocene and support the initial observation that glacial pCO2 declined across the MPT [Hönisch et al., 2009; Chalk et al., 2017]. While the collapse of North Atlantic deep-water formation around 900 ka coincides with this decrease in glacial pCO2 [e.g., Pena and Goldstein, 2014], our atmospheric estimates do not allow us to determine the cause of the pCO2 drawdown. However, the covariation of ocean circulation and pCO2 shifts over the MPT to longer, colder glacial intervals highlights the close association of ocean dynamics, atmospheric circulation, and climate in the Pleistocene [e.g., Clark et al., 2006].
Glacial pCO2 was higher in the early Pleistocene than the late Pleistocene, just as glacial temperatures were also 2–2.2 K warmer in the period 1.2–1.8 Ma than in the period 0–0.5 Ma based on multiple compilations of temperature change between the early and late Pleistocene [Herbert et al., 2010; Martínez-Botí et al., 2015a; Snyder, 2016]. If average surface temperature is due to the radiative pCO2 forcing alone, an equilibrium response of global temperature to such forcing can be calculated. At its most basic, this calculation is commonly given in the form of S[CO2] = ΔT/ΔF[CO2] where ΔT (change in global average surface temperature) and ΔF[CO2] (change in radiative forcing due to pCO2) are millennial-scale averages and all other forcing mechanisms are regarded as internal feedbacks. Here the canonical radiative forcing due to CO2 is used, where ΔF[CO2] = 5.35 * ln(C/278 μatm) and C is atmospheric pCO2 [Myhre et al., 1998]. For the early Pleistocene we thus calculate an equilibrium temperature response to radiative CO2 forcing as S[CO2] = 0.75 (±0.5) °C/Wm−2. For the late Pleistocene, the parallel calculation yields S[CO2] = 1.75 (±0.6) °C/Wm−2 (Figure 6). To the first order, these relationships imply that climate sensitivity to radiative greenhouse gas forcing increased by a factor of ~2 between 1.2 and 0.5 Ma, largely driven by lower glacial-interglacial temperature amplitude in the early Pleistocene.
Figure 6.
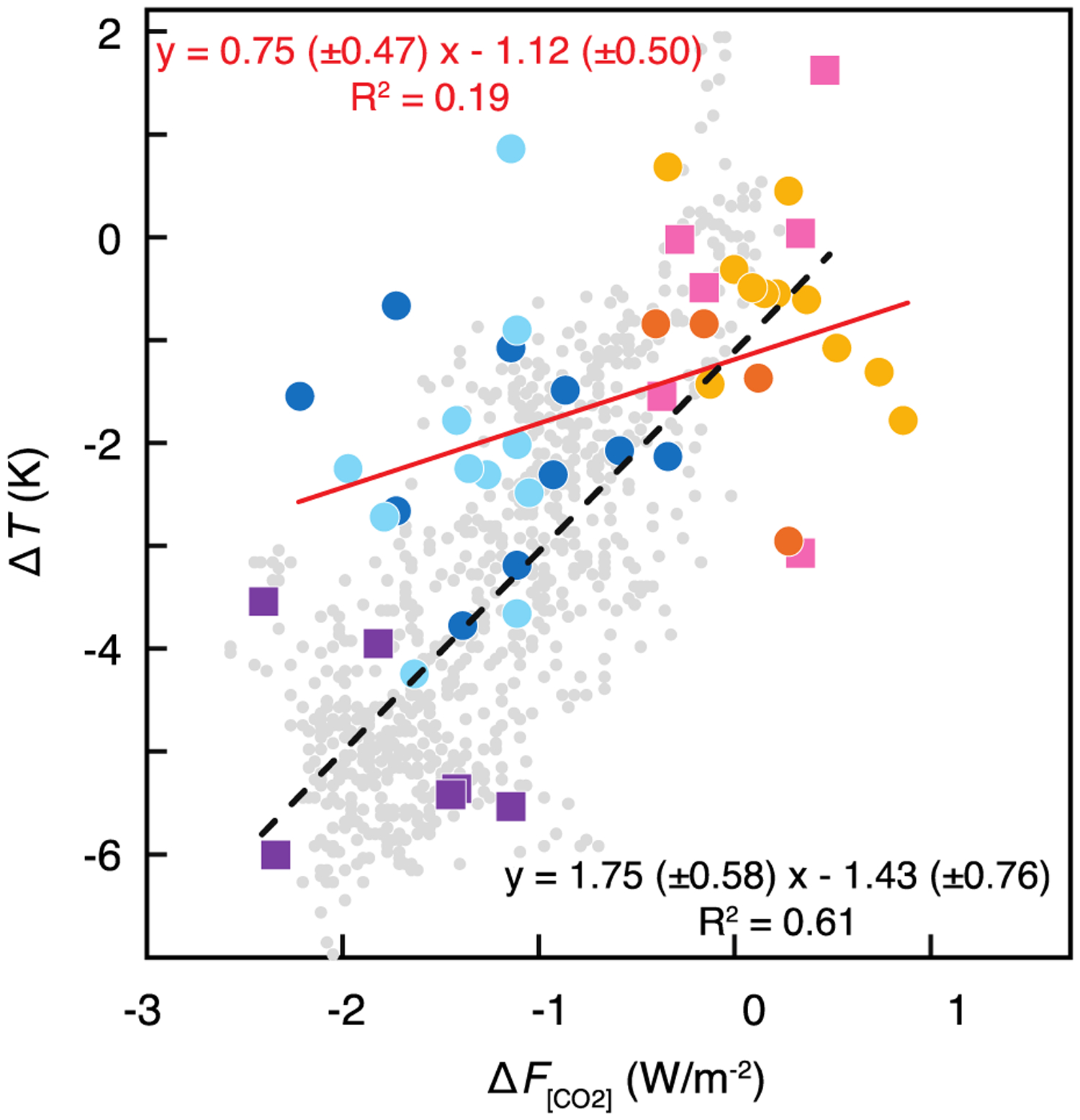
Cross plot of the change in global average surface temperature [ΔT; Snyder 2016] with the change in radiative pCO2 forcing (ΔF[CO2]; calculated after Myhre et al. [1998], relative to pre-industrial pCO2 of 278 ppm). Colors are the same as in Figure 5, with fit through early Pleistocene (1.0–1.8 Ma) in red and fit through late Pleistocene data (0–0.8 Ma) in black. Note that this framework considers the contributions of other feedbacks as internal to the Pleistocene climate system.
In reality, however, climate feedbacks such as ice sheets and ocean circulation also amplify the range of global average temperature [e.g., Köhler et al., 2015; von der Heydt et al., 2016]. Recent studies have pointed out that in order to project the climate effects of rising pCO2 levels in a warmer world with smaller ice sheets, climate sensitivity must account for the temperature response to each climate parameter separately (i.e., CO2, ice sheets, etc.) [e.g., Köhler et al., 2015; Schmidt et al., 2017]. The lower early Pleistocene climate sensitivity calculated above is a result of comparing between time periods in which, at minimum, ice dynamics have changed [Raymo et al., 2006; Shakun et al., 2016]. Though this dataset does not allow us to quantify changes in albedo and atmospheric circulation, increased glacial ice volume (and vertical growth of ice sheets) at the MPT is an important change in the earth system that could have amplified glacial-interglacial temperature variability. Increased ice volume cools global temperature through two pathways: by increasing ice sheet extent, which increased global albedo, and through taller ice sheets, which deflected cold northern hemisphere atmospheric jets southward and compressed the subtropical atmospheric bands [Manabe and Broccoli, 1985; Shinn and Barron, 1989; Clark et al., 1999]. Qualitatively, then, these additional ice sheet and ocean feedbacks, in conjunction with lower atmospheric pCO2, worked to cool glacial temperatures across the mid-Pleistocene transition.
4.3. Model comparison
We evaluate these reconstructions of early and late Pleistocene pCO2 with a simple numerical box model that allows for an initial exploration of Pleistocene climate sensitivity given the available surface temperature and pCO2 data. This model has three coupled components: ice sheets, temperature, and carbon dioxide levels. We force this model with atmospheric insolation over glacial cycles to explore the effect of Laurentide ice sheet changes for climate sensitivity. This model has been documented elsewhere [Schmidt et al., 2017]; briefly, this model includes coupling between ice sheet volume (L) and global average surface temperature (T) and between surface temperature and carbon dioxide levels (C) but does not incorporate 3D geography or topography. The model is initialized at the following mid-glacial values: T0 = 285 K, C0 = 230 μatm, L0 = −60 m (meters of sea level equivalent). The sensitivity of ice sheets to temperature (a) and the non-Planck feedback (λ) are tested via 20×20 experiments (varying a = [0,30] mSL K−1 and λ = [2.8,4.6] Wm−2 K−1). These parameters are consistent with the full range of late Pleistocene observations [Köhler et al., 2010].
We use this model to explore the qualitative impact of increasing ice volume on global climate at the MPT. Geochemical evidence and ice sheet models suggest that the Laurentide ice sheet of the early Pleistocene was thinner due to a “slippery” layer of weathered regolith over solid bedrock [Clark and Pollard, 1998; Clark et al., 2006], but that the spatial extent of land ice was similar to the late Pleistocene ice ages [Roy et al., 2004; Balco and Rovey, 2010]. Thus, the radiative forcing due to ice sheet albedo was roughly similar in the early and late Pleistocene, but the ice volume required (in equivalent meters of sea level fall) to acquire that forcing was smaller prior to ~1.0 Ma. We test the effect of radiative albedo forcing per ice sheet volume (μ) at 0.025 Wm−2 mSL−1 for the late Pleistocene such that late Pleistocene ice sheets cooled the global climate by 1 Wm−2 for each 40 meters of equivalent sea level fall, consistent with what is assumed for ice-albedo cooling at the LGM [Köhler et al., 2010]. If the early Pleistocene Laurentide ice sheet was comparably expansive as in the late Pleistocene, but thinner, it can be thought of as more ‘efficient’ at radiative albedo cooling per unit volume of ice. We investigate this idea by testing the parameter μ (defined above) for the early Pleistocene at double and triple the late Pleistocene value: at 0.05 and 0.075 Wm−2 mSL−1. This exercise is not meant to fully simulate the climate system, but to explore elemental system sensitivity to a realistic range of radiative CO2, insolation, and ice-sheet albedo forcing.
The results of this model suggest that if the efficiency of ice cooling (μ) were higher in the past, the amplitude of temperature variability and atmospheric CO2 variability would also have been higher. When μ is doubled (or tripled), which simulates the large, but relatively thinner early Pleistocene northern hemisphere ice sheets, temperature and pCO2 amplitude are ~40 (~80% for tripled μ) larger. However, we do not observe this phenomenon in the early Pleistocene geochemical datasets. Instead we observe lower temperature variability in the early Pleistocene, and a similar amplitude of atmospheric CO2 variability as in the late Pleistocene. These observations could be a consequence of only considering northern hemisphere insolation and the impact on Laurentide ice. Out-of-phase insolation in the southern hemisphere could have driven Antarctic ice in the opposite direction and dampened overall atmospheric pCO2 levels by modulating the Southern Ocean dynamics [Raymo et al., 2006]. Interhemispheric insolation differences tend to diminish the greater temperature variability that would be implied by greater northern hemisphere ice sheet efficiency in the early Pleistocene.
The simple model described above is also designed to explore the effect of changing Laurentide ice sheet dynamics on climate sensitivity. As the efficiency of ice sheet cooling per unit volume of ice was greater for the early Pleistocene conditions (model μ is increased from 0.025 to 0.075 Wm−2mSL−1) the model suggests that earth system sensitivity (ESS) must be higher as well. This particular example indicates that a three-fold increase in μ would increase ESS from 3.0 to 5.1 K for a doubling of atmospheric CO2. As stated previously, we observe the opposite in geochemical reconstructions of the early Pleistocene: because the early Pleistocene pCO2 amplitude is only slightly smaller than the late Pleistocene pCO2 amplitude, the associated temperature change with a climate sensitivity of 5.1 K per doubling of CO2 would predict a temperature amplitude of ~4 K. In contrast, we observe a temperature amplitude of ~3 K and a correspondingly lower ESS in the early Pleistocene than in the late Pleistocene. This result simply suggests that earth’s surface temperature in the early Pleistocene is not solely determined by northern hemisphere ice sheets and CO2 feedbacks. Rather, other forcing factors, which are not explicitly taken into account in this model (e.g., tropical and Southern Ocean dynamics, southern hemisphere insolation, Antarctic ice sheets) must be responsible for helping to dampen glacial-interglacial temperature amplitude in the early Pleistocene.
4.4. Species-specific differences; implications for Plio-Pleistocene pCO2 drawdown
As the boron isotope proxy gains maturity and acceptance in the scientific community, the number of δ11B-based datasets has increased, offering an opportunity to glean more detailed insight into the interplay between pCO2 and climate. In Figure 7 we compile new pCO2 estimates alongside ice-core records of late Pleistocene pCO2 [Bereiter et al., 2015; Higgins et al., 2015] and six published records of δ11B-based pCO2. As described above, the δ11 B-based pCO2 data [Hönisch et al., 2009; Seki et al., 2010; Bartoli et al., 2011; Henehan et al., 2013; Martínez-Botí et al., 2015a; Chalk et al., 2017], have been recalculated to minimize differences in the chemical and physical boundary conditions estimated for each record (see Section 2.7). It is worth reiterating that the resulting pCO2 values are primarily dependent on the published δ11B measurements and that our minor revisions in boundary condition treatment do not shift average pCO2 estimates outside of the published 2σ uncertainty bands.
Figure 7.
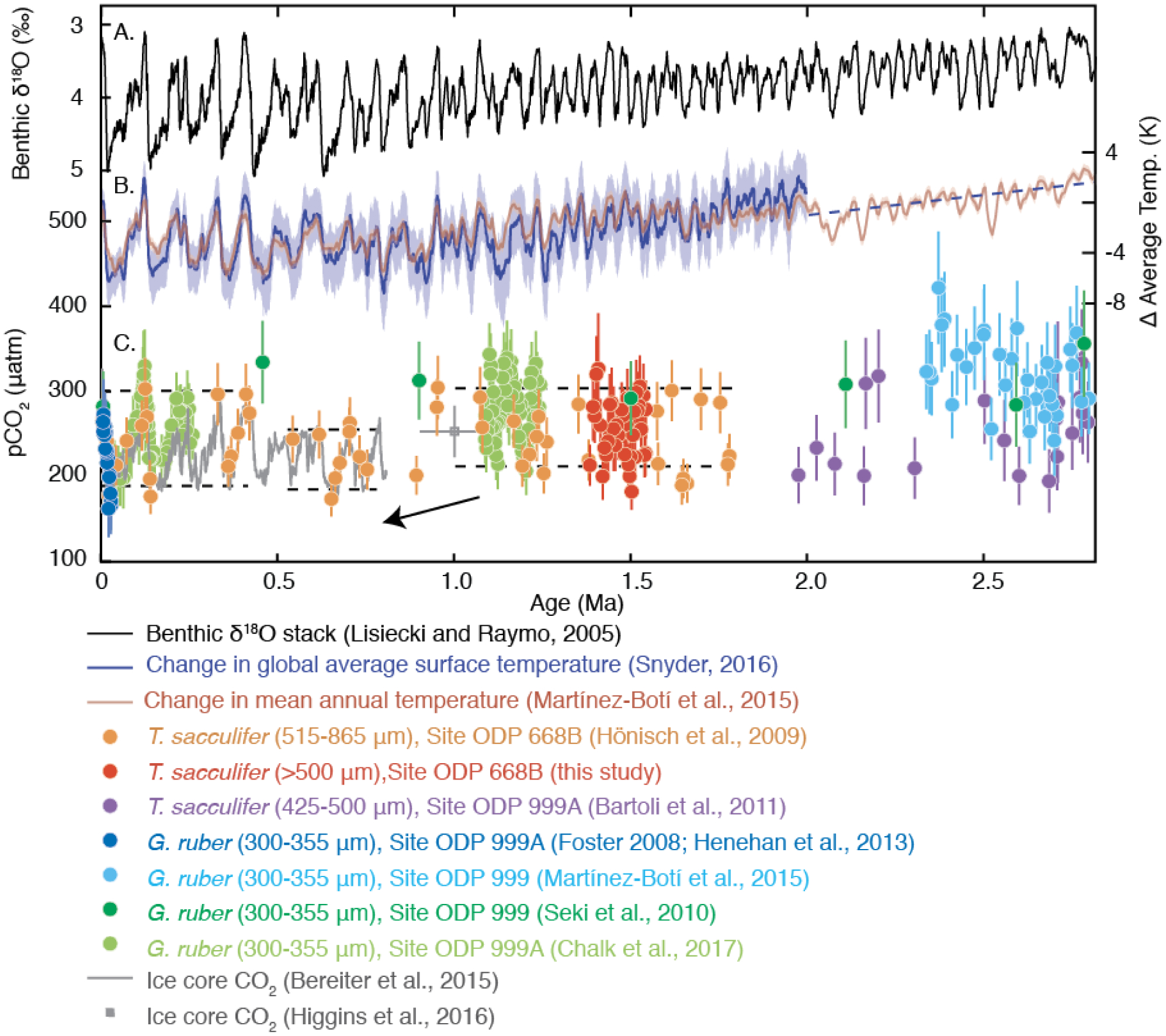
(A) Benthic δ18O [Lisiecki and Raymo, 2005] and (B) change in global average surface temperature (ΔGAST) [Snyder, 2016] compared with (C) pCO2 derived from ice cores [Bereiter et al., 2015; Higgins et al., 2015] and boron isotope reconstructions [Hönisch et al., 2009; Seki et al., 2010; Bartoli et al., 2011; Henehan et al., 2013; Martínez-Botí et al., 2015a; Chalk et al., 2017]. Published pCO2 records have been recalculated using consistent methods (see Methods) from the original δ11B data. Late Pleistocene boron-based pCO2 records from Sites 999A and 668B are in the same range as ice-core-based pCO2 measurements. The largest resulting difference is the record of Bartoli et al. [2011], to which new temperature data have been applied and pCO2 is calculated by pairing pH with alkalinity estimates rather than [CO32-].
Paleo-pCO2 estimates based on δ11B values of either T. sacculifer or G. ruber [Bartoli et al., 2011; Martínez-Botí et al., 2015a] suggest that pCO2 declined by 50–100 μatm between 2.8 and 2.6 Ma (Figure 8). This pCO2 drawdown, coincident with the onset of northern hemisphere glaciation [Raymo, 1994; Haug et al., 1999] is thought to be the result of increased glacial dust load and greater biological productivity in high-nitrate low-chlorophyll regions [Bailey et al., 2011] or polar stratification which sequestered carbon in the deep ocean in both the north Pacific [e.g., Haug et al., 1999; Sigman et al., 2004] and the Southern Ocean [Hodell and Venz-Curtis, 2006; Waddell et al., 2009; Martínez-Garcia et al., 2011]. The pCO2 initial drawdown at 2.8–2.6 Ma is implicated in the increased glaciation of Greenland and the northern hemisphere more broadly [Lunt et al., 2008].
Figure 8.
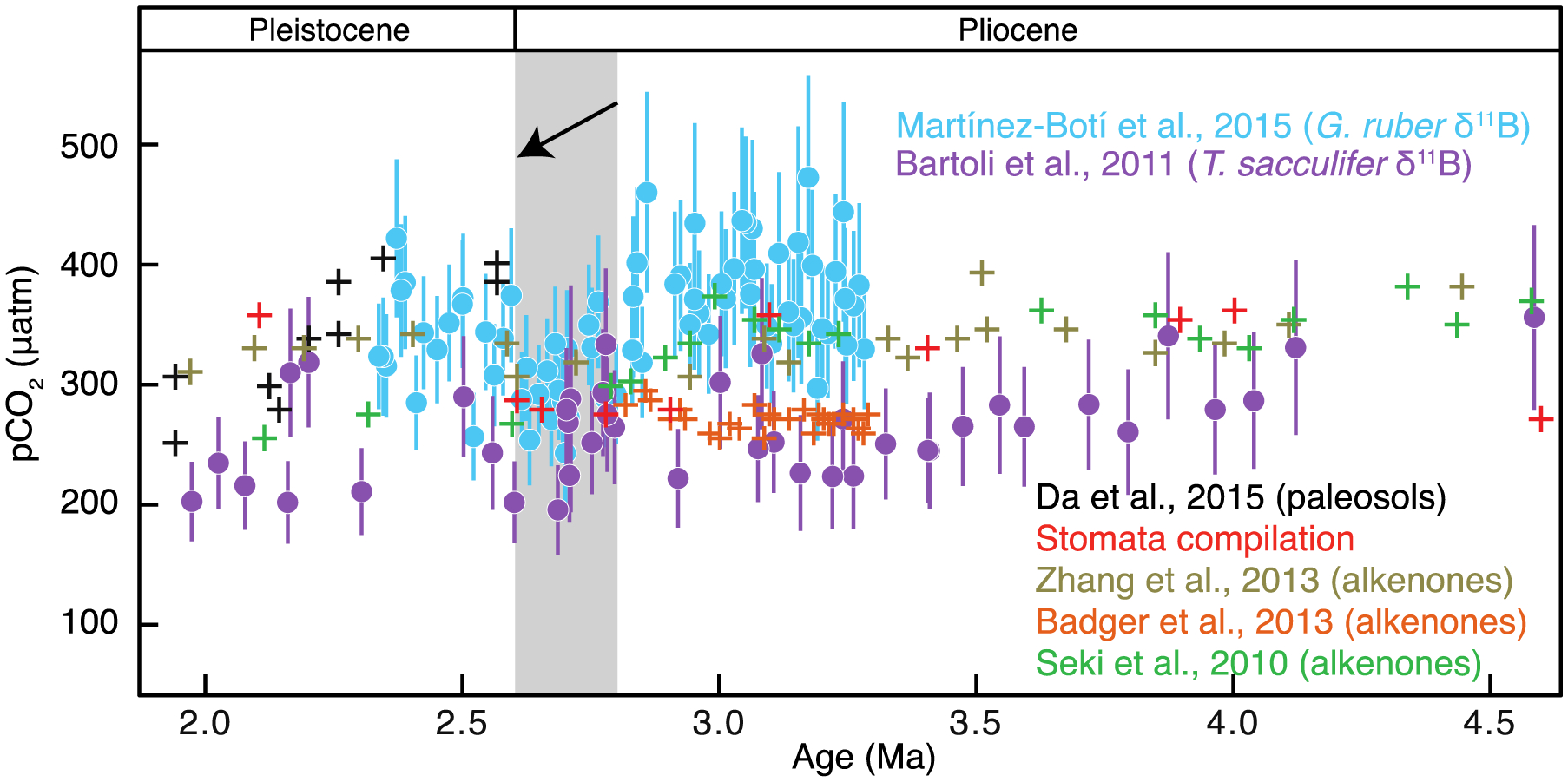
Revised pCO2 values using δ11 B from T. sacculifer [Bartoli et al., 2011] (purple) compared with pCO2 from G. ruber [Martínez-Botí et al., 2015a] (blue). In the interval 2.8–2.6 Ma, G. ruber-based pCO2 declines by >100 μatm, coincident with the onset of northern hemisphere glaciation (gray band, black arrow). Throughout the Pliocene, the T. sacculifer-based pCO2 record is lower than G. ruber-based pCO2 but also shifts to lower values at 2.7 Ma. The text discusses potential offsets due to evolutionary changes in foraminiferal physiology and consequent species-differences between pH and pCO2 reconstructions. Paleo-pCO2 reconstructions from paleosols [Da et al., 2015], stomatal indices [Kürschner et al., 1996; Retallack, 2009; Stults et al., 2011; Wang et al., 2015], and alkenone δ13C [Seki et al., 2010; Badger et al., 2013; Zhang et al., 2013] are intermediate between the estimates of Martínez-Botí et al. [2015a] and Bartoli et al. [2011].
Nonetheless, a distinct mismatch exists between the two δ11B-based pCO2 estimates from Site 999A (Figures 7, 8) for much of the late Pliocene. Where these two records overlap (from 3.3–2.3 Ma), the G. ruber-based pCO2 estimates are on average 90 μatm higher than the T. sacculifer estimates (Figures 7, 8). This difference is apparent to a small degree even where G. ruber and T. sacculifer records overlap in the late Pleistocene, and as a mismatch between G. ruber and ice core records (Figure S8), although the species difference is larger further back in time. We can rule out discrepancies in environmental temperature as impacting the equilibrium constants because the Mg/Ca-based temperature reconstructions from the two datasets are indistinguishable (Figure S7–B). As we have already precluded inconsistent treatment of the physicochemical boundary conditions, the difference is more likely rooted in the underlying δ11B data of G. ruber and T. sacculifer, where δ11BG. ruber decreases more strongly between the last glacial cycle and the late Pliocene than δ11BT. sacculifer. It is important to explore this discrepancy, which could be explained a biologically-mediated shift in the δ11Bcalcite to δ11Bborate calibration (Figure S9), as differential pCO2 results have important implications for Plio-Pleistocene climate research [e.g., Martínez-Botí et al., 2015a].
Differences in the pCO2 reconstructions from these two surface dwelling foraminifera could in theory result from diagenetic alteration or incomplete sample cleaning. However, in the specific case of the late Pliocene data, evaluation of these factors is partly constrained by the fact that both species have been analyzed from the same sediment core. The first of these arguments relates to the potential bias due to partial dissolution, which typically lowers the δ11B value by preferential removal of higher-δ11B calcite [e.g., Hönisch and Hemming, 2004]. Both Pliocene data sets were measured using material from the same shallow core, so diagenetic alteration due to regional changes in the lysocline can reasonably be excluded. Could dissolution susceptibility be species-specific [e.g., Berger, 1967]? Higher pCO2 estimates are based on relatively lower δ11B values, suggesting that G. ruber would have to be relatively more dissolved that T. sacculifer. Shell-weight evidence indeed suggests that G. ruber is slightly more susceptible to partial dissolution than T. sacculifer based on in situ and laboratory work [e.g., Thunell and Honjo, 1981]. In actuality, however, studies of foraminiferal δ11B from depth transects have suggested that G. ruber δ11B values may be more robust and not greatly affected by partial dissolution [Ni et al., 2007; Seki et al., 2010; Henehan et al., 2013] and that the foraminiferal δ11B signature is preserved even when the shell is visibly recrystallized [Edgar et al., 2015]. Another factor which could lower δ11B values is contamination from pelagic clays, whose δ11B values typically fall in the −7 to +5‰ range [Ishikawa and Nakamura, 1993] and are thus much lower than δ11B of foraminiferal calcite. For the G. ruber pCO2 estimates to agree with the T. sacculifer estimates, clay contamination would have to explain the −1‰ discrepancy in δ11BG. ruber. This would require the G. ruber samples to have contained ~5 wt % clay, which is unlikely given the robust cleaning protocol applied to remove impurities and the careful contaminant screening for aluminum and iron [e.g., Martínez-Botí et al., 2015a; Chalk et al., 2017].
At this point we have reasonably ruled out dissolution differences and clay contamination in addition to differential temperature calibrations, δ11Bsw values, salinity, and alkalinity estimates. It is therefore more likely that the difference is biological. Although both foraminifera species and instrumental techniques have been calibrated using modern cultured and core top specimens and shown to replicate the ice-core pCO2 record of the last glacial cycle [Hönisch and Hemming, 2004; 2005; Foster, 2008; Henehan et al., 2013; Chalk et al., 2017], one or both of the studied foraminifer species could have evolved prior to the last glacial cycle. One line of evidence for such an evolutionary shift has been described by Bartoli et al. [2011], who could not find enough Pliocene T. sacculifer specimens in the >500 μm test size fraction and instead substituted specimens in the 425–500 μm range. Hönisch and Hemming [2004] observed no significant difference in δ11BT. sacculifer between the 425–500 and >500 μm test size fraction, suggesting that the depth habitat and ecology is the same for both of the large size fractions. Indeed, if the overall smaller specimens of the largest size fraction in the early Pleistocene sediments had inhabited a deeper water depth, T. sacculifer should have recorded lower δ11B, which would imply lower pH and elevated pCO2. Instead, we observe the opposite pattern: T. sacculifer pCO2 estimates are lower than those based on G. ruber [Seki et al., 2010; Martínez-Botí et al., 2015a; Chalk et al., 2017].
Could the response of G. ruber to ocean pH have evolved over the Plio-Pleistocene? Interestingly, in the modern Caribbean the pink variety of G. ruber is quite abundant, whereas the culture and global core top calibration of Henehan et al. [2013] is based on the white variety. The canonical G. ruber morphospecies represents up to five genetically distinct varieties each with non-unique ecological and biogeographic preferences. In contrast, T. sacculifer does not exhibit such cryptic diversity [Darling and Wade, 2008; André et al., 2012]. Confidence in the δ11B proxy of pCO2 in the Pliocene and early Pleistocene is strengthened by good proxy replication of pCO2 with the ice core record (past 800 ka). In comparing the last two glacial cycles, average G. ruber δ11B values are ~0.3‰ lower in MIS 6 even as pCO2 derived from ice cores is nearly identical in MIS 2 and 6 [Chalk et al., 2017]. Using a consistent method for estimating temperature, in this case via Evans and Müller [2012], the pCO2 calculated from G. ruber δ11B is ~40 μatm higher in MIS 6 than in MIS 2. This result suggests that evolutionary differences in G. ruber could be as young as ~200 ka. Once again, the Mg/Ca-based temperatures derived from these two species do not diverge in the Plio-Pleistocene (3.3–2.3 Ma), which implies that the difference in reconstructed pCO2 is less likely due to changes in habitat depth or seasonal preferences.
Finally, pCO2 estimates from other proxy archives are at odds with the highest Pliocene G. ruber-based pCO2 estimates (Figure 8). For example, evidence from the alkenone δ13C proxy suggests relatively lower (<350 μatm) levels of late Pliocene pCO2, whether derived from Site 999A, (high resolution data: 270±40) [Badger et al., 2013], (low resolution data: 330±40) [Seki et al., 2010], or from Site 925 in eastern tropical Atlantic (320±40) [Zhang et al., 2013]. While different proxies have different uncertainties, the larger amplitude of Pliocene pCO2 change in the G. ruber-based record (~150 μatm as opposed to ~100 μatm in the T. sacculifer-based record) is difficult to justify given the smaller amplitude of glacial-interglacial cycles in the Pliocene [Lisiecki and Raymo, 2005]. In summary, although we cannot unequivocally state which species may have changed behavior, it will be important to cross-calibrate the δ11B proxy in the Pliocene with regard to potential physiological distinctions.
Importantly, both G. ruber and T. sacculifer record a pCO2 decline through the Plio-Pleistocene (Figures 7, 8), but the extent of the pCO2 decline is larger for G. ruber reconstructions and this has critical implications for estimates of climate sensitivity from these two species. If the Pliocene G. ruber-based pCO2 estimate is correct, then late Pliocene (3.3–2.8 Ma) pCO2 levels were relatively high (>350 μatm), and highly variable, and then dramatically declined at the onset of northern hemisphere glaciation. Martínez-Botí et al. [2015a] use this data to infer low sensitivity to radiative pCO2 forcing in the Pliocene and higher sensitivity in the Pleistocene. They call out increased continental ice-albedo as the primary mechanism for this change in sensitivity and use, as one line of evidence, the inflection point at ~275 μatm in the relationship between benthic δ18O and forcing due to pCO2 changes [Martínez-Botí et al., 2015a]. Here we replot this figure using all available datasets discussed herein, but we also separate the two species (Figure 9). Martínez-Botí et al. [2015a] originally excluded the data of Bartoli et al. [2011] from their plot to improve clarity, but it now emerges that the change in slope of the relationship observed by Martínez-Botí et al. [2015a] is only true for G. ruber but not for T. sacculifer (Figure 9). Explaining the larger Plio-Pleistocene CO2 drawdown inferred by the G. ruber δ11B record requires significant increases in ocean stratification, dust fertilization, and/or (potentially) silicate rock weathering since the Pliocene (e.g., Martinez-Garcia et al., 2011).
Figure 9.
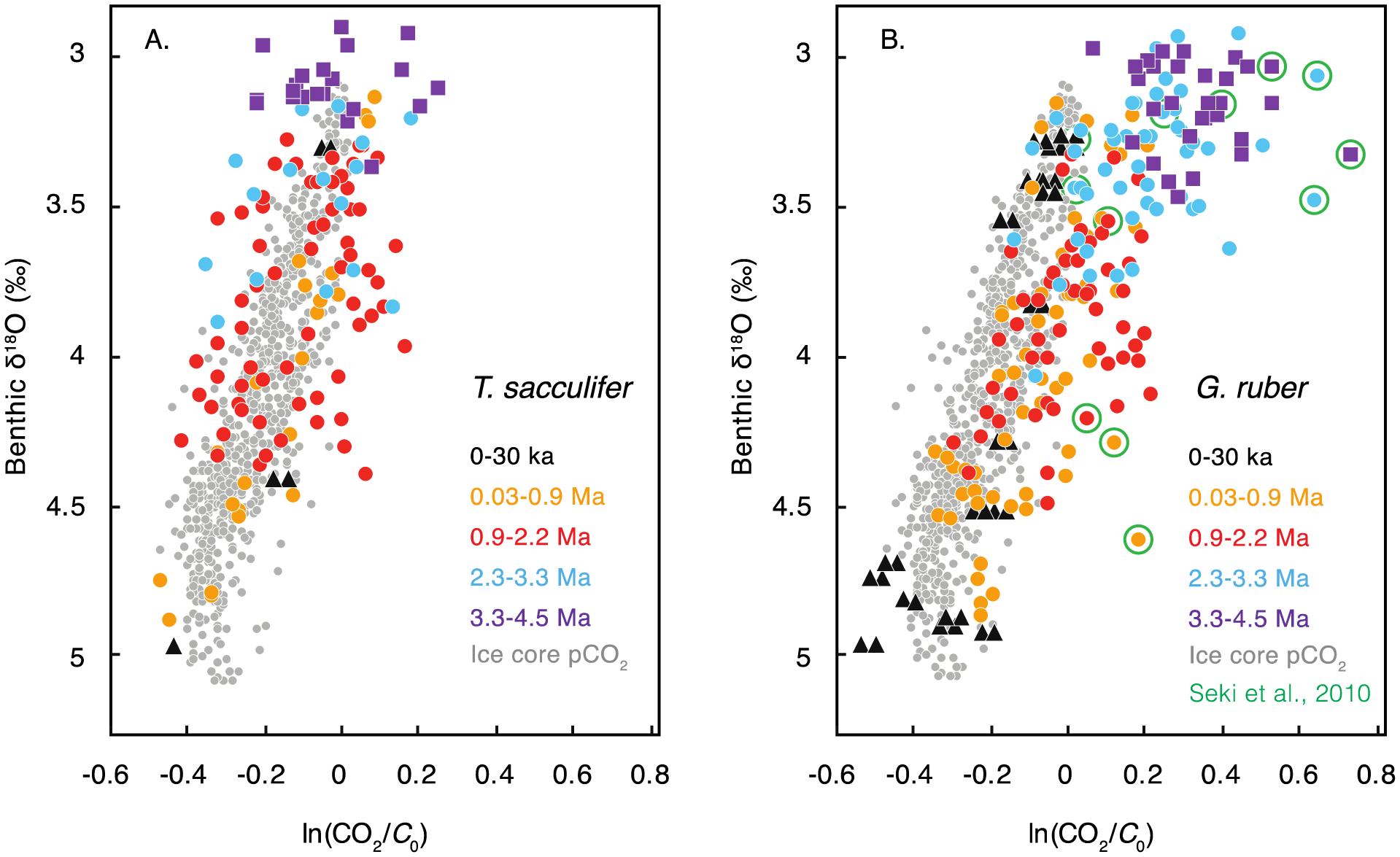
Relationship between benthic δ18O and climate forcing due to radiative CO2 forcing (ln(CO2/ C0), where C0 is 278 μatm), including all six δ11B-based pCO2 records presented in Figure 7. (A) Forcing derived from δ11BT. sacculifer-based pCO2 compared with forcing from pCO2 values derived from ice cores (gray). Time periods are coded by color, yet the overall benthic δ18O-to-ln(CO2/C0) relationship for boron-based pCO2 data falls along the same relationship for pCO2 as that derived from ice cores. (B) The corresponding relationship of boron-based CO2 forcing compared with pCO2 values from ice cores, this time using δ11B values from G. ruber. Although Holocene and LGM pCO2 values based on the foraminifer G. ruber are consistent with the ice-core pCO2, the estimates deviate in earlier time periods, when pCO2 values are higher than expected based on the general benthic δ18O : ln(CO2/C0) relationship derived from ice cores. This gives rise to the question of whether G. ruber and its vital effects evolved over time. Values derived from one early study are circled in green; these data are based on unusually low boron isotope values [Seki et al., 2010].
If, on the other hand, the Pliocene T. sacculifer-based pCO2 estimates are correct, Pliocene pCO2 was less elevated relative to early Pleistocene pCO2 values and the linear relationship between benthic δ18O and pCO2 forcing extended into the Pliocene (Figure 9A). The T. sacculifer-based estimates, with pCO2 likely <350–400 μatm, support the evidence for lower Pliocene pCO2 from other proxies (Figure 8): alkenone δ13C, stomatal indices, and paleosols [e.g., Badger et al., 2013; Da et al., 2015; Wang et al., 2015] and implies that atmospheric pCO2 was relatively similar between the late Pliocene and early Pleistocene, despite the descent into cooler polar temperatures. If correct, this record implies that Arctic temperatures declined at the Plio-Pleistocene transition due to factors other than direct radiative pCO2 forcing, such as the thickening of northern-hemisphere ice sheets or reduced poleward oceanic heat transport. Further work is warranted to improve our understanding of foraminiferal vital effects on δ11B, and thereby improve confidence in paleo-pCO2 estimates from this proxy.
5. Conclusions
Glacial-interglacial climate cycles in the early Pleistocene (>1 Ma) were shorter and less severe than the iconic 100-kyr cycles of the last 0.5 million years. Our new high-resolution record of atmospheric pCO2 over three glacial cycles in the early Pleistocene (1.38–1.54 Ma) is closely linked with obliquity pacing, with an average pCO2 amplitude of 92 (±13) μatm. Over the MPT, glacial pCO2 declined, alongside glacial SST cooling in both the tropics and high latitudes. In contrast to an earlier assessment of climate sensitivity from δ11B in G. ruber, our compilation of pCO2 estimates from δ11 B in T. sacculifer suggests apparent climate sensitivity in the early Pleistocene was lower compared to the late Pleistocene; the difference is likely due to changing ocean or ice sheet dynamics over the mid-Pleistocene. Using a simple model, we suggest that the lower climate sensitivity in the early Pleistocene was not directly related to the thinner, yet extensive, northern-hemisphere terrestrial ice sheets, but is rather a result of changing ocean dynamics in the southern hemisphere or tropics. The observed discrepancy in late Pliocene pCO2 estimates based on G. ruber and T. sacculifer requires further study of potentially evolving species-specific vital effects in the past, so that paleo-pCO2 estimates from the proxy can be appreciated with confidence.
Supplementary Material
Key points:
Early Pleistocene pCO2 roughly varied with obliquity cycles.
Interglacial pCO2 was similar in the early and late Pleistocene; glacial pCO2 declined over the mid-Pleistocene transition.
Discrepancies between δ11B values and corresponding pCO2 estimates from G. ruber and T. sacculifer are observed and may indicate evolving vital effects.
Acknowledgements
We thank the International Ocean Drilling Program repository for providing samples for these analyses and Carina Fish, Peter deMenocal and Angela Dial for assistance with the new trace element analyses. This work was funded by the National Science Foundation grant EAR-1349616 to KD and a gift from Gerry Lenfest to BH. Upon publication, all original data from this study will be archived at NOAA’s National Center for Environmental Information at https://www.ncdc.noaa.gov/paleo/study/25490 as a part of the Research Coordination Network grant to BH and Pratigya Polissar (OCE 16-36005).
Footnotes
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process which may lead to differences between this version and the Version of Record.
References
- Allen KA, Hönisch B, Eggins SM, and Rosenthal Y (2012), Environmental controls on B/Ca in calcite tests of the tropical planktic foraminifer species Globigerinoides ruber and Globigerinoides sacculifer, Earth and Planetary Science Letters, 351–352(C), 270–280, doi: 10.1016/j.epsl.2012.07.004. [DOI] [Google Scholar]
- Anand P, Elderfield H, and Conte MH (2003), Calibration of Mg/Ca thermometry in planktonic foraminifera from a sediment trap time series, Paleoceanography, 18(2), 1050, doi: 10.1029/2002PA000846. [DOI] [Google Scholar]
- Anderson RF, Ali S, Bradtmiller LI, Nielsen S, Fleisher MQ, Anderson BE, and Burckle LH (2009), Wind-driven upwelling in the Southern Ocean and the deglacial rise in atmospheric CO2, Science, 323(5920), 1443–1448, doi: 10.1126/science.1167441. [DOI] [PubMed] [Google Scholar]
- Badger MPS, Schmidt DN, Mackensen A, and Pancost RD (2013), High-resolution alkenone palaeobarometry indicates relatively stable pCO2 during the Pliocene (3.3–2.8 Ma), Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences, 371(2001), 20130094–20130094, doi: 10.1016/j.gca.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Bailey I, Liu Q, Swann GEA, Jiang Z, Sun Y, Zhao X, and Roberts AP (2011), Iron fertilisation and biogeochemical cycles in the sub-Arctic northwest Pacific during the late Pliocene intensification of northern hemisphere glaciation, Earth and Planetary Science Letters, 307(3–4), 253–265, doi: 10.1016/j.epsl.2011.05.029. [DOI] [Google Scholar]
- Balco G, and Rovey CW (2010), Absolute chronology for major Pleistocene advances of the Laurentide Ice Sheet, Geology, 38(9), 795–798, doi: 10.1130/G30946.1. [DOI] [Google Scholar]
- Barker S, Greaves M, and Elderfield H (2003), A study of cleaning procedures used for foraminiferal Mg/Ca paleothermometry, Geochemistry Geophysics Geosystems, 4(9), doi: 10.1029/2003GC000559. [DOI] [Google Scholar]
- Bartoli G, Hönisch B, and Zeebe RE (2011), Atmospheric CO2 decline during the Pliocene intensification of Northern Hemisphere glaciations, Paleoceanography, 26(4), PA3206, doi: 10.1029/2010PA002055. [DOI] [Google Scholar]
- Bemis BE, Spero HJ, Bijma J, and Lea DW (1998), Reevaluation of the oxygen isotopic composition of planktonic foraminifera: Experimental results and revised paleotemperature equations, Paleoceanography, 13(2), 150–160, doi: 10.1029/98PA00070. [DOI] [Google Scholar]
- Bereiter B, Eggleston S, Schmitt J, Nehrbass-Ahles C, Stocker TF, Fischer H, Kipfstuhl S, and Chappellaz J (2015), Revision of the EPICA Dome C CO2 record from 800 to 600kyr before present, Geophysical Research Letters, 42(2), 542–549, doi: 10.1002/2014GL061957. [DOI] [Google Scholar]
- Berger WH (1967), Foraminiferal Ooze - Solution at Depths, Science, 156(3773), 383–385, doi: 10.1126/science.156.3773.383. [DOI] [PubMed] [Google Scholar]
- Bé AWH (1980), Gametogenic calcification in a spinose planktonic foraminifer, Globigerinoides sacculifer (Brady), Marine Micropaleontology, 5, 283–310, doi: 10.1016/0377-8398(80)90014-6. [DOI] [Google Scholar]
- Bibby T, Putkonen J, Morgan D, Balco G, and Shuster DL (2016), Million year old ice found under meter thick debris layer in Antarctica, Geophysical Research Letters, doi: 10.1002/2016GL069889. [DOI] [Google Scholar]
- Bintanja R, and van de Wal RSW (2008), North American ice-sheet dynamics and the onset of 100,000-year glacial cycles, Nature, 454(7206), 869–872, doi: 10.1038/nature07158. [DOI] [PubMed] [Google Scholar]
- Bird MI, and Cali JA (1998), A million-year record of fire in sub-Saharan Africa, Nature, 394(6695), 767–769, doi: 10.1038/29507. [DOI] [Google Scholar]
- Bird MI, and Cali JA (2002), A revised high-resolution oxygen-isotope chronology for ODP-668B: implications for Quaternary biomass burning in Africa, Global and Planetary Change, 33(1–2), 73–76, doi: 10.1016/S0921-8181(02)00062-0. [DOI] [Google Scholar]
- Boyle EA (1988), The Role of Vertical Chemical Fractionation in Controlling Late Quaternary Atmospheric Carbon-Dioxide, J. Geophys. Res. Oceans, 93(C12), 15701–15714, doi: 10.1029/JC093iC12p15701. [DOI] [Google Scholar]
- Boyle EA, and Keigwin LD (1985), Comparison of Atlantic and Pacific paleochemical records for the last 215,000 years: Changes in deep ocean circulation and chemical inventories, Earth and Planetary Science Letters, 76(1–2), 135–150, doi: 10.1016/0012-821X(85)90154-2. [DOI] [Google Scholar]
- Branson O, Kaczmarek K, Redfern SAT, Misra S, Langer G, Tyliszczak T, Bijma J, and Elderfield H (2015), The coordination and distribution of B in foraminiferal calcite, Earth and Planetary Science Letters, 416(C), 67–72, doi: 10.1016/j.epsl.2015.02.006. [DOI] [Google Scholar]
- Brennan ST, Lowenstein TK, and Cendon DI (2013), The major-ion composition of Cenozoic seawater: The past 36 million years from fluid inclusions in marine halite, American Journal of Science, 313(8), 713–775, doi: 10.2475/08.2013.01. [DOI] [Google Scholar]
- Broecker WS (1971), Calcite accumulation rates and glacial to interglacial changes in oceanic mixing, in The Late Cenozoic glacial ages, edited by Turekian KK, pp. 239–265, Yale University. [Google Scholar]
- Broecker WS, and Peng T-H (1987), The role of CaCO3 compensation in the glacial to interglacial atmospheric CO2 change, GBC, 1(1), 15–29, doi: 10.1029/GB001i001p00015. [DOI] [Google Scholar]
- Chalk TB et al. (2017), Causes of ice age intensification across the Mid-Pleistocene Transition, PNAS, 114(50), 13114–13119, doi: 10.1073/pnas.1702143114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark PU, and Pollard D (1998), Origin of the middle Pleistocene transition by ice sheet erosion of regolith, Paleoceanography, 13(1), 1–9, doi: 10.1029/97pa02660. [DOI] [Google Scholar]
- Clark PU, Archer D, Pollard D, Blum JD, Rial JA, Brovkin V, Mix AC, Pisias NG, and Roy M (2006), The middle Pleistocene transition: characteristics, mechanisms, and implications for long-term changes in atmospheric pCO2, Quaternary Science Reviews, 25(23–24), 3150–3184, doi: 10.1016/j.quascirev.2006.07.008. [DOI] [Google Scholar]
- Clark PU, Alley RB, and Pollard D (1999), Northern Hemisphere Ice-Sheet Influences on Global Climate Change, Science, 286(5442), 1104–1111, doi: 10.1126/science.286.5442.1104. [DOI] [Google Scholar]
- Da J, Zhang YG, Wang H, Balsam W, and Ji J (2015), An Early Pleistocene atmospheric CO2 record based on pedogenic carbonate from the Chinese loess deposits, Earth and Planetary Science Letters, 426(C), 69–75, doi: 10.1016/j.epsl.2015.05.053. [DOI] [Google Scholar]
- Dai Y, Yu J, and Johnstone HJH, 2016, Distinct responses of planktonic foraminiferal B/Ca to dissolution on seafloor: Geochemistry Geophysics Geosystems, v. 17, no. 4, p. 1339–1348, doi: 10.1002/2015GC006199. [DOI] [Google Scholar]
- de Villiers S, 2005, Foraminiferal shell-weight evidence for sedimentary calcite dissolution above the lysocline: Deep Sea Research Part I: Oceanographic Research Papers, v. 52, no. 5, p. 671–680, doi: 10.1016/j.dsr.2004.11.014. [DOI] [Google Scholar]
- Dekens PS, Lea DW, Pak DK, and Spero HJ (2002), Core top calibration of Mg/Ca in tropical foraminifera: Refining paleotemperature estimation, Geochemistry Geophysics Geosystems, 3, 1022, doi: 10.1029/2001GC000200. [DOI] [Google Scholar]
- Delaney ML, Bé AWH, and Boyle EA (1985), Li, Sr, Mg, and Na in foraminiferal calcite shells from laboratory culture, sediment traps, and sediment cores, Geochimica et Cosmochimica Acta, doi: 10.1016/0016-7037(85)90284-4. [DOI] [Google Scholar]
- Dickson AG (1990), Thermodynamics of the dissociation of boric acid in synthetic seawater from 273.15 to 318.15 K, Deep-Sea Research II, 37, 755–766, doi: 10.1021/je00061a009. [DOI] [Google Scholar]
- Dyez K, and Ravelo AC (2013), Late Pleistocene tropical Pacific temperature sensitivity to radiative greenhouse gas forcing, Geology, 41(1), 23–26, doi: 10.1130/G33425.1. [DOI] [Google Scholar]
- Dyez K, and Ravelo AC (2014), Dynamical changes in the tropical Pacific warm pool and zonal SST gradient during the Pleistocene, Geophysical Research Letters, 41, 7626–7633, doi: 10.1002/2014GL061639. [DOI] [Google Scholar]
- Edgar KM, Anagnostou E, Pearson PN, and Foster GL (2015), Assessing the impact of diagenesis on δ11B, δ13C, δ18O, Sr/Ca and B/Ca values in fossil planktic foraminiferal calcite, Geochimica et Cosmochimica Acta, 166(C), 189–209, doi: 10.1016/j.gca.2015.06.018. [DOI] [Google Scholar]
- Evans D, and Müller W (2012), Deep time foraminifera Mg/Ca paleothermometry: Nonlinear correction for secular change in seawater Mg/Ca, Paleoceanography, 27(4), PA4205, doi: 10.1029/2012PA002315. [DOI] [Google Scholar]
- Evans D, Brierley CM, Raymo ME, Erez J, and Müller W (2016), Planktic foraminifera shell chemistry response to seawater chemistry: Pliocene–Pleistocene seawater Mg/Ca, temperature and sea level change, Earth and Planetary Science Letters, 438(C), 139–148, doi: 10.1016/j.epsl.2016.01.013. [DOI] [Google Scholar]
- Fantle MS, and DePaolo DJ (2005), Variations in the marine Ca cycle over the past 20 million years, Earth and Planetary Science Letters, 237(1–2), 102–117. [Google Scholar]
- Fantle MS, and DePaolo DJ (2006), Sr isotopes and pore fluid chemistry in carbonate sediment of the Ontong Java Plateau: Calcite recrystallization rates and evidence for a rapid rise in seawater Mg over the last 10 million years, Geochimica et Cosmochimica Acta, 70(15), 3883–3904. [Google Scholar]
- Farmer EC, Kaplan A, de Menocal PB, and Lynch-Stieglitz J (2007), Corroborating ecological depth preferences of planktonic foraminifera in the tropical Atlantic with the stable oxygen isotope ratios of core top specimens, Paleoceanography, 22(3), doi: 10.1029/2006PA001361. [DOI] [Google Scholar]
- Farmer JR, Hönisch B, and Uchikawa J (2016), Single laboratory comparison of MCICP-MS and N-TIMS boron isotope analyses in marine carbonates, Chemical Geology, 1–10, doi: 10.1016/j.chemgeo.2016.11.008. [DOI] [Google Scholar]
- Farrell JW, and Prell WL (1991), Pacific CaCO3 Preservation and δ18O Since 4 Ma: Paleoceanic and Paleoclimatic Implications, Paleoceanography, 6(4), 485–498, doi: 10.1029/91PA00877. [DOI] [Google Scholar]
- Fischer H et al. (2010), The role of Southern Ocean processes in orbital and millennial CO2 variations - A synthesis, Quaternary Science Reviews, 29(1–2), 193–205, doi: 10.1016/j.quascirev.2009.06.007. [DOI] [Google Scholar]
- Fischer H et al. (2013), Where to find 1.5 million yr old ice for the IPICS “Oldest Ice” ice core, Clim. Past Discuss, 9(3), 2771–2815, doi: 10.5194/cpd-9-2771-2013. [DOI] [Google Scholar]
- Foster GL (2008), Seawater pH, pCO2 and [CO3−2] variations in the Caribbean Sea over the last 130 kyr: A boron isotope and B/Ca study of planktic foraminifera, Earth and Planetary Science Letters, 271(1–4), 254–266, doi: 10.1016/j.epsl.2008.04.015. [DOI] [Google Scholar]
- Foster GL, Hönisch B, Paris G, Dwyer GS, Rae JWB, Elliott T, Gaillardet J, Hemming NG, Louvat P, and Vengosh A (2013), Interlaboratory comparison of boron isotope analyses of boric acid, seawater and marine CaCO3 by MC-ICPMS and NTIMS, Chemical Geology, 358(C), 1–14, doi: 10.1016/j.chemgeo.2013.08.027. [DOI] [Google Scholar]
- Foster GL, Pogge von Strandmann PAE, and Rae JWB (2010), Boron and magnesium isotopic composition of seawater, Geochemistry Geophysics Geosystems, 11(8), doi: 10.1029/2010GC003201. [DOI] [Google Scholar]
- Greenop R, Hain MP, Sosdian SM, Oliver KIC, Goodwin P, Chalk TB, Lear CH, Wilson PA, and Foster GL (2017), A record of Neogene seawater δ11B reconstructed from paired δ11B analyses on benthic and planktic foraminifera, Climate of the Past, 13(2), 149–170, doi: 10.5194/cp-13-149-2017. [DOI] [Google Scholar]
- Groeneveld J (2005), Effect of the Pliocene closure of the Panamanian Gateway on Caribbean and east Pacific sea surface temperatures and salinities by applying combined Mg/Ca and, 1–165 pp. Christian Albrechts University, 20 October. [Google Scholar]
- Hansen J et al. (2005), Efficacy of climate forcings, J. Geophys. Res. Atmos, 110(D18), doi: 10.1029/2005JD005776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J, Lacis A, Rind D, Russell G, Stone P, Fung IY, Ruedy R, and Lerner J (1984), Climate sensitivity: Analysis of feedback mechanisms, in Climate Processes and Climate Sensitivity, vol. 29, edited by Hansen JE and Takahashi T, American Geophysical Union. [Google Scholar]
- Haug GH, Sigman DM, Tiedemann R, Pedersen TF, and Sarnthein M (1999), Onset of permanent stratification in the subarctic Pacific Ocean, Nature, 401(6755), 779–782. [Google Scholar]
- Hays J, Imbrie J, and Shackleton NJ (1976), Variations in the earth’s orbit: pacemaker of the ice ages, Science, 194(4270), 1121–1132, doi: 10.1126/science.194.4270.1121. [DOI] [PubMed] [Google Scholar]
- Hemming NG, and Hanson GN (1992), Boron isotopic composition and concentration in modern marine carbonates, Geochimica et Cosmochimica Acta, 56(1), 537–543. [Google Scholar]
- Henehan MJ et al. (2013), Calibration of the boron isotope proxy in the planktonic foraminifera Globigerinoides ruber for use in palaeo-CO2 reconstruction, Earth and Planetary Science Letters, 364, 111–122, doi: 10.1016/j.epsl.2012.12.029. [DOI] [Google Scholar]
- Herbert TD, Peterson LC, Kucera MT, and Liu Z (2010), Tropical Ocean Temperatures Over the Past 3.5 Million Years, Science, 328, 1530–1534, doi: 10.1126/science.1185435. [DOI] [PubMed] [Google Scholar]
- Heydt, von der AS. et al. (2016), Lessons on climate sensitivity from past climate changes, Curr Clim Change Rep, 2(4), 1–11, doi: 10.1007/s40641-016-0049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JA, Kurbatov AV, Spaulding NE, Brook EJ, Introne DS, Chimiak LM, Yan Y, Mayewski PA, and Bender ML (2015), Atmospheric composition 1 million years ago from blue ice in the Allan Hills, Antarctica, PNAS, 112(22), 6887–6891, doi: 10.1073/pnas.1420232112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodell DA, and Venz-Curtis KA (2006), Late Neogene history of deepwater ventilation in the Southern Ocean, Geochemistry Geophysics Geosystems, 7(9), doi: 10.1029/2005GC001211. [DOI] [Google Scholar]
- Horita J, Zimmermann H, and Holland HD (2002), Chemical evolution of seawater during the Phanerozoic: Implications from the record of marine evaporites, Geochimica et Cosmochimica Acta, 66(21), 3733–3756, doi: 10.1016/S0016-7037(01)00884-5. [DOI] [Google Scholar]
- Hönisch B, and Hemming NG (2004), Ground-truthing the boron isotope-paleo-pH proxy in planktonic foraminifera shells: Partial dissolution and shell size effects, Paleoceanography, 19(4), PA4010, doi: 10.1029/2004PA001026. [DOI] [Google Scholar]
- Hönisch B, and Hemming NG (2005), Surface ocean pH response to variations in pCO2 through two full glacial cycles, Earth and Planetary Science Letters, 236(1–2), 305–314, doi: 10.1016/j.epsl.2005.04.027. [DOI] [Google Scholar]
- Hönisch B, Allen KA, Lea DW, Spero HJ, Eggins SM, Arbuszewski JA, deMenocal PB, Rosenthal Y, Russell AD, and Elderfield H (2013), The influence of salinity on Mg/Ca in planktic foraminifers - Evidence from cultures, core-top sediments and complementary δ18O, Geochimica et Cosmochimica Acta, 121(C), 196–213, doi: 10.1016/j.gca.2013.07.028. [DOI] [Google Scholar]
- Hönisch B, Hemming NG, Archer D, Siddall M, and McManus JF (2009), Atmospheric carbon dioxide concentration across the mid-Pleistocene transition, Science, 324(5934), 1551, doi: 10.1126/science.1171477. [DOI] [PubMed] [Google Scholar]
- Huybers P (2007), Glacial variability over the last two million years: an extended depth-derived agemodel, continuous obliquity pacing, and the Pleistocene progression, Quaternary Science Reviews, 26(1–2), 37–55, doi: 10.1016/j.quascirev.2006.07.013. [DOI] [Google Scholar]
- Imbrie J et al. (1992), On the structure and origin of major glaciation cycles 1. Linear responses to Milankovitch forcing, Paleoceanography, 7(6), 701–738, doi: 10.1029/92pa02253. [DOI] [Google Scholar]
- Imbrie J, Hays JD, Martinson DG, McIntyre A, Mix AC, Morley J, Pisias NG, Prell WL, and Shackleton NJ (1984), The orbital theory of Pleistocene climate: Support from a revised chronology of the marine δ18O record, edited by Berger AL, Imbrie J, Hays J, Kukla G, and Saltzman B, pp. 269–305, Milankovitch and Climate. [Google Scholar]
- Ishikawa T, and Nakamura E (1993), Boron Isotope Systematics of Marine-Sediments, Earth and Planetary Science Letters, 117(3–4), 567–580, doi: 10.1016/0012-821X(93)90103-G. [DOI] [Google Scholar]
- Joannin S et al. (2010), Early Pleistocene climate cycles in continental deposits of the Lesser Caucasus of Armenia inferred from palynology, magnetostratigraphy, and 40Ar/39Ar dating, Earth and Planetary Science Letters, 291(1–4), 149–158, doi: 10.1016/j.epsl.2010.01.007. [DOI] [Google Scholar]
- Jouzel J et al. (2007), Orbital and Millennial Antarctic Climate Variability over the Past 800,000 Years, Science, 317(5839), 793–796, doi: 10.1126/science.1141038. [DOI] [PubMed] [Google Scholar]
- Key RM, Kozyr A, Sabine CL, Lee K, Wanninkhof R, Bullister JL, Feely RA, Millero F, Mordy C, and Peng TH (2004), A global ocean carbon climatology: Results from Global Data Analysis Project (GLODAP), GBC, 18(4), doi: 10.1029/2004GB002247. [DOI] [Google Scholar]
- Klochko K, Kaufman AJ, Yao W, Byrne RH, and Tossell JA (2006), Experimental measurement of boron isotope fractionation in seawater, Earth and Planetary Science Letters, 248(1–2), 276–285, doi: 10.1016/j.epsl.2006.05.034. [DOI] [Google Scholar]
- Köhler P, and Bintanja R (2008), The carbon cycle during the Mid Pleistocene Transition: the Southern Ocean Decoupling Hypothesis, Climate of the Past, 4(4), 311–332, doi: 10.5194/cp-4-311-2008. [DOI] [Google Scholar]
- Köhler P, de Boer B, von der Heydt AS, Stap LB, and van de Wal RSW (2015), On the state dependency of the equilibrium climate sensitivity during the last 5 million years, Climate of the Past, 11(12), 1801–1823, doi: 10.5194/cp-11-1801-2015. [DOI] [Google Scholar]
- Köhler P, Bintanja R, Fischer H, Joos F, Knutti R, Lohmann G, and Masson-Delmotte V (2010), What caused Earth’s temperature variations during the last 800,000 years? Databased evidence on radiative forcing and constraints on climate sensitivity, Quaternary Science Reviews, 29(1–2), 129–145, doi: 10.1016/j.quascirev.2009.09.026. [DOI] [Google Scholar]
- Kürschner WM, vanderBurgh J, Visscher H, and Dilcher DL (1996), Oak leaves as biosensors of late Neogene and early Pleistocene paleoatmospheric CO2 concentrations, Marine Micropaleontology, 27(1–4), 299–312, doi: 10.1016/0377-8398(95)00067-4. [DOI] [Google Scholar]
- Laskar J, Robutel P, Joutel F, Gastineau M, Correia ACM, and Levrard B (2004), A long-term numerical solution for the insolation quantities of the Earth, Astronomy and Astrophysics, 428(1), 261–285, doi: 10.1051/0004-6361:20041335. [DOI] [Google Scholar]
- Lawrence K, Herbert TD, Brown CM, Raymo ME, and Haywood AM (2009), High-amplitude variations in North Atlantic sea surface temperature during the early Pliocene warm period, Paleoceanography, 24(2), 1–15, doi: 10.1029/2008PA001669. [DOI] [Google Scholar]
- Lea DW (2004), The 100 000-yr cycle in tropical SST, greenhouse forcing, and climate sensitivity, Journal of Climate, 17(11), 2170–2179, doi:. [DOI] [Google Scholar]
- Lee K, Kim T-W, Byrne RH, Millero FJ, Feely RA, and Liu Y-M (2010), The universal ratio of boron to chlorinity for the North Pacific and North Atlantic oceans, Geochimica et Cosmochimica Acta, 74(6), 1801–1811, doi: 10.1016/j.gca.2009.12.027. [DOI] [Google Scholar]
- Legrande AN, and Schmidt GA (2006), Global gridded data set of the oxygen isotopic composition in seawater, Geophysical Research Letters, 33, doi: 10.1029/2006GL026011. [DOI] [Google Scholar]
- Lemarchand D, Gaillardet J, Lewin E, and Allegre CJ (2000), The influence of rivers on marine boron isotopes and implications for reconstructing past ocean pH, Nature, 408(6815), 951–954, doi: 10.1038/35050058. [DOI] [PubMed] [Google Scholar]
- Lisiecki LE (2010), A benthic δ13C-based proxy for atmospheric pCO2 over the last 1.5 Myr, Geophysical Research Letters, 37(21), L21708, doi: 10.1029/2010GL045109. [DOI] [Google Scholar]
- Lisiecki LE, and Raymo ME (2005), A Pliocene-Pleistocene stack of 57 globally distributed benthic δ18O records, Paleoceanography, 20, doi: 10.1029/2004PA001071. [DOI] [Google Scholar]
- Locarnini RA et al. (2013), World Ocean Atlas 2013, Vol. 1: Temperature, in NOAA Atlas NESDIS 73, edited by Levitus S and Mishonov A, p. 40. [Google Scholar]
- Lueker TJ, Dickson AG, and Keeling CD (2000), Ocean pCO2 calculated from dissolved inorganic carbon, alkalinity, and equations for K1 and K2: validation based on laboratory measurements of CO2 in gas and seawater at equilibrium, Marine Chemistry, 70(1–3), 105–119, doi: 10.1016/S0304-4203(00)00022-0. [DOI] [Google Scholar]
- Lunt DJ, Foster GL, Haywood AM, and Stone EJ (2008), Late Pliocene Greenland glaciation controlled by a decline in atmospheric CO2 levels, Nature, 454(7208), 1102–1105, doi: 10.1038/nature07223. [DOI] [PubMed] [Google Scholar]
- Lüthi D et al. (2008), High-resolution carbon dioxide concentration record 650,000–800,000 years before present, Nature, 453(7193), 379–382, doi: 10.1038/nature06949. [DOI] [PubMed] [Google Scholar]
- Manabe S, and Broccoli AJ (1985), The Influence of Continental Ice Sheets on the Climate of an Ice-Age, J. Geophys. Res. Atmos, 90(ND1), 2167–2190, doi: 10.1029/JD090iD01p02167. [DOI] [Google Scholar]
- Martin PA, and Lea DW (2002), A simple evaluation of cleaning procedures on fossil benthic foraminiferal Mg/Ca, Geochemistry Geophysics Geosystems, 3(10), 1–8, doi: 10.1029/2001GC000280. [DOI] [Google Scholar]
- Martínez-Botí MA, Foster GL, Chalk TB, Rohling EJ, Sexton PF, Lunt DJ, Pancost RD, Badger MPS, and Schmidt DN (2015a), Plio-Pleistocene climate sensitivity evaluated using high-resolution CO2 records, Nature, 518(7537), 49–54, doi: 10.1038/nature14145. [DOI] [PubMed] [Google Scholar]
- Martínez-Botí MA, Marino G, Foster GL, Ziveri P, Henehan MJ, Rae JWB, Mortyn PG, and Vance D (2015b), Boron isotope evidence for oceanic carbon dioxide leakage during the last deglaciation, Nature, 518(7538), 219–222, doi: 10.1038/nature14155. [DOI] [PubMed] [Google Scholar]
- Martínez-Garcia A, Rosell-Melé A, McClymont EL, Gersonde R, and Haug GH (2010), Subpolar link to the emergence of the modern Equatorial Pacific cold tongue, Science, 328(5985), 1550, doi: 10.1126/science.1184480. [DOI] [PubMed] [Google Scholar]
- Martínez-Garcia A, Rosell-Melé A, Jaccard SL, Geibert W, Sigman DM, and Haug GH (2011), Southern Ocean dust-climate coupling over the past four million years, Nature, 476(7360), 312–315, doi: 10.1038/nature10310. [DOI] [PubMed] [Google Scholar]
- Mashiotta T, Lea DW, and Spero HJ (1999), Glacial-interglacial changes in Subantarctic sea surface temperature and δ18O-water using foraminiferal Mg, Earth and Planetary Science Letters, 170(4), 417–432, doi: 10.1016/S0012-821X(99)00116-8. [DOI] [Google Scholar]
- McClymont EL, Rosell-Melé A, Haug GH, and Lloyd JM (2008), Expansion of subarctic water masses in the North Atlantic and Pacific oceans and implications for mid-Pleistocene ice sheet growth, Paleoceanography, 23(4), PA4214, doi: 10.1029/2008PA001622. [DOI] [Google Scholar]
- Medina-Elizalde M, and Lea DW (2005), The mid-Pleistocene transition in the Tropical Pacific, Science, 310(5750), 1009, doi: 10.1126/science.1115933. [DOI] [PubMed] [Google Scholar]
- Medina-Elizalde M, Lea DW, and Fantle MS (2008), Implications of seawater Mg/Ca variability for Plio-Pleistocene tropical climate reconstruction, Earth and Planetary Science Letters, 269(3–4), 585–595, doi: 10.1016/j.epsl.2008.03.014. [DOI] [Google Scholar]
- Millero FJ (1995), Thermodynamics of the carbon dioxide system in the oceans, Geochimica et Cosmochimica Acta, 59(4), 661–677, doi: 10.1016/0016-7037(94)00354-o. [DOI] [Google Scholar]
- Myhre G, Highwood EJ, Shine KP, and Stordal F (1998), New estimates of radiative forcing due to well mixed greenhouse gases, Geophysical Research Letters, 25(14), 2715–2718, doi: 10.1029/98GL01908. [DOI] [Google Scholar]
- Ni Y, Foster GL, Bailey T, Elliott T, Schmidt DN, Pearson PN, Haley BA, and Coath C (2007), A core top assessment of proxies for the ocean carbonate system in surface-dwelling foraminifers, Paleoceanography, 22(3), PA3212, doi: 10.1029/2006PA001337. [DOI] [Google Scholar]
- O’Brien CL, Foster GL, Martínez-Botí MA, Abell R, Rae JWB, and Pancost RD (2014), High sea surface temperatures in tropical warm pools during the Pliocene, Nature Geoscience, 7(8), 606–611, doi: 10.1038/ngeo2194. [DOI] [Google Scholar]
- Paillard D, Labeyrie L, and Yiou P (1996), Macintosh Program performs time-series analysis, Eos Trans. AGU, 77(39), 379–379, doi: 10.1029/96EO00259. [DOI] [Google Scholar]
- Pearson PN, and Palmer M (2000), Atmospheric carbon dioxide concentrations over the past 60 million years, Nature, 406(6797), 695–699, doi: 10.1038/35021000. [DOI] [PubMed] [Google Scholar]
- Pena LD, and Goldstein SL (2014), Thermohaline circulation crisis and impacts during the mid-Pleistocene transition, Science, doi: 10.1126/science.1249770. [DOI] [PubMed] [Google Scholar]
- Perez FF, and Fraga F (1987), The pH measurements in seawater on the NBS scale, Marine Chemistry, 21(4), 315–327, doi: 10.1016/0304-4203(87)90054-5. [DOI] [Google Scholar]
- Petit J-R, Jouzel J, Raynaud D, Barkov NI, Barnola J-M, Basile I, Bender ML, Chappellaz J, Davis M, and Delaygue G (1999), Climate and atmospheric history of the past 420,000 years from the Vostok ice core, Antarctica, Nature, 399(6735), 429–436. [Google Scholar]
- Pierrot DEL, Wallace DWR, and Lewis E (2006), MS Excel program developed for CO2 system calculations, Carbon Dioxide Information Analysis Center. [Google Scholar]
- Raitzsch M, and Hönisch B (2013), Cenozoic boron isotope variations in benthic foraminifers, Geology, 41(5), 591–594, doi: 10.1130/G34031.1. [DOI] [Google Scholar]
- Ravelo AC, and Fairbanks RG (1992), Oxygen Isotopic Composition of Multiple Species of Planktonic Foraminifera: Recorders of the Modern Photic Zone Temperature Gradient, Paleoceanography, 7(6), 815–831, doi: 10.1029/92PA02092. [DOI] [Google Scholar]
- Raymo ME (1994), The initiation of Northern Hemisphere glaciation, Annual Review of Earth and Planetary Sciences, 22(1), 353–383, doi: 10.1146/annurev.earth.22.1.353. [DOI] [Google Scholar]
- Raymo ME, and Nisancioglu KH (2003), The 41 kyr world: Milankovitch’s other unsolved mystery, Paleoceanography, 18(1), 1–6, doi: 10.1029/2002PA000791. [DOI] [Google Scholar]
- Raymo ME, Lisiecki LE, and Nisancioglu K (2006), Plio-Pleistocene ice volume, Antarctic climate, and the global δ18O record, Science, 313(5786), 492. [DOI] [PubMed] [Google Scholar]
- Regenberg M, Nürnberg D, Steph S, Groeneveld J, Garbe-Schönberg D, Tiedemann R, and Dullo W-C (2006), Assessing the effect of dissolution on planktonic foraminiferal Mg/Ca ratios: Evidence from Caribbean core tops, Geochemistry Geophysics Geosystems, 7(7), doi: 10.1029/2005GC001019. [DOI] [Google Scholar]
- Retallack GJ (2009), Greenhouse crises of the past 300 million years, Geological Society of America Bulletin, 121(9–10), 1441–1455, doi: 10.1130/B26341.1. [DOI] [Google Scholar]
- Roy M, Clark PU, Raisbeck GM, and Yiou F (2004), Geochemical constraints on the regolith hypothesis for the middle Pleistocene transition, Earth and Planetary Science Letters, 227(3), 281–296, doi: 10.1016/j.epsl.2004.09.001. [DOI] [Google Scholar]
- Ruddiman WF, Raymo ME, Martinson DG, Clement BM, and Backman J (1989), Pleistocene evolution: Northern hemisphere ice sheets and North Atlantic Ocean, Paleoceanography, 4(4), 353–412, doi: 10.1029/PA004i004p00353. [DOI] [Google Scholar]
- Russon T, Elliot M, Sadekov AY, Cabioch G, Corrège T, and De Deckker P (2010), Inter-hemispheric asymmetry in the early Pleistocene Pacific warm pool, Geophysical Research Letters, 37(11), doi: 10.1029/2010GL043191. [DOI] [Google Scholar]
- Sanyal A, Bijma J, Spero HJ, and Lea DW (2001), Empirical relationship between pH and the boron isotopic composition of Globigerinoides sacculifer: Implications for the boron isotope paleo-pH proxy, Paleoceanography, 16(5), 515–519, doi: 10.1029/2000pa000547. [DOI] [Google Scholar]
- Schiebel R, and Hemleben C (2005), Modern planktic foraminifera, Paläontol. Z, 79(1), 135–148, doi: 10.1007/BF03021758. [DOI] [Google Scholar]
- Schlitzer R (2000), Electronic atlas of WOCE hydrographic and tracer data now available, Eos, Transactions American Geophysical Union, 81(5), 45–45, doi: 10.1029/00EO00028. [DOI] [Google Scholar]
- Schlitzer R (2017), Ocean Data View, http://www.odv.awi.de, 4 ed.
- Schmidt GA et al. (2017), Overestimate of committed warming, Nature, 547(7662), E16–E17, doi: 10.1038/nature22803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki O, Foster GL, Schmidt DN, Mackensen A, Kawamura K, and Pancost RD (2010), Alkenone and boron-based Pliocene pCO2 records, Earth and Planetary Science Letters, 292(1–2), 201–211, doi: 10.1016/j.epsl.2010.01.037. [DOI] [Google Scholar]
- Shackleton NJ, Berger AL, and Peltier W (1990), An alternative astronomical calibration of the lower Pleistocene timescale based on ODP Site 677, Trans. R. Soc. Edinburgh Earth Sci, 81, 251–261, doi: 10.1017/s0263593300020782. [DOI] [Google Scholar]
- Shakun JD, Raymo ME, and Lea DW (2016), An early Pleistocene Mg/Ca-18O record from the Gulf of Mexico: Evaluating ice sheet size and pacing in the 41-kyr world,, 1–17, doi: 10.1002/(ISSN)1944-9186. [DOI] [Google Scholar]
- Shakun JD, Clark PU, He F, Marcott SA, Mix AC, Liu Z, Otto-Bliesner BL, Schmittner A, and Bard E (2012), Global warming preceded by increasing carbon dioxide concentrations during the last deglaciation, Nature, 484(7392), 49–54, doi: 10.1038/nature10915. [DOI] [PubMed] [Google Scholar]
- Shinn RA, and Barron EJ (1989), Climate Sensitivity to Continental Ice-Sheet Size and Configuration, Journal of Climate, 2(12), 1517–1537, doi:. [DOI] [Google Scholar]
- Shipboard Scientific Party (1988), Site 668, in Proceedings of the Ocean Drilling Program, Initial Reports, vol. 108, edited by Ruddiman W, Sarnthein M, Baldauf J, et al. , pp. 931–946, Ocean Drilling Program, College Station, TX. [Google Scholar]
- Sigman DM, Jaccard SL, and Haug GH (2004), Polar ocean stratification in a cold climate, Nature, 428(6978), 59–63, doi: 10.1038/nature02357. [DOI] [PubMed] [Google Scholar]
- Snyder CW (2016), Evolution of global temperature over the past two million years, Nature, 538, 226–228, doi: 10.1038/nature19798. [DOI] [PubMed] [Google Scholar]
- Spero HJ, Mielke KM, Kalve EM, Lea DW, and Pak DK (2003), Multispecies approach to reconstructing eastern equatorial Pacific thermocline hydrography during the past 360 kyr, Paleoceanography, 18(1), doi: 10.1029/2002PA000814. [DOI] [Google Scholar]
- Stults DZ, Wagner-Cremer F, and Axsmith BJ (2011), Atmospheric paleo-CO2 estimates based on Taxodium distichum (Cupressaceae) fossils from the Miocene and Pliocene of Eastern North America, Palaeogeography Palaeoclimatology Palaeoecology, 309(3–4), 327–332, doi: 10.1016/j.palaeo.2011.06.017. [DOI] [Google Scholar]
- Takahashi T et al. (2009), Climatological mean and decadal change in surface ocean pCO2, and net sea–air CO2 flux over the global oceans, Deep-Sea Research II, 56(8–10), 554–577, doi: 10.1016/j.dsr2.2008.12.009. [DOI] [Google Scholar]
- Thunell RC, and Honjo S (1981), Calcite dissolution and the modification of planktonic foraminiferal assemblages, Marine Micropaleontology, 6(2), 169–182, doi: 10.1016/0377-8398(81)90004-9. [DOI] [Google Scholar]
- Tripati AK, Roberts CD, Eagle RA, and Li G (2011), A 20 million year record of planktic foraminiferal B/Ca ratios: Systematics and uncertainties in pCO2 reconstructions, Geochimica et Cosmochimica Acta, 75(10), 2582–2610, doi: 10.1016/j.gca.2011.01.018. [DOI] [Google Scholar]
- Tyrrell T, and Zeebe RE (2004), History of carbonate ion concentration over the last 100 million years, Geochimica et Cosmochimica Acta, 68(17), 3521–3530, doi: 10.1016/j.gca.2004.02.018. [DOI] [Google Scholar]
- Tziperman E, and Gildor H (2003), On the mid-Pleistocene transition to 100-kyr glacial cycles and the asymmetry between glaciation and deglaciation times, Paleoceanography, 18(1), 1–1–1–8, doi: 10.1029/2001pa000627. [DOI] [Google Scholar]
- Waddell LM, Hendy IL, Moore TC, and Lyle MW (2009), Ventilation of the abyssal Southern Ocean during the late Neogene: A new perspective from the subantarctic Pacific, Paleoceanography, 24(3), 1769–15, doi: 10.1029/2008PA001661. [DOI] [Google Scholar]
- Wang Y, Momohara A, Wang L, Lebreton-Anberrée J, and Zhou Z (2015), Evolutionary History of Atmospheric CO2 during the Late Cenozoic from Fossilized Metasequoia Needles, edited by Wong WO, PLOS ONE, 10(7), 1–15, doi: 10.1371/journal.pone.0130941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weldeab S, Schneider RR, and Kölling M (2006), Comparison of foraminiferal cleaning procedures for Mg/Ca paleothermometry on core material deposited under varying terrigenous-input and bottom water conditions, Geochemistry Geophysics Geosystems, 7(4), doi: 10.1029/2005GC000990. [DOI] [Google Scholar]
- Witze A (2015), Super-fast drills hunt for oldest ice, Nature, 526, 618–619, doi: 10.1038/526618a. [DOI] [PubMed] [Google Scholar]
- York D, Evensen NM, Martıńez ML, and De Basabe Delgado J (2004), Unified equations for the slope, intercept, and standard errors of the best straight line, American Journal of Physics, 72(3), 367–375, doi: 10.1119/1.1632486. [DOI] [Google Scholar]
- Zhang YG, Pagani M, Liu Z, Bohaty SM, and DeConto R (2013), A 40-million-year history of atmospheric CO2, Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences, 371, 20130096–20130096, doi: 10.1098/rsta.2013.0096. [DOI] [PubMed] [Google Scholar]
- Zweng MM et al. (2013), World Ocean Atlas 2013, Vol. 2: Salinity, in NOAA Atlas NESDIS 73, edited by Levitus S and Mishonov A, p. 39. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


